Abstract
In this case study, a man at the onset of Alzheimer’s disease (AD) was enrolled in a cognitive treatment program based upon spatial navigation in a virtual reality (VR) environment. We trained him to navigate to targets in a symmetric, landmark-less virtual building. Our research goals were to determine whether an individual with AD could learn to navigate in a simple VR navigation (VRN) environment and whether that training could also bring real-life cognitive benefits. The results show that our participant learned to perfectly navigate to desired targets in the VRN environment over the course of the training program. Furthermore, subjective feedback from his primary caregiver (his wife) indicated that his skill at navigating while driving improved noticeably and that he enjoyed cognitive improvement in his daily life at home. These results suggest that VRN treatments might benefit other people with AD.
Keywords: virtual reality, Alzheimer, dementia, cognitive rehabilitation, spatial navigation
Introduction
“Use it or Lose it” has widely been adopted as the battle cry of doctors and health care professionals around the world with regard to muscle atrophy, cardiovascular health, and, more recently, neurocognitive health. Alzheimer’s disease (AD) causes accelerated destruction of neurons and interneuronal links in the brain, which compromises the affected individual’s memory and other cognitive abilities. AD is often characterized by buildups of amyloid plaques and neurofibrillary tangles in the brain; however, in some cases, this pathology may be present without corresponding neurocognitive decline.1 It is thought that these inconsistencies occur when a person has a large number of redundant neural pathways or neural functionality, otherwise called a “cognitive reserve”. This cognitive reserve is thought to be built up by pursuing intellectually stimulating activities including education, staying abreast of current events, frequent socializing, and reading.1 It would seem that learning to navigate in new environments can also strengthen cognitive reserve. A study done with laboratory mice showed that performing navigational training tasks could reduce AD pathology in the hippocampus,2 the area of the brain that is thought to be important for spatial memory and navigation.3 We wondered whether navigational exercises strengthened the cognitive reserve of the mice and hypothesized that similar exercises could help individuals with AD to improve/maintain their cognition, in general, and their spatial cognition (specifically, spatial navigation) in particular. To investigate this hypothesis, we refined our existing virtual reality navigation (VRN) task4 to be used for neurocognitive treatment of an individual at the onset stage of AD.
The idea of testing for neurological damage (as opposed to treating it) using VRN tools is not new; it has been studied by our team4,5,6 as well as other groups.3,7,8 The Morris water task was popularized in spatial navigation experiments using rodents9 and is a popular model for VR-based spatial navigation assessments. This is because it has been shown to be sensitive to damage to the hippocampus.3 In general, VR-based experiments are viewed as screening tools for emerging AD and may be combined with pen-and-paper-based screening tools8 such as the Montreal Cognitive Assessment (MoCA)10 or Mini Mental State Examination (MMSE).11 Such screening tools are usually used to determine whether more in-depth diagnostic assessments are needed. Both the MoCA and the MMSE are questionnaire-style assessments that probe language, memory, attention, orientation, and visuospatial ability. Both assessments score participants out of a maximum of 30 points, with lower numbers indicating increasingly severe cognitive difficulty. We use the MoCA as a quantifier of cognitive ability in our other works4,5,6 because it has been found to be more sensitive to mild impairments than the MMSE.12
In addition to testing, virtual environments have been used for functional cognitive rehabilitation. Kober et al13 used VRN training to treat patients with focal brain lesions during five training sessions. The patients in that work had a wide variety of lesions, but there were four particular patients with some combination of memory impairment, dementia, and/or hippocampus damage. Three of those cases exhibited improvement in spatial cognition following treatment.
