Abstract
Objective
Plasminogen activator inhibitor-1 (PAI-1), a serine protease inhibitor that promotes and inhibits cell migration, plays a complex and important role in adverse vascular remodeling. Little is known about the effects of pharmacological PAI-1 inhibitors, an emerging drug class, on migration of vascular smooth muscle cells (SMCs) and endothelial cells (ECs), crucial mediators of vascular remodeling. We investigated the effects of PAI-039 (tiplaxtinin), a specific PAI-1 inhibitor, on SMC and EC migration in vitro and vascular remodeling in vivo.
Approach and Results
PAI-039 inhibited SMC migration through collagen gels, including those supplemented with vitronectin and other extracellular matrix proteins, but did not inhibit migration of PAI-1-deficient SMCs, suggesting that its anti-migratory effects were PAI-1-specific and physiologically relevant. However, PAI-039 did not inhibit EC migration. PAI-039 inhibited phosphorylation and nuclear translocation of STAT-1 in SMCs, but had no discernable effect on STAT-1 signaling in ECs. Expression of LDL receptor-related protein 1 (LRP1), a motogenic PAI-1 receptor that activates JAK/STAT-1 signaling, was markedly lower in ECs than in SMCs. Notably, PAI-039 significantly inhibited intimal hyperplasia and inflammation in murine models of adverse vascular remodeling, but did not adversely affect re-endothelialization after endothelium-denuding mechanical vascular injury.
Conclusions
PAI-039 inhibits SMC migration and intimal hyperplasia, while having no inhibitory effect on ECs, which appears to be due to differences in PAI-1-dependent LRP1/JAK/STAT-1 signaling between SMCs and ECs. These findings suggest that PAI-1 may be an important therapeutic target in obstructive vascular diseases characterized by neointimal hyperplasia.
Keywords: plasminogen activators, inhibitors, muscle, smooth, remodeling, pharmacology
Plasminogen activator inhibitor-1 (PAI-1) is a serine protease inhibitor expressed by vascular endothelial cells, vascular smooth muscle cells (SMCs), and several other cell types.1 PAI-1 is present in plasma and the extracellular matrix (ECM) of blood vessels, where it rapidly inhibits tissue-type plasminogen activator (t-PA) and urinary-type plasminogen activator (u-PA), which down-regulates plasmin formation and fibrinolysis. Complete deficiency of PAI-1 produces a bleeding disorder,2 while elevated plasma levels of PAI-1 are associated with thrombotic disorders.3–5 Although its primary function appears to be the regulation of fibrinolysis, PAI-1 also regulates cell migration, which plays a critical role in vascular remodeling, including pathological neointimal hyperplasia.6 PAI-1 decreases cell migration by inhibiting pericellular plasmin formation and binding to vitronectin (VN) in the ECM, thereby blocking VN’s binding interactions with αVβ3 integrin and the u-PA receptor (uPAR).7–10 Conversely, PAI-1 exerts a pro-migratory effect by binding to low-density lipoprotein receptor-related protein 1 (LRP1) and activating a Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway.11 Consistent with its opposing effects on cell migration in vitro, PAI-1 has been shown to either inhibit12–14 or promote15–19 neointima formation in vivo. While PAI-1 affects vascular remodeling via multiple pathways, including SMC proliferation and apoptosis,20–22 ECM synthesis and clearance,23 and inflammatory signaling pathways,24–26 its effects on SMC migration are of major importance in vascular remodeling.6
Several small-molecular-weight inhibitors of PAI-1 have been developed.27 These compounds inhibit thrombosis, macrophage migration, and vascular senescence.28–30 However, little is known about their effects on SMC and endothelial cell (EC) migration in vitro and vascular wall remodeling in vivo. Given the potential of PAI-1 to either promote or inhibit SMC migration, and considering the central role of SMC migration in pathological vascular remodeling, including arterial restenosis and vein graft intimal hyperplasia, it is critical to determine the effects of pharmacological PAI-1 inhibition on SMC migration. It is equally important to determine the effects of pharmacological PAI-1 inhibition on endothelial cell migration, as an intact, functional endothelium is essential to maintain vascular health. Therefore, the goals of this study were to examine the effects of PAI-039 (tiplaxtinin), a highly specific and well characterized inhibitor of PAI-1,28 on SMC and EC migration under physiologically relevant conditions in vitro and arterial and venous remodeling in vivo.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
PAI-039 inhibits SMC migration
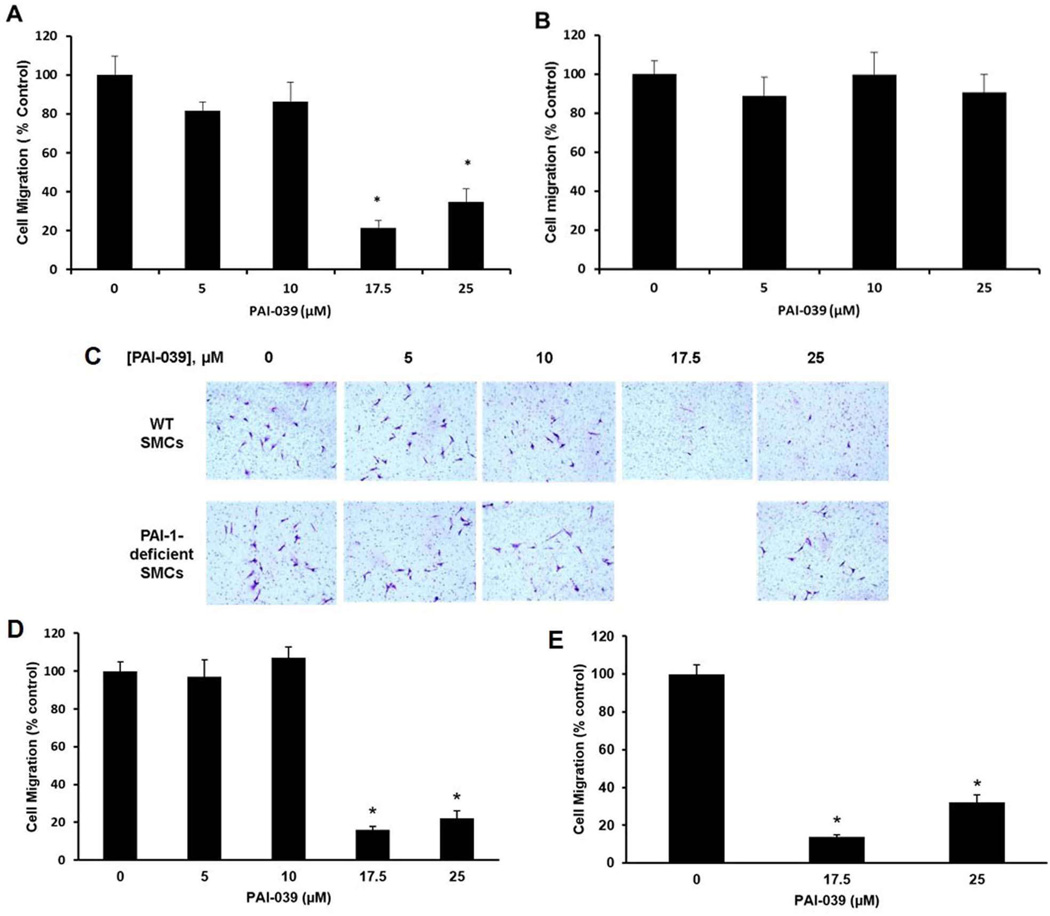
We studied the migration of SMCs through 3-dimensional collagen matrices in the presence or absence or PAI-039. PAI-039 significantly inhibited migration of wild-type murine SMCs when present in concentrations ≥17.5 µM (Fig. 1A, C). PAI-039 also inhibited the migration of human coronary artery SMCs through collagen (Supplementary Fig. IA in online data supplement). However, PAI-039 did not inhibit migration of SMCs isolated from pai1−/− mice (Fig. 1B, C), suggesting that the anti-migratory effect of PAI-039 was due specifically to PAI-1 inhibition. PAI-039 had no significant effect on proliferation of wild-type SMCs (Supplementary Fig. IB in online data supplement), suggesting that the reduced migration of PAI-039-treated cells was not mediated by an anti-mitogenic effect. Addition of a mixture of ECM proteins (MaxGel ECM) or purified VN to collagen gels increased SMC migration (Supplementary Fig. II in online data supplement), but did not blunt the anti-migratory effect of PAI-039 (Fig. 1 D–E). These results suggest that PAI-039 inhibits SMC migration under physiological conditions, including in the presence of VN, which binds PAI-1 and reduces its susceptibility to inhibition by PAI-039.31
Figure 1.
