The role of the cerebellum in behavior has classically been confined to the control of movement. However, the cerebellum projects to nonmotor areas, and neuroimaging studies show neural changes in the cerebellum during perception and language tasks. This paper provides initial evidence in healthy humans that alterations of the cerebellum impair the timing of perceptual decisions in speech without impacting the outcome of perceptual decisions.
Keywords: cerebellum, perceptual learning, speech perception, transcranial direct current stimulation, timing
Abstract
Neuroimaging studies suggest that the cerebellum might play a role in both speech perception and speech perceptual learning. However, it remains unclear what this role is: does the cerebellum help shape the perceptual decision, or does it contribute to the timing of perceptual decisions? To test this, we used transcranial direct current stimulation (tDCS) in combination with a speech perception task. Participants experienced a series of speech perceptual tests designed to measure and then manipulate (via training) their perception of a phonetic contrast. One group received cerebellar tDCS during speech perceptual learning, and a different group received sham tDCS during the same task. Both groups showed similar learning-related changes in speech perception that transferred to a different phonetic contrast. For both trained and untrained speech perceptual decisions, cerebellar tDCS significantly increased the time it took participants to indicate their decisions with a keyboard press. By analyzing perceptual responses made by both hands, we present evidence that cerebellar tDCS disrupted the timing of perceptual decisions, while leaving the eventual decision unaltered. In support of this conclusion, we use the drift diffusion model to decompose the data into processes that determine the outcome of perceptual decision-making and those that do not. The modeling suggests that cerebellar tDCS disrupted processes unrelated to decision-making. Taken together, the empirical data and modeling demonstrate that right cerebellar tDCS dissociates the timing of perceptual decisions from perceptual change. The results provide initial evidence in healthy humans that the cerebellum critically contributes to speech timing in the perceptual domain.
NEW & NOTEWORTHY
The role of the cerebellum in behavior has classically been confined to the control of movement. However, the cerebellum projects to nonmotor areas, and neuroimaging studies show neural changes in the cerebellum during perception and language tasks. This paper provides initial evidence in healthy humans that alterations of the cerebellum impair the timing of perceptual decisions in speech without impacting the outcome of perceptual decisions.
the role of the cerebellum in behavior has classically been confined to the control of movement. The cerebellum is known, for instance, to be involved in motor control through the detection and correction of movement errors (Izawa et al. 2012; Panouillères et al. 2015; Rabe et al. 2009; Smith and Shadmehr 2005; Wolpert et al. 1998). However, the cerebellum projects to nonmotor areas (Strick et al. 2009), and several studies suggest a cerebellar contribution to behaviors such as perception, language, and memory (Desmond and Fiez 1998; Durisko and Fiez 2010; Lesage et al. 2012; Mathiak et al. 2002). A host of neuroimaging studies have noted activity changes in the cerebellum during speech-sound classification, word recognition, and language tasks (Ackermann et al. 2007; Stoodley and Schmahmann 2009; Xiang et al. 2003). Furthermore, recent evidence has linked neural changes in the cerebellum to perceptual learning during both speech and nonspeech behaviors (Guediche et al. 2015; Vahdat et al. 2014). To date, direct interventional studies of the cerebellum's role in speech perception and perceptual learning are lacking. Here we use transcranial direct current stimulation (tDCS) to provide an initial test of the role of the cerebellum in speech perception.
Neuroimaging meta-analysis suggests that areas in the right cerebellum are active during speech perception (Stoodley and Schmahmann 2009), but the nature of this activity remains unclear. One possibility is that the cerebellum contributes to perceptual decision-making. This contribution might be most meaningful during times of perceptual change. Indeed, at least three neuroimaging studies suggest that the right cerebellum is involved in perceptual learning. Callan et al. (2003) examined neural changes in native Japanese speakers following feedback-driven perceptual learning on a difficult English phonetic contrast. Increases in neural activity were observed in Crus I and lobule VI of the right cerebellum, areas active during motor and language tasks (Stoodley and Schmahmann 2009). More recent neuroimaging studies provide further evidence that the cerebellum is involved in perceptual learning. In the first case, Guediche at al. (2015) linked increased activation in the cerebellum to a task involving adaptation to distorted speech; and, in the second, Vahdat et al. (2014) examined changes in neural connectivity following perceptual learning related to the position of the right arm during reaching movements. In this case, learning was driven via explicit feedback (as in Callan et al. 2003), and perceptual-learning-related changes in functional connectivity were observed between the supplementary motor area and right Crus I and lobule VI in the cerebellum. This work presents the intriguing possibility that the cerebellum's known role in motor learning might be mirrored in the perceptual domain.
There are, of course, other explanations for neural changes in the cerebellum associated with speech perception. A long line of research suggests that the cerebellum plays a role in the timing of subsecond behaviors (Spencer and Ivry 2013). For instance, patients with cerebellar ataxia show deficits in movement timing, such as tapping in sync with a metronome (Franz et al. 1996; Spencer et al. 2003), deficits not observed in basal ganglia disorders such as Parkinson's patients (Ivry and Keele 1989). Noninvasive brain stimulation studies support a role for the cerebellum in movement timing. To give one example, repetitive transcranial magnetic stimulation (TMS) applied to the cerebellum can cause increased variability in the pacing of movements (Koch et al. 2007; Théoret et al. 2001). A smaller amount of research has examined the role of the cerebellum in the timing of nonmotor behaviors. Repetitive TMS of the right cerebellum drove participants to perceive subsecond time intervals as longer (Koch et al. 2007). Patients with cerebellar degeneration have trouble discriminating between speech sounds distinguished by their voice onset time (Ackermann et al. 2007), and cerebellar tDCS delivered to the right cerebellum has been shown to alter response times and, in some cases, measures of accuracy, associated with working memory tasks, difficult serial subtraction, and linguistic prediction (Ferrucci et al. 2008; Miall et al. 2016; Pope and Miall 2012). Intriguingly, besides deficits in the timing of behaviors, cerebellar damage seems to leave other aspects of behaviors, such as movement trajectory and accuracy, relatively unscathed (Spencer and Ivry 2013).
To test the role of the right cerebellum in speech perception, we used tDCS to alter the cerebellum during a speech perceptual learning task. tDCS was used (as opposed to TMS) because it can be applied throughout perceptual learning. Anodal stimulation was used (i.e., the anode was placed over the cerebellum) because it has been shown to alter the functioning of the cerebellum and influence behavior in both the motor and cognitive domain (Ferrucci et al. 2008; Galea et al. 2009; Galea et al. 2011).
