Abstract
Metabolomic studies have identified several metabolites associated with type 2 diabetes (T2D) in populations of European ancestry. East Asians, a population of particular susceptibility to T2D, were generally not included in previous studies. We examined the associations of plasma metabolites with risk and prevalence of T2D in 976 Chinese men and women (40–74 years of age) who were participants of two prospective cohort studies and had no cardiovascular disease or cancer at baseline. Sixty-eight prevalent and 73 incident T2D cases were included. Non-targeted metabolomics was conducted that detected 689 metabolites with known identities and 690 unknown metabolites. Multivariable logistic and Cox regressions were used to evaluate the associations of standardized metabolites with diabetes risk and prevalence. We identified 36 known metabolites and 10 unknown metabolites associated with prevalent and/or incident T2D at false discovery rate <0.05. The known metabolites are involved in metabolic pathways of glycolysis/gluconeogenesis, branched-chain amino acids, other amino acids, fatty acids, glycerophospholipids, androgen, and bradykinin. Six metabolites showed independent associations with incident T2D: 1,5-anhydroglucitol, mannose, valine, 3-methoxytyrosine, docosapentaenoate (22:5n3), and bradykinin-hydroxy-pro(3). Each standard deviation increase in these metabolites was associated with a 40–150 % change in risk of developing diabetes (30–80 % after further adjustment for glucose). Risk prediction was significantly improved by adding these metabolites in addition to known T2D risk factors, including central obesity and glucose. These findings suggest that hexoses, branched-chain amino acids, and yet to be validated novel plasma metabolites may improve risk prediction and mechanistic understanding of T2D in Chinese populations.
Keywords: Metabolomics, Type 2 diabetes, Epidemiology, Prospective cohort study, Chinese populations
1 Introduction
Type 2 diabetes (T2D) is an increasing global threat to public health and economic growth, particularly in developing countries (Hu 2011). Fueled by rapid urbanization and changes in diet and lifestyle, its prevalence has increased dramatically in China during recent decades (Ma et al. 2014). In 1980, less than 1 % of the Chinese population was affected by T2D; whereas, the rate increased to 5.5 % in 2001 and reached an alarming 11.6 % in 2010 (Xu et al. 2013b). With another 500 million adults being pre-diabetic, the prevalence of T2D in China is expected to have a continuous and substantial increase (The World Bank 2011). Diet and lifestyle modifications have been shown to effectively slow the development and improve the complications of diabetes (Li et al. 2008); however, recommendations for behavioral modifications to prevent T2D in the general population have not yet yielded measurable success (Chan et al. 2014). Therefore, understanding the biological mechanism(s) in the T2D pathogenesis and identifying high risk populations are critically important to curtail the growing burden of diabetes in China and worldwide.
A few population-based, prospective cohort studies have assessed T2D risk using established risk factors and emerging biomarkers (Noble et al. 2011). By measuring thousands of small molecules in biological samples simultaneously, metabolomics draws a comprehensive picture of genotypic and phenotypic variations and external exposures to food, microorganisms, medications, and other environmental factors, as well as their interactions (Bain et al. 2009). It is a high-throughput, high-sensitivity, and multi-factorial approach with great potential to discover new markers reflecting metabolic perturbations inside the human body. Using this technology, several metabolites, such as branched-chain amino acids (BCAAs), hexoses, and certain lipids, have been found to be associated with T2D risk (Lu et al. 2013; Suhre 2014). These metabolites provide information beyond traditional risk predictors and bring novel insights into diabetes pathogenesis.
Racial/ethnic differences exist in the susceptibility and pathophysiology of T2D (Ma et al. 2014). For example, Chinese people develop diabetes at a relatively low body mass index (BMI) and young age (Ma and Chan 2013). East Asians have higher insulin sensitivity but a much lower insulin response than Caucasians and Africans (Kodama et al. 2013). To date, most metabolomic studies have been conducted in populations with European ancestry. A few studies conducted in Singapore Chinese and South Asian adults, mostly among men, suggested that perturbance in amino acid homeostasis may underlie insulin resistance and diabetes onset in these Asian populations (Tai et al. 2010; Xu et al. 2013a; Tillin et al. 2015). To our knowledge, no studies have investigated the global metabolomic profiling in relation to T2D risk in a longitudinal study among East Asian populations.
Using a non-targeted metabolomics approach, we systematically examined the associations of plasma metabolites with both prevalent and incident T2D in 976 Chinese men and women between 40 and 74 years of age, who had no cardiovascular disease or cancer at baseline.
2 Subjects and methods
2.1 Study population
Participants of our study were drawn from the Shanghai Women’s Health Study (SWHS) and the Shanghai Men’s Health Study (SMHS), two population-based prospective cohort studies conducted in urban communities in Shanghai, China. The SWHS recruited 74,941 women during 1997–2000 (response rate: 93 %) and the SMHS recruited 61,482 men during 2002–2006 (response rate: 75 %) (Shu et al. 2015; Zheng et al. 2005). Similar methods and questionnaires were used in both studies. At baseline in-person interviews, information on sociodemographics, diet, lifestyle, and medical history was obtained; height, body weight, and waist circumference were measured. In both studies, a 10 ml blood sample was collected using an EDTA tube. After collection, blood samples were kept at 4 °C and processed within 6 h, and then plasma was aliquoted to 2 ml vials for long-term storage at −70 °C until analysis. The studies were approved by the Institutional Review boards of the Shanghai Cancer Institute and Vanderbilt University. Informed consent was obtained from all participants.