PAI-039 inhibits SMC migration. (A) PAI-039 inhibits migration of wild-type (WT) murine venous SMCs through collagen. (B) PAI-039 does not inhibit migration of PAI-1-deficienct SMCs. (C) Representative images of SMCs that migrated through collagen gel to lower-chamber side of transwell membranes. (D) PAI-039 inhibits SMC migration through more complex collagen gels containing a mixture of extracellular matrix proteins (MaxGel ECM). (E) PAI-039 inhibits SMC migration through collagen gels supplemented with purified vitronectin (10 µg/mL). Data represent mean of 3 independent experiments. *P<0.05 vs. control group lacking PAI-039.
PAI-039 does not inhibit EC migration
We next studied the effect of PAI-039 on EC migration in vitro, utilizing a 2-dimensional scratch assay. Contrary to its inhibitory effect on SMCs, PAI-039 had no significant effect on human aortic EC (AEC) migration (Supplementary Fig. III in online data supplement).
PAI-039 inhibits STAT-1 signaling in SMCs
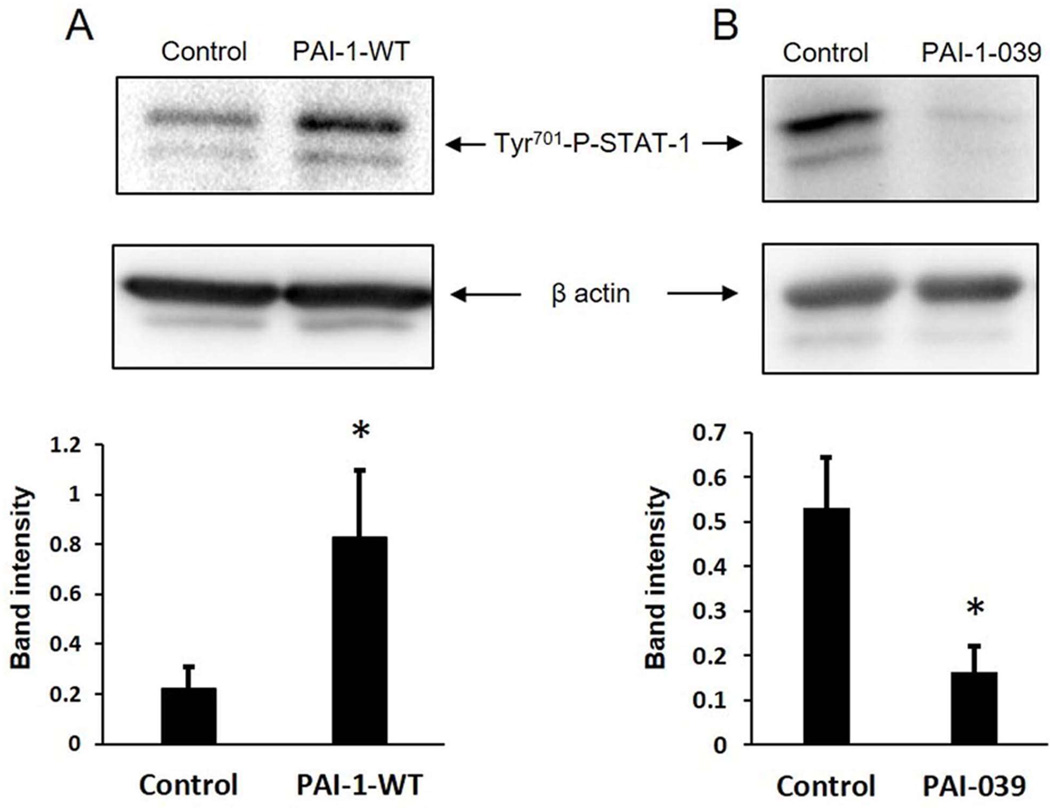
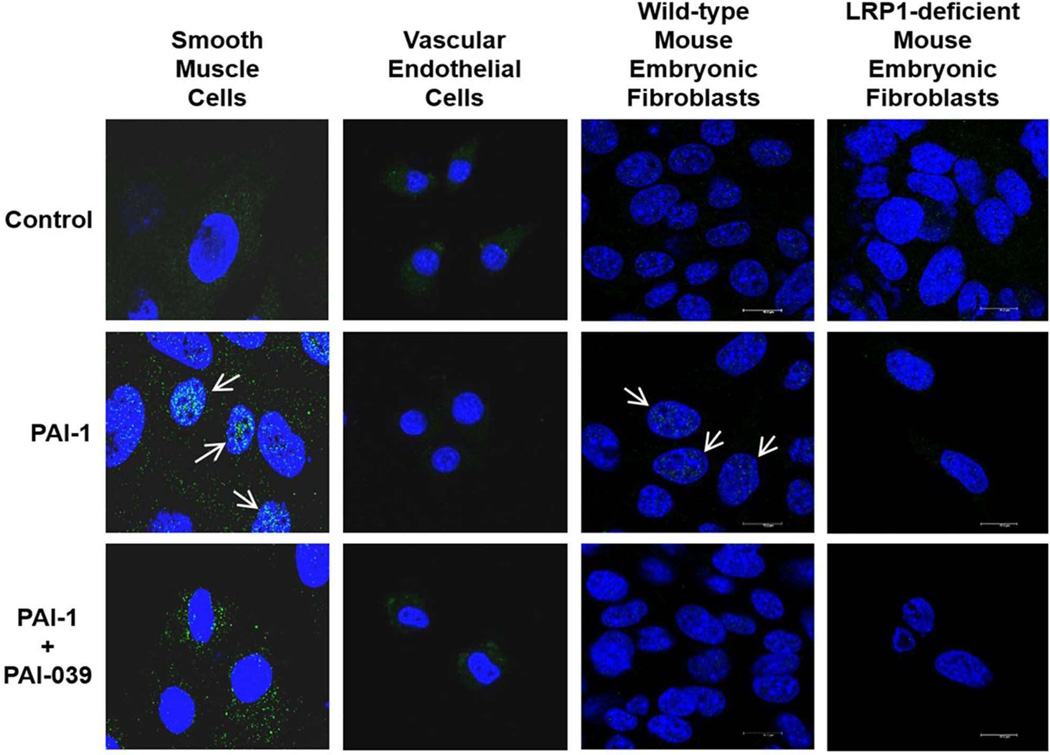
PAI-1 induces cell migration via an LRP1-dependent pathway involving STAT-1 nuclear translocation.11 However, the effects of PAI-1 and PAI-1 inhibition on STAT-1 phosphorylation have not been investigated previously. Therefore, human coronary artery SMCs were incubated in the presence or absence of recombinant wild-type PAI-1 (PAI-1-WT) and STAT-1 phosphorylation was analyzed by Western blotting. PAI-1-WT significantly increased STAT-1 phosphorylation at Tyr701 (Fig. 2A). We also studied the effect of PAI-1 inhibition on STAT-1 phosphorylation by incubating human coronary artery SMCs in the presence or absence of PAI-039. PAI-039 significantly decreased the level of constitutive STAT-1 phosphorylation in SMCs (Fig. 2B). To explore effects of PAI-039 on STAT-1 intracellular trafficking we treated murine arterial SMCs and AECs with PAI-1-WT and analyzed STAT-1 nuclear translocation by fluorescence immuno-histochemistry/confocal scanning laser microscopy. PAI-1-WT induced nuclear translocation of STAT-1 in SMCs, but not in AECs (Fig. 3). PAI-039 blocked the capacity of PAI-1-WT to induce STAT-1 nuclear translocation in SMCs, but had no discernable effect on STAT-1 signaling in AECs. LRP1 gene and protein expression were markedly lower in AECs than in SMCs (Fig. IV in online Data Supplement), suggesting that the resistance of ECs to the anti-migratory effect of PAI-039 may be explained by a very low level of expression of LRP1. PAI-039 blocked PAI-1-WT-induced STAT-1 nuclear translocation in wild-type mouse embryonic fibroblasts (MEFs), but there was no discernable effect of PAI-1-WT or PAI-039 on STAT-1 nuclear translocation in LRP1-deficient