Abstract
Context/Objective
Traumatic damage to the cervical spinal cord is usually associated with a disruption of the autonomic nervous system (ANS) and impaired cardiovascular control both during and following exercise. The magnitude of the cardiovascular dysfunction remains unclear. The aim of the current study was to compare cardiovascular responses to peak voluntary exercise in individuals with tetraplegia and able-bodied participants.
Design
A case-control study.
Subjects
Twenty males with cervical spinal cord injury (SCI) as the Tetra group and 27 able-bodied males as the Control group were included in the study.
Outcome Measures
Blood pressure (BP) response one minute after the peak exercise, peak heart rate (HRpeak), and peak oxygen consumption (VO2peak) on an arm crank ergometer were measured. In the second part of the study, 17 individuals of the Control group completed the Tetra group's workload protocol with the same parameters recorded.
Results
There was no increase in BP in response to the exercise in the Tetra group. Able-bodied individuals exhibited significantly increased post-exercise systolic BP after the maximal graded exercise test (123±16%) and after completion of the Tetra group's workload protocol (114±11%) as compared to pre-exercise. The Tetra group VO2peak was 59% and the HRpeak was 73% of the Control group VO2peak and HRpeak, respectively.
Conclusions
BP did not increase following maximal arm crank exercise in males with a cervical SCI unlike the increases observed in the Control group. Some males in the Tetra group appeared to be at risk of severe hypotension following high intensity exercise, which can limit the ability to progressive increase and maintain high intensity exercise.
Keywords: Cardiovascular system, Blood pressure, Exercise, Spinal cord trauma, Tetraplegia
Introduction
Significant limitation of skeletal muscle control, impaired sympathetic cardiovascular innervation, physical inactivity, and eventual respiratory complications, are associated with reduced physical fitness and increased cardiovascular morbidity in individuals with tetraplegia.1–4 Traumatic damage to the cervical spinal cord typically disrupts the Autonomic Nervous System (ANS), specifically the sympathetic pathways.5–7 This disruption is manifested by long-term hypotension and reduced chronotropic and inotropic effects on the heart during physical activity (PA).8,9 Additionally, an insufficient skeletal muscle pump for venous return and the disruption of sympathetic vascular innervation may result in pooling of blood in the lower extremities and reduced efficiency of splanchnic circulation.10,11 Some studies attribute reductions in peak oxygen consumption in tetraplegic individuals partly to impaired sympathetic innervation.9,12 There are limited reports in the literature on the blood pressure (BP) response to peak voluntary exercise in individuals with tetraplegia.13,14 Claydon et al. reported transient post-exercise hypotension in individuals with cervical SCI, but not in those with thoracic SCI. A reference of able-bodied individuals was not included.13 Dela et al. demonstrated a significant decrease in BP in six individuals with tetraplegia during electrically induced exercise, while individuals with paraplegia demonstrated a non-significant decrease and able-bodied controls showed no change.14 The aim of the current study was to describe the differences in the BP response between able-bodied individuals and individuals with cervical SCI following a graded exercise test. The study further aimed to report the differences in peak heart rate (HRpeak), peak oxygen consumption (VO2peak), and minute ventilation (VEpeak) between the two groups. The maximal voluntary effort is associated with a dramatically lower peak workload in tetraplegic individuals, a potential confounding factor for the case-control study design. Therefore, the cardiovascular responses of the tetraplegic participants during maximal voluntary exercise were compared to the responses of the able-bodied individuals during maximal voluntary exercise and during the average tetraplegic workload protocol. The second aim of the current study was to evaluate whether there were any significant differences in HRpeak, VO2peak, and BP response between participants with clinically complete and incomplete spinal cord injury. This knowledge is useful for PA interventions, therapy modifications during sports training, and also for the understanding of ANS's influence on the cardiovascular system, both in able-bodied individuals and individuals with cervical SCI.
Methods
In total, 47 males participated in the study. Twenty males with a cervical SCI (Tetra group) and 27 able-bodied males (Control group) were included. Table 1 provides the Tetra group characteristics and Control group anthropometrics. All participants were White/Caucasian. Exclusion criteria included use of drugs affecting ANS function, cardiovascular or any other serious illness, prior upper extremity injury affecting the ability to complete the graded exercise test, and any acute disease. In addition, the Tetra group was at least one year post-injury. All individuals of the Tetra group were examined according to the International Standards for Neurological Classification of Spinal Cord injury (ISNCSCI).15
Table 1.