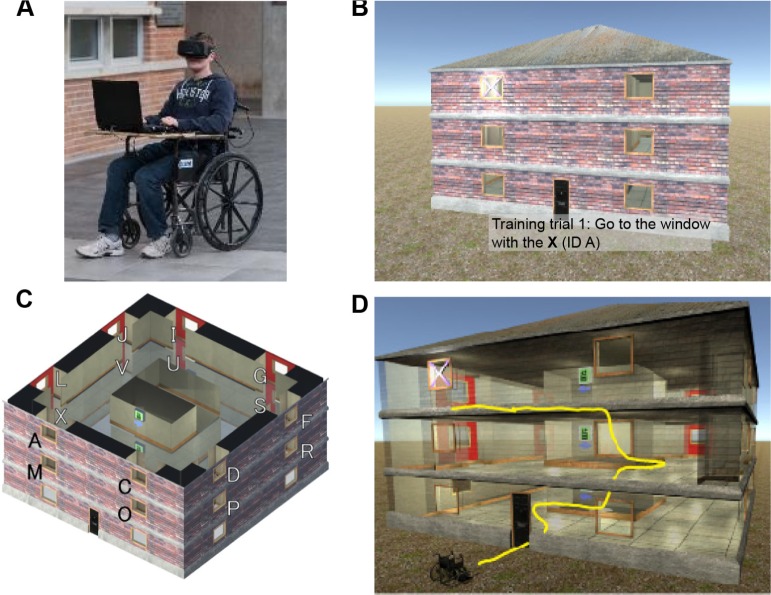
Nearly all VR systems in the literature use standard interaction devices such as a joystick (or keyboard input) and desktop display,3,7,8,14–17 but this interaction paradigm has been shown to baffle elderly people, since they are generally inexperienced with using such devices.18 To avoid biasing the results of VRN training (ie, assuming a person has navigation difficulties when they merely were confused by the input device), we designed a custom input system based on a wheelchair.18 Our custom wheelchair captures a user’s motion in the real world and translates it to the VRN environment. This makes interaction with the VRN environment natural and intuitive; to move in the VRN environment, the user simply sits in the chair and shuffles around; the head-mounted display (HMD) allows the user to look around and feel immersed in the VRN environment. This apparatus is illustrated in Figure 1A.
Figure 1.
VRN Building spatial navigation exercise.
Notes: A) Author Paul White uses our custom wheelchair, which captures a user’s real-world motion and translates it to the VRN environment, without the need of a joystick. B) The target window is marked with an X. C) Each of the 16 second- and third-floor windows is assigned a unique letter ID. Window A is the left-hand window at the front of the house on the third floor. D) The elevators to move between floors force participants to turn around. This perturbs participants’ cognitive map.
Another possible side benefit of using our custom wheelchair as an input device may be elevated levels of brain-derived neurotrophic factor (BDNF). BDNF is a protein that has been associated with improved cognitive functions such as synaptic plasticity and memory formation and has been found in reduced quantities in AD patients as compared to healthy controls.19 BDNF levels in the hippocampus have also been shown to increase during exercise in laboratory mice,20 which means that physically moving through a virtual environment could promote cognitive processes more effectively than using a standard input device, in addition to being more intuitive.
In this paper, we report on our pilot study results of using a VRN environment for training an individual at the onset stage of AD over the course of 7 consecutive weeks. We measured changes in spatial navigation by using our VRN task and real-world feedback from the individual’s wife regarding his navigation while driving. We also measured general cognition changes using a neuropsychological assessment (MoCA) and further subjective feedback from the individual’s wife. This research was approved by the University of Manitoba Health Research Ethics Board, and conducted in accordance with the principles of the Declaration of Helsinki. The study participant gave written, informed consent to participate in the experiment.
Methods
The VRN environment used in this work was created using the Unity 5 game engine, and displayed using an Oculus Rift DK2 (runtime 0.6.0.1). It ran on a laptop with an NVIDIA GTX 970 m graphics processor. The participant interacted with the VRN environment by using our custom wheelchair.18 The treatment protocol consisted of 45-minute training sessions, three times per week for 7 consecutive weeks. We evaluated the participant’s performance by means of the MoCA and by studying the trajectories we recorded while he navigated in the VRN environment. Our VRN environment that detects deficits in spatial navigation is described in our previous works,4,6 but since we used the system in this work with some modifications, it is described here briefly.
