Abstract
Rationale:
Altered cardiac energetics is known to play an important role in the progression toward heart failure. A noninvasive method for imaging metabolic markers that could be used in longitudinal studies would be useful for understanding therapeutic approaches that target metabolism.
Objective:
To demonstrate the first hyperpolarized 13C metabolic magnetic resonance imaging of the human heart.
Methods and Results:
Four healthy subjects underwent conventional proton cardiac magnetic resonance imaging followed by 13C imaging and spectroscopic acquisition immediately after intravenous administration of a 0.1 mmol/kg dose of hyperpolarized [1-13C]pyruvate. All subjects tolerated the procedure well with no adverse effects reported ≤1 month post procedure. The [1-13C]pyruvate signal appeared within the chambers but not within the muscle. Imaging of the downstream metabolites showed 13C-bicarbonate signal mainly confined to the left ventricular myocardium, whereas the [1-13C]lactate signal appeared both within the chambers and in the myocardium. The mean 13C image signal:noise ratio was 115 for [1-13C]pyruvate, 56 for 13C-bicarbonate, and 53 for [1-13C]lactate.
Conclusions:
These results represent the first 13C images of the human heart. The appearance of 13C-bicarbonate signal after administration of hyperpolarized [1-13C]pyruvate was readily detected in this healthy cohort (n=4). This shows that assessment of pyruvate metabolism in vivo in humans is feasible using current technology.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov. Unique identifier: NCT02648009.
Keywords: heart failure, magnetic resonance imaging, metabolic imaging, metabolism, mitochondria
Understanding the role of altered intermediary metabolism in driving the transition from functional compensation to decompensated heart failure remains a promising avenue for the development of new therapies.1–4 The foundations of cardiac metabolism research are built on experiments in vitro and in the isolated perfused heart.5,6 However, to reproduce the effects of insulin and other hormones, plasma substrate levels, and workload/energy demand, in vivo assessment of cardiac metabolism is of paramount importance.
Editorial, see p 1146
In This Issue, see p 1145
Existing clinical imaging modalities for studying cardiac metabolism include positron emission tomography (PET) and magnetic resonance spectroscopy. Although providing unique insights into metabolism, both techniques suffer limitations. Magnetic resonance spectroscopy is only able to detect a limited range of biochemical reactions because of the inherent low sensitivity of this technique, and PET gives no information about the metabolic fate of the substrate beyond its cellular uptake. Furthermore, PET tracers deliver a dose of ionizing radiation, thus limiting repeated application. New methods for noninvasively probing the dynamics of cardiac metabolism in patients are still needed to augment the information currently available to the clinician.
Hyperpolarized carbon-13 (13C) magnetic resonance imaging (MRI) is promising in this regard because it can give images showing the uptake of metabolic substrates and subsequent intracellular conversion into downstream products.7–10 The method relies on rapid dissolution dynamic nuclear polarization, which can provide a signal enhancement of >4 orders of magnitude.7 Measurements based on these images may give new information about the metabolic state of the heart in individual patients. In addition to its scientific value as a powerful tool for investigating normal cardiac metabolism,11 developments in preclinical models support the potential clinical value of 13C MRI in evaluating pathologies of the human heart, including myocardial viability after acute ischemia/reperfusion injury,12 early- and late-onset metabolic changes in the failing heart,13 and diabetic cardiomyopathy.14
Using the substrate [1-13C]pyruvate, which is an important intermediate of cellular metabolism, this study demonstrates the feasibility of observing, in a single examination, the following 4 different single-step enzyme-catalyzed reactions: pyruvate dehydrogenase complex (PDC), alanine aminotransferase, lactate dehydrogenase, and carbonic anhydrase. Of particular interest is the 13C-bicarbonate signal that can be measured within the myocardium after an intravenous injection of [1-13C]pyruvate, which is proportional to flux of pyruvate through PDC on the mitochondrial membrane.8 Because 13C MRI can be integrated into a conventional cardiovascular magnetic resonance (CMR) workup with only a small addition to the scan duration (eg, 10 minutes), the translation of this new form of MRI to patient studies is readily achievable, particularly where cardiac MRI is already used clinically. The first images of hyperpolarized 13C MRI in the human heart are presented in this pilot study in 4 healthy volunteers.