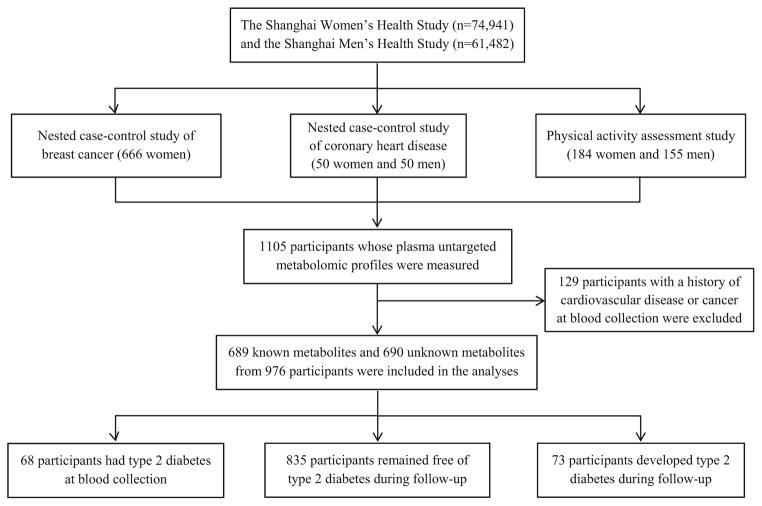
The present analysis included men and women originally selected for three research projects within the SWHS and SMHS: a nested case–control study of breast cancer, of coronary heart disease (CHD), and a methodological study for physical activity measurement. The breast cancer study consists of 333 incident post-menopausal breast cancer cases and 333 controls, individually matched by age, baseline menopausal status, and date and time of blood draw. The CHD study includes 25 men and 25 women who developed incident CHD and 50 controls, individually matched by sex, age, and date and time of blood draw. The physical activity study included 155 men and 184 women who participated in a 1-year study that collected four weekly accelerometer measures and two blood samples. Blood samples drawn at study enrollment, i.e., prior to diagnosis of cancer or CHD, were used in the current study. Participants with a history of any cardiovascular disease or cancer at blood collection were excluded, leaving a total of 976 participants for the present study. A flowchart of study sampling is shown in Fig. 1.
Fig. 1.
Flowchart of study sampling
2.2 Diabetes assessment
Participants were asked about their diabetes diagnosis at baseline and at subsequent home visits conducted every 2–3 years. Diabetes was defined if the participants reported having been diagnosed with T2D by a physician and also reported meeting at least one of the following criteria: (1) fasting glucose ≥7.0 mmol/L; (2) oral glucose tolerance test ≥11.1 mmol/L; (3) use of antidiabetic medication. Two participants who did not report a diabetes diagnosis at baseline but had significant detectable metformin in their plasma [beyond 3 standard deviations (SDs) of log-transformed mean] were also considered prevalent cases.
2.3 Metabolomics measurement
Plasma metabolites were measured by Metabolon Inc. Its platform and protocol have been described elsewhere (Evans et al. 2009). Ultra-high-performance liquid chromatography and gas chromatography coupled with tandem mass spectrometry were used. The mass spectra peaks were searched in a chemical reference library including 2500 standard metabolites to identify the corresponding metabolite and assess the relative quantity. Approximately 30 samples (one batch) were assayed every day. To account for batch differences, metabolite concentrations were standardized by the median of non-missing values in each batch. The concentrations below detectable limits were imputed with the minimum of non-missing values. We previously reported the reproducibility of the metabolomics platform used in the current study (Sampson et al. 2013; Moore et al. 2014). The median of technical intra-class correlations was 0.84 (interquartile range 0.63, 0.94) and the median of intra-assay coefficients of variation was 0.14 (interquartile range 0.09, 0.21).
In the breast cancer study, 505 metabolites with known identities and 215 metabolites with unknown identities were detected. In the CHD study, 312 known and 305 unknown metabolites were detected. The physical activity study contains two waves of measurements. There were 274 known and 192 unknown metabolites in the 1st wave; and 603 known and 479 unknown metabolites in the 2nd wave. All together, these four rounds of measurements captured 689 known metabolites and 690 unknown metabolites. Among them, 333 metabolites were available in all four measurements; 155 in three; 297 in two; and 594 were found in one measurement. We analyzed all known and unknown metabolites using the following statistical methods.
2.4 Statistical analysis
Metabolite concentrations were log-transformed and normalized by the mean and SD within each round of measurement: . Baseline characteristics were compared between non-diabetes and prevalent diabetic participants at baseline and between participants developing incident diabetes and those remaining diabetes free at the end of follow-up using student t and χ2 tests for continuous and categorical variables, respectively.
For risk of prevalent diabetes, we performed logistic regression, stratified by study and adjusted for age, sex, cigarette smoking, waist circumference, history of hypertension, time of blood draw, time interval since last meal, and assay batch. Further adjustment for education, alcohol consumption, BMI, and breast cancer and CHD case status barely changed the association; thus these variables were not included. For risk of incident diabetes, we excluded participants with prevalent diabetes and performed Cox regression using age as the time scale, stratified by study and adjusted for the same covariates listed above. P values were corrected for multiple testing by controlling the false discovery rate (FDR). Pearson correlation between metabolites was assessed. To identify metabolites independently associated with diabetes, we conducted stepwise selection among metabolites that showed significant associations after further adjustment for glucose. The Harrell’s C-index was used to evaluate the ability of selected metabolites to predict diabetes risk in addition to known diabetes risk factors. Model improvement was assessed via the likelihood ratio test. Sensitivity analysis was performed by excluding those who developed diabetes, CHD, or cancer during the first two years of follow-up. Interactions between metabolites and sex were tested by likelihood ratio test of the Cox model with and without the interaction term. Two-sided P < 0.05 or FDR < 0.05 was considered statistically significant. Analyses were conducted on SAS 9.4 (SAS Institute, Cary, NC) and R 2.15.1.
3 Results
Baseline characteristics of study participants are shown in Table 1. Compared with non-diabetes (n = 835), participants with prevalent T2D (n = 68) or those who developed incident T2D after baseline (n = 73), were older, had higher BMI and waist circumference, and were more likely to have a history of hypertension.
Table 1.