Overview of VRN Building
The VRN Building (also referred to in our previous work as the VRN House)4 assessment is used to study human navigation in a landmark-less, symmetric three-story building in a series of eight trials. At the beginning of each trial, the participant is shown an external view of the building, where a randomly selected window is marked with an X (Fig. 1B). The participant is instructed to enter the building and find the target window from the inside. When the participant enters the building, the X on the target window is made invisible to prevent participants from rediscovering the target window if they have forgotten its location or become disoriented. When the participant enters the correct room, the X reappears, the participant is congratulated, and the trial ends. The layout of the building intentionally forces participants to make turns to get to higher floors; this perturbs the participant’s cognitive map and makes the assessment more challenging, especially for people with navigation impairments. As can be seen in Figure 1C and D, the participant needs to turn around 180 degrees to reach the third floor from the second floor, which adds an extra layer of complexity. Figure 1D illustrates an example of a path to get to a room on the third floor.
In our regular assessment, we use a custom algorithm6 to calculate an “error score” for the participant’s sequence of window visits; higher scores indicate increasing difficulty in spatial navigation. The error score is computed by classifying the navigation errors the participant makes, that is, choosing incorrect windows.
In this work, we analyzed the number of errors made by the participant during his treatment sessions and looked for negative trends to show improvement. We also looked at the specific types of errors described in our other work:
Wall errors: selected window is on an incorrect wall (eg, participant incorrectly chooses the North wall instead of the West wall). We observed this type of error more frequently than the others6 and believe that it indicates that the participant is disoriented by the floor transitions.
Floor errors: selected window is on an incorrect floor (eg, third floor instead of second floor).
Left/right errors: selected window is on opposite side of the correct target (eg, if the target window is Window A in Fig. 1C, Window F would count as a left/right error because it is on the right half of its wall, while Window A is on the left half of its wall).
Treatment protocol
Our participant in this work was a retired 74-year-old male, who lived with his wife. He had received a Master’s degree in Social work and had worked in that field throughout his career. In the time before our study, he was physically and socially active in his life and capable of living independently. He was diagnosed with mild cognitive impairment (MCI) with probable development of AD. He reported symptoms including short-term memory loss and an increased difficulty remembering directions while driving. Furthermore, he had scored 24 on the MoCA v7.1. In the time leading up to our treatment regimen, he was only comfortable driving his vehicle in familiar areas. He had a family history of AD on his father’s side.
We recruited the participant from our ongoing VRN Building assessment study,4 which he had previously volunteered for twice within the previous 2 years. His first error score in 2013 was 66%, and his MoCA score was 28/30. Two years later, during his second assessment, his error score increased to 72% and his MoCA score dropped to 24/30. These data, collected 6 weeks prior to beginning treatment, served as our baseline. It is important to note that we consider an initial error score of more than 50% in the VRN Building assessment combined with an increase in error score to be a warning sign of AD (normally, people’s error scores decrease in subsequent assessments). Since the participant’s MoCA score also decreased, we referred him to a neuropsychiatrist who upon further assessment diagnosed the participant with MCI, with probable development of AD. We have consistently found that the VRN Building assessment is very difficult for AD patients; in our experience, all participants diagnosed with AD fail to find any of the targets in the VRN Building. Since the participant was still at a very early stage of the disease progression and was struggling with the VRN Building assessment, we decided to use it as a treatment for this work.
The treatment period was split into two phases: Supported Training and Independent Training. These phases differed simply in that the Supported Training phase was restricted in certain ways, while the Independent Training phase was repeated applications of the standard VRN Building assessment. The Supported Training phase was restricted in the following ways:
We restricted the targets to only those on the second floor to avoid the second rotational perturbation associated with going to the third floor.
Each window was visited twice each trial. The first time, we gave the participant hints and guidance during his navigation; the second time he was not assisted unless the examiner believed he had clearly become lost. As we progressed through the Supported Training phase, we decided to swap the ordering, so that the first window would be un-assisted and the second one would be assisted (if needed). During the third week, once the participant was more comfortable with the second-floor windows, we began including target windows on the third floor.
