Our study demonstrates that resveratrol exhibits protective effects in experimental autoimmune encephalomyelitis therapy through its anti-inflammation and antioxidant activities by protecting the basement membrane tight junction proteins to improve blood-brain barrier integrity.
Keywords: encephalomyelitis, blood-brain barrier, resveratrol, multiple sclerosis
Abstract
The mouse autoimmune encephalomyelitis (EAE), an experimental model of multiple sclerosis (MS), is primarily characterized as dysfunction of the blood-brain barrier (BBB). Resveratrol exhibits anti-inflammatory, antioxidative, and neuroprotective activities. We investigated the beneficial effects of resveratrol in protecting the integrity of the BBB in EAE mice and observed improved clinical outcome in the EAE mice after resveratrol treatment. Evans blue (EB) extravasation was used to detect the disruption of BBB. Western blot were used to detected the tight junction proteins and adhesion molecules zonula occludens-1 (ZO-1), occludin, ICAM-1, and VCAM-1. Inflammatory factors inducible nitric oxide synthase (iNOS), IL-1β, and arginase 1 were evaluated by quantitative RT-PCR (qPCR) and IL-10 by ELISA. NADPH oxidase (NOX) levels were evaluated by qPCR, and its activity was analyzed by lucigenin-derived chemiluminescence. Resveratrol at doses of 25 and 50 mg/kg produced a dose-dependent decrease in EAE paralysis and EB leakage, ameliorated EAE-induced loss of tight junction proteins ZO-1, occludin, and claudin-5, as well as repressed the EAE-induced increase in adhesion proteins ICAM-1 and VCAM-1. In addition, resveratrol suppressed the EAE-induced overexpression of proinflammatory transcripts iNOS and IL-1β and upregulated the expression of anti-inflammatory transcripts arginase 1 and IL-10 cytokine in the brain. Furthermore, resveratrol downregulated the overexpressed NOX2 and NOX4 in the brain and suppressed NADPH activity. Resveratrol ameliorates the clinical severity of MS through maintaining the BBB integrity in EAE mice.
NEW & NOTEWORTHY
Our study demonstrates that resveratrol exhibits protective effects in experimental autoimmune encephalomyelitis therapy through its anti-inflammation and antioxidant activities by protecting the basement membrane tight junction proteins to improve blood-brain barrier integrity.
multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS) that could manifest as a variable course of neurological disabilities (Gao et al. 2014; Glenn et al. 2014). Experimental autoimmune encephalomyelitis (EAE) is widely employed as an animal model to study the clinical, immunological, and histopathological features of MS, including demyelinating lesions in spinal cord as well as optic nerve (Guo et al. 2013; Hou et al. 2013; Jones et al. 2013; Kassis et al. 2013). In both MS and EAE, activated autoaggressive immune cells are thought to break down the blood-brain barrier (BBB) and invade the CNS, where they initiate immunological cascades resulting in demyelination, neuroinflammation, and neuronal damage (Wang et al. 2014; Wolburg et al. 2004; Yang et al. 2016). The BBB is composed of basement membrane, interendothelial tight junctions (TJs), and perivascular astrocytes. TJs, composed of large multiprotein complexes, seal the gaps between biological barriers. Reactive oxygen species (ROS), which could induce severe CNS damage, also play essential roles in the pathogenesis of MS (Seo et al. 2015).
Resveratrol is a naturally occurring polyphenol with numerous reported beneficial effects, including cardioprotections, anticancer, antioxidation, anti-inflammation, and neuroprotective effects (Csiszar 2011; Das and Das 2007; de la Lastra and Villegas 2005; Imler and Petro 2009; Labinskyy et al. 2006; Udenigwe et al. 2008; Wood et al. 2010). SRT501, a pharmaceutical formulation of resveratrol, was reported to delay EAE onset in mice and prevent neuronal damage (Fonseca-Kelly et al. 2012). Since the chronic autoimmune inflammatory disorder could damage the integrity of the BBB, resulting in its dysfunction (Flugel et al. 2001; Zhang et al. 2013), we hereby speculate that the anti-inflammation and antioxidative stress properties of resveratrol could be exploited in the EAE mouse model to protect the BBB integrity.
MATERIALS AND METHODS
Experimental animals.
Two-month-old female C57BL/L6 mice were purchased from Model Animal Institute (Nanjing, China). Housing and treatment of animals conformed to the Institutional Animal Care and Use Committee Guidelines. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal protocols were approved by the Institutional Animal Care and Use Committee of The Second Hospital of Hebei Medical University. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
Induction and scoring of EAE model.
