Abstract
The receptor for advanced glycation end products (RAGE) is a multi-ligand receptor. Alternative splicing and enzymatic shedding produce soluble forms that protect against damage by ligands including Advanced Glycation End products (AGEs). A link between RAGE and oxygen levels is evident from studies showing RAGE-mediated injury following hyperoxia. The effect of hypoxia on pulmonary RAGE expression and circulating sRAGE levels is however unknown. Therefore mice were exposed to chronic hypoxia for 21d and expression of RAGE, sheddases in lungs and circulating sRAGE were determined. In addition, accumulation of AGEs in lungs and expression of the AGE detoxifying enzyme GLO1 and receptors were evaluated.
In lung tissue gene expression of total RAGE, variants 1 and 3 was elevated in mice exposed to hypoxia, whereas mRAGE and sRAGE protein levels were decreased. In the hypoxic group plasma sRAGE levels were enhanced. Although the levels of pro-ADAM10 were elevated in lungs of hypoxia exposed mice, the relative amount of the active form was decreased and gelatinase activity unaffected. In the lungs, the RAGE ligand HMGB1 was decreased and of the AGEs, only LW-1 was increased by chronic hypoxia. Gene expression of AGE receptors 2 and 3 was significantly upregulated.
Chronic hypoxia is associated with downregulation of pulmonary RAGE protein levels, but a relative increase in sRAGE. These alterations might be part of the adaptive and protective response mechanism to chronic hypoxia and are not associated with AGE formation except for the fluorophore LW-1 which emerges as a novel marker of tissue hypoxia.
Keywords: Hypoxia, RAGE expression, shedding, AGEs, detoxification
Introduction
The receptor for advanced glycation end products (RAGE) is a transmembrane, multi-ligand, pattern-recognition receptor belonging to the immunoglobulin super family of cell surface receptors [1, 2]. The wide variety of ligands include pathogen and damage-associated molecular pattern molecules (PAMPs and DAMPs) such as high mobility group B1 (HMGB1) and advanced glycation end products (AGEs) after which it was named [3]. AGEs are a class of compounds formed by non-enzymatic glycation and oxidation of proteins and lipids. Binding of AGEs and other ligands to RAGE causes activation of downstream signaling pathways, leading to the activation of the pro-inflammatory transcription factor nuclear factor kappa B (NF-κB) [4]. Ligand accumulation and engagement in turn upregulates RAGE expression [5].
Structurally RAGE consists of an extracellular, a transmembrane, and cytosolic domain (mRAGE). In addition, soluble forms exist, which act as decoy receptors since they display similar affinity for ligands, and thereby limit their availability to mRAGE [6]. Soluble forms are generated by two distinct mechanisms. Firstly, by alternative splicing of the AGER gene, which leads to the formation of endogenous soluble RAGE (esRAGE) [7, 8]. Secondly, ectodomain shedding of mRAGE by sheddases like Disintegrin and Metalloproteinase domain Containing protein-10 (ADAM10) and matrix metalloproteinase 9 (MMP9) generates soluble RAGE (sRAGE), which lacks both the transmembrane and cytosolic domain [6, 9].
RAGE is highly expressed in the mature lung by type I pneumocytes (ATI) compared to other lung cells and other tissues [10]. As a pattern recognition receptor, RAGE has been implicated in the pathogenesis of multiple (chronic) pulmonary inflammatory conditions such as chronic obstructive pulmonary disease (COPD), acute lung injury (ALI)/adult respiratory distress syndrome (ARDS) and lung cancer [11-16]. Specific associations with lung development, immune responses, inflammation and tissue remodeling have been described. For instance embryonic overexpression of RAGE in mice leads to under development of the lungs, while overexpression in adult animals was shown to lead to airspace enlargement and a pro-inflammatory state [17, 18].
In lungs of healthy smokers, patients with COPD and mice chronically exposed to cigarette smoke, increased expression of RAGE was shown [19-21]. Bronchoalveolar lavage fluid (BALF) levels of sRAGE were also found to be increased in COPD [22]. Increased BALF sRAGE levels were also shown in ARDS, a disease associated with damage to the ATI cell, but here in the absence of expression changes [23, 24]. Plasma levels of sRAGE were found to be affected as well in COPD and ARDS, albeit in COPD in the opposite direction as at the local level [15, 23]. Plasma sRAGE measurements are currently receiving ample attention as a potential biomarker of disease in general and of lung damage in particular.
Systemic hypoxia is a consequence of a number of pulmonary diseases. Links between RAGE and oxygen levels are evident from a study that showed enhanced RAGE expression in lung tissue by hyperoxia and protection from hyperoxia-induced inflammation, injury and mortality in RAGE deficient mice compared to wild type mice [25]. Furthermore, in our studies patients with COPD treated with oxygen displayed lower plasma sRAGE levels compared to patients not receiving this treatment [15, 26]. In contrast to enhanced pulmonary RAGE expression in response to hyperoxia, it is unknown if hypoxia influences lung RAGE expression, and if this might reflect in alterations in circulating sRAGE levels. Understanding the effect of different insults on RAGE expression in lung tissue is important, given the fact that RAGE plays an important role in pulmonary physiology as well as pathophysiology. Moreover, plasma levels of sRAGE are considered as biomarkers in various pulmonary conditions. A recent study furthermore showed that acute hypoxia induced the generation of AGEs by endothelial cells, which by engagement with RAGE elicited tissue injury [27].
The present study thus investigated the effect of chronic hypoxia on plasma sRAGE levels and the expression of RAGE, its splice variants and sheddases as well as on the accumulation of AGEs in lung tissue of mice.
Materials and methods
Animal handling and treatment
Fifty two week old male C57BL/6J mice (Charles River Laboratories) were exposed to ambient air (normoxia, n = 8) or chronic hypoxia (n = 8) for 21 days. Mice of this age were used as it is a more relevant age to study the effects of hypoxia in relation to lung diseases which occur at middle to advanced age in humans. All mice were housed in experimental chambers at 21°C with a 12-h dark/light cycle. Mice received standard chow (V1534–000 ssniff R/M-H, ssniff Spezialdiäten, Soest, Germany) and water ad libitum. Using the proOX P110 (BioSpherix, Lacona, NY, USA) system, O2 was replaced by N2 in a stepwise manner to create normobaric oxygen levels of 12% (day 1), 10% (day 2), and finally 8% (60.8 mmHg) on day 3. The later oxygen concentration was maintained until day 21. Three to four mice were housed per cage. Daily food intake was determined per cage, and mice were weighed daily. On day 21, mice were anesthetized with isoflurane gas, the abdominal cavity was opened and aortic blood was collected into a heparin-coated 1ml syringe (Becton Dickinson, Breda, The Netherlands). Blood gas and pH were measured immediately using the ABL 510 Blood Gas Analyzer (Radiometer; Diamond Diagnostics, Holliston, MA) and blood cell count was determined with the Coulter Ac T Diff hematology Analyzer (Beckman Coulter, Woerden, The Netherlands). Plasma was stored at −80°C until further analyses. The lungs were snap frozen in liquid nitrogen and stored immediately at −80°C. The protocol was approved by the Committee for Animal Care and Use of Maastricht University (project 2009-151).
Quantitative polymerase chain reaction
RNA from total lung tissue was isolated using the RNeasy Mini kit (Qiagen, CA, USA) and form cells using the High Pure RNA isolation kit (Roche). An equal amount of RNA was reverse transcribed to cDNA using the transcriptor cDNA synthesis kit (Roche Applied Sciences, Mannheim, Germany). PCR reactions were performed on an ABI 7900HT real time PCR (ABI 7900HT, Applied Biosystems, Foster city, CA, USA) using Power SYBR® Green PCR Master Mix (Applied Biosystems) and primers listed in Table 1. Relative mRNA expression was calculated using the ΔΔct method with RPL13a as reference gene.