In the study, participants made perceptual decisions about a series of stimuli that spanned the phonetic contrast between the words “head” and “had.” Feedback was given to manipulate the point of perceptual uncertainty between the two words, a paradigm recently shown to cause learned changes in perception that persist for a week (Lametti et al. 2014). This perceptual learning task was ideal for two reasons. 1) Reflecting the cerebellum's role in motor learning, we reasoned that cerebellar involvement in the outcome of speech perceptual decisions might be greatest during times of perceptual change. 2) The learning task perturbed the timing of perceptual decisions; this allowed for the cerebellum's role in perceptual timing to also be assessed. We compared the acquisition, transfer, and retention of this type of perceptual speech learning between two groups: one that received tDCS to the right cerebellum throughout learning and another that was given sham tDCS during the same task. We also compared the timing of perceptual decisions between the groups by examining gross changes in reaction times throughout the task. Finally, we used the drift diffusion model to decompose reaction times into processes related to perceptual decision-making and unrelated processes such as behavioral timing. We hypothesized that, if tDCS effectively altered the functioning of the cerebellum, changes would be observed in processes unrelated to the outcome of decision making during speech perceptual learning.
METHODS
Participants and apparatus.
Thirty-six neurologically healthy native English speakers participated in the experiments (age range = 18–35 yr); 21 were female. (One of the 36 participants was excluded from the final analysis because his/her reaction times differed by >2.5 SDs from the group mean.) Participants wore headphones (Bose) and responded to speech stimuli from the headphones by pressing keys on a keyboard. A direct current stimulator (NeuroConn) was used to apply tDCS to the cerebellum. Participants gave their informed consent, and the local ethics committee approved the experiments.
Procedure.
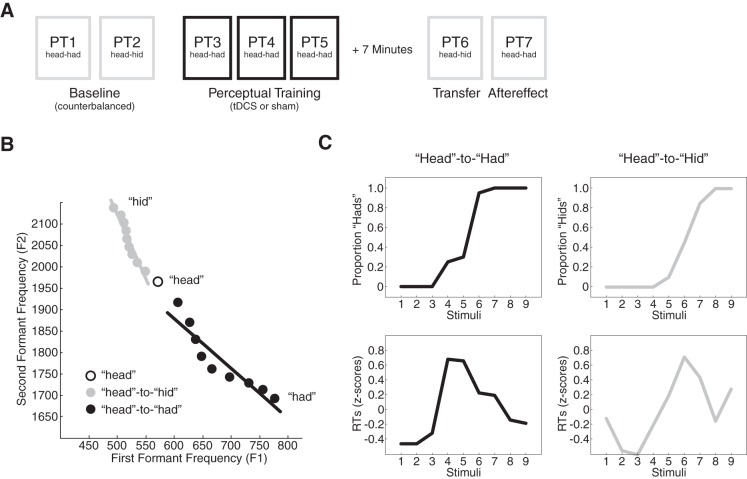
Figure 1A shows the procedure. The experiment began with two perceptual tests designed to measure perception of the words head and had and the words head and “hid” (PT1 and PT2, respectively). The order of the tests was balanced between participants. Participants then performed a learning task in which their perception of the phonetic contrast between the words head and had was manipulated (PT3 to PT5: see Perceptual Learning).
Fig. 1.
Experimental methods, stimuli, and data analysis. A: the experiment involved 7 perceptual tests. Baseline perceptual tests (PT1 and PT2) were followed by perceptual training (PT3 to PT5), a transfer test (PT6), and an aftereffect test (PT7). The order of the baseline tests was balanced across participants. B: the perceptual continua used in the experiment are depicted by their first formant (F1) and second formant (F2) values. One continua spanned the distinction between “head” and “had” (black dots), and one spanned the distinction between head and “hid” (gray dots). C: perceptual change was assessed by measuring the proportion of had and hid responses for each stimulus in each perceptual test (top). Perceptual change was also examined by measuring the time it took participants to respond to the stimuli (bottom). Reaction times were log normalized and displayed as z-scores.
During perceptual learning, subjects received either 15 min (“real”) or 30 s (“sham”) of tDCS (see Transcranial Direct Current Stimulation). Perceptual learning was followed by a 5-min break and two more perceptual tests. The first was a head-to-hid perceptual test that examined whether learning transferred to a different phonetic contrast (PT6); the second was a head-to-had perceptual test that measured aftereffects associated with learning (PT7). The transfer test always followed learning; it was included to assess whether the effects of cerebellar tDCS on speech perception were global or limited to trained speech sounds.
Measuring speech perception.
Speech perception was assessed using two perceptual tests, one that measured the distinction between head and had and a second that measured the distinction between head and hid. Each perceptual test used nine speech stimuli. Figure 1B depicts the stimuli by their first and second formant frequency values (F1 and F2). The stimuli were created in Matlab by altering F1 and F2 in 10 steps from formant values associated with the word head to those associated with had or hid (Lametti et al. 2014). An English-speaking male provided the root word head and the continua endpoints, had or hid. The root word was not included in either continuum. Stimuli were 0.430 s long and started with 0.05 s of silence.
During each perceptual test, the entire set of nine stimuli was played from the headphones in a random order, one word at a time. After each stimulus, participants were prompted by text on a computer screen to indicate whether they heard head or had (in the case of the head-to-had perceptual test) or head or hid (in the case of the head-to-hid perceptual test). If participants thought they heard head they pressed “s” on the keyboard with their left hand; if they thought they heard had or hid they pressed “l” with their right hand. Participants were instructed to respond accurately and quickly. The entire stimulus set was repeated 20 times in each perceptual test yielding 180 perceptual decisions per test. Each perceptual test took about 5 min.
The proportion of had or hid responses was found for each test. Psychometric functions were fit to these values using “glmfit” in Matlab. The perceptual boundary, that is, the point on the continua where had or hid was reported 50% of the time, was computed from the functions. The locations on the continua where participants perceived had/hid 25% and 75% was also computed from the psychometric functions. The distance between these values was used as a measure of perceptual acuity as in Vahdat et al. (2014) (e.g., a smaller distance indicates a steeper psychometric function).
Perceptual learning.