Characteristics of all subjects
| Tetra group (n = 20) |
||||||||
|---|---|---|---|---|---|---|---|---|
| ISNCSCI NLI | AIS | Period post-injury | AD history | AD in last year | Age (yr) | Weight (kg) | Stature (cm) | Body fat (%) |
| C5 | B | 4.1 | yes | yes | 35.5 | 75 | 175 | 19.2 |
| C5 | B | 1.8 | yes | yes | 33.9 | 90 | 196 | 24.6 |
| C5 | A | 12.8 | yes | yes | 33.0 | 50 | 184 | 21.0 |
| C5 | A | 11.5 | yes | yes | 36.2 | 84 | 174 | 29.2 |
| C5 | B | 4.8 | yes | yes | 19.6 | 68 | 186 | 14.1 |
| C5 | A | 2.3 | yes | yes | 21.6 | 62 | 192 | 11.5 |
| C6 | A | 11.0 | yes | yes | 31.0 | 80 | 183 | 13.2 |
| C6 | A | 14.5 | yes | no | 33.8 | 79 | 176 | 21.2 |
| C6 | A | 3.0 | yes | yes | 24.9 | 90 | 190 | 13.6 |
| C6 | B | 23.3 | yes | no | 39.7 | 81.5 | 185 | 17.3 |
| C6 | A | 8.3 | yes | no | 30.1 | 95 | 185 | 23.6 |
| C6 | B | 5.6 | yes | no | 22.4 | 55.5 | 196 | 17.9 |
| C6 | A | 11.5 | yes | yes | 31.1 | 90 | 189 | 31.1 |
| C6 | A | 10.0 | yes | no | 32.0 | 81.5 | 179 | 22.7 |
| C6 | A | 3.7 | yes | no | 27.5 | 77 | 185 | 16.4 |
| C7 | A | 13.3 | yes | yes | 30.2 | 90 | 185 | 16.8 |
| C7 | A | 7.5 | no | no | 34.3 | 70 | 173 | 22.3 |
| C7 | A | 11.4 | yes | no | 38.2 | 58 | 177 | 16.6 |
| C7 | C | 2.1 | yes | yes | 37.3 | 88 | 178 | 23.6 |
| C7 | B | 1.0 | yes | no | 27.4 | 65.5 | 178 | 17.1 |
| Mean | 8.2 ± 5.5 | 31.0 ± 5.5 | 76.5 ± 12.8 | 183.3 ± 6.8 | 19.7 ± 5.1 | |||
| Median | 7.9 | 31.6 | 79.5 | 184.5 | 18.6 | |||
| Normal distribution | yes | yes | yes | yes | yes | |||
| Subgroup AIS A (n = 13) | ||||||||
| Mean | 9.3 ± 3.9 | 31.1 ± 4.3 | 77.5 ± 13.3 | 182.5 ± 5.9 | 19.9 ± 5.8 | |||
| Median | 11.0 | 31.1 | 80.0 | 184.0 | 21.0 | |||
| Normal distribution | yes | yes | yes | yes | yes | |||
| Subgroup AIS B (n = 6) | ||||||||
| Mean | 6.8 ± 7.6 | 29.7 ± 7.2 | 72.6 ± 11.2 | 186.0 ± 8.0 | 18.4 ± 3.2 | |||
| Median | 4.5 | 30.6 | 71.5 | 185.5 | 17.6 | |||
| Normal distribution* | no | yes | yes | yes | yes | |||
| *due to small “n” tested by Kolmogorov-Smirnov normality test | ||||||||
| Control group (n = 27) | ||||||||
| Mean | 30.9 ± 8.1 | 82.7 ± 9.1 | 181.2 ± 8.2 | 15.9 ± 6.7 | ||||
| Median | 27.8 | 76.5 | 183.0 | 14.8 | ||||
| Normal distribution | yes | no | yes | no | ||||
| Control group submax (n = 17) | ||||||||
| Mean | 32.3 ± 8.7 | 79.0 ± 10.4 | 178.8 ± 7.8 | 14.3 ± 1.5 | ||||
| Median | 29.7 | 80.0 | 179.5 | 6.8 | ||||
| Normal distribution | yes | yes | yes | yes | ||||
Neurological level of injury (NLI) and clinical completeness of the injury (AIS) according to the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI); period post spinal cord injury (SCI); history of autonomic dysreflexia (AD) and incidence of AD in the last year; mean ± standard deviation (SD), body fat estimate as provided by the Bodystat® Quad Scan 4000
Height was measured while standing in the Control group and while lying supine in the Tetra group. The Electronic Wheelchair Weigher 7805 (Soehnle Professional, Backnang, Germany) was used for body weight measurement. Body composition was analyzed using whole-body measures by Bodystat® Quad Scan 4000 (Bodystat Ltd., Douglas, Isle of Man, UK).16 The history of autonomic dysreflexia symptoms was recorded for the Tetra group. Tetra group training status was evaluated with the Leisure Time Physical Activity Questionnaire (LTPAQ) for People with Spinal Cord Injury (LTPAQ-SCI).17 This questionnaire is similar to frequently used, brief self-report measures in the able-bodied population. Therefore the same questions on amount of mild, moderate and heavy PA were used for the Control group. All participants were asked to evaluate both their present activity status and their physical activity behaviors during a typical week in the previous six months.
Resting HR (HRrest) by electrocardiography (BTL-08, BTL, UK) and resting BP (BPrest) by calibrated sphygmomanometer (Chirana t-Injecta, A.S., Stará Turá, Slovakia) were measured after 1) five minutes lying supine and 2) five minutes seated rest with the trunk at 60°.
Graded exercise test
The graded exercise test consisted of handcycling on an electronically braked arm ergometer (Ergometrics 900, ergoline GMBH, Bitz, Germany) with synchronous cranks. Due to the impaired gripping ability in the Tetra group, the participants’ (Control and Tetra groups) forearms were firmly fixed to the handles. Participants sat on a handbike seat and were fixed by a wide belt across the abdomen with the trunk supported at 60°.