Table 1.
Primer sequences used
| Gene name | Forward 5’-3’ | Reverse 3’-5’ |
|---|---|---|
| mouse | ||
| VEGF-A | CTGTACCTCCACCATGCCAAGT | TCGCTGGTAGACATCCATGAACT |
| VEGF-B | TGCCCATGAGTTCCATGC | CCCAGTTTGATGGCCCCA |
| VEGF-C | TTTAAGGAAGCACTTCTGTGTGT | GTAAAAACAAACTTTTCCCTAATTC |
| VEGF-D | GGTGCTGAATGAGATCTCCC | GCAAGACGAGACTCCACTGC |
| Total RAGE | ACTACCGAGTCCGAGTCTACC | GTAGCTTCCCTCAGACACACA |
| RAGE Variant 1 | AAGCCCTCCTGTCAGCATCAG | GGCCATCACACAGGCTCGAT |
| RAGE Variant 3 | ACCCACCCTAGCCACGGAC | GTCCCCCGGCACCATTCTC |
| ADAM10 | AATCCAAAGTTGCCTCCTCCTAA | GGGTTGCTGAATGGGCTGT |
| GLO1 | ATGACGAGACTCAGAGTTACCACAA | TAGACATCAGGAACGGCAAATCC |
| OST-48 (AGE R1) | CCCCTTCGTGAGGACCTTCCT | CGTCAGGCAGCTTGAACTGGA |
| 80K-H (AGE R2) | TCTACCGGCTTTGCCCCTTC | CTTGTCATGATCAGGGCCAGC |
| Galectin-3(AGE R3) | TTATGGTGTCCCCGCTGGAC | AGGCAAGGGCAGGTCATAGGG |
| RPL13a | CCTGGAGGAGAAGAGGAAAGAGA | TTGAGGACCTCTGTGTATTTGTCAA |
| human | ||
| Total RAGE | AACACCAGCCGTGTGAGTTCA | CCGAGTCCGTGTCTACCAGAT |
| esRAGE | GGCCAACTGCAGGTGAG | TTTTCTGGGGCCTTCCATTC |
| RPL13a | CCTGGAGGAGAAGAGGAAAGAGA | TTGAGGACCTCTTGTGTATTTGTCAA |
Western blot
Lung tissue was lysed in 1xRIPA buffer (Cell signaling technology, Denver, MA, USA) and the total protein content was determined with the DC protein kit (BIO-RAD laboratories Inc, Hercules, CA, USA). Equal amounts of protein were separated on 4-12% SDS PAGE gels, and transferred to a nitrocellulose membrane. The membrane was blocked with 5% nonfat milk in Tris Buffered Saline (TBS) containing 0.1% tween (T), at room temperature (RT) for 1h. Membranes were then incubated overnight with primary antibodies raised against RAGE (1:1000, H300, Santa Cruz Biotechnology, Santa Cruz, CA, USA), ADAM10 (1:1000, generous gift from S. Rose-John, Christian-Albrechts-University), HMGB1 (1:2500, Cell Signaling Technologies) or GAPDH (1:2500, Cell Signaling Technology) in 1% milk dissolved in TBST. Blots were washed 3 times with TBST, and incubated with respective peroxidase-conjugated secondary antibodies at RT for 1 h. After 3 washes with TBST, conjugated peroxidase was detected by chemiluminescence using the Pierce ECL Western blotting substrate (Thermo scientific, Rockford, IL, USA). Densitometric quantitation of Western blots was performed using Quantity One software (BIO-RAD laboratories).
Gelatinase activity assay
0.5mg of protein containing RIPA buffer lung lysate was used from each animal for the measurement of gelatinase activity using the gelatinase assay kit according to the manufacturer's instructions (Enz check, Life Technologies, Leusden, NL).
Determination of Advanced Glycation Endproducts
Insoluble lung proteins (IP) were prepared by homogenization in Chelex-100 (Bio-Rad) treated PBS followed by extraction with each of 2:1 chloroform-methanol, 1 M sodium chloride, 0.5 M acetic acid and 5 μg pepsin/ml 0.5 M acetic acid as previously described for skin (Sell et al., Proc. Natl. Acad. Sci., 93:485-490, 1996). Heme was removed by the procedure of Chang et al. (J. Biol. Chem. 260:7970-7974, 1985) and each pellet was freeze-dried. Approximately 2 mg portion of each IP preparation was enzymatically digested into free amino acids by sequential digestion at 37°C for 24 hrs with each of 0.07 units (1%) elastase (porcine pancrease, Sigma), 56 units collagenase (Type VII, Sigma), 0.7 units pronase (Roche), 1 unit enterokinase (Sigma) and 5 units prolidase (Sigma). The protein content of each digest was determined by the ninhydrin assay assuming 1 mg protein ≈ 10 μmoles leucine equivalents (Moore and Stein, J. Biol. Chem. 211:907-913, 1954). Each digest ~100 μg protein was spiked with isotopocially-labeled internal standards and analyzed (~40 μg) by high pressure liquid chromatography-mass spectrometry (HPLC-MS/MS) as previously described (Fan et al., Free Rad. Biol. Med. 49:847-856, 2010) for glyoxal (G-H1) and methylglyoxal (MG-H1) hydroimidazolones, fructose-lysine (FL), carboxymethyl-lysine (CML) and carboxyethyl-lysine (CEL). Long wavelength fluorophore (LW-1) was assayed by HPLC with fluorescence detection as previously described [28].
ELISA
Plasma sRAGE levels were determined by a mouse specific sandwich ELISA from R&D systems (Minneapolis, MN, USA) according to the manufacturer's instructions.
Cell culture
A549 cells with stable knockdown of Hif1α or control were used as described previously [29].
Statistical analysis
Data were analyzed using the Mann-Whitney U test (SPSS 19) and were expressed as mean ±SD. Differences were considered significant if p<0.05.
Results
Blood gas and hematologic adaptations induced by exposure to chronic hypoxia
Table 2 summarizes the results of the blood gas analyses and the hematological profile from mice exposed to chronic hypoxia compared to normoxia. As expected, PaO2 and SaO2 were significantly decreased in hypoxia compared to normoxia exposed mice. Mice exposed to hypoxia exhibited a lower blood pH and a lower HCO3 concentration, but a normal PaCO2 level. Furthermore, the hematocrit, hemoglobin concentration, erythrocyte count and mean corpuscular volume were found to be significantly increased in mice exposed to chronic hypoxia. Some of these data have been published previously [30].
Table 2.
Overview of blood analyses of normoxia and hypoxia exposed mice
| Normoxia (n=8) | Hypoxia (n=6) | p-value | |
|---|---|---|---|
| Arterial blood gas analyses | |||
| pH | 7.3 (0.03) | 7.1 (0.06) | <0.001 |
| PaO2, mmHg | 128.2 (8.4) | 34.1 (3.9) | <0.001 |
| PaCO2, mmHg | 35.4 (3.7) | 35.8 (3.1) | 0.90 |
| HCO3, mmol/L | 15.9 (2.2) | 11.3 (1.8) | <0.001 |
| SaO2, % | 100.6 (0.9) | 24.3 (5.8) | <0.001 |
| Hematological adaptations | |||
| Hematocrit, % | 0.45 (0.03) | 0.76 (0.16) | <0.001 |
| Hemoglobin, mmol/L | 9.0 (0.6) | 14.6 (0.3) | <0.001 |
| Erythrocytes, ×106 | 10.0 (0.6) | 13.6 (0.4) | <0.001 |
| MCV, fL | 45.5 (0.70) | 56.3 (1.8) | <0.001 |
| MCH, pg | 0.91 (0.18) | 1.08 (0.04) | <0.001 |
| MCHC, g/dl | 19.8 (0.43) | 19.1 (0.7) | 0.028 |
Data expressed as mean ± SD. Abbreviations; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration.