The treatment advanced to the Independent Training phase when the participant demonstrated a high degree of mastery of the trials in the Supported Training phase. During the Independent Training phase, we performed the standard VRN Building assessment (ie, pseudorandomly selected windows on both the second and the third floors with no repetitions) as many times as we could within our 45-minute session. This worked out to approximately 12–15 targets. Once the participant was able to find eight targets consecutively with no errors, we decided to limit the training to eight target windows to reduce the participant’s time commitment (the regular VRN Building assessment uses eight target windows). In order to track performance across the Independent Training phase and the Supported Training phase, we compared the number of errors, rather than the error score, since the error score may not be applicable to data collected during the Supported Training phase. A benefit of the two-phase treatment system is that withholding the third-floor windows allows us to control for practice effects and see if training solely on the second floor allowed the participant to navigate more accurately on the third floor.
In addition to tracking navigation errors, we tracked the participant’s progress using the MoCA. We performed three MoCA assessments during the course of treatment and two follow-ups: once before starting, then during the program at the 4-week mark, and at the end of the seventh week. We performed follow-up evaluations 5 weeks and 28 weeks after the end of the program. We also asked the participant’s wife to keep a log of his daily activities and driving performance.
In summary, we used four performance metrics to track spatial navigation and overall cognition in this work:
We tracked the participant’s navigation errors in the VRN Building over the duration of the treatment and looked for changes in the overall number of errors, as well as particular types of errors (wall errors, floor errors, and left/right errors).
We tracked his ability to navigate to third-floor windows before treatment, after training only on the second floor, and during two follow-up sessions.
We scored his overall cognition using the MoCA at various points during the treatment and during two follow-up sessions.
The participant’s wife kept a journal commenting on the participant’s navigation while driving and on his “real-world” cognitive health at home.
Results
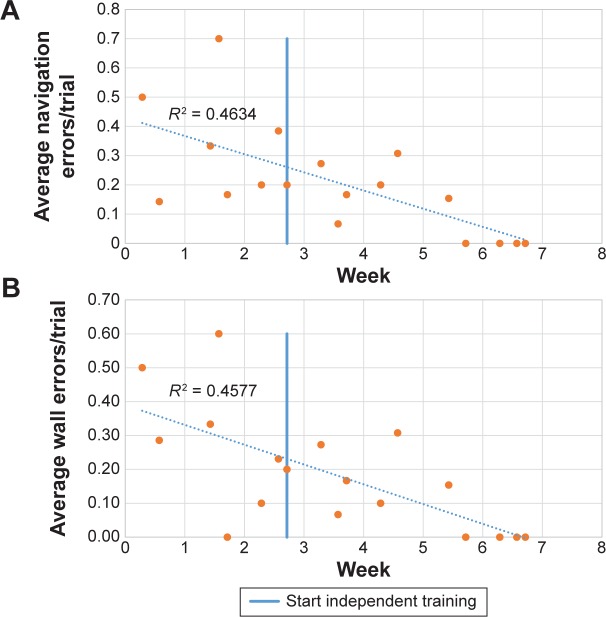
The participant showed a substantial improvement in navigating in the VRN Building assessment. This can be clearly observed by comparing the baseline assessment (where the participant could not reach more than one target window without error) with the end of the training program, when he could reliably locate eight randomly selected windows with no errors. Figure 2 shows the steady improvement of the participant’s spatial navigation by plotting the number of navigation errors for each session. Since each session had a different number of trials, in Figure 2, the number of errors is plotted as the number of navigation errors divided by the total number of trials for that session. Figure 2A shows a plot of the total navigation errors, and Figure 2B specifically shows wall-type navigation errors.
Figure 2.
The participant’s navigation errors over time. The number of errors is divided by the number of trials during each session, because as the treatment progressed, the participant visited more windows within the one-hour time slot. Note a ceiling effect toward the end of training, where the participant was able to find all target windows with no errors. (A) Total navigation errors. (B) Incorrect wall-type navigation errors.
Table 1 shows the participant’s navigation errors at certain milestones, when navigating to four specific windows on the third floor of the VRN Building. These window IDs correspond to the window IDs shown in Figure 1C. After training on the second floor, the participant was able to correctly locate third-floor windows that he had not navigated to since the baseline assessment, and a follow-up assessment showed that this effect persisted 5 weeks after ending the training sessions. However, this improvement appeared to have worn off 28 weeks after training.
Table 1.