The graded exercise test protocol consisted of a four-minute warm-up followed by a graded exercise with increasing loads. The aim was to keep the rating of perceived exertion according to Borg18 (RPE) at a rating of 9–11 (very light to fairly light) during the warm-up, and to reach the voluntary maximum between the 8th and 12th minute of the graded exercise test. The participants were asked to maintain a cadence of 50–70 revolutions per minute (rpm) during the exercise test and 30–70 rpm during the last minute of the test if there was continual rise in VO2. Previous research suggests increasing workloads 2–6 W·min−1 in order to achieve the highest VO2 in participants with tetraplegia.19 Therefore, in the current study, the workload was increased by 5 W·min−1 for the Tetra group and adequately faster for the Control group (5–10 W/ 15–30 s) in order to finish the test within 8 to 12 minutes. If the peak voluntary workload occurred outside of this time range, the test was considered invalid. The general protocol for both groups is shown in Figure 1. The test continued until the participant was too exhausted to continue, the cadence fell below 30 rpm, or if test termination was indicated according to the American College of Cardiology and American Heart Association guidelines. Expired gases were measured using a Jaeger Oxycon Pro metabolic system (Viasys, Frankfurt, Germany). Expired gases and HR was measured continuously throughout the graded exercise test and for the five minutes following the completion of the test. BP was measured 1 minute after test termination (BPpost). VO2peak was averaged over a 30-second period when the highest values occurred. The three shortest R-R intervals excluding arrhythmias, as recorded by ECG during the peak exercise, were used for the HRpeak calculation. The Control group also completed a graded exercise test on a cycle ergometer (Ergoselect 400, ergoline GMBH, Bitz, Germany) beginning with four minute warm-up and finishing within 8 to 12 minutes in order to assess their cardiovascular endurance. This test was performed beyond the main aim of the current study in order to report the general fitness of able-bodied participants.
Figure 1.
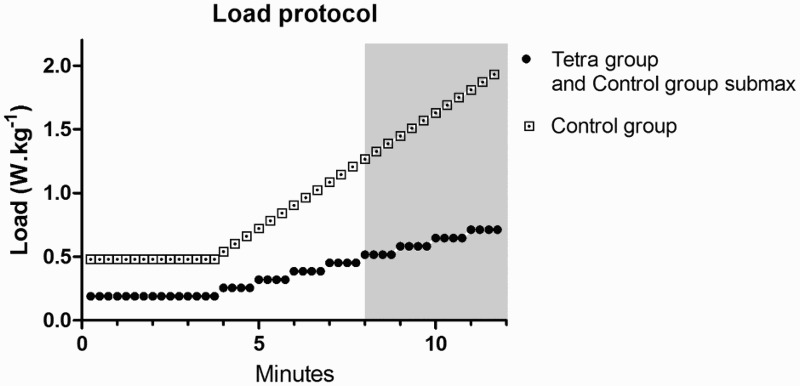
Workload protocol of the arm crank exercise test as expressed in mean W·kg−1; peak voluntary stop between the 8 and 12 minutes (grey area).
In order to control for the potential confounding influence of differing maximal workloads between the Control and Tetra groups on the metabolic responses, Control group participants completed a second handcycling graded exercise test using the average workload protocol of the Tetra group. In order to appropriately design the protocol, the data collection could not start until all participants of the Tetra group completed the graded exercice test. The protocol was based on the workloads (W) of the Tetra group and consisted of a 4-minute warm-up at the same workload (W·kg−1) followed by the graded exercise test that increased in intensity by 5 W every minute until the average peak workload (W·kg−1) of the Tetra group was obtained. The test was terminated after 35 seconds of handcycling at the average peak workload and was therefore not a maximal exercise test for the Control group. The primary aim of this exercise test was to compare the systolic BP (SBP) response after exposure to similar workloads.
Descriptive statistics were calculated for all variables (mean, standard deviation, median). Normal distribution was assessed using D'Agostino-Pearson omnibus test (alpha = 0.05). The unpaired t-test was used for inter-group analysis with normal data distribution. The Mann-Whitney test was used in instances of non-Gaussian data distribution. The paired t-test was used for intra-group analysis. P < 0.05 was considered significant. The effect size was determined by Cohen's d.20
The project was approved by the local ethics committee and participants gave their written informed consent.
Results
Thirteen males with complete and 7 males with incomplete SCI participated in the study. According to the ISNCSCI,15 all Tetra group participants were evaluated as having grade 5 elbow flexor motor function with the exception of one individual who was indicated to have grade 4 elbow flexor motor function. All Control group participants demonstrated grade 5 elbow flexor function. The characteristics of all participants as presented in Table 1 are normally distributed with the exception of Control group weight (kg) and body fat (%) and period post-injury in the Subgroup AIS B. Further analysis revealed these deviations were caused by single outliers. Seventeen participants of the Control group completed the second part of the study – characteristics of these participants are presented in Table 1 as Control group submax.
Cardiovascular parameters at rest
Table 2 summarizes the main cardiovascular parameters of all participants at rest following five minutes of lying supine and five minutes seated rest with the trunk supported at 60°. Resting heart rate, diastolic BPrest (DBPrest), and systolic BPrest (SBPrest) were lower in the Tetra group. Twelve participants in Tetra group had a lower seated BPrest as compared to lying supine; however, this difference was not significant between the two body positions (P > 0.05).
Table 2.