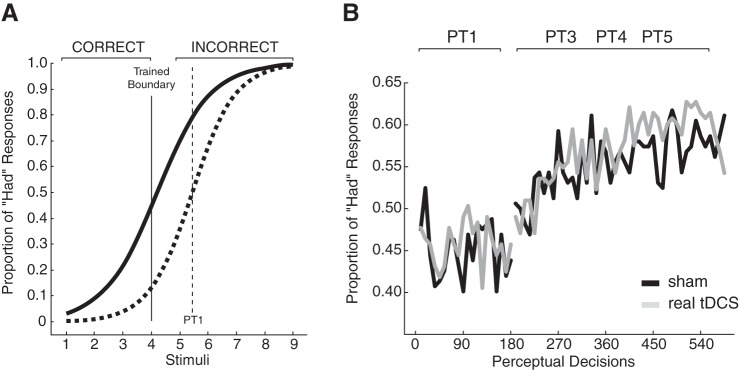
The perceptual distinction between the words head and had was manipulated using feedback as described previously (Lametti et al. 2014). Briefly, the perceptual boundary between head and had was computed from the baseline phase of the experiment. For the real tDCS group, this boundary averaged 5.39; for the sham group, it averaged 5.45. This difference was not significant (P = 0.8). A new perceptual boundary was then set two stimuli lower than the original, rounded-to-the-nearest integer, perceptual boundary. Feedback was delivered around this new boundary immediately following each perceptual decision. Figure 2A shows how the feedback would be applied based on the average baseline psychometric function (broken line curve) for the head-to-had continuum. If, for instance, a participant's baseline perceptual boundary was computed as 5.50, a new perceptual boundary was set at stimulus 4 for training purposes. After this, a response of head for stimuli 1–4 and had for stimuli 5–9 resulted in “CORRECT” being displayed on the computer screen. A had response for stimuli 1–4 or a head response for stimuli 5–9 resulted in the appearance of “INCORRECT” on the screen and the addition of one point to an error counter at the bottom of the screen. Perceptual learning consisted of three perceptual tests in a row with this feedback. Perceptual tests with feedback had 135 perceptual decisions (15 blocks of the 9 stimuli instead of 20 as in the baseline, transfer, and aftereffect tests). There was a 30-s break between perceptual tests. During the break, the error counter was zeroed, and participants were instructed to reduce their errors. Perceptual learning lasted for about 17 min (16.81 mean, 1.16 SD).
Fig. 2.
Feedback altered perceptual responses. A: during perceptual training, feedback was delivered around a new perceptual boundary (solid vertical line) that was set lower than the perceptual boundary (broken vertical line) measured during the baseline head-to-had perceptual test. In this example, “CORRECT” was displayed on the screen if the participant perceived stimuli 1–4 as head, and “INCORRECT” was displayed if the stimuli were perceived as had. CORRECT was displayed on the screen if participants perceived stimuli 5–9 as had, and INCORRECT was displayed on the screen if they were perceived as head. B: the proportion of had responses (y-axis) was computed for blocks of 9 stimuli for the baseline head-to-had perceptual test (PT1) and during perceptual training (PT3-PT5). The introduction of feedback led to a learned increase in the proportion of had responses. The gray lines represent the group that received transcranial direct current stimulation (tDCS); the black lines represent the group that received sham stimulation.
Transcranial direct current stimulation.
tDCS was applied to the right cerebellum during learning. Following the baseline phase of the experiment, the anode was placed in a 25-cm2 saline-soaked sponge and positioned 3 cm lateral to the inion on the right side of the scalp. The cathode was placed in a 25-cm2 saline-soaked sponge and positioned in the center of the right buccinator muscle. This tDCS electrode configuration has previously been shown to influence behavior attributed to the right cerebellum and cause neural changes associated with alterations of the right cerebellum (Galea et al. 2009; Galea et al. 2011; and see Grimaldi et al. 2016 for a review of the impact of tDCS on the cerebellum).
Participants were divided into two groups. A real stimulation group (n = 17) received 15 min of stimulation during perceptual learning and a sham group (n = 18) received 30 s of stimulation at the start of learning. In each case, the current was ramped up to 2.0 mA over 30 s and ramped down to 0 over 30 s. The electrodes were removed from the scalp during the break that followed training. Participants were blind to the stimulation condition.
Data analysis.
The proportion of had or hid responses was computed for each perceptual test on a per subject basis (Fig. 1C, top). Training-related changes in this proportion were found by comparing postlearning perceptual tests with prelearning perceptual tests. These changes were then averaged across participants within each group. To visualize perceptual learning (as in Fig. 2B), the proportion of had responses was computed for each of the 65 blocks of 9 perceptual decisions that made up the baseline head-to-had perceptual test and the training perceptual tests. These proportions were then averaged across participants within each group.
The time it took participants to come to a perceptual decision by pressing s or l on the keyboard was examined. Reaction times were measured from the start of each stimulus. The idea behind measuring reaction times was that they would peak near the category boundary, or the point where participants were the most uncertain about whether they heard had, head, or hid (Niziolek and Guenther 2013). In this case, learning-related changes in the perceptual boundary should also be reflected by reaction time changes.
Across stimuli and groups, the mean reaction time was 0.638 s (0.161 SD) before training and 0.602 s (0.172 SD) after training. Reaction times >1,250 ms were discarded (∼5% of the data). The reaction time data were positively skewed. To correct for this, reaction times were log normalized (using the natural logarithm). Reaction times were also converted into z-scores on a per perceptual test and subject basis (Fig. 1C, bottom). Average z-scores were then computed for each stimulus in each perceptual test. To examine gross changes in reaction time between the groups, for each perceptual test, log-normalized reaction times were averaged across stimuli. This was done first within subjects and then across groups. tDCS-related changes in reaction time were visualized (as in Fig. 5) by averaging log-normalized reaction times across the blocks of nine stimuli that made up each perceptual test.
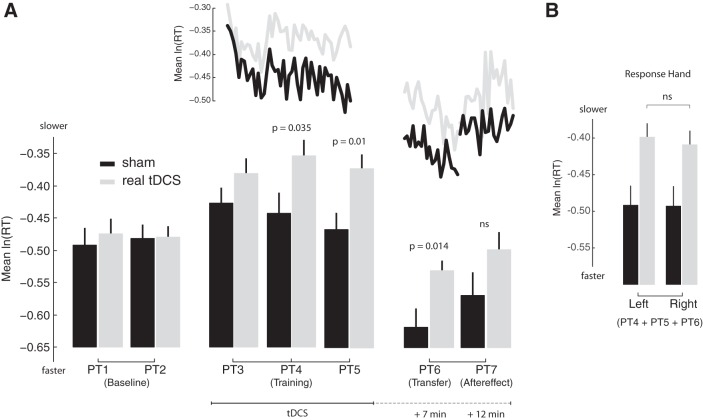
Fig. 5.
Cerebellar tDCS slowed reaction times. A: the mean (log-normalized) reaction time is displayed for each perceptual test. The gray bars represent the group that received cerebellar tDCS. The black bars represent the group that received sham tDCS. The approximate timing of the transfer, aftereffect, and retention tests in relation to tDCS and perceptual learning is indicated on the bottom. The application of cerebellar tDCS caused a reaction time difference between the groups (PT3–PT5). This difference was still present during the transfer test that occurred 7 min after tDCS. To visualize how reaction times evolved during training and transfer and the aftereffect test, log-normalized reaction times associated with blocks of 9 perceptual decisions were averaged and joined via the gray lines (real stimulation) and black lines (sham stimulation) at the top. B: average reaction times from PT4, PT5, and PT6 were pooled for left and right hand responses and compared between the groups. A similar tDCS-related difference in reaction time was observed for left and right hand responses.