Methods
Healthy subjects (n=4) were recruited and gave written informed consent under a protocol approved by the Sunnybrook Research Ethics Board and approved by Health Canada as a Clinical Trial Application. Subjects were instructed to eat as they normally would, and an oral carbohydrate load was administered ≈1 hour before the pyruvate injection as 35 g of Gatorade powder in water, containing 34 g of sucrose and dextrose, to be consistent with a protocol previously established for preclinical cardiac imaging.15 A 20-gauge intravenous catheter was placed in the left arm before each subject was positioned supine and feet first within a 13C volume transmit coil system (GE Healthcare, Cleveland, OH) installed on a GE MR750 3.0 T MRI scanner (GE Healthcare, Waukesha, WI). The 13C receiver coil system consisted of 2 separate paddles each containing 4 receiver elements (channels).16 One paddle was positioned on the anterior chest wall over the heart, with the other paddle under the upper left back. The left arm was fully extended, positioned directly by the side of the subject, and supported with padding to prevent direct contact with the transmit coil. A pulse oximeter was placed on the left index finger for cardiac triggering.
The investigational product, designated with the generic name Hyperpolarized (13)C Pyruvate Injection, was prepared using a General Electric SPINLab system17 equipped with the quality control module, which provides automated measurement and display of release parameters. Each dose was compounded from 1.47±0.05 g of [1-13C]pyruvic acid (Sigma Aldrich, St. Louis, MO) combined with AH111501 [Tris(8-carboxy-2,2,6,6 (tetra(methoxyethyl) benzo-[1,2–4,5′]bis-(1,3)dithiole-4- yl)methyl sodium salt] (Syncom, Groningen, The Netherlands) in a 49:1 weight by weight ratio, respectively. Once frozen in the polarizer, each sample was exposed to microwave irradiation for ≈3 hours to achieve maximum steady-state polarization. The other components within the sterile fluid pathway were 19.0±0.5 mL of sterile water for injection, which is heated and used to rapidly dissolve the frozen sample, and a buffered base and chelating solution in the receiver vessel made by mixing 17.5±0.5 mL of a stock solution (600 mmol/L NaOH, 333 mmol/L Tris base, and 333 mg/L disodium EDTA) with 19.0±0.5 mL of sterile water for injection.
Short-axis cardiac-gated cine images were acquired from slices covering the left ventricle (LV) using the body coil for both transmit and receive, with a separate breath-hold performed for each slice. After completion of the anatomic scanning, a prescan calibration of the 13C receive frequency and transmit power was performed using the signal from a 1.5-cm diameter spherical phantom containing an ≈8 mol/L solution of 13C-urea, which was fixed on top of the anterior receiver coil housing. The dissolution of the polarized sample was initiated by the operator, and the quality control parameters were evaluated by the study pharmacist to ensure that the parameters were within specifications. On release, the dose syringe was rapidly loaded onto a Spectris Solaris power injector (Medrad, Indianola, PA), and the volume corresponding to a 0.1 mmol/kg dose was injected at 5 mL/s followed by a 25 mL saline flush at 5 mL/s.
The 13C imaging data acquisition was initiated at the end of the saline flush and was preceded by the same automated breath-hold instructions as used for the anatomic scanning. The data acquisition consisted of slice-selective spectral-spatial excitation of the 13C-bicarbonate resonance in 6 slices of 1-cm thickness covering the LV.15 As a cardiac-gated sequence, the heart rate affects the scan time and sets an upper limit on the number of slices that can be acquired per heartbeat; the breath-hold duration sets the lower limit. To maintain a reasonable breath-hold duration, 3 slices were sequentially excited and imaged within each cardiac cycle (taking 2 cardiac cycles to complete all 6), with the cardiac trigger delay set such that the acquisition window was aligned with diastole. These 6 slices were then imaged at the [1-13C]lactate and [1-13C]pyruvate resonances, and the whole process was repeated 3×, taking 18 cardiac cycles to complete. The nominal spatial resolution was 8.8 × 8.8 mm in-plane with a 48-cm field-of-view.