Cardiovascular parameters at rest
| HR rest(bpm) | SBP rest(mmHg) | DBP rest(mmHg) | |
|---|---|---|---|
| Following 5 minutes lying supine | |||
| Tetra group | |||
| Mean ± SD | 55.1 ± 9.2 | 109.0 ± 11.1 | 67.5 ± 19.8 |
| Median | 56 | 110 | 75 |
| Normal distribution | yes | yes | no |
| Control group | |||
| Mean ± SD | 62.0 ± 8.6 | 125.0 ± 9.8 | 76.5 ± 11.8 |
| Median | 62 | 130 | 80 |
| Normal distribution | yes | yes | no |
| Tetra vs. Control | |||
| Cohen's d | 0.8 | 1.5 | 0.6 |
| p | * | *** | |
| Control group submax | |||
| Mean ± SD | 60.5 ± 9.5 | 122.9 ± 10.3 | 80.6 ± 10.6 |
| Median | 58 | 120 | 80 |
| Normal distribution | yes | yes | yes |
| Following 5 minutes seated rest with trunk supported at 60° | |||
| Tetra group | |||
| Mean ± SD | 59.9 ± 9.9 | 102.8 ± 15.3 | 62.3 ± 20.0 |
| Median | 59 | 100 | 60 |
| Normal distribution | yes | yes | no |
| Control group | |||
| Mean ± SD | 64.7 ± 9.1 | 125.4 ± 12.9 | 80.9 ± 9.0 |
| Median | 63.5 | 125 | 80 |
| Normal distribution | yes | yes | yes |
| Tetra vs. Control | |||
| Cohen's d | 0.5 | 1.6 | 1.2 |
| p | *** | *** | |
| Control group submax | |||
| Mean ± SD | 63.7 ± 10.9 | 126.2 ± 8.9 | 82.2 ± 10.2 |
| Median | 60.5 | 125 | 80 |
| Normal distribution | yes | yes | yes |
*p < 0.05**p < 0.01***p < 0.001 Resting heart rate (HRrest), systolic blood pressure (SBPrest) and diastolic blood pressure (DBPrest) after five minutes of lying supine and after five minutes of seated rest with trunk supported at 60°; differences between the Tetra group and the Control group are presented as Cohen's d and the p value symbol; mean ± standard deviation (SD)
Graded exercise test
Table 3 presents the arm crank graded exercise test results and associated physiological measures. Figure 2 provides a comparison of SBPrest and SBPpost as a response to the peak voluntary arm crank test in both groups. The Control group SBP response to the Tetra group's workload is also presented in Figure 2.
Table 3.
Cardiovascular response to arm crank peak exercise test
| VO2peak | VEpeak | HRpeak | Loadpeak | RPEpeak | SBPpost | DBPpost | |
|---|---|---|---|---|---|---|---|
| (mL·min−1·kg−1) | (L·min−1) | (bpm) | (W) | (mmHg) | (mmHg) | ||
| Tetra group | |||||||
| Mean ± SD | 14.2 ± 4.8 | 41.4 ± 23.1 | 112.4 ± 13.6 | 52.8 ± 15.0 | 18.0 ± 1.0 | 103.8 ± 19.3 | 60.9 ± 20.3 |
| Median | 13.3 | 33.5 | 110 | 52.5 | 18 | 100 | 60.0 |
| Normal distribution | no | no | yes | yes | yes | yes | no |
| Control group | |||||||
| Mean ± SD | 24.2 ± 4.4 | 81.2 ± 27.4 | 154.1 ± 14.5 | 117.9 ± 22.6 | 18.0 ± 1.0 | 154.0 ± 14.5 | 65.8 ± 23.8 |
| Median | 24.3 | 72.8 | 155 | 115.0 | 18 | 150 | 60 |
| Normal distribution | yes | no | yes | yes | yes | yes | no |
| Tetra vs. Control | |||||||
| Cohen's d | 2.2 | 1.6 | 3.0 | 3.4 | 0 | 2.9 | 0.2 |
| P | *** | *** | *** | *** | *** |
Caption: Peak oxygen consumption (VO2peak), minute ventilation (VEpeak), peak heart rate (HRpeak), peak load, ratio of perceived exertion (RPEpeak); differences between the Tetra group and the Control group are presented as Cohen's d and the P-value symbol; mean ± standard deviation (SD).
Figure 2.
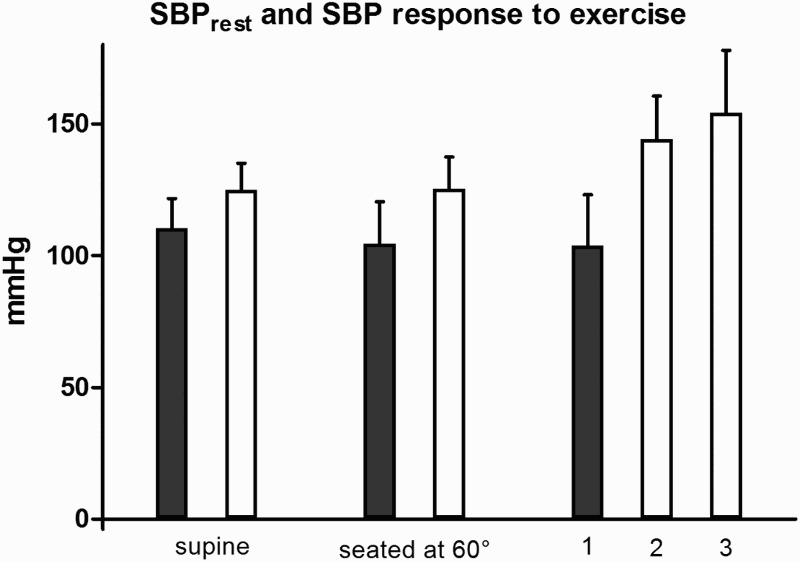
Systolic blood pressure (SBP) following five minutes lying supine, following five minutes seated rest with the trunk supported at 60° and one minute after the peak voluntary exercise on an arm crank ergometer in the Tetra group (black), in the Control group (white), and in the Control group submax (white); mean ± standard deviation (SD). 1 Tetra group; after the peak voluntary arm exercise (0.7 W·kg−1). 2 Control group submax; after the submaximal voluntary arm exercise (0.7 W·kg−1). 3 Control group; after the peak voluntary arm exercise (1.4 W·kg−1).
