Abstract
CHARGE syndrome (Coloboma of the eye, Heart defects, Atresia of the choanae, Retardation of growth and/or development, Genital and/or urinary anomalies, and Ear malformations, including deafness and vestibular disorders) is a genetic condition characterized by a specific and recognizable pattern of features. Heterozygous pathogenic variants in the chromodomain helicase DNA-binding protein 7 (CHD7) are the major cause of CHARGE syndrome, and have been identified in 70–90% of individuals fulfilling clinical diagnostic criteria. Since 2004, when CHD7 was discovered as the causative gene for CHARGE syndrome, the phenotypic spectrum associated with pathogenic CHD7 variants has expanded. Predicted pathogenic CHD7 variants have been identified in individuals with isolated features of CHARGE including autism and hypogonadotropic hypogonadism. Here we present genotype and phenotype data from a cohort of 28 patients who were considered for a diagnosis of CHARGE syndrome, including one patient with atypical presentations and a pathogenic CHD7 variant. We also summarize published literature on pathogenic CHD7 variant positive individuals who have atypical clinical presentations. Lastly, we propose a revision to current clinical diagnostic criteria, including broadening of the major features associated with CHARGE syndrome and addition of pathogenic CHD7 variant status as a major criterion.
Keywords: human development, genetic condition, congenital anomalies, clinical variability, diagnosis
INTRODUCTION
CHARGE syndrome is an autosomal dominant genetic condition characterized by a nonrandom association of clinical features. First coined as an acronym by Pagon et al. [Pagon et al., 1981], the main features of CHARGE are: Coloboma, Heart malformations, Atresia of the choanae, Retardation of growth or development, Genital anomalies, and Ear malformations, including deafness and vestibular disorders. The prevalence of CHARGE syndrome is estimated to be between 1 in 10,000 [Issekutz et al., 2005] and 1 in 15,000, depending on the region and diagnostic practices [Janssen et al., 2012]. Initially considered to be an association, CHARGE was later recognized as a condition likely to have a single unifying mechanistic explanation, qualifying it as a syndrome [Graham 2001]. In 2004, CHD7 was identified as the gene responsible for CHARGE syndrome [Vissers et al., 2004]. CHD7 encodes a member of the Chromodomain Helicase DNA binding (CHD) protein family, whose members are involved in tissue specific regulation of gene expression during development [Woodage et al., 1997]. Pathogenic CHD7 variants have been identified in 70–90% of suspected cases of CHARGE syndrome [Jongmans et al., 2006; Zentner et al., 2010]; however, when strict clinical diagnostic criteria are met, a pathogenic CHD7 variant is present in over 90% of cases [Bergman et al., 2011]. Diagnostic laboratories have reported a low yield (35%) for CHD7 testing, suggesting that there is a trend of referral bias for “rule-out” diagnoses, although it is impossible to know if the patients fulfilled diagnostic criteria [Bartels et al., 2010].
The spectrum of clinical features associated with CHARGE syndrome has expanded since the original description in 1981, resulting in several iterative changes to published clinical diagnostic criteria. Following the original description, Blake and colleagues updated diagnostic criteria to include cranial nerve dysfunction and visceral malformations [Blake et al., 1998]. In 2005, Verloes added semicircular canal hypoplasia to the major criteria and developed formal definitions for partial and atypical CHARGE syndrome [Verloes 2005]. Blake and Prasad later noted that choanal atresia occurs at much lower frequencies than the other major diagnostic criteria, and suggested that cleft palate may be used in its place when absent, as these features rarely co-occur [Blake et al., 2006]. These updates to clinical diagnostic criteria have added to our knowledge about the major and minor features associated with CHARGE syndrome, and reflect the wide variability in phenotypic severity that has been reported since the discovery of CHD7. The use of typical vs. atypical and major vs. minor criteria for a clinical diagnosis of CHARGE also reflects the complex phenotypes commonly observed, and raise the very important question of whether individuals with isolated features such as autism spectrum disorder or hypogonadotropic hypogonadism and putative pathogenic or proven pathogenic CHD7 variants should be considered as having CHARGE.
Since the discovery of CHD7 as the causative gene for CHARGE syndrome, clinical testing for CHD7 variant status has been widely implemented [Bartels et al., 2010; Janssen et al., 2012; Jongmans et al., 2006]. In addition, application of whole exome sequencing has expanded the phenotypic spectrum of individuals with pathogenic CHD7 variants, with many reports of presumed pathogenic CHD7 variants in individuals lacking the full spectrum of CHARGE clinical features. CHD7 variants have been reported in individuals with isolated features including Autism Spectrum Disorder [Jiang et al., 2013; O’Roak et al., 2012] or gonadotropin-releasing hormone deficiency [Balasubramanian et al., 2014], but not cardiac defects [Corsten-Janssen et al., 2014] or cleft palate [Felix et al., 2006]. These observations raise the important question of whether CHARGE syndrome should be considered as a clinical diagnosis, a molecular diagnosis, or both.
Here, we present a genotype-phenotype study from our own cohort of 28 patients, including one patient with atypical presentation and a pathogenic CHD7 variant. We also review published literature and summarize previously reported atypical features. We suggest that pathogenic CHD7 variant status should be considered as a major feature for assignment of a CHARGE syndrome diagnosis. These newly revised clinical diagnostic criteria are intended to help clarify diagnostic assignment for individuals with atypical features and phenotypes. This should be especially useful in familial cases where reduced penetrance and clinical variability are common.
MATERIALS AND METHODS
All human subject research was performed with approval of the University of Michigan Medical School Institutional Review Board (IRBMED). Individuals who were evaluated in The University of Michigan Pediatric Genetics Clinic from August 2003–August 2014 for features of CHARGE syndrome were identified through a search of the scheduling database. To identify individuals who were likely considered for a diagnosis of CHARGE, we used search terms associated with major diagnostic criteria. These search terms included: CHARGE, coloboma, choanal atresia, sensorineural hearing loss, and hearing loss, based on major criteria used for diagnosis. Each individual who was identified through the search and consented to participate was assessed for CHD7 variant status. Patients who had a clinical diagnosis of CHARGE but had not undergone CHD7 testing were excluded. Additional eligible patients were identified from previously published data [Green et al., 2014]. A literature review was conducted using PubMed. Search terms included CHARGE, CHD7, CHARGE phenotype, atypical CHARGE, and CHD7 phenotype.
Statistical significance of differences between pathogenic CHD7 variant positive and variant negative cohorts was assessed using Fisher’s exact test.
RESULTS
Genotype-Phenotype Correlations
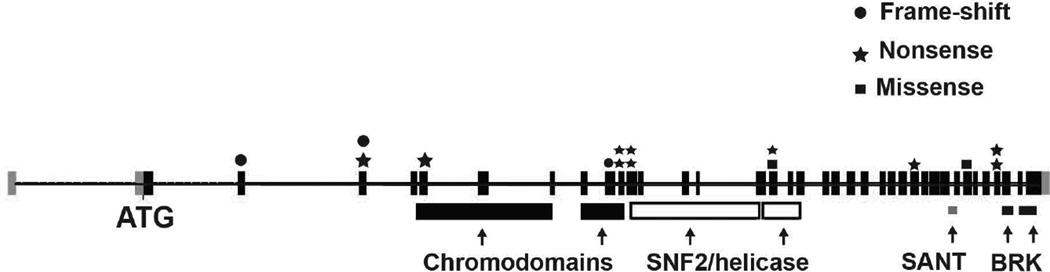
We identified 28 individuals in our clinic that had been considered to have a possible clinical diagnosis of CHARGE and had undergone CHD7 sequencing. Among these, 16 were identified to carry a pathogenic CHD7 variant; 15 of these variants were unique and were evenly distributed throughout the CHD7 coding region; 10 were nonsense, 3 were frame-shift, and two were missense variants [Fig. 1]. Clinical features of these 16 individuals with pathogenic CHD7 variants are listed in Table 1. The remaining 12 individuals tested negative for CHD7 variants or deletions/duplications.
Figure 1.
Schematic of human CHD7 gene with pathogenic variants identified in our 16 patients. Shown in boxes are coding exons 2–38, starting with exon 2 and the ATG translation start site. Functional chromodomains, SNF2/helicase, SANT, and BRK domains are shown below corresponding exons. Shown in above specific exons are frame-shift variants (circles), nonsense variants (stars), and missense variants (squares). Clinical and variant details are provided in Table 1.
Table 1.