Immediately after the 13C imaging, the residual hyperpolarized magnetization was used to acquire MR spectroscopic data from the whole heart. For the first subject, a slice-selective radiofrequency pulse was used (nominal flip angle=30°, 10-cm axial slab covering the heart), and the acquisition was not cardiac gated (repetition time=3 s). For the 3 subsequent subjects, a 200-µs nonselective radiofrequency pulse was used (nominal flip angle=18°), and the acquisition was cardiac gated, so the repetition times were ≈3 s (3–4 interbeat intervals, depending on heart rate). The shorter radiofrequency pulse was intended to excite the 13CO2 resonance, which was not observable in the data from the first subject. Whole-heart spectroscopy was used to provide adequate signal, given that much of the magnetization had likely been consumed by the preceding 13C imaging.
Results
The 4 subjects recruited were male with no known cardiac history. Their mean age was 41 years (range 28–48). The mean LV ejection fraction was 61% (range 55%–66%). The LV end-diastolic and end-systolic volumes indexed to body surface area were 104 (range 82–115) and 41 (range 30–51) mL/m2, respectively. The mean LV mass (indexed to body surface area) was 84 g/m2 (range 62–93; Table).
Table.
Subject Characteristics and Scan Details
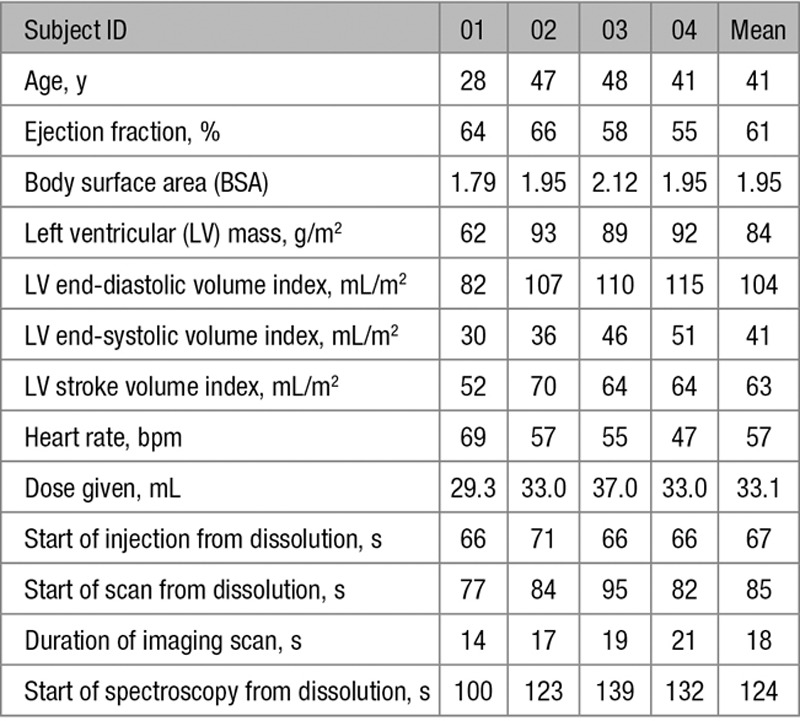
Of the 4 doses injected, the mean polarization was 28% (range 17%–35%), pH was 7.35 (range 7.3–7.4), temperature was 36.4°C (range 36.0–37.0°C). The duration from dissolution to the start of imaging ranged from 66 to 71 s. Imaging scan duration ranged from 14 to 21 s. The injected volume of [1-13C]pyruvate ranged from 29 to 37 mL to achieve 0.1 mmol/kg dose.
All subjects tolerated the procedure well. Two subjects noted a sweet taste after [1-13C]pyruvate injection, which dissipated shortly afterward. No serious adverse effects were noted. During injection, there was no change in heart rate and no reported change in respiration. Noninvasive blood pressure measured before the CMR examination and post [1-13C]pyruvate injection did not vary significantly. No subject reported any adverse effects ≤1 month post [1-13C]pyruvate injection. For subject 04, the 13C imaging acquisition failed because of a scanner malfunction, but the subsequent 13C spectroscopy acquisition was successful.