Diffusion modeling.
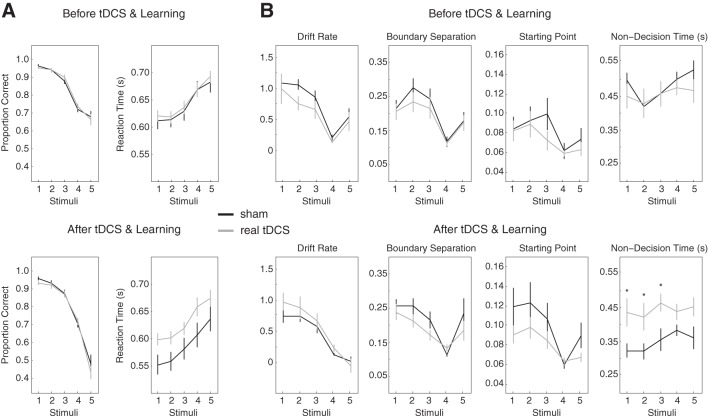
The drift diffusion model was fit to participant responses and reaction times using the Diffusion Model Analysis Toolbox in Matlab (Vandekerckhove and Tuerlinckx 2008). The model assumes that one decision reflects a correct response and the other reflects an incorrect response. Given that perceptual boundaries before and after learning were not statistically different from the stimulus in the middle of the continua (5.24 on average, 1.0 SD), the data were grouped by stimulus quality or coherence. Stimulus 9 (“had/hid”) was made equivalent to stimulus 1 (head), 8 was made equivalent to 2, 7 was made equivalent to 3, and 6 was made equivalent to 4. A response of head was considered to be correct under this transformation. This left five stimuli that differed in stimulus quality such that the proportion of correct responses decreased as the quality of the stimuli decreased (see Fig. 6A).
Fig. 6.
Drift diffusion modeling. A: data were grouped by stimulus coherence. Right, proportion of correct responses. Left, reaction times for each of the transformed stimuli. Top, measures before tDCS and learning. Bottom, the same measures after tDCS and learning. B: a drift diffusion model was fit to the data shown in A. The first three boxes on the top and bottom show the parameters that account for the outcome of perceptual decisions. The fourth box shows the parameter that accounts for processes unrelated to perceptual decision making. Top, parameters before tDCS. Bottom, parameters after tDCS. Cerebellar tDCS caused a difference in the parameter that accounts for processes unrelated to perceptual decision making. *Parameters statistically different at P < 0.05.
To further increase the sample size used for modeling, data from PT1 (head-to-had continuum) were combined with PT2 (head-to-hid continuum) to create a before tDCS dataset, and data from PT6 (head-to-had continuum) were combined with PT7 (head-to-hid continuum) to create an after tDCS dataset. The model was then fit to the before tDCS and after tDCS datasets on a per subject basis, and the model's parameters were compared between the sham and real tDCS groups. Approximately 5% of the parameters estimated from individual subject data were >2 SDs from the group mean; these values were not included in the final analysis.
Statistical analysis.
Between- and within-group comparisons of the measures described above were performed using split-plot or repeated-measures ANOVA. Where appropriate, post hoc comparisons were performed using two-tailed t-tests. The significance level for all statistical tests was 0.05; this value was corrected for multiple comparisons using the Bonferroni method.
RESULTS
The aim of the experiment was to test the competing hypotheses that the cerebellum might influence the outcome of speech perceptual decisions vs. playing a role in the timing of decisions. To do this, a group of participants received tDCS to the cerebellum while they performed a speech perceptual learning task, a task that altered both speech perception and the timing of speech perceptual decisions. Their performance during training, on a transfer test, and on an aftereffect test was compared with participants who received sham tDCS (see Fig. 1A).
Feedback drove a learned change in response to the stimuli. Figure 2B shows the proportion of had responses during the baseline phase of the experiment (PT1) and during perceptual learning (PT3, PT4, and PT5). Feedback caused a change in response such that the proportion of had responses increased during learning [F(1, 64) = 13.79, P < 0.0001: main effect of block]. Across the 45 blocks of perceptual decisions that made up the training phase of the study, a block-by-block comparison revealed no significant differences between the sham and real tDCS groups (P > 0.05 in every case), and there was no interaction between blocks and the presence or absence of tDCS [F(1, 44) = 1.03, P > 0.4]. This model-free analysis suggests that cerebellar tDCS did not alter the rate and amount of speech perceptual learning.
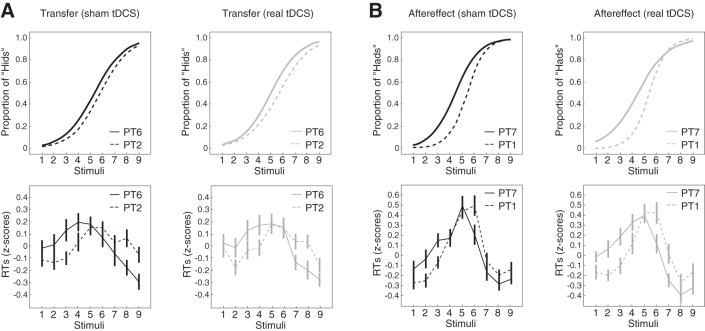
Following learning, participants experienced a transfer test (PT6). Figure 3A shows the average psychometric function (top) and log-normalized reaction times at each stimulus (bottom) for the head-to-hid continuum before and after speech perceptual learning on the head-to-had continuum (PT2 vs. PT6). Figure 3A thus depicts the transfer of learning from one phonetic contrast to another. Figure 3A, left, shows the sham tDCS group, and Fig. 3A, right, shows the real tDCS group. Compared with baseline, training on the head-to-had continuum altered how participants responded during the head-to-hid transfer test. Specifically, the psychometric functions shifted toward head such that participants reported perceiving more “hids.” This change in perception was reflected by a change in reaction times for some of the stimuli [F(8,26) = 5.96, P < 0.001: interaction between stimuli and experimental phase]. Reaction times increased for stimuli 3 and 4 in the case of the sham group and stimuli 2 in the case of the real group (P < 0.05 in each case). This suggests that participants became less certain about whether these stimuli were head or hid. On the other hand, reaction times decreased for stimuli 8 and 9 in the case of the sham group and stimuli 7 and 8 in the case of the real tDCS group (P < 0.05 in each case), that is, participants became faster to perceive and label these stimuli as head or hid. These reaction time changes are consistent with a shift in the perceptual boundary (the point of greatest perceptual uncertainty) toward head. Crucially, the pattern of reaction times following learning did not differ between the sham and real tDCS groups [F(8,26) = 0.27, P > 0.95: interaction between stimuli and group]. Thus, perceptual learning on the head-to-had continuum altered participants' perception of the head-to-hid continuum, and this alternation was not changed by cerebellar tDCS applied during learning.