MEFs (Fig. 3). Furthermore, recombinant PAI-1-AK, a recombinant PAI-1 mutant that promotes cell migration by binding to LRP1, but not VN,32 significantly increased migration of wild-type MEFs, but not LRP1-deficient MEFs (Supplementary Fig. V in online Data Supplement). Together, these results suggest that the differential effects of pharmacological PAI-1 inhibition on SMC vs. EC migration are mediated by a distinct difference in the expression of LRP1 and downstream JAK/STAT-1 signaling between these two cell types.
Figure 2.
PAI-1 and PAI-039 regulate STAT-1 phosphorylation in SMCs. (A) Recombinant PAI-1 stimulates STAT-1 phosphorylation. Human coronary artery SMCs were incubated with vehicle control or PAI-1-WT (0.1 µg/mL) for 20 min, after which cells were lysed and STAT-1 phosphorylation was assessed by Western blotting and quantified by band intensity analysis. Data shown are from 12 experiments. *P<0.05 vs. control. Representative images from an individual experiment shown are. (B) PAI-039 inhibits STAT-1 phosphorylation. Human coronary artery SMCs were incubated with PAI-039 (10 µM) or vehicle control for 24 hrs, after which cell lysates were prepared and analyzed by Western blotting. Quantitative analysis of STAT-1 phosphorylation is shown (n=4 experiments/group; *P<0.01 vs. control), along with representative images from an individual experiment.
Figure 3.
Effects of PAI-1 and PAI-039 on STAT-1 trafficking, assessed by fluorescence immuno-histochemistry. PAI-1-WT (0.1 µg/mL) induces translocation of STAT-1 (green) from cell cytoplasm to nuclei (blue) in murine arterial SMCs and wild-type mouse embryonic fibroblasts (arrows), which is inhibited by PAI-039 (25 µM). In contrast, PAI-1 does not induce nuclear translocation of STAT-1 in murine endothelial cells or LRP1-deficient mouse embryonic fibroblasts. Scale bar equals 14 µm. All images shown are representative of data from at least 2 independent experiments.
PAI-039 inhibits intimal hyperplasia
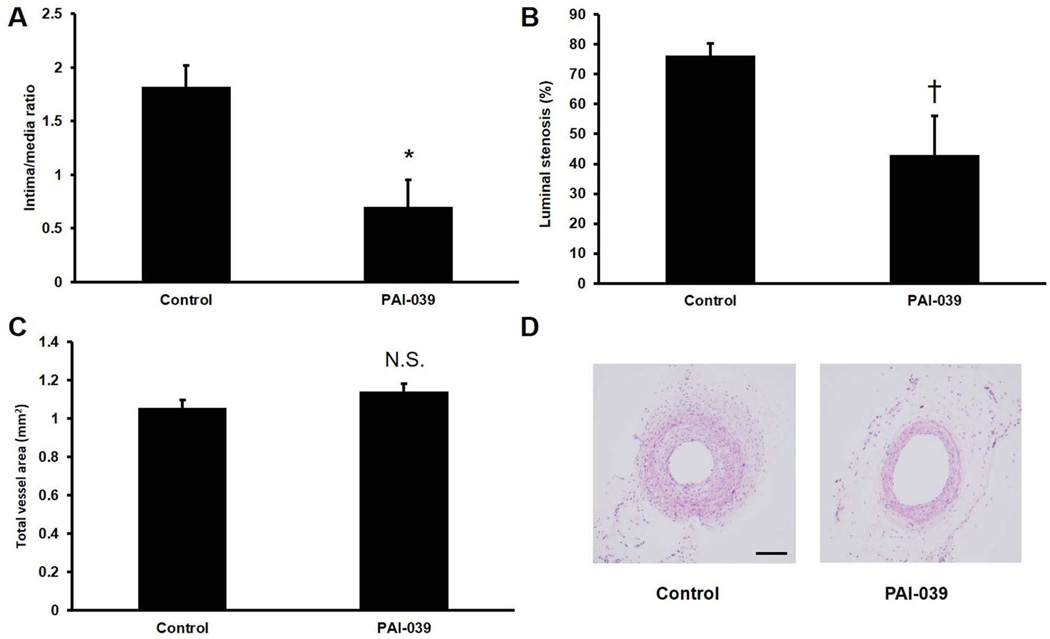
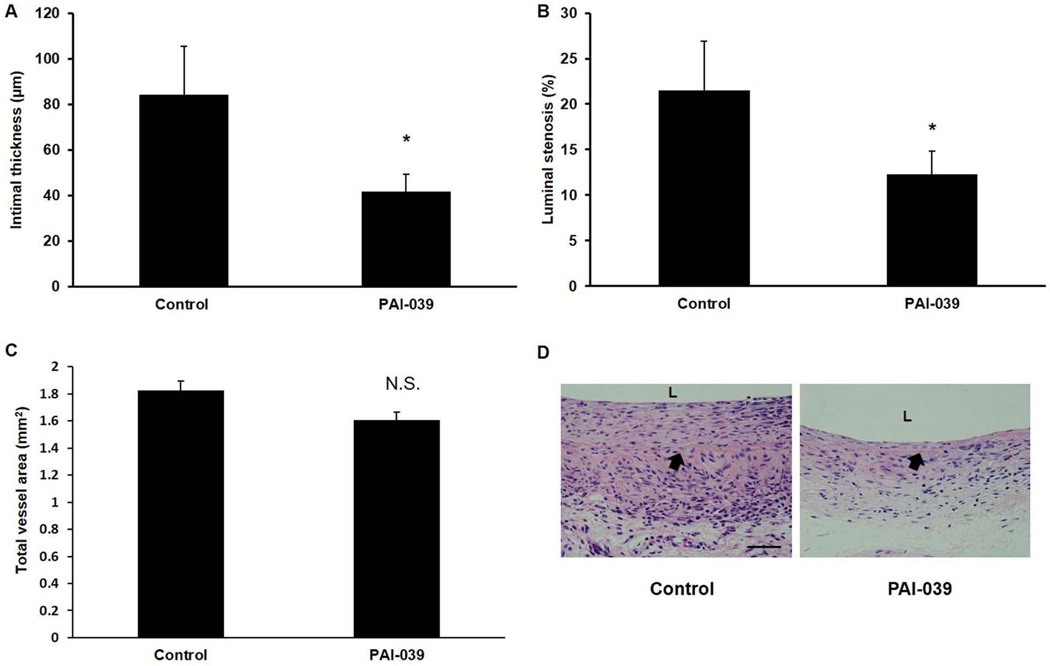
To examine the effects of PAI-039 in vivo, we subjected mice to carotid artery ligation, which induces formation of a SMC-rich neointima proximal to the site of vascular occlusion.33 We used pluronic gel to deliver PAI-039 (n=5) or vehicle control (n=5) to the surface of ligated carotid arteries. In addition, mice received PAI-039 or vehicle control by intraperitoneal (IP) injection for 4 weeks after surgery. PAI-039 significantly inhibited neointima formation and percent lumen stenosis, but not vessel total cross-sectional area (Fig. 4). Immunohistochemical analysis revealed that there was negligible macrophage (Mac-3-positive cell) invasion