EAE was induced in C57BL/6 mice by immunization with myelin oligodendroglial glycoprotein (MOG) peptide 35-55 (MOG35-55; Invitrogen, Carlsbad, CA) according to the published method (Hou et al. 2013; Imler and Petro 2009). Briefly, the mice were injected subcutaneously at two sites with a total of 200 μg of MOG35-55 emulsified in complete Freund adjuvant (CFA; Difco, Detroit, MI) containing 6 mg/ml Mycobacterium tuberculosis (Difco). Sham control mice were injected with an equal volume of phosphate-buffered saline (PBS) and CFA. EAE and sham control mice received 100 ng of pertussis toxin (List Biological Laboratories, Campbell, CA) in 0.1 ml of PBS by intraperitoneal injection 2 and 24 h after MOG35-55 injection.
Paralysis as clinical evidence of EAE was scored daily by a blinder observer starting on day 5 after immunization, when all of the mice were still clinically normal. Clinically, animals were scored as follows: 0, no clinical signs; 1, limp tail; 2, partial hind-leg paralysis; 3, complete hind-leg paralysis; 4, complete hind-leg paralysis and partial front-leg paralysis; and 5, moribund or dead.
Resveratrol treatment.
Purified resveratrol purchased from Sigma (St. Louis, MO) was suspended in PBS. All treatments started on the day of immunization. Resveratrol was administered intraperitoneally once daily for 20 consecutive days with 10, 25, and 50 mg/kg. Mice treated with PBS alone served as vehicle control. Ten mice were included in each group.
Evaluation of BBB leakage.
BBB leakage was assessed using Evans blue (EB) dye as previously described (Wang et al. 2014). Four mice of every group were randomly assigned for the evaluation of BBB permeability. One day after last administration of resveratrol (on day 21 postimmunization), the EB dye was injected (2% in PBS, 5 ml/kg; Sigma) into the mouse tail vein and was allowed in circulation for 1.5 h. The mice were then perfused with saline (250 ml) through the left ventricle at 110 mmHg pressure until colorless perfusion fluid was obtained from the right atrium. The brains were removed and snap-frozen. Sections (20 μm) were then photographed for qualitative analysis. Five randomly picked sections per animal were used to quantify the percentage of EB-positive area for the whole hemisphere.
Western blot.
Three mice of each group were killed 24 h after the last resveratrol injection. Brain tissue proteins were extracted by homogenizing with the RIPA Lysis Buffer (Beyotime, Jiangsu, China). After separated on 12% SDS-PAGE and transferred onto nitrocellulose membrane (Bio-Rad), the membranes were incubated at 4°C overnight with one of the primary antibodies anti-ZO-1 (1:200; sc-10804; Santa Cruz Biotechnology), anti-occludin (1:500; ab31721; Abcam), anti-claudin-5 (1:200; Santa Cruz Biotechnology), anti-ICAM-1 (1:500; sc-8439; Santa Cruz Biotechnology), or anti-VCAM-1 (1:500; 11444-1-AP; Proteintech, Wuhan, China), and anti-β-actin antibody (dilution 1:1,000; sc-69879; Santa Cruz Biotechnology) was used as normalization for relative protein quantification. After incubating with the appropriate primary antibody, the membrane was washed three times with TBS-Tween 20 (TBST) buffer for 10 min at room temperature. The membrane was then incubated with appropriate horseradish peroxidase-conjugated secondary antibodies (dilution 1:2,000; ZSGB-BIO, Beijing, China) for 1 h followed by washing three times with TBST buffer for 10 min at room temperature. Images were captured and qualified by a ChemiQ 3650mini fluorescence and chemiluminescence imaging system (Bioshine, Shanghai, China). The densitometry of the bands was analyzed semiquantitatively using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD).
RT-PCR analysis.
Brain tissues were taken 24 h after the last resveratrol injection (on day 21 postimmunization, 3 mice per group). Total cellular RNA from these tissues was isolated using TRIzol reagent (Beijing TransGen Biotech, Beijing, China) following the standard protocol. Single-stranded cDNA synthesis was performed by reverse transcription using a Promega Reverse Transcription System. Expression levels of inducible nitric oxide synthase (iNOS), IL-1β, arginase 1, and NOX genes were investigated using real-time quantitative RT-PCR (qPCR). Real-time qPCR analysis was performed using a LightCycler 480 SYBR Green I Master (Roche Applied Science). To determine the relative quantification of target gene expression, we used the gel imaging analysis system (Dalian Jingmai Biotech, Liaoning, China). All of the reactions were performed in triplicate. β-Actin was used as a housekeeping endogenous control gene. The primers were as follows: β-actin, 5′-CCAGAGCAAGAGAGGTATCC-3′ and 5′-CTGTGGTGGTGAAGCTGTAG-3′; iNOS, 5′-GTGTTCCACCAGGAGATGTTG-3′ and 5′-CTCCTGCCCACTGAGTTCGTC-3′; IL-1β, 5′-TGTAATGAAAGACGGCACACC-3′ and 5′-TCTTCTTTGGGTATTGCTTGG-3′; arginase 1, 5′-AAGAAAAGGCCGATTCACCT-3′ and 5′-CACCTCCTCT GCTGTCTTCC-3′; NOX1, 5′-AATGCCCAGGATCGAGGT-3′ and 5′-GATGGAAGCAAAGGGAGTGA-3′; NOX2, 5′-CCCTTTGGTACAGCCAGTGAAGAT-3′ and 5′-CAATCCCGGCTCCCACTA AC ATCA-3′; and NOX4, 5′-GGATCACAGAAGGTCCCTAGCAG-3′ and 5′-GCGGCTACAGCACACCTGAG AA-3′.