Upregulation of pulmonary expression of VEGFs
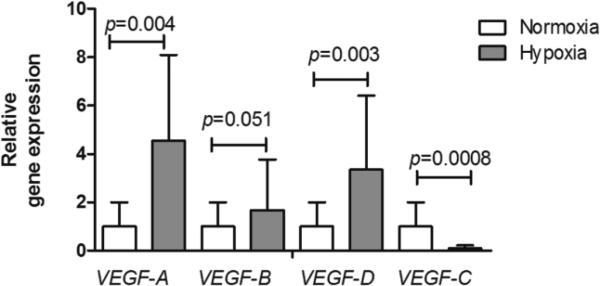
In addition to the hematological adaptations, we assessed whether hypoxia affected the lungs of mice in our model. As expected, chronic hypoxia induced gene expression of vascular endothelial growth factor (VEGF)-A, a hypoxia-inducible gene. Also VEGF-D expression was significantly enhanced in response to hypoxia and for VEGF-B a trend to increase was observed. Interestingly, VEGF-C was found to be downregulated in lung tissue upon chronic hypoxia exposure (Fig. 1).
Fig 1.
Chronic hypoxia-induced changes in VEGF gene expression. VEGF-A, VEGF-B, VEGF-D and VEGF-C mRNA levels were analyzed by QPCR and data expressed as mean relative gene expression corrected for RPL13a ± SD; normoxia n=8, hypoxia n=7; the Mann-Whitney U test was used for statistical analyses.
Hypoxia associated alterations in pulmonary RAGE expression
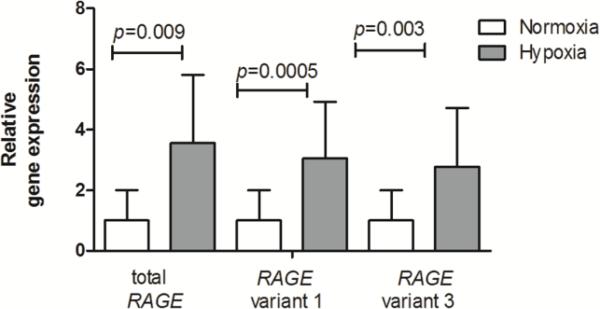
We next assessed the impact of chronic hypoxia exposure on pulmonary expression of RAGE and its variants at the mRNA and protein level. As shown in Fig. 2, gene expression of total RAGE was significantly enhanced in mice exposed to hypoxia compared to mice at normoxia. Furthermore, RAGE splice variants 1 and 3 which translate into an endogenous soluble protein capable of scavenging RAGE ligands were examined. As for total RAGE, significantly increased mRNA levels of variant 1 and 3 were observed in hypoxia exposed mice.
Fig 2.
Significant increases in gene expression of RAGE and its variants 1 and 3 in lungs of hypoxia exposed mice. Data are expressed as mean relative gene expression corrected for RPL13a ± SD; normoxia n=8, hypoxia n=7; the Mann-Whitney U test was used for statistical analyses.
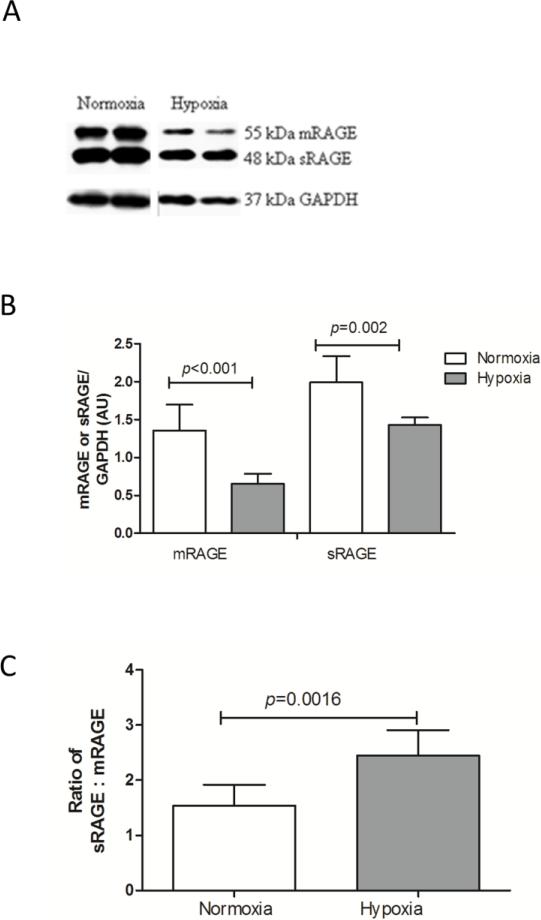
In contrast, at the protein level significantly decreased levels of both mRAGE and sRAGE were found by Western blotting in hypoxia exposed mice (Fig. 3A and 3B). Furthermore, the ratio of soluble to membrane RAGE was significantly enhanced in response to hypoxia (Fig. 3C).
Fig 3.
Decreased membrane and soluble RAGE protein levels in lung tissue of mice exposed to chronic hypoxia. A) Representative Western blots of mRAGE and sRAGE on lung tissue lysates. B) Quantification of mRAGE and sRAGE signals on Western blot, using GAPDH as a loading control. Data are expressed as mean arbitrary units ± SD; normoxia n=8, hypoxia n=5. C) Ratio of sRAGE to mRAGE expression from (B). The Mann-Whitney U test was used for statistical analyses.
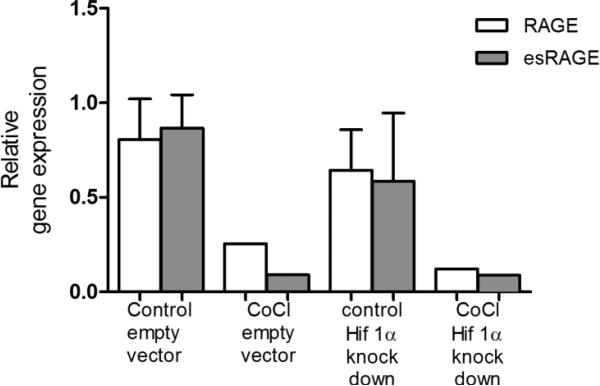
No role for HIF1α in the regulation of RAGE mRNA expression in vitro
To assess whether HIF1α is implicated in the regulation of RAGE mRNA expression at baseline and in response to hypoxia, A549, alveolar epithelial type II cells with stable knockdown of HIF1α were treated with the hypoxia mimetic CoCl for 48 hours and gene expression of RAGE was analyzed. A similar dose of CoCl was found to lead to a similar induction of HIF1α protein levels as culture of A549 cells under 1% O2 [31]. First, this model of acute hypoxia in vitro caused a downregulation of total RAGE as well as esRAGE gene expression. Secondly, knockdown of HIF1α did not affect basal expression of RAGE or its soluble transcript variant and it did not modulate the effects of CoCl (Fig. 4).
Fig 4.
No role for HIF1α in the decreased mRNA expression of total and esRAGE after CoCl treatment in vitro. A549 cells stably transfected with shRNA against HIF1α or control were treated with 200μM CoCl for 48h after which gene expression of total and esRAGE were determined by QPCR corrected for RPL13a.