Figure 1 shows time-integrated [1-13C]pyruvate, 13C-bicarbonate and [1-13C]lactate images from 2 of the 3 normal healthy volunteers with successful imaging acquisitions. These images were reconstructed from only the 4 channels on the anterior chest wall because the posterior channels gave insignificant signal. From the spatial distributions observed in the metabolite images, the 13C-bicarbonate signal appeared mainly confined to the LV myocardium, whereas the [1-13C]pyruvate appeared within the chambers (in the blood pool) but not within the muscle. In contrast, the [1-13C]lactate signal appeared both within the chambers and in the myocardium.
Figure 1.
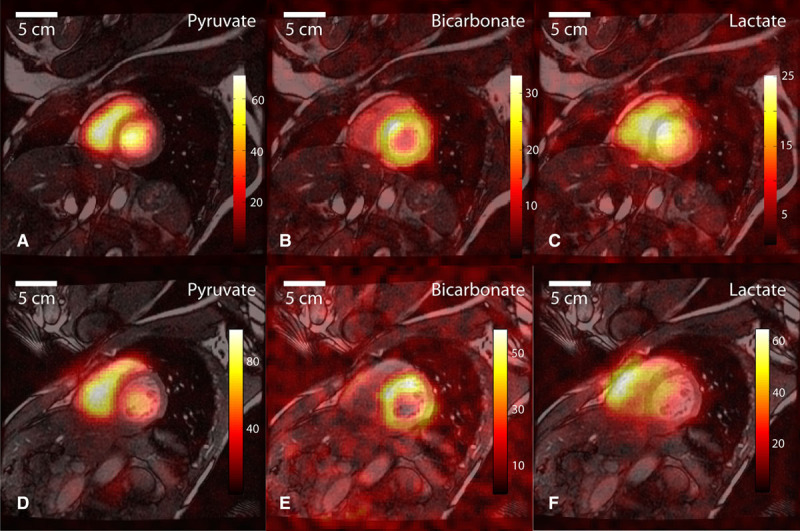
Representative 13C images displayed as color overlays on top of grayscale anatomic images in a midleft ventricle (LV) slice from subject 01(A–C) and subject 03(D–F). The [1-13C]pyruvate substrate was seen mainly in the blood pool within the cardiac chambers (A and D). Flux of pyruvate through the pyruvate dehydrogenase complex is reflected in the 13C-bicarbonate images (B and E), with signal predominantly in the wall of the LV. The [1-13C]lactate signal (C and F) appeared with a diffuse distribution covering the muscle and chambers.
The maximum image signal:noise ratio (SNR) for each 13C-labeled metabolite across the subjects is plotted in Figure 2. This was calculated as the maximum pixel value for the corresponding metabolite image divided by the SD of the noise in the image background and serves as a benchmark for the image SNR that can be obtained in humans for this particular spatial resolution and at the polarization achievable currently. However, as the SNR in MRI varies linearly with the voxel volume (holding other parameters equal), this benchmark will be useful for the design of future experiments in patients. The modest spatial resolution used here (8.8×8.8 mm in-plane and 10 mm through plane) resulted in readily detectable metabolic conversion within the tissue and enabled observations about the spatial distribution of the substrate and products. The bicarbonate signal:noise in the right ventricular myocardium was insufficient for reliable assessment with the chosen spatial resolution and coil configuration.
Figure 2.