into the neointima of either experimental group at this time point (data not shown), consistent with a prior study.33 We also studied the effects of pharmacological PAI-1 inhibition on the intimal hyperplasia that develops in segments of inferior vena cava (IVC) after they are grafted into the transected carotid arteries of recipient mice.34 RT-PCR analysis revealed that PAI-1 gene expression was increased greater than 2-fold in carotid artery vein grafts harvested 4 weeks after surgery as compared to naïve IVC segments (Supplementary Fig. VI in online Data Supplement). Similar to our arterial study, PAI-039 significantly inhibited vein graft intimal hyperplasia and lumen stenosis, while having no significant effect on total vessel cross-sectional area (Fig. 5). In addition, PAI-039 significantly decreased macrophage invasion into vein grafts, as well as SMC content within vein graft neointima (Supplementary Fig. VII in online Data Supplement).
Figure 4.
PAI-039 inhibits intimal hyperplasia in murine carotid arteries. Wild-type mice were subjected to carotid ligation surgery and treated with PAI-039 (n=5) or vehicle control (n=5). Four weeks after surgery arterial (A) intima/media ratio, (B) % lumen stenosis, and (C) total area were measured. *P<0.01 vs. vehicle control; †P<0.05 vs. vehicle control; N.S. = difference between groups was not statistically significant (P>0.05). (D) Representative images of arteries from control and PAI-039-treated mice. Scale bar = 100 µm.
Figure 5.
PAI-039 inhibits intimal hyperplasia in murine vein grafts. Wild-type mice were subjected to vein graft surgery and treated with PAI-039 (n=8) or vehicle control (n=8), as described in Methods. Four weeks after surgery vein graft (A) intimal thickness, (B) % lumen stenosis, and (C) total area were measured. *P<0.03 vs. vehicle control; N.S. = difference between groups was not statistically significant (P>0.05). (D) Representative images of vein grafts from control and PAI-039-treated mice. Scale bar = 50 µm. L = lumen. Arrow denotes internal elastic lamina.
PAI-039 does not inhibit endothelial repopulation in vivo
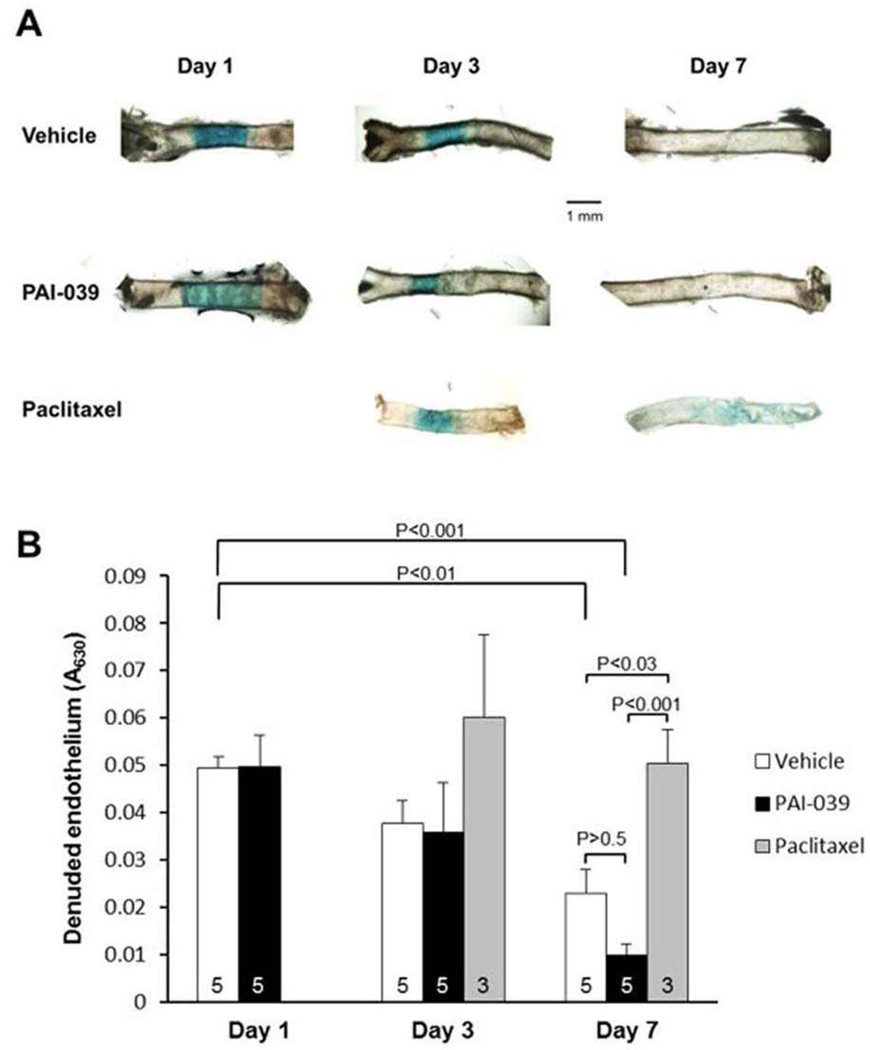
To determine if the lack of an inhibitory effect of PAI-039 on EC migration in vitro was also observed in vivo, we denuded the endothelium in mouse carotid arteries by mechanical injury and used the Evans Blue dye method to quantify the effects of PAI-039 on re-endothelialization, which is mediated by EC migration from the adjacent segments of normal artery into the denuded zone.35 PAI-039 had no significant inhibitory effect on EC migration in vivo (Fig. 6). In contrast, paciltaxel, which inhibits intimal hyperplasia,36 significantly inhibited EC recovery.
