Retrotrapezoid nucleus (RTN) neurons encode brain Pco2 and activate breathing. We show by unit recording that these central respiratory chemoreceptors are strongly activated by somatic afferent stimulation, like the neighboring presympathetic neurons. This finding, in anesthetized rats, underscores that RTN neurons integrate multiple types of information besides monitoring the surrounding Pco2. Inputs from somatic afferents to RTN may contribute to the hyperpnea of exercise or mediate respiratory stimulation elicited by activation of nociceptive or thermosensitive afferents.
Keywords: exercise hyperpnea, nociception, retrotrapezoid nucleus, somatic input
Abstract
Retrotrapezoid nucleus (RTN) neurons sustain breathing automaticity. These neurons have chemoreceptor properties, but their firing is also regulated by multiple synaptic inputs of uncertain function. Here we test whether RTN neurons, like neighboring presympathetic neurons, are excited by somatic afferent stimulation. Experiments were performed in Inactin-anesthetized, bilaterally vagotomized, paralyzed, mechanically ventilated Sprague-Dawley rats. End-expiratory CO2 (eeCO2) was varied between 4% and 10% to modify rate and amplitude of phrenic nerve discharge (PND). RTN and presympathetic neurons were recorded extracellularly below the facial motor nucleus with established criteria. Sciatic nerve stimulation (SNstim, 1 ms, 0.5 Hz) slightly increased blood pressure (6.6 ± 1.6 mmHg) and heart rate and, at low eeCO2 (<5.5%), entrained PND. Ipsi- and contralateral SNstim produced the known biphasic activation of presympathetic neurons. SNstim evoked a similar but weaker biphasic response in up to 67% of RTN neurons and monophasic excitation in the rest. At low eeCO2, RTN neurons were silent and responded more weakly to SNstim than at high eeCO2. RTN neuron firing was respiratory modulated to various degrees. The phasic activation of RTN neurons elicited by SNstim was virtually unchanged at high eeCO2 when PND entrainment to the stimulus was disrupted. Thus RTN neuron response to SNstim did not result from entrainment to the central pattern generator. Overall, SNstim shifted the relationship between RTN firing and eeCO2 upward. In conclusion, somatic afferent stimulation increases RTN neuron firing probability without altering their response to CO2. This pathway may contribute to the hyperpnea triggered by nociception, exercise (muscle metabotropic reflex), or hyperthermia.
NEW & NOTEWORTHY
Retrotrapezoid nucleus (RTN) neurons encode brain Pco2 and activate breathing. We show by unit recording that these central respiratory chemoreceptors are strongly activated by somatic afferent stimulation, like the neighboring presympathetic neurons. This finding, in anesthetized rats, underscores that RTN neurons integrate multiple types of information besides monitoring the surrounding Pco2. Inputs from somatic afferents to RTN may contribute to the hyperpnea of exercise or mediate respiratory stimulation elicited by activation of nociceptive or thermosensitive afferents.
the retrotrapezoid nucleus (RTN) is a cluster of respiratory chemoreceptors that regulate breathing automaticity and contribute to the stability of arterial Pco2 (Guyenet et al. 2016). By our definition, RTN consists of ∼2,000 CO2-activated neurons (in rats; 800 in mice) that express a specific and seemingly unique combination of markers (Phox2b, VGLUT2, NK1R, GPR4, TASK-2) (Guyenet et al. 2016). When activated these glutamatergic neurons increase lung ventilation massively, and their inhibition has the opposite effect (Abbott et al. 2009; Basting et al. 2015; Guyenet et al. 2016; Kanbar et al. 2010). RTN neurons are activated by hypercapnia via their intrinsic proton sensitivity and astrocyte-dependent local effects of CO2/pH (Forsberg et al. 2016; Gourine et al. 2010; Guyenet et al. 2016; Huckstepp et al. 2010; Kumar et al. 2015). Genetic lesions of RTN or deletion of two genes expressed by RTN and implicated in pH sensing reduces the central respiratory chemoreflex by 67–90% (Kumar et al. 2015; Ramanantsoa et al. 2011).
The central respiratory chemoreflex dampens arterial Pco2 fluctuations by fine-tuning lung ventilation, but this reflex is neither the only nor even the principal mechanism that underlies arterial Pco2 stability (Forster et al. 2012; Guyenet 2014; Nattie 2006; Nattie and Li 2012). This point is dramatically illustrated by dynamic exercise during which ventilation is greatly elevated, arterial Po2 is normal, and arterial Pco2 is unchanged or even slightly reduced (Forster et al. 2012). The ventilatory adjustments to exercise are attributed to “central command,” a term referring to the activation of the breathing network by some undefined portion of the CNS or spinal cord locomotor circuitry and to sensory feedback from exercising muscles (Amann et al. 2010; Eldridge et al. 1981; Forster et al. 2012; Gariepy et al. 2012; Kaufman 2012; McCord and Kaufman 2010). The more subtle increase in metabolic rate accompanying posture, emotions, being awake as opposed to asleep, and thermoregulation are also associated with measured increases in ventilation that keep arterial Pco2 constant. The breathing adjustments associated with these behaviors or physiological states presumably also operate in a feedforward manner, i.e., via inputs from sensory afferents and a hierarchy of brain regions.
RTN could be one of a few nodal points where these various feedforward mechanisms are integrated with chemoreceptor information to maintain the balance between the metabolic production of CO2 and its excretion via the lungs. RTN is adjacent to the portion of the ventrolateral medulla that performs a parallel integrative role with respect to circulatory control and blood pressure (BP) homeostasis (Guyenet et al. 2013). The dendrites of RTN neurons are covered with asymmetric, likely glutamatergic synapses (Lazarenko et al. 2009), and physiological evidence that RTN performs a complex integrative function besides detecting local Pco2 is mounting (Guyenet et al. 2016).
A fraction of RTN neurons express Fos after moderate dynamic exercise in rodents (Barna et al. 2012, 2014). Arterial Pco2 presumably does not increase under such conditions; therefore RTN activation likely derives from central command, sensory feedback, or both. Supporting the first possibility, RTN neurons are robustly activated when the hypothalamus is stimulated (Fortuna et al. 2009; Waldrop et al. 1988). One of these hypothalamic inputs originates from orexinergic neurons (Dias et al. 2009; Fortuna et al. 2009; Lazarenko et al. 2011), many of which discharge in association with movement (Lee et al. 2005). RTN neurons could also receive excitatory inputs from an ascending neural pathway originating in the spinal cord and activated by afferents from exercising muscles (Kaufman 2012; McCord and Kaufman 2010). This possibility is compatible with existing anatomical evidence: the ventrolateral medulla inclusive of the RTN region receives input from lamina I of the dorsal horn, which in turn receives input from nociceptive afferents and afferents (type III and IV) activated by contracting muscles (Craig and Kniffki 1985; Kaufman 2012).
In the present study we sought physiological evidence that RTN neurons receive excitatory input from somatic afferents. To do so, we recorded from single RTN neurons in anesthetized rats and examined their response to sciatic nerve stimulation.
METHODS
Animal use was conducted according to protocols reviewed and approved by the University of Virginia Animal Care and Use Committee. Experiments were conducted on 19 male Sprague-Dawley rats weighing between 300 and 350 g.
Physiological preparation.
Surgical procedures were performed under isoflurane (5% for induction and 2.5% for maintenance) in 100% oxygen. Rectal temperature was maintained at 37.5 ± 0.5°C. The following procedures were performed sequentially in all rats: tracheostomy, mechanical ventilation, bilateral vagotomy, insertion of venous and arterial femoral catheters for intravenous injections and arterial BP recording, respectively, exposure of the left and right mandibular branches of the facial nerve for antidromic mapping of the facial motor nucleus, dissection of the left phrenic nerve in the neck, and exposure of both sciatic nerves. The intact sciatic and cut phrenic nerves were mounted on bipolar electrodes and isolated in biocompatible polymer (World Precision Instruments, Sarasota, FL) for later stimulation (sciatic nerve) or multifiber recording (phrenic nerve). Rats were placed prone in a Kopf stereotaxic frame with the bite bar set at negative 3.5 mm. A small portion of the occipital plate was removed on either side to allow transcerebellar access to the rostral medulla oblongata with glass recording pipettes.
End-expiratory CO2 (eeCO2) was continuously monitored with a microcapnometer (Columbus Instruments, Columbus, OH) and set between 4.0% and 10% by adding CO2 to the inspired mixture. While we were searching for RTN neurons, eeCO2 was maintained at least 1.5% above the apneic threshold (typically 5.5%). Phenylephrine (5 μg/kg) was injected intravenously to raise BP.
Upon completion of surgical procedures, isoflurane was withdrawn gradually while an isotonic solution of Inactin (thiobutabarbital) was slowly administered (Sigma; initial dose of 80–100 mg/kg iv with additional doses of 10 mg/kg as required) (Nakamura and Morrison 2011). Boosters were required every 3–4 h. Usually two boosters were administered. Rats were also paralyzed with a muscle relaxant (pancuronium 1 mg/kg iv with additional doses of 0.2 mg/kg when needed). In the absence of pancuronium, adequacy of anesthesia was monitored by the absence of withdrawal reflex and, under paralysis, by the limited increase in BP (<10 mmHg) and lack of activation of the phrenic nerve in response to a firm paw pinch.
Single-unit recordings.