Clinical features in 16 CHD7 mutation positive and negative patients
| Patient | Sex | Variant | Variant type |
MT/PP/S prediction |
Coloboma | Choanal atresia |
Inner ear anomalies |
Ext ear anomalies | Hearing loss |
Heart defect |
Genital anomalies |
Renal anomalies |
Cleft lip/ palate |
TEF | Growth deficiency |
DD | CN dysfunction |
Skeletal anomalies |
Brain anomalies |
Feeding difficulties |
Verloes criteria |
Proposed criteria |
Bergman rec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 1774C>T | NS | + | − | + | + | + | − | + | − | + | − | − | + | NA | NA | − | − | T | Y | seq CHD7 | |
| 2 | M | 6322G>A | MS | DC/PrD/D | + | − | + | + | + | − | + | − | − | − | − | + | + | NA | − | − | T | Y | seq CHD7 |
| 3 | M | 2254A>T | NS | + | + | NA | + | + | − | + | + | + | − | + | + | + | + | − | + | T | Y | seq CHD7 | |
| 4 | F | 2764C>T | NS | + | − | + | + | + | + | NA | NA | − | − | NA | NA | + | NA | + | + | T | Y | seq CHD7 | |
| 5 | M | 3881T>C | MS | DC/PrD/D | + | + | NA | + | + | + | NA | + | − | − | + | + | NA | + | + | NA | T | Y | seq CHD7 |
| 6 | F | 2839C>T | NS | − | − | + | + | + | + | − | NA | + | + | + | + | + | + | − | + | T† | Y | seq CHD7 | |
| 7 | M | 5458C>T | NS | + | − | + | + | + | + | + | − | − | − | + | + | + | + | − | + | T | Y | seq CHD7 | |
| 8 | M | 729delC | FS | + | − | + | + | + | + | + | + | − | − | + | + | + | − | NA | + | T | Y | seq CHD7 | |
| 9 | F | 2724G>A | NS | − | + | NA | + | + | + | − | − | − | − | + | + | + | + | + | + | * | Y | seq CHD7 | |
| 10 | M | 7282C>T | NS | − | − | + | + | + | − | + | − | − | − | + | + | + | + | + | − | A | Y | seq CHD7 | |
| 11 | F | 7447G>T | NS | + | + | + | + | + | + | − | − | − | − | − | + | + | NA | + | + | T | Y | seq CHD7 | |
| 12 | F | 1925dupA | FS | − | − | NA | + | + | + | − | + | − | − | + | + | + | + | − | + | * | Y | seq CHD7 | |
| 13 | F | 2689dupC | FS | + | + | + | + | + | + | − | + | − | + | + | + | NA | − | − | + | T | Y | seq CHD7 | |
| 14 | M | 2839C>T | NS | + | + | + | + | − | + | + | − | + | − | − | + | + | NA | + | + | T | Y | seq CHD7 | |
| 15 | M | 4164G>A | NS | + | − | NA | + | + | + | + | + | + | + | + | + | + | NA | + | + | T† | Y | seq CHD7 | |
| 16 | M | 4164G>A | NS | + | + | + | + | + | + | + | NA | − | − | + | + | NA | + | NA | + | T | Y | seq CHD7 | |
| Total number affected | 12/16 | 7/16 | 11/11 | 16/16 | 15/16 | 12/16 | 9/14 | 6/13 | 5/16 | 3/16 | 11/15 | 15/15 | 12/12 | 8/10 | 7/14 | 12/15 | |||||||
| 1 | F | + | + | + | − | + | + | − | + | − | − | + | + | NA | NA | NA | + | T | Y | seq CHD7 | |||
| 2 | M | + | − | + | + | + | + | + | + | + | − | + | + | NA | + | NA | + | T | Y | seq CHD7 | |||
| 3 | M | + | − | + | + | + | + | + | + | + | − | − | + | NA | + | − | + | A | Y | seq CHD7 | |||
| 4 | F | NA | − | + | + | − | − | − | + | − | + | + | + | + | NA | + | + | * | * | seq CHD7 | |||
| 5 | F | NA | − | NA | − | + | − | − | NA | + | − | + | + | NA | NA | NA | + | * | * | temp CT | |||
| 6 | F | − | − | + | − | + | − | − | NA | − | − | − | + | − | NA | NA | − | N | N | seq CHD7 | |||
| 7 | M | + | − | + | + | + | + | + | − | − | − | + | + | NA | NA | NA | + | T | Y | seq CHD7 | |||
| 8 | F | + | − | + | + | + | + | − | − | − | − | + | + | + | NA | − | + | T | Y | seq CHD7 | |||
| 9 | M | − | + | NA | + | + | + | + | + | − | − | + | + | + | NA | + | + | * | Y | seq CHD7 | |||
| 10 | F | + | − | + | + | + | + | − | + | − | − | − | + | + | + | + | + | T | Y | seq CHD7 | |||
| 11 | M | NA | + | NA | + | + | + | + | − | − | + | − | + | NA | + | − | − | * | Y | seq CHD7 | |||
| 12 | F | − | − | − | + | + | + | − | − | − | − | − | + | NA | − | − | + | N | N | seq CHD7 | |||
| Total number affected | 6/9 | 3/12 | 8/9 | 9/12 | 11/12 | 9/12 | 5/12 | 6/10 | 3/12 | 2/12 | 7/12 | 12/12 | 4/5 | 4/5 | 3/7 | 10/12 | |||||||
MT, Mutation Taster; PP, PolyPhen2; S, SIFT; DC, disease causing; PrD, probably damaging; D, deleterious; TEF, tracheoesophageal fistula; CN, cranial nerve; diff, difficulties; NS, nonsense; MS, missense; FS, frameshift; T, typical CHARGE; rec, recommendation; A, atypical CHARGE; N, does not meet criteria
substitution of cleft palate for choanal atresia as major criterion; temp CT, temporal CT scan; NA , not assessed
cannot be determined due to lack of temporal bone imaging or eye exam
We compared the clinical features of pathogenic CHD7 variant positive and CHD7 variant negative cohorts and found a similar frequency for most features assessed [Table 2]. Although we observed a higher frequency of choanal atresia in pathogenic CHD7 variant positive individuals (7/16 (44%) vs. 3/12 (25%)), and a lower frequency of renal anomalies in pathogenic CHD7 variant positive individuals (6/13 (46%) vs. 6/10 (60%)), these differences were not statistically significant. Genital anomalies have historically been considered as a minor clinical diagnostic criterion for CHARGE [Blake et al., 1998]. Interestingly, in our cohort, genital anomalies were only reported in male patients. Indeed, 9 of 10 males with pathogenic CHD7 variants and all 5 males without pathogenic CHD7 variants were found to have a genital anomaly.
Table 2.
Frequency of clinical features in pathogenic CHD7 variant positive vs. negative patients
| Clinical Features |
Hale | Lalani 2005 | Zentner 2010 |
Bergman 2011 CHD7+ |
Sum | ||||
|---|---|---|---|---|---|---|---|---|---|
| CHD7+ | CHD7− | CHD7+ | CHD7− | CHD7+ | CHD7− | CHD7+ | CHD7− | ||
| Coloboma | 12/16 (75%) |
6/9 (67%) |
55/62 (89%) |
30/43 (70%) |
190/253 (75%) |
74/114 (65%) |
189/234 (81%) |
446/567 (79%) |
110/166 (66%) |
| Choanal atresia | 7/16 (44%) |
3/12 (25%) |
34/57 (60%) |
23/39 (59%) |
95/247 (38%) |
52/110 (47%) |
99/179 (55%) |
235/499 (47%) |
78/161 (48%) |
| Inner ear anomalies |
11/11 (100%) |
8/9 (89%) |
21/22 (95%) |
9/10 (90%) |
94/96 (98%) |
21/28 (75%) |
110/117 (94%) |
236/246 (96%) |
38/47 (81%) |
| External ear anomalies |
16/16 (100%) |
9/12 (75%) |
59/62 (95%) |
39/42 (93%) |
214/235 (91%) |
46/51 (90%) |
224/231 (97%) |
513/544 (94%) |
94/105 (90%) |
| Hearing loss | 15/16 (94%) |
11/12 (92%) |
54/59 (92%) |
36/38 (95%) |
198/223 (89%) |
83/103 (86%) |
267/298 (90%) |
130/153 (85%) |
|
| Heart defect | 12/16 (75%) |
9/12 (75%) |
54/59 (92%) |
30/42 (71%) |
193/250 (77%) |
86/120 (72%) |
191/252 (76%) |
450/577 (78%) |
125/174 (72%) |
| Genital anomalies |
9/14 (64%) 9/9 ♂ (100%♂) |
5/12 (42%) 5/5 ♂ (100%♂) |
29/53 (55%) |
26/39 (67%) |
116/187 (62%) |
46/66 (70%) |
118/145 (81%) |
272/399 (68%) |
77/117 (66%) |
| Renal anomalies |
6/13 (46%) |
6/10 (60%) |
|||||||
| Cleft lip/palate | 5/16 (31%) |
3/12 (25%) |
18/60 (30%) |
9/41 (22%) |
79/242 (33%) |
34/119 (29%) |
79/163 (48%) |
181/481 (38%) |
46/172 (27%) |
| TEF | 3/16 (19%) |
2/12 (17%) |
10/55 (18%) |
3/40 (8%) |
35/185 (19%) |
8/45 (18%) |
42/146 (29%) |
90/402 (22%) |
15/97 (15%) |
| Growth Deficiency |
11/15 (73%) |
7/12 (58%) |
101/141 (72%) |
31/33 (94%) |
35/94 (37%) |
147/250 (59%) |
38/45 (84%) |
||
| Developmental delay |
15/15 (100%) |
12/12 (100%) |
107/141 (76%) |
44/47 (94%) |
147/149 (99%) |
269/305 (88%) |
56/59 (95%) |
||
| CN dysfunction | 12/12 (100%) |
4/5 (80%) |
72/187 (39%) |
19/102 (19%) |
173/174 (99%) |
257/373 (69%) |
23/107 (21%) |
||
| Skeletal anomalies |
8/10 (80%) |
4/5 (80%) |
|||||||
| Brain anomalies | 7/14 (50%) |
3/7 (43%) |
|||||||
| Feeding difficulty |
12/15 (80%) |
10/12 (83%) |
90/110 (82%) |
102/125 (82%) |
|||||
| Immuno- deficiency |
0/1 (0%) |
1/2 (50%) |
|||||||
| Endocrine dysfunction |
4/7 (57%) |
3/5 (60%) |
|||||||
| Behavioral issues |
4/4 (100%) |
2/2 (100%) |
|||||||
Abbreviations: CN, cranial nerve; TEF, tracheo-esophageal fistula.