Baseline characteristics of non-diabetic participants and participants with prevalent or incident type 2 diabetes in the Shanghai Women’s and Men’s Health Studies
| Baseline characteristics | Non-diabetics (n = 835) | Prevalent diabetes (n = 68) | Incident diabetes (n = 73) |
|---|---|---|---|
| Age (year) | 57.4 ± 7.9 | 60.7 ± 6.9* | 58.5 ± 6.8 |
| Male (%) | 21.2 | 18.2 | 10.8† |
| Education > 12 years (%) | 17.5 | 13.2a | 16.4 |
| Smoke cigarettes (%) | 17.7 | 13.2 | 11.0 |
| Body mass index (kg/m2) | 24.1 ± 3.5 | 25.2 ± 3.8* | 26.5 ± 3.6† |
| Waist circumference (cm) | 80.3 ± 8.9 | 84.8 ± 9.5* | 86.2 ± 9.4† |
| History of hypertension (%) | 26.6 | 50.0* | 54.8† |
| Blood draw in the morning (%) | 42.4 | 32.4 | 53.4 |
| Time interval since last meal (h) | 4.1 ± 3.3 | 4.4 ± 3.2 | 4.8 ± 4.1 |
Data were mean ± SD or percentage
P < 0.05 between non-diabetes and prevalent diabetes.
P <0.05 between non-diabetes and incident diabetes
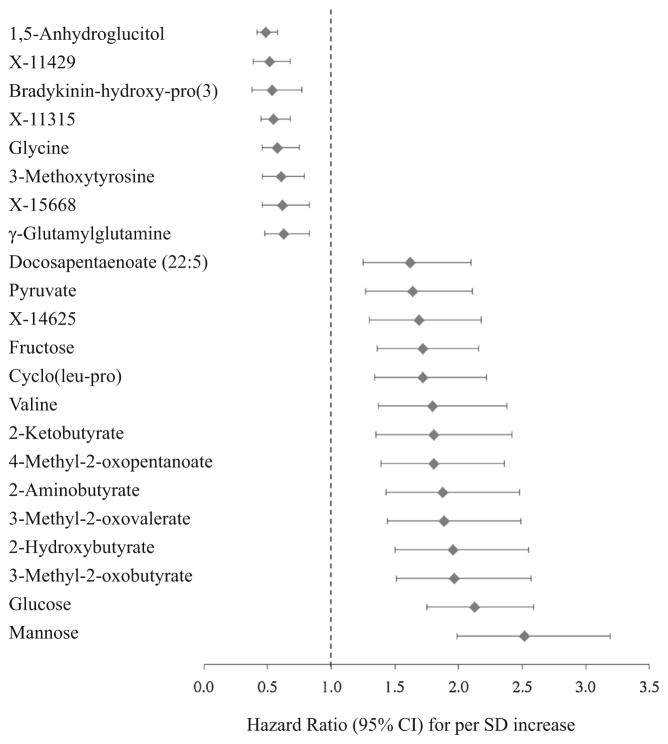
After adjustment for potential confounders and at the FDR <0.05, 24 metabolites with known identities and 10 unknown metabolites were associated with prevalent diabetes (Table 2); 18 metabolites with known identities and 4 unknown metabolites were associated with incident diabetes (Table 2 and Fig. 2). Twenty-three metabolites showed associations with both prevalent and incident T2D, including five carbohydrates (mainly hexoses, e.g. 1,5-anhydroglucitol [1,5-AG], mannose, and glucose), 10 amino acids (e.g. 2-hydroxybutyrate, 2-aminobutyrate, and glycine), one peptide (γ-glutamylglutamine), one nucleotide (pseudouridine), and six unknown metabolites (X-11315, X-14625, X-11429, X-11537, X-19437, and X-15668). The largest effect sizes and the smallest P values were observed for 1,5-AG [for per SD increase, OR (95 % CI) 0.42 (0.34, 0.51), HR (95 % CI) 0.49 (0.42, 0.58)] and mannose [OR (95 % CI) 3.03 (2.34, 3.91), HR (95 % CI) 2.52 (1.99, 3.19)]. Generally, the association with prevalent diabetes was stronger than that with incident diabetes, except for 2-aminobutyrate, 2-ketobutyrate, glycine, 3-methoxytyrosine, and γ-glutamylglutamine, whose associations with incident diabetes were more evident.
Table 2.
Metabolites associated with risk of type 2 diabetes at FDR <0.05 in the Shanghai Women’s and Men’s Health Studies
| Metabolites | Chemical class | HMDB ID | Odds ratio (95 % CI) for prevalent diabetesa | Hazard ratio (95 % CI) for incident diabetesb | Previously reported as associated with diabetes or pre-diabetes |
|---|---|---|---|---|---|
| Associated with both prevalent and incident diabetes | |||||
| 1,5-Anhydroglucitol | Carbohydrate | HMDB02712 | 0.42 (0.34, 0.51)* | 0.49 (0.42, 0.58)* | TwinsUK, OR 0.38 (0.28, 0.50), 0.77 (0.65, 0.92) for IFG |
| Mannose | Carbohydrate | HMDB00169 | 3.03 (2.34, 3.91)* | 2.52 (1.99, 3.19)* | TwinsUK, OR 3.36 (2.44, 4.62), 1.83 (1.49, 2.24) for IFG EPIC, HR 2.36 (2.06, 2.71) for total hexose |
| Glucose | Carbohydrate | HMDB00122 | 2.43 (1.93, 3.05)* | 2.13 (1.75, 2.59)* | |
| Fructose | Carbohydrate | HMDB00660 | 1.77 (1.41, 2.23)* | 1.72 (1.36, 2.16)* | TwinsUK, OR 2.21 (1.68, 2.92), 1.55 (1.27, 1.89) for IFG |
| Gluconate | Carbohydrate | HMDB00625 | 2.65 (1.96, 3.58)* | 1.54 (1.18, 2.01) | |
| 3-Hydroxypyruvate | Amino acid | HMDB01352 | 2.39 (1.66, 3.44)* | 1.67 (1.22, 2.29) | |
| 2-Hydroxybutyrate | Amino acid | HMDB00008 | 2.02 (1.55, 2.63)* | 1.96 (1.50, 2.55)* | TwinsUK, OR 1.62 (1.26, 2.08), 1.67 (1.39, 2.02) for IFG |
| 2-Aminobutyrate | Amino acid | HMDB00650 | 1.77 (1.34, 2.33)* | 1.88 (1.43, 2.48)* | |
| 2-Ketobutyrate | Amino acid | HMDB00005 | 1.43 (1.06, 1.92) | 1.81 (1.35, 2.42)* | |
| 3-Hydroxyisobutyrate | Amino acid | HMDB00336 | 1.64 (1.25, 2.14)* | 1.52 (1.17, 1.97) | |
| 3-Hydroxypropanoate | Amino acid | HMDB00700 | 1.61 (1.24, 2.08)* | 1.38 (1.04, 1.82) | |
| Pyroglutamine | Amino acid | HMDB00267 | 0.52 (0.38, 0.69)* | 0.66 (0.51, 0.86) | |