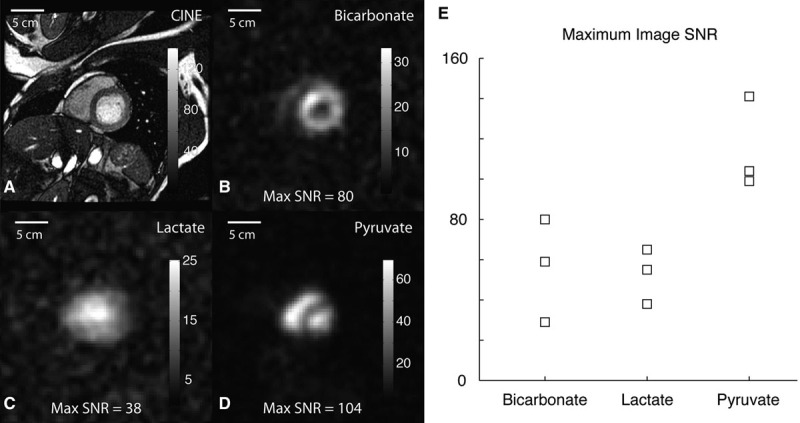
Grayscale anatomic (A) and 13C images(B–D) from subject 01 are shown separately with the calculated maximum signal:noise ratio (SNR). A summary of maximum image SNR across the different subjects is shown on the right (E).
Representative dynamic 13C spectra from one of the subjects are shown in Figure 3. Despite starting the spectroscopic acquisition after the completion of 13C imaging (and at least 90 s after dissolution), major metabolites from [1-13C]pyruvate were all observed in the spectra (Figure 3A and 3B). The peaks observed correspond to [1-13C]lactate (185 ppm), [1-13C]alanine (179 ppm), [1-13C]pyruvate (172 ppm), and 13C-bicarbonate (162 ppm). From one of the data sets using the nonselective radiofrequency pulse, 13CO2 was also observed, and the pH calculated from the 13C-bicarbonate and 13CO2 signals using the Henderson–Hasselbalch equation was 7.1. For 3 subjects with cardiac-gated spectroscopic acquisitions, the bicarbonate:pyruvate ratios remained relatively stable during the time points with sufficient SNR for quantification, ranging from 20 to 70 s. In contrast, the lactate:pyruvate ratio increased over a similar time frame for all subjects (Figure 3C and 3D).
Figure 3.
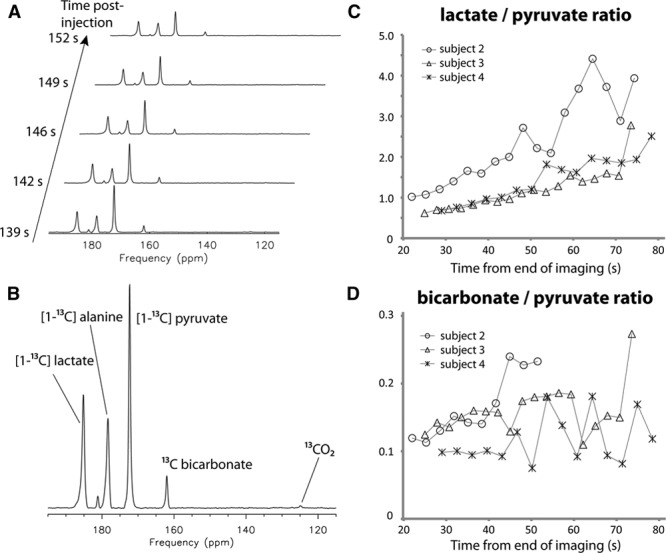
Representative dynamic 13C spectra acquired using a nonselective excitation pulse from one of the subjects are shown in A and B. The spectrum in B is the sum of the 5 consecutive time points shown in A. Lactate and bicarbonate to pyruvate ratios from the spectroscopic data are shown in C and D, respectively. For the 3 subjects with cardiac-gated spectroscopic acquisitions, the lactate:pyruvate ratios increased over time, whereas the bicarbonate:pyruvate ratios remained relatively stable during the time before the signal:noise ratio became too low for quantification.
Discussion
To the best of the authors’ knowledge, these results represent the first hyperpolarized 13C images of the human heart. [1-13C]pyruvate metabolism in the LV myocardium, as indicated by the generation of the 13C-bicarbonate signal was readily detected in this healthy cohort. This suggests that this technology may one day allow a direct measure of flux through the PDC in the myocardium in vivo in humans. The opposing patterns observed in Figure 1 for the [1-13C]pyruvate substrate and the 13C-bicarbonate product are consistent with rapid [1-13C]pyruvate flux through the LV myocardial PDC (consuming the [1-13C]pyruvate in the muscle). The diffuse appearance of the [1-13C]lactate signal was consistent with previous data from a porcine model, where the [1-13C]lactate signal seemed diffuse in both the blood and the tissue before ischemia.12 Understanding the more diffuse appearance of the [1-13C]lactate signal would require further experiments, but it is clear that the largest component is in the blood pool (at the time points imaged here) and that any interpretation of whole-heart 13C spectra must take this into account.
Rationalization of the temporal evolution of the lactate:pyruvate and bicarbonate:pyruvate ratios observed in Figure 3C and 3D requires some degree of speculation. These data were acquired ≈1 minute after the initial bolus, so the spatial distribution of metabolites seen in Figure 1 had likely changed. A steady state was not observed for the lactate:pyruvate ratio, perhaps as a result of lactate dehydrogenase–mediated label exchange between the 13C-enriched pyruvate pool and the larger pre-existing lactate pool,18 resulting in the [1-13C]lactate:[1-13C]pyruvate ratio continuing to approach the endogenous lactate:pyruvate ratio. Unlike the reversible label exchange between pyruvate and lactate, the labeling of 13C-bicarbonate is the result of an irreversible forward flux, resulting in enrichment of 13CO2 and 13C-bicarbonate. Through the normal physiology of aerobic respiration, intracellular 13CO2 is continually exported and transported away via the blood, draining the 13C-bicarbonate pool. This negative feedback on the 13C-bicarbonate pool may account for the more stable trend in the 13C-bicarbonate:[1-13C]pyruvate ratio over this time frame.
Importantly, no adverse events were recorded (except for the taste experienced by 2 subjects), and the injection and 13C imaging were well tolerated. With this tool to assess in vivo PDC flux in a rapid, safe and well-tolerated manner, longitudinal studies in humans incorporating this metabolic assessment of the heart become feasible. The metabolic information comes along with the detailed assessment of cardiac structure and function from the conventional CMR assessment done during the same scanning session. This augmented form of CMR is anticipated to provide novel insights into how metabolic changes relate to the process of functional decompensation leading to heart failure.
There are several limitations to the current technique and to this study. To apply this method, specialized equipment in the form of the SPINLab and 13C cardiac coils are required, limiting the number of sites that are currently able to do these studies. With the current polarization level and imaging method, spatial resolution was limited because of a trade-off with SNR (these will certainly improve in the future as polarization levels increase and coils are improved). Thus, imaging the ischemic heart to assess myocardial viability, or other structures in the heart, such as the right ventricle, remain technically challenging. Finally, this study only included normal volunteers, so the feasibility in a population with disease remains to be shown.
The clinical potential for this technique remains considerable. As demonstrated by our previous work in porcine models of heart failure,12 as well as work by other groups,19 the ability to perform repeated assessments of PDC flux will provide important insights into disease pathogenesis that can potentially facilitate treatment strategies in the form of PDC modulators. A significant body of work implicates PDC activity as an important determinant of cardiac function, particularly in states where insulin resistance occurs.20 For instance, in insulin-resistant ob/ob mice, enhanced fatty acid metabolism at the expense of glucose oxidation is associated with impaired contractile function.21 Indeed, there is a growing body of evidence to suggest that improving contractile function may be associated with the normalization of PDC flux. Changes in PDC activity may not only be a marker for abnormal cellular metabolism and increased oxidative stress it may also serve as a therapeutic target to prevent the development of heart failure in states where PDC activity is impaired.22 However, as the relative utilization of fatty acids and carbohydrates shifts depending on the pathogenesis and stage of disease,3 the appropriate therapy will require individualization for the particular patient, and this is where CMR plus 13C MRI may be clinically valuable.
Conclusions
These results represent the first 13C images of the human heart. The appearance of hyperpolarized signals of both 13CO2 and 13C-bicarbonate from [1-13C]pyruvate suggests that this technology may one day allow a direct measure of flux through the PDC in the myocardium in vivo in humans.
Acknowledgments
We thank Tracey Rideout, Sergio DeFigueiredo, and Stephanie Vidotto for pharmacy technician support for this study, Julie Green for coordinating the study, and Ruby Endre and Garry Detzler for MR technician support.
Sources of Funding
This study was supported by the Heart and Stroke Foundation of Canada and Canadian Institutes of Health Research MOP133-504. K.A. Connelly is supported by a CIHR New Investigator award.
Disclosures
A.P. Chen is an employee of GE Healthcare. The other authors report no conflicts.
Nonstandard Abbreviations and Acronyms
- CMR
- cardiac magnetic resonance
- LV
- left ventricle
- MRI
- magnetic resonance imaging
- PDC
- pyruvate dehydrogenase complex
- SNR
- signal:noise ratio
In August 2016, the average time from submission to first decision for all original research papers submitted to Circulation Research was 13.98 days.
Brief UltraRapid Communications are designed to be a format for manuscripts that are of outstanding interest to the readership, report definitive observations, but have a relatively narrow scope. Less comprehensive than Regular Articles but still scientifically rigorous, BURCs present seminal findings that have the potential to open up new avenues of research. A decision on BURCs is rendered within 7 days of submission.
Novelty and Significance
What Is Known?
Altered metabolism plays a role in heart failure progression.
Therapy that targets metabolism will require individualization for the particular patient.
Hyperpolarized 13C magnetic resonance imaging holds the potential to provide a noninvasive metabolic assessment.
What New Information Does This Article Contribute?
The first hyperpolarized 13C images of the human heart.
Demonstrated the feasibility of an integrated structural/functional/metabolic magnetic resonance imaging assessment.
A hyperpolarized 13C image-quality benchmark in the human heart using current technology.
Altered cardiac metabolism has long been known to play a role in progression of heart failure. Although there are many methods for making in vivo measurements related to metabolism, a noninvasive test that can be applied longitudinally in patients has remained an unmet need. In this study, an augmented form of magnetic resonance imaging that enables imaging of the metabolic conversion of pyruvate, an important metabolic intermediate, into its downstream metabolic products is demonstrated for the first time in humans. These results represent the first 13C metabolic images of the human heart and open the door to future studies in patients.
References
- 1.Stanley WC, Chandler MP. Energy metabolism in the normal and failing heart: potential for therapeutic interventions. Heart Fail Rev. 2002;7:115–130. doi: 10.1023/a:1015320423577. [DOI] [PubMed] [Google Scholar]
- 2.Ashrafian H, Frenneaux MP, Opie LH. Metabolic mechanisms in heart failure. Circulation. 2007;116:434–448. doi: 10.1161/CIRCULATIONAHA.107.702795. doi: 10.1161/CIRCULATIONAHA.107.702795. [DOI] [PubMed] [Google Scholar]
- 3.Ardehali H, Sabbah HN, Burke MA, Sarma S, Liu PP, Cleland JG, Maggioni A, Fonarow GC, Abel ED, Campia U, Gheorghiade M. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Fail. 2012;14:120–129. doi: 10.1093/eurjhf/hfr173. doi: 10.1093/eurjhf/hfr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. 2013;113:709–724. doi: 10.1161/CIRCRESAHA.113.300376. doi: 10.1161/CIRCRESAHA.113.300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garlick PB, Radda GK, Seeley PJ. Phosphorus NMR studies on perfused heart. Biochem Biophys Res Commun. 1977;74:1256–1262. doi: 10.1016/0006-291x(77)91653-9. [DOI] [PubMed] [Google Scholar]
- 6.Bailey IA, Gadian DG, Matthews PM, Radda GK, Seeley PJ. Studies of metabolism in the isolated, perfused rat heart using 13C NMR. FEBS Lett. 1981;123:315–318. doi: 10.1016/0014-5793(81)80317-1. [DOI] [PubMed] [Google Scholar]
- 7.Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merritt ME, Harrison C, Storey C, Jeffrey FM, Sherry AD, Malloy CR. Hyperpolarized 13C allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proc Natl Acad Sci U S A. 2007;104:19773–19777. doi: 10.1073/pnas.0706235104. doi: 10.1073/pnas.0706235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golman K, Petersson JS, Magnusson P, Johansson E, Akeson P, Chai CM, Hansson G, Månsson S. Cardiac metabolism measured noninvasively by hyperpolarized 13C MRI. Magn Reson Med. 2008;59:1005–1013. doi: 10.1002/mrm.21460. doi: 10.1002/mrm.21460. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder MA, Cochlin LE, Heather LC, Clarke K, Radda GK, Tyler DJ. In vivo assessment of pyruvate dehydrogenase flux in the heart using hyperpolarized carbon-13 magnetic resonance. Proc Natl Acad Sci U S A. 2008;105:12051–12056. doi: 10.1073/pnas.0805953105. doi: 10.1073/pnas.0805953105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schroeder MA, Clarke K, Neubauer S, Tyler DJ. Hyperpolarized magnetic resonance: a novel technique for the in vivo assessment of cardiovascular disease. Circulation. 2011;124:1580–1594. doi: 10.1161/CIRCULATIONAHA.111.024919. doi: 10.1161/CIRCULATIONAHA.111.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lau AZ, Chen AP, Barry J, Graham JJ, Dominguez-Viqueira W, Ghugre NR, Wright GA, Cunningham CH. Reproducibility study for free-breathing measurements of pyruvate metabolism using hyperpolarized (13) C in the heart. Magn Reson Med. 2013;69:1063–1071. doi: 10.1002/mrm.24342. doi: 10.1002/mrm.24342. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder MA, Lau AZ, Chen AP, Gu Y, Nagendran J, Barry J, Hu X, Dyck JR, Tyler DJ, Clarke K, Connelly KA, Wright GA, Cunningham CH. Hyperpolarized (13)C magnetic resonance reveals early- and late-onset changes to in vivo pyruvate metabolism in the failing heart. Eur J Heart Fail. 2013;15:130–140. doi: 10.1093/eurjhf/hfs192. doi: 10.1093/eurjhf/hfs192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, Carr CA, Heather LC, Tyler DJ. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: A Combined 13C Hyperpolarized Magnetic Resonance and Echocardiography Study. Diabetes. 2015;64:2735–2743. doi: 10.2337/db14-1560. doi: 10.2337/db14-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau AZ, Chen AP, Ghugre NR, Ramanan V, Lam WW, Connelly KA, Wright GA, Cunningham CH. Rapid multislice imaging of hyperpolarized 13C pyruvate and bicarbonate in the heart. Magn Reson Med. 2010;64:1323–1331. doi: 10.1002/mrm.22525. doi: 10.1002/mrm.22525. [DOI] [PubMed] [Google Scholar]
- 16.Tropp J, Calderon P, Carvajal L, Robb F, Larson PEZ, Shin P, Vigneron DB, Nelson SJ. Proceedings of the 20th Annual Meeting of ISMRM. Melbourne, Australia: 2012. A carbon receive array of 8 elements, interoperable with proton scanning, for human temporal lobe. Abstract 2658. [Google Scholar]
- 17.Ardenkjaer-Larsen JH, Leach AM, Clarke N, Urbahn J, Anderson D, Skloss TW. Dynamic nuclear polarization polarizer for sterile use intent. NMR Biomed. 2011;24:927–932. doi: 10.1002/nbm.1682. [DOI] [PubMed] [Google Scholar]
- 18.Witney TH, Kettunen MI, Brindle KM. Kinetic modeling of hyperpolarized 13C label exchange between pyruvate and lactate in tumor cells. J Biol Chem. 2011;286:24572–24580. doi: 10.1074/jbc.M111.237727. doi: 10.1074/jbc.M111.237727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rider OJ, Tyler DJ. Clinical implications of cardiac hyperpolarized magnetic resonance imaging. J Cardiovasc Magn Reson. 2013;15:93. doi: 10.1186/1532-429X-15-93. doi: 10.1186/1532-429X-15-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taegtmeyer H, McNulty P, Young ME. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 21.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 22.Lewis AJ, Neubauer S, Tyler DJ, Rider OJ. Pyruvate dehydrogenase as a therapeutic target for obesity cardiomyopathy. Expert Opin Ther Targets. 2016;20:755–766. doi: 10.1517/14728222.2016.1126248. doi: 10.1517/14728222.2016.1126248. [DOI] [PubMed] [Google Scholar]