ELISA.
Mouse IL-10 Quantikine ELISA Kit (R&D Systems) was used to measure serum IL-10 of mice on day 21 after immunization. ELISA was performed according to the provided protocol of the kit. In brief, 2-fold diluted serum was incubated in the precoated 96-well plates for 4 h followed by conjugated antibody incubation and color development. The optical density of each well was determined by using a microplate reader at 450 nm.
NADPH oxidase activity determination.
NADPH oxidase activity was measured by lucigenin assay as described previously (Vallejo et al. 2014). Briefly, the brains from EAE mice were homogenized in lysis buffer (pH 7.0) containing 50 mM KH2PO4, 1 mM EGTA, and 150 mM sucrose at 4°C. The assay was performed in PBS containing 5 mM lucigenin and 100 mM NADPH as substrate. Luminescence was then measured every 10 s for 5 min in a microplate luminometer (Orion II Microplate Luminometer; Berthold Technologies). For every sample, NADPH oxidase activity was calculated as the difference between the activities obtained in the presence and in the absence of NADPH. The enzymatic activity was expressed as relative light units (RLU) per milligram of protein per minute.
Statistics.
Data were analyzed by one- or two-way ANOVA followed by a Tukey post hoc test. Statistical significance was determined when P < 0.05.
RESULTS
Resveratrol treatment attenuates severity of clinical EAE.
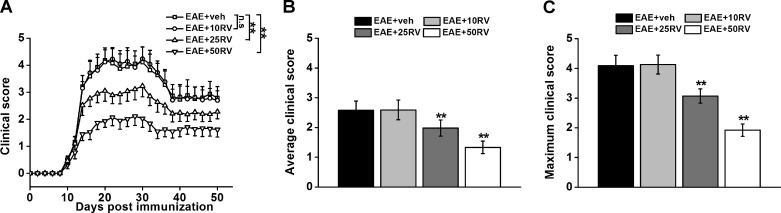
We first investigated the effects of resveratrol treatment on the paralysis symptoms of the EAE mice. All groups of mice started to develop clinical signs, including tail and hindlimb paralysis, at ∼12 days after MOG sensitization. Mice treated with PBS developed a typical course of chronic EAE, as evidenced by ascending paralysis beginning at ∼12 days after immunization, which peaked several days later and then persisted (Fig. 1A) until the end of the experiment. Treatment with 10 mg/kg resveratrol failed to suppress EAE, whereas both 25 and 50 mg/kg resveratrol significantly attenuated the paralysis of EAE during 50 days observation period, although by 35 days postimmunization EAE mice began to recover (Fig. 1). During the chronic phase of EAE, the average and maximum clinical scores of 25 and 50 mg/kg resveratrol-treated mice were significantly lower than the PBS treatment group, showing a dose-dependent response (Fig. 1, B and C). During the chronic phase of EAE, 25 and 50 mg/kg resveratrol produced a dose-related decrease in the average and maximal clinical scores.
Fig. 1.
Resveratrol treatment attenuates clinical EAE severity in mice. A: mean daily clinical scores in resveratrol (RV)-treated mice at different doses (10, 25, and 50 mg/kg) over 50 days postimmunization. Resveratrol was administrated for 20 consecutive days after immunization once daily by intraperitoneal injection. B: the average clinical score was assessed from day 1 until day 50 after immunization. C: the maximum clinical score for each mouse over the entire observation period was recorded. N = 10 in each group. Experiments were repeated in triplicate. Data are presented as means ± SD. **P < 0.01 vs. EAE+veh group. veh, Vehicle control; n.s, not significant.
Resveratrol treatment protects BBB integrity in EAE mice.
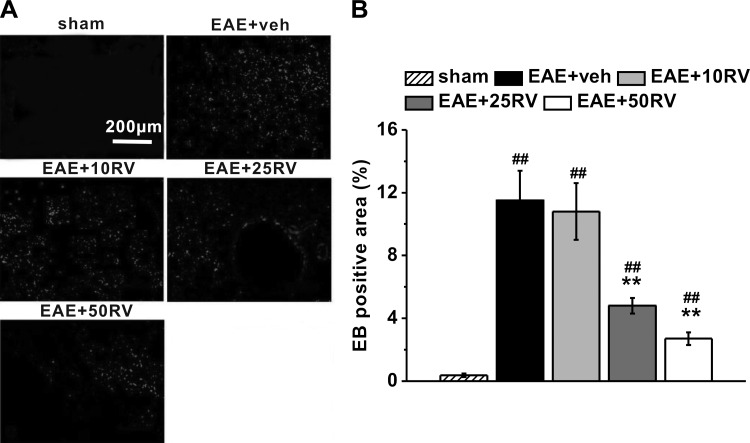
We next examined the extravasation of EB dye in the mouse brains to determine the effect of resveratrol on BBB integrity. As shown in Fig. 2A, comparing with sham control (non-EAE) mice, EB leakage into brain area was observed in the untreated vehicle control EAE mice on day 21 postimmunization. Meanwhile, quantitative results showed lower EB leakage detected in brains of resveratrol-treated EAE mice at the doses of 25 and 50 mg/kg compared with vehicle control, whereas there was no significant difference between 10 mg/kg resveratrol treatment group and the untreated vehicle control group (Fig. 2B). These results indicated that resveratrol successfully prevented EB leakage through the BBB.