Figure 6.
PAI-039 does not inhibit re-endothelialization after mechanical carotid artery injury. Murine carotid arteries were injured and treated with PAI-039, vehicle control, or paclitaxel. At 1, 3, and 7 days after injury, absence of an intact endothelium was determined with Evans blue dye, which stains denuded endothelium. (A) Representative images of carotid arteries. (B) Quantitative analysis of denuded endothelium. The number of mice in each treatment group at each time point is indicated within each bar (a total of 36 mice were used in the experiment). Statistical analyses are shown (one-way analysis of variance with pairwise multiple comparison procedures).
Discussion
SMC migration from the tunica media to the intima results in neointima formation and is a key process in atherosclerosis, restenosis after coronary angioplasty/stent implantation, and vein graft disease. PAI-1 plays an important role in regulating SMC migration, with both pro- and anti-migratory effects being reported.8, 11 In this study we sought to determine the effects of pharmacological inhibition of PAI-1 on SMC migration under conditions that are relevant to human cardiovascular disease. We showed that pharmacological inhibition of PAI-1 with PAI-039 attenuates SMC, but not EC, migration in vitro. Our data suggest that PAI-039 inhibits SMC migration by down-regulating LRP1-dependent STAT-1 activation. Furthermore, PAI-039 inhibits arterial and venous intimal hyperplasia and vascular inflammation without adversely affecting re-endothelialization after vascular injury.
VN is present in the vascular wall and its expression is increased during neointima formation.37–39 VN binds PAI-1 and regulates its function.32 Previous studies have shown that PAI-039 effectively inhibits free, but not VN-bound, PAI-1.28, 31, 40 However, we have shown in this study that PAI-039 retains its anti-migratory effects on SMCs in vitro in collagen matrices supplemented with VN, and inhibits neointima formation in vivo. In a dynamic system involving living cells or in vivo it is likely that microenvironments exist in which free (i.e. PAI-039-susceptible) PAI-1 is present, due to either molar excess over VN or transient dissociation from VN, as occurs in an equilibrium binding interaction. The capacity of PAI-039 to selectively inhibit free PAI-1, while “bypassing” active, VN-bound PAI-1, is intriguing and could be advantageous in the context of adverse vascular remodeling, as VN-bound PAI-1 inhibits cell migration by blocking VN binding interactions with its cellular receptors.8, 9 VN-bound PAI-1 is also inhibited from binding to LRP1, a motogenic PAI-1 receptor.41 Therefore, PAI-039 does not inhibit the anti-migratory function of PAI-1, but does inhibit its pro-migratory effect by preventing its functional interaction with LRP1.
JAK/STAT-1 signaling plays an important role in regulating SMC activation and intimal hyperplasia.42–44 PAI-1 promotes cell migration by binding LRP1 and inducing nuclear translocation of STAT-1.11 In this study we have shown that PAI-1 induces, while PAI-039 decreases, STAT-1 phosphorylation in SMCs. We also have demonstrated that PAI-039 inhibits PAI-1-induced STAT-1 nuclear translocation in a LRP1-dependent manner. Together with our in vitro cell migration and in vivo vascular remodeling data, our results support the hypothesis that PAI-039 exerts important inhibitory effects on LRP1/JAK/STAT-1 activation in SMCs that down-regulate intimal hyperplasia. Our results, however, do not exclude the possibility that PAI-039 also targets other molecular pathways to inhibit neointima formation.
EC migration plays an essential role in restoring an intact vascular endothelium after vascular injury. In contrast to its inhibitory effect on SMC migration, PAI-039 did not significantly affect EC migration. This differential effect could be clinically significant – i.e. while it is desirable to inhibit SMC migration to reduce intimal hyperplasia and vascular stenosis, concomitant inhibition of EC recovery, as occurs with currently employed anti-restenosis drugs, promotes thrombosis and other adverse vascular effects. Our data suggest that the non-responsiveness of ECs to the anti-migratory effect of PAI-039 may be due to their very low expression level of LRP1, a finding that is consistent with published reports.11, 45
In a previous study we demonstrated that mice with complete PAI-1 deficiency exhibit increased vein graft intimal hyperplasia compared to WT controls.46 We hypothesized that the enhanced neointima formation in the absence of PAI-1 resulted from 1) enhanced availability of ECM VN to its cellular receptors (due to loss of binding of PAI-1 to VN), which would favor cell migration, and 2) enhanced activity of proteases that are susceptible to inhibition by PAI-1, such as plasmin and thrombin, which promote intimal hyperplasia.7, 46 Thus, it would seem paradoxical that pharmacological inhibition of PAI-1 would decrease intimal hyperplasia in the same model. Several factors may explain our results. It is known that PAI-1 has motogenic and anti-motogenic effects,8, 9, 11 both of which would be affected by genetic PAI-1 deficiency. However, it is likely that PAI-039 has a significantly greater inhibitory effect on the motogenic pool of free PAI-1 than on the anti-motogenic pool of VN-bound PAI-1.31 Such a differential effect of PAI-039 could potentially produce a different net effect on intimal hyperplasia than complete genetic PAI-1 deficiency. Consistent with this hypothesis, we showed previously that PAI-1-deficient SMCs exhibit enhanced migration compared to WT SMCs,32 yet, as we demonstrate in the current study, PAI-039 inhibits SMC migration. Other factors may also contribute to the differential effects of genetic- vs. pharmacologically-induced reductions in PAI-1 activity on vascular remodeling. First, it is possible that genetic deficiency of PAI-1 produces compensatory changes in the expression of other proteins that affect vein graft remodeling. Second, PAI-1 regulates enzyme activity not only in the extracellular space, but also within the intracellular environment.20 While genetic deficiency would affect both compartments of PAI-1, it is not known if PAI-039 inhibits intracellular PAI-1. Lastly, it is possible that complete genetic deficiency of PAI-1 might produce different phenotypic effects than partial or near-complete inhibition of PAI-1, which would be expected from a pharmacological inhibitor. Of note, genetic deficiency of soluble epoxide hydrolase produced an opposite effect on cardiac fibrosis than that produced by a pharmacological inhibitor.47 Thus, there is a precedent for genetic deficiency and pharmacological inhibition of a factor producing opposing phenotypes.