Single-unit recordings were made with glass pipettes filled with 2 M NaCl (8–14 MΩ). The caudal and lower boundaries of the facial motor nucleus were identified by mapping antidromic field potentials evoked by stimulating the facial branch of the facial nerve (Brown and Guyenet 1985; Mulkey et al. 2004). Unit recordings were made 0–500 μm rostral to the caudal end of the facial motor nucleus, 1.8–2.4 mm lateral to the midline, and from 0 to 400 μm ventral to the lower edge of the facial motor nucleus. We focused on this specific brain region because it contains the highest concentration of RTN neurons intermixed with presympathetic neurons (Abbott et al. 2009; Stornetta et al. 2006). No histology was performed. The location of the recorded units was identified with sufficient precision by their location relative to the caudal and ventral boundaries of the facial motor nucleus.
The term “presympathetic neurons,” aka bulbospinal cardiovascular neurons (Brown and Guyenet 1985), aka reticulospinal vasomotor neurons (Morrison et al. 1989), refers to cells that are silenced by activation of arterial baroreceptors and cluster within the rostral ventrolateral medullary nucleus (definition after Ross et al. 1984). Virtually all these neurons have a spinal axon with terminal branches located within the intermediolateral cell column (Morrison et al. 1988). The majority are C1 cells (Milner et al. 1988; Schreihofer and Guyenet 1997). These presympathetic neurons provide a major excitatory drive to the sympathetic efferents that control the circulation and are viewed as primarily responsible for BP stabilization by the baroreflex (Guyenet et al. 2013).
RTN neurons were identified by three criteria: location under the facial motor nucleus, insensitivity to changes in BP, and activation by CO2 (Mulkey et al. 2004).
Sciatic nerve electrical stimulation.
The nerve was stimulated at low frequency (0.5–1 Hz) with 1-ms square-wave pulses with bipolar electrodes made of silver wire. The intensity ranged from 1 to 10 mA. These parameters were used because of prior evidence that such stimuli activate all fiber types (Stornetta et al. 1989). Electrical stimulation produced an artifact in the phrenic nerve discharge (PND) and single-unit recording signals, which was removed post hoc with the script “artrem” downloaded from the Cambridge Electronic Design (CED) Spike2 website. We used this script to clamp 4–6 ms worth of AC recording at 0 mV. The blanked segments were triggered at the onset of the stimulation pulse.
Both contralateral and ipsilateral stimulation of the sciatic nerve were performed whenever possible. RTN neuron response was also tested under low (<5.5% eeCO2)- and high (>5.5% eeCO2)-CO2 conditions.
In a few experiments (7 RTN neurons), the stimulus was delivered exclusively during the inspiratory phase and then exclusively during the expiratory phase of the central respiratory cycle. To that effect, we wrote a custom-built script on Spike2 that identified the rising phase of the PND and produced TTL pulses at preset times after PND onset via a Micro1401 digitizer (CED, Cambridge, UK). The TTL pulses triggered the stimulator. The sciatic nerve could thus be stimulated during inspiration only (TTL ∼20 ms after PND onset) or during expiration (TTL pulse 0.6–1 s after PND onset depending on the period of the breathing cycle).
Data acquisition and analysis.
All analog data were acquired via a Micro1401 digitizer (CED) and were processed off-line with Spike5 software (CED) (Guyenet et al. 2005). Integrated phrenic nerve discharge (PNDr) was obtained by rectifying and smoothing (τ = 0.05 s) the original 100- to 1,000-Hz band-pass AC signal. PND amplitude was defined as the area under the curve of the rectified and smoothed phrenic bursts, and these values were normalized by assigning a value of 100 to the maximum value recorded at high eeCO2 in the absence of any nerve stimulation and a value of 0 to the noise recorded between bursts. The noise was determined as the average electrical signal present between PNDs or the electrical signal recorded when the animal was sufficiently hyperventilated to eliminate the phrenic bursts.
Event-triggered histograms of neuronal activity were constructed to analyze the effect of sciatic stimulation on the discharge rate of the neurons of interest. Neuronal activation was calculated by subtracting the spike frequency observed prior to the electrical stimulus from that present during the activation phase(s). In general, activation occurred in two phases with short vs. long latency and each response was analyzed separately. Occasionally the late peak was also multiphasic, but this response was also analyzed as a single event.
Statistics.
All data sets were tested for normality with the Shapiro-Wilk test, and equal variances for ANOVAs were determined with Bartlett's test. If the criteria of normality and equal variance, as appropriate, were satisfied, we evaluated statistical significance with either a two-tailed paired t-test or one-way ANOVA or ANCOVA, as appropriate. When the criteria of normality were not met, we used a nonparametric statistical test (2-tailed Wilcoxon signed-rank test for paired values, 2-tailed Mann-Whitney U for unpaired values, or Kruskal-Wallis with post hoc comparisons using Dunnett's correction for multiple comparisons). Values are expressed as means ± SE if the data set is normally distributed, or with nonnormally distributed data we report the medians and 95% confidence intervals (CIs). Statistical significance is set at P < 0.05. All statistics were performed with GraphPad Prism v.7 software or the R statistical package (R Core Team 2013).
RESULTS
Neuron characterization and location.
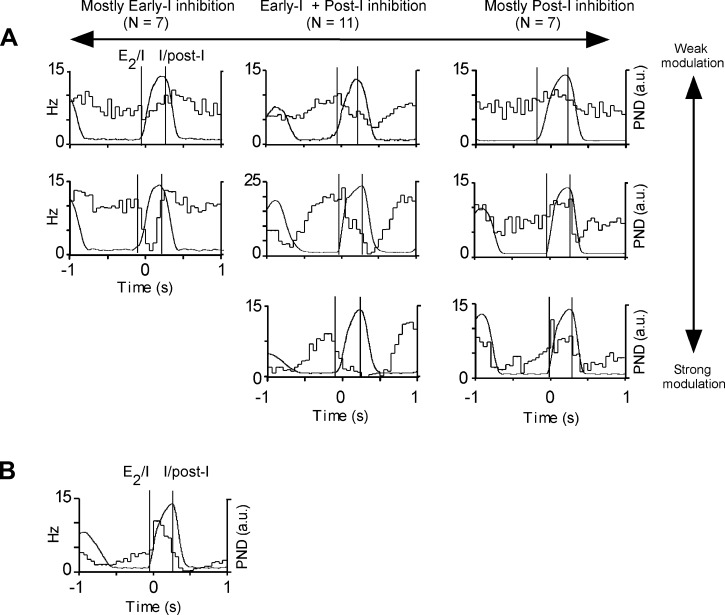
Recordings were made under Inactin anesthesia. We first verified that presympathetic and RTN neurons could be unambiguously identified under these anesthetic conditions. We recorded from 58 active neurons located under the caudal edge of the facial motor nucleus in 19 rats (location: see Fig. 1). The neurons could be readily classified into two types based on their differential response to CO2 and baroreceptor activation. The first category (presympathetic; n = 29) were highly barosensitive but virtually insensitive to changes of eeCO2 (Fig. 2). Their discharge was strongly pulse modulated but weakly entrained to respiration (Fig. 2, C and D). The rest of the neurons (RTN; n = 29) were insensitive to BP changes (7 neurons tested during phenylephrine injection) and strongly CO2 sensitive, and their discharge, unlike that of the presympathetic neurons, had no pulse modulation (29 neurons analyzed; Fig. 3). These neurons were respiratory modulated to various degrees (Fig. 3C and Fig. 4). Three main respiratory patterns could be identified on the basis of the timing of the neuronal discharge probability nadir(s) in relation to the breathing cycle (Fig. 4): early-I inhibition (n = 7), early-I and post-I inhibition (n = 11), and post-I inhibition (n = 7). This nomenclature is based on the plausible but unproven assumption that periods of reduced discharge probability denote the presence of inhibitory volleys. Another four cells were also inhibited during both I and post-I phases, but their activity increased abruptly during the early part of inspiration (Fig. 4B). CO2-stimulated neurons exclusively active during late expiration were not encountered.
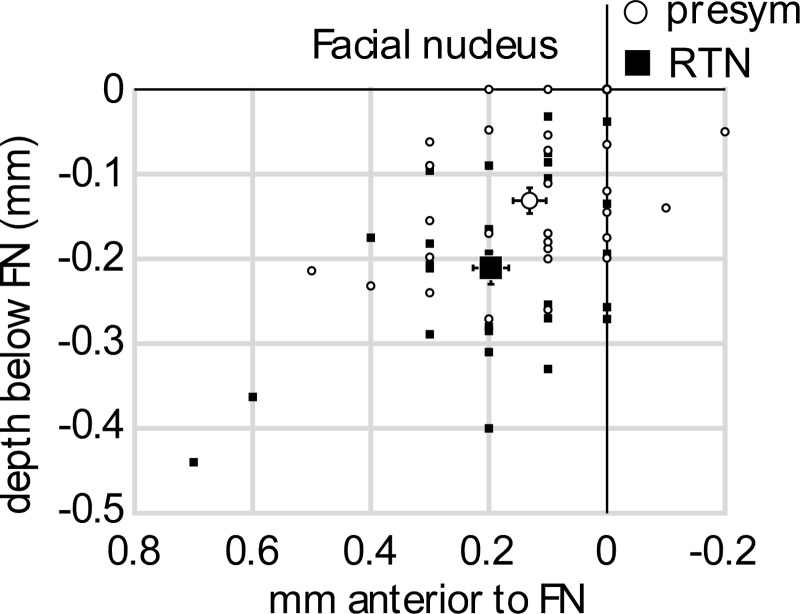
Fig. 1.