Fig. 2.
Resveratrol treatment protects blood-brain barrier integrity in EAE mice. A: representative images of brain sections with Evans blue (EB) staining in the experimental groups. B: quantification of the percentage of EB-positive area comparing the entire section. N = 10 in each group. Experiments were repeated in triplicate. Data are presented as means ± SD. ##P < 0.01 vs. sham group, **P < 0.01 vs. EAE+veh group.
Resveratrol treatment ameliorates EAE-induced loss of tight junction proteins.
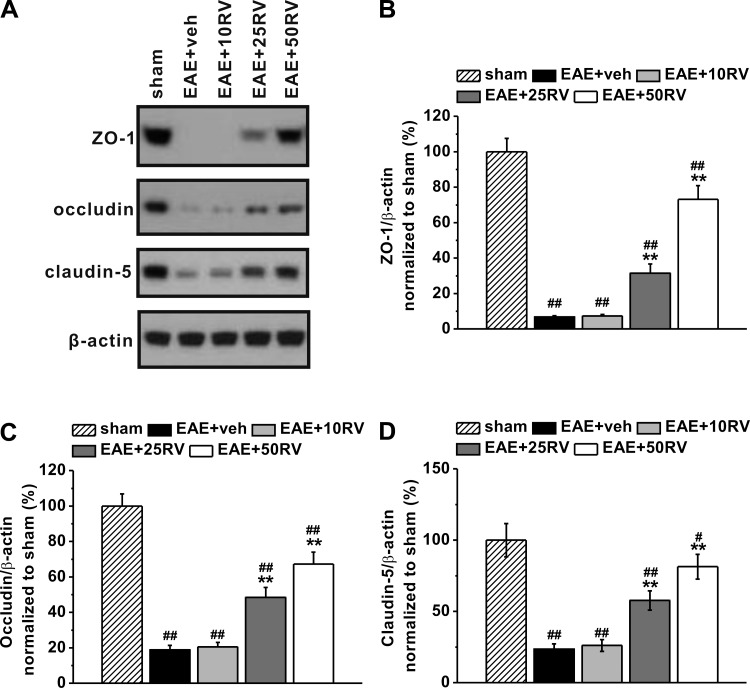
TJs are specialized cell-cell adhesion structures and critical components of the BBB for which normal distributions have previously been shown to be disrupted in MS tissue (Wolburg et al. 2004). To explore further the protective effects of resveratrol on EAE-induced disruption of the BBB basement membrane, we examined the transmembrane tight junction proteins ZO-1, occludin, and claudin-5 on day 21 postimmunization using Western blot analysis (Fig. 3A). The expressions of ZO-1, occludin, and claudin-5 significantly declined in EAE mice compared with the sham control mice (Fig. 3, B–D, respectively; all P < 0.01). Treatment with 25 and 50 mg/kg resveratrol produced a marked, dose-related reduction in the loss of ZO-1, occludin, and claudin-5.
Fig. 3.
Resveratrol treatment ameliorates EAE-induced loss of tight junction proteins in EAE mice. A: representative images of Western blot analysis of ZO-1, occludin, and claudin-5. β-Actin was used as internal control. Histogram of relative expression of ZO-1 (B), occludin (C), and claudin-5 (D) in the experimental groups, normalized to that of sham. Experiments were repeated in triplicate. Data are presented as means ± SD. ##P < 0.01 vs. sham group, **P < 0.01 vs. EAE+veh group.
Effects of resveratrol on the expressions of ICAM-1 and VCAM-1.