| Glycine | Amino acid | HMDB00123 | 0.67 (0.52, 0.87) | 0.58 (0.46, 0.75)* | EPIC, HR 0.73 (0.64, 0.83) |
| SABRE, OR 0.76 (0.61, 0.94) for European men and OR 0.98 (0.81, 1.19) for South Asian men | |||||
| 3-Methoxytyrosine | Amino acid | HMDB01434 | 0.72 (0.55, 0.94) | 0.61 (0.46, 0.79)* | |
| O-Sulfo-L-tyrosine | Amino acid | – | 0.64 (0.49, 0.84)* | 0.78 (0.61, 1.00) | |
| γ-Glutamylglutamine | Peptide | HMDB11738 | 0.71 (0.54, 0.94) | 0.63 (0.48, 0.83)* | |
| Pseudouridine | Nucleotide | HMDB00767 | 0.57 (0.43, 0.75)* | 0.66 (0.50, 0.87) | |
| Associated with prevalent diabetes | |||||
| Kynurenine | Amino acid | HMDB00684 | 0.57 (0.43, 0.76)* | 0.92 (0.71, 1.20) | |
| Dimethylarginine | Amino acid | HMDB01539 HMDB03334 | 0.65 (0.51, 0.83)* | 0.94 (0.72, 1.21) | TwinsUK, OR 0.55 (0.41, 0.74), 0.94 (0.79, 1.12) for IFG |
| Citrulline | Amino acid | HMDB00904 | 0.66 (0.50, 0.85)* | 0.98 (0.77, 1.27) | TwinsUK, OR 0.54 (0.42, 0.71), 0.83 (0.79, 0.99) for IFG |
| Imidazole propionate | Amino acid | HMDB02271 | 1.49 (1.17, 1.89)* | 0.99 (0.73, 1.35) | |
| 2-Arachidonoylglycerol | Lipid | HMDB04666 | 0.53 (0.42, 0.69)* | 0.88 (0.67, 1.15) | |
| 1-Palmitoyl-glycerophosphoinositol | Lipid | HMDB61695 | 0.60 (0.45, 0.79)* | 0.98 (0.77, 1.26) | |
| 1-Oleoyl-glycerophosphoinositol | Lipid | HMDB61693 | 0.60 (0.44, 0.81)* | 0.94 (0.70, 1.25) | |
| Androsterone sulfate | Lipid | HMDB02759 | 0.67 (0.53, 0.85)* | 0.81 (0.64, 1.03) | |
| Epiandrosterone sulfate | Lipid | HMDB00365 | 0.67 (0.53, 0.85)* | 0.84 (0.66, 1.07) | |
| Pelargonate (9:0) | Lipid | HMDB00847 | 0.65 (0.50, 0.84)* | 0.97 (0.75, 1.24) | TwinsUK, OR 0.57 (0.44, 0.75), 1.01 (0.84, 1.20) for IFG |
| N6-Carbamoyl-threonyl-adenosine | Nucleotide | – | 0.61 (0.46, 0.81)* | 0.93 (0.71, 1.21) | |
| Associated with incident diabetes | |||||
| Pyruvate | Carbohydrate | HMDB00243 | 1.26 (0.96, 1.65) | 1.64 (1.27, 2.11)* | |
| Valine | Amino acid | HMDB00883 | 1.19 (0.90, 1.58) | 1.80 (1.37, 2.38)* | Framingham, HR 1.57 (1.17, 2.09) |
| EPIC, HR 1.27 (1.16, 1.40) | |||||
| SABRE, OR 1.00 (0.80, 1.27) for European men and OR 1.24 (1.00, 1.54) for South Asian men | |||||
| 3-Methyl-2-oxobutyrate | Amino acid | HMDB00019 | 1.10 (0.84, 1.44) | 1.97 (1.51, 2.57)* | TwinsUK, OR 1.45 (1.15, 1.83), 1.54 (1.30, 1.82) for IFG |
| 3-Methyl-2-oxovalerate | Amino acid | HMDB03736 | 1.26 (0.96, 1.65) | 1.89 (1.44, 2.49)* | TwinsUK, OR 2.18 (1.68, 2.84), 1.65 (1.39, 1.95) for IFG |
| 4-Methyl-2-oxopentanoate | Amino acid | HMDB00695 | 1.25 (0.96, 1.62) | 1.81 (1.39, 2.36)* | TwinsUK, OR 2.00 (1.54, 2.59), 1.58 (1.33, 1.87) for IFG |
| Cyclo(leu-pro) | Peptide | HMDB34276 | 1.11 (0.84, 1.45) | 1.72 (1.34, 2.22)* | |
| Docosapentaenoate (22:5n3) | Lipid | HMDB06528 | 0.96 (0.73, 1.26) | 1.62 (1.25, 2.10)* | |
| Bradykinin-hydroxy-pro(3) | Peptide | HMDB11728 | 0.93 (0.68, 1.26) | 0.54 (0.38, 0.77)* | |
Metabolites associated with risk of prevalent or incident type 2 diabetes at the FDR<0.05 and with known identities are shown in the table. Ten unidentified metabolites were associated with prevalent and/or incident diabetes: X-11315, X-14625, X-11429, X-11537, X-19437, X-15668, X-19411, X-16394, X-11687, and X -14626
EPIC is short for European Prospective Investigation into Cancer and Nutrition (Floegel et al. 2013); Framingham is short for the Framingham Offspring Study (Wang et al. 2011); SABRE is short for the Southall And Brent REvisited Study (Tillin et al. 2015); TwinsUK (Menni et al. 2013)
CI confidence interval, FDR false discover rate, HMDB the human metabolome database, IFG impaired fasting glucose
Logistic regression model was stratified by study and adjusted for age, sex, smoking, waist circumference, history of hypertension, time of blood draw, time interval since last meal, and assay batch. * FDR <0.05
Participants with baseline diabetes were excluded. Cox regression model using age as the time scale was stratified by study and adjusted for the same covariates. * FDR <0.05
Fig. 2.