Additionally, brain and skeletal/limb anomalies are considered minor features of CHARGE, having been reported in single cases and small cohort studies of individuals with pathogenic CHD7 variant positive CHARGE syndrome [Alazami et al., 2008; Brock et al., 2003; Doyle et al., 2005; Jongmans et al., 2006; Pagon et al., 1981; Van de Laar et al., 2007; Wright et al., 2009; Yu et al., 2013]. Previously reported brain and skeletal/limb anomalies in pathogenic CHD7 variant positive individuals are listed in Table 3. In our patients, brain and skeletal/limb anomalies were observed at higher frequencies (50% and 80%, respectively) than previously published.
Table 3.
Skeletal, limb and brain anomalies in pathogenic CHD7 variant positive patients
| Patient Number |
Skeletal/limb anomaly | Brain anomaly |
|---|---|---|
| 1 | NA | None |
| 2 | NA | None |
| 3 | Thoracic kyphosis;
cervical lordosis, failure of fusion of the posterior elements of C6 |
None |
| 4 | NA | Small splenium of the
corpus callosum |
| 5 | Fused vertebrae, scoliosis | Chiari I malformation |
| 6 | Scoliosis | None |
| 7 | Neuromuscular scoliosis | None |
| 8 | None | NA |
| 9 | Kyphoscoliosis | Mild dysmorphic brain with
prominent ventricular system including the fourth and third ventricles, small pons |
| 10 | Fused vertebrae,
congenital scoliosis and kyphosis |
Dysmorphic cerebellar
structures wrapping around the lateral aspects of the brainstem |
| 11 | NA | Hypoplasia of the inferior
cerebellar vermis and thin corpus callosum |
| 12 | Polysyndactyly of bilateral
5th and 6th toes |
None |
| 13 | None | None |
| 14 | NA | Hypoplasia of the inferior
cerebellar vermis |
| 15 | NA | Hypoplasia of the inferior
cerebellar vermis |
| 16 | Bilateral hypoplasia of 5th
finger phalanges |
NA |
NA, not assessed
Review of Previously Published Atypical Patients
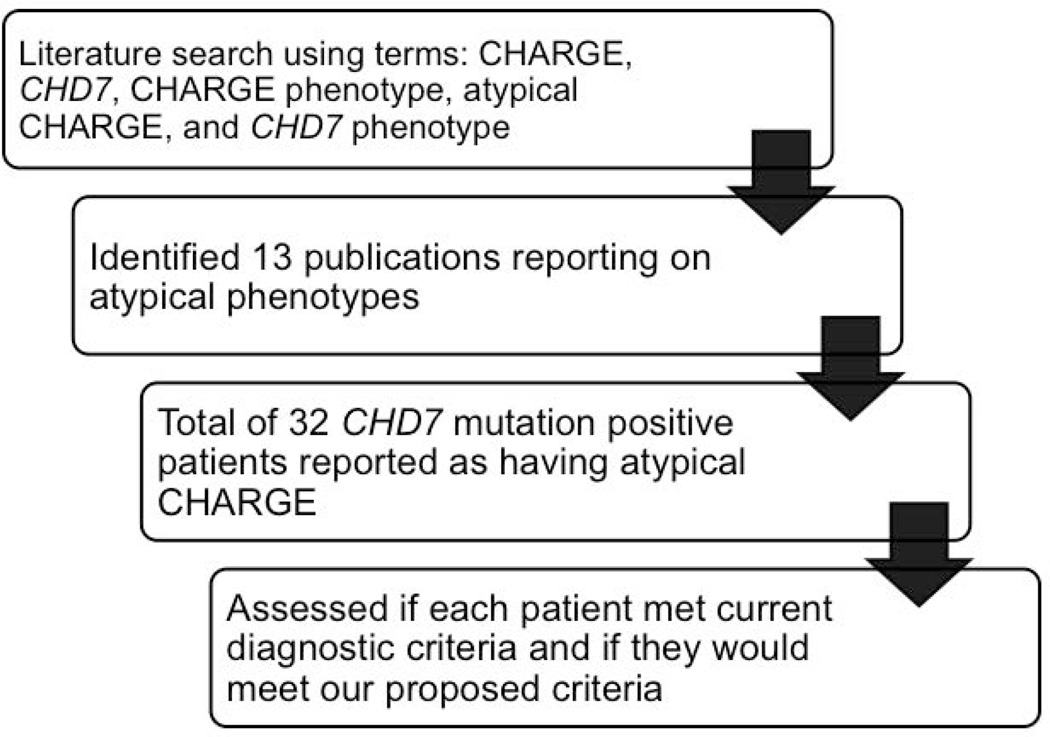
Since the discovery of CHD7 as the major gene for CHARGE, 32 individuals with atypical presentations have been reported in several large patient cohorts and unique case studies [Bergman et al., 2011; Cappuccio et al., 2014; Delahaye et al., 2007; Hughes et al., 2014; Jain et al., 2011; Jongmans et al., 2008; Jongmans et al., 2009; Michelucci et al., 2010; Palumbo et al., 2013; Randall et al., 2009; Wincent et al., 2008]. We identified these 32 individuals by conducting a literature review as described in Figure 2. Their clinical features and associated variation are presented in Table 2. All patients with atypical presentations lacked coloboma or choanal atresia, both of which are major Verloes diagnostic criteria for CHARGE. In addition, Vissers et al. described one individual with an atypical phenotype who presented without coloboma or choanal atresia, but was found to have many characteristic features of CHARGE including semicircular canal agenesis, hearing loss, facial nerve palsy, genital hypoplasia, and restriction of growth and development [Vissers et al., 2004]. Under Verloes criteria, the individual described by Vissers et al meets 1 of 3 major criteria, and would be considered to have a diagnosis of atypical CHARGE syndrome.
Figure 2.
Flowchart describing the literature review process. A PubMed search was conducted using various search terms related to CHARGE syndrome, CHD7, and atypical phenotypes. Each publication identified in the search was reviewed to identify patients reported to have atypical or partial CHARGE, or to not meet diagnostic criteria. Clinical features were noted for each patient. We then assessed whether each patient would meet current diagnostic criteria as well as our proposed diagnostic criteria.
