Abstract
Background.
Information about the contribution of chronic conditions to disability in the sub-Saharan African older persons is derived from implicit data. We investigated the association of chronic conditions with incident and persistent disability among community-dwelling elderly Nigerians.
Methods.
We followed disability-free participants in a household cluster randomized sample of 2,149 Nigerians, aged 65 years or older, in three waves over 5 years (2003–2009). Disability was measured using culturally adapted tools. Dementia and depression were ascertained using validated interviewer-administered measures. The presence of pain in six sites, angina, systemic hypertension, diabetes, heart and respiratory disease, and vision and hearing impairment were assessed using standardized self-report of clinician diagnoses. Independent predictors of disability were investigated using separate multivariate binomial and multinomial regression models with Bonferroni corrections.
Results.
Among 1,887 disability-free participants, 457 (24.2%) had incident disability over 5 years; there were 234 (12.4%), 177 (9.4%), and 106 (5.6%) new cases in each of the waves. A total of 181 (10.0%) persons had disability persistently. Having a pain condition (relative risk ratio [RRR] = 4.7, 95% confidence interval [CI] = 2.0–11.0), especially when nonlocalizing (RRR = 1.5, 95% CI = 1.0–2.2), was the main predictor of incident disability in the study. Dementia was associated with cumulative deaths over 5 years (RRR = 3.5, 95% CI = 2.3–5.3). There were no significant associations between having a chronic condition and persistent disability following correction for false discovery rates.
Conclusion.
Using direct measurements, musculoskeletal pain appears to be the most disabling condition in this sub-Saharan African elderly cohort surviving for up to 5 years with chronic conditions. Dementia may be associated with early death.
Keywords: Disease burden, Low- and middle-income countries, Disability-adjusted life–years, Years of life lost, Years lived with disability.
The 1990 projections from the World Health Organization (WHO) Global Burden of Disease (GBD) studies (1) suggest that by 2020, an older person living in low- and middle-income countries (LMICs) can expect to spend 50% of their remaining life in disability. Improved data in 2012 (2) and 2015 (3) have not led to a substantial change in the earlier forecast. However, these recent calculations now suggest that the global and regional impact of some conditions, such as dementia and musculoskeletal pain, on disability in older people may have been underestimated previously.
The GBD studies are based on implied, rather than measured, disability indicators extrapolated mostly from studies of incidence and duration of the relevant diseases from across many countries (1). Epidemiological studies estimating the impact chronic physical and neuropsychiatric conditions on directly measured disability in the elderly in LMICs, especially those in sub-Saharan Africa, are growing, but still relatively few (3,4). Therefore, extrapolating results derived from a global pool of studies to LMICs may mask the true state of the experience of older people living with a chronic condition in those countries. Key information from studies of the associations of chronic conditions with measured disability in LMICs is presented in Supplementary Table 1.
An earlier report examining the cross-sectional association of a number of chronic health conditions on self-reported disability suggests that, compared with several physical health problems, depression was associated with a higher level of disability among community elderly Nigerians aged 65 years and older (5,6). That report however did not examine disorder-specific associations of these conditions with new onset or persistent disability. In the present report, we followed the same cohort of elderly Nigerians, who were participants in the Ibadan Study of Aging (ISA), for about 5 years, thereby taking advantage of increased accrual of information. We aimed to estimate the association of several chronic medical, neuropsychiatric, and pain conditions with incident and persistent disability measured using culturally validated tools.
Methods
Sample Selection, Recruitment, and Follow-up
The ISA is a multiwave community-based investigation of the health and well-being of elderly persons living in households spread across the Yoruba speaking communities in South-Western Nigeria. Its methodology has been fully described (7). Briefly, individuals were selected using stratified multistage cluster sampling from eight contiguous states in South-Western Nigeria, inhabited by about 22% of the national population at the time of the study. From 15 strata, based on state and urban versus rural locations, 43 Local Government Areas were selected as primary sampling units. Four secondary sampling units, each consisting of 50–70 housing units, were systematically selected from each primary sampling unit (172 secondary sampling units in total). A census was then conducted within each of the selected secondary sampling units to identify households with persons aged 65 years or older. A random sample of 17 households with at least one such person was selected from each secondary sampling unit. For households with multiple eligible individuals (aged 65 years or older and fluent in Yoruba—the local language), one prospective respondent was selected using a Kish grid (8). Up to five possible calls were made to contact the selected individual for assessment and there was no replacement for those who could not be contacted or who refused to participate in the study.
Baseline assessments were conducted between November 3, 2003 and August 27, 2004. Three annual follow-up waves were implemented in 2007, 2008, and 2009.
Measures
We describe only the assessments in the ISA data collection protocol (7) that are the focus of the present study.
Participants were asked about their age in years, marital status, and the number of years of completed education. We used an inventory of 21 household and personal items such as chairs, radio, television sets, cookers, and iron to classify the socioeconomic status of the participants. Each participant’s status was determined by relating the number of their household possessions to the median number of possessions of the overall sample. Thus, a respondent’s economic status was classified as low if the number of possessions was less than or equal to 0.5 of the median, low-average if it was greater than 0.5–1.0, high-average if more than 1.0–2.0, and high if more than 2.