Note: three participants of the Tetra group, in which the BP was not detectable after the peak exercise, were excluded from this statistics.
Control group SBPpost significantly increased to 123 ± 16% of seated SBPrest (P < 0.0001; d = 1.5). On the contrary, the SBP of the Tetra group showed no significant change in response to the peak exercise (P ˃ 0.05; d = 0.06). Six Tetra group participants demonstrated a decrease in BP. The BPpost was not detectable in three individuals in the Tetra group. As a result, values of these three participants were not included in the blood pressure analyses. Their seated BPrest were 90/60, 95/50 and 95/60, respectively.
The VOmax measured on the bike ergometer in the Control group was 43.9 ± 8.3 mL·kg−1·min−1.
Control group exposed to the Tetra group's workload protocol
The average Tetra group warm-up workload was 0.2 ± 0.1 W·kg−1 and the peak workload was 0.7 W·kg−1, thus these values established the protocol for the Control group submax in the second part of the study. VO2peak (15.0 ± 1.5 mL·min−1·kg−1) and HRpeak (112.6 ± 15.0 bpm) were similar between the Control group submax and the Tetra group. Control group submax reported an RPE of 13 ± 0.6 and the SBPpost significantly increased to 144 ± 16 mmHg (114 ± 11% seated SBPrest; P < 0.0001; d = 1.3).
Clinical completeness of SCI and its impact on cardiovascular function
Only one participant was evaluated as AIS C, therefore, only males with AIS A and AIS B were included in the comparison. Supine HRrest was 56.9 ± 8.5 bpm in participants with AIS A and 51.0 ± 9.9 bpm in those with AIS B. Supine SBPrest was 110.4 ± 10.8 mmHg and 105.8 ± 12.0 mmHg in AIS A and AIS B, respectively. Seated HRrest was 60.2 ± 10.4 bpm in AIS A and 59.5 ± 9.6 bpm in AIS B, while the seated SBPrest was 106.2 ± 15.5 mmHg and 94.2 ± 12.4 mmHg, respectively. None of the resting measures were significantly different between groups (P > 0.05). There was a trend with a large effect size towards a lower seated SBPrest in AIS B (P = 0.13; d = 0.86).
There were no significant differences in HRpeak (P > 0.05; d = 0.5), VO2peak (P > 0.05; d = 0.06) and Loadpeak (P > 0.05, d = 0.2) between participants with AIS A (HRpeak = 114.2 ± 11.8 bpm; VO2peak = 14.1 ± 3.9 mL·min−1·kg−1; Loadpeak = 53.5 ± 16.0) and participants with AIS B lesions (HRpeak = 107.2 ± 16.3 bpm; VO2peak = 14.4 ± 6.5 mL·min−1·kg−1; Loadpeak = 50.8 ± 14.0). The SBPpost was 108.3 ± 17.6 (100 ± 15% of its pre-exercise values) in participants with AIS A and 85 ± 11.2 (93 ± 23% of its pre-exercise values) in participants with AIS B and was significantly different between the groups (P < 0.05; d = 1.6). The BPpost was not detectable in one participant with AIS A and two participants with AIS B.
Training status
The activity status, as evaluated by LTPAQ, showed high intra-group variability with a non-gaussian distribution in the both groups. Therefore, medians are reported for the statistical description. At the time of the study, the Tetra group self-reported more mild (420 min/week) and moderate (255 min/week) leisure time physical activity (LTPA) than the Control group (150 and 120 min/week, respectively). Heavy LTPA time was similar between the two groups (Tetra group: 22.5 min/week, Control group: 20 min/week). Similar to current physical activity reports, during a typical week in the previous six months, the Tetra group reported participating in more mild (420 min/week) and moderate (255 min/week) LTPA than the Control group (120 and 120 min/week, respectively) with no differences in heavy LTPA (Tetra group: 37.5 min/week, Control group: 30 min/week). Two participants in the Tetra group did not complete the questionnaire.
Discussion
Sympathetic neurons for the upper body arteries and the heart leave the spinal cord thoracic levels T1–T6 and neurons for splanchnic bed at T5-lumbar (L) 2.11,21 In this study, all participants had a SCI at the cervical level (C5–C7) allowing us to evaluate the effect of impaired ANS descending control on cardiovascular function. The sympathetic pathways are disrupted in a vast majority of patients with a cervical SCI. This is consistent with the history of the autonomic dysreflexia in 19 participants (95%) of the Tetra group. Nine of these patients reported no symptoms of the autonomic dysreflexia in the preceding year. This is not due to regeneration of the autonomic pathways, but is likely related to the avoidance of potential triggering factors. These findings are in accordance with Claydon et al. who found a very minimal sympathetic skin response in only one of ten tested individuals with cervical SCI.13
Heart rate and blood pressure response to graded exercise
Other ANS disorders are known to cause hypotension in response to intense PA.22 To our knowledge, there are no studies examining the BP response to peak voluntary exercise in individuals with cervical SCI as compared to the responses of an able-bodied control group.