In our cohort, 13 of the 16 pathogenic CHD7 variant positive patients met Verloes criteria for a clinical diagnosis of CHARGE, one met Verloes criteria for an atypical diagnosis of CHARGE, and two could not be fully assessed due to lack of temporal bone imaging [Table 1]. Among our 12 CHD7 variant negative patients, five patients met Verloes diagnostic criteria , one met Verloes criteria for an atypical diagnosis of CHARGE, four did not have comprehensive clinical evaluations to assess for the presence of coloboma or semicircular canal hypoplasia, and two did not meet clinical criteria for a diagnosis of CHARGE, yet underwent CHD7 sequencing due to presence of hearing loss with various additional minor features. A single pathogenic CHD7 variant positive patient in our cohort met criteria for an atypical CHARGE diagnosis and lacked coloboma or choanal atresia, but had characteristic inner ear abnormalities. This patient also displayed external ear anomalies, hearing loss, cranial nerve dysfunction, growth deficiency, and developmental delay, consistent with the highly diagnostic nature of these features in CHARGE.
Proposal of new diagnostic criteria
Current clinical diagnostic criteria for CHARGE were published ten years ago [Verloes 2005]. More recently, efforts have been made to establish guidelines for clinical circumstances in which CHD7 testing should be pursued [Bergman et al., 2011]. In their paper, Bergman et al. divided all features associated with CHARGE into cardinal and supportive features, and provided guidelines for when CHD7 testing should be pursued depending on the combination of features present [Bergman et al., 2011]. Under these guidelines, 27 of 28 patients in our cohort would be recommended for CHD7 analysis (see Table 1).
In assessing the clinical data available for our 16 pathogenic CHD7 variant positive patients, we found that the most common features were inner ear anomaly, external ear anomaly, hearing loss, cranial nerve dysfunction and developmental delay. As in previous studies, other features were also seen at high frequencies in our cohort, including coloboma, heart defects, and feeding difficulties. While choanal atresia is typically considered a major feature of CHARGE syndrome, in our cohort it was observed in less than half of pathogenic CHD7 variant positive individuals. Substitution of cleft palate according to revisions to Verloes criteria by Blake and Prasad elevated two patients to full CHARGE diagnoses [Blake et al., 2006].
As a result of our analysis and review of existing literature, we propose an update to the clinical diagnostic criteria for CHARGE syndrome. To account for individuals with milder phenotypes (including instances of inherited CHD7 variants), we suggest inclusion of pathogenic CHD7 variant status as a major feature (Table 4). Under these new criteria, pathogenic CHD7 variant status plus one major feature would be sufficient for a diagnosis of CHARGE syndrome. We also propose broadening the description of supportive features associated with CHARGE syndrome, as listed in Table 4. Previously, recommendations were made to aid decisions for molecular testing [Bergman et al., 2011]. We agree with Bergman et al [Bergman et al., 2011] that CHD7 variant testing is indicated for individuals presenting with more than a single major or minor criterion. We also acknowledge that in the current “genotype first” environment of expanded clinical genetic sequencing, a pathogenic or suspected pathogenic variant in CHD7 should warrant careful clinical correlation and examination of major diagnostic criteria.
Table 4.
Comparison of Verloes (2005) and Proposed Clinical Diagnostic Criteria for CHARGE Syndrome
|
Verloes
(2005) |
Proposed Criteria |
|---|---|
Major criteria:
|
Major criteria:
|
Minor criteria:
|
Minor criteria:
|
| Inclusion rule: | Inclusion rule: |
| Typical CHARGE: 3 major OR 2 major + 2
minor Partial CHARGE: 2 major + 1 minor Atypical CHARGE: 2 major + 0 minor OR 1 major + 3 minor |
CHARGE: 2 major + any number of minor |
DISCUSSION
Here we identified 28 individuals who had been considered for a clinical diagnosis of CHARGE syndrome and were tested for CHD7 variants. We summarized the clinical features reported in these patients, and noted the high prevalence of skeletal and brain anomalies in this cohort [Tables 1, 2, 3]. Of the 16 pathogenic CHD7 variant positive patients, one was found to meet an atypical CHARGE diagnosis (by Verloes criteria), while two others had insufficient clinical assessments to provide a definitive diagnosis.
Phenotype frequencies between pathogenic CHD7 variant positive vs negative patients in our cohort differed slightly from previously published cohorts [Bergman et al., 2011; Lalani et al., 2006; Zentner et al., 2010]. In both Lalani et al and Zentner et al, there were significant differences between pathogenic CHD7 variant positive and negative individuals in the frequencies of coloboma, heart defects, growth delay, developmental delay, and temporal bone anomalies (Table 2). The clinical features with the largest variations in frequencies across all four cohorts were choanal atresia, cranial nerve dysfunction, growth deficiency, and genital anomalies. In our cohort, the high prevalence of genital anomalies in males may reflect referral patterns by Pediatricians and Endocrinologists and the relative difficulty of identifying genital hypoplasia in females, especially prior to the onset of puberty. Together, these observations also raise the question of whether genital anomalies are a useful minor diagnostic criterion for females with CHARGE.
We acknowledge ascertainment bias in the small cohort size of our analysis. The individuals included in our study were identified by review of a clinical database of patients seen at The University of Michigan Pediatric Genetics clinic. Importantly, the purpose of this study was to evaluate the cases seen in our own clinic and not to provide prevalence or incidence information. In addition, our sample size was intentionally restricted to those who had undergone CHD7 variant testing, and therefore our study does capture the multiple factors that influence whether a given individual has CHD7 variant testing performed, including insurance coverage, parental motivation, and access to clinical genetics services.
We identified 32 previously published pathogenic CHD7 variant positive patients with atypical presentations. Together with our patient, these cases highlight the great variability in presence and severity of features associated with pathogenic CHD7 variants, and the need for more comprehensive clinical diagnostic criteria. Our data and review of the literature on atypical presentations also suggest that the presence of one major clinical feature of CHARGE, along with a pathogenic CHD7 variant, with or without other supportive features (e.g. hearing loss, external ear anomalies, and developmental delay) could be considered sufficient to establish the diagnosis.
It is important to note that the interpretation of variation in CHD7 remains dependent on the variant type. Nonsense and frameshift variants with predicted protein truncation and nonsense mediated decay are typically considered pathogenic, whereas missense variants are not considered pathogenic unless reported de novo in another individual with CHARGE. The importance of variant recurrence in assigning pathogenic status is illustrated by a report from Jain and colleagues of an individual identified with a missense CHD7 variant (2230G>A) and a unique phenotype not consistent with CHARGE by Verloes criteria (Table 5) [Jain et al., 2011]. The patient was an 18-year-old man initially seen for refractory hypocalcemia. He was born at 25 weeks of gestation and during infancy underwent surgery to correct a ventricular septal defect and right eyelid coloboma. He also had congenital hypothyroidism, bilateral sensorineural hearing loss, global developmental delay, flexion deformity of the right thumb, short stature, and bilateral multi-cystic dysplastic kidneys. CT of the head showed calcifications consistent with chronic hypocalcemia. The missense CHD7 variant (2230G>A) reported in this patient was previously described as a polymorphism, although no functional testing was done [Vuorela et al., 2007]. This variant encodes for an amino acid that is highly conserved and exists in the population at a minor allele frequency of 0.005 according to the 1000 Genomes variant browser. This amino acid change is predicted to be disease causing by MutationTaster, probably damaging by PolyPhen2, and tolerated according to SIFT. If the variant identified in this patient is found to be present in another patient with CHARGE, then according to our newly proposed criteria, a diagnosis of CHARGE would be made.
Table 5.