Metabolites associated with incident type 2 diabetes in the Shanghai Women’s and Men’s Health Studies
We also identified 11 known metabolites associated only with prevalent diabetes, including metabolites of tryptophan, arginine, and histidine, lipids, glycerophospholipids, and androgen sulfates. Eight metabolites were primarily associated with incident diabetes, including pyruvate, valine, 3 branched-chain keto-acids, cyclo(leu-pro), docosapentaenoate (DPA, 22:5n3), and bradykinin-hydroxy-pro(3). We observed some moderate-to-strong correlations between significant unknown metabolites and known metabolites; for example, X-11315 was correlated with1,5-AG (r = 0.43), X-14625 with glucose (r = 0.63), X-11429 with 3-methoxytyrosine (r = 0.40) and pseudouridine (r = 0.83), and X-15668 with 1-linoleoylglyc-erophosphocholine (r = 0.48).
Among metabolites associated with incident diabetes, we further adjusted for glucose; 16 metabolites remained significantly associated with diabetes risk but six metabolites became non-significant (fructose, 2-ketobutyrate, γ-glutamylglutamine, pyruvate, X-14625, and X-15668). We then conducted stepwise regression analyses among these 16 significant metabolites. Six metabolites, present in all participants, were selected in the final model: 1,5-AG, mannose, valine, 3-methoxytyrosine, DPA, and bradykinin-hydroxy-pro(3). Except that mannose was moderately correlated with 1,5-AG (r = −0.22) and DPA (r = 0.25), other metabolites were weakly or not correlated with one another (r = 0.02–0.13). Adding these metabolites improved the risk prediction of diabetes. The C-index increased from 0.767 in the model with traditional risk factors (age, sex, smoking, waist circumference, hypertension, and glucose) to 0.781–0.789 after adding one of the six metabolites (all P < 0.05). The C-index was 0.792 after adding both 1,5-AG and mannose, 0.813 after further adding valine, and 0.839 in the model adding six metabolites (all P < 0.05).
Excluding participants who developed diabetes, CHD, or cancer during the first two years after blood collection did not substantially change the results for most FDR-significant metabolites except that the association for pyruvate became marginally significant. We did not find significant interactions between metabolites and sex at the FDR <0.05 in the present study.
4 Discussion
Using a non-targeted metabolomics platform, we identified 46 plasma metabolites associated with the prevalence and/or incidence of T2D in Chinese adults. These significant metabolites are involved in glycolysis/gluconeogenesis and metabolisms of BCAAs, other amino acids, lipids, glycerophospholipids, androgen, and bradykinin, revealing multiple perturbed pathways at different stages of diabetes development and suggesting potential novel biomarkers for diabetes risk prediction.
As far as we know, this is the first population-based, longitudinal metabolomic study for T2D in Chinese adults. We found that many previously reported metabolites associated with diabetes in European, US, Singapore, and South Asian populations were similarly associated with diabetes risk in the present study (Ferrannini et al. 2013; Floegel et al. 2013; Gall et al. 2010; Menni et al. 2013; Suhre et al. 2010; Wang-Sattler et al. 2012; Wang et al. 2011; Xu et al. 2013a; Tillin et al. 2015). The most significant changes were observed in carbohydrate metabolites both for prevalent and incident diabetes, indicating that impaired glucose metabolism occurred many years before clinical diagnosis. Certain hexoses, such as 1,5-AG and mannose, showed stronger associations with diabetes than glucose and their associations remained significant after adjustment for glucose [HR (95 % CI) for 1-SD increase was 0.62 (0.50, 0.78) for 1,5-AG and 1.82 (1.25, 2.66) for mannose]. This is in agreement with findings of the European Prospective Investigation into Cancer and Nutrition (EPIC) study (Floegel et al. 2013) and the Cooperative Health Research in the Region of Augsburg (KORA) study (Wang-Sattler et al. 2012), although in the KORA study, the association between total hexose and diabetes disappeared after adjusting for glucose, insulin, and hemoglobin A1c. 1,5-AG is a sensitive marker for postprandial hyperglycemia and short-term glycemic control (Dungan et al. 2006; Lu et al. 2013). Its concentration decreases when glucose exceeds 10 mmol/l. Recently, 1,5-AG test has been approved by the US Food and Drug Administration and its saliva level may be used as a non-invasive marker to facilitate diabetes screenings (Mook-Kanamori et al. 2014).
Another group of altered metabolites is related to BCAAs, which may be the most frequently and consistently reported metabolites for T2D (Lynch and Adams 2014; Newgard et al. 2009; Wang et al. 2011; Tillin et al. 2015). The magnitude of associations was comparable among Chinese and individuals mostly of European descent. In the Framingham Offspring Study, each SD increase in plasma BCAAs predicted an approximately 60 % higher risk of diabetes over 12 years of follow-up, independent of baseline BMI and glucose (Wang et al. 2011). We observed increased concentrations for all three BCAAs at baseline that was an average of 10 years before the diabetes diagnoses [HR (95 % CI) for 1-SD increase in valine, leucine, and isoleucine was 1.55 (1.16, 2.08), 1.37 (1.03, 1.82), and 1.23 (0.93, 1.64) after further adjustment for glucose, respectively]. We also observed highly significant associations of elevated BCAA metabolites [branched-chain keto-acid (BCKA)] with diabetes incidence, consistent with the TwinsUK study showing that BCKA 3-methyl-2-oxovalerate was the strongest marker for impaired fasting glucose among 447 metabolites (Menni et al. 2013). Although evidence has supported the association of higher BCAAs with T2D, the causality of this association remains unclear. As essential amino acids, dietary or supplemental BCAAs seem to have beneficial effects on body weight, lean body mass, and diabetes prevention (Nagata et al. 2013; Qin et al. 2011). It has been proposed that elevated BCAA metabolites due to reduced BCAA catabolism or increased tissue protein breakdown, not BCAAs per se, cause insulin resistance and diabetes (Lynch and Adams 2014; Mayers et al. 2014; Tai et al. 2010). Supporting this, genetic defects in BCAA catabolism have been associated with obesity and T2D (Taneera et al. 2012; Tiffin et al. 2006). Exercise, known to improve insulin sensitivity, has been shown to promote BCAA catabolism in skeletal muscle (Shimomura et al. 2004). The totality of the evidence suggests that elevated BCAAs or BCKAs may serve as early markers of T2D and point toward new therapeutic targets.