Measurement of Disability
The Katz Index of Independence in Activities of Daily Living (9,10) was used to assess the ability of the ISA participants to perform activities of daily living independently. It rates the participants’ functional status by the adequacy of performance of six functions: bathing, dressing, toileting, transferring, continence, and feeding.
Instrumental activities of daily living was evaluated by the ability of the participants to perform seven functions in the following areas: climbing a flight of stairs, reaching above the head to carry something weighing about 4.5kg, stooping, gripping small objects with hands, shopping, and activities such as sweeping the floor with a broom or cutting grass. These adapted (11,12) seven items are similar to those described in the International Classification of Functioning, Disability, and Health (ICF) (13). Each of the activities in the two domains was rated: (i) can do without difficulty, (ii) can do with some difficulty, (iii) can do only with assistance, and (iv) unable to do activity. We classified as disabled, any respondent with a rating of 3 or 4 on any item. A subgroup of 37 respondents was assessed twice, about 7 days apart, to assess test–retest reliability of these disability markers. Agreement was generally very good to excellent, with a κ range of 0.65–1.0. Both instruments were translated into the local Yoruba language using the iterative back-translation method. As part of the translation process, they were subjected to cultural adaptation. Thus, for example, in describing 4.5kg in the functional assessment, a tuber of yam (a local staple) of such weight was used.
Ascertainment of Chronic Conditions
Depression was assessed with the fully structured WHO Composite International Diagnostic Interview (CIDI) (14). Diagnosis was made on the basis of the criteria of the Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition (DSM-IV) (15).
For a diagnosis of probable dementia, the adapted 10-Word Delayed Recall Test learning list (10-WDRT) was used to screen at baseline and follow-up. For the learning phase of this test, a list of 10 words, adapted as previously described (16), was read to the participant, who was then asked to list all of the words that they could remember. The test was repeated for a total of three administrations to allow for adequate learning. After approximately 5 minutes, participants were requested to repeat as many of the words as they could recall. A diagnosis of probable dementia was made at baseline in persons who were unable to recall two or more items.
Vision was assessed with the use of self-report questions derived from the WHO multicountry World Health Survey questionnaire (17). Respondents were asked whether “in the past month, and with the use of spectacles, if they wore any, they had difficulty in seeing and recognizing somebody known to them, across the road” (distant vision) or “reading/seeing something held at arm’s length” (near vision). Possible responses were: no difficulty, some difficulty, and marked difficulty. In this report, persons with marked difficulty are classified as being visually impaired. For hearing impairments, respondents were asked to give a “yes” or “no” response to questions about whether they had “difficulty hearing clearly.” For this purpose, interviewers were also required to complete a set of questions reflecting their observation on the participants during the entire ISA interview. One of the items was whether difficulty of hearing had been noted.
Other chronic medical or pain conditions were assessed using a checklist (18). Respondents were asked whether, in the previous 12 months, they had any chronic respiratory conditions (asthma, tuberculosis, and other causes of chronic cough), cardiovascular conditions (high blood pressure or heart disease), angina, and diabetes mellitus. The presence of chronic pain, which was a persistent pain present most of the time for a period of 6 months or more during the previous year, was also ascertained. These included pains in different sites—such as back or neck, chest, stomach or abdomen, joints, headaches, and a residual category of pain which may be nonlocalizing.
Statistical Analyses
Elderly persons who had no disability at baseline (2003/2004) constituted the sample for this analysis. A participant was considered to have been followed up successfully in each of three waves (2007, 2008, and 2009) if they were located and re-assessed for disability. An individual with disability in a preceding wave was excluded from the succeeding wave in exploring incident disability. The characteristics of individuals who were successfully followed up to 2009 were compared with the characteristics of those who were not followed to this end point using the chi-squared test for categorical variables.
We examined the associations between the presence of a chronic condition in 2003/2004 and incident disability during follow-up through multinomial regression models using the entire disability-free participants at baseline. Not having a disability at any of the follow-up waves (2007, 2008, and 2009) was the reference outcome category in each model, while all new onset disability, death, and loss to follow-up were the other outcome categories.
Similarly, we conducted three sets of multinomial regression analyses, one set for incident disability in every wave of follow-up. For these analyses, not having a disability in each wave (2007, 2008, and 2009) was the reference outcome. Age, sex, residence (urban, semi-urban, or rural), educational attainment, and economic status were included as covariates in all models. The exponentials of the coefficients in the multinomial regression analyses, denoted as the relative risk ratios (RRRs) by convention (19,20), represent the risk of the outcome between those with and those without chronic conditions at baseline.
Next, we investigated the relationship between having a chronic condition at baseline and having persistent disability throughout the follow-up waves (2007, 2008–2009). Persistent disability was defined as having disability for the first time in the 2007 wave and having the same or higher ratings of disability in the 2008 and 2009 waves. For this objective, we conducted a multiple logistic regression analysis with persistent disability as the dependent variable. Age, sex, residence, educational attainment, and economic status were also included as covariates in the model. Odds ratios with 95% confidence intervals (CIs) for this analysis are presented.
Data were analyzed using Stata version 13.0 (21). The “svy: mlogit” and “svy: logit” commands were used for the analyses, and a level of alpha less than .05 was set to control for family-wise Type I error rate using the simple Bonferroni corrections. For the corrections, tests involving pain (18 comparisons) were considered one family in each regression model and medical conditions (33 comparisons) were one family.