Previously published cases of pathogenic CHD7 variant positive atypical CHARGE
| Sex | Variant | Variant type |
MT/PP/S Prediction |
Coloboma | Choanal atresia |
Inner ear anomalies |
Ext ear anomalies |
Hearing loss |
Heart defect |
Genital anomalies |
Renal anomalies | CL/P | TEF | Growth deficiency |
DD | CN dysfunction |
Skeletal anomalies |
Brain anomalies |
Feeding difficulties |
Verloes Criteria |
Proposed Criteria |
Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 1714C>T | NS | − | − | + | + | − | + | + | + | + | + | T† | Y | [1] | |||||||
| F | 5833C>T | NS | − | − | + | + | + | + | − | + | + | A | Y | [2] | ||||||||
| M | 2254A>T | NS | + | + | + | + | + | + | + | + | + | T† | Y | [3] | ||||||||
| F | 2501C>T | MS | DC/PrD/D | + | − | + | + | + | − | * | Y | [3] | ||||||||||
| M | 469C>T | NS | + | + | + | + | + | + | + | + | * | Y | [3] | |||||||||
| M | 469C>T | NS | − | + | − | − | − | * | Y | [3] | ||||||||||||
| F | 3297G>A | NS | − | − | + | + | + | − | + | − | + | + | A | Y | [4] | |||||||
| M | 2442+5G>C | SS | − | − | + | + | + | − | + | − | − | − | − | + | A | Y | [5] | |||||
| M | 2442+5G>C | SS | − | − | + | + | + | − | + | − | + | − | − | + | T† | Y | [5] | |||||
| F | 6322G>A | MS | DC/PrD/D | − | − | + | + | + | + | + | * | Y | [5] | |||||||||
| F | 6322G>A | MS | DC/PrD/D | − | − | + | + | + | − | − | − | − | N | Y | [5] | |||||||
| M | 6322G>A | MS | DC/PrD/D | + | + | * | Y | [5] | ||||||||||||||
| F | 6322G>A | MS | DC/PrD/D | + | * | Y | [5] | |||||||||||||||
| M | 8803G>T | NS | + | + | * | * | [6] | |||||||||||||||
| M | 6347T>A | MS | DC/PrD/D | + | + | + | * | Y | [6] | |||||||||||||
| M | Del8q12.1- q12.2 |
Del | − | − | + | − | + | + | + | * | Y | [7] | ||||||||||
| M | 2230G>A | MS | DC/PrD/T | + | + | + | + | − | + | + | + | * | Y | [8] | ||||||||
| F | 4406A>G | MS | DC/PrD/D | − | + | + | − | − | * | Y | [9] | |||||||||||
| M | 7769delC | FS | + | − | + | * | Y | [9] | ||||||||||||||
| M | 6322G>A | MS | DC/PrD/D | + | − | + | + | * | Y | [9] | ||||||||||||
| F | Del8q12.1- q12.3 |
Del | − | − | − | + | + | + | − | + | − | + | N | Y | [10] | |||||||
| M | 6290A>C | MS | DC/PrD/D | + | + | + | + | T† | Y | [11] | ||||||||||||
| M | 6290A>C | MS | DC/PrD/D | + | + | + | + | + | + | T† | Y | [11] | ||||||||||
| F | 6290A>C | MS | DC/PrD/D | + | * | Y | [11] | |||||||||||||||
| M | 6290A>C | MS | DC/PrD/D | + | + | + | + | * | * | [11] | ||||||||||||
| F | 6290A>C | MS | DC/PrD/D | + | * | * | [11] | |||||||||||||||
| M | 6290A>C | MS | DC/PrD/D | + | + | * | Y | [11] | ||||||||||||||
| F | 6290A>C | MS | DC/PrD/D | + | * | * | [11] | |||||||||||||||
| F | 6290A>C | MS | DC/PrD/D | + | + | * | Y | [11] | ||||||||||||||
| M | 4353+3A>G | SS | + | + | + | + | − | + | + | + | + | + | − | T† | Y | [12] | ||||||
| F | 8077- 10T>A |
SS | − | + | + | + | + | + | * | Y | [12] | |||||||||||
| F | 2954_2956 delACA |
Del | − | + | + | + | + | + | − | − | + | + | + | * | Y | [13] |
major criteria not assessed, so criteria cannot be applied
N, does not meet criteria
Blank, not reported
MS, missense; NS=nonsense; SS=splice-site; FS=frameshift; Del=deletion
substitution of cleft palate for choanal atresia as major criterion
Vissers, L.E.L.M.; van Ravenswaaij, C.M.A.; Admiraal, R.; Hurst, J.A.; de Vries, B.B.A.; Janssen, I.M.; van der Vliet, W.A.; Huys, E.H.L.P.G.; de Jong, P.J.; Hamel, B.C.J., et al. Mutations in a new member of the chromodomain gene family cause charge syndrome. Nat Genet 2004, 36, 955–957.
Jongmans, M.C.J.; Admiraal, R.J.; van der Donk, K.P.; Vissers, L.E.L.M.; Baas, A.F.; Kapusta, L.; van Hagen, J.M.; Donnai, D.; de Ravel, T.J.; Veltman, J.A., et al. Charge syndrome: The phenotypic spectrum of mutations in the chd7 gene. J Med Genet 2006, 43, 306–314.
Delahaye, A.; Sznajer, Y.; Lyonnet, S.; Elmaleh-Berges, M.; Delpierre, I.; Audollent, S.; Wiener-Vacher, S.; Mansbach, A.L.; Amiel, J.; Baumann, C., et al. Familial charge syndrome because of chd7 mutation: Clinical intra- and interfamilial variability. Clin Genet 2007, 72, 112–121.
Wincent, J.; Holmberg, E.; Stromland, K.; Soller, M.; Mirzaei, L.; Djureinovic, T.; Robinson, K.; Anderlid, B.; Schoumans, J. Chd7 mutation spectrum in 28 swedish patients diagnosed with charge syndrome. Clin Genet 2008, 74, 31–38.
Jongmans, M.C.J.; Hoefsloot, L.H.; van der Donk, K.P.; Admiraal, R.J.; Magee, A.; van de Laar, I.; Hendriks, Y.; Verheij, J.B.G.M.; Walpole, I.; Brunner, H.G., et al. Familial charge syndrome and the chd7 gene: A recurrent missense mutation, intrafamilial recurrence and variability. Am J Med Genet A 2008, 146A, 43–50.
Jongmans, M.C.J.; van Ravenswaaij-Arts, C.M.A.; Pitteloud, N.; Ogata, T.; Sato, N.; Claahsen-van der Grinten, H.L.; van der Donk, K.; Seminara, S.; Bergman, J.E.H.; Brunner, H.G., et al. Chd7 mutations in patients initially diagnosed with kallmann syndrome - the clinical overlap with charge syndrome. Clin Genet 2009, 75, 65–71.
Randall, V.; McCue, K.; Roberts, C.; Kyriakopoulou, V.; Beddow, S.; Barrett, A.N.; Vitelli, F.; Prescott, K.; Shaw-Smith, C.; Devriendt, K., et al. Great vessel development requires biallelic expression of chd7 and tbx1 in pharyngeal ectoderm in mice. J Clin Invest 2009, 119, 3301–3310.
Jain, S.; Kim, H.G.; Lacbawan, F.; Meliciani, I.; Wenzel, W.; Kurth, I.; Sharma, J.; Schoeneman, M.; Ten, S.; Layman, L.C., et al. Unique phenotype in a patient with charge syndrome. Int J Pediatr Endocrinol 2011, 2011, 11.
Bergman, J.E.H.; Janssen, N.; Hoefsloot, L.H.; Jongmans, M.C.J.; Hofstra, R.M.W.; van Ravenswaaij-Arts, C.M.A. Chd7 mutations and charge syndrome: The clinical implications of an expanding phenotype. J Med Genet 2011, 48, 334–342.
Palumbo, O.; Palumbo, P.; Stallone, R.; Palladino, T.; Zelante, L.; Carella, M. 8q12.1q12.3 de novo microdeletion involving the chd7 gene in a patient without the major features of charge syndrome: Case report and critical review of the literature. Gene 2013, 513, 209–213.
Hughes, S.S.; Welsh, H.I.; Safina, N.P.; Bejaoui, K.; Ardinger, H.H. Family history and clefting as major criteria for charge syndrome. Am J Med Genet A 2014, 164A, 48–53.
Cappuccio, G.; Ginocchio, V.M.; Maffe, A.; Ungari, S.; Andria, G.; Melis, D. Identification of two novel splice-site mutations in chd7 gene in two patients with classical and atypical charge syndrome phenotype. Clin Genet 2014, 85, 201–202.
Michelucci, A.; Ghirri, P.; Iacopetti, P.; Conidi, M.E.; Fogli, A.; Baldinotti, F.; Lunardi, S.; Forli, F.; Moscuzza, F.; Berrettini, S., et al. Identification of three novel mutations in the chd7 gene in patients with clinical signs of typical or atypical charge syndrome. Int J Pediatr Otorhinolaryngol 2010, 74, 1441–1444.
Because the majority of pathogenic variants in CHD7 associated with CHARGE syndrome are nonsense or predicted loss of function [Janssen et al., 2012], haploinsufficiency of CHD7 is thought to be the major pathogenic mechanism underlying CHARGE syndrome. To date, seven patients have been reported to carry deletions involving CHD7 [Arrington et al., 2005; Hurst et al., 1991; Palumbo et al., 2013; Randall et al., 2009; Vissers et al., 2004; Wincent et al., 2008]. Of these seven, two do not currently meet diagnostic criteria for CHARGE syndrome (Table 5) [Palumbo et al., 2013; Randall et al., 2009]. However, under our proposed criteria, both patients with CHD7 deletions would be given a diagnosis of CHARGE. One of these patients was reported to have a de novo 8q12.1q12.3 deletion involving the entire CHD7 gene [Palumbo et al., 2013]. This patient presented with facial asymmetry, failure to thrive, developmental delay, gastro-esophageal reflux disease, external ear anomalies, and normal middle and inner ears. This patient’s deletion on chromosome 8 also included several other candidate genes, which could influence the patient’s phenotype.