Following a SCI, muscle mass is significantly reduced and the sympathetic reflex loop is disturbed.5,6 In contrast to able bodied and paraplegic individuals, participants with tetraplegia are not able to control the splanchnic bed pooling and the blood distribution in the legs. Furthermore, in response to intense physical activity, peripheral vasodilatation is not accompanied by an adequate increase in cardiac output (CO). These factors combined can lead to exercise-induced hypotension.23 Presumably, this is the reason why SBP measured shortly after intense PA in the Tetra group was not greater than SBPrest as was observed in the Control group. In six of the participants, the post-exercise blood pressure was lower and in three participants, it was not measurable likely due to very low, non-detectable values. Importantly, the significant SBP rise was evident in able-bodied participants not only after the maximal voluntary arm exercise, but also after completing the Tetra group's protocol. This between-group difference in the BP response to the same physical loading reflects a severe ANS dysfunction in participants of the Tetra group. The current study proposes that the pathological BP dynamics is the important reason for subjective discomfort, loss of power, and nausea often reported by individuals with tetraplegia participating in high intensity physical activity. These symptoms can limit progressive increases in exercise intensity, minimizing the beneficial metabolic adaptations of exercise for conditions such as insulin resistance syndrome and heart disease. Additionally, the reduced compliance especially of small arteries is associated with heart disease. Small artery compliance can be improved by regular PA in able-bodied participants and also in participants with SCI.3
In the current study, the HRpeak (112.4 ± 13.6 bpm) in the Tetra group was very similar to the HRpeak in the Control group submax (112.6 ± 15.0 bpm) at the peak average workload of the Tetra group. This indicates that the chronotropic impairment was probably not the key limitation of the peak physical effort in the Tetra group. The impaired BP regulation combined with less muscle mass involvement appears to have a greater influence.
Potential arm crank exercise modifications for people with cervical SCI facilitate blood flow to reduce blood pooling, thereby improving oxygen delivery to the working muscles. This can be achieved by an external compression of the core and lower extremities (abdomen and legs), elevating the lower extremities or a more horizontal body position during arm crank training, concomitant passive leg movement, functional electrical stimulation, or use of an oral alpha-sympathomimetic agent as suggested by Nieshoff et al.14,24–27 A self-induction of autonomic dysreflexia, so called “boosting”, has to be avoided due to severe health risks.28 The general recommendation remains to avoid exercise in a warm environment and to maintain adequate hydration.
Oxygen uptake response to graded exercise
In the current study, the VO2peak of the Tetra group was 59% and 32% of the Control group VO2peak on the handcrank ergometer and cycle ergometer, respectively. The VO2peak of the Tetra group is equivalent to 4 Metabolic Equivalents of Task (METs) activity or calm walking in able-bodied males.29 Less muscle mass is involved during exercise in individuals with tetraplegia, which is associated with lower oxygen demands and subsequently fewer health-related adaptations to the cardiovascular system. In addition, upper extremity cardiovascular training can have a slightly lower metabolic response than exercise involving the legs when oxygen extraction is normalized to working muscle mass.30 It should be noted that the mean intensity suitable for prolonged exercise must be lower than 4 METs. Individuals with tetraplegia need to increase their physical activity duration in order to achieve the energy expenditure associated with health-related adaptations. The increased exercise duration will require effective psychological support and available exercise opportunities.
The VO2peak and HRpeak results of this study are similar to the findings of Schmid et al.9 in a group of 25 tetraplegic patients that completed a wheelchair ergometer exercise protocol. Their study did not state the VO2peak normalized to body weight, but if the reported mean VO2peak is divided by the mean weight of their participants, a VO2peak of 13.7 mL·min−1·kg−1 is calculated, similar to the results of the current study.
Impact of SCI completeness on cardiovascular function
The association between the neurological level of SCI and cardiovascular functions has already been described.31 However, the relationship between the clinically evaluated completeness of SCI and ANS function remains unclear. The sensorimotor impairment as evaluated by ISNCSCI15 does not correspond to the integrity of the autonomic pathways. Claydon and Krassioukov found that by using sympathetic skin response examinations, 40% of the participants with AIS A, total sensorimotor lesion, had residual functions of autonomic pathways.32 In the same study, 30% of the individuals with AIS B–D degrees had complete autonomic lesion. The current study confirmed the lack of relationship between AIS degree and cardiovascular responses to peak voluntary PA. Paradoxically, participants with an incomplete lesion evaluated as AIS B exhibited a trend towards a lower seated BPrest and even lower BPpost than those with “complete” lesion AIS A, indicating larger dysfunction of ANS. As an opposite example, the participant with the highest HRpeak (142) in this study was evaluated as a patient with “complete” lesion (AIS A) at C6. The reason for this discrepancy is most likely due to different topographic preservation of anatomical pathways. The ISNCSCI15 evaluates the damage of corticospinal, spinothalamic, and dorsal column tracts, however, the descending vasomotor fibers are located in other dorsolateral parts of spinal cord.33
Training status
The Tetra group reported a higher amount of mild and moderate LTPA than the Control group, whereas heavy LTPA was similar between the groups. It should be emphasized that the amount of PA subjectively measured by using LTPAQ-SCI and parallel questionnaires do not evaluate the actual energy expenditure and therefore do not provide an appropriate comparison of energy expenditure between groups. In this study, the same RPE subjectively reported by participants in both groups was related to a significantly lower workload in the Tetra group. The VO2max of the Control group on the cycle ergometer fell within able-bodied population norms.34
Limitations
In the current study, the research design did not allow for the measurement of BP during peak PA. The BP measurement by sphygmomanometer would not be reliable during the cyclic arm movement. At the same time, invasive techniques could increase the risk of reactive hypotension.35 As a result, the authors of the current study decided to measure the BP exactly one minute following cessation of the graded exercise test. To ensure the valid measurement of BP, all measures were taken by experienced healthcare personnel. Nevertheless, in three tetraplegic participants, BP could not be measured after the peak exercise, which probably resulted from very low, non-detectable BP values.