Besides well-recognized hexoses and BCAAs, we identified three other metabolites associated with diabetes risk: 3-methoxytyrosine, DPA, and bradykinin-hydroxy-pro(3). Their associations were independent of central obesity, glucose, and reported metabolites. 3-Methoxyty-rosine is a major metabolite of levodopa (Goldstein et al. 2003). Levodopa is a neurotransmitter precursor and used to treat Parkinson’s disease (Connolly and Lang 2014). Levodopa or its metabolites have not been associated with T2D in previous metabolomic studies. However, bromocriptine, a dopamine receptor agonist and a drug for Parkinson’s disease, has been approved for T2D as well (DeFronzo 2011). On the other hand, antidiabetic drugs, such as exenatide, may have neuroprotective effects (Aviles-Olmos et al. 2013). Although the mechanisms linking neurotransmitters and diabetes remain unclear, some neurological and mental disorders (e.g. Parkinson’s disease, schizophrenia, and depression) and lifestyles that disrupt circadian rhythm (e.g. night shift work) have been associated with higher T2D risk (Pan et al. 2010, 2011; Schoepf et al. 2012). Our results suggest that neurotransmitter dysregulation might play a role in T2D pathogenesis, a hypothesis that needs to be further evaluated. DPA (22:5n3) is a long-chain n-3 polyunsaturated fatty acid, whose association with T2D remains controversial. Most studies that had measured circulating fatty acids did not find a significant association between DPA and T2D, including a few large prospective cohort studies (Patel et al. 2010; Kröger et al. 2011; Hodge et al. 2007). However, our finding is consistent with results from a few Asian studies showing that DPA concentration was associated with insulin resistance and other T2D risk factors (Kusunoki et al. 2007; Huang et al. 2010; Zhang et al. 2012). The associations of fatty acids with T2D may be population-specific because populations differ in dietary sources of fatty acids and also genetic variations of fatty acid metabolism. Bradykinin-hydroxy-pro(3) is an analog of bradykinin with the 3rd proline replaced by hydroxyproline. The kinins are vasodilators and exert cardiac and renal protective effects through regulating blood pressure, inflammation, and smooth muscle tone (Marcondes and Antunes 2005). No metabolomic studies have reported reduced circulating kinins in relation to incident T2D, but several animal and human studies have suggested the beneficial roles of bradykinin in diabetes (Tomita et al. 2012). For example, stimulating bradykinin generation improved glucose utilization and insulin sensitivity (Kolodka et al. 2014; Montanari et al. 2005); inhibiting its degradation protected against diabetic nephropathy (Tomita et al. 2012); and polymorphisms in bradykinin receptor gene that cause a reduced signaling, were associated with hyperglycemia and higher T2D risk (Alvim et al. 2012). However, since no other known metabolite from the same pathway showed a significant association, a chance finding cannot be ruled out. Future validation studies are needed.
Our present study has several limitations. First, it is a secondary data analysis and each set of samples was measured separately. To make metabolites more comparable across studies, we standardized and analyzed metabolites within each study, and adjusted for study as well as a wide range of possible confounders. Further adjustment for breast cancer and CHD status or exclusion of participants who developed cancer or CHD within two years after blood draw did not materially change the results. Second, because the largest contributing study for the current analysis was originally designed for breast cancer, male participants were under-represented. However, a large-scale study, the EPIC, found no evidence of effect modification by sex (Floegel et al. 2013). We also did not observe significant interactions of metabolites with sex. Moreover, we replicated several findings from studies that included mostly men (Tai et al. 2010; Suhre et al. 2010; Xu et al. 2013a). Third, we did not require participants to fast before blood draw. Food consumption could influence some circulating metabolites. However, previous studies did not observed considerable effect modifications of fasting status on metabolite-diabetes associations (Floegel et al. 2013). To minimize the potential influence of fasting status, we adjusted for time since last meal in our analysis. Fourth, T2D cases were self-reported and may be subject to misclassification. Under-diagnosis is the main concern. These misclassifications are more likely to underestimate the associations. Despite these methodological limitations, we replicated the associations for many of the established T2D-related metabolites and for the known T2D risk factors, which provide strong evidence supporting the validity of our findings.
In conclusion, using a non-targeted metabolomics approach, we identified 46 plasma metabolites reflecting multiple metabolic abnormalities that were associated with prevalent and/or incident diabetes in middle-aged and older Chinese men and women. Six metabolites were independently associated with incident diabetes, including two hexoses, a BCAA, a long-chain polyunsaturated fatty acid, a neurotransmitter metabolite, and an analog of bradykinin. The consistent findings for hexoses and BCAAs in our study population, uncovered in previous studies, strengthen the evidence for their possible causal associations, but the novel metabolites we observed need to be further validated in well-designed, large-scale studies. Meanwhile, future collaborative studies are warranted to examine the differences and similarities between populations regarding the human metabolome and type 2 diabetes.
Acknowledgments
We thank Dr. Joshua Sampson for his comments on the paper. We thank Ms. Nancy Kennedy for her assistance on preparing the manuscript. We thank the research team and participants of the Shanghai Women’s Health Study and the Shanghai Men’s Health Study for their foundation work for this study.
Footnotes
Compliance with Ethical Standards
Conflict of interest
The authors, including Danxia Yu, Steven C. Moore, Charles E. Matthews, Yong-Bing Xiang, Xianglan Zhang, Yu-Tang Gao, Wei Zheng, and Xiao-Ou Shu, have no conflict of interest to declare. The study uses existing data and specimens that have already been collected by the parent studies, i.e., the Shanghai Women’s Health Study and the Shanghai Men’s Health Study. All study participants provided informed consent to the parent studies.