The participant’s VRN Building navigation errors for four of the third-floor windows.
| WINDOW ID (FIG. 1C) | PRE-TRAINING (6 WEEKS PRIOR TO TREATMENT) | AFTER SECOND FLOOR TRAINING (MIDDLE OF WEEK 3) | INDEPENDENT TRAINING (FINAL DAY) | 5 WEEKS AFTER TRAINING | 28 WEEKS AFTER TRAINING |
|---|---|---|---|---|---|
| C | 3 | 0 | 0 | 0 | 0 |
| D | 0 | 0 | 0 | 0 | 3 |
| G | 3 | 0 | 0 | 0 | 3 |
| J | 1 | 2 | 0 | 0 | 0 |
Notes: Each column contains the number of navigation errors the participant made the first time he navigated to a window following a particular milestone. The “Pre-training” column contains the four third-floor windows that the participant navigated to during the baseline assessment. We ensured that during the follow-up trials, the same windows were visited as during the baseline assessment.
The participant’s MoCA scores remained relatively consistent during the treatment. This progression is illustrated in Table 2. The MoCA defines scores of 25 or less to be indicative of cognitive impairment, and since the participant’s scores were at or below the borderline of 25, he was classified as having MCI. Since the participant was still at a relatively high level of cognitive ability, most assessments were unable to capture any changes in him due to the ceiling effect. In fact, Table 2 shows that the only area where the participant had difficulty on the MoCA test was the “Delayed Recall” (or “Memory”) section. We did not expect to see much improvement in that area because the focus of our training was mainly in spatial navigation. Therefore, in order to determine whether the treatment resulted in any real-life improvements, we asked the participant’s wife to keep a log of her observations.
Table 2.
The participant’s MoCA scores.
| WEEK | MoCA VARIANT | OVERALL MoCA SCORE | VISUOSPATIAL/EXECUTIVE (/5) | DELAYED RECALL (/5) | ALL OTHER (/20) | ||
|---|---|---|---|---|---|---|---|
| NO CUE | CATEGORY CUE | MULTIPLE CHOICE CUE | |||||
| Baseline (6 weeks prior to start) | 7.1 original | 24 | 5 | 0 | 2 | 3 | 19 |
| Week 4 | 7.1 original | 25 | 4 | 0 | 2 | 2 | 19 |
| Week 7 | 7.1 original | 26 | 5 | 2 | 2 | 1 | 19 |
| 5-week follow up | 7.2 variant | 25 | 5 | 0 | 3 | 2 | 20 |
| 28-week follow up | 7.1 original | 23 | 5 | 1 | 1 | 3 | 17 |
The participant’s wife noted improvements in his daily living functions, in particular in his orientation skills while driving, as well as his mood. Furthermore, we subjectively noted that during the earliest treatment session, the participant would make self-deprecating comments concerning his mental state (eg, “my dopey brain”), but as treatment progressed and his scores improved, these comments ceased.
Discussion
The VRN treatment, presented in this paper, has shown some benefits for one person at an early stage of AD, but the benefits of such a treatment protocol cannot be generalized until they are confirmed in a number of other individuals at the early stages of the AD. The main benefit of the presented VRN system here as compared with other designs is that it addresses some limitations inherent to the so-called “ambulatory” VR systems.
The overall results of this case study suggest that people at the early stages of AD can learn to navigate paths in a suitably immersive VR system, and the learned paths may translate to overall real-world spatial navigation skill, as indicated by our participant’s wife. Furthermore, the results illustrated in Table 1 suggest that a training period as short as 4 weeks might be enough to achieve significant improvement, and Figure 2B shows that the participant’s wall-type errors decreased after the conclusion of the Supported Training phase. The participant was able to translate the orientation skills (ie, correctly identify the different walls) he learned on the second floor to the third floor, which he had not navigated on since the baseline assessment nearly 2 months previously. That being said, 4 weeks may be overly aggressive, since it actually took 6 weeks until the participant could complete the assessment with 0 errors.
Although Table 1 shows that the participant’s spatial navigation performance had not deteriorated even 5 weeks after training, we observed decline in his spatial navigation performance at 28 weeks. Performance could possibly be maintained by having periodic “booster” sessions to maintain and reinforce any cognitive reserve improvements.