Conclusions
The SBP of individuals with cervical SCI did not increase in response to maximal voluntary exercise unlike the responses of the Control group. Some males in the Tetra group appeared to be at risk of severe hypotension following the high intensity exercise. This can be an important factor limiting a progressive rise in and the maintenance of a higher exercise intensity. This study also found that the injury completeness, expressed as the AIS degree,15 does not correspond to cardiovascular function/dysfunction controlled by the ANS. Thus, the findings of this study are in line with other studies proposing the need to focus on a specific ANS examination and its incorporation into clinical practice.33,36 Exercise modifications for people with cervical SCI should focus on blood flow facilitation principles, which have a potential to increase and maintain higher exercise intensities that are associated with health-related adaptations.
Acknowledgments
The project was supported by PRVOUK P38.
References
- 1.Haisma JA, van der Woude LH, Stam HJ, Bergen MP, Sluis TA, Bussmann JB. Physical capacity in wheelchair-dependent persons with a spinal cord injury: a critical review of the literature. Spinal Cord 2006;44(11):642–52. doi: 10.1038/sj.sc.3101915 [DOI] [PubMed] [Google Scholar]
- 2.Dallmeijer AJ, van der Woude LH, Hollander PA, Angenot EL. Physical performance in persons with spinal cord injuries after discharge from rehabilitation. Med Sci Sports Exerc 1999;31(8):1111–7. doi: 10.1097/00005768-199908000-00006 [DOI] [PubMed] [Google Scholar]
- 3.Wong SC, Bredin SS, Krassioukov AV, Taylor A, Warburton DE. Effects of training status on arterial compliance in able-bodied persons and persons with spinal cord injury. Spinal Cord 2013;51(4):278–81. doi: 10.1038/sc.2012.151 [DOI] [PubMed] [Google Scholar]
- 4.Warburton DE, Eng JJ, Krassioukov A, Sproule S, the SRT . Cardiovascular health and exercise rehabilitation in spinal cord injury. Top Spinal Cord Inj Rehabil 2007;13(1):98–122. doi: 10.1310/sci1301-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunten DC, Warner AL, Brunnemann SR, Segal JL. Heart rate variability is altered following spinal cord injury. Clin Auton Res 1998;8(6):329–34. doi: 10.1007/BF02309623 [DOI] [PubMed] [Google Scholar]
- 6.Furlan JC, Fehlings MG. Cardiovascular complications after acute spinal cord injury: pathophysiology, diagnosis, and management. Neurosurg Focus 2008;25(5):E13. doi: 10.3171/FOC.2008.25.11.E13 [DOI] [PubMed] [Google Scholar]
- 7.Koh J, Brown TE, Beightol LA, Ha CY, Eckberg DL. Human autonomic rhythms: vagal cardiac mechanisms in tetraplegic subjects. J Physiol 1994;474(3):483–95. doi: 10.1113/jphysiol.1994.sp020039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ravensbergen HJ, de Groot S, Post MW, Slootman HJ, van der Woude LH, Claydon VE. Cardiovascular function after spinal cord injury: prevalence and progression of dysfunction during inpatient rehabilitation and 5 years following discharge. Neurorehabil Neural Repair 2014;28(3):219–29. doi: 10.1177/1545968313504542 [DOI] [PubMed] [Google Scholar]
- 9.Schmid A, Huonker M, Barturen JM, Stahl F, Schmidt-Trucksass A, Konig D, et al. Catecholamines, heart rate, and oxygen uptake during exercise in persons with spinal cord injury. J Appl Physiol 1998;85(2):635–41. [DOI] [PubMed] [Google Scholar]
- 10.Krassioukov A. Autonomic dysreflexia: current evidence related to unstable arterial blood pressure control among athletes with spinal cord injury. Clin J Sport Med 2012;22(1):39–45. [DOI] [PubMed] [Google Scholar]
- 11.Krassioukov A, Claydon VE. The clinical problems in cardiovascular control following spinal cord injury: an overview. Prog Brain Res 2006;152:223–9. doi: 10.1016/S0079-6123(05)52014-4 [DOI] [PubMed] [Google Scholar]
- 12.Coutts KD, Rhodes EC, McKenzie DC. Maximal exercise responses of tetraplegics and paraplegics. J Appl Physiol 1983;55(2):479–82. [DOI] [PubMed] [Google Scholar]
- 13.Claydon VE, Hol AT, Eng JJ, Krassioukov AV. Cardiovascular responses and postexercise hypotension after arm cycling exercise in subjects with spinal cord injury. Arch Phys Med Rehabil 2006;87(8):1106–14. doi: 10.1016/j.apmr.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 14.Dela F, Mohr T, Jensen CM, Haahr HL, Secher NH, Biering-Sorensen F, et al. Cardiovascular control during exercise: insights from spinal cord-injured humans. Circulation 2003;107(16):2127–33. doi: 10.1161/01.CIR.0000065225.18093.E4 [DOI] [PubMed] [Google Scholar]