Funding
This work was supported, in part, by the US National Institutes of Health [R37 CA070867 and UM1 CA182910 to Dr. W. Zheng, UM1 CA173640, R01 HL079123 and NO2-CP11010-66 to Dr. X.O. Shu]. This work was also supported, in part, by the Breast Cancer Research Stamp Fund, awarded through competitive peer review and the Intramural Research Program of the National Cancer Institute, National Institutes of Health.
References
- Alvim RO, Santos PCJL, Nascimento RM, Coelho GLLM, Mill JG, et al. BDKRB2 +9/− 9 polymorphism is associated with higher risk for diabetes mellitus in the Brazilian general population. Journal of Diabetes Research. 2012;2012:e480251. doi: 10.1155/2012/480251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson’s disease. Journal of Clinical Investigation. 2013;123(6):2730–2736. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research moving from information to knowledge. Diabetes. 2009;58(11):2429–2443. doi: 10.2337/db09-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JCN, Zhang Y, Ning G. Diabetes in China: A societal solution for a personal challenge. The Lancet Diabetes & Endocrinology. 2014;2(12):969–979. doi: 10.1016/S2213-8587(14)70144-5. [DOI] [PubMed] [Google Scholar]
- Connolly BS, Lang AE. Pharmacological treatment of parkinson disease: A review. JAMA. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Bromocriptine: A sympatholytic, D2-dopamine agonist for the treatment of type 2 diabetes. Diabetes Care. 2011;34(4):789–794. doi: 10.2337/dc11-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dungan KM, Buse JB, Largay J, Kelly MM, Button EA, Kato S, Wittlin S. 1,5-Anhydroglucitol and postprandial hyperglycemia as measured by continuous glucose monitoring system in moderately controlled patients with diabetes. Diabetes Care. 2006;29(6):1214–1219. doi: 10.2337/dc06-1910. [DOI] [PubMed] [Google Scholar]
- Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Analytical Chemistry. 2009;81(16):6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62(5):1730–1737. doi: 10.2337/db12-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes. 2013;62(2):639–648. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, et al. α-Hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE. 2010;5(5):e10883. doi: 10.1371/journal.pone.0010883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein DS, Eisenhofer G, Kopin IJ. Sources and significance of plasma levels of catechols and their metabolites in humans. Journal of Pharmacology and Experimental Therapeutics. 2003;305(3):800–811. doi: 10.1124/jpet.103.049270. [DOI] [PubMed] [Google Scholar]
- Hodge AM, English DR, O’Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: Interpreting the role of linoleic acid. The American Journal of Clinical Nutrition. 2007;86(1):189–197. doi: 10.1093/ajcn/86.1.189. [DOI] [PubMed] [Google Scholar]
- Hu FB. Globalization of diabetes the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–1257. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Wahlqvist ML, Xu T, Xu A, Zhang A, Li D. Increased plasma n-3 polyunsaturated fatty acid is associated with improved insulin sensitivity in type 2 diabetes in China. Molecular Nutrition & Food Research. 2010;54(S1):S112–S119. doi: 10.1002/mnfr.200900189. [DOI] [PubMed] [Google Scholar]
- Kodama K, Tojjar D, Yamada S, Toda K, Patel CJ, Butte AJ. Ethnic differences in the relationship between insulin sensitivity and insulin response a systematic review and meta-analysis. Diabetes Care. 2013;36(6):1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodka T, Charles ML, Raghavan A, Radichev IA, Amatya C, Ellefson J, et al. Preclinical characterization of recombinant human tissue kallikrein-1 as a novel treatment for type 2 diabetes mellitus. PLoS ONE. 2014;9(8):e103981. doi: 10.1371/journal.pone.0103981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Döring F, et al. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Potsdam Study. The American Journal of Clinical Nutrition. 2011;93(1):127–142. doi: 10.3945/ajcn.110.005447. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Tsutsumi K, Nakayama M, Kurokawa T, Nakamura T, Ogawa H, et al. Relationship between serum concentrations of saturated fatty acids and unsaturated fatty acids and the homeostasis model insulin resistance index in Japanese patients with type 2 diabetes mellitus. The Journal of Medical Investigation. 2007;54(3,4):243–247. doi: 10.2152/jmi.54.243. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet. 2008;371(9626):1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- Lu J, Xie G, Jia W, Jia W. Metabolomics in human type 2 diabetes research. Frontiers of Medicine. 2013;7(1):4–13. doi: 10.1007/s11684-013-0248-4. [DOI] [PubMed] [Google Scholar]
- Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nature Reviews Endocrinology. 2014;10(12):723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma RCW, Chan JCN. Type 2 diabetes in East Asians: Similarities and differences with populations in Europe and the United States. Annals of the New York Academy of Sciences. 2013;1281(1):64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma RCW, Lin X, Jia W. Causes of type 2 diabetes in China. The Lancet Diabetes & Endocrinology. 2014;2(12):980–991. doi: 10.1016/S2213-8587(14)70145-7. [DOI] [PubMed] [Google Scholar]
- Marcondes S, Antunes E. The plasma and tissue kininogen-kallikrein-kinin system: Role in the cardiovascular system. Current Medicinal Chemistry—Cardiovascular & Hematological Agents. 2005;3(1):33–44. doi: 10.2174/1568016052773351. [DOI] [PubMed] [Google Scholar]
- Mayers JR, Wu C, Clish CB, Kraft P, Torrence ME, Fiske BP, et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nature Medicine. 2014;20(10):1193–1198. doi: 10.1038/nm.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C, Fauman E, Erte I, Perry JRB, Kastenmüller G, Shin SY, et al. Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes. 2013;62(12):4270–4276. doi: 10.2337/db13-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanari D, Yin H, Dobrzynski E, Agata J, Yoshida H, Chao J, Chao L. Kallikrein gene delivery improves serum glucose and lipid profiles and cardiac function in streptozotocin-induced diabetic rats. Diabetes. 2005;54(5):1573–1580. doi: 10.2337/diabetes.54.5.1573. [DOI] [PubMed] [Google Scholar]