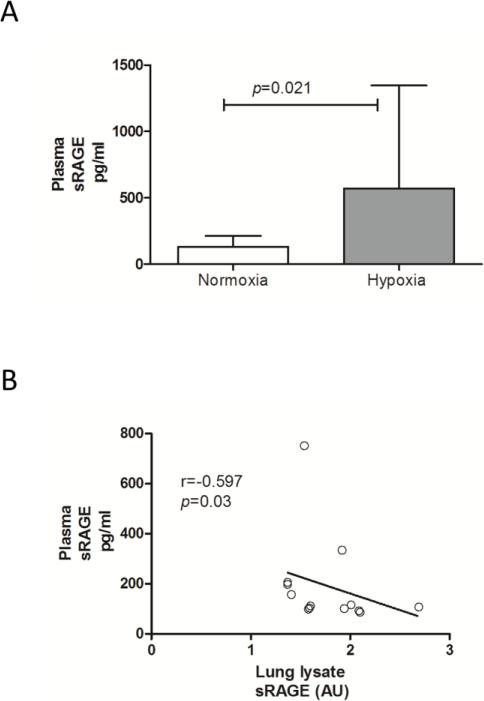
Chronic hypoxia increases circulating sRAGE levels
In some pulmonary diseases plasma levels of sRAGE are altered and could serve as biomarkers because of their correlation with lung function. We thus assessed here whether chronic hypoxia affected plasma sRAGE levels. As shown in Fig. 5A, chronic hypoxia significantly enhanced sRAGE levels in plasma of hypoxia treated mice compared to mice at normoxia (normoxia: 132.0 ± 82.4; hypoxia: 571.4 ± 774.0; pg/ml; p=0.021). Furthermore, a negative association between plasma sRAGE and lung sRAGE was observed (r=−0.597, p=0.03, Fig. 5B).
Fig 5.
Hypoxia induces significant increases in plasma sRAGE levels (A), which shows a negative association with lung lysate sRAGE (B). Data are expressed as mean ± SD and spearman rho correlation coefficient respectively; normoxia n=8, hypoxia n=7.
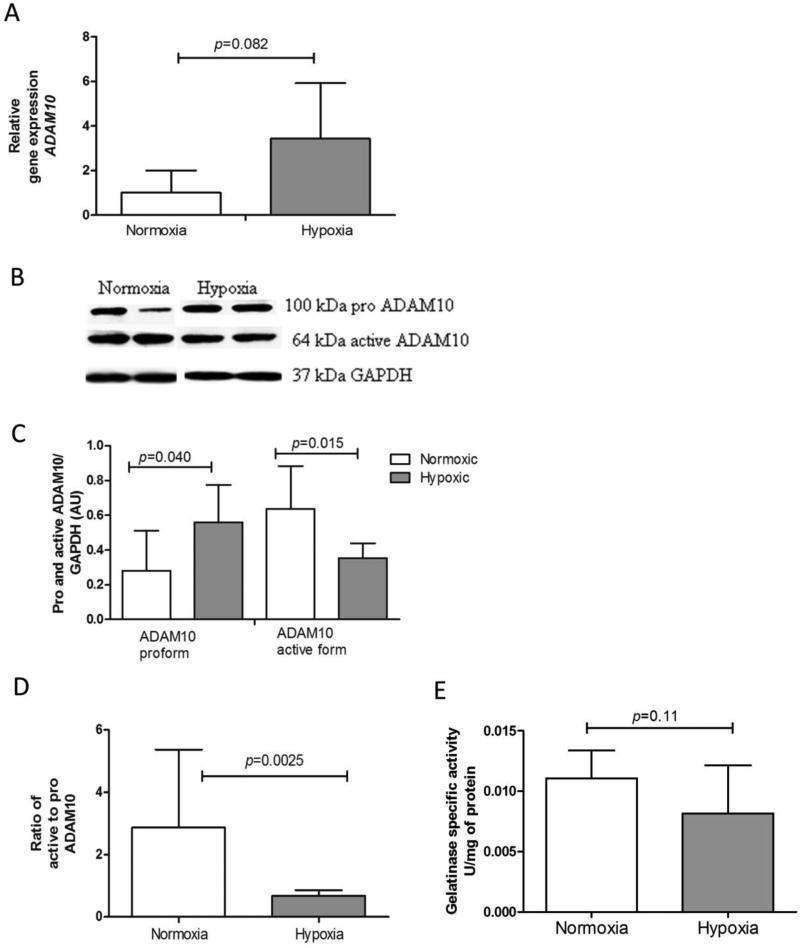
RAGE sheddases
Although the relative amount of sRAGE was increased in lung tissue of hypoxia exposed mice, the net amount of sRAGE decreased. Because these observations are likely not explained by the increased mRNA expression of variants 1 and 3, we analyzed whether hypoxia affected shedding through effects on gelatinases, which include MMP9 and ADAM10 in total lung tissue. Hypoxia exposed mice showed a trend to increased ADAM10 mRNA levels compared to mice at normoxia (Fig. 5A). Furthermore protein levels of the 100kDa pro-form of ADAM10 were found to be significantly enhanced in hypoxia treated mice, whereas the 64kDa active form was found to be downregulated (Fig. 5B and 5C). Since both the net amount of active ADAM10 and the ratio of active to pro-ADAM10 (Fig. 5D) are significantly decreased after chronic exposure to hypoxia, it is unlikely that elevated soluble RAGE levels are due to enhanced cleavage of the membrane form by this sheddase. Furthermore, we analyzed total lung tissue gelatinase activity as mRNA expression of MMP9, another important sheddase, were too low to obtain reliable data (data not shown). Results in Fig. 6E show that chronic hypoxia did not significantly affect lung gelatinase activity.
Fig 6.
Effects of chronic hypoxia on RAGE sheddase ADAM10 and gelatinase activity. A) Trend of increased ADAM10 gene expression in hypoxia exposed mice. Data are expressed as mean relative gene expression corrected for RPL13a ± SD; normoxia n=8, hypoxia n=7. B) Representative Western blots for pro ADAM10 and active ADAM10 on lung tissue lysates. C) Quantification of pro ADAM10 and active ADAM10 from Western blot, using GAPDH as an internal control. Data are expressed as mean arbitrary units ± SD; normoxia n=8, hypoxia n=5; the Mann-Whitney U test was used for statistical analyses. D) Ratio of active to pro ADAM10 from (C). E). Gelatinase activity was assessed in whole lung lysates and expressed as units/mg protein; normoxia n=8, hypoxia n=7; the Mann-Whitney U test was used for statistical analyses.
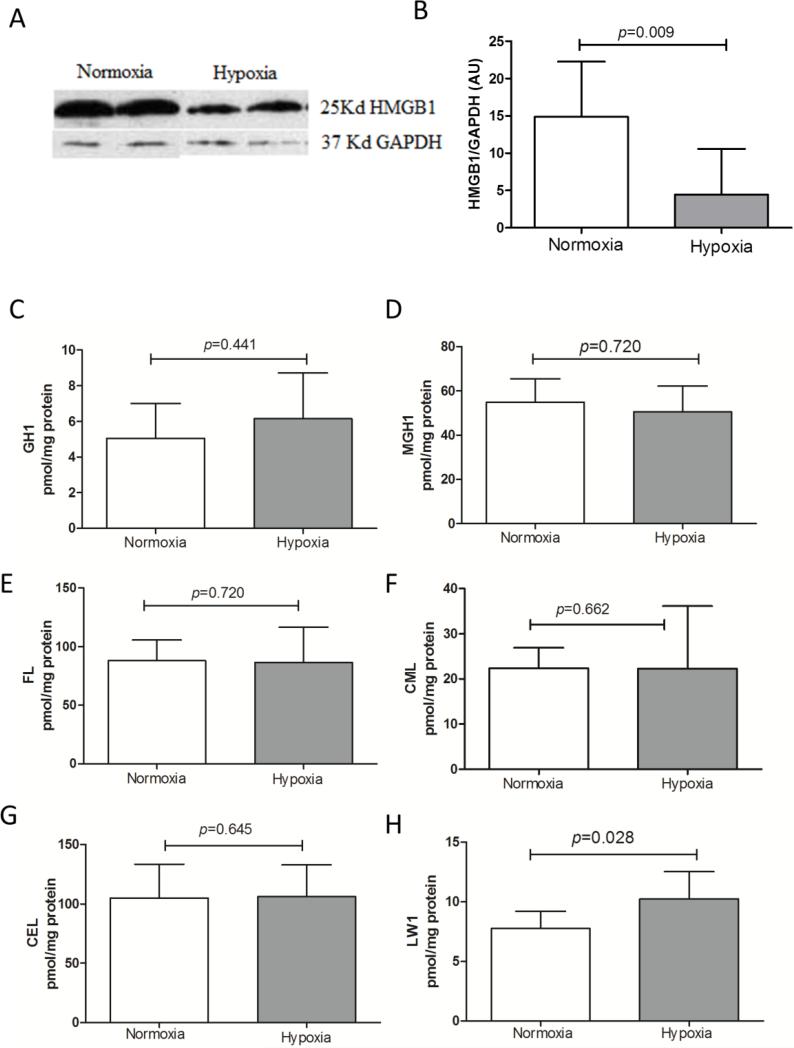
Pulmonary levels of HMGB1 and AGEs are not affected by chronic hypoxia
The observed alterations in RAGE homeostasis in mice exposed to chronic hypoxia could, next to direct effects of hypoxia on expression of RAGE and sheddases, be associated with differential levels of RAGE ligands. We thus analyzed the major pulmonary RAGE ligand HMGB1, and found that chronic hypoxia markedly attenuated HMGB1 protein levels (Fig. 7A and B). Secondly, we analyzed a panel of AGEs including GH1, MGH1, FL, CML, CEL, and LW1 in the insoluble protein fraction of lung tissue of both mouse groups. As indicated in Fig. 7C-H, chronic hypoxia only significantly enhanced LW1 levels.