Location of recorded units. The stereotaxic coordinates of the recorded neurons [29 RTN (small filled squares) and 29 presympathetic neurons (small open circles)] are plotted in 2 dimensions i.e., relative to the ventral and caudal edges of the facial motor nucleus (solid lines). The reference planes (level 0) were determined by antidromic mapping of the field potential evoked by stimulation of the mandibular branch of the facial nerve. Average (±SE) position of RTN (large filled square) and presympathetic neurons (large open circle) is also indicated.
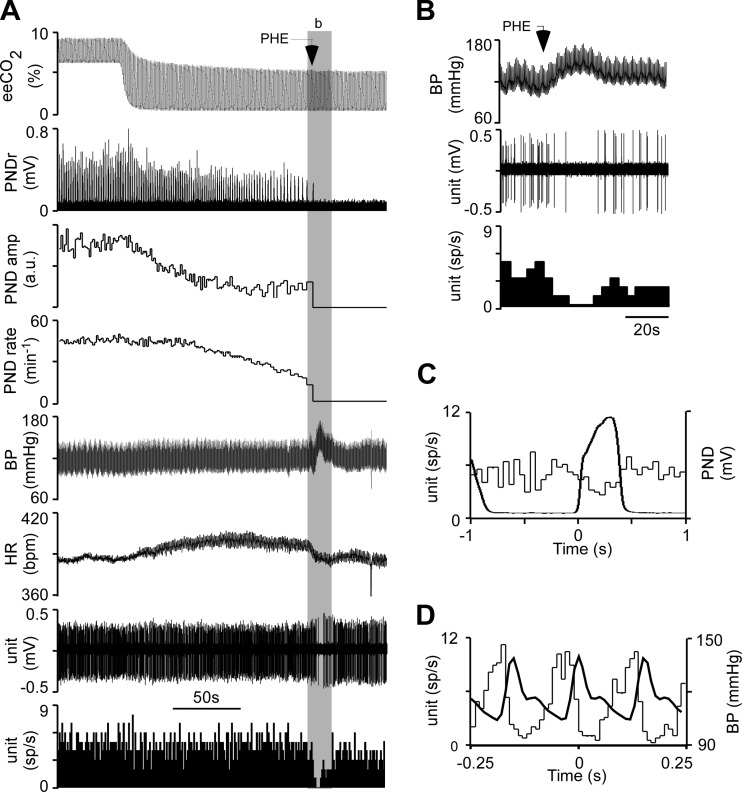
Fig. 2.
Identification of presympathetic neurons. A: from top to bottom: end-expiratory CO2 (eeCO2), rectified and integrated phrenic nerve discharge (PND), PND amplitude, PND rate, blood pressure (BP; femoral artery), heart rate (HR), single RTN unit (extracellular recording), and RTN unit discharge rate (action potentials binned every 1 s and expressed as spikes/s). In this excerpt, eeCO2 was decreased by withdrawing the CO2 added to the inhalation mixture. Phenylephrine (PHE) was administered intravenously (5 μg/kg) to transiently raise BP. B: expanded timescale excerpt (from shaded area b in A) illustrating the effect of PHE on the discharge of the recorded neurons (BP, top; recorded single unit, middle; discharge rate of single unit binned in 4-s intervals, bottom). C: event-triggered histogram of the cell's discharge. The histogram (thin line) was triggered at time 0 by the onset of the PND. The waveform-averaged PND is also represented (thick line). Note the weak respiratory modulation with reduced firing probability of the unit during early inspiration and peak discharge probability during the postinspiratory phase. D: histogram of the cell's discharge triggered at time 0 by the BP pulse. Note the strong pulse modulation of the neuron's discharge. a.u., Arbitrary units.
Fig. 3.
RTN neuron characterization. A: original recording showing the effect of CO2 and phenylephrine (PHE; 5 μg/kg iv) on the discharge of a single RTN neuron. Traces presented as in Fig. 2. The unit was silent below 5% eeCO2 but unresponsive to BP elevation. B: expanded timescale excerpt (from shaded period in A) illustrating the lack of effect of PHE. C: PND-triggered histogram of the activity of the unit represented in A. Note weak respiratory modulation with activity nadir during postinspiration. D: pulse-triggered activity histogram (thin trace) and superimposed averaged pressure pulse illustrating the absence of pulse modulation of this unit.
Fig. 4.
Respiratory patterns of RTN neurons. Respiratory patterns were classified according to the timing of the periods of reduced firing probability relative to the phrenic nerve discharge (PND). A: 3 most commonly encountered patterns, i.e., early-inspiratory (early-I) inhibition, twin inhibition during early-I and post-I phases, and post-inspiratory (post-I) inhibition; 2–3 examples of each class of neuron are represented to illustrate variations in the amplitude of the respiratory modulation. B: atypical RTN neurons (type 2) exhibiting maximum firing probability during early inspiration and nadir during postinspiration. Vertical lines identify the onset and termination of the inspiratory burst (expiration-inspiration and inspiration-postinspiration transitions) taking into consideration the smoothing effect (t = 0.05 s) of the PND integration procedure.
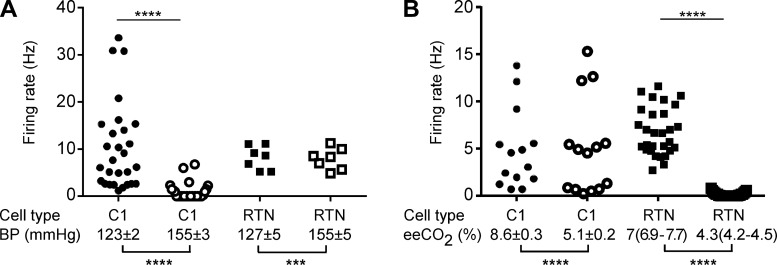
On average (Fig. 5), increasing mean BP with intravenous phenylephrine (from 123 ± 2 mmHg to 155 ± 3 mmHg; n = 28, t = 18.9, df = 27, P < 0.0001) reduced the firing rate of the presympathetic neurons from a median value of 6.9 spikes/s (95% CI 6.7–13.9) to a median value of 0 (95% CI 0.27–1.6) (W = −406, n = 28, P < 0.0001), i.e., silencing them (Fig. 2, A and B, and Fig. 5A). Similar increases in BP (from 127 ± 5 mmHg to 155 ± 5 mmHg; n = 7, t = 7.4, df = 6, P < 0.0003) had no effect on the firing rate of RTN neurons (from 8.2 ± 0.9 Hz to 7.9 ± 0.9 Hz; n = 7, t = 0.637 df = 6, P = 0.5475; Fig. 3, A and B, and Fig. 5A). Decreasing eeCO2 (from a median value of 7%, 95% CI 6.9–7.7% to a median value of 4.3%, 95% CI 4.2–4.5%; W = −435, n = 29, P < 0.0001) silenced RTN cells (from a median value of 6.7 Hz, 95% CI 5.9–7.8% to 0 Hz, 95% CI 0–0.2%, W = −435, n = 29, P < 0.0001; Fig. 3A and Fig. 5B). A similar change in eeCO2 (from 8.6 ± 0.3% to 5.1 ± 0.2%, n = 14; t-test, t = 10.2, df = 13, P < 0.0001) had no effect on the discharge rate of the presympathetic neurons (from median 3.8 Hz, 95% CI 1.3–9.2 Hz to 4.7 Hz, 95% CI 0.69–12.2 Hz, W = −11, n = 14, P = 0.7609; Fig. 2A and Fig. 5B).
Fig. 5.
Effect of BP or end-expiratory CO2 on the firing rate of presympathetic and RTN neurons. A: discharge rate (spikes/s; scatterplot) of 28 presympathetic and 7 RTN neurons recorded at high and low BP (mean ± SE indicated below graph). B: discharge rate of 14 presympathetic and 29 RTN neurons at high (>5.5%) and low (<5.5%) eeCO2. Statistical significance: ***P < 0.0003, ****P < 0.0001. See text for further details regarding statistics.
Presympathetic and RTN neurons were largely intermingled within the restricted brain region that was sampled (Fig. 1). On average, RTN neurons (n = 29) were located significantly deeper than presympathetic neurons (211 ± 19 μm below the facial motor nucleus vs. 131 ± 15 μm; t = 3.184, df = 56, P = 0.0024). This was expected because RTN neurons reside in general deeper than C1 cells, which are the predominant type of presympathetic neurons located in this region of the brain (Stornetta et al. 2006). The average rostrocaudal location of both classes of neurons was the same [RTN median value 200 μm (95% CI 134–260 μm) rostral to the caudal edge of the facial motor nucleus vs. C1 median 100 μm (95% CI 73–190 μm) (Mann-Whitney U = 331, P = 0.156)]. This result merely reflects that the sampled region was purposefully restricted to the region with the greatest overlap between C1 and RTN neurons (Stornetta et al. 2006). Both types of cells were commonly encountered in the same animals (11/19 preparations).
In summary, presympathetic and RTN neurons have discharge characteristics under Inactin anesthesia similar to those under other anesthetics (halothane, chloralose-urethane) (Guyenet et al. 2005; Schreihofer and Guyenet 1997).
Sciatic nerve stimulation increases cardiorespiratory parameters.