Results
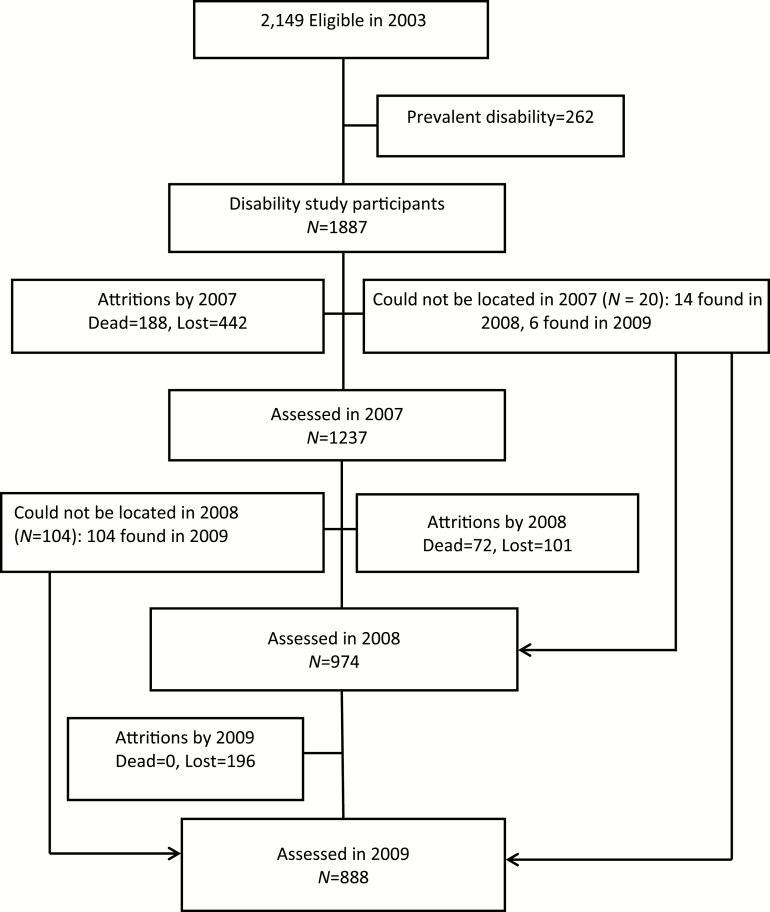
Between November 2003 and August 2004, 2,873 individuals aged 65 years or older were selected for inclusion in the study, with 2,149 (74%) providing data. A total of 1,887 of these participants were free of disability. Figure 1 provides details of study recruitment and follow-up. It also includes the number of eligible participants at each data collection wave, those who died and those who were lost to follow-up. The mean age of those who were followed up was 72.1 years.
Figure 1.
Flow chart for the study.
Table 1 describes the characteristics of participants who were followed up compared with those who were not followed up to the last assessments in 2009.
Table 1.
Characteristics of Subjects by 2009 Follow-up Status
| Characteristics | Not Followed up to 2009 (N = 999) | Followed up to 2009 (N = 888) | χ2 | df | p Value |
|---|---|---|---|---|---|
| Age group | 2.44 | 1 | .086 | ||
| 65–69 | 41.6 | 37.9 | |||
| 70–74 | 26.7 | 32.6 | |||
| 75–79 | 17.0 | 18.2 | |||
| 80+ | 14.7 | 11.3 | |||
| Gender | 0.96 | 1 | .343 | ||
| Male | 57.9 | 60.9 | |||
| Female | 42.1 | 39.1 | |||
| Site | 0.80 | 2 | .460 | ||
| Urban | 25.9 | 23.2 | |||
| Semi-urban | 41.5 | 42.8 | |||
| Rural | 32.6 | 34.0 | |||
| Economic status | 4.14 | 3 | .014 | ||
| Low | 26.9 | 20.1 | |||
| Low-average | 34.3 | 33.8 | |||
| High-average | 27.0 | 30.0 | |||
| High | 11.8 | 16.2 | |||
| Education | 0.57 | 3 | .593 | ||
| 0 | 54.9 | 54.0 | |||
| 1–6 | 24.8 | 25.4 | |||
| 7–12 | 11.7 | 13.4 | |||
| 13+ | 8.7 | 7.1 | |||
| Occupation attained | 0.51 | 2 | .605 | ||
| Elementary | 47.2 | 44.0 | |||
| Trade | 39.4 | 40.8 | |||
| Semiskilled/higher | 13.4 | 15.2 | |||
| Good social engagement | 88.0 | 89.0 | 0.38 | 1 | .546 |
| Weight | 2.72 | 3 | .062 | ||
| Normal weight | 49.9 | 47.6 | |||
| Under weight | 9.1 | 4.6 | |||
| Overweight | 26.6 | 30.0 | |||
| Obese | 14.5 | 17.9 | |||
| Self-reported health | 0.00 | 1 | .953 | ||
| Poor | 4.8 | 4.7 | |||
| Good | 95.2 | 95.3 | |||
| Ever smoked | 43.5 | 44.9 | 0.23 | 1 | .638 |
| Ever drank | 49.0 | 48.8 | 0.01 | 1 | .930 |
Table 2 shows the proportion of chronic conditions in the sample. Also shown in the same table are the weighted proportions of participants with pairs of comorbid medical or pain conditions in the study. Sensory impairments (vision and hearing) and probable dementia were the least comorbid with other medical conditions, while heart disease was the most comorbid with other medical conditions. Among comorbidities investigated, the co-occurrence of heart disease and angina was the most common (Table 2). Arthritis and back/neck pain were the most comorbid with other pain conditions.