Recent studies have suggested that pharmacological PAI-1 inhibition has considerable potential as a therapeutic strategy to prevent and treat vascular diseases by pathways that are independent of fibrinolysis. TM5441, a small molecule PAI-1 inhibitor, inhibits vascular senescence and other organ dysfunction associated with aging.30, 48 TM5275, another PAI-1 inhibitor, inhibits macrophage migration.29 Consistent with this study, we found that PAI-039 decreased macrophage invasion into vein grafts. In contrast to our findings, Leik et al showed that PAI-039 promoted SMC migration in vitro.40 In this study purified PAI-1 was pre-incubated with PAI-039, then added to SMCs migrating on a 2-dimensional, VN-coated surface. These experimental conditions, while informative regarding the potential of PAI-039 to inhibit PAI-1, probably did not adequately reproduce the physiological conditions under which SMCs migrate in vivo. A recent study showed that PAI-039 induces apoptosis of SMCs, thereby producing an anti-proliferative effect,49, which is consistent with the known anti-apoptotic properties of PAI-1.20, 22 In addition, another recent study demonstrated that PAI-039 improved dermal wound closure in diabetic mice,50 which is intriguing because vascular remodeling has been considered as a form of wound repair.51
In conclusion, we have shown that pharmacological inhibition of PAI-1 with PAI-039 inhibits SMC migration and intimal hyperplasia without adversely affecting EC migration or vascular re-endothelialization. These differential effects appear to be due to the marked difference in LRP1 expression between SMCs and ECs, with the low level of LRP1 expression in ECs shielding them from the effects of PAI-1 and PAI-039 on LRP1-induced JAK/STAT-1 signaling and cell migration.11 Our study suggests that pharmacological targeting of PAI-1 with PAI-039, and perhaps other PAI-1 inhibitors, may be an effective strategy to treat and prevent vascular disease without disrupting EC function, which is a major limitation of currently employed anti-restenosis drugs. Additional studies that assess the effects of pharmacological PAI-1 inhibitors on adverse vascular remodeling in the setting of diabetes mellitus and obesity, which are associated with increased PAI-1 expression,52, 53 and preexisting atherosclerosis are warranted. Such studies could lead to clinical trials to determine if pharmacological PAI-1 inhibition exerts beneficial effects on human atherosclerosis, restenosis, vein graft disease, and other obstructive vascular diseases characterized by increased PAI-1 expression.54, 55
Supplementary Material
Highlights.
PAI-039, a small molecule, specific inhibitor of plasminogen activator inhibitor-1 (PAI-1), decreases vascular SMC migration.
PAI-039 does not inhibit endothelial cell (EC) migration.
The differential effect of PAI-039 on SMCs vs. ECs appears to be mediated by a marked difference in the expression level of LDL receptor-related protein-1 (LRP1) between cell types, and consequently, in PAI-1-dependent activation of the LRP1/JAK/STAT-1 signaling pathway.
In mice, PAI-039 inhibits intimal hyperplasia, but not re-endothelialization after vascular injury, suggesting that PAI-1 may be an important drug target for preventing and treating obstructive vascular diseases.
Acknowledgments
We thank Alexander Jurkevich, PhD, Molecular Cytology Core, University of Missouri, for technical assistance with performing laser scanning confocal microscopy.
Sources of Funding
This work was supported by NIH grants HL57346 and HL095951 (WPF), a Department of Veterans Affairs Merit Review Award (CARA-007-12S; WPF), the Missouri Life Sciences Research Board (WPF), and an American Heart Association Scientist Development Grant (JW).
Nonstandard Abbreviations and Acronyms
- AEC
aortic endothelial cell
- ECM
extracellular matrix
- JAK
Janus kinase
- LRP1
low density lipoprotein receptor-related protein 1
- PAI-1
plasminogen activator inhibitor-1
- SMC
smooth muscle cell
- STAT
signal transducers and activators of transcription
- VN
vitronectin
Footnotes
Disclosures
The authors have no financial or other conflicts of interests.
References
- 1.Declerck PJ, Gils A. Three decades of research on plasminogen activator inhibitor-1: a multifaceted serpin. Semin Thromb Hemost. 2013;39:356–364. doi: 10.1055/s-0033-1334487. [DOI] [PubMed] [Google Scholar]
- 2.Fay WP, Shapiro AD, Shih JL, Schleef RR, Ginsburg D. Complete Deficiency of Plasminogen-Activator Inhibitor Type 1 Due to a Frame-Shift Mutation. New England Journal of Medicine. 1992;327:1729–1733. doi: 10.1056/NEJM199212103272406. [DOI] [PubMed] [Google Scholar]
- 3.Hamsten A, Wiman B, De Faire U, Blomback M. Increased plasma levels of a rapid inhibitor of tissue plasminogen activator in young survivors of myocardial infarction. New England Journal of Medicine. 1985;313:1557–1563. doi: 10.1056/NEJM198512193132501. [DOI] [PubMed] [Google Scholar]
- 4.Tuttolomondo A, Pinto A, Corrao S, Di Raimondo D, Fernandez P, Di Sciacca R, Arnao V, Licata G. Immuno-inflammatory and thrombotic/fibrinolytic variables associated with acute ischemic stroke diagnosis. Atherosclerosis. 2009;203:503–508. doi: 10.1016/j.atherosclerosis.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Juhan-Vague I, Valadier J, Alessi MC, Aillaud MF, Ansaldi J, Philip-Joet C, Holvoet P, Serradimigni A, Collen D. Deficient t-PA release and elevated PA inhibitor levels in patients with spontaneous or recurrent deep venous thrombosis. Thromb Haemost. 1987;57:67–72. [PubMed] [Google Scholar]
- 6.Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000;190:300–309. doi: 10.1002/(SICI)1096-9896(200002)190:3<300::AID-PATH596>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P, Moons L, Ploplis V, Plow E, Collen D. Impaired arterial neointima formation in mice with disruption of the plasminogen gene. Journal of Clinical Investigation. 1997;99:200–208. doi: 10.1172/JCI119148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stefansson S, Lawrence DA. The serpin PAI-1 inhibits cell migration by blocking integrin alpha V beta 3 binding to vitronectin. Nature. 1996;383:441–443. doi: 10.1038/383441a0. [DOI] [PubMed] [Google Scholar]