Fig 7.
Fluorescent AGE LW-1 was selectively increased by hypoxia in lung tissue matrix. A) Representative Western blots for HMGB1 on lung tissue lysates. B) Quantification of HMGB1 from Western blot, using GAPDH as an internal control; normoxia n=8, hypoxia n=7; the Mann-Whitney U test was used for statistical analyses. Insoluble lung AGEs C) GH1, D) MGH1, E) FL, F) CML, G) CEL and H) LW1 levels expressed as mean ± SD; normoxia n=8, hypoxia n=8; the Mann-Whitney U test was used for statistical analyses.
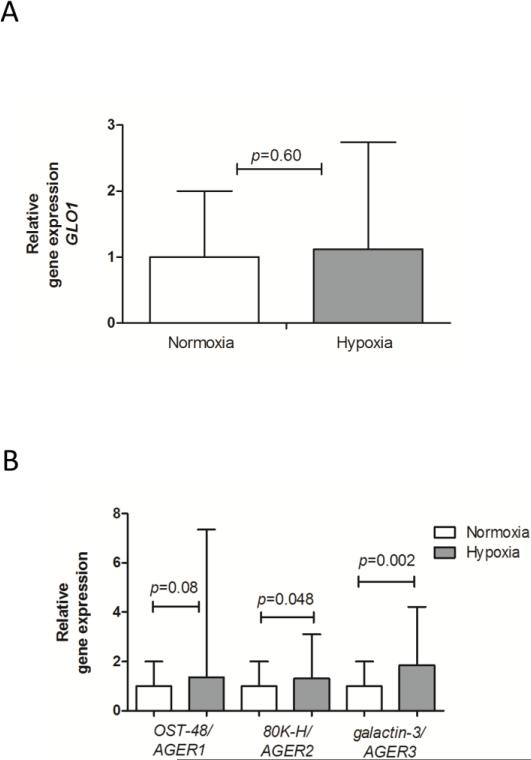
This observation led us to further investigate AGE detoxifying mechanisms, which could have limited accumulation of AGEs. No effect of hypoxia on the AGE detoxifying enzyme glyoxalase 1 was observed in Fig 8A. We also examined the influence of chronic hypoxia on the expression levels of receptors which bind and remove AGEs. 80K-H/AGER2 and galactin-3/AGER3 were found to be significantly increased in the hypoxia compared to the normoxia exposed mouse group (p=0.048, p=0.002 respectively). The lack of effect of hypoxia on oligosaccharyl transerase (OST)-48/AGER1 expression was likely due to the large variability (Fig. 8B). Furthermore, based on the average CT values of these three AGERs under normoxic conditions it appears that AGER1 is the most abundantly expressed at the mRNA level in the lungs, and AGER2 the least (AGER1: 23.34±0.39; AGER2: 26.76±0.52; AGER3 25.12±0.56, average SD).
Fig 8.
Chronic hypoxic induced AGER2 and AGER3 expression, but did not affect GLO1, or AGER1 gene expression. A) GLO1, B) AGER1, AGER2 and AGER3 mRNA expression represented as mean relative gene expression corrected for RPL13a ± SD; normoxia n=8, hypoxia n=7; the Mann-Whitney U test was used for statistical analyses.
Discussion
Chronic hypoxia was shown to decrease lung protein levels of both mRAGE and sRAGE, with an increased sRAGE to mRAGE ratio. These alterations were accompanied by increased mRNA expression of 2 isoforms that code for soluble RAGE, but also by decreased protein levels of the active form of the sheddase ADAM10. Hypoxia furthermore increased plasma sRAGE concentrations, which negatively correlated with pulmonary sRAGE levels. Although these alterations in the lungs and plasma are in opposing directions, indicating that plasma sRAGE levels are not a reflection of sRAGE alterations in the lungs, the significant correlation between both compartments hints to a common mechanism behind the observed effects in the respective compartments. Only the AGE LW1 was found to be increased in lung tissue by chronic hypoxia. The enhanced gene expression of AGE receptors 2 and 3 in response to chronic hypoxia could explain the absence of accumulation of other AGEs investigated.
Chronic hypoxia treatment induced hypoxemia in blood which was evidenced by the decreased levels of oxygen tension and saturation, decreased pH and compensatory increase in the RBC numbers and parameters in hypoxia exposed mice. In addition, local effects of hypoxia in lung tissue were evidenced by the upregulation of VEGF-A, a hypoxia sensitive gene. In the present study we also examined the effect of chronic hypoxia on other members of the VEGF family which share structural features, but display different biological activities [32]. The biological function of VEGF-B is similar to that of VEGF-A, but as reported is not significantly influenced by hypoxia. The absence of a significant difference in VEGF-B gene expression between hypoxia and normoxia exposed mice is in line with this literature [33]. VEGF-C and VEGF-D on the other hand control the growth of lymphatic vessel in lungs [34, 35]. Although hypoxia has been shown not to induce VEGF-C [33] we here observed decreased VEGF-C expression. Effects of hypoxia on VEGF-D have not been reported, but an induction was evident in our study. The overall effects of hypoxia on pulmonary lymphatic vessels and the role of these 2 VEGF isoforms herein remain to be investigated.
HIF1-α is the main molecular effector of hypoxia signaling. It has been shown that HIF1-α is able to bind the HIF-1α binding site present in the RAGE promoter region and activate gene transcription [36]. In line herewith we observed up-regulated gene expression of RAGE as well as its splice variants that code for soluble forms by chronic hypoxia in murine lung tissue. In vitro, a model of acute hypoxia in contrast attenuated total and esRAGE gene expression in a cancer-derived lung epithelial cell line, and we did not find evidence for a role of HIF1α herein. A study in pancreatic tumor cells also found RAGE expression not to be modulated by HIF1α in response to hypoxia, but instead identified a role for NF-κB [37], for which a binding site in the RAGE promotor is present as well.