Table 2.
The Proportion of Chronic Conditions and the Population With Pairs of Comorbid Conditions in the Sample
| Medical Conditions | Unweighted Number of Observations | No Comorbidity (%) | Plus Any of the Following Medical Conditions (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Angina | Heart Disease | Hypertension | Depression | Probable Dementia | Diabetes | Respiratory Disease | Vision Impairment | Hearing Impairment | |||
| No disease | 913 | — | — | — | — | — | — | — | — | — | — |
| Angina | 250 | 48.0 | — | 5.4 | 11.5 | 14.8 | 3.6 | 2.0 | 17.9 | 19.3 | 2.1 |
| Heart disease | 25 | 6.7 | 57.2 | — | 55.0 | 36.8 | 7.1 | 5.7 | 39.8 | 9.0 | 0.0 |
| Hypertension | 178 | 40.3 | 14.4 | 6.6 | — | 12.5 | 8.6 | 12.1 | 12.4 | 30.6 | 0.5 |
| Depression | 120 | 38.7 | 28.2 | 6.6 | 19.0 | — | 6.1 | 2.6 | 16.5 | 24.7 | 1.0 |
| Probable dementia | 196 | 53.1 | 5.8 | 1.1 | 11.0 | 5.1 | — | 4.2 | 13.7 | 29.0 | 3.9 |
| Diabetes | 41 | 20.1 | 10.8 | 2.9 | 52.1 | 7.5 | 14.3 | — | 12.8 | 37.1 | 1.8 |
| Respiratory disease | 133 | 29.7 | 32.5 | 6.9 | 18.0 | 15.8 | 15.6 | 4.3 | — | 25.8 | 1.5 |
| Vision impairment | 407 | 52.4 | 12.4 | 0.5 | 15.7 | 8.3 | 11.7 | 4.4 | 9.1 | — | 2.6 |
| Hearing impairment | 45 | 51.4 | 14.5 | 0.0 | 2.9 | 3.5 | 16.6 | 2.3 | 5.4 | 27.1 | — |
| Pain Conditions | Unweighted Number of Observations | No Comorbidity (%) | Plus Any of the Following Pain Conditions (%) | ||||
| Arthritis | Back/ Neck Pain | Headaches | Chest Pain | Nonspecific Pain | |||
| No pain | 410 | — | — | — | — | — | — |
| Arthritis | 1,283 | 27.3 | — | 66.4 | 27.3 | 19.2 | 12.4 |
| Back/neck | 953 | 6.4 | 88.6 | — | 34.6 | 25.7 | 15.5 |
| Headaches | 422 | 6.6 | 83.8 | 79.6 | — | 36.9 | 22.2 |
| Chest pain | 280 | 3.1 | 85.6 | 85.9 | 53.5 | — | 26.9 |
| Nonspecific | 201 | 10.9 | 78.2 | 73.5 | 45.7 | 38.2 | — |
Note: “No disease/pain” means the participant did not have any of the conditions in this table. “No comorbidity” means the participant had only the disease in the row of the table and not the diseases in the column. Proportions are for participants with the row diseases.
The association between having comorbid medical and pain conditions with incident or persistent disability is shown in Tables 3 and 4 (also see Supplementary Table 2). By 2007, 234, or 12.4%, of persons who were free of disability at baseline had become disabled (Table 3). In the same table, 117 and 106 incident disability was ascertained in the 2008 and 2009 waves, respectively. Having a pain condition was associated with incident disability in 2008 (RRR = 4.7, 95% CI = 2.0–11.0). Table 4 contains the result of the assessments of incident disability in a single wave of 5 years (2003/2004–2009). In that measurement, we identified 457, or 24.2%, of the participants with incident disability. Having a nonspecific pain condition was the main predictor of disability (Table 4). There were no significant associations between having a chronic disease and persistent disability after Bonferroni corrections (see Supplementary Table 2).
Table 3.