- 15.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med 2011;34(6):535–46. doi: 10.1179/204577211X13207446293695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun G, French CR, Martin GR, Younghusband B, Green RC, Xie YG, et al. Comparison of multifrequency bioelectrical impedance analysis with dual-energy X-ray absorptiometry for assessment of percentage body fat in a large, healthy population. The American journal of clinical nutrition. 2005;81(1):74–8. [DOI] [PubMed] [Google Scholar]
- 17.Martin Ginis KA, Phang SH, Latimer AE, Arbour-Nicitopoulos KP. Reliability and validity tests of the leisure time physical activity questionnaire for people with spinal cord injury. Arch Phys Med Rehabil 2012;93(4):677–82. doi: 10.1016/j.apmr.2011.11.005 [DOI] [PubMed] [Google Scholar]
- 18.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14(5):377–81. doi: 10.1249/00005768-198205000-00012 [DOI] [PubMed] [Google Scholar]
- 19.Lasko-McCarthey P, Davis JA. Protocol dependency of VO2max during arm cycle ergometry in males with quadriplegia. Med Sci Sports Exerc 1991;23(9):1097–101. doi: 10.1249/00005768-199109000-00016 [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd Edition). Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 21.Bonica JJ. Autonomic innervation of the viscera in relation to nerve block. Anesthesiology 1968;29(4):793–813. doi: 10.1097/00000542-196807000-00023 [DOI] [PubMed] [Google Scholar]
- 22.Low DA, da Nobrega AC, Mathias CJ. Exercise-induced hypotension in autonomic disorders. Auton Neurosci 2012;171(1–2):66–78. doi: 10.1016/j.autneu.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 23.Krediet CT, Wilde AA, Wieling W, Halliwill JR. Exercise related syncope, when it's not the heart. Clin Auton Res 2004;14 Suppl 1:25–36. doi: 10.1007/s10286-004-1005-1 [DOI] [PubMed] [Google Scholar]
- 24.Hopman MT, Dueck C, Monroe M, Philips WT, Skinner JS. Limits to maximal performance in individuals with spinal cord injury. Int J Sports Med 1998;19(2):98–103. doi: 10.1055/s-2007-971889 [DOI] [PubMed] [Google Scholar]
- 25.Davis GM, Hamzaid NA, Fornusek C. Cardiorespiratory, metabolic, and biomechanical responses during functional electrical stimulation leg exercise: health and fitness benefits. Artif Organs 2008;32(8):625–9. doi: 10.1111/j.1525-1594.2008.00622.x [DOI] [PubMed] [Google Scholar]
- 26.Griffin L, Decker MJ, Hwang JY, Wang B, Kitchen K, Ding Z, et al. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J Electromyogr Kinesiol 2009;19(4):614–22. doi: 10.1016/j.jelekin.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Nieshoff EC, Birk TJ, Birk CA, Hinderer SR, Yavuzer G. Double-blinded, placebo-controlled trial of midodrine for exercise performance enhancement in tetraplegia: a pilot study. J Spinal Cord Med 2004;27(3):219–25. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson AK. Autonomic dysreflexia. Spinal Cord 1999;37(6):383–91. doi: 10.1038/sj.sc.3100867 [DOI] [PubMed] [Google Scholar]
- 29.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, et al. Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43(8):1575–81. doi: 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 30.Calbet JA, Holmberg HC, Rosdahl H, van Hall G, Jensen-Urstad M, Saltin B. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol 2005;289(5):R1448–58. doi: 10.1152/ajpregu.00824.2004 [DOI] [PubMed] [Google Scholar]
- 31.West CR, Mills P, Krassioukov AV. Influence of the neurological level of spinal cord injury on cardiovascular outcomes in humans: a meta-analysis. Spinal Cord 2012;50(7):484–92. doi: 10.1038/sc.2012.17 [DOI] [PubMed] [Google Scholar]
- 32.Claydon VE, Krassioukov AV. Orthostatic hypotension and autonomic pathways after spinal cord injury. J Neurotrauma 2006;23(12):1713–25. doi: 10.1089/neu.2006.23.1713 [DOI] [PubMed] [Google Scholar]
- 33.West CR, Bellantoni A, Krassioukov AV. Cardiovascular function in individuals with incomplete spinal cord injury: a systematic review. Top Spinal Cord Inj Rehabil 2013;19(4):267–78. doi: 10.1310/sci1904-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Máček M, Vávra J. Fyziologie a patofyziologie tělesné zátěže. Praha: Avicenum 1988;353 p. [Google Scholar]
- 35.Stevens PM. Cardiovascular dynamics during orthostasis and the influence of intravascular instrumentation. Am J Cardiol 1966;17(2):211–8. doi: 10.1016/0002-9149(66)90354-7 [DOI] [PubMed] [Google Scholar]
- 36.West CR, Wong SC, Krassioukov AV. Autonomic cardiovascular control in Paralympic athletes with spinal cord injury. Med Sci Sports Exerc 2014;46(1):60–8. doi: 10.1249/MSS.0b013e31829e46f3 [DOI] [PubMed] [Google Scholar]