In contrast to the mRNA data however, downregulation of mRAGE and sRAGE protein levels were found in lung tissue of mice after chronic exposure to hypoxia in the current study. We did unfortunately not collect lung tissue for immunohistochemical localization of RAGE and the existing literature on effects of acute hypoxia on RAGE expression in lung tissue did also not examine in which compartments it was altered. In human IPF samples RAGE protein levels were reported to be attenuated, which was associated with weaker staining in bronchial and alveolar epithelium, as well as fibroblasts [38]. In COPD and cigarette smoke exposed mice on the other hand, enhanced RAGE protein levels were mainly derived from increased expression in alveolar epithelium [21, 39]. It remains to be examined if chronic hypoxia causes a homogenous downregulation of RAGE protein levels in the lungs, or whether this is confined to alveolar regions.
Attenuated rates of translation, decreased protein stability or enhanced receptor turnover are possible mechanisms that could underlie the discrepancy between mRNA and protein expression. Regardless, the ratio of sRAGE to mRAGE was significantly enhanced in lung tissue, as were sRAGE levels in the circulation of hypoxia treated mice compared to mice under normoxia. The increased mRNA expression of variants 1 and 3 could likely not explain the relative increase in sRAGE since the net amount of pulmonary sRAGE protein was decreased. We therefore speculated that hypoxia might have activated sheddases. Two possible mechanisms causing shedding of membrane RAGE have been reported. One is by sheddases including MMP9 and ADAM10 [40]; the other is by G-protein coupled receptor mediated shedding [41]. In the present study mRNA expression and levels of inactive pro-ADAM10 were upregulated, whereas protein levels of the active form of the enzyme were decreased by hypoxia. It is therefore unlikely that the relative increase in sRAGE levels is due to ADAM10-mediated shedding. Unfortunately effects of hypoxia on MMP9 specifically could not be assessed in the current study because expression levels were below the detection limit. Total lung gelatinase activity was on the other hand not affected by chronic hypoxia, data which are in line with a study reporting no significant effect on MMP9 gene and protein expression by chronic hypobaric hypoxia [42]. To exclude a role for shedding, other sheddases remain to be investigated.
Enhanced sRAGE levels could also be a reflection of mere damage to pneumocytes. Indeed, acute hypoxia, 7% O2 for 6h, induced a slight increase in sRAGE levels in the BALF, which was shown to be due to damage to type I pneumocytes [43]. Hyperoxia treatment on the other hand has also been shown to lead to increased pulmonary and BALF sRAGE levels. The enhanced BALF and plasma sRAGE levels in models of hypoxia as well as hyperoxia fit with the proposed role of sRAGE as marker of pneumocyte damage, as both models are associated with alveolar damage. Unfortunately, we did not collect BALF to measure sRAGE in the present study.
In addition to the potential role of sRAGE as a damage marker, the observed alterations in RAGE homeostasis in response to chronic hypoxia are likely to play a functional role in oxygen sensing and adaptation to varied oxygen levels. This is indeed not only implied by its localization to the basolateral side of type I cells and role thereby in cell spreading, thinning and adherence, but also by the observed protection from hyperoxia-induced inflammation, damage and mortality in RAGE knock-out mice [25]. It is proposed that the absence of RAGE limits both the induction and amplification of inflammatory responses otherwise induced by the multitude of ligands. Furthermore the protection offered by exogenous administration of sRAGE in models of lung ischemia-reperfusion injury [44], as well as hypoxia [45] indicates that the RAGE alterations observed in our model could be part of an adaptive and protective response mechanism to chronic hypoxia. Studies aimed at further unraveling this hypothesis might have to distinguish between the function of RAGE as an adhesion molecule in alveolar regions vs its function as pattern recognition receptor in airways. In this regard, HMGB1, a RAGE ligand acting as a danger signal, has been shown to be an early mediator of the response to acute hypoxia/ischemia [46]. HMGB1 was furthermore found to be implicated in the pathogenesis of pulmonary arterial hypertension using a mouse model of a 2 day exposure to 10% O2. This study demonstrated enhanced levels of HMGB1 in lung lysates as well as BALF [47]. In our model of chronic hypoxia on the other hand, we found lower levels of HMGB1 in lung lysates, which again could represent an adaptive mechanism, which might be linked to the lower levels of RAGE. It remains to be investigated whether BALF levels of HMGB1 are attenuated as well.
For several years it has been known that high blood glucose concentrations promote the formation of AGEs, intra- as well as extracellularly [48]. In addition, high dietary intake of AGEs enhances organ AGE levels as well RAGE expression, including in lung tissue [49-51]. Oxidative stress and inflammation may also play a role in the formation of AGEs in vivo through a glucose independent pathway [52, 53]. Although hypoxia induces oxidative stress in several organs including lungs [54] and induction of AGEs has been observed by ischemia/reperfusion injury in the heart [55-57], neither glycoxidation nor lipoxidation, as reflected by CML and CEL levels, were increased. Similarly, no changes in the levels of hydroimidazolones MGH1 and GH1 were observed, implying no effect of hypoxia on methylglyoxal and glyoxal levels, respectively. Also, furosine levels were unchanged, thereby reflecting no impact of hypoxia on glucose levels and protein glycation by glucose.
The lack of accumulation of the other AGEs investigated was likely not due to compensatory enzymatic detoxification since GLO1 expression not found to be affected by hypoxia. On the other hand, mRNA expression of AGER2 and AGER3, two receptors which are involved in the detoxification and removal of AGEs was induced in lung tissue by chronic hypoxia.
We investigated the effect of the same hypoxia regimen in mice of 12 weeks of age on certain, but not all parameters reported in this manuscript. RAGE and ADAM data were in agreement with the older mice, but in these younger mice, despite attenuated GLO-1 mRNA expression in response to chronic hypoxia, none of the AGEs investigated was increased, including LW-1 (data not shown). In older mice however, a novel finding of major interest is that the lung fluorescent AGE LW-1 level was specifically and significantly increased by hypoxia. The rational for determining LW1 in this study was to test the hypothesis that hypoxia would increase its levels. This hypothesis grew out from previous observations where LW1 levels were found elevated in insoluble collagen of skin obtained at autopsy from non-diabetic individuals suffering from chronic lung disease [28, 58]. No mechanism in its formation can be currently inferred based upon LW1 structure of which we only know that it includes an aromatic ring coupled to a lysine residue and a sugar of undetermined nature. Thus, this study independently and for the first time confirms the suggested role of hypoxia in LW-1 formation.
In summary, chronic hypoxia is associated with downregulation of pulmonary RAGE protein levels, but a relative increase in sRAGE, which is reflected in increased plasma sRAGE levels. These alterations might be part of the adaptive and protective response mechanism to chronic hypoxia and do not appear to be associated with AGEs formation, except for the fluorescent AGE LW-1 which emerges as a novel tissue marker of hypoxia.
Supplementary Material
Highlights.
Chronic hypoxia
- decreased pulmonary mRAGE and sRAGE protein levels but increased the ratio of sRAGE to mRAGE
- enhanced plasma sRAGE levels
- decreased active ADAM10 levels
- decreased HMGB1 levels, and increased the AGE LW1 which could be a new biomarker of tissue hypoxia
Acknowledgements
We would like to thank Prof. CG Schalkwijk, Maastricht University, Maastricht, the Netherlands for the mouse specific GLO1 primers and drs. M Donners, Maastricht University, and S. Rose-John, Christian-Albrechts-University, Germany for the mouse specific ADAM10 antibody.