Multivariate Multinomial Regression Showing the Impact of Chronic Conditions on New Onset Disability in Each Follow-up Wave After Censoring Participants With Disability in 2003 and Successive Waves
| RRR (95% CI) for New Onset Disability in Each Wave | ||||||||
|---|---|---|---|---|---|---|---|---|
| Chronic Diseases | 2007 | 2008 | 2009* | |||||
| Disability (N = 234) | Death (N = 188) | Loss (N = 462) | Disability (N = 117) | Death (N = 52) | Loss (N = 225) | Disability (N = 106) | Loss (N = 185) | |
| Pain conditions | ||||||||
| Any pain condition | 0.9 (0.6–1.4) | 1.1 (0.8–1.8) | 1.0 (0.7–1.4) | 4.7 (2.0–11.0)† | 0.6 (0.3–1.3) | 1.0 (0.6–1.6) | 2.2 (0.9–5.3) | 1.6 (0.8–3.2) |
| Arthritis | 0.9 (0.6–1.2) | 1.2 (0.7–1.8) | 1.0 (0.8–1.3) | 2.4 (1.3–4.3) | 0.6 (0.2–1.3) | 0.9 (0.6–1.2) | 1.8 (0.9–3.5) | 1.5 (1.0–2.1) |
| Back/neck pain | 1.1 (0.6–1.9) | 0.9 (0.6–1.3) | 1.1 (0.8–1.5) | 1.6 (0.8–3.4) | 1.0 (0.5–2.1) | 1.0 (0.7–1.5) | 1.2 (0.7–2.1) | 1.2 (0.7–2.1) |
| Headache | 1.0 (0.5–2.0) | 0.9 (0.5–1.6) | 0.9 (0.6–1.3) | 0.7 (0.4–1.2) | 0.3 (0.1–0.9) | 1.1 (0.6–2.0) | 1.7 (0.9–3.3) | 1.6 (1.0–2.5) |
| Chest pain | 1.0 (0.6–1.8) | 1.0 (0.6–1.8) | 0.8 (0.5–1.1) | 0.9 (0.5–1.7) | 0.3 (0.1–0.9) | 0.8 (0.4–1.7) | 0.9 (0.3–2.6) | 1.7 (0.9–3.3) |
| Nonspecific pain | 1.9 (1.2–3.1) | 1.3 (0.8–2.1) | 1.2 (0.7–2.2) | 1.4 (0.6–3.5) | 0.4 (0.1–1.2) | 0.8 (0.3–1.9) | 1.5 (0.8–2.8) | 1.5 (0.8–2.8) |
| Medical conditions | ||||||||
| Any medical condition | 1.0 (0.6–1.8) | 1.7 (1.2–2.6) | 1.0 (0.6–1.5) | 1.0 (0.4–2.2) | 0.5 (0.2–1.1) | 0.8 (0.4–1.3) | 1.7 (0.7–4.0) | 1.8 (1.0–3.1) |
| Angina | 0.9 (0.5–1.8) | 1.0 (0.5–2.0) | 1.0 (0.5–1.8) | 1.1 (0.6–2.3) | 0.3 (0.1–1.0) | 0.6 (0.4–0.9) | 2.1 (0.9–4.8) | 1.4 (0.7–3.0) |
| Heart disease | 0.5 (0.1–2.9) | 1.2 (0.3–4.4) | 2.0 (0.4–9.7) | 2.3 (0.6–8.5) | 1.1e-4‡ | 0.2 (0.0–1.9) | 1.6 (0.3–8.5) | 1.2 (0.4–4.0) |
| Hypertension | 1.1 (0.5–2.4) | 1.9 (1.1–3.3) | 1.6 (1.0–2.6) | 1.0 (0.3–3.2) | 0.9 (0.4–2.5) | 0.8 (0.4–1.5) | 3.5 (1.6–7.6) | 2.3 (1.5–5.1) |
| Depression | 1.1 (0.4–3.0) | 1.9 (0.5–7.5) | 1.4 (0.7–3.1) | 2.0 (0.7–5.1) | 0.3 (0.0–2.0) | 1.4 (0.7–3.1) | 0.9 (0.2–3.7) | 1.0 (0.5–2.4) |
| Probable dementia | 1.6 (0.8–3.5) | 2.9 (1.6–5.4) | 1.5 (0.9–2.4) | 1.2 (0.7–2.0) | 2.4 (0.4–9.2) | 1.1 (0.5–2.8) | 2.1 (0.5–8.9) | 2.9 (0.5–5.5) |
| Diabetes | 1.5 (0.4–6.7) | 3.9 (1.3–12.1) | 1.3 (0.5–3.3) | 2.2 (0.3–15.4) | 4.3e-6‡ | 0.5 (0.1–3.9) | 3.1 (0.9–10.5) | 7.1 (1.4–37.5) |
| Respiratory disease | 0.6 (0.2–1.4) | 2.1 (0.8–5.1) | 0.6 (0.2–1.8) | 0.8 (0.2–2.4) | 0.2 (0.0–1.6) | 0.7 (0.4–1.3) | 0.7 (0.2–3.5) | 0.4 (0.2–1.1) |
| Vision impairment | 1.7 (1.1–2.7) | 1.8 (1.0–3.3) | 0.9 (0.7–1.3) | 0.7 (0.3–1.6) | 0.4 (0.2–1.0) | 1.3 (0.8–2.0) | 0.9 (0.5–1.7) | 1.3 (0.7–2.3) |
| Hearing impairment | 0.7 (0.3–1.9) | 0.9 (0.2–4.5) | 0.8 (0.3–1.9) | 2.4 (0.5–12.5) | 5.1 (0.6–43.2) | 1.9 (0.5–7.4) | 0.1 (0.0–1.3) | 1.0 (0.4–2.6) |
| Comorbid pain and medical conditions§ | 0.9 (0.5–1.9) | 2.0 (1.1–3.6) | 0.9 (0.5–1.7) | 4.1 (1.0–16.4) | 0.2 (0.0–0.7) | 0.8 (0.4–1.4) | 3.3 (0.9–12.0) | 2.6 (1.0–6.6) |
Notes: Each row (including for each wave of follow-up) represents a separate multinomial model; age, gender, education, site, and socioeconomic status were covariates in each model. CI = confidence interval; RRR = relative risk ratio.