The phenotypic spectrum of pathogenic CHD7 variation has recently expanded to include individuals with a diagnosis of Kallmann syndrome. In one Kallmann syndrome cohort, three individuals were identified as having CHD7 variants, two of which had additional features suggestive of atypical CHARGE syndrome (Table 5) [Jongmans et al., 2009]. Additional reports have confirmed the presence of CHD7 variants in a subset of patients clinically diagnosed with Kallmann syndrome [Bergman et al., 2012; Marcos et al., 2014]. Individuals with Kallmann syndrome often have one or more supportive features of CHARGE syndrome, yet do not fulfill clinical diagnostic criteria for CHARGE. CHD7 variants identified in these individuals are commonly missense variants than frameshift, nonsense, or deletion variants, which may explain the milder CHARGE phenotypes. Under our newly proposed criteria, one of the two atypical patients described by Jongmans and colleagues would also be considered as having CHARGE syndrome, and the other would not be assigned a diagnosis due to incomplete clinical assessment [Jongmans et al., 2009].
In a comprehensive study by Bergman and colleagues in 2011, 17% (22/124) of pathogenic CHD7 variant positive patients could not be clinically diagnosed with CHARGE syndrome (based on Verloes criteria) due to the presence of none or only one major feature (Table 5) [Bergman et al., 2011]. Of these 22 patients, three mildly affected pathogenic CHD7 variant positive individuals were described. One had characteristic external ear anomalies, normal semicircular canals, normal cranial nerve function, and normal pubertal development. The second pathogenic CHD7 variant positive patient came to clinical attention only after he had severely affected children. His clinical features consisted of mild semicircular canal anomalies and mild hearing loss. The third pathogenic CHD7 variant positive patient in Bergman et al. was initially diagnosed with Kallmann syndrome and sensorineural hearing loss, but after CHD7 testing, temporal bone CT was performed and revealed hypoplasia of the semicircular canals [Bergman et al., 2011]. With our newly proposed diagnostic criteria, all three of these patients with atypical presentations would be considered as having a diagnosis of CHARGE syndrome.
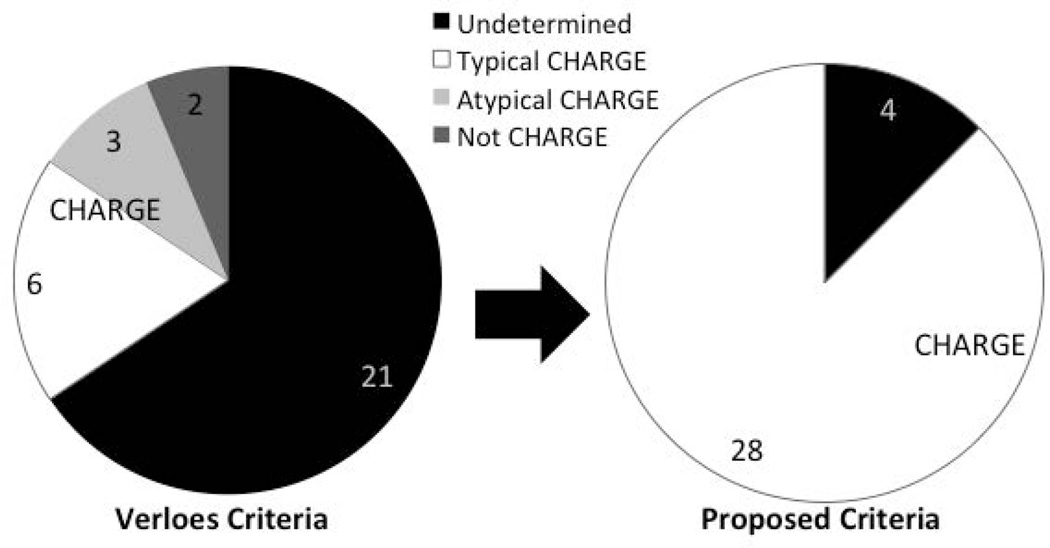
Interestingly, of the 32 pathogenic CHD7 variant positive individuals previously reported as having atypical CHARGE or not meeting diagnostic criteria, 28 would be assigned a diagnosis of CHARGE under our proposed criteria (Figure 3). 21 of the 32 individuals cannot be assigned a diagnosis of CHARGE using Verloes criteria because temporal bone imaging or other critical clinical evaluations have not been performed (Table 5). In addition, the three pathogenic CHD7 variant positive patients (one with atypical CHARGE and two with incomplete clinical information) described here would be given a diagnosis of CHARGE under our proposed criteria, due to the presence of at least one other major feature.
Figure 3.
Application of new CHARGE diagnostic criteria. Pie charts show numbers of published cases with undetermined, atypical, or no CHARGE diagnosis, both before and after application of the new diagnostic criteria.
These expanded diagnostic criteria should allow for inclusion of individuals formerly described as having “partial” or “atypical” CHARGE, and will allow for potential assignment of other diagnoses in individuals who present with CHD7 variants and subsets of features such as Kallmann or Autism Spectrum Disorder. We highlight the important of a thorough clinical assessment including CT of the temporal bones, full ophthalmological exam, nasal endoscopy, audiometry, cardiac evaluation, brain imaging, and renal ultrasound as early as possible. Many features associated with CHARGE syndrome can be missed unless specifically explored. For example, the high frequency of skeletal/limb, renal, and brain anomalies present in our cohort demonstrates the need to assess for these features.
Notably, the most atypical and mild phenotypes reported in association with pathogenic CHD7 variants have been described in cases of inherited CHARGE syndrome. Jongmans et al. reported three different families with inherited pathogenic CHD7 variants and mild phenotypes observed in affected individuals [Jongmans et al., 2008]. Inherited cases of CHARGE syndrome have also been demonstrated in families with as many as 3 generations of family members found to carry a pathogenic CHD7 variant (Table 5) [Hughes et al., 2014]. The phenotypes in pathogenic variant positive family members ranged from unilateral hearing loss in one individual to bilateral cleft lip/palate, bilateral coloboma, growth deficiency, and external ear anomaly in another individual. As a result of the wide phenotypic variability associated with a pathogenic CHD7 variant in this family, the authors proposed that a positive family history, i.e. any first degree relative with at least one major feature of CHARGE, should be considered as a clinical diagnostic criterion for CHARGE syndrome. Application of the diagnostic criteria such as those we propose in Table 4 would allow for these milder phenotypes to be considered as consistent with CHARGE syndrome. However, not all of the family members described by Hughes et al. currently meet our proposed diagnostic criteria. Importantly, none of the family members have undergone temporal bone imaging. Structure of the semicircular canals is a critical piece of clinical information in making a diagnosis of CHARGE; without this information, a diagnosis of CHARGE syndrome would not be possible in the absence of other major features.
The addition of pathogenic CHD7 variant status to clinical diagnostic criteria is not a new approach in clinical genetics. Notable examples of other genetic conditions where pathogenic variant status is specifically included in diagnostic criteria include Marfan and Stickler syndromes [Loeys et al., 2010; Rose et al., 2005]. In addition, wide phenotypic variability upon expanded molecular testing has been observed for other genetic conditions. This has led to replacement of the word “syndrome” for broader terms that reflect the underlying molecular basis of the condition, such as MECP2-Related disorders, MED12-Related Disorders, COL4A1-Related Disorders and RASopathies [Graham et al., 2013; Kuo et al., 2012; Neul et al., 2010; Tidyman et al., 2009]. The term “CHD7-related disorders” may be appropriate for individuals with pathogenic CHD7 variants and subsets of CHARGE features.
Additional CHARGE related genes may also be identified, in which case additional revision of these criteria may become necessary. It may become useful to use the term CHD7-Related disorders, in the same way that EZH2-Related Weaver Syndrome has replaced the traditional syndromic name. We suggest that while the designation of typical vs. atypical CHARGE denotes a difference in phenotype, it may not fully capture phenotypic severity. In addition, it relies on diagnostic algorithms that can be complex and difficult to remember. Instead, patients and providers may consider using the qualifiers “mild” or “severe” rather than typical or atypical as a reflection of clinical severity.
Broadening of the CHARGE clinical diagnostic criteria as proposed here could help clarify, for clinicians and families, whether individuals with milder features who carry a pathogenic CHD7 variant should be considered as having CHARGE. It could also provide a basis for assessing risk of other CHARGE features in children of individuals who have pathogenic CHD7 variants and only minor CHARGE features, such as hypogonadotropic hypogonadism, intellectual disability, or Autism Spectrum Disorder. Our goal is not to diminish the importance of accurate clinical classification and careful phenotyping. On the contrary, we believe that detailed attention to clinical features, both major and minor, that occur in association with CHARGE and/or pathogenic CHD7 variants, will ultimately determine how best to diagnose, counsel, and care for affected individuals.