Grants
This study was supported by the Lung Foundation Netherlands (3.2.09.049) and in part by NIH/NIDDK grants DK-79432 to DRS and Juvenile Diabetes Foundation grants 17-2010-318 to VMM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, Elliston K, Stern D, Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267(21):14998–15004. [PubMed] [Google Scholar]
- 2.Kumano-Kuramochi M, Ohnishi-Kameyama M, Xie Q, Niimi S, Kubota F, Komba S, Machida S. Minimum stable structure of the receptor for advanced glycation end product possesses multi ligand binding ability. Biochem Biophys Res Commun. 2009;386(1):130–134. doi: 10.1016/j.bbrc.2009.05.142. [DOI] [PubMed] [Google Scholar]
- 3.Sparvero LJ, Asafu-Adjei D, Kang R, Tang D, Amin N, Im J, Rutledge R, Lin B, Amoscato AA, Zeh HJ, et al. RAGE (Receptor for Advanced Glycation Endproducts), RAGE ligands, and their role in cancer and inflammation. J Transl Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexiou P, Chatzopoulou M, Pegklidou K, Demopoulos VJ. RAGE: a multi-ligand receptor unveiling novel insights in health and disease. Curr Med Chem. 2010;17(21):2232–2252. doi: 10.2174/092986710791331086. [DOI] [PubMed] [Google Scholar]
- 5.Clynes R, Moser B, Yan SF, Ramasamy R, Herold K, Schmidt AM. Receptor for AGE (RAGE): weaving tangled webs within the inflammatory response. Curr Mol Med. 2007;7(8):743–751. doi: 10.2174/156652407783220714. [DOI] [PubMed] [Google Scholar]
- 6.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). Faseb J. 2008;22(10):3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 7.Hudson BI, Carter AM, Harja E, Kalea AZ, Arriero M, Yang H, Grant PJ, Schmidt AM. Identification, classification, and expression of RAGE gene splice variants. Faseb J. 2008;22(5):1572–1580. doi: 10.1096/fj.07-9909com. [DOI] [PubMed] [Google Scholar]
- 8.Kalea AZ, Reiniger N, Yang H, Arriero M, Schmidt AM, Hudson BI. Alternative splicing of the murine receptor for advanced glycation end-products (RAGE) gene. Faseb J. 2009;23(6):1766–1774. doi: 10.1096/fj.08-117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamakawa N, Uchida T, Matthay MA, Makita K. Proteolytic release of the receptor for advanced glycation end products from in vitro and in situ alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300(4):L516–525. doi: 10.1152/ajplung.00118.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demling N, Ehrhardt C, Kasper M, Laue M, Knels L, Rieber EP. Promotion of cell adherence and spreading: a novel function of RAGE, the highly selective differentiation marker of human alveolar epithelial type I cells. Cell Tissue Res. 2006;323(3):475–488. doi: 10.1007/s00441-005-0069-0. [DOI] [PubMed] [Google Scholar]
- 11.Mauri T, Masson S, Pradella A, Bellani G, Coppadoro A, Bombino M, Valentino S, Patroniti N, Mantovani A, Pesenti A, et al. Elevated plasma and alveolar levels of soluble receptor for advanced glycation endproducts are associated with severity of lung dysfunction in ARDS patients. The Tohoku journal of experimental medicine. 2010;222(2):105–112. doi: 10.1620/tjem.222.105. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MJ, Carles M, Brohi K, Calfee CS, Rahn P, Call MS, Chesebro BB, West MA, Pittet JF. Early release of soluble receptor for advanced glycation endproducts after severe trauma in humans. The Journal of trauma. 2010;68(6):1273–1278. doi: 10.1097/TA.0b013e3181db323e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing R, Cui M, Wang J, Wang H. Receptor for advanced glycation end products (RAGE) soluble form (sRAGE): a new biomarker for lung cancer. Neoplasma. 2010;57(1):55–61. doi: 10.4149/neo_2010_01_055. [DOI] [PubMed] [Google Scholar]
- 14.Smith DJ, Yerkovich ST, Towers MA, Carroll ML, Thomas R, Upham JW. Reduced soluble receptor for advanced glycation end-products in COPD. Eur Respir J. 2011;37(3):516–522. doi: 10.1183/09031936.00029310. [DOI] [PubMed] [Google Scholar]
- 15.Gopal P, Rutten EP, Dentener MA, Wouters EF, Reynaert NL. Decreased plasma sRAGE levels in COPD: influence of oxygen therapy. Eur J Clin Invest. 2012;42(8):807–814. doi: 10.1111/j.1365-2362.2012.02646.x. [DOI] [PubMed] [Google Scholar]
- 16.Miniati M, Monti S, Basta G, Cocci F, Fornai E, Bottai M. Soluble receptor for advanced glycation end products in COPD: relationship with emphysema and chronic cor pulmonale: a case-control study. Respir Res. 2011;12:37. doi: 10.1186/1465-9921-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stogsdill JA, Stogsdill MP, Porter JL, Hancock JM, Robinson AB, Reynolds PR. Embryonic overexpression of receptors for advanced glycation end-products by alveolar epithelium induces an imbalance between proliferation and apoptosis. Am J Respir Cell Mol Biol. 2012;47(1):60–66. doi: 10.1165/rcmb.2011-0385OC. [DOI] [PubMed] [Google Scholar]
- 18.Stogsdill MP, Stogsdill JA, Bodine BG, Fredrickson AC, Sefcik TL, Wood TT, Kasteler SD, Reynolds PR. Conditional overexpression of receptors for advanced glycation end-products in the adult murine lung causes airspace enlargement and induces inflammation. Am J Respir Cell Mol Biol. 2013;49(1):128–134. doi: 10.1165/rcmb.2013-0013OC. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds PR, Cosio MG, Hoidal JR. Cigarette smoke-induced Egr-1 upregulates proinflammatory cytokines in pulmonary epithelial cells. Am J Respir Cell Mol Biol. 2006;35(3):314–319. doi: 10.1165/rcmb.2005-0428OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006;19(11):1437–1445. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Ma L, Nicholson LF, Black PN. Advanced glycation end products and its receptor (RAGE) are increased in patients with COPD. Respir Med. 2011;105(3):329–336. doi: 10.1016/j.rmed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Sukkar MB, Postma DS. Receptor for advanced glycation end products and soluble receptor for advanced glycation end products: a balancing act in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2013;188(8):893–894. doi: 10.1164/rccm.201308-1489ED. [DOI] [PubMed] [Google Scholar]
- 23.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173(9):1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X, Lee JW, Matthay ZA, Mednick G, Uchida T, Fang X, Gupta N, Matthay MA. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol. 2007;37(2):186–192. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reynolds PR, Schmitt RE, Kasteler SD, Sturrock A, Sanders K, Bierhaus A, Nawroth PP, Paine R, 3rd, Hoidal JR. Receptors for advanced glycation end-products targeting protect against hyperoxia-induced lung injury in mice. Am J Respir Cell Mol Biol. 2010;42(5):545–551. doi: 10.1165/rcmb.2008-0265OC. [DOI] [PubMed] [Google Scholar]
- 26.Gopal P, Reynaert NL, Scheijen JL, Schalkwijk CG, Franssen FM, Wouters EF, Rutten EP. Association of plasma sRAGE, but not esRAGE with lung function impairment in COPD. Respir Res. 2014;15(1):24. doi: 10.1186/1465-9921-15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang JS, Wendt T, Qu W, Kong L, Zou YS, Schmidt AM, Yan SF. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res. 2008;102(8):905–913. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- 28.Sell DR, Nemet I, Monnier VM. Partial characterization of the molecular nature of collagen-linked fluorescence: role of diabetes and end-stage renal disease. Arch Biochem Biophys. 2010;493(2):192–206. doi: 10.1016/j.abb.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramaekers CH, van den Beucken T, Meng A, Kassam S, Thoms J, Bristow RG, Wouters BG. Hypoxia disrupts the Fanconi anemia pathway and sensitizes cells to chemotherapy through regulation of UBE2T. Radiother Oncol. 2011;101(1):190–197. doi: 10.1016/j.radonc.2011.05.059. [DOI] [PubMed] [Google Scholar]