*No death in 2009.
†Critical p <.003 after Bonferroni corrections.
‡Numbers too small for meaningful interpretation.
§Participants with at least one medical and one pain condition occurring together.
Table 4.
Multivariate Multinomial Regression Showing the Impact of Chronic Conditions on All New Onset Disability by 2009 After Censoring Participants With Disability at Baseline Only
| Chronic Conditions | Incident Disability by 2009 (N = 457) | Deaths by 2009 (N = 237) | Loss to Follow-up by 2009 (N = 537) |
|---|---|---|---|
| RRR (95% CI) | |||
| Any pain condition | 1.78 (1.20–2.64) | 1.28 (0.87–1.88) | 1.15 (0.80–1.67) |
| Arthritis | 1.48 (1.16–1.88) | 1.21 (0.82–1.77) | 1.13 (0.87–1.47) |
| Back/neck pain | 1.34 (0.94–1.91) | 1.05 (0.82–1.35) | 1.23 (0.93–1.62) |
| Headache | 0.98 (0.61–1.58) | 0.88 (0.57–1.34) | 0.86 (0.56–1.33) |
| Chest pain | 0.87 (0.56–1.33) | 0.77 (0.46–1.26) | 0.66 (0.44–0.98) |
| Nonspecific pain | 1.93 (1.47–2.54) | 1.14 (0.72–1.80) | 1.51 (1.02–2.23)* |
| Any medical conditions | 1.12 (0.67–1.87) | 1.30 (0.84–2.00) | 0.79 (0.57–1.11) |
| Angina | 1.22 (0.68–2.21) | 0.81 (0.43–1.50) | 1.04 (0.65–1.64) |
| Heart disease | 1.06 (0.29–3.80) | 0.91 (0.22–3.76) | 1.90 (0.37–9.76) |
| Hypertension | 1.51 (0.87–2.62) | 1.74 (1.06–2.87) | 1.45 (0.88–2.39) |
| Depression | 1.40 (0.66–2.99) | 1.89 (0.57–6.30) | 1.68 (0.80–3.53) |
| Probable dementia | 1.84 (0.87–3.87) | 3.49 (2.31–5.26)* | 1.72 (1.06–2.80) |
| Diabetes | 1.66 (0.60–4.59) | 2.52 (0.81–7.82) | 0.82 (0.34–1.97) |
| Respiratory disease | 0.62 (0.29–1.33) | 1.38 (0.60–3.16) | 0.53 (0.25–1.10) |
| Vision impairment | 1.13 (0.78–1.64) | 1.29 (0.76–2.19) | 0.92 (0.66–1.27) |
| Hearing impairment | 0.85 (0.32–2.23) | 1.67 (0.38–7.39) | 1.17 (0.44–3.08) |
| Comorbid pain and medical condition† | 1.86 (1.02–3.37) | 1.67 (0.86–3.22) | 0.93 (0.21–2.56) |
Each row represents a separate multinomial model; age, gender, education, site, and socioeconomic status were covariates in each model. CI = confidence interval; RRR = relative risk ratio.
*Critical p <.002 after Bonferroni corrections.
†Participants with at least one medical and one pain condition occurring together.
Discussion
This was a large multiwave investigation of the associations of several chronic conditions with disability in the elderly living in a sub-Saharan African community. We found that having a chronic pain condition, especially when nonlocalizing, was the main predictor of incident disability in the study. According to our findings, dementia, which is often associated with disability in elderly populations across the world, was associated with death within the 5-year period of this study.
These results are consistent with the projected burden of chronic conditions in the elderly living in sub-Saharan Africa (2–4,22–25), with suggestions that musculoskeletal pain may be among the chronic conditions most likely to be associated with years of healthy life lost due to disability, while dementia may be associated with years of life lost due to early death of the elderly in this population. When using “implicit societal weights” and projecting to 2020, the GBD studies suggested that musculoskeletal pain, along with sensory impairment (vision and hearing) may be the highest ranking disorders in terms of years lived with disability in the elderly living in the region (1). On the other hand, a review of surveys, all cross-sectional, using direct measurements of disability across a total of nine LMICs (4,26–31), as well as the previous cross-sectional analyses in the ISA cohort (5,6) suggest that conditions such as depression may also be associated with high levels of disability in countries such as Nigeria.
The plausible effect of reverse causality between, for example, depression and disability in cross-sectional analyses may result in larger sizes of association between these conditions. Similarly, the impact of conditions such as rheumatoid pain, which may be associated with disability overtime (26,31), may be underestimated in cross-sectional investigations. This is because cross-sectional analyses are inadequate in providing robust evidence for the direction of association between the relevant health conditions with disability overtime. In the present study, we have investigated the association of several chronic conditions on functional limitation assessed in multiple waves over a period of 5 years. We think that this approach, which may be qualitatively similar to the conceptual framework of the “years lived with disability” as approximated in the GBD studies, can provide a more direct estimate of the differential impact of those health conditions on disability in the short and medium terms. For instance, short-term relapse and remitting disability, which occurs in some categories of depression (32), may be qualitatively different from the chronically unremitting disability that may be associated with many causes of back pain.