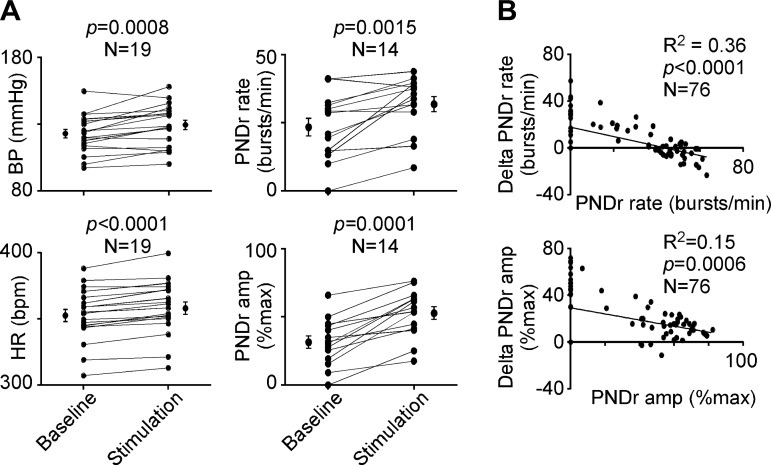
The effect of sciatic nerve electrical stimulation was evaluated by averaging the effect of multiple 30- to 60-s periods of stimulation at 0.5 Hz for each rat (Fig. 6A). Sciatic nerve stimulation increased BP slightly (from 123 ± 3 mmHg to 129 ± 4 mmHg, n = 19 rats; 2-tailed paired t-test: t = 4.018, df = 18,P = 0.0008), produced a slight tachycardia [heart rate from 405 ± 9 beats/min (bpm) to 416 ± 9 bpm, n = 19 rats; 2-tailed paired t-test t = 7.477, df = 18, P < 0.0001], and increased PND rate (from 23 ± 3 bursts/min to 32 ± 3 bursts/min, n = 14 rats, paired t-test: t = 3.988, df = 13, P = 0.0015) and amplitude (from 31% to 53% of maximum recorded amplitude, n = 14 rats, Wilcoxon matched-pairs signed-rank test: P = 0.0001). Since PND rate is a CO2-dependent variable, the relation between the increase in PND rate and the baseline value of this parameter was examined (Fig. 6B). A similar analysis was performed with PNDr amplitude, for the same reason. There was a significant inverse relationship between baseline PND frequency and amplitude and the effect of stimulation on these parameters. The effect of sciatic nerve stimulation tended to be more pronounced at lower baseline values and decreased as baseline PND rate and amplitude increased. In addition, when sciatic stimulation frequency was lower than baseline PND frequency, the frequency tended to decrease (Fortuna et al. 2009).
Fig. 6.
Cardiorespiratory responses to sciatic nerve stimulation. A: effect of sciatic nerve stimulation (0.5 Hz) on mean blood pressure (BP), heart rate (HR), PND rate, and amplitude of rectified PND (PNDr) expressed as % of the largest amplitude recorded in each preparation (typically at 10% eeCO2) for the number of animals indicated. Each point represents the average of multiple periods of rest or stimulation for a given rat. The mean (±SE) value is also shown. B: relationship between the degree of respiratory stimulation elicited by sciatic nerve stimulation (delta PND rate and delta PND amplitude) and the baseline value of these variables. The regression coefficient, significance value, and number of measurements are indicated.
Sciatic nerve stimulation activates presympathetic neurons.
We examined the responses of 29 presympathetic neurons to sciatic nerve stimulation. Contralateral (n = 25) and ipsilateral (n = 16) sciatic nerves were stimulated at 0.5 Hz for 5 min (1-ms pulses). All neurons responded to both ipsi- and contralateral nerve stimulation; the response typically consisted of an early and a late excitation, followed by a period of reduced activity (Fig. 7, B and C).
Fig. 7.
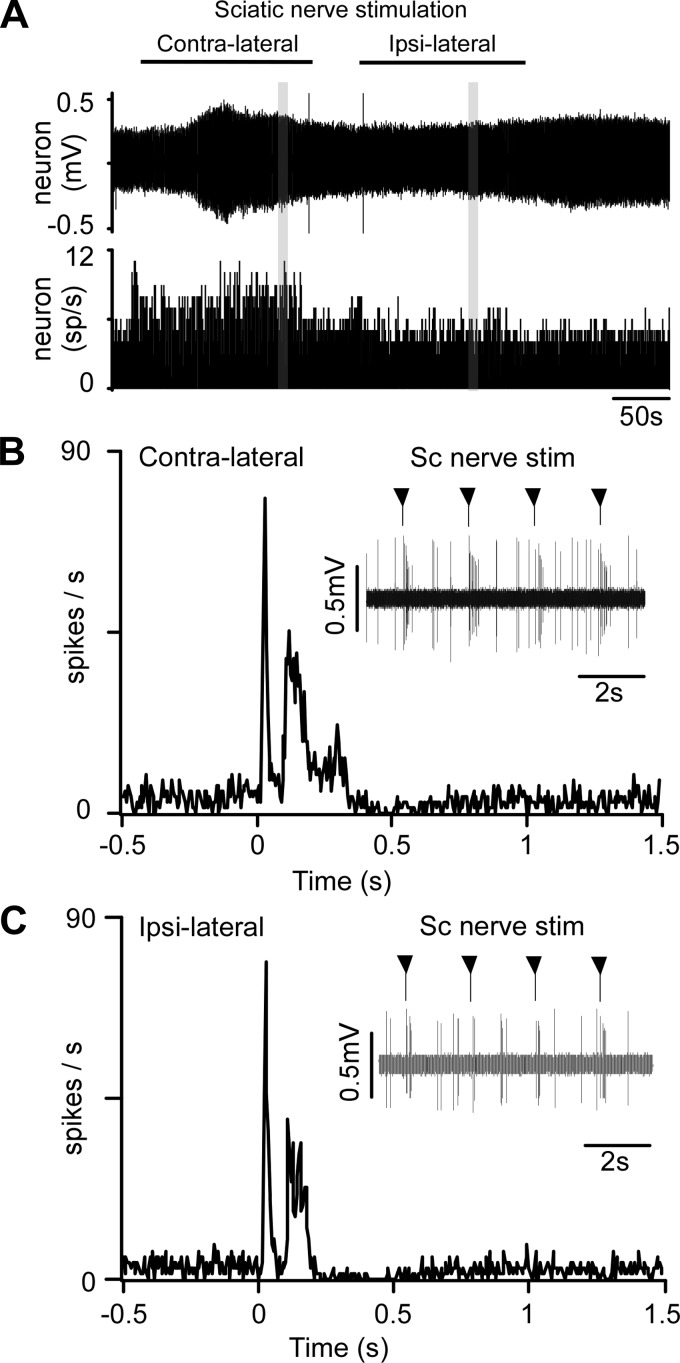
Activation of a presympathetic neuron by sciatic nerve stimulation. A, top: original trace of recorded single-unit (stimulation artifacts eliminated). Individual action potentials cannot be discriminated at this slow timescale. Bottom: integrated rate histogram showing the minimal effect of sciatic nerve stimulation on the mean discharge rate of the unit. B, top: expanded timescale excerpt of the unit's response to contralateral sciatic nerve stimulation (artifact suppressed, stimuli delivered at arrowheads). Bottom: poststimulus time histogram (stimuli delivered at time 0, artifacts eliminated) showing bimodal activation of neuron. C: response of the same unit to stimulation of the ipsilateral sciatic nerve (presentation as in B).
Every presympathetic neuron was activated by contralateral sciatic stimulation. In most neurons (20/25) a short-onset latency response (<40 ms) was observed. In the rest (5/25) the onset of the earliest response was between 70 and 90 ms. Most cells (22/25 subjected to contralateral stimulation and 14/16 subjected to ipsilateral stimulation) also had a second, longer-latency activation (Table 1). Occasionally the late response was itself multiphasic (Fig. 7B), but we analyzed it as one. The short-latency activation was generally less intense and shorter lasting than the long-latency activation (Table 1). Contra- and ipsilateral sciatic stimulation evoked very similar responses in terms of latency and amplitude (Fig. 7, B and C; Table 1). The degree of activation (maximum evoked firing rate during a given peak of activation) was positively correlated with the resting firing rate for peak 1 (contralateral stimulation r2 = 0.56, P < 0.0001 and ipsilateral stimulation r2 = 0.47, P = 0.0033) and peak 2 (contralateral r2 = 0.26, P = 0.0144 and ipsilateral r2 = 0.61, P = 0.001). The latency, duration, and intensity of the responses were the same when sciatic nerve stimulation was applied during inspiration or expiration (data not shown).
Table 1.