Fig. 3.
Training altered speech perception. A: top, psychometric functions were fit to the proportion of hid responses before (PT2, broken lines) and after (PT6, solid lines) perceptual training. Prior training on the head-to-had continuum altered the proportion of hid responses on the head-to-hid continuum such that participants were more likely to report hearing hid. Bottom, log-normalized reaction times were computed and displayed as z-scores for each stimulus before (PT2, broken lines) and after (PT6, solid lines) perceptual training. Changes in the perceptual boundary were mirrored by changes in reaction times to some of the stimuli. B: top, psychometric functions were fit to the proportion of had responses before (PT1, broken lines) and after (PT7, solid lines) perceptual training. Following training, participants were more likely to report hearing had. Bottom, log-normalized reaction times were computed and displayed as z-scores for each stimulus before (PT1, broken lines) and after (PT7, solid lines) perceptual training. Changes in the psychometric function were mirrored by changes in reaction times. Error bars represent ± SE.
The transfer test was followed by an aftereffect test (PT7). Figure 3B depicts average psychometric functions for the head-to-had continuum and associated reaction times before and after learning (PT1 vs. PT7) for the sham and real tDCS groups. Figure 3B thus depicts aftereffects associated with speech perceptual learning. Compared with baseline, perceptual learning altered how subjects responded during the head-to-had perceptual test even after the feedback was removed. Consistent with the trained perceptual boundary, the psychometric functions moved toward head, indicating that subjects reported perceiving more “hads.” This change in perception was, again, reflected by a change in reaction times to some of the stimuli [F(8,26) = 3.40, P < 0.01: interaction between stimuli and experimental phase]. In the case of the sham group, reaction times increased for stimuli 2 and 3 and decreased for stimulus 6 (P < 0.01 in each case). In the case of the real group, reaction times increased for stimuli 2 and 3 and decreased for stimuli 6 and 7 (P < 0.05 in each case). The reaction time changes agree with a learning-related shift in the perceptual boundary on the head-to-had continuum toward head. Following learning, the pattern of reaction times did not differ between the sham and real tDCS groups [F(8,26) = 0.78, P > 0.62: interaction between stimuli and group]. This suggests that the aftereffects of perceptual learning were not altered by cerebellar tDCS.
The learning-related changes in the psychometric functions shown in Fig. 3 are quantified in Fig. 4. Specifically, Fig. 4 shows changes in the proportion of had or hid responses from baseline and the impact of cerebellar tDCS on these changes. During the transfer test, perceptual learning caused an increase in the proportion of hid responses for both the sham and real tDCS groups (P = 0.018 and 0.011, respectively). However, there was no difference in this change between the two groups (P = 0.84). During the aftereffect test, perceptual learning caused an increase in the proportion of had responses for both groups (P < 0.0001 in both cases). Again, there was no difference in these changes between the two groups (P = 0.94). Finally, we examined changes in the acuity of the psychometric function (i.e., the steepness of the curves depicted in Fig. 3) across baseline, perceptual training, transfer, and aftereffect tests. Cerebellar tDCS did not have an impact on perceptual acuity [F(6,27) = 1.23, P = 0.319: interaction between acuity and group]. In combination with the reaction time measures, this demonstrates that cerebellar tDCS did not have an impact on either the transfer or retention of speech perceptual learning.
Fig. 4.
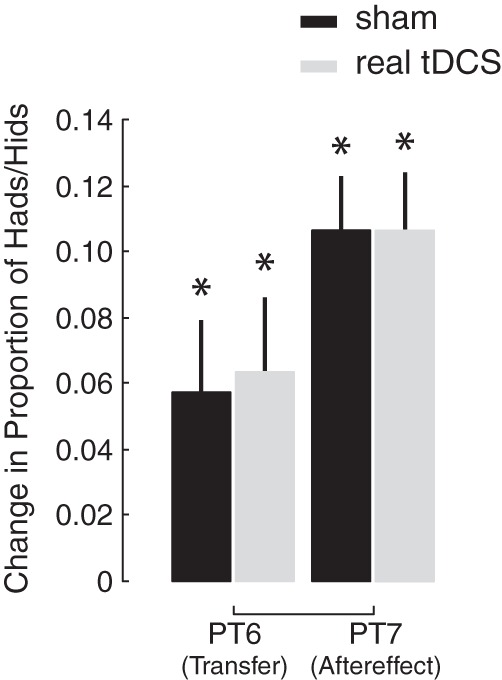
Training-related changes in the proportion of hid and had responses were computed for the transfer (PT6 − PT2) and aftereffect (PT7 − PT1) tests. Training caused an increase (*P < 0.05) in the proportion of hid and had responses during these perceptual tests. Training-related changes in the proportion of hid and had responses did not differ between the sham (black bars) and real (gray bars) stimulation groups.
The perceptual data demonstrate that cerebellar tDCS does not have an impact on the outcome of speech perceptual decision-making for either trained or untrained speech stimuli. We next examined whether the cerebellum might play a more general role in speech perception related to the timing of perceptual decisions.
The z-scores depicted in Fig. 3 give a measure of how perception changed across the stimuli. However, because the z-scores were computed on a per perceptual test and subject basis, they mask overall differences in mean reaction time between tests and groups, differences that could provide evidence for changes in the timing of decisions.
Figure 5A shows average (but still log normalized) reaction times for each perceptual test over the course of the experiment. The squiggly lines shows how average reaction times evolved during the training, transfer, and aftereffect tests. Cerebellar tDCS drove significant between-group differences in average reaction time over the course of the experiment [F(6,28) = 2.65, P = 0.037: interaction between perceptual tests and group]. There was no difference in average reaction time between the groups during the baseline phase of the experiment (PT1 and PT2). The introduction of feedback at the start of perceptual learning led to an increase in reaction time (P < 0.05 in each case). The group that received sham stimulation decreased their response times over the course of perceptual learning (PT3 vs. PT5: P = 0.012) until reaction times did not differ from baseline responses. A similar decrease was not observed for the group that received real stimulation (PT3 vs. PT5: P = 0.73). Indeed, by the middle of learning and tDCS (PT4), the sham group was responding to the stimuli faster than the real group (P = 0.035). This tDCS-related change in reaction times was also observed at the end of learning (PT5, P = 0.01) and 7 min after stimulation during the transfer test (PT6), a test that involved responses to untrained stimuli (P = 0.014). Twelve minutes after tDCS during the retention test (PT7), there was no longer a difference in average reaction times between the two groups (P = 0.155). The difference in reaction time thus grew with stimulation and wore off when stimulation was removed. In combination with the lack of a difference in the perceptual measures (as depicted in Figs. 3 and 4), this suggests that, independent of the outcome of perceptual decision-making, right cerebellar tDCS impaired the timing of speech perceptual decisions.