During the treatment, the participant’s MoCA increased by 1–2 points; however, we caution against considering this to be a significant improvement because it is normal for repeated evaluations to vary by small amounts.10,21 We suspect that this improvement could also be explained by learning effects, since the three consecutive MoCA tests in Table 2 used the same MoCA variant with the same memory words; as a case in point, in the first follow-up MoCA test, we used a different variant with different memory words and noted that the participant’s score remained similar. Table 2 also shows that most of the points that the participant lost were in the Delayed Recall section, which tests memory. We reiterate that this was not the focus of our training program; our focus was on spatial cognition, but the MoCA questions that assessed visuospatial reasoning showed a ceiling effect for the participant.
Due to a lack of other widely accepted objective assessments that would not suffer from ceiling effects when measuring spatial cognition at the time of this study, we opted to document the various subjective benefits that our treatment may have caused. During the course of his treatment, the participant’s wife kept a journal of his behavior at home and noted that he appeared to be happier and more confident in his day-to-day activities, particularly in driving and remembering directions, and was no longer asking his wife for direction after beginning his treatments. His mood was also significantly improved to the extent that he adopted a healthier life style, for example, reducing alcohol intake and practicing brain exercises.22
Since using an HMD with our VRN system removes the typical bias exhibited by elderly people inexperienced with using computer systems, it allows us to study spatial navigation more effectively than other VR designs. Our wheelchair paradigm allows people to physically move about, which substantially reduces simulator sickness,18 especially in comparison to stationary VR systems.7,8,23,24 Indeed, the vestibular stimulation that our system provides is a key differentiator among other works, and this additional stimulation may contribute to path integration and environment encoding at a low level.3
We have observed quantitative and qualitative benefits in this case study, which encourage further investigations with larger samples.
Conclusion
We examined the effectiveness of a VR-based spatial navigation exercise in a simple case study of a 74-year old male at the onset stage of AD and found evidence to suggest that there may be cognitive benefits to VR navigational training. We observed quantitative improvement in our participant’s ability to navigate in the designed VRN environment over a period of approximately 7 weeks of training; also, the participant’s wife observed qualitative improvements in his navigation while driving. This type of treatment is noninvasive and leverages natural learning strategies to reinforce neural pathways, thus possibly creating a larger cognitive reserve.
Acknowledgments
Thanks to Ahmad Byagowi for his design of the VRN wheelchair, and thanks to our volunteer for participating in the case study.
Footnotes
ACADEMIC EDITOR: Lora Talley Watts, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1619 words, excluding any confidential comments to the academic editor.
FUNDING: This work was supported by the National Science and Engineering Research Council of Canada (NSERC). The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert single-blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: ZM. Analyzed the data: PJFW. Wrote the first draft of the manuscript: PJFW. Contributed to the writing of the manuscript: ZM, PJFW. Agree with manuscript results and conclusions: ZM. Jointly developed the structure and arguments for the paper: ZM, PJFW. Made critical revisions and approved final version: ZM. Both authors reviewed and approved the final manuscript.