- 9.Deng G, Curriden SA, Wang S, Rosenberg S, Loskutoff DJ. Is plasminogen activator inhibitor-1 the molecular switch that governs urokinase receptor-mediated cell adhesion and release? The Journal of Cell Biology. 1996;134:1563–1571. doi: 10.1083/jcb.134.6.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown SL, Lundgren CH, Nordt T, Fujii S. Stimulation of migration of human aortic smooth muscle cells by vitronectin: implications for atherosclerosis. Cardiovascular Research. 1994;28:1815–1820. doi: 10.1093/cvr/28.12.1815. [DOI] [PubMed] [Google Scholar]
- 11.Degryse B, Neels JG, Czekay RP, Aertgeerts K, Kamikubo Y, Loskutoff DJ. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Moons L, Lijnen R, Janssens S, Lupu F, Collen D, Gerard RD. Inhibitory Role of Plasminogen Activator Inhibitor-1 in Arterial Wound Healing and Neointima Formation. A Gene Targeting and Gene Transfer Study in Mice. 1997;96:3180–3191. doi: 10.1161/01.cir.96.9.3180. [DOI] [PubMed] [Google Scholar]
- 13.Otsuka G, Agah R, Frutkin AD, Wight TN, Dichek DA. Transforming growth factor beta-1 induces neointima formation through plasminogen activator inhibitor-1-dependent pathways. Arterioscler Thromb Vasc Biol. 2006;26:737–743. doi: 10.1161/01.ATV.0000201087.23877.e1. [DOI] [PubMed] [Google Scholar]
- 14.de Waard V, Arkenbout EK, Carmeliet P, Lindner V, Pannekoek H. Plasminogen Activator Inhibitor 1 and Vitronectin Protect Against Stenosis in a Murine Carotid Artery Ligation Model. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:1978–1983. doi: 10.1161/01.atv.0000042231.04318.e6. [DOI] [PubMed] [Google Scholar]
- 15.Peng L, Bhatia N, Parker AC, Zhu Y, Fay WP. Endogenous vitronectin and plasminogen activator inhibitor-1 promote neointima formation in murine carotid arteries. Arterioscler Thromb Vasc Biol. 2002;22:934–939. doi: 10.1161/01.atv.0000019360.14554.53. [DOI] [PubMed] [Google Scholar]
- 16.Ploplis VA, Cornelissen I, Sandoval-Cooper MJ, Weeks L, Noria FA, Castellino FJ. Remodeling of the Vessel Wall after Copper-Induced Injury Is Highly Attenuated in Mice with a Total Deficiency of Plasminogen Activator Inhibitor-1. The American Journal of Pathology. 2001;158:107–117. doi: 10.1016/S0002-9440(10)63949-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Y, Farrehi PM, Fay WP. Plasminogen activator inhibitor type 1 enhances neointima formation after oxidative vascular injury in atherosclerosis-prone mice. Circulation. 2001;103:3105–3110. doi: 10.1161/01.cir.103.25.3105. [DOI] [PubMed] [Google Scholar]
- 18.Schäfer K, Müller K, Hecke A, Mounier E, Goebel J, Loskutoff DJ, Konstantinides S. Enhanced Thrombosis in Atherosclerosis-Prone Mice Is Associated With Increased Arterial Expression of Plasminogen Activator Inhibitor-1. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23:2097–2103. doi: 10.1161/01.ATV.0000097766.36623.DF. [DOI] [PubMed] [Google Scholar]
- 19.DeYoung MB, Tom C, Dichek DA. Plasminogen Activator Inhibitor Type 1 Increases Neointima Formation in Balloon-Injured Rat Carotid Arteries. Circulation. 2001;104:1972–1971. doi: 10.1161/hc4101.097110. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Kelm RJ, Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspase-3 in vascular smooth muscle cells by plasminogen activator inhibitor type-1. J Cellular Biochem. 2004;92:178–188. doi: 10.1002/jcb.20058. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Budd RC, Kelm RJ, Jr, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- 22.Schneider DJ, Chen Y, Sobel BE. The effect of plasminogen activator inhibitor type 1 on apoptosis. Thromb Haemost. 2008;100:1037–1040. [PubMed] [Google Scholar]
- 23.Luttun A, Lupu F, Storkebaum E, Hoylaerts MF, Moons L, Crawley J, Bono F, Poole AR, Tipping P, Herbert JM, Collen D, Carmeliet P. Lack of plasminogen activator inhibitor-1 promotes growth and abnormal matrix remodeling of advanced atherosclerotic plaques in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:499–505. doi: 10.1161/hq0302.104529. [DOI] [PubMed] [Google Scholar]
- 24.Cao C, Lawrence DA, Li Y, Von Arnim CA, Herz J, Su EJ, Makarova A, Hyman BT, Strickland DK, Zhang L. Endocytic receptor LRP together with tPA and PAI-1 coordinates Mac-1-dependent macrophage migration. Embo j. 2006;25:1860–1870. doi: 10.1038/sj.emboj.7601082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corgosinho FC, de Piano A, Sanches PL, Campos RM, Silva PL, Carnier J, Oyama LM, Tock L, Tufik S, de Mello MT, Damaso AR. The role of PAI-1 and adiponectin on the inflammatory state and energy balance in obese adolescents with metabolic syndrome. Inflammation. 2012;35:944–951. doi: 10.1007/s10753-011-9397-2. [DOI] [PubMed] [Google Scholar]
- 26.Renckens R, Roelofs JJTH, De Waard V, Florquin S, Lijnen HR, Carmeliet P, Van Der Poll T. The role of plasminogen activator inhibitor type 1 in the inflammatory response to local tissue injury. Journal of Thrombosis and Haemostasis. 2005;3:1018–1025. doi: 10.1111/j.1538-7836.2005.01311.x. [DOI] [PubMed] [Google Scholar]
- 27.Fortenberry YM. Plasminogen activator inhibitor-1 inhibitors: a patent review (2006-present) Expert Opin Ther Patents. 2013;23:801–815. doi: 10.1517/13543776.2013.782393. [DOI] [PubMed] [Google Scholar]
- 28.Elokdah H, Abou-Gharbia M, Hennan JK, McFarlane G, Mugford CP, Krishnamurthy G, Crandall DL. Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: design, synthesis, and preclinical characterization. J Med Chem. 2004;47:3491–3494. doi: 10.1021/jm049766q. [DOI] [PubMed] [Google Scholar]
- 29.Ichimura A, Matsumoto S, Suzuki S, Dan T, Yamaki S, Sato Y, Kiyomoto H, Ishii N, Okada K, Matsuo O, Hou FF, Vaughan DE, van Ypersele de Strihou C, Miyata T. A Small Molecule Inhibitor to Plasminogen Activator Inhibitor 1 Inhibits Macrophage Migration. Arterioscler Thromb Vasc Biol. 2013;33:935–942. doi: 10.1161/ATVBAHA.113.301224. [DOI] [PubMed] [Google Scholar]
- 30.Boe AE, Eren M, Murphy SB, Kamide CE, Ichimura A, Terry D, McAnally D, Smith LH, Miyata T, Vaughan DE. Plasminogen Activator Inhibitor-1 Antagonist TM5441 Attenuates N-Nitro-l-Arginine Methyl Ester-Induced Hypertension and Vascular Senescence. Circulation. 2013;128:2318–2324. doi: 10.1161/CIRCULATIONAHA.113.003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorlatova NV, Cale JM, Elokdah H, Li D, Fan K, Warnock M, Crandall DL, Lawrence DA. Mechanism of inactivation of plasminogen activator inhibitor-1 by a small molecule inhibitor. J Biol Chem. 2007;282:9288–9296. doi: 10.1074/jbc.M611642200. [DOI] [PubMed] [Google Scholar]