- 30.van den Borst B, Schols AM, de Theije C, Boots AW, Kohler SE, Goossens GH, Gosker HR. Characterization of the inflammatory and metabolic profile of adipose tissue in a mouse model of chronic hypoxia. J Appl Physiol. 1985;2013114(11):1619–1628. doi: 10.1152/japplphysiol.00460.2012. [DOI] [PubMed] [Google Scholar]
- 31.Li QF, Wang XR, Yang YW, Lin H. Hypoxia upregulates hypoxia inducible factor (HIF)-3alpha expression in lung epithelial cells: characterization and comparison with HIF-1alpha. Cell Res. 2006;16(6):548–558. doi: 10.1038/sj.cr.7310072. [DOI] [PubMed] [Google Scholar]
- 32.Veikkola T, Alitalo K. VEGFs, receptors and angiogenesis. Semin Cancer Biol. 1999;9(3):211–220. doi: 10.1006/scbi.1998.0091. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Eriksson U. Novel VEGF family members: VEGF-B, VEGF-C and VEGF-D. Int J Biochem Cell Biol. 2001;33(4):421–426. doi: 10.1016/s1357-2725(01)00027-9. [DOI] [PubMed] [Google Scholar]
- 34.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A. 1998;95(2):548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamada Y, Nezu J, Shimane M, Hirata Y. Molecular cloning of a novel vascular endothelial growth factor, VEGF-D. Genomics. 1997;42(3):483–488. doi: 10.1006/geno.1997.4774. [DOI] [PubMed] [Google Scholar]
- 36.Pichiule P, Chavez JC, Schmidt AM, Vannucci SJ. Hypoxia-inducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia/ischemia. J Biol Chem. 2007;282(50):36330–36340. doi: 10.1074/jbc.M706407200. [DOI] [PubMed] [Google Scholar]
- 37.Kang R, Hou W, Zhang Q, Chen R, Lee YJ, Bartlett DL, Lotze MT, Tang D, Zeh HJ. RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell Death Dis. 2014;5:e1480. doi: 10.1038/cddis.2014.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Queisser MA, Kouri FM, Konigshoff M, Wygrecka M, Schubert U, Eickelberg O, Preissner KT. Loss of RAGE in pulmonary fibrosis: molecular relations to functional changes in pulmonary cell types. Am J Respir Cell Mol Biol. 2008;39(3):337–345. doi: 10.1165/rcmb.2007-0244OC. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds PR, Kasteler SD, Schmitt RE, Hoidal JR. Receptor for advanced glycation end-products signals through Ras during tobacco smoke-induced pulmonary inflammation. Am J Respir Cell Mol Biol. 2011;45(2):411–418. doi: 10.1165/rcmb.2010-0231OC. [DOI] [PubMed] [Google Scholar]
- 40.Raucci A, Cugusi S, Antonelli A, Barabino SM, Monti L, Bierhaus A, Reiss K, Saftig P, Bianchi ME. A soluble form of the receptor for advanced glycation endproducts (RAGE) is produced by proteolytic cleavage of the membrane-bound form by the sheddase a disintegrin and metalloprotease 10 (ADAM10). FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22(10):3716–3727. doi: 10.1096/fj.08-109033. [DOI] [PubMed] [Google Scholar]
- 41.Metz VV, Kojro E, Rat D, Postina R. Induction of RAGE shedding by activation of G protein-coupled receptors. PLoS One. 2012;7(7):e41823. doi: 10.1371/journal.pone.0041823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estrada KD, Chesler NC. Collagen-related gene and protein expression changes in the lung in response to chronic hypoxia. Biomech Model Mechanobiol. 2009;8(4):263–272. doi: 10.1007/s10237-008-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang SX, Miller JJ, Stolz DB, Serpero LD, Zhao W, Gozal D, Wang Y. Type I epithelial cells are the main target of whole-body hypoxic preconditioning in the lung. Am J Respir Cell Mol Biol. 2009;40(3):332–339. doi: 10.1165/rcmb.2008-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharma AK, LaPar DJ, Stone ML, Zhao Y, Kron IL, Laubach VE. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury. Am J Transplant. 2013;13(9):2255–2267. doi: 10.1111/ajt.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iannitti RG, Casagrande A, De Luca A, Cunha C, Sorci G, Riuzzi F, Borghi M, Galosi C, Massi-Benedetti C, Oury TD, et al. Hypoxia promotes danger-mediated inflammation via receptor for advanced glycation end products in cystic fibrosis. Am J Respir Crit Care Med. 2013;188(11):1338–1350. doi: 10.1164/rccm.201305-0986OC. [DOI] [PubMed] [Google Scholar]
- 46.Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, et al. High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation. 2008;117(25):3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- 47.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med. 2012;18:1509–1518. doi: 10.2119/molmed.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brownlee M. Negative consequences of glycation. Metabolism. 2000;49(2 Suppl 1):9–13. doi: 10.1016/s0026-0495(00)80078-5. [DOI] [PubMed] [Google Scholar]
- 49.Diamanti-Kandarakis E, Piperi C, Korkolopoulou P, Kandaraki E, Levidou G, Papalois A, Patsouris E, Papavassiliou AG. Accumulation of dietary glycotoxins in the reproductive system of normal female rats. J Mol Med (Berl) 2007;85(12):1413–1420. doi: 10.1007/s00109-007-0246-6. [DOI] [PubMed] [Google Scholar]
- 50.Smit PJ, Guo WA, Davidson BA, Mullan BA, Helinski JD, Knight PR., 3rd Dietary advanced glycation end-products, its pulmonary receptor, and high mobility group box 1 in aspiration lung injury. J Surg Res. 2014;191:214–223. doi: 10.1016/j.jss.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartling B, Fuchs C, Somoza V, Niemann B, Silber RE, Simm A. Lung level of HMBG1 is elevated in response to advanced glycation end product-enriched food in vivo. Mol Nutr Food Res. 2007;51(4):479–487. doi: 10.1002/mnfr.200600223. [DOI] [PubMed] [Google Scholar]
- 52.Anderson MM, Heinecke JW. Production of N(epsilon)-(carboxymethyl)lysine is impaired in mice deficient in NADPH oxidase: a role for phagocyte-derived oxidants in the formation of advanced glycation end products during inflammation. Diabetes. 2003;52(8):2137–2143. doi: 10.2337/diabetes.52.8.2137. [DOI] [PubMed] [Google Scholar]
- 53.Miyata T, van Ypersele de Strihou C, Kurokawa K, Baynes JW. Alterations in nonenzymatic biochemistry in uremia: origin and significance of “carbonyl stress” in long-term uremic complications. Kidney Int. 1999;55(2):389–399. doi: 10.1046/j.1523-1755.1999.00302.x. [DOI] [PubMed] [Google Scholar]
- 54.Quintero M, Gonzalez-Martin MD, Vega-Agapito V, Gonzalez C, Obeso A, Farre R, Agapito T, Yubero S. The effects of intermittent hypoxia on redox status, NFkappaB activation, and plasma lipid levels are dependent on the lowest oxygen saturation. Free Radic Biol Med. 2013 doi: 10.1016/j.freeradbiomed.2013.08.180. [DOI] [PubMed] [Google Scholar]
- 55.Bucciarelli LG, Kaneko M, Ananthakrishnan R, Harja E, Lee LK, Hwang YC, Lerner S, Bakr S, Li Q, Lu Y, et al. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation. 2006;113(9):1226–1234. doi: 10.1161/CIRCULATIONAHA.105.575993. [DOI] [PubMed] [Google Scholar]
- 56.Aleshin A, Ananthakrishnan R, Li Q, Rosario R, Lu Y, Qu W, Song F, Bakr S, Szabolcs M, D'Agati V. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am J Physiol Heart Circ Physiol. 2008;294(4):H1823–1832. doi: 10.1152/ajpheart.01210.2007. [DOI] [PubMed] [Google Scholar]
- 57.Yan SF, Ramasamy R, Schmidt AM. The receptor for advanced glycation endproducts (RAGE) and cardiovascular disease. Expert Rev Mol Med. 2009;11:e9. doi: 10.1017/S146239940900101X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.