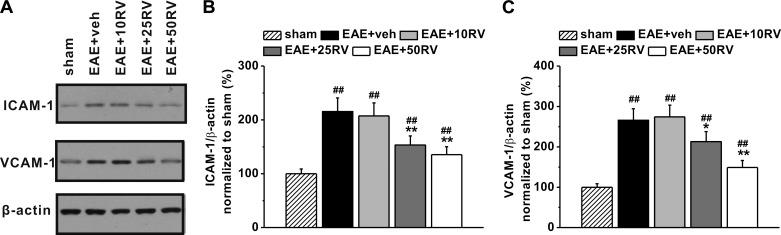
Furthermore, protein expressions of intercellular cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion protein-1 (VCAM-1) in the experimental groups were analyzed by Western blot (Fig. 4A). As shown in Fig. 4, ICAM-1 and VCAM-1 were significantly increased in brain tissues of EAE mice. Although treatment with 10 mg/kg resveratrol did not change the levels of these two adhesion factors (Fig. 4, B and C), mice treated with 25 and 50 mg/kg resveratrol showed a dose-dependent repression in the EAE-induced expressions of ICAM-1 and VCAM-1 in the brain compared with the vehicle control group. These results suggested that resveratrol inhibited the upregulation of both ICAM-1 and VCAM-1 in the brain tissues of EAE mice.
Fig. 4.
Effects of resveratrol on the expression of ICAM-1 and VCAM-1. A: representative images of Western blot analysis of ICAM-1 and VCAM-1. β-Actin was used as internal control. Histogram of relative expression of ICAM-1 (B) and VCAM-1 (C) in the experimental groups, normalized to that of sham. Experiments were repeated in triplicate. Data are presented as means ± SD. ##P < 0.01 vs. sham group, *P < 0.05 and **P < 0.01 vs. EAE+veh group.
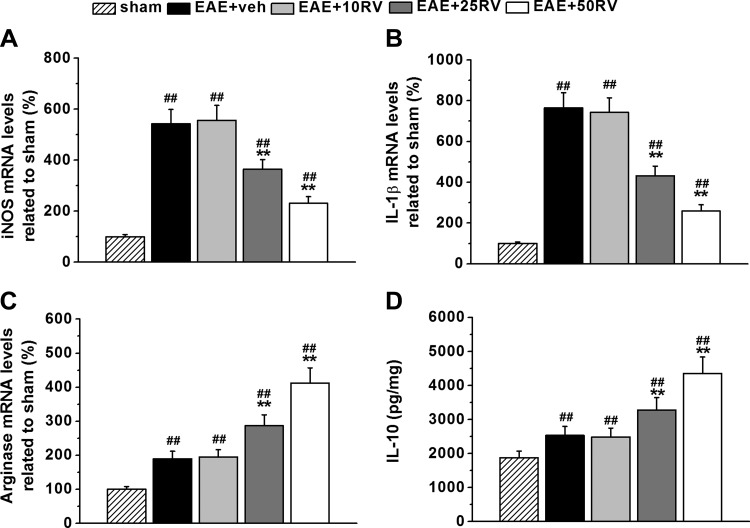
Resveratrol suppresses local inflammation in the brains.
Considering the close relationship between inflammation and BBB integrity, we then examined the inflammatory responses to unravel the possible mechanisms underlying the protective effects of resveratrol. Brain proinflammatory genes iNOS and IL-1β, anti-inflammatory gene arginase 1, and anti-inflammation cytokine IL-10 were analyzed to evaluate local inflammation in EAE mice. As shown in Fig. 5, A and B, markedly elevated iNOS and IL-1β were detected in brain samples of EAE mice, which were then significantly suppressed by 25 and 50 mg/kg resveratrol treatment. Local anti-inflammatory factors arginase 1 and IL-10 were slightly upregulated in untreated vehicle EAE mice (Fig. 5, C and D) but greatly upregulated in EAE mice after 25 and 50 mg/kg resveratrol treatment. Consistently, we found that 10 mg/kg resveratrol treatment did not affect these two factors. Of note, 25 and 50 mg/kg resveratrol treatments also attenuated the production of proinflammatory cytokines TNF-α and IL-1β in the serum of EAE mice. These results indicated that resveratrol suppressed local inflammation in the brains of EAE mice.
Fig. 5.
Resveratrol treatment suppresses local inflammation in the brains of EAE mice. A–C: qPCR analysis of mRNA expressions of proinflammatory genes iNOS, IL-1β, and anti-inflammation-associated gene arginase 1. D: the anti-inflammation cytokine IL-10 in the experimental groups was characterized by ELISA. N = 10 in each group. Experiments were repeated in triplicate. Data are presented as means ± SD. ##P < 0.01 vs. sham group, **P < 0.01 vs. EAE+veh group.
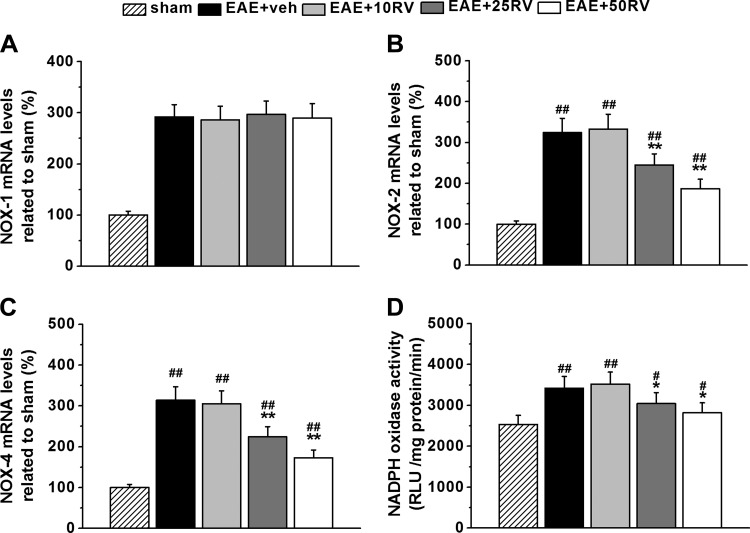
Resveratrol inhibits NADPH oxidase expression and activity in the brain.