Acknowledgments
We thank the patients and their families for participating in this research. This work was supported by NIH R01 DC009410 and the Donita B. Sullivan, MD Professorship to DMM, NIH T32 GM007863 to ANN and DMM, and by a Rackham Graduate School fellowship to CAH.
REFERENCES
- Alazami AM, Alzahrani F, Alkuraya FS. Expanding the “E” in CHARGE. Am J Med Genet A. 2008;146A:1890–1892. doi: 10.1002/ajmg.a.32376. [DOI] [PubMed] [Google Scholar]
- Arrington CB, Cowley BC, Nightingale DR, Zhou H, Brothman AR, Viskochil DH. Interstitial deletion 8q11.2-q13 with congenital anomalies of CHARGE association. Am J Med Genet A. 2005;133:326–330. doi: 10.1002/ajmg.a.30562. [DOI] [PubMed] [Google Scholar]
- Balasubramanian R, Choi JH, Francescatto L, Willer J, Horton ER, Asimacopoulos EP, Stankovic KM, Plummer L, Buck CL, Quinton R, Nebesio TD, Mericq V, Merino PM, Meyer BF, Monies D, Gusella JF, Al Tassan N, Katsanis N, Crowley WF., Jr Functionally compromised CHD7 alleles in patients with isolated GnRH deficiency. Proc Natl Acad Sci U S A. 2014;111:17953–17958. doi: 10.1073/pnas.1417438111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels CF, Scacheri C, White L, Scacheri PC, Bale S. Mutations in the CHD7 gene: the experience of a commercial laboratory. Genet Test Mol Biomarkers. 2010;14:881–891. doi: 10.1089/gtmb.2010.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman JEH, Janssen N, Hoefsloot LH, Jongmans MCJ, Hofstra RMW, van Ravenswaaij-Arts CMA. CHD7 mutations and CHARGE syndrome: the clinical implications of an expanding phenotype. J of Med Genet. 2011;48:334–342. doi: 10.1136/jmg.2010.087106. [DOI] [PubMed] [Google Scholar]
- Bergman JEH, Janssen N, van der Sloot AM, de Walle HEK, Schoots J, Rendtorff ND, Tranebjaerg L, Hoefsloot LH, van Ravenswaaij-Arts CMA, Hofstra RMW. A novel classification system to predict the pathogenic effects of CHD7 missense variants in CHARGE syndrome. Hum Mutat. 2012;33:1251–1260. doi: 10.1002/humu.22106. [DOI] [PubMed] [Google Scholar]
- Blake KD, Davenport SL, Hall BD, Hefner MA, Pagon RA, Williams MS, Lin AE, Graham JM., Jr CHARGE association: an update and review for the primary pediatrician. Clin Pediatr. 1998;37:159–173. doi: 10.1177/000992289803700302. [DOI] [PubMed] [Google Scholar]
- Blake KD, Prasad C. CHARGE syndrome. Orphanet J Rare Dis. 2006;1:34. doi: 10.1186/1750-1172-1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock KE, Mathiason MA, Rooney BL, Williams MS. Quantitative analysis of limb anomalies in CHARGE syndrome: correlation with diagnosis and characteristic CHARGE anomalies. Am J Med Genet A. 2003;123A:111–121. doi: 10.1002/ajmg.a.20526. [DOI] [PubMed] [Google Scholar]
- Cappuccio G, Ginocchio VM, Maffe A, Ungari S, Andria G, Melis D. Identification of two novel splice-site mutations in CHD7 gene in two patients with classical and atypical CHARGE syndrome phenotype. Clin Genet. 2014;85:201–202. doi: 10.1111/cge.12115. [DOI] [PubMed] [Google Scholar]
- Corsten-Janssen N, du Marchie Sarvaas GJ, Kerstjens-Frederikse WS, Hoefsloot LH, van Beynum IM, Kapusta L, van Ravenswaaij-Arts CM. CHD7 mutations are not a major cause of atrioventricular septal and conotruncal heart defects. Am J Med Genet A. 2014 doi: 10.1002/ajmg.a.36747. [DOI] [PubMed] [Google Scholar]
- Delahaye A, Sznajer Y, Lyonnet S, Elmaleh-Berges M, Delpierre I, Audollent S, Wiener-Vacher S, Mansbach AL, Amiel J, Baumann C, Bremond-Gignac D, Attie-Bitach T, Verloes A, Sanlaville D. Familial CHARGE syndrome because of CHD7 mutation: clinical intra- and interfamilial variability. Clin Genet. 2007;72:112–121. doi: 10.1111/j.1399-0004.2007.00821.x. [DOI] [PubMed] [Google Scholar]
- Doyle C, Blake K. Scoliosis in CHARGE: a prospective survey and two case reports. Am J Med Genet A. 2005;133:340–343. doi: 10.1002/ajmg.a.30564. [DOI] [PubMed] [Google Scholar]
- Felix TM, Hanshaw BC, Mueller R, Bitoun P, Murray JC. CHD7 gene and non-syndromic cleft lip and palate. Am J Med Genet A. 2006;140:2110–2114. doi: 10.1002/ajmg.a.31308. [DOI] [PubMed] [Google Scholar]
- Graham JM., Jr A recognizable syndrome within CHARGE association: Hall-Hittner syndrome. Am J Med Genet. 2001;99:120–123. doi: 10.1002/1096-8628(2000)9999:999<00::aid-ajmg1132>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Graham JM, Jr, Schwartz CE. MED12 related disorders. Am J Med Genet A. 2013;161A:2734–2740. doi: 10.1002/ajmg.a.36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green GE, Huq FS, Emery SB, Mukherji SK, Martin DM. CHD7 mutations and CHARGE syndrome in semicircular canal dysplasia. Otol Neurotol. 2014;35:1466–1470. doi: 10.1097/MAO.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SS, Welsh HI, Safina NP, Bejaoui K, Ardinger HH. Family history and clefting as major criteria for CHARGE syndrome. Am J Med Genet A. 2014;164A:48–53. doi: 10.1002/ajmg.a.36192. [DOI] [PubMed] [Google Scholar]
- Hurst JA, Meinecke P, Baraitser M. Balanced t(6;8)(6p8p;6q8q) and the CHARGE association. J Med Genet. 1991;28:54–55. doi: 10.1136/jmg.28.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz KA, Graham JM, Jr, Prasad C, Smith IM, Blake KD. An epidemiological analysis of CHARGE syndrome: preliminary results from a Canadian study. Am J Med Genet A. 2005;133A:309–317. doi: 10.1002/ajmg.a.30560. [DOI] [PubMed] [Google Scholar]
- Jain S, Kim HG, Lacbawan F, Meliciani I, Wenzel W, Kurth I, Sharma J, Schoeneman M, Ten S, Layman LC, Jacobson-Dickman E. Unique phenotype in a patient with CHARGE syndrome. Int J Pediatr Endocrinol. 2011;2011:11. doi: 10.1186/1687-9856-2011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen N, Bergman JE, Swertz MA, Tranebjaerg L, Lodahl M, Schoots J, Hofstra RM, van Ravenswaaij-Arts CM, Hoefsloot LH. Mutation update on the CHD7 gene involved in CHARGE syndrome. Hum Mutat. 2012;33:1149–1160. doi: 10.1002/humu.22086. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Yuen RK, Jin X, Wang M, Chen N, Wu X, Ju J, Mei J, Shi Y, He M, Wang G, Liang J, Wang Z, Cao D, Carter MT, Chrysler C, Drmic IE, Howe JL, Lau L, Marshall CR, Merico D, Nalpathamkalam T, Thiruvahindrapuram B, Thompson A, Uddin M, Walker S, Luo J, Anagnostou E, Zwaigenbaum L, Ring RH, Wang J, Lajonchere C, Wang J, Shih A, Szatmari P, Yang H, Dawson G, Li Y, Scherer SW. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome sequencing. Am J Hum Genet. 2013;93:249–263. doi: 10.1016/j.ajhg.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MCJ, Admiraal RJ, van der Donk KP, Vissers LELM, Baas AF, Kapusta L, van Hagen JM, Donnai D, de Ravel TJ, Veltman JA, van Kessel AG, De Vries BBA, Brunner HG, Hoefsloot LH, van Ravenswaaij CMA. CHARGE syndrome: the phenotypic spectrum of mutations in the CHD7 gene. J Med Genet. 2006;43:306–314. doi: 10.1136/jmg.2005.036061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongmans MCJ, Hoefsloot LH, van der Donk KP, Admiraal RJ, Magee A, van de Laar I, Hendriks Y, Verheij JBGM, Walpole I, Brunner HG, van Ravenswaaij CMA. Familial CHARGE syndrome and the CHD7 gene: A recurrent missense mutation, intrafamilial recurrence and variability. Am J Med Genet A. 2008;146A:43–50. doi: 10.1002/ajmg.a.31921. [DOI] [PubMed] [Google Scholar]