Characteristics of C1 and RTN neuronal responses to sciatic nerve stimulation
| C1 Neurons |
RTN Neurons |
|||||
|---|---|---|---|---|---|---|
| Contralateral stim | Ipsilateral stim | Contralateral stim | Contralateral stim | Ipsilateral stim | Ipsilateral stim | |
| eeCO2, % | 7.6 ± 0.4 | 7.5 ± 0.5 | 4.2 ± 0.1 | 6.8 ± 0.3 | 4.3 ± 0.2 | 7.0 ± 0.3 |
| n cells recorded | 25 | 16 | 19 | 15 | 7 | 6 |
| Baseline frequency, Hz | 6.0 ± 1.1 | 5.8 ± 1.3 | 1.0 ± 0.4 | 6.9 ± 1.4 | 1.0 ± 0.5 | 6.7 ± 2.6 |
| n cells with first peak (%) | 25 (100%) | 16 (100%) | 15 (79%) | 15 (100%) | 5 (71%) | 6 (100%) |
| Onset, ms | 28 ± 5 | 27 ± 6 | 88 ± 30 | 55 ± 14 | 79 ± 63 | 64 ± 26 |
| Duration, ms | 39 ± 4 | 37 ± 4 | 123 ± 36 | 121 ± 42 | 116 ± 52 | 133 ± 46 |
| Maximum time, ms | 44 ± 7 | 38 ± 8 | 122 ± 41 | 85 ± 25 | 121 ± 95 | 93 ± 31 |
| Maximum, Hz | 56 ± 9 | 47 ± 9 | 25 ± 7 | 26 ± 4 | 53 ± 23 | 24 ± 10 |
| Activation, % | 87 ± 16 | 67 ± 13 | 56 ± 12 | 77 ± 27 | 55 ± 26 | 53 ± 19 |
| n cells with second peak (%) | 22 (88%) | 14 (88%) | 6 (32%) | 7 (47%) | 4 (57%) | 4 (67%) |
| Onset, ms | 113 ± 12* | 117 ± 15* | 113 ± 25 | 136 ± 33 | 101 ± 3 | 141 ± 46 |
| Duration, ms | 243 ± 33* | 277 ± 64* | 219 ± 75 | 234 ± 59 | 270 ± 64 | 241 ± 109 |
| Maximum time, ms | 179 ± 13* | 178 ± 34* | 138 ± 23 | 184 ± 36 | 184 ± 45 | 213 ± 93 |
| Maximum, Hz | 49 ± 8 | 48 ± 9 | 46 ± 15 | 32 ± 8 | 40 ± 16 | 27 ± 12 |
| Activation, % | 555 ± 111* | 461 ± 93 | 215 ± 94 | 179 ± 67 | 159 ± 39 | 255 ± 200 |
Percent activation was calculated by subtracting the number of spikes during peak activation from the number of spikes during baseline for the same duration and dividing by the number of sweeps (stimulation). When possible, cells were tested under low and high end-expiratory CO2 (eeCO2) level and with contra- and ipsilateral sciatic stimulation. In certain cases, the first peak has a delayed onset; nevertheless, it was grouped with the first peak data.
P < 0.05 vs. peak 1.
Sciatic nerve stimulation activates RTN neurons.
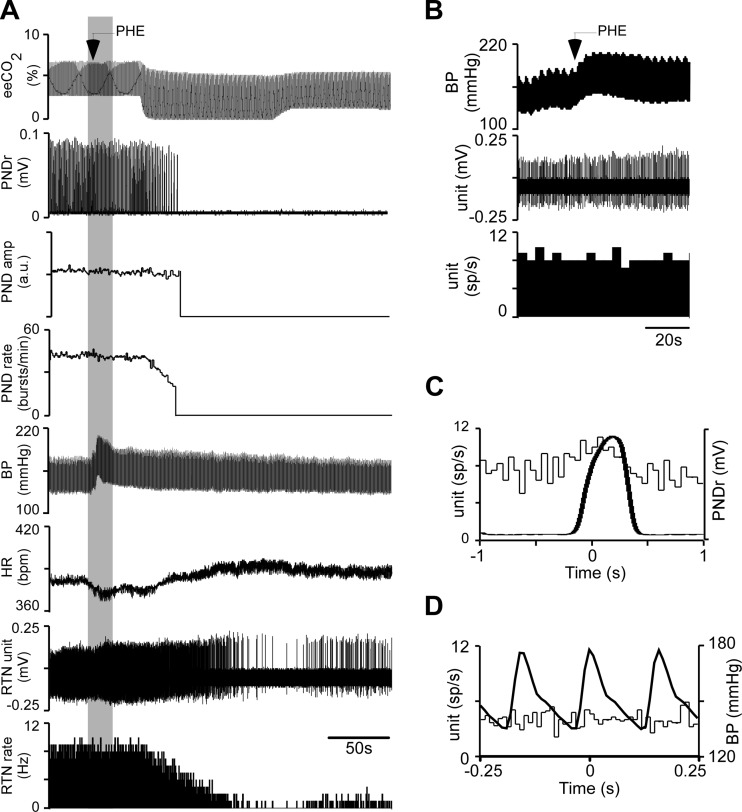
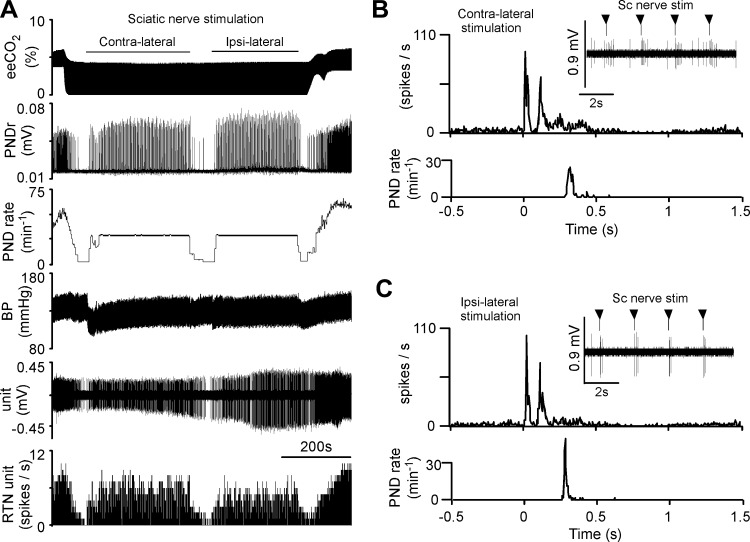
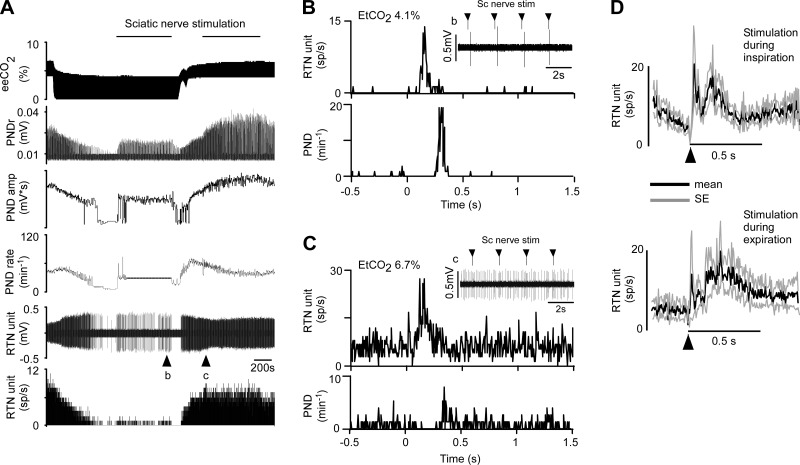
Every RTN neuron tested (n = 29) was activated by sciatic nerve stimulation (contralateral stimulation, n = 25; ipsilateral stimulation, n = 11). The neuron illustrated in Fig. 8 was silenced by lowering eeCO2 before stimulation was applied. This neuron was activated about equally by stimulating the ipsilateral or contralateral sciatic nerves. Characteristically for animals in central apnea, each stimulus elicited a single phrenic burst, resulting in a flat PND rate at the stimulation rate of 30 min−1 (0.5 Hz; Fig. 8A). At low eeCO2 PND onset occurred 391 ± 51 ms after the stimulus (n = 13 rats; 4.3 ± 0.1%). Sciatic nerve stimulation elicited in this neuron a biphasic response (Fig. 8, B and C). This type of response was observed in a majority of RTN neurons, particularly when eeCO2 was elevated and RTN neurons were active in the absence of stimulation (Table 1); in the other neurons, the evoked response consisted of a single peak (Fig. 9; Table 1).
Fig. 8.
Activation of an RTN neuron by sciatic nerve stimulation. A: sciatic nerve stimulation during period of low eeCO2. From top to bottom: eeCO2, rectified/smoothed PND, PND rate (bursts/min), BP, RTN unit, and unit's discharge rate. Contra- and ipsilateral sciatic nerve stimulated at 0.5 Hz. B, from top to bottom: higher-resolution excerpt of the unit's response to contralateral sciatic nerve stimulation (stimuli at arrowheads), poststimulus time histogram (stimuli delivered at time 0; stimulus artifacts eliminated) of the neuronal activity showing bimodal activation (latencies: 40 ms for early peak, 100 ms for late peak), and poststimulus time histogram of the onset of the PND evoked by nerve stimulation. C: response of the same unit to stimulation of the ipsilateral sciatic nerve (presentation as in B; 45-ms and 95-ms latency for early and late peaks, respectively).
Fig. 9.
Effect of sciatic nerve stimulation on RTN neuron firing rate at low and high CO2. A: traces from top to bottom and abbreviations as in Fig. 8. Sciatic nerve stimulation (0.5 Hz) was delivered during a period of low (4%) and high (6.4%) eeCO2. At 4% eeCO2, the neuron was silent in the absence of stimulation. B: from top to bottom: higher-resolution excerpt of the unit's response to contralateral sciatic nerve stimulation (stimuli at arrowheads; stimulus artifacts eliminated), poststimulus time histogram (stimuli delivered at time 0) of the neuronal activity showing bimodal activation, and poststimulus time histogram of the onset of the PND evoked by nerve stimulation. C: response of the same unit to sciatic nerve stimulation during period of elevated eeCO2 (presentation as in B). D: average response (mean ± SE) evoked in 7 RTN neurons by sciatic nerve stimulation during inspiration (top) or during expiration (bottom). Stimulus delivered at arrowhead; stimulation artifacts eliminated.