- Mook-Kanamori DO, Selim MMED, Takiddin AH, Al-Homsi H, Al-Mahmoud KAS, Al-Obaidli A, et al. 1,5-Anhydroglucitol in saliva is a noninvasive marker of short-term glycemic control. The Journal of Clinical Endocrinology & Metabolism. 2014;99(3):E479–E483. doi: 10.1210/jc.2013-3596. [DOI] [PubMed] [Google Scholar]
- Moore SC, Matthews CE, Sampson JN, Stolzenberg-Solomon RZ, Zheng W, Cai Q, et al. Human metabolic correlates of body mass index. Metabolomics. 2014;10(2):259–269. doi: 10.1007/s11306-013-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata C, Nakamura K, Wada K, Tsuji M, Tamai Y, Kawachi T. Branched-chain amino acid intake and the risk of diabetes in a japanese community the takayama study. American Journal of Epidemiology. 2013;178(8):1226–1232. doi: 10.1093/aje/kwt112. [DOI] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabolism. 2009;9(4):311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: Systematic review. BMJ. 2011;343:d7163. doi: 10.1136/bmj.d7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Lucas M, Sun Q, et al. BIdirectional association between depression and type 2 diabetes mellitus in women. Archives of Internal Medicine. 2010;170(21):1884–1891. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: A pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)–Norfolk cohort. The American Journal of Clinical Nutrition. 2010;92(5):1214–1222. doi: 10.3945/ajcn.2010.29182. [DOI] [PubMed] [Google Scholar]
- Qin LQ, Xun P, Bujnowski D, Daviglus ML, Horn LV, Stamler J, He K. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged east asian and western adults. The Journal of Nutrition. 2011;141(2):249–254. doi: 10.3945/jn.110.128520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson JN, Boca SM, Shu XO, Stolzenberg-Solomon RZ, Matthews CE, Hsing AW, et al. Metabolomics in epidemiology: Sources of variability in metabolite measurements and implications. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology. 2013;22(4):631–640. doi: 10.1158/1055-9965.EPI-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepf D, Potluri R, Uppal H, Natalwala A, Narendran P, Heun R. Type-2 diabetes mellitus in schizophrenia: Increased prevalence and major risk factor of excess mortality in a naturalistic 7-year follow-up. European Psychiatry. 2012;27(1):33–42. doi: 10.1016/j.eurpsy.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA. Exercise promotes BCAA catabolism: Effects of BCAA supplementation on skeletal muscle during exercise. The Journal of Nutrition. 2004;134(6):1583S–1587S. doi: 10.1093/jn/134.6.1583S. [DOI] [PubMed] [Google Scholar]
- Shu X-O, Li H, Yang G, Gao J, Cai H, Takata Y, et al. Cohort profile: The shanghai men’s health study. International Journal of Epidemiology. 2015:dyv013. doi: 10.1093/ije/dyv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhre K. Metabolic profiling in diabetes. Journal of Endocrinology. 2014;221(3):R75–R85. doi: 10.1530/JOE-14-0024. [DOI] [PubMed] [Google Scholar]
- Suhre K, Meisinger C, Döring A, Altmaier E, Belcredi P, Gieger C, et al. Metabolic footprint of diabetes: A multiplatform metabolomics study in an epidemiological setting. PLoS ONE. 2010;5(11):e13953. doi: 10.1371/journal.pone.0013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai ES, Tan MLS, Stevens RD, Low YL, Muehlbauer MJ, Goh DLM, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53(4):757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneera J, Lang S, Sharma A, Fadista J, Zhou Y, Ahlqvist E, et al. A Systems genetics approach identifies genes and pathways for type 2 diabetes in human islets. Cell Metabolism. 2012;16(1):122–134. doi: 10.1016/j.cmet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- The World Bank. Toward a healthy and harmonious life in china: Stemming the rising tide of non-communicable diseases. 2011 (No. World Bank Report Number 62318-CN) [Google Scholar]
- Tiffin N, Adie E, Turner F, Brunner HG, van Driel MA, Oti M, et al. Computational disease gene identification: A concert of methods prioritizes type 2 diabetes and obesity candidate genes. Nucleic Acids Research. 2006;34(10):3067–3081. doi: 10.1093/nar/gkl381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillin T, Hughes AD, Wang Q, Würtz P, Ala-Korpela M, Sattar N, et al. Diabetes risk and amino acid profiles: Cross-sectional and prospective analyses of ethnicity, amino acids and diabetes in a South Asian and European cohort from the SABRE (Southall And Brent REvisited) Study. Diabetologia. 2015;58(5):968–979. doi: 10.1007/s00125-015-3517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita H, Sanford RB, Smithies O, Kakoki M. The kallikrein–kinin system in diabetic nephropathy. Kidney International. 2012;81(8):733–744. doi: 10.1038/ki.2011.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, et al. Metabolite profiles and the risk of developing diabetes. Nature Medicine. 2011;17(4):448–453. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Sattler R, Yu Z, Herder C, Messias AC, Floegel A, He Y, et al. Novel biomarkers for pre-diabetes identified by metabolomics. Molecular Systems Biology. 2012;8(1):615. doi: 10.1038/msb.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Tavintharan S, Sum CF, Woon K, Lim SC, Ong CN. Metabolic signature shift in type 2 diabetes mellitus revealed by mass spectrometry-based metabolomics. The Journal of Clinical Endocrinology & Metabolism. 2013a;98(6):E1060–E1065. doi: 10.1210/jc.2012-4132. [DOI] [PubMed] [Google Scholar]
- Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013b;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- Zhang G, Sun Q, Hu FB, Ye X, Yu Z, Zong G, et al. Erythrocyte n-3 fatty acids and metabolic syndrome in middle-aged and older Chinese. The Journal of Clinical Endocrinology and Metabolism. 2012;97(6):E973–E977. doi: 10.1210/jc.2011-2997. [DOI] [PubMed] [Google Scholar]
- Zheng W, Chow WH, Yang G, Jin F, Rothman N, Blair A, et al. The Shanghai Women’s Health Study: Rationale, study design, and baseline characteristics. American Journal of Epidemiology. 2005;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]