Abstract
Background and Aims
Liver stiffness (LS) and spleen stiffness (SS) are two most widely accessible non-invasive parameters for predicting esophageal varices (EV), but the reported accuracy of the two predictors have been inconsistent across studies. This meta-analysis aims to evaluate the diagnostic performance of LS and SS measurement for detecting EV in patients with chronic liver disease (CLD), and compare their accuracy.
Methods
Pubmed/Medline, Embase, Cochrane Library and Ovid were searched for all studies assessing SS and LS simultaneously in EV diagnosis. A total of 16 studies including 1892 patients were included in this meta-analysis, and the pooled statistical parameters were calculated using the bivariate mixed effects models.
Results
In detection of any EV, for LS measurement, the summary sensitivity was 0.83 (95% confidence interval [CI]: 0.78–0.87), and the specificity was 0.66 (95% CI: 0.60–0.72). While for SS measurement, the pooled sensitivity and specificity was 0.88 (95% CI: 0.83–0.92) and 0.78 (95% CI: 0.73–0.83). The summary receiver operating characteristic (SROC) curve values of LS and SS were 0.81 (95% CI: 0.77–0.84) and 0.88 (95% CI: 0.85–0.91) respectively, and the results had statistical significance (P<0.01). The diagnostic odds ratio (DOR) of SS (25.73) was significantly higher than that of LS (9.54), with the relative DOR value was 2.48 (95%CI: 1.10–5.60), P<0.05.
Conclusions
Under current techniques, SS is significantly superior to LS for identifying the presence of EV in patients with CLD. SS measurement may help to select patients for endoscopic screening.
Introduction
Esophageal varices (EV) are mainly induced by portal hypertension [1], which is one of the most common consequences of chronic liver disease (CLD). Variceal bleeding from rupture of EV is associated with high mortality [2]. According to the most recent guidelines [3], all patients with newly diagnosed cirrhosis are recommended to undergo screening esophagogastroduodenoscopy (EGD) for identifying varices. However, the invasive nature of EGD leads to significant healthcare costs and patient discomfort [4]. There is thus considerable interest in developing non-invