Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) has been claimed as a liver phenotype of metabolic syndrome, which in turn is associated with male hypogonadism. We assessed whether an independent association between NAFLD and androgen deficiency could be revealed in men with chronic spinal cord injury (SCI), who exhibit a high prevalence of biochemical androgen deficiency and a combination of risk factors for metabolic syndrome.
Design
Fifty-five consecutive men with chronic SCI admitted to a rehabilitation program underwent clinical/biochemical evaluations and liver ultrasonography.
Results
NAFLD was diagnosed in 27 patients (49.1%). Men with NAFLD were older and exhibited significantly higher body mass index, Homeostatic model assessment of insulin resistance, triglycerides and gamma-glutamyl transpeptidase values, lower total and free testosterone levels and they were engaged in a significantly poorer weekly leisure time physical activity (LTPA). At the multiple logistic regression analysis, only total and free testosterone levels exhibited a significant independent association with NAFLD. The risk of having NAFLD increased indeed of 1% for each decrement of 1 ng/dL of total testosterone and of 3% for each decrement of 1 pg/mL of free testosterone, after adjustment for confounders. In men with total testosterone < 300 ng/dL (36.4%) the prevalence of NAFLD reached 85%: they had a risk of having NAFLD significantly higher (∼12-fold) than those with total testosterone ≥ 300 ng/dL, after adjustment for confounders.
Conclusion
The evidence of an independent association between NAFLD and low testosterone is strongly reinforced by its demonstration in men with chronic SCI, in spite of the many confounders peculiar to this population.
Keywords: Androgen deficiency, Steatosis, Hypogonadism, Paraplegia, Tetraplegia
Introduction
Non-alcoholic fatty liver disease (NAFLD) refers to fat deposition in the liver that is not caused by excessive use of alcohol.1 Its prevalence has increased substantially in recent decades, reaching 10–24% in the overall population1 and 75% in patients with type 2 diabetes.2 This condition is currently recognized as an important public health problem in developed countries. In fact, NAFLD is associated with visceral fat accumulation and other components of metabolic syndrome,3,4 with which NAFLD shares a high risk of cardiovascular (CV) disease.5
In men, an association of low serum testosterone levels with obesity and metabolic syndrome has been well established.6 The pathogenetic mechanisms appear to be complex and often multi-directional:7 visceral obesity can probably be considered a relevant cause of androgen deficiency8,9 but, at the same time, androgen deficiency could be a cause of obesity and insulin resistance, leading to metabolic syndrome and consequently establishing a vicious cycle.10
Due to the relationship of obesity/insulin resistance/metabolic syndrome with both low testosterone levels and NAFLD, an association between NAFLD and low testosterone levels might be expected. Actually, it was explored only by Kim et al.,11 who reported that low total testosterone levels were associated with NAFLD, but, interestingly, this association persisted after adjustment for visceral adipose tissue and insulin resistance, thus indicating an independent association. The claimed independent association between NAFLD and low testosterone should be demonstrable, regardless of confounders, in different study populations, including men who exhibit a high prevalence of biochemical androgen deficiency as well as a combination of risk factors for metabolic syndrome. In this light, men with spinal cord injury (SCI) could represent a suitable clinical model of study. A biochemical androgen deficiency has been reported in up to ∼40% of men with chronic SCI,12–15 in whom an increased prevalence of obesity, metabolic syndrome, diabetes and CV disease has been also reported when compared to able-bodied individuals.16,17 Nevertheless, studies focusing on NAFLD in spinal cord-injured population are lacking.
Therefore, in this study we explored whether an independent association between NAFLD and low testosterone levels would be demonstrable in men with chronic SCI after adjusting for the many confounding factors, which are highly prevalent in this population.
Subjects and methods
Study population
Fifty-five consecutive male patients, aged 46.6 ± 17.3 years, admitted to a rehabilitation program at the San Raffaele Institute of Sulmona because of traumatic SCI, were included in this study. All patients had a documented history of neurologically stable SCI for more than 1 year. No patient had a history of communication or cognitive disorders (mental retardation, organic mental disorders). No patient had acute illness hindering the rehabilitative program. Coexisting chronic illness, including diabetes and dyslipidemia, were registered. No patient had a medical history of autoimmune hepatitis and chronic viral liver disease, cholestasis, and other metabolic liver diseases, or had previously used steatogenic medications including antiretroviral drugs, antiarrhythmic drugs and anticancer drugs. No patient received testosterone replacement therapy. Subjects who consumed ≥140 g of alcohol per week or >14 standard drinks/week (n = 3) were excluded from the study. The study was approved by the local ethics committee and all enrolled subjects signed an informed consent.
Neurological examination
Patients underwent detailed neurological examination according to the guidelines of the International Standards for Neurological Examination and Functional Classification of Spinal Cord Injury18 and the American Spinal Injury Association (ASIA) protocol was used to define both level and completeness of the lesion.18 According to the ASIA impairment scale, patients with complete lesion and no sensory or motor function preserved in the lowest sacral segment were categorized as A, whereas patients with incomplete lesion were categorized as B–D. Category B indicated sensory incomplete lesion (including segments S4–S5); category C indicated sensory and motor incomplete lesion where more than half of the 10 pairs of key muscles have strength of less than 3 on a scale of 0–5; category D indicated sensory and motor incomplete lesion with at least half of the key muscles having strength greater than or equal to 3.
Functional independence at admission was assessed by the Spinal Cord Independence Measure (SCIM). This is a 19-item instrument to measure the degree of functional independence attained in activities of daily living: the SCIM weighs each function separately, giving a final score that ranges from 0 (totally dependent) to 100 (totally independent).19
Anthropometric measures
Weight was taken with patients wearing light clothing, using a professional mechanical chair scale (Wunder SA BI Srl, Monza, Italy). After placing the patient in a bed, his legs were straightened, his head was positioned in the Frankfurt plane, and his feet were placed in dorsal flexion. Height was determined by an elastic tape, measuring segmentally the heel to knee, the knee to hip and the hip to head distances. The body mass index (BMI) was calculated in kilograms per square meter (kg/m2).
Assessment of leisure time physical activity
Leisure time physical activity (LTPA) includes physical exertion-related activities, that people choose to do in their free time, and in case of patients with SCI it includes walking or wheeling and certain sports played in a gym. Leisure time physical activity was quantified using the LTPA Questionnaire for people with Spinal Cord Injury (LTPAQ-SCI).20 This is a SCI-specific measure of minutes of mild, moderate, and heavy intensity LTPA performed over the previous 7 days, according to the physical activity guidelines for people with SCI.21 The questionnaire takes less than 5 minutes to complete and can be self-administered. Participants used an intensity classification chart to distinguish between mild, moderate, and heavy intensity LTPA, based on perceived psychophysical effort. For each intensity level, participants recalled the number of days, over the past 7 days, that they performed LTPA at each intensity. Next, they recalled how many minutes/day they usually spent doing LTPA at that intensity. The scale was scored by calculating the total number of weekly minutes of activity performed at each intensity (number of days of activity × number of minutes of activity). Total weekly minutes were divided by 60 to obtain the number of hours of activity performed over the past week. Due to the high correlation of total LTPAQ-SCI scores with mild, moderate and heavy sub-scores,15 only total LTPAQ-SCI scores were used for analyses.
Hormones, biochemistry and hematology
A single fasting morning venous blood sample was obtained from each subject between 8.00 and 9.00 a.m. Serum levels of total testosterone were measured by chemiluminescence immunoassay, using kits from Ortho-Clinical Diagnostics (Johnson & Johnson, New Brunswick, NJ, USA). The lower detection limit for testosterone quantitation was 0.03 nmol/L; the within- and between-assay coefficients of variation of testosterone measurements were 2.5% and 4.9%, respectively. Sex hormone binding globulin (SHBG), luteinizing hormone (LH) and insulin levels were assessed by a chemiluminescence immunoassay, using kits from Medical Systems (Genova, Italy). Free testosterone levels were derived from total hormone, SHBG, and albumin concentrations as previously described,22 using a web-based calculator (http://www.issam.ch/freetesto.htm). All the other biochemical/hematological measurements were performed using standard methods and commercial kits (Instrumentation Laboratory Company, Lexington, MA, USA). Insulin resistance was assessed using the homeostatic model (HOMA-IR) according to the formula: insulin (mU/L) × glucose (m/dL)/405.23
A total testosterone level below 300 ng/dL (<10.4 nmol/L) indicated a biochemical androgen deficiency, according to the Endocrine Society guidelines.24
Abdominal ultrasonography
The diagnosis of NAFLD was based on abdominal ultrasonography performed by experienced physicians using a 5 MHz transducer and a high-resolution instrument (MyLab 50; Esaote, Florence, Italy). The examiners were blinded to the medical information and the laboratory parameters of the participants. Fatty infiltration of the liver was defined by the presence of hepatorenal contrast and brightness of liver parenchyma.1 Patients showing fatty liver in the absence of other potential causes of hepatitis and excessive alcohol consumption (≥140 g/week) were considered to be affected by NAFLD.1
Statistical analysis
Statistical analysis was performed using the R statistical software (version 2.15.2, 2012, The R Foundation for Statistical Computing, Vienna, Austria). After assessing the distribution of data with Shapiro–Wilk test, Wilcoxon rank-sum test and unpaired two-sided Student's t test were used, as appropriate, for data analysis. Proportional differences were assessed by the χ2 test or the Fisher exact test as appropriate. A multiple logistic regression analysis was performed to reveal independent associations with NAFLD. Significance of differences in the prevalence of NAFLD from the 1st to the 3rd tertile of increasing total testosterone levels were assessed by χ2 test for trend (Mantel-Haenszel test). A logistic regression analysis was performed after dichotomizing total testosterone values in order to calculate or of having NAFLD in patients with biochemical androgen deficiency (total testosterone < 300 ng/dL) compared to those with normal testosterone levels (≥300 ng/dL), after adjusting for confounding factors.
Results
Non-alcoholic fatty liver disease was found in 27 out of 55 patients (49.1%). Table 1 shows the characteristics of the study population categorized by the presence of NAFLD. Patients with NAFLD were older and exhibited significantly higher BMI, insulin, HOMA-IR, triglycerides and gamma-glutamyl transpeptidase (γ-GT) values, lower total and free testosterone levels and they were engaged in a significantly lower number of hours/week of LTPA.
Table 1.
Characteristics of the study population categorized by the presence of NAFLD
| NAFLD (n = 27) | no NAFLD (n = 28) | P value | |
|---|---|---|---|
| Physiological measures | |||
| Age (yr) | 52.0 [23.0−78.0] | 36.5 [20.0−83.0] | 0.02 |
| Systolic blood pressure (mmHg) | 113.4 ± 14.3 | 111.1 ± 9.4 | 0.5 |
| Diastolic blood pressure (mmHg) | 70.9 ± 6.4 | 71.3 ± 7.2 | 0.8 |
| Lifestyle variables | |||
| Current smokers–no. (%) | 8 (29.6) | 14 (50.0) | 0.2 |
| Alcohol intake ≥ 10 g/week–no. (%) | 21 (77.8) | 20 (71.4) | 0.8 |
| LTPA (hours/week) | 5.0 [0.5−19.0] | 11.0 [0.0−20.5] | 0.003 |
| Anthropometric measures | |||
| Height (cm) | 170.0 [160.0−187.0] | 173.0 [160.0−194.0] | 0.4 |
| Weight (Kg) | 80.6 ± 15.1 | 68.1 ± 12.6 | 0.001 |
| Body mass index (Kg/m2) | 26.8 ± 4.3 | 22.3 ± 3.8 | 0.0001 |
| Hormones | |||
| Total testosterone (ng/dL) | 261.6 ± 159.5 | 505.7 ± 185.6 | < 0.0001 |
| Free testosterone (pg/mL) | 77.4 ± 51.7 | 143.0 ± 54.3 | < 0.0001 |
| LH (U/L) | 4.0 [0.7−23.8] | 3.7 [1.1−58.8] | 0.8 |
| SHBG (nmol/L) | 16.4 [2.8−64.0] | 18.3 [5.7−51.0] | 0.4 |
| Biochemistry | |||
| Glucose (mg/dL) | 87.0 [56.0−145.0] | 84.5 [71.0−102.0] | 0.2 |
| Insulin (mU/L) | 8.4 [2.7−26.5] | 5.3 [1.9−30.7] | 0.001 |
| HOMA-IR | 1.9 [0.4−8.1] | 1.0 [0.3−7.7] | 0.0005 |
| LDL-c (mg/dL) | 112.2 [69.0−205.6] | 100.7 [53.6−141.8] | 0.2 |
| HDL-c (mg/dL) | 39.5 ± 9.4 | 39.8 ± 8.6 | 0.9 |
| Triglycerides (mg/dL) | 155.0 [59.0−289.0] | 110.0 [42.0−276.0] | 0.03 |
| AST (U/L) | 18.0 [8.0−83.0] | 15.5 [9.0−36.0] | 0.3 |
| ALT (U/L) | 19.0 [6.0−88.0] | 14.5 [5.0−49.0] | 0.4 |
| γ-GT (U/L) | 32.0 [13.0−80.0] | 22.0 [12.0−69.0] | 0.005 |
| Uric acid (mg/dL) | 5.1 [3.8−9.6] | 5.3 [3.3−8.5] | 0.8 |
| Injury-related characteristics | |||
| Distance from lesion (yr) | 6.3 [1.0−25.0] | 11.1 [1.2−48.0] | 0.1 |
| Level of lesion–no (%) | |||
| Cervical spine | 14 (51.9) | 11 (39.3) | 0.5 |
| Thoracic spine | 11 (40.7) | 15 (53.6) | 0.5 |
| Lumbar spine | 2 (7.4) | 2 (7.1) | 1 |
| Lesion completeness–no (%) | |||
| ASIA score A (complete) | 11 (40.7) | 18 (64.3) | 0.1 |
| ASIA score B-D (incomplete) | 16 (59.3) | 10 (35.7) | 0.1 |
| SCIM score | 53.9 ± 21.5 | 51.1 ± 20.2 | 0.7 |
| Coexisting illness–no. (%) | |||
| Diabetes | 2 (7.4) | 2 (7.1) | 1 |
| Dyslipidemia | 4 (14.8) | 6 (21.4) | 0.7 |
| CRP > 5 mg/L–no. (%) | 9 (33.3) | 5 (17.8) | 0.3 |
| Drug intake-no. (%) | |||
| Hypolipidemic drugs | 4 (14.8) | 4 (14.3) | 1 |
| Metformin | 2 (7.4) | 2 (7.1) | 1 |
| Acetaminophen | 1 (3.7) | 1 (3.6) | 1 |
Data were expressed as mean ± standard deviation when normally distributed, as median [min-max] for parameters with non-normal distribution and as percentages when categorical.
LTPA, leisure time physical activity; LH, Luteinizing hormone; SHBG, Sex hormone binding globulin; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transpeptidase; ASIA, american spinal injury association; SCIM, spinal cord independence measure; CRP, C-reactive protein.
*To convert the values for total testosterone to nmol/L, multiply by 0.03467; to convert the values for free testosterone to pmol/L, multiply by 3.46.
At the multiple logistic regression analysis, only total and free testosterone levels exhibited a significant independent association with NAFLD. The risk of having NAFLD increased indeed of 1% for each decrement of 1 ng/dL of total testosterone and of 3% for each decrement of 1 pg/mL of free testosterone, after adjustment for age, BMI, HOMA-IR, triglycerides and weekly LTPA (Table 2). These significant associations remained unchanged also after further adjustment for dichotomic variables including coexisting diabetes and inflammatory status, as indicated by a C-reactive protein value > 5 mg/L (not shown).
Table 2.
Association of NAFLD with serum total and free testosterone levels: multiple logistic regressions
| OR (95%CI) | P | OR (95%CI) | P | ||
|---|---|---|---|---|---|
| Total testosterone | 0.99 (0.98−0.99) | 0.01 | Free testosterone | 0.97 (0.94−0.99) | 0.01 |
| Age | 1.02 (0.97−1.08) | 0.31 | Age | 1.03 (0.98−1.08) | 0.26 |
| LTPA | 1.13 (0.89−1.43) | 0.29 | LTPA | 1.16 (0.92−1.47) | 0.21 |
| BMI | 1.18 (0.96−1.46) | 0.11 | BMI | 1.20 (0.98−1.47) | 0.07 |
| HOMA–IR | 1.19 (0.68−2.06) | 0.53 | HOMA–IR | 1.40 (0.79−2.48) | 0.24 |
| Triglycerides | 1.00 (0.99−1.01) | 0.66 | Triglycerides | 1.00 (0.99−1.01) | 0.65 |
Total testosterone levels and free testosterone levels were expressed as ng/dL and pg/mL, respectively. OR, odds ratio; CI, confidence interval. LTPA, leisure time physical activity (hours/week); BMI, body mass index (Kg/m2); HOMA–IR, homeostatic model assessment of insulin resistance.
As shown in Fig. 1A, the prevalence of NAFLD significantly decreased as the serum total testosterone levels increased from the 1st tertile to the 3rd tertile. Twenty patients (36.4%) exhibited biochemical androgen deficiency (total testosterone < 300 ng/dL). They showed an 85% prevalence of NAFLD (Fig. 1B), with an OR of having NAFLD 12.2-fold higher than those with total testosterone ≥ 300 ng/dL (95%CI: 1.4–170.8), after adjustment for age, BMI, HOMA-IR, triglycerides, weekly LTPA, coexisting diabetes and inflammatory status.
Figure 1.
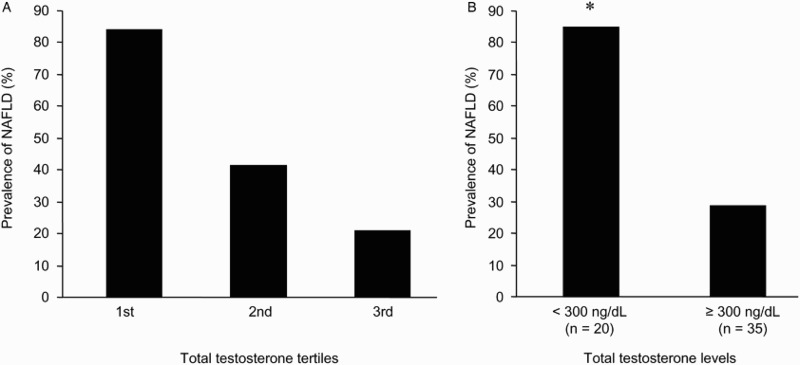
Prevalence of non-alcoholic fatty liver disease (NAFLD) by serum total testosterone levels in men with chronic spinal cord injury. (A) Prevalence of NAFLD in tertiles of increasing total testosterone levels; range of total testosterone tertiles (ng/dL): 1st tertile ≤269 (range: 25–269), 2nd tertile 270–453 (range: 290–447), 3rd tertile > 453 (range: 456–948). χ2 test for trend: χ2 = 15.16, P-value = 0.0001. (B) Prevalence of NAFLD in men with and without biochemical androgen deficiency (total testosterone < 300 ng/dL); *P < 0.0001 (Fisher exact test). To convert the values for total testosterone to nmol/L, multiply by 0.03467.
Discussion
To date, NAFLD had not yet been investigated in men with chronic SCI, although metabolic and lifestyle related risk factors for NAFLD are highly prevalent in this population. In the present study, NAFLD was exhibited by 49.1% of men with chronic SCI and it showed an independent association with low total and free testosterone levels.
The first datum would suggest a prevalence of NAFLD in patients with SCI higher (up to two-fold) than in general population,1,2 and, although rather expected, it should also suggest the advisability of a confirmation in larger controlled studies.
As far as the second datum is concerned (independent association of NAFLD with low total and free testosterone), an independent association with low total testosterone had been previously reported in one study on general population.11 The mechanisms underlying this association still remain speculative. As NAFLD has been regarded as the liver phenotype of metabolic syndrome,4 it might be interpreted as a component of the claimed interplay between low testosterone and visceral obesity/insulin resistance. On the one hand, as testosterone inhibits adipogenic differentiation25 and reduces the lipoprotein lipase activity in adipose tissue,26 androgen deficiency may increase the risk of visceral obesity,10 which in turn favours systemic insulin resistance.6 Accordingly, there is evidence that testosterone replacement therapy improves visceral adiposity and insulin resistance in hypogonadal men with type 2 diabetes.27 An excess of visceral fat mass also results in liver exposure to high levels of free fatty acids, a process which is involved in the pathogenesis of both NAFLD and liver insulin resistance.2 On the other hand, visceral obesity and metabolic syndrome can favour androgen deficiency as suggested by prospective studies,8,9 and also supported by the evidence that testosterone levels increase following weight loss in men with metabolic syndrome.28
The association between fat mass, insulin resistance and low testosterone appears to be much more evident in men with chronic SCI,15 who exhibit a very high prevalence of biochemical androgen deficiency,12–15 as well as a combination of risk factors for metabolic syndrome. They exhibit a deep alteration in body composition. Lean tissue mass is dramatically reduced below the level of lesion after SCI and this muscle wasting results in decreased energy expenditure, ultimately leading to increased adiposity and deleterious metabolic consequences.29 Low energy expenditure and increased adiposity with metabolic consequences are also related to poor physical activity. People with SCI are considered the most physically inactive members of society: it has been reported that they spend less than 2% of their waking time engaged in any LTPA.30
In the present study, patients with NAFLD exhibited significantly higher BMI and HOMA-IR, and were engaged in a significantly poorer weekly LTPA. However, the significant inverse association between testosterone levels and NAFLD persisted after adjustment for the effect of BMI, insulin resistance, and inflammatory status, suggesting a direct link between androgen deficiency and liver fat deposition. Some interesting evidence for a direct impact of androgens upon liver physiology has been obtained in animal models. Both hepatic androgen receptor (AR) KO mice31 and 5α-reductase type 1 KO mice32 developed higher degree of hepatic steatosis and inflammation than wild type controls. It has been hypothesized that androgens could modulate liver fatty acid β-oxidation and de novo lipid synthesis.31 In an orchidectomized rat model the treatment with dihydrotestosterone was associated with decreased lipid accumulation and cholesterol synthesis in the liver by increasing expression of carnitine palmitotyltransferase-1 and phosphorylation of 3-hydroxy-3-methyl-glutaryl-CoA reductase via an AR-mediated pathway.33 These findings only arise from animal models and their relevance to the relationship between low testosterone and NAFLD in the human remains to be determined.
A limitation of the present study is the use of ultrasonography to assess liver infiltration. Although ultrasonography is the diagnostic tool of choice for NAFLD, because it is non-invasive, safe, sensitive, and specific in identifying fatty infiltration,34 liver biopsy is regarded as the gold standard for detecting hepatic steatosis.1 Nevertheless, biopsy is reserved for patients with clinically relevant liver disease and it was not practicable for this population-based study. Another limitation of our study is represented by its transversal design: only longitudinal intervention studies could strengthen cause-effect relationships. In this regard, testosterone treatment ameliorated liver lipid accumulation in animal models, such as castrated rat models,33,35 and a rabbit model with metabolic syndrome.36 Interestingly, in this latter model, testosterone treatment also exerted an anti-inflammatory effect both in liver36 and prostate.37 However, recent randomized controlled trials reported conflicting results in the human. In a study by Hoyos et al.,38 a 18-week treatment with testosterone significantly reduced liver fat content in obese men; on the contrary, Huang et al.39 reported that testosterone administration for 6 months was not associated with a reduction in hepatic fat in older men with mobility limitation and low testosterone levels. Differences in the characteristics of study populations and in diagnosis methods could partially explain these conflicting results.
Nevertheless, this study also presents some strengths. The evidence of a direct link between low testosterone and NAFLD, which was reported in one study on the general population,11 is here strongly reinforced by its demonstration in men with chronic SCI, in whom the association persisted after adjustment for the many confounding factors which are highly prevalent in this population. Moreover, this study provides the first demonstration of the independent association between NAFLD and low free other than low total testosterone, thereby ruling out confounding effects of serum levels of SHBG, which might be variably affected by metabolic syndrome as well as liver disease.
Conclusion
This study strongly reinforces the evidence of an independent association between NAFLD and low testosterone by its demonstration in men with chronic SCI, in spite of the many confounders peculiar to this population.
Acknowledgments
This work was supported by Ministero dell'Università e della Ricerca (MIUR), Italy.
Disclaimer statements
Contributors AB designed the research, conducted the study, analyzed data and interpreted results, performed statistical analysis and wrote the manuscript; MRCV and MC analyzed data; GF and SF revised the article critically and approved its final version; FF designed the research, analyzed data and interpreted results, wrote the manuscript, revised the article critically and approved its final version.
Funding None
Conflicts of interest The authors declare no conflicts of interest
Ethics approval The study was approved by ethics committee of ASL 01 Avezzano-Sulmona-L‘Aquila.
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346(16):1221–31. doi: 10.1056/NEJMra011775 [DOI] [PubMed] [Google Scholar]
- 2.Medina J, Fernandez-Salazar LI, Garcia-Buey L, Moreno-Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care 2004;27(8):2057–66. doi: 10.2337/diacare.27.8.2057 [DOI] [PubMed] [Google Scholar]
- 3.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol 2007;102(12):2708–15. doi: 10.1111/j.1572-0241.2007.01526.x [DOI] [PubMed] [Google Scholar]
- 4.Kotronen A, Yki-Jarvinen H. Fatty liver: a novel component of the metabolic syndrome. Arterioscler Thromb Vasc Biol 2008;28(1):27–38. doi: 10.1161/ATVBAHA.107.147538 [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363(14):1341–50. doi: 10.1056/NEJMra0912063 [DOI] [PubMed] [Google Scholar]
- 6.Traish AM, Guay A, Feeley R, Saad F. The dark side of testosterone deficiency: I. Metabolic syndrome and erectile dysfunction. J Androl 2009;30(1):10–22. doi: 10.2164/jandrol.108.005215 [DOI] [PubMed] [Google Scholar]
- 7.Corona G, Rastrelli G, Vignozzi L, Mannucci E, Maggi M. Testosterone, cardiovascular disease and the metabolic syndrome. Best Pract Res Clin Endocrinol Metab 2011;25(2):337–53. doi: 10.1016/j.beem.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 8.Laaksonen DE, Niskanen L, Punnonen K, Nyyssonen K, Tuomainen TP, Valkonen VP, et al. The metabolic syndrome and smoking in relation to hypogonadism in middle-aged men: a prospective cohort study. J Clin Endocrinol Metab 2005;90(2):712–9. doi: 10.1210/jc.2004-0970 [DOI] [PubMed] [Google Scholar]
- 9.Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab 2007;92(2):549–55. doi: 10.1210/jc.2006-1859 [DOI] [PubMed] [Google Scholar]
- 10.Laaksonen DE, Niskanen L, Punnonen K, Nyyssönen K, Tuomainen TP, Valkonen VP, et al. Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care 2004;27(5):1036–41. doi: 10.2337/diacare.27.5.1036 [DOI] [PubMed] [Google Scholar]
- 11.Kim S, Kwon H, Park JH, Cho B, Kim D, Oh SW, et al. A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol 2012;12:69. doi: 10.1186/1471-230X-12-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostovski E, Iversen PO, Birkeland K, Torjesen PA, Hjeltnes N. Decreased levels of testosterone and gonadotrophins in men with long-standing tetraplegia. Spinal Cord 2008;46(8):559–64. doi: 10.1038/sc.2008.3 [DOI] [PubMed] [Google Scholar]
- 13.Durga A, Sepahpanah F, Regozzi M, Hastings J, Crane DA. Prevalence of testosterone deficiency after spinal cord injury. PM R 2011;3(10):929–32. doi: 10.1016/j.pmrj.2011.07.008 [DOI] [PubMed] [Google Scholar]
- 14.Bauman WA, Fountaine MF, Spungen AM. Age-related prevalence of low testosterone in men with spinal cord injury. J Spinal Cord Med 2014;37(1):32–9. doi: 10.1179/2045772313Y.0000000122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barbonetti A, Vassallo MRC, Pacca F, Cavallo F, Costanzo M, Felzani G, et al. Correlates of low testosterone in men with chronic spinal cord injury. Andrology 2014;2(5):721–8. doi: 10.1111/j.2047-2927.2014.00235.x [DOI] [PubMed] [Google Scholar]
- 16.Bauman WA, Spungen AM. Carbohydrate and lipid metabolism in chronic spinal cord injury. J Spinal Cord Med 2001;24(4):266–77. [DOI] [PubMed] [Google Scholar]
- 17.Jones LM, Legge M, Goulding A. Healthy body mass index (BMI) values often underestimate body fat in spinal cord injured males. Arch Phys Med Rehabil 2003;84(7):1068–71. doi: 10.1016/S0003-9993(03)00045-5 [DOI] [PubMed] [Google Scholar]
- 18.Maynard FM Jr, Bracken MB, Creasey G, Ditunno JF Jr, Donovan WH, Ducker TB, et al. International standards for neurological and functional classification of spinal cord injury. Spinal Cord 1997;35(5):266–74. doi: 10.1038/sj.sc.3100432 [DOI] [PubMed] [Google Scholar]
- 19.Catz A, Itzkovich M, Agranov E, Ring H, Tamir A. SCIM–spinal cord independence measure: a new disability scale for patients with spinal cord lesions. Spinal Cord, 1997;35(12):850–6. doi: 10.1038/sj.sc.3100504 [DOI] [PubMed] [Google Scholar]
- 20.Martin Ginis KA, Phang SH, Latimer AE, Arbour-Nicitopoulos KP. Reliability and validity tests of the leisure time physical activity questionnaire for people with spinal cord injury. Arch Phys Med Rehabil 2012;93(4):677–82. doi: 10.1016/j.apmr.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Ginis KA, Hicks AL, Latimer AE, Warburton DE, Bourne C, Ditor DS, et al. The development of evidence-informed physical activity guidelines for adults with spinal cord injury. Spinal Cord 2011;49(11):1088–96. doi: 10.1038/sc.2011.63 [DOI] [PubMed] [Google Scholar]
- 22.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84(10):3666–72. doi: 10.1210/jcem.84.10.6079 [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28(7):412–9. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 24.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, et al. Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95(6):2536–59. doi: 10.1210/jc.2009-2354 [DOI] [PubMed] [Google Scholar]
- 25.Singh R, Artaza JN, Taylor WE, Braga M, Yuan X, Gonzalez-Cadavid NF, et al. Testosterone inhibits adipogenic differentiation in 3T3-L1 cells: nuclear translocation of androgen receptor complex with beta-catenin and T-cell factor 4 may bypass canonical Wnt signaling to down-regulate adipogenic transcription factors. Endocrinology 2006;147(1):141–54. doi: 10.1210/en.2004-1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez ME, McMurry MP, Wiebke GA, Felten KJ, Ren K, Meikle AW, et al. Evidence for sex steroid inhibition of lipoprotein lipase in men: comparison of abdominal and femoral adipose tissue. Metabolism 1997;46(2):179–85. doi: 10.1016/S0026-0495(97)90299-7 [DOI] [PubMed] [Google Scholar]
- 27.Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006;154(6):899–906. doi: 10.1530/eje.1.02166 [DOI] [PubMed] [Google Scholar]
- 28.Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight los and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004;6(3):208–15. doi: 10.1111/j.1462-8902.2004.00335.x [DOI] [PubMed] [Google Scholar]
- 29.Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr 2003;77(2):371–8. [DOI] [PubMed] [Google Scholar]
- 30.Latimer AE, Ginis KA, Craven BC, Hicks AL. The physical activity recall assessment for people with spinal cord injury: validity. Med Sci Sports Exerc 2006;38(2):208–16. doi: 10.1249/01.mss.0000183851.94261.d2 [DOI] [PubMed] [Google Scholar]
- 31.Lin HY, Yu IC, Wang RS, Chen YT, Liu NC, Altuwaijri S, et al. Increased hepatic steatosis and insulin resistance in mice lacking hepatic androgen receptor. Hepatology 2008;47(6):1924–35. doi: 10.1002/hep.22252 [DOI] [PubMed] [Google Scholar]
- 32.Dowman JK, Hopkins LJ, Reynolds GM, Armstrong MJ, Nasiri M, Nikolaou N, et al. Loss of 5a-reductase Type 1 accelerates the development of hepatic steatosis but protects against hepatocellular carcinoma in male mice. Endocrinology 2013;154(12):4536–47. doi: 10.1210/en.2013-1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H, Liu Y, Wang L, Li Z, Zhang H, Wu J, et al. Differential effects of estrogen/androgen on the prevention of nonalcoholic fatty liver disease in the male rat. J Lipid Res 2013;54(2):345–57. doi: 10.1194/jlr.M028969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol 2003;15(5):539–43. [DOI] [PubMed] [Google Scholar]
- 35.Nikolaenko L, Jia Y, Wang C, Diaz-Arjonilla M, Yee JK, French SW, et al. Testosterone replacement ameliorates nonalcoholic fatty liver disease in castrated male rats. Endocrinology 2014;155(2):417–28. doi: 10.1210/en.2013-1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vignozzi L, Filippi S, Comeglio P, Cellai I, Sarchielli E, Morelli A, et al. Nonalcoholic steatohepatitis as a novel player in metabolic syndrome-induced erectile dysfunction: An experimental study in the rabbit. Mol Cell Endocrinol 2014;384(1–2):143–54. doi: 10.1016/j.mce.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 37.Vignozzi L, Morelli A, Sarchielli E, Comeglio P, Filippi S, et al. Testosterone protects from metabolic syndrome-associated prostate inflammation: an experimental study in rabbit. J Endocrinol 2012;212(1):71–84. doi: 10.1530/JOE-11-0289 [DOI] [PubMed] [Google Scholar]
- 38.Hoyos CM, Yee BJ, Phillips CL, Machan EA, Grunstein RR, Liu PY. Body compositional and cardiometabolic effects of testosterone therapy in obese men with severe obstructive sleep apnea: a randomized placebo-controlled trial. Eur J Endocrinol 2012;167(4):531–41. doi: 10.1530/EJE-12-0525 [DOI] [PubMed] [Google Scholar]
- 39.Huang G, Bhasin S, Tang ER, Aakil A, Anderson SW, Jara H, et al. Effect of testosterone administration on liver fat in older men with mobility limitation: results from a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013;68(8):954–9. doi: 10.1093/gerona/gls259 [DOI] [PMC free article] [PubMed] [Google Scholar]