- Jongmans MCJ, van Ravenswaaij-Arts CMA, Pitteloud N, Ogata T, Sato N, Claahsen-van der Grinten HL, van der Donk K, Seminara S, Bergman JEH, Brunner HG, Crowley WF, Hoefsloot LH. CHD7 mutations in patients initially diagnosed with Kallmann syndrome - the clinical overlap with CHARGE syndrome. Clin Genet. 2009;75:65–71. doi: 10.1111/j.1399-0004.2008.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo DS, Labelle-Dumais C, Gould DB. COL4A1 and COL4A2 mutations and disease: insights into pathogenic mechanisms and potential therapeutic targets. Hum Mol Genet. 2012;21:R97–R110. doi: 10.1093/hmg/dds346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani SR, Safiullah AM, Fernbach SD, Harutyunyan KG, Thaller C, Peterson LE, McPherson JD, Gibbs RA, White LD, Hefner M, Davenport SL, Graham JM, Bacino CA, Glass NL, Towbin JA, Craigen WJ, Neish SR, Lin AE, Belmont JW. Spectrum of CHD7 Mutations in 110 Individuals with CHARGE Syndrome and Genotype-Phenotype Correlation. Am J Hum Genet. 2006;78:303–314. doi: 10.1086/500273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys BL, Dietz HC, Braverman AC, Callewaert BL, De Backer J, Devereux RB, Hilhorst-Hofstee Y, Jondeau G, Faivre L, Milewicz DM, Pyeritz RE, Sponseller PD, Wordsworth P, De Paepe AM. The revised Ghent nosology for the Marfan syndrome. J Med Genet. 2010;47:476–485. doi: 10.1136/jmg.2009.072785. [DOI] [PubMed] [Google Scholar]
- Marcos S, Sarfati J, Leroy C, Fouveaut C, Parent P, Metz C, Wolczynski S, Gerard M, Bieth E, Kurtz F, Verier-Mine O, Perrin L, Archambeaud F, Cabrol S, Rodien P, Hove H, Prescott T, Lacombe D, Christin-Maitre S, Touraine P, Hieronimus S, Dewailly D, Young J, Pugeat M, Hardelin JP, Dode C. The prevalence of CHD7 missense versus truncating mutations is higher in patients with Kallmann syndrome than in typical CHARGE patients. J Clin Endocrinol Metab. 2014;99:E2138–E2143. doi: 10.1210/jc.2014-2110. [DOI] [PubMed] [Google Scholar]
- Michelucci A, Ghirri P, Iacopetti P, Conidi ME, Fogli A, Baldinotti F, Lunardi S, Forli F, Moscuzza F, Berrettini S, Boldrini A, Simi P, Pellegrini S. Identification of three novel mutations in the CHD7 gene in patients with clinical signs of typical or atypical CHARGE syndrome. Int J Pediatr Otorhinolaryngol. 2010;74:1441–1444. doi: 10.1016/j.ijporl.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Neul JL, Kaufmann WE, Glaze DG, Christodoulou J, Clarke AJ, Bahi-Buisson N, Leonard H, Bailey ME, Schanen NC, Zappella M, Renieri A, Huppke P, Percy AK, RettSearch C. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944–950. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, Karakoc E, Krumm N, Coe BP, Levy R, Ko A, Lee C, Smith JD, Turner EH, Stanaway IB, Vernot B, Malig M, Baker C, Reilly B, Akey JM, Borenstein E, Rieder MJ, Nickerson DA, Bernier R, Shendure J, Eichler EE. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagon RA, Graham JM, Jr, Zonana J, Yong SL. Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J Pediatr. 1981;99:223–227. doi: 10.1016/s0022-3476(81)80454-4. [DOI] [PubMed] [Google Scholar]
- Palumbo O, Palumbo P, Stallone R, Palladino T, Zelante L, Carella M. 8q12.1q12.3 de novo microdeletion involving the CHD7 gene in a patient without the major features of CHARGE syndrome: case report and critical review of the literature. Gene. 2013;513:209–213. doi: 10.1016/j.gene.2012.09.132. [DOI] [PubMed] [Google Scholar]
- Randall V, McCue K, Roberts C, Kyriakopoulou V, Beddow S, Barrett AN, Vitelli F, Prescott K, Shaw-Smith C, Devriendt K, Bosman E, Steffes G, Steel KP, Simrick S, Basson MA, Illingworth E, Scambler PJ. Great vessel development requires biallelic expression of Chd7 and Tbx1 in pharyngeal ectoderm in mice. J Clin Invest. 2009;119:3301–3310. doi: 10.1172/JCI37561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose PS, Levy HP, Liberfarb RM, Davis J, Szymko-Bennett Y, Rubin BI, Tsilou E, Griffith AJ, Francomano CA. Stickler syndrome: clinical characteristics and diagnostic criteria. Am J Med Genet A. 2005;138A:199–207. doi: 10.1002/ajmg.a.30955. [DOI] [PubMed] [Google Scholar]
- Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19:230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Laar I, Dooijes D, Hoefsloot L, Simon M, Hoogeboom J, Devriendt K. Limb anomalies in patients with CHARGE syndrome: an expansion of the phenotype. Am J Med Genet A. 2007;143A:2712–2715. doi: 10.1002/ajmg.a.32008. [DOI] [PubMed] [Google Scholar]
- Verloes A. Updated diagnostic criteria for CHARGE syndrome: a proposal. Am J Med Genet A. 2005;133:306–308. doi: 10.1002/ajmg.a.30559. [DOI] [PubMed] [Google Scholar]
- Vissers LELM, van Ravenswaaij CMA, Admiraal R, Hurst JA, de Vries BBA, Janssen IM, van der Vliet WA, Huys EHLPG, de Jong PJ, Hamel BCJ, Schoenmakers EFPM, Brunner HG, Veltman JA, van Kessel AG. Mutations in a new member of the chromodomain gene family cause CHARGE syndrome. Nat Genet. 2004;36:955–957. doi: 10.1038/ng1407. [DOI] [PubMed] [Google Scholar]
- Vuorela P, Ala-Mello S, Saloranta C, Penttinen M, Poyhonen M, Huoponen K, Borozdin W, Bausch B, Botzenhart EM, Wilhelm C, Kaariainen H, Kohlhase J. Molecular analysis of the CHD7 gene in CHARGE syndrome: identification of 22 novel mutations and evidence for a low contribution of large CHD7 deletions. Genet Med. 2007;9:690–694. doi: 10.1097/gim.0b013e318156e68e. [DOI] [PubMed] [Google Scholar]
- Wincent J, Holmberg E, Stromland K, Soller M, Mirzaei L, Djureinovic T, Robinson K, Anderlid B, Schoumans J. CHD7 mutation spectrum in 28 Swedish patients diagnosed with CHARGE syndrome. Clin Genet. 2008;74:31–38. doi: 10.1111/j.1399-0004.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- Woodage T, Basrai MA, Baxevanis AD, Hieter P, Collins FS. Characterization of the CHD family of proteins. Proc Natl Acad Sci U S A. 1997;94:11472–11477. doi: 10.1073/pnas.94.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright EM, O’Connor R, Kerr BA. Radial aplasia in CHARGE syndrome: a new association. Eur J Med Genet. 2009;52:239–241. doi: 10.1016/j.ejmg.2009.03.017. [DOI] [PubMed] [Google Scholar]
- Yu T, Meiners LC, Danielsen K, Wong MT, Bowler T, Reinberg D, Scambler PJ, van Ravenswaaij-Arts CM, Basson MA. Deregulated FGF and homeotic gene expression underlies cerebellar vermis hypoplasia in CHARGE syndrome. Elife. 2013;2:e01305. doi: 10.7554/eLife.01305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentner GE, Layman WS, Martin DM, Scacheri PC. Molecular and phenotypic aspects of CHD7 mutation in CHARGE syndrome. Am J Med Genet A. 2010;152A:674–686. doi: 10.1002/ajmg.a.33323. [DOI] [PMC free article] [PubMed] [Google Scholar]