Given these possibilities, the absence of an association between, for example, depression and disability in the present analyses may be understandable in terms of the heterogeneous nature of the syndrome. Some clinical depressions may be transient, others may be recurrent, while another fraction may be chronic or intractable (32). Therefore, since we have investigated the association of the conditions with disability over a period of 3–5 years, we may have systematically underestimated the effect of depression occurring for a shorter period of time. In the same way, we may have identified larger associations for chronic pain conditions which are often associated with more sustained levels of disability (33).
There are several possible reasons for our inability to demonstrate an association between the chronic conditions in this study and persistent disability. First, because of high numbers of those who died or were lost to follow-up, only 10% of the disability-free sample met criteria for persistent disability. This may have affected the power to find a difference between the group with persistent disability and those that could not be described as having such dimensions of disability in the present study. In line with this observation, we note that while dementia was not statistically associated with new onset or persistent disability, it was associated with the cumulative deaths over the 5-year period of the study. Perhaps many of the participants with dementia had died before they were due for follow-up assessments for functional limitations.
Second, we have considered the association of chronic diseases ascertained at baseline only, with persistence of disability over the follow-up period. A high incidence of some of the chronic conditions in the studied population, for example, depression (34), which is also known to be associated with disability, may reduce the power to find a statistical difference in disability ratings during follow-up, between those with or without such diseases at baseline. This is also true for conditions such as heart diseases, which may be characterized by sudden and unexpected onset and disability during follow-up. In this way, many participants not reporting conditions characterized by high incidence rates in 2003/2004 may still end up developing those conditions and, perhaps, the associated disability during the follow-up waves.
We have identified some of the chronic conditions in this cohort by the method of clinical assessments, and others by self-reports of clinician diagnoses. While the use of self-reports is a valid methodology (18), we note that in settings such as sub-Saharan Africa where access to quality healthcare may not be universal, individuals who are better educated and have higher economic power may be more likely to obtain information about their health status and therefore provide more reliable self-report of their health. On the other hand, the possibility exists in these settings that a greater disease burden may be present in the more socioeconomically deprived participants, reflecting differences in access to quality health care. Whereas, we did not find a systematic difference in educational attainment between those who were followed up to the end point of 2009 and those who were not, those who dropped out belonged to the lowest socioeconomic category. In all, the combination of our reliance on self-report for some of the conditions as well as the problem of differential attrition may have led to an underestimation of the disability associated with many of the diseases investigated. However, because we did not find a significant difference between the self-reported health status of those who were followed up to the end point of the study and those who were not, we were unable to make accurate inferences about the direction and magnitude of any biases that might have been due to differential attrition in the present study. On the other hand, our sample selection procedure, spread over a wide geographical area, would suggest that the findings from this study may be generalizable to other sub-Saharan African communities.
In conclusion, musculoskeletal pain appears to be the most disabling among chronic conditions in older persons surviving for up to 5 years in these sub-Saharan African communities. Dementia, which has been associated with disability in elderly populations across the world, led to early death in this sample. Thus, reducing the contribution of dementia to years of healthy life lost due to disability in this population, but increasing its contribution to years of life lost due to earlier death in older adults. This would suggest that musculoskeletal pain and dementia carry a greater level of composite burden among chronic conditions in the Nigerian older persons. This finding is in keeping with the projected impact of chronic conditions in the sub-Saharan African elderly people, and unlikely to be different from what would be obtained in other communities in the region.
Supplementary Material
Supplementary data is available at The Journals of Gerontology, Series A: Biomedical Sciences and Medical Sciences online.
Funding
This work was supported by the Wellcome Trust (grant no: WT079662MF)
Conflict of Interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Supplementary Material
Acknowledgments
We would like to thank the participants for their involvement in this study, the data collection team, and three anonymous reviewers for their helpful comments and suggestions.