To rule out the possibility that the reaction time delay observed in PT4, PT5, and PT6 could be explained by a perturbation of the motor system, we examined average reaction times from left and right hand responses separately. Because the right cerebellum projects to frontal lobe motor areas in the left hemisphere, we reasoned that a perturbation of the motor system caused by right cerebellar tDCS should have a larger (if not exclusive) impact on right hand responses. To increase the sample size and the likelihood of seeing an interaction between the response hand and tDCS-related changes, reaction times from PT4, PT5, and PT6 were pooled into left and right hand responses. Figure 5B shows that right cerebellar tDCS slowed perceptual responses regardless of the hand used to indicate perception [F(1,33) = 0.59, P = 0.45: interaction between the hand used to respond and group]. This result does not fit with a perturbation of the motor system originating in the right cerebellum.
To further explore the impact (or lack thereof) of cerebellar tDCS on perceptual decision-making, we fit a drift diffusion model to the reaction times and associated perceptual decisions. Diffusion models have been shown to account for reaction times in a wide range of simple perceptual decisions such as those in this study (Gold and Shadlen 2007). The model has four key parameters that break down reaction times and associated perceptual responses into different aspects of perceptual processing: boundary separation reflects the decision criteria; starting point reflects the bias for one of two perceptual decisions; and drift rate relates to the rate of evidence accumulation. In combination, these three parameters define the speed of perceptual decisions, while the fourth parameter, nondecision time, accounts for the time required for processes unrelated to perceptual decision-making (Ratcliff and McKoon 2008). Cerebellar tDCS could have impaired one or a combination of these parameters, leading to the observed reaction time delay. However, if tDCS spared processes related to perceptual decision-making, only a difference in the nondecision time parameter should be observed between the groups.
To allow the effect of tDCS on reaction times to be carried by one or more of the parameters, we let all four vary when fitting the data. Figure 6A shows the transformed stimulus categories (see methods) and associated perceptual decisions and reactions times to which the model was fit. Figure 6A, top, shows the transformed data before tDCS and perceptual learning, and Fig. 6A, bottom, shows the transformed data after tDCS and perceptual learning. Similarly, Fig. 6B, top, shows the parameters before tDCS and perceptual learning, and Fig. 6B, bottom, shows the parameters after tDCS and perceptual learning.
Cerebellar tDCS caused a clear difference in nondecision time between the sham and real tDCS groups [main effect of group: F(1,30) = 7.76, P < 0.01; Fig. 6B, bottom right]. A difference between the sham and real stimulation groups was not observed for any of the other parameters (i.e., there were no other significant main effects or interactions following tDCS). Fitting the model with fewer free parameters yielded results that were qualitatively and, in most cases, quantitatively similar. This provides additional evidence that, during speech perceptual decisions, disruptions of the cerebellum spare the perceptual decision making process.
DISCUSSION
Motivated by fMRI studies showing activity changes in the cerebellum during both speech perception and perceptual learning, we used tDCS to test whether the cerebellum is involved in speech perceptual learning vs. the timing of perceptual behaviors. The empirical data and modeling of the perceptual decision-making process support the second hypothesis (with caveats discussed below). In short, cerebellar tDCS significantly altered the time it took participants to come to a speech perceptual decision without changing the outcome of their decision.
In the experiments, feedback was used to drive a change in the perception of the phonetic contrast between the words head and had while tDCS was applied to the right cerebellum. This task caused an alteration in both perception and the timing of perceptual decisions. For both groups, the induced change in perception was identical and robust; it was reflected by changes in perceptual responses and normalized patterns of reaction times across the stimuli, and it transferred to a different phonetic contrast. Compared with sham stimulation, cerebellar tDCS significantly increased the time it took participants to respond to the speech stimuli. The alteration in response time grew as tDCS was applied, it wore off after stimulation came to an end, and it altered the timing of both trained and untrained speech perceptual decisions. Taken together, the behavioral results show a tDCS-related dissociation between perceptual change in speech and the timing of perceptual decisions, implicating the right cerebellum in perceptual timing during speech.
Learning, whether for motor or perceptual tasks, typically involves a practice-dependent change in the timing of behaviors (Spencer and Ivry 2013). As the trial and error process of learning progresses behaviors become better timed. In the present study, the introduction of feedback at the start of learning caused an increase in reaction time. The sham group reduced reaction time as learning progressed, whereas the group receiving cerebellar stimulation did not. Both groups achieved the same amount of perceptual change, but a disruption of a practice-dependent change in response time during the task was only observed in the stimulated group. A disruption in response time was also observed during the transfer task, which involved untrained stimuli. Our interpretation of the result is that the cerebellum does not play a direct role in perceptual decision-making in speech. However, by perturbing response time, a role for the cerebellum in the timing of when perceptual decisions are initiated or, possibly, when they are used in behavior was revealed.
If the cerebellum is involved in the timing of speech decisions, as the empirical data suggest, it leaves open the possibility that the cerebellum might have a greater impact on perceptual change when perceptual learning places a greater reliance on timing. Speech perceptual learning can be driven by both externally generated feedback (as in this study) and internally generated error signals. In the case of the latter, learning is presumably caused by a mismatch between a predicted speech sound and what was actually perceived (Guediche et al. 2015). There is a large amount of evidence from the motor control literature that the cerebellum plays a role in motor learning driven by errors in prediction (Izawa et al. 2012; Rabe et al. 2009; Smith and Shadmehr 2005; Wolpert et al. 1998). The cerebellum might play a larger role in the outcome of perceptual learning when learning relies on similar temporal predictions (Spencer and Ivry 2013). Indeed, the cerebellum has a known role in other forms of learning that depend on temporal predictions. For instance, lesions of the cerebellum in animal models and humans disrupt classical conditioning (Hoffland et al. 2012; McCormick and Thompson 1984), which critically depends on the correct timing between unconditioned responses and conditioned stimuli (Pavlov 1926). It thus remains to be tested whether repeating this study with a perceptual learning paradigm involving a time-dependent error signal would reveal an impact of cerebellar tDCS on the outcome of perception. Such an outcome would support our interpretation of the results presented here.
Using the drift diffusion model, we broke down participants' decisions into processes related to the outcome of speech perception vs. unrelated processes. Cerebellar tDCS only impacted the latter (i.e., “nondecision time”). Importantly, the nondecision time parameter altered by tDCS includes other processes besides the timing of perceptual decisions, such as the motor act of indicating perception (but see the next paragraph). Nevertheless, the computational results provide additional evidence that cerebellar tDCS entirely spared the perceptual decision making process in speech.