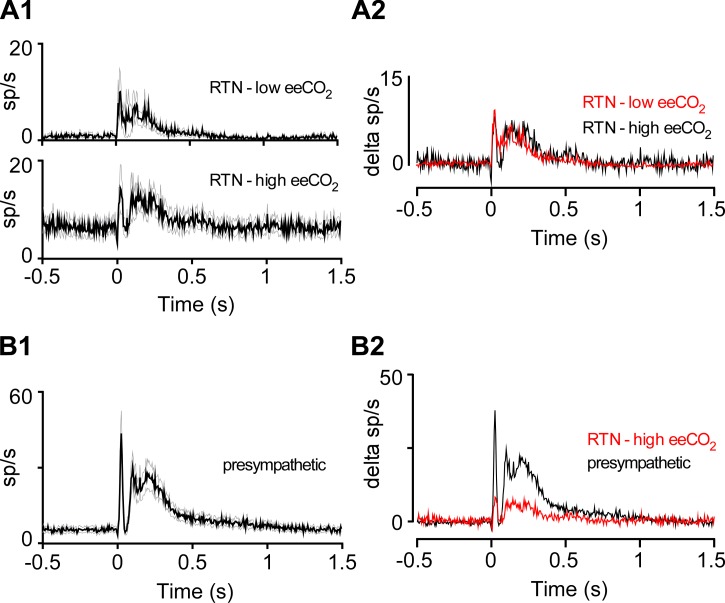
Although sciatic nerve stimulation commonly entrained the PND, the neuronal response evoked by sciatic nerve stimulation seemed too brief and sharply delineated (Fig. 8 and Fig. 9) to be caused by the respiratory modulation of these RTN neurons. To test this assertion, we compared the responses evoked by sciatic nerve stimulation during periods of low (∼4%) vs. high (∼6.7%) eeCO2. In the first case, nerve stimulation faithfully entrained the PND (Fig. 8B and Fig 9B). By contrast, during hypercapnia the stimulation rate was below the resting frequency of the central pattern generator, which disrupted and slowed the breathing rhythm and resulted in poor PND entrainment to the stimuli (Fig. 9C). Yet, under such conditions, sciatic nerve stimulation of RTN neurons still produced a sharp evoked response with peak and latency characteristics very similar to those observed under hypocapnic conditions (Fig. 9). The high vs. low eeCO2 test was carried out in nine RTN neurons. In five cases, the cell was activated to the same extent at high and low eeCO2; the remaining four neurons were only activated at high eeCO2. The rest of the RTN neurons were tested only at low (n = 10) or high (n = 6) eeCO2. The average response evoked at low eeCO2 by contralateral sciatic nerve stimulation in 19 RTN neurons is represented in Fig. 10A and compared to the average response elicited in 15 neurons recorded at high eeCO2. Despite a higher activity at rest, the averaged response evoked by sciatic nerve stimulation was unchanged in hypercapnia. The averaged response evoked in 25 presympathetic neurons is also shown in Fig. 10B to highlight its similarity with the response of RTN neurons to the same stimulus. Additional details regarding the latencies, amplitude, and duration of the responses evoked in RTN and presympathetic neurons by sciatic nerve stimulation are found in Table 1.
Fig. 10.
Sciatic nerve stimulation produces larger evoked responses in presympathetic than RTN neurons. A1: average responses (black trace and gray lines: mean and SE) evoked by contralateral sciatic nerve stimulation (delivered at time 0) in RTN neurons at high (n = 15) vs. low (n = 19) eeCO2. A2: evoked responses at high and low eeCO2 superimposed to emphasize similarity. y-Axis indicates change in discharge rate relative to prestimulation baseline. B1: average response evoked in 25 presympathetic neurons by contralateral sciatic nerve stimulation. B2: responses evoked in 25 presympathetic neurons and 19 RTN neurons (high eeCO2, from A1) superimposed to emphasize differences in magnitude of response. y-Axis indicates change in discharge rate relative to prestimulation baseline.
Finally, we examined whether sciatic nerve stimulation evoked a different response in RTN neurons (n = 7) if the stimulus was delivered during the inspiratory phase (∼20 ms after PND onset) as opposed to the expiratory phase of the central pattern generator (0.6–1 s after PND onset). During these tests eeCO2 was 6.9 ± 0.5% and the resting activity of the seven RTN neurons was 5.5 ± 1.4 spikes/s. The biphasic neuronal response was similar regardless of the timing of the stimulus (Fig. 9D).
Sciatic nerve stimulation and CO2 have approximatively additive effects on RTN neuron discharge rate.
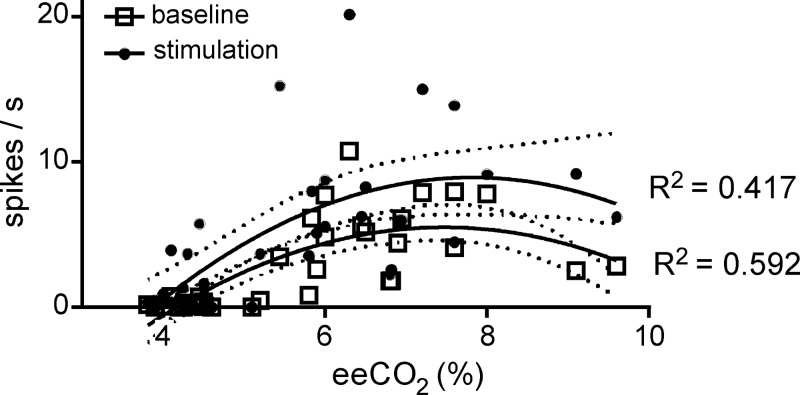
The relation between RTN neuron firing rate and eeCO2 could be modeled by a second-order quadratic equation (y = a + bx + cx2) both at rest (R2 = 0.592) and during sciatic nerve stimulation (R2 = 0.417; Fig. 11), suggesting that the discharge of the neurons is a saturable function of Pco2. The likelihood ratio test (χ2 of 13.02, 2 df, P = 0.00149) revealed that the polynomial model was a better fit for the data than a linear model.
Fig. 11.
Sciatic nerve stimulation shifts the relationship between RTN firing rate and eeCO2 upward. Firing rate of all recorded RTN neurons before (open squares) and during 5-min sciatic nerve stimulation at 0.5 Hz (filled circles) plotted as a function of eeCO2. A nonlinear second-order polynomial curve was fitted with the least squares method. Coefficients of regression are indicated, and 95% confidence intervals are depicted by dotted lines.
To test that the mean discharge rate of the neurons was elevated by the stimulation, we performed a Wilcoxon matched-pairs signed-rank test (2 tailed) with all data points (N = 36 pairs of recordings at low and high CO2). This test revealed a highly significant effect of stimulation on RTN neuron mean firing rate [W = 638; median neuronal firing rate in the absence of stimulation: 1.308 (95% CI = 1.663–3.745); median firing rate during 5-min stimulation: 3.795 (95% CI = 3.264–6.649); P < 0.0001].
To test whether there was an interaction between effects of CO2 and sciatic nerve stimulation on RTN neuron firing, we did a square root transform of the firing rate data to meet the data distribution requirements for ANCOVA. This analysis confirmed that there was a significant effect of both CO2 [F(1,33) = 42.88, P < 0.002] and stimulation [F(2,33) = 30.53, P < 0.002], but there was no interaction between these two manipulations on firing rate [F(2,33) = 0.333, P = 0.7192]. This lack of interaction suggests that somatic nerve stimulation may not change the sensitivity of RTN neurons to CO2.
DISCUSSION
This study provides the following novel findings: 1) Sciatic nerve electrical stimulation activates RTN chemoreceptors in anesthetized rats. 2) The activation is weaker than that of nearby presympathetic neurons, but its pattern is similar. 3) RTN activation is not mediated via the respiratory pattern generator. 4) The relationship between RTN firing rate and CO2 level is shifted upward by somatic afferent input.
In short, RTN neurons can be activated by somatic inputs as well as by changes in local tissue Pco2, and we briefly discuss the physiological circumstances under which these somatic inputs could contribute to breathing.
Properties of RTN neurons under Inactin anesthesia.
RTN neurons were readily identifiable under Inactin anesthesia, and their properties were virtually identical to prior descriptions in rats anesthetized with halothane (Guyenet et al. 2005; Mulkey et al. 2004) or chloralose-urethane (Takakura et al. 2006). These defining properties include location under the facial motor nucleus, activation by hypercapnia (recruitment threshold close to 4% eeCO2), insensitivity to acute BP changes, and, in vagotomized preparations, respiratory modulation with activity nadir predominantly in early expiration and/or postinspiration (Guyenet et al. 2005; Mulkey et al. 2004). These cells were intermingled with presympathetic neurons, identified here by their strong barosensitivity and modest respiratory modulation.
The present electrophysiological results are consistent with prior evidence that the majority of active neurons located within the limited region of interest are either C1 bulbospinal neurons or neurons previously defined as RTN based on histological markers (VGLUT2, Phox2B, NK1R receptors, galanin), responses to CO2, and projection pattern (Guyenet et al. 2016; Stornetta et al. 2006). The region probably contains other types of neurons, but these cells were either silent or did not generate recordable action potentials in the preparation used.
Activation of presympathetic neurons by somatic input.