We also explored whether oxidative stress was altered by resveratrol treatment in the EAE mice. Brain local oxidation status was evaluated by NADPH oxidase (NOX) expression and NADPH activity. The EAE mice showed overexpressed NOX1, NOX2, and NOX4 (Fig. 6, A–C). Interestingly, resveratrol did not affect NOX1 expression compared with vehicle control (Fig. 6A). However, the upregulated NOX2 and NOX4 were significantly suppressed by resveratrol treatment in a dose-dependent manner (Fig. 6, B and C). The increased NADPH activity in EAE mice was also significantly suppressed by resveratrol at the doses of 25 and 50 mg/kg (Fig. 6D).
Fig. 6.
Resveratrol inhibits NADPH oxidase (NOX) expression and activity in the brains of EAE mice. A–C: mRNA expressions of NOX1, NOX2, and NOX4 related to those in sham brain. D: NOX activity in the experimental groups. N = 10 in each group. Experiments were repeated in triplicate. Data are presented as means ± SD. #P < 0.05 and ##P < 0.01 vs. sham group, *P < 0.05 and **P < 0.01 vs. EAE+veh group.
DISCUSSION
MS and its EAE animal model are widely used in studies involving T cell-mediated inflammatory diseases, characterized by lymphocyte infiltration, demyelination, and axonal injury (Eberle et al. 2015; Guo et al. 2013). Dysfunction of the BBB is one of the major features in the progression of EAE and MS, in which proinflammatory cells and toxic molecules migrate into the brain via the damaged BBB, resulting in cerebral edema, demyelination, and neural cell death (Wang et al. 2014; Yang et al. 2016; Zhang et al. 2013). In the present study, we investigated the potential protective effects of resveratrol on the paralysis symptoms of EAE mice (Fig. 1). We also provided evidence that the therapeutic effects of resveratrol on EAE are, at least partially, mediated through its strengthening of BBB integrity as well as its anti-inflammation and antioxidant properties (Fischer et al. 2013).
TJs, composed of large multiprotein complexes, are one of the major components of the BBB, and zonula occludens-1 (ZO-1) is the primary cytoplasmic protein associated with TJs. ZO-1 functions to link the COOH termini of occludin and claudins to the underlying actin cytoskeleton, and reductions in ZO-1 or occludin expressions were reported to result in increased BBB permeability (Zhang et al. 2013).
EB dye is widely used to detect BBB leakage since it conjugates with serum albumin to form a large molecular complex incapable of crossing the intact BBB under normal physiological circumstances. We therefore quantified the extravasation of EB dye into the brain as an indicator of BBB permeability. Our study showed that EB leakage was markedly increased in the EAE mice compared with sham control mice, which was also associated with decreased tight junction proteins ZO-1, occludin, and claudin-5 in vehicle control-treated EAE mice. Resveratrol treatment significantly decreased EB content in the brain compared with vehicle control-treated EAE mice (Figs. 2 and 3). Together, these results indicated resveratrol could prevent the disruption of BBB in the EAE mouse model.
Besides the breakdown of the BBB, adhesion factors ICAM-1 and VCAM-1 have long been implicated in the pathogenesis of MS (Chaudhary et al. 2006). VCAM-1 mediates the capture of pathogenic CD4+ T cells on the BBB endothelium, whereas ICAM-1 arrests T cells on the BBB and assists with T cell diapedesis across the BBB. In our current study, the upregulated ICAM-1 and VCAM-1 in EAE mice was attenuated by resveratrol treatment. The expression of proinflammatory genes iNOS and IL-1β as well as anti-inflammatory gene arginase 1 and cytokine IL-10 were also significantly increased in EAE mice (Figs. 4 and 5). Our results again demonstrated the anti-inflammatory effect of resveratrol, consistent with previous reports (de la Lastra and Villegas 2005). Current literature reveals that a variety of immune cells, such as T cells and macrophages, are likely to be the target of the anti-inflammatory properties of resveratrol (Ma et al. 2015; Shindler et al. 2010). However, it may be difficult to identify the exact target cell type of resveratrol, since many types of immune cells are able to produce TNF-α, IL-1β, and IL-10. In this context, a highly selective pharmacological inhibition on a specific type of immune cells is necessary to carefully dissect the target(s) of resveratrol. In addition, evidence from in vitro and in vivo studies suggest that the possible mechanisms underlying the anti-inflammatory effect of resveratrol include activating sirtuin 1 (Shindler et al. 2010), inducing apoptosis in activated T cells (Shindler et al. 2010) and modulating NF-κB and Janus kinase/STAT signaling pathways (Ma et al. 2015). However, since the aim of our current study is to investigate the protective effect of resveratrol on BBB integrity, we did not attempt to search intensively for the possible signaling pathways resveratrol may modulate in the EAE mouse model. Nevertheless, studies to unravel the molecular target and the signaling pathway are currently underway for a better understanding of the therapeutic effects of resveratrol.