References
- 1. Murray C, Lopez A. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard School of Public Health; 1996. [Google Scholar]
- 2. Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi:10.1016/S0140-6736(12)61689-4 [DOI] [PubMed] [Google Scholar]
- 3. Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi:10.1016/S0140-6736(15)60692-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sousa RM, Ferri CP, Acosta D, et al. Contribution of chronic diseases to disability in elderly people in countries with low and middle incomes: a 10/66 Dementia Research Group population-based survey. Lancet. 2009;374:1821–1830. doi:10.1016/S0140-6736(09)61829-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gureje O, Ademola A, Olley BO. Depression and disability: comparisons with common physical conditions in the Ibadan Study of Aging. J Am Geriatr Soc. 2008;56:2033–2038. doi:10.1111/j.1532-5415.2008.01956.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gureje O, Ogunniyi A, Kola L, Afolabi E. Functional disability in elderly Nigerians: results from the Ibadan Study of Aging. J Am Geriatr Soc. 2006;54:1784–1789. doi:10.1111/j.1532-5415.2006.00944.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gureje O, Kola L, Afolabi E. Epidemiology of major depressive disorder in elderly Nigerians in the Ibadan Study of Ageing: a community-based survey. Lancet. 2007;370:957–964. doi:10.1016/S0140-6736(07)61446-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kish L. A procedure for objective respondent selection within the household. J Am Stat Assoc. 1949;247:380–387. [Google Scholar]
- 9. Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/14044222 [DOI] [PubMed] [Google Scholar]
- 10. Ciesla JR, Shi L, Stoskopf CH, Samuels ME. Reliability of Katz's Activities of Daily Living Scale when used in telephone interviews. Eval Health Prof. 1993;16:190–203. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10125776 [DOI] [PubMed] [Google Scholar]
- 11. Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–145. [DOI] [PubMed] [Google Scholar]
- 12. Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/137366 [PubMed] [Google Scholar]
- 13. World Health Organization. International Classification of Functioning Disability and Health. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 14. Kessler RC, Ustün TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res. 2004;13:93–121. Retrieved from http://www.ncbi.nlm.nih.gov/ pubmed/15297906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders: DSM-IV Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 16. Gureje O, Ogunniyi A, Kola L, Abiona T. Incidence of and risk factors for dementia in the Ibadan Study of Aging. J Am Geriatr Soc. 2011;59:869–874. doi:10.1111/j.1532-5415.2011.03374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ustun TB, Chatterji S, Mechbal A, Murray CJL; WHS Collaborating Group. The World Health Surveys. In: Murray CJL, Evans DB, eds. Health Systems Performance Assessment: Debates, Methods, Empiricism. Geneva, Switzerland: World Health Organization; 2003:310. [Google Scholar]
- 18. National Health Statistics. Evaluation of National Health Interview Survey diagnostic reporting. Vital Health Stat. 1994;120:1–116. [PubMed] [Google Scholar]
- 19. Train K. Discreet Choice Methods With Simulation. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 20. Blizzard L, Hosmer DW. The log multinomial regression model for nominal outcomes with more than two attributes. Biom J. 2007;49:889–902. doi:10.1002/bimj.200610377 [DOI] [PubMed] [Google Scholar]
- 21. Stata Corp. Stata Statistical Software. College Station, TX: StataCorp LP; 2013. [Google Scholar]
- 22. Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–1442. doi:10.1016/S0140-6736(96)07495-8 [DOI] [PubMed] [Google Scholar]
- 23. GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;385:117–171. doi:10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Norman R, Gaziano T, Laubscher R, Steyn K, Bradshaw D; South African Comparative Risk Assessment Collaborating Group. Estimating the burden of disease attributable to high blood pressure in South Africa in 2000. S Afr Med J. 2007;97(8 Pt 2):692–698. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17952226 [PubMed] [Google Scholar]
- 25. Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385:549–562. doi:10.1016/S0140-6736(14)61347-7 [DOI] [PubMed] [Google Scholar]
- 26. Patel KV, Peek MK, Wong R, Markides KS. Comorbidity and disability in elderly Mexican and Mexican American adults: findings from Mexico and the southwestern United States. J Aging Health. 2006;18:315–329. doi:10.1177/0898264305285653 [DOI] [PubMed] [Google Scholar]
- 27. Acosta D, Rottbeck R, Rodríguez JG, et al. The prevalence and social patterning of chronic diseases among older people in a population undergoing health transition. A 10/66 Group cross-sectional population-based survey in the Dominican Republic. BMC Public Health. 2010;10:344. doi:10.1186/1471-2458-10-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arias-Merino ED, Mendoza-Ruvalcaba NM, Ortiz GG, Velázquez-Brizuela IE, Meda-Lara RM, Cueva-Contreras J. Physical function and associated factors in community-dwelling elderly people in Jalisco, Mexico. Arch Gerontol Geriatr. 2012;54:e271–e278. doi:10.1016/j. archger.2012.02.010 [DOI] [PubMed] [Google Scholar]
- 29. Llibre Jde J, Valhuerdi A, Calvo M, et al. Dementia and other chronic diseases in older adults in Havana and Matanzas: the 10/66 study in Cuba. MEDICC Rev. 2011;13:30–37. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22143605 [DOI] [PubMed] [Google Scholar]
- 30. Hairi NN, Bulgiba A, Mudla I, Said MA. Chronic diseases, depressive symptoms and functional limitation amongst older people in rural Malaysia, a middle income developing country. Prev Med. 2011;53:343–346. doi:10.1016/j.ypmed.2011.07.020 [DOI] [PubMed] [Google Scholar]
- 31. Duba AS, Rajkumar AP, Prince M, Jacob KS. Determinants of disability among the elderly population in a rural south Indian community: the need to study local issues and contexts. Int Psychogeriatr. 2012;24:333–341. doi:10.1017/S1041610211001669 [DOI] [PubMed] [Google Scholar]
- 32. Nuevo R, Leighton C, Dunn G, et al. Impact of severity and type of depression on quality of life in cases identified in the community. Psychol Med. 2010;40:2069–2077. doi:10.1017/S0033291710000164 [DOI] [PubMed] [Google Scholar]
- 33. Bryant LL, Grigsby J, Swenson C, Scarbro S, Baxter J. Chronic pain increases the risk of decreasing physical performance in older adults: the San Luis Valley Health and Aging Study. J Gerontol A Biol Sci Med Sci. 2007;62:989–996. Retrieved from http://www.ncbi.nlm.nih.gov/ pubmed/17895437 [DOI] [PubMed] [Google Scholar]
- 34. Gureje O, Oladeji B, Abiona T. Incidence and risk factors for late-life depression in the Ibadan Study of Ageing. Psychol Med. 2011;41:1897–1906. doi:10.1017/S0033291710002643 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.