The somato-sympathetic “pressor” reflex elicited under anesthesia is largely mediated by activation of rostral ventrolateral medulla presympathetic neurons (Stornetta et al. 1989). As reported previously (Morrison and Reis 1989), we found that the majority of the presympathetic neurons had a biphasic response to sciatic nerve stimulation. Also as reported previously (McMullan et al. 2008), a fraction of the barosensitive neurons lacked the long-latency response; these putative presympathetic neurons may be specialized in controlling sympathetic efferents traveling within the cervical nerve (McMullan et al. 2008).
RTN activation by somatic input stimulation.
The main new observation of the present study is that RTN neurons were activated by sciatic nerve stimulation. The biphasic pattern of activation was very similar in RTN and presympathetic neurons, although the magnitude of the response, measured as the number of spikes elicited per stimulus, was smaller in RTN neurons.
The discharge of RTN neurons is not respiratory phasic, but it is respiratory modulated and this modulation can be quite pronounced, especially when the respiratory pattern generator is strongly activated (Connelly et al. 1990; Guyenet et al. 2005; present results). Because somatic nerve stimulation entrains the respiratory pattern generator (Potts et al. 2005; present study), the activation of RTN neurons that follows each stimulus delivered to the sciatic nerve could a priori have resulted from such entrainment. However, this was clearly not the case because somatic stimulation evoked the same short-latency responses in RTN neurons when sciatic stimulation did not entrain the respiratory pattern generator. This condition was met when eeCO2 was elevated and the duration of the respiratory cycle was shorter than 2 s, the interval between sciatic nerve stimuli. Indeed, entrainment of the respiratory pattern generator to somatic stimuli requires that such stimuli be delivered at a rate greater than the resting frequency of the respiratory network (Potts et al. 2005). The entrainment is disrupted by blocking synaptic transmission in the parabrachial region (Potts et al. 2005), a region that receives heavy projections from laminae I and II of the spinal cord (Bernard et al. 1995; Craig 1995). This result has been logically interpreted as evidence that somatic afferents pace the respiratory generator via projections to this region of the brain. Such projections certainly exist, but the dorsal horn of the spinal cord also innervates the entire ventrolateral medulla (Craig 1995) and the respiratory generator can be robustly entrained by phasic optogenetic activation of RTN neurons (Abbott et al. 2011). Respiratory entrainment by RTN or sciatic nerve stimulation has similar characteristics, requiring that the stimulus be delivered during the expiratory phase of the cycle (Abbott et al. 2011; Potts et al. 2005). In the present series of experiments RTN activation preceded the PND and occurred during the expiratory phase of the cycle (e.g., Fig. 8 and Fig. 9). Accordingly, PND entrainment to sciatic nerve stimulation could conceivably be mediated via RTN activation. Given the high degree of interconnection between the medullary and pontine portions of the brain stem respiratory generator, a conservative interpretation of the available data might be that somatic afferent stimulation entrains the respiratory cycle via several routes simultaneously, one of which may be through RTN.
Potential physiological role of somatic input to RTN neurons.
Our results are compatible with the possibility that RTN receives excitatory inputs from muscle metabotropic receptors and contributes to th e hyperpnea of exercise (Forster et al. 2012; Kaufman 2012). Supporting this notion, lamina I/II neurons receive input from muscle sensory afferents and innervate the ventrolateral medulla inclusive of the RTN region (Craig 1995; Craig and Mense 1983; Jankowski et al. 2013; Kaufman 2012; Wilson et al. 2002). This pathway projects about equally to both sides of the medulla oblongata, which is consistent with RTN responding identically to stimulation of the right or left sciatic nerves. However, this pathway could just as well mediate the breathing stimulation elicited by nociceptive afferents, which also terminate in the most superficial regions of the dorsal horn (Andrew et al. 2003; Basbaum et al. 2009).
The expression of Fos by 18% of RTN neurons (Sagar et al. 1988) after dynamic exercise in rats (Barna et al. 2012, 2014; Iwamoto and Waldrop 1996) gives credence to the hypothesis that RTN could receive input from muscle metabotropic receptors. However, “central command” could provide an alternate explanation (Forster et al. 2012). The central command hypothesis states that a CNS locomotor generator, possibly located within the hypothalamus, activates the lower brain stem respiratory network to produce the hyperpnea of exercise (Eldridge et al. 1981; Forster et al. 2012). RTN neurons receive a strong excitatory input from the hypothalamus, at least partly orexinergic in nature (Fortuna et al. 2009; Lazarenko et al. 2011; Li and Nattie 2010).
In a neonatal brain stem-spinal cord preparation, Phox2b+ “pfRG” neurons, the probable neonatal version of the neurons that we define as RTN (Guyenet et al. 2016; Onimaru et al. 2014), can be entrained by the spinal cord locomotor generator (Le Gal et al. 2014). It is therefore also conceivable that sciatic nerve stimulation activates RTN neurons via this circuit in adult rats.
Finally, the response of RTN neurons to sciatic nerve stimulation could be mediated via a subset of presympathetic neurons. Indeed, selective optogenetic activation of the rostral C1 cells produces a small activation of RTN neurons in anesthetized rats (Burke et al. 2014), and PNMT/VGlut2+ boutons are in close proximity to RTN dendrites (Rosin et al. 2006). A direct or indirect connection between C1 and RTN neurons could explain the similarity between the response of these two neuronal populations to sciatic nerve stimulation.
Conclusion and perspectives.
RTN neurons, like nearby presympathetic neurons, can be strongly activated by stimulating somatic afferents. Multiple types of sensory afferents may contribute to this response, and the pathway(s) recruited by these afferents to excite RTN remain(s) to be identified. A pauci-synaptic connection between somatic sensory afferents and RTN, featuring a direct spinoreticular connection between the dorsal-most laminae of the spinal cord and RTN, probably exists, but more indirect routes via the parabrachial nuclei, periaqueductal gray matter, C1 cells, and/or the nucleus of the solitary tract could also account for the present observations.
Regardless of the exact nature of the connections, the existence of somatic inputs to RTN further underscores that these “central respiratory chemoreceptors” integrate multiple types of information besides responding, directly or in paracrine manner, to the surrounding Pco2 (Gourine et al. 2005; Kumar et al. 2015; Meigh et al. 2013). Local tissue Pco2 likely provides the dominant excitatory drive to RTN neurons under anesthesia and in unanesthetized mammals during non-REM sleep (Basting et al. 2015; Burke et al. 2015; Guyenet et al. 2005, 2016). However, this may not be the case when the metabolic production of CO2 is higher as during waking as opposed to sleeping, during behaviors such as eating or grooming, during cold-induced thermogenesis (Morrison 2011), under the influence of negative emotions, and, above all, while exercising (Forster et al. 2012; Paterson 2014). Such conditions require greater lung ventilation to maintain the equilibrium between CO2 production and elimination. An elevated baseline activity of RTN neurons driven by synaptic inputs, rather than CO2, could potentially contribute to this equilibrium. RTN receives excitatory input from appropriate sources such as the spinal locomotor pattern generator, hypothalamus, sensory afferents (carotid bodies and present results), and brain regions involved in emotional behavior or metabolic regulations (Brust et al. 2014; Fortuna et al. 2009; Guyenet et al. 2016; Kuwaki and Zhang 2010; Le Gal et al. 2014). To minimize the fluctuations of arterial Pco2, RTN should remain highly responsive to CO2 regardless of the absolute level of alveolar ventilation. Such fine-tuning requires that the sensitivity of RTN neurons to CO2 be relatively unaffected by changes in the baseline activity of these neurons, as is indeed observed when the hypothalamus is stimulated (Fortuna et al. 2009) or, in the present case, during somatic nerve stimulation. Consistent with these observations, the pH sensitivity of RTN neurons in vitro is unaffected by the addition of excitatory transmitters such as serotonin, ACh, or orexin (Hawryluk et al. 2012; Lazarenko et al. 2011; Sobrinho et al. 2016).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant RO1 HL-074011 (P. G. Guyenet). R. Kanbar was supported by funding from the Lebanese American University (LAU-2015-04).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
R.K., R.L.S., and P.G.G. conception and design of research; R.K. performed experiments; R.K., R.L.S., and P.G.G. analyzed data; R.K., R.L.S., and P.G.G. interpreted results of experiments; R.K. and R.L.S. prepared figures; R.K., R.L.S., and P.G.G. drafted manuscript; R.K., R.L.S., and P.G.G. edited and revised manuscript; R.L.S. and P.G.G. approved final version of manuscript.
ACKNOWLEDGMENTS
Ben Holloway, University of Virginia, wrote the script to trigger stimulation during expiration and inspiration. We thank Clay Ford, University of Virginia Library, for help with the statistical analysis.