One possible explanation for the observed reaction time delay (an explanation that would agree with the modeling results) is that tDCS simply impaired the motor system. After careful consideration, we believe this conclusion to be unlikely for at least two reasons. In the study, tDCS was applied to the right cerebellum. The right cerebellum interacts with speech, language, and motor areas in the left hemisphere. In particular, the right cerebellum projects to left hemisphere motor areas that control movements of the right hand (Kelly and Strick 2003). One would thus expect impairments in this motor circuit to only impact right hand responses. On the other hand, word recognition is largely lateralized to the left hemisphere (DeWitt and Rauschecker 2012). An impairment related to the timing of word perception should thus be observed in responses from both hands, and this is precisely what we saw.
Does tDCS focally stimulate the cerebellum? This question, which is of paramount importance to the interpretation of this study, can be addressed by examining the results of studies that pair tDCS and TMS (Grimaldi et al. 2016). When a conditioning TMS pulse is applied to the cerebellum 5–7 ms before a test TMS pulse is applied to motor cortex, a reduction in the ensuing motor-evoked potential is observed. This phenomenon is known as cerebellar inhibition (Daskalakis et al. 2004; Pinto and Chen 2001), and it is thought to be caused by inhibitory output from cerebellar Purkinje cells on cortical motor areas. Importantly, cerebellar inhibition is altered by both anodal and cathodal cerebellar tDCS (Galea et al. 2009). The direction of the alteration depends on the polarity of the stimulation. Cerebellar tDCS does not seem to alter the excitability of adjacent areas, a result supported by behavioral work and studies that model the flow of direct current applied to the brain (Galea et al. 2011; Rampersad et al. 2014; and see Fig. 3 in Grimaldi et al. 2016). Thus, neurophysiological investigations, behavioral work, and computational modeling suggest that cerebellar tDCS focally alters the functioning of the cerebellum. Nevertheless, as Grimaldi et al. (2016) point out, more work is needed to determine the precise impact of tDCS on cerebellar neurons and the locations within the cerebellum that tDCS affects (e.g., cerebellar cortex or Purkinje cells).
Why did anodal tDCS impair behavior in this study? There are many examples of studies examining the impact of anodal tDCS on motor behavior that have observed isolated behavioral improvements. These results, which have mainly focused on the effects of tDCS when applied to the cerebral cortex, have led to the oversimplified idea that anodal tDCS ought to improve behavior, whereas cathodal tDCS should inhibit it. However, we know of no established mechanistic framework that would support this, and, given the complexity and nonlinear dynamics of cortical and cerebellar processing, it is increasingly clear that the heuristic of a sliding scale rationale is overly simplistic (Bestmann et al. 2015; de Berker et al. 2013; Rahman et al. 2015). Indeed, anodal tDCS can impair behavior and cathodal tDCS can improve behavior, and this seems especially true when applied to the cerebellum. To give two examples of particular relevance to the current study, Ferrucci et al. (2008) applied anodal tDCS to the right cerebellum and found that practice-dependent changes in reaction time associated with a working memory task were impaired. Also, in more recent work, Pope and Miall (2012) applied cathodal tDCS to the cerebellum and observed improvements in performance on a difficult serial subtraction task. In explanation, Pope and Miall speculate based on the neurophysiology of cerebellar-cortical connections that cathodal stimulation led to a decrease in inhibitory output from the cerebellum and, by consequence, a release of cognitive resources. Although there is some evidence that seems to counter this idea (e.g., Boehringer et al. 2013 report impairments to cognition following cathodal cerebellar tDCS), the results presented here in combination with neurophysiological investigations of the impact of tDCS on the cerebellum complement Pope and Miall's hypothesis. In Galea et al. (2009), for instance, anodal cerebellar tDCS was observed to increase inhibitory output from the cerebellum on motor cortex, whereas cathodal tDCS was observed to decrease it. Thus, if the present study were repeated with cathodal cerebellar tDCS, one might predict an improvement in the timing of perceptual behavior compared with sham stimulation. Of course, the lack of a cathodal group does not subtract from this paper's main finding: alterations of the cerebellum dissociate the timing of perceptual decisions from perceptual change in speech.
Ambiguous speech sounds are often encountered during conversation (most notably when talking with a foreign-accented speaker), and we rapidly adapt our perception of speech in these situations (Bradlow and Bent 2008; Reinisch and Holt 2014). During conversation, external feedback related to the meaning of ambiguous speech is readily available via body language, contextual information, or explicit clarification. Here we demonstrate that simple external feedback can drive changes in the perception of ambiguous speech sounds, and these changes are transferable. The timing of this perceptual behavior critically depends on the integrity of the right cerebellum. More generally, the work supports a growing body of evidence that the cerebellum plays a role in the timing of behaviors beyond the motor domain.
GRANTS
D. R. Lametti was supported by fellowships from the Les Fonds Québécois de la Recherche sur la Nature et les Technologies, Québéc, and the British Academy. S. Bestmann and J. Bonaiuto are supported by the European Research Council (ActSelectContext; 260424).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.R.L. and J.C.R. conception and design of research; D.R.L. performed experiments; D.R.L. analyzed data; D.R.L., L.O.W., J.B., S.B., and J.C.R. interpreted results of experiments; D.R.L. prepared figures; D.R.L. drafted manuscript; D.R.L., L.O.W., J.B., S.B., and J.C.R. edited and revised manuscript; D.R.L., L.O.W., J.B., S.B., and J.C.R. approved final version of manuscript.