- 32.Garg N, Goyal N, Strawn TL, Wu J, Mann KM, Lawrence DA, Fay WP. Plasminogen activator inhibitor-1 and vitronectin expression level and stoichiometry regulate vascular smooth muscle cell migration through physiological collagen matrices. J Thromb Haemost. 2010;8:1847–1854. doi: 10.1111/j.1538-7836.2010.03907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar A, Lindner V. Remodeling with neointima formation in the mouse carotid artery after cessation of blood flow. Arterioscler Thromb Vasc Biol. 1997;17:2238–2244. doi: 10.1161/01.atv.17.10.2238. [DOI] [PubMed] [Google Scholar]
- 34.Zou Y, Dietrich H, Hu Y, Metzler B, Wick G, Xu Q. Mouse model of venous bypass graft arteriosclerosis. Am J Pathol. 1998;153:1301–1310. doi: 10.1016/S0002-9440(10)65675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kikuchi S, Umemura K, Kondo K, Saniabadi AR, Nakashima M. Photochemically Induced Endothelial Injury in the Mouse as a Screening Model for Inhibitors of Vascular Intimal Thickening. Arteriosclerosis, Thrombosis, and Vascular Biology. 1998;18:1069–1078. doi: 10.1161/01.atv.18.7.1069. [DOI] [PubMed] [Google Scholar]
- 36.Grube E, Silber S, Hauptmann KE, Mueller R, Buellesfeld L, Gerckens U, Russell ME. TAXUS I: Six- and Twelve-Month Results From a Randomized, Double-Blind Trial on a Slow-Release Paclitaxel-Eluting Stent for De Novo Coronary Lesions. Circulation. 2003;107:38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- 37.van Aken EB, Seiffert D, Thinnes T, Loskutoff JD. Localization of vitronectin in the normal and atherosclerotic human vessel wall. Histochemistry and Cell Biology. 1997;107:313–320. doi: 10.1007/s004180050116. [DOI] [PubMed] [Google Scholar]
- 38.Stoop AA, Lupu F, Pannekoek H. Colocalization of Thrombin, PAI-1, and Vitronectin in the Atherosclerotic Vessel Wall: A Potential Regulatory Mechanism of Thrombin Activity by PAI-1/Vitronectin Complexes. Arteriosclerosis, Thrombosis, and Vascular Biology. 2000;20:1143–1149. doi: 10.1161/01.atv.20.4.1143. [DOI] [PubMed] [Google Scholar]
- 39.Dufourcq P, Couffinhal T, Alzieu P, Daret D, Moreau C, Duplàa C, Bonnet J. Vitronectin is up-regulated after vascular injury and vitronectin blockade prevents neointima formation. Cardiovascular Research. 2002;53:952–962. doi: 10.1016/s0008-6363(01)00547-8. [DOI] [PubMed] [Google Scholar]
- 40.Leik CE, Su EJ, Nambi P, Crandall DL, Lawrence DA. Effect of pharmacologic plasminogen activator inhibitor-1 inhibition on cell motility and tumor angiogenesis. J Thromb Haemost. 2006;4:2710–2715. doi: 10.1111/j.1538-7836.2006.02244.x. [DOI] [PubMed] [Google Scholar]
- 41.Kamikubo Y, Neels JG, Degryse B. Vitronectin inhibits plasminogen activator inhibitor-1-induced signalling and chemotaxis by blocking plasminogen activator inhibitor-1 binding to the low-density lipoprotein receptor-related protein. Int J Biochem Cell Biol. 2009;41:578–585. doi: 10.1016/j.biocel.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Kirchmer MN, Franco A, Albasanz-Puig A, Murray J, Yagi M, Gao L, Dong ZM, Wijelath ES. Modulation of vascular smooth muscle cell phenotype by STAT-1 and STAT-3. Atherosclerosis. 2014;234:169–175. doi: 10.1016/j.atherosclerosis.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Madamanchi NR, Li S, Patterson C, Runge MS. Reactive Oxygen Species Regulate Heat-Shock Protein 70 via the JAK/STAT Pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2001;21:321–326. doi: 10.1161/01.atv.21.3.321. [DOI] [PubMed] [Google Scholar]
- 44.Seki Y, Kai H, Shibata R, Nagata T, Yasukawa H, Yoshimura A, Imaizumi T. Role of the JAK/STAT Pathway in Rat Carotid Artery Remodeling After Vascular Injury. Circulation Research. 2000;87:12–18. doi: 10.1161/01.res.87.1.12. [DOI] [PubMed] [Google Scholar]
- 45.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL Receptor-Related Protein 1: Unique Tissue-Specific Functions Revealed by Selective Gene Knockout Studies. Physiological Reviews. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Y, Strawn TL, Grunz EA, Stevenson MJ, Lohman AW, Lawrence DA, Fay WP. Multifaceted Role of Plasminogen Activator Inhibitor-1 in Regulating Early Remodeling of Vein Bypass Grafts. Arterioscler Thromb Vasc Biol. 2011;31:1781–1787. doi: 10.1161/ATVBAHA.111.228767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Li N, Pang W, Zhang X, Hammock BD, Ai D, Zhu Y. Opposite Effects of Gene Deficiency and Pharmacological Inhibition of Soluble Epoxide Hydrolase on Cardiac Fibrosis. PLoS One. 2014;9:e94092. doi: 10.1371/journal.pone.0094092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eren M, Boe AE, Murphy SB, Place AT, Nagpal V, Morales-Nebreda L, Urich D, Quaggin SE, Budinger GRS, Mutlu GkM, Miyata T, Vaughan DE. PAI-1–regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci USA. 2014;111:7090–7095. doi: 10.1073/pnas.1321942111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simone TM, Higgins SP, Archambeault J, Higgins CE, Ginnan RG, Singer H, Higgins PJ. A small molecule PAI-1 functional inhibitor attenuates neointimal hyperplasia and vascular smooth muscle cell survival by promoting PAI-1 cleavage. Cellular Signalling. 2015 doi: 10.1016/j.cellsig.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rebalka IA, Raleigh MJ, ΔGÇÖSouza DM, Coleman SK, Rebalka AN, Hawke TJ. Inhibition of PAI-1 Via PAI-039 Improves Dermal Wound Closure in Diabetes. Diabetes. 2015;64:2593–2602. doi: 10.2337/db14-1174. [DOI] [PubMed] [Google Scholar]
- 51.Marx SO, Totary-Jain H, Marks AR. Vascular Smooth Muscle Cell Proliferation in Restenosis. Circulation: Cardiovascular Interventions. 2011;4:104–111. doi: 10.1161/CIRCINTERVENTIONS.110.957332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Juhan-Vague I, Alessi MC. PAI-1, obesity, insulin resistance and risk of cardiovascular events. Thromb Haemost. 1997;78:656–660. [PubMed] [Google Scholar]
- 53.Taeye BD, Smith LH, Vaughan DE. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol. 2005;5:149–154. doi: 10.1016/j.coph.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Schneiderman J, Sawdey MS, Keeton MR, Bordin GM, Bernstein EF, Dilley RB, Loskutoff DJ. Increased type 1 plasminogen activator inhibitor gene expression in atherosclerotic human arteries. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:6998–7002. doi: 10.1073/pnas.89.15.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kauhanen P, Sirén V, Carpén O, Vaheri A, Lepäntalo M, Lassila R. Plasminogen Activator Inhibitor-1 in Neointima of Vein Grafts - Its Role in Reduced Fibrinolytic Potential and Graft Failure. Circulation. 1997;96:1783–1789. doi: 10.1161/01.cir.96.6.1783. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.