REFERENCES
- Abbott SB, Stornetta RL, Coates MB, Guyenet PG. Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci 31: 16410–16422, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci 29: 5806–5819, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol 109: 966–976, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D, Krout KE, Craig AD. Differentiation of lamina I spinomedullary and spinothalamic neurons in the cat. J Comp Neurol 458: 257–271, 2003. [DOI] [PubMed] [Google Scholar]
- Barna BF, Takakura AC, Moreira TS. Pontomedullary and hypothalamic distribution of Fos-like immunoreactive neurons after acute exercise in rats. Neuroscience 212: 120–130, 2012. [DOI] [PubMed] [Google Scholar]
- Barna BF, Takakura AC, Moreira TS. Acute exercise-induced activation of Phox2b-expressing neurons of the retrotrapezoid nucleus in rats may involve the hypothalamus. Neuroscience 258: 355–363, 2014. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell 139: 267–284, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basting TM, Burke PG, Kanbar R, Viar KE, Stornetta DS, Stornetta RL, Guyenet PG. Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosis. J Neurosci 35: 527–543, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JF, Dallel R, Raboisson P, Villanueva L, Le Bars D. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol 353: 480–505, 1995. [DOI] [PubMed] [Google Scholar]
- Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res 56: 359–369, 1985. [DOI] [PubMed] [Google Scholar]
- Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM. Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep 9: 2152–2165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PG, Abbott SB, Coates MB, Viar KE, Stornetta RL, Guyenet PG. Optogenetic stimulation of adrenergic C1 neurons causes sleep state-dependent cardiorespiratory stimulation and arousal with sighs in rats. Am J Respir Crit Care Med 190: 1301–1310, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PG, Kanbar R, Basting TM, Hodges WM, Viar KE, Stornetta RL, Guyenet PG. State-dependent control of breathing by the retrotrapezoid nucleus. J Physiol 593: 2909–2926, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CA, Ellenberger HH, Feldman JL. Respiratory activity in retrotrapezoid nucleus in cat. Am J Physiol Lung Cell Mol Physiol 258: L33–L44, 1990. [DOI] [PubMed] [Google Scholar]
- Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol 361: 225–248, 1995. [DOI] [PubMed] [Google Scholar]
- Craig AD, Kniffki KD. Spinothalamic lumbosacral lamina I cells responsive to skin and muscle stimulation in the cat. J Physiol 365: 197–221, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD, Mense S. The distribution of afferent fibers from the gastrocnemius-soleus muscle in the dorsal horn of the cat, as revealed by the transport of horseradish peroxidase. Neurosci Lett 41: 233–238, 1983. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie EE. Antagonism of orexin receptor 1 (OX1R) in the retrotrapezoid nucleus (RTN) inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol 587: 2059–2067, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge FL, Millhorn DE, Waldrop TG. Exercise hyperpnea and locomotion: parallel activation from the hypothalamus. Science 211: 844–846, 1981. [DOI] [PubMed] [Google Scholar]
- Forsberg D, Horn Z, Tserga E, Smedler E, Silberberg G, Shvarev Y, Kaila K, Uhlen P, Herlenius E. CO2-evoked release of PGE2 modulates sighs and inspiration as demonstrated in brainstem organotypic culture. Elife 5: e14170, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster HV, Haouzi P, Dempsey JA. Control of breathing during exercise. Compr Physiol 2: 743–777, 2012. [DOI] [PubMed] [Google Scholar]
- Fortuna MG, Stornetta RL, West GH, Guyenet PG. Activation of the retrotrapezoid nucleus by posterior hypothalamic stimulation. J Physiol 587: 5121–5138, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy JF, Missaghi K, Chevallier S, Chartre S, Robert M, Auclair F, Lund JP, Dubuc R. Specific neural substrate linking respiration to locomotion. Proc Natl Acad Sci USA 109: E84–E92, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436: 108–111, 2005. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. Regulation of breathing and autonomic outflows by chemoreceptors. Compr Physiol 4: 1511–1562, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Bayliss DA, Stornetta RL, Ludwig MG, Kumar NN, Shi Y, Burke PG, Kanbar R, Basting TM, Holloway BB, Wenker IC. Proton detection and breathing regulation by the retrotrapezoid nucleus. J Physiol 594: 1529–1551, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA. Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25: 8938–8947, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body's EMTs. Am J Physiol Regul Integr Comp Physiol 305: R187–R204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV, Mulkey DK. KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J Neurosci 32: 16943–16952, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588: 3901–3920, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto GA, Waldrop TG. Lateral tegmental field neurons sensitive to muscular contraction: a role in pressor reflexes? Brain Res Bull 41: 111–120, 1996. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol 109: 2374–2381, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbar R, Stornetta RL, Cash DR, Lewis SJ, Guyenet PG. Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am J Respir Crit Care Med 182: 1184–1194, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP. The exercise pressor reflex in animals. Exp Physiol 97: 51–58, 2012. [DOI] [PubMed] [Google Scholar]
- Kumar NN, Velic A, Soliz J, Shi Y, Li K, Wang S, Weaver JL, Sen J, Abbott SB, Lazarenko RM, Ludwig MG, Perez-Reyes E, Mohebbi N, Bettoni C, Gassmann M, Suply T, Seuwen K, Guyenet PG, Wagner CA, Bayliss DA. Regulation of breathing by CO2 requires the proton-activated receptor GPR4 in retrotrapezoid nucleus neurons. Science 348: 1255–1260, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T, Zhang W. Orexin neurons as arousal-associated modulators of central cardiorespiratory regulation. Respir Physiol Neurobiol 174: 43–54, 2010. [DOI] [PubMed] [Google Scholar]
- Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol 517: 69–86, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko RM, Stornetta RL, Bayliss DA, Guyenet PG. Orexin A activates retrotrapezoid neurons in mice. Respir Physiol Neurobiol 175: 283–287, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gal JP, Juvin L, Cardoit L, Thoby-Brisson M, Morin D. Remote control of respiratory neural network by spinal locomotor generators. PLoS One 9: e89670, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci 25: 6716–6720, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol 588: 2935–2944, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Kaufman MP. Reflex autonomic responses evoked by group III and IV muscle afferents. In: Translational Pain Research: From Mouse to Man, edited by Kruger L, Light AR. Boca Raton, FL: CRC/Taylor & Francis, 2010. [PubMed] [Google Scholar]
- McMullan S, Pathmanandavel K, Pilowsky PM, Goodchild AK. Somatic nerve stimulation evokes qualitatively different somatosympathetic responses in the cervical and splanchnic sympathetic nerves in the rat. Brain Res 1217: 139–147, 2008. [DOI] [PubMed] [Google Scholar]
- Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N. CO2 directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife 22: e01213, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner TA, Morrison SF, Abate C, Reis DJ. Phenylethanolamine N-methyltransferase-containing terminals synapse directly on sympathetic preganglionic neurons in the rat. Brain Res 448: 205–222, 1988. [DOI] [PubMed] [Google Scholar]
- Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J Appl Physiol 110: 1137–1149, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Callaway J, Milner TA, Reis DJ. Glutamate in the spinal sympathetic intermediolateral nucleus: localization by light and electron microscopy. Brain Res 503: 5–15, 1989. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Milner TA, Reis DJ. Reticulospinal vasomotor neurons of the rat rostral ventrolateral medulla: relationship to sympathetic nerve activity and the C1 adrenergic cell group. J Neurosci 8: 1286–1301, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SF, Reis DJ. Reticulospinal vasomotor neurons in the RVL mediate the somatosympathetic reflex. Am J Physiol Regul Integr Comp Physiol 256: R1084–R1097, 1989. [DOI] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7: 1360–1369, 2004. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. Central efferent pathways for cold-defensive and febrile shivering. J Physiol 589: 3641–3658, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. Why do we have both peripheral and central chemoreceptors? J Appl Physiol 100: 9–10, 2006. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li A. Central chemoreceptors: locations and functions. Compr Physiol 2: 221–254, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Ikeda K, Mariho T, Kawakami K. Cytoarchitecture and CO2 sensitivity of Phox2b-positive parafacial neurons in the newborn rat medulla. Prog Brain Res 209: 57–71, 2014. [DOI] [PubMed] [Google Scholar]
- Paterson DJ. Defining the neurocircuitry of exercise hyperpnoea. J Physiol 592: 433–444, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts JT, Rybak IA, Paton JF. Respiratory rhythm entrainment by somatic afferent stimulation. J Neurosci 25: 1965–1978, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C. Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci 31: 12880–12888, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing, 2013. [Google Scholar]
- Rosin DL, Chang DA, Guyenet PG. Afferent and efferent connections of the rat retrotrapezoid nucleus. J Comp Neurol 499: 64–89, 2006. [DOI] [PubMed] [Google Scholar]
- Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci 4: 474–494, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science 240: 1328–1330, 1988. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J Comp Neurol 387: 524–536, 1997. [DOI] [PubMed] [Google Scholar]
- Sobrinho CR, Kuo FS, Barna BF, Moreira TS, Mulkey DK. Cholinergic control of ventral surface chemoreceptors involves Gq/inositol 1,4,5-trisphosphate-mediated inhibition of KCNQ channels. J Physiol 594: 407–419, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Morrison SF, Ruggiero DA, Reis DJ. Neurons of rostral ventrolateral medulla mediate somatic pressor reflex. Am J Physiol Regul Integr Comp Physiol 256: R448–R462, 1989. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Moreira TS, Colombari E, West GH, Stornetta RL, Guyenet PG. Peripheral chemoreceptor inputs to retrotrapezoid nucleus (RTN) CO2-sensitive neurons in rats. J Physiol 572: 503–523, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldrop TG, Bauer RM, Iwamoto GA. Microinjection of GABA antagonists into the posterior hypothalamus elicits locomotor activity and a cardiorespiratory activation. Brain Res 444: 84–94, 1988. [DOI] [PubMed] [Google Scholar]
- Wilson LB, Andrew D, Craig AD. Activation of spinobulbar lamina I neurons by static muscle contraction. J Neurophysiol 87: 1641–1645, 2002. [DOI] [PubMed] [Google Scholar]