REFERENCES
- Ackermann H, Mathiak K, Riecker A. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum 6: 202–213, 2007. [DOI] [PubMed] [Google Scholar]
- Bestmann S, de Berker AO, Bonaiuto J. Understanding the behavioural consequences of noninvasive brain stimulation. Trends Cogn Sci 19: 13–20, 2015. [DOI] [PubMed] [Google Scholar]
- Boehringer A, Macher K, Dukart J, Villringer A, Pleger B. Cerebellar tran scranial direct current stimulation modulates verbal working memory. Brain Stimul 6: 649–653, 2013. [DOI] [PubMed] [Google Scholar]
- Bradlow AR, Bent T. Perceptual adaptation to non-native speech. Cognition 106: 707–729, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callan DE, Tajima K, Callan AM, Kubo R, Masaki S, Akahane-Yamada R. Learning-induced neural plasticity associated with improved identification performance after training of a difficult second-language phonetic contrast. Neuroimage 19: 113–124, 2003. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol 557: 689–700, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Berker AO, Bikson M, Bestmann S. Predicting the behavioral impact of transcranial direct current stimulation: issues and limitations. Front Hum Neurosci 7: 613, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trends Cogn Sci 2: 355–362, 1998. [DOI] [PubMed] [Google Scholar]
- DeWitt I, Rauschecker JP. Phoneme and word recognition in the auditory ventral stream. Proc Natl Acad Sci USA 109: E505–14, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durisko C, Fiez JA. Functional activation in the cerebellum during working memory and simple speech tasks. Cortex 46: 896–906, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci R, Marceglia S, Vergari M, Cogiamanian F, Mrakic-Sposta S, Mameli F, Zago S, Barbieri S, Priori A. Cerebellar transcranial direct current stimulation impairs the practice-dependent proficiency increase in working memory. J Cogn Neurosci 20: 1687–1697, 2008. [DOI] [PubMed] [Google Scholar]
- Franz EA, Ivry RB, Helmuth LL. Reduced Timing Variability in Patients with Unilateral Cerebellar Lesions during Bimanual Movements. J Cogn Neurosci 8: 107–118, 1996. [DOI] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29: 9115–9122, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, de Xivry JJO, Celnik P. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex 21: 1761–1770, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci 30: 535–574, 2007. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, Edwards DJ, Ferrucci R, Fregni F, Galea JM, Hamada M, Manto M, Miall RC, Morales-Quezada L, Pope PA, Priori A, Rothwell J, Tomlinson SP, Celnik P. Cerebellar transcranial direct current stimulation (ctDCS): a novel approach to understanding cerebellar function in health and disease. Neuroscientist 22: 83–97, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guediche S, Holt LL, Laurent P, Lim SJ, Fiez JA. Evidence for cerebellar contributions to adaptive plasticity in speech perception. Cereb Cortex 25: 1867–1877, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffland BS, Bologna M, Kassavetis P, Teo JTH, Rothwell JC, Yeo CH, van de Warrenburg BP, Edwards MJ. Cerebellar theta burst stimulation impairs eyeblink classical conditioning. J Physiol 590: 887–897, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci 1: 136–152, 1989. [DOI] [PubMed] [Google Scholar]
- Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar contributions to reach adaptation and learning sensory consequences of action. J Neurosci 32: 4230–4239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23: 8432–8444, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Oliveri M, Torriero S, Salerno S, Lo Gerfo E, Caltagirone C. Repetitive TMS of cerebellum interferes with millisecond time processing. Exp Brain Res 179: 291–299, 2007. [DOI] [PubMed] [Google Scholar]
- Lametti DR, Krol SA, Shiller DM, Ostry DJ. Brief periods of auditory perceptual training can determine the sensory targets of speech motor learning. Psychol Sci 25: 1325–1336, 2014a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lametti DR, Rochet-Capellan A, Neufeld E, Shiller DM, Ostry DJ. Plasticity in the human speech motor system drives changes in speech perception. J Neurosci 34: 10339–10346, 2014b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage E, Morgan BE, Olson AC, Meyer AS, Miall RC. Cerebellar rTMS disrupts predictive language processing. Curr Biol 22: R794–R795, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak K, Hertrich I, Grodd W, Ackermann H. Cerebellum and speech perception: a functional magnetic resonance imaging study. J Cogn Neurosci 14: 902–912, 2002. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Thompson RF. Cerebellum: essential involvement in the classically conditioned eyelid response. Science 223: 296–299, 1984. [DOI] [PubMed] [Google Scholar]
- Miall RC, Antony J, Goldsmith-Sumner A, Harding SR, McGovern C, Winter JL. Modulation of linguistic prediction by TDCS of the right lateral cerebellum. Neuropsychologia 86: 103–109, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niziolek CA, Guenther FH. Vowel category boundaries enhance cortical and behavioral responses to speech feedback alterations. J Neurosci 33: 12090–12098, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panouillères MTN, Miall RC, Jenkinson N. The role of the posterior cerebellum in saccadic adaptation: a transcranial direct current stimulation study. J Neurosci 35: 5471–5479, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. New York, NY: Dover, 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto AD, Chen R. Suppression of the motor cortex by magnetic stimulation of the cerebellum. Exp Brain Res 140: 505–510, 2001. [DOI] [PubMed] [Google Scholar]
- Pope PA, Miall RC. Task-specific facilitation of cognition by cathodal transcranial direct current stimulation of the cerebellum. Brain Stimul 5: 84–94, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe K, Livne O, Gizewski ER, Aurich V, Beck A, Timmann D, Donchin O. Adaptation to visuomotor rotation and force field perturbation is correlated to different brain areas in patients with cerebellar degeneration. J Neurophysiol 101: 1961–1971, 2009. [DOI] [PubMed] [Google Scholar]
- Rahman A, Lafon B, Bikson M. Multilevel computational models for predicting the cellular effects of noninvasive brain stimulation. Prog Brain Res 222: 25–40, 2015. [DOI] [PubMed] [Google Scholar]
- Rampersad SM, Janssen AM, Lucka F, Aydin Ü, Lanfer B, Lew S, Wolters CH, Stegeman DF, Oostendorp TF. Simulating transcranial direct current stimulation with a detailed anisotropic human head model. IEEE Trans Neural Syst Rehabil Eng 22: 441–452, 2014. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, McKoon G. The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput 20: 873–922, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinisch E, Holt LL. Lexically guided phonetic retuning of foreign-accented speech and its generalization. J Exp Psychol Hum Percept Perform 40: 539–555, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington's disease but not cerebellar degeneration. J Neurophysiol 93: 2809–2821, 2005. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Ivry RB. Cerebellum and Timing. In: Handbook of the Cerebellum and Cerebellar Disorders, edited by Manto M, Schmahmann JD, Rossi F, Gruol DL, Koibuchi N. Rotterdam, The Netherlands: Springer, 2013, p. 1201–1219. [Google Scholar]
- Spencer RMC, Zelaznik HN, Diedrichsen J, Ivry RB. Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science 300: 1437–1439, 2003. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage 44: 489–501, 2009. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci 32: 413–434, 2009. [DOI] [PubMed] [Google Scholar]
- Théoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett 306: 29–32, 2001. [DOI] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Ostry DJ. Structure of plasticity in human sensory and motor networks due to perceptual learning. J Neurosci 34: 2451–2463, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J, Tuerlinckx F. Diffusion model analysis with MATLAB: a DMAT primer. Behav Res Methods 40: 61–72, 2008. [DOI] [PubMed] [Google Scholar]
- Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci 2: 338–347, 1998. [DOI] [PubMed] [Google Scholar]
- Xiang H, Lin C, Ma X, Zhang Z, Bower JM, Weng X, Gao JH. Involvement of the cerebellum in semantic discrimination: an fMRI study. Hum Brain Mapp 18: 208–214, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]