The production of reactive oxygen species (ROS) and the activation of signaling pathways leading to neuroinflammation were reported by Svajger and Jeras (2012) and Zhang et al. (2010). ROS are produced by NOX isoforms in a variety of cells and tissues in response to stimulation with various growth factors or cytokines. Here, we investigated expressions of NOX genes in the brains of resveratrol-treated EAE mice. Upregulated NOX1, NOX2, and NOX4 and increased NOX activity were observed in all EAE mice, indicating higher oxidative stress in the CNS. Resveratrol showed inhibitory effects on expressions of NOX2 and NOX4 and NADPH activity, suggesting that the antioxidative activity of resveratrol may be involved in the observed neuroprotection in the EAE mice.
Conclusions.
In summary, our study demonstrates that resveratrol exhibits protective effects in EAE therapy through its anti-inflammation and antioxidant activities by protecting the basement membrane tight junction proteins to improve BBB integrity.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.W. and L.G. conception and design of research; D.W., S.-P.L., and J.-S.F. performed experiments; S.-P.L., J.-S.F., S.Z., and L.B. analyzed data; S.-P.L., S.Z., and L.B. interpreted results of experiments; D.W., S.-P.L., J.-S.F., S.Z., L.B., and L.G. prepared figures; D.W. and L.G. drafted manuscript; L.G. edited and revised manuscript; D.W., S.-P.L., J.-S.F., S.Z., L.B., and L.G. approved final version of manuscript.
REFERENCES
- Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol 175: 87–96, 2006. [DOI] [PubMed] [Google Scholar]
- Csiszar A. Anti-inflammatory effects of resveratrol: possible role in prevention of age-related cardiovascular disease. Ann NY Acad Sci 1215: 117–122, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Das DK. Anti-inflammatory responses of resveratrol. Inflamm Allergy Drug Targets 6: 168–173, 2007. [DOI] [PubMed] [Google Scholar]
- de la Lastra CA, Villegas I. Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res 49: 405–430, 2005. [DOI] [PubMed] [Google Scholar]
- Eberle M, Ebel P, Mayer CA, Barthelmes J, Tafferner N, Ferreiros N, Ulshofer T, Henke M, Foerch C, de Bazo AM, Grosch S, Geisslinger G, Willecke K, Schiffmann S. Exacerbation of experimental autoimmune encephalomyelitis in ceramide synthase 6 knockout mice is associated with enhanced activation/migration of neutrophils. Immunol Cell Biol 93: 825–836, 2015. [DOI] [PubMed] [Google Scholar]
- Fischer HJ, Schweingruber N, Luhder F, Reichardt HM. The potential role of T cell migration and chemotaxis as targets of glucocorticoids in multiple sclerosis and experimental autoimmune encephalomyelitis. Mol Cell Endocrinol 380: 99–107, 2013. [DOI] [PubMed] [Google Scholar]
- Flugel A, Matsumuro K, Neumann H, Klinkert WE, Birnbacher R, Lassmann H, Otten U, Wekerle H. Anti-inflammatory activity of nerve growth factor in experimental autoimmune encephalomyelitis: inhibition of monocyte transendothelial migration. Eur J Immunol 31: 11–22, 2001. [DOI] [PubMed] [Google Scholar]
- Fonseca-Kelly Z, Nassrallah M, Uribe J, Khan RS, Dine K, Dutt M, Shindler KS. Resveratrol neuroprotection in a chronic mouse model of multiple sclerosis. Front Neurol 3: 84, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Wen Q, Xia Y, Yang J, Gao P, Zhang N, Li H, Zou S. Osthole augments therapeutic efficiency of neural stem cells-based therapy in experimental autoimmune encephalomyelitis. J Pharm Sci 124: 54–65, 2014. [DOI] [PubMed] [Google Scholar]
- Glenn JD, Smith MD, Calabresi PA, Whartenby KA. Mesenchymal stem cells differentially modulate effector CD8+ T cell subsets and exacerbate experimental autoimmune encephalomyelitis. Stem Cells 32: 2744–2755, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Chan KH, Lai WH, Siu CW, Kwan SC, Tse HF, Wing-Lok Ho P, Wing-Man Ho J. Human mesenchymal stem cells upregulate CD1dCD5(+) regulatory B cells in experimental autoimmune encephalomyelitis. Neuroimmunomodulation 20: 294–303, 2013. [DOI] [PubMed] [Google Scholar]