REFERENCES
- 1.Stern Y. Cognitive reserve. Neuropsychologia. 2010;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billings LM, Green KN, McGaugh JL, LaFerla FM. Learning decreases Aß*56 and tau pathology and ameliorates behavioral decline in 3xTg-AD mice. J Neurosci. 2007;27(4):751–761. doi: 10.1523/JNEUROSCI.4800-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132(1):77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 4.Byagowi A, Moussavi Z. Design of a virtual reality navigational (VRN) experiment for assessment of egocentric spatial cognition. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:4812–4815. doi: 10.1109/EMBC.2012.6347070. [DOI] [PubMed] [Google Scholar]
- 5.Zen D, Byagowi A, Garcia M, Kelly DM, Lithgow B, Moussavi Z. The perceived orientation in people with and without Alzheimer’s; International IEEE/EMBS Conference on Neural Engineering, NER; San Diego, CA: IEEE; 2013. pp. 460–463. [Google Scholar]
- 6.Pouya OR, Byagowi A, Kelly DM, Moussavi Z. Introducing a new age and cognition-sensitive measurement for assessing spatial orientation using a landmark-less virtual reality navigational task. Q J Exp Psychol. 2016:1–35. doi: 10.1080/17470218.2016.1187181. [DOI] [PubMed] [Google Scholar]
- 7.Lee J-Y, Kho S, Bin YH, et al. Spatial memory impairments in amnestic mild cognitive impairment in a virtual radial arm maze. Neuropsychiatr Dis Treat. 2014;10:653–660. doi: 10.2147/NDT.S58185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werner P, Rabinowitz S, Klinger E, Korczyn AD, Josman N. Use of the virtual action planning supermarket for the diagnosis of mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27(4):301–309. doi: 10.1159/000204915. [DOI] [PubMed] [Google Scholar]
- 9.Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12(2):239–260. [Google Scholar]
- 10.Nasreddine ZS. MoCA Montreal—Cognitive Assessment. [Accessed March 1, 2016]. Available at: http://www.mocatest.org/
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Lessig S, Nie D, Xu R, Corey-Bloom J. Changes on brief cognitive instruments over time in Parkinson’s disease. Mov Disord. 2012;27(9):1125–1128. doi: 10.1002/mds.25070. [DOI] [PubMed] [Google Scholar]
- 13.Kober SE, Wood G, Hofer D, Kreuzig W, Kiefer M, Neuper C. Virtual reality in neurologic rehabilitation of spatial disorientation. J Neuroeng Rehabil. 2013;1999;10:17. doi: 10.1186/1743-0003-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71(12):888–895. doi: 10.1212/01.wnl.0000326262.67613.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weniger G, Ruhleder M, Lange C, Wolf S, Irle E. Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia. 2011;49(3):518–527. doi: 10.1016/j.neuropsychologia.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann M, Ro A, Schwarz W, et al. Interactive computer-training as a therapeutic tool in Alzheimer’s disease. Compr Psychiatry. 2003;44(3):213–219. doi: 10.1016/S0010-440X(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 17.Klinger E, Chemin I, Lebreton S, Marie RM. A virtual supermarket to assess cognitive planning. Annu Rev CyberTherapy Telemed. 2004;2:49–57. [Google Scholar]
- 18.Byagowi A, Mohaddes D, Moussavi Z. Design and application of a novel virtual reality navigational technology (VRNChair) J Exp Neurosci. 2014;8:7–14. doi: 10.4137/JEN.S13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin XY, Cao C, Cawley NX, et al. Decreased peripheral brain-derived neurotrophic factor levels in Alzheimer’s disease: a meta-analysis study (N = 7277) Mol Psychiatry. 2016:1–9. doi: 10.1038/mp.2016.62. [DOI] [PubMed] [Google Scholar]
- 20.Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife. 2016;5:1–21. doi: 10.7554/eLife.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman TTA, El Gaafary MM. Montreal cognitive assessment Arabic version: reliability and validity prevalence of mild cognitive impairment among elderly attending geriatric clubs in Cairo. Geriatr Gerontol Int. 2009;9(1):54–61. doi: 10.1111/j.1447-0594.2008.00509.x. [DOI] [PubMed] [Google Scholar]
- 22.Tere Garcia-Campuzano M, Virues-Ortega J, Smith S, Moussavi Z. Effect of cognitive training targeting associative memory in the elderly: a small randomized trial and a longitudinal evaluation. J Am Geriatr Soc. 2013;61(12):2252–2254. doi: 10.1111/jgs.12574. [DOI] [PubMed] [Google Scholar]
- 23.Zakzanis KK, Quintin G, Graham SJ, Mraz R. Age and dementia related differences in spatial navigation within an immersive virtual environment. Med Sci Monit. 2009;15(4):CR140–CR150. [PubMed] [Google Scholar]
- 24.Brooks JO, Goodenough RR, Crisler MC, et al. Simulator sickness during driving simulation studies. Accid Anal Prev. 2010;42(3):788–796. doi: 10.1016/j.aap.2009.04.013. [DOI] [PubMed] [Google Scholar]