- Hou Y, Ryu CH, Park KY, Kim SM, Jeong CH, Jeun SS. Effective combination of human bone marrow mesenchymal stem cells and minocycline in experimental autoimmune encephalomyelitis mice. Stem Cell Res Ther 4: 77, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler TJ Jr, Petro TM. Decreased severity of experimental autoimmune encephalomyelitis during resveratrol administration is associated with increased IL-17+IL-10+ T cells, CD4(−) IFN-gamma+ cells, and decreased macrophage IL-6 expression. Int Immunopharmacol 9: 134–143, 2009. [DOI] [PubMed] [Google Scholar]
- Jones MV, Nguyen TT, Ewaleifoh O, Lebson L, Whartenby KA, Griffin JW, Calabresi PA. Accelerated axon loss in MOG35-55 experimental autoimmune encephalomyelitis (EAE) in myelin-associated glycoprotein-deficient (MAGKO) mice. J Neuroimmunol 262: 53–61, 2013. [DOI] [PubMed] [Google Scholar]
- Kassis I, Petrou P, Halimi M, Karussis D. Mesenchymal stem cells (MSC) derived from mice with experimental autoimmune encephalomyelitis (EAE) suppress EAE and have similar biological properties with MSC from healthy donors. Immunol Lett 154: 70–76, 2013. [DOI] [PubMed] [Google Scholar]
- Labinskyy N, Csiszar A, Veress G, Stef G, Pacher P, Oroszi G, Wu J, Ungvari Z. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem 13: 989–996, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Wang Y, Dong L, Li M, Cai W. Anti-inflammatory effect of resveratrol through the suppression of NF-kappaB and JAK/STAT signaling pathways. Acta Biochim Biophys Sin (Shanghai) 47: 207–213, 2015. [DOI] [PubMed] [Google Scholar]
- Seo JE, Hasan M, Han JS, Kang MJ, Jung BH, Kwok SK, Kim HY, Kwon OS. Experimental autoimmune encephalomyelitis and age-related correlations of NADPH oxidase, MMP-9, and cell adhesion molecules: the increased disease severity and blood-brain barrier permeability in middle-aged mice. J Neuroimmunol 287: 43–53, 2015. [DOI] [PubMed] [Google Scholar]
- Shindler KS, Ventura E, Dutt M, Elliott P, Fitzgerald DC, Rostami A. Oral resveratrol reduces neuronal damage in a model of multiple sclerosis. J Neuroophthalmol 30: 328–339, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svajger U, Jeras M. Anti-inflammatory effects of resveratrol and its potential use in therapy of immune-mediated diseases. Int Rev Immunol 31: 202–222, 2012. [DOI] [PubMed] [Google Scholar]
- Udenigwe CC, Ramprasath VR, Aluko RE, Jones PJ. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr Rev 66: 445–454, 2008. [DOI] [PubMed] [Google Scholar]
- Vallejo S, Palacios E, Romacho T, Villalobos L, Peiró C, Sánchez-Ferrer CF. The interleukin-1 receptor antagonist anakinra improves endothelial dysfunction in streptozotocin-induced diabetic rats. Cardiovasc Diabetol 13: 158, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XS, Fang HL, Chen Y, Liang SS, Zhu ZG, Zeng QY, Li J, Xu HQ, Shao B, He JC, Hou ST, Zheng RY. Idazoxan reduces blood-brain barrier damage during experimental autoimmune encephalomyelitis in mouse. Eur J Pharmacol 736: 70–76, 2014. [DOI] [PubMed] [Google Scholar]
- Wolburg H, Wolburg-Buchholz K, Engelhardt B. Involvement of tight junctions during transendothelial migration of mononuclear cells in experimental autoimmune encephalomyelitis. Ernst Schering Res Found Workshop 47: 17–38, 2004. [DOI] [PubMed] [Google Scholar]
- Wood LG, Wark PA, Garg ML. Antioxidant and anti-inflammatory effects of resveratrol in airway disease. Antioxid Redox Signal 13: 1535–1548, 2010. [DOI] [PubMed] [Google Scholar]
- Yang X, Yan J, Feng J. Treatment with tanshinone IIA suppresses disruption of the blood-brain barrier and reduces expression of adhesion molecules and chemokines in experimental autoimmune encephalomyelitis. Eur J Pharmacol 771: 18–28, 2016. [DOI] [PubMed] [Google Scholar]
- Zhang F, Liu J, Shi JS. Anti-inflammatory activities of resveratrol in the brain: role of resveratrol in microglial activation. Eur J Pharmacol 636: 1–7, 2010. [DOI] [PubMed] [Google Scholar]
- Zhang S, Kan QC, Xu Y, Zhang GX, Zhu L. Inhibitory effect of matrine on blood-brain barrier disruption for the treatment of experimental autoimmune encephalomyelitis. Mediators Inflamm 2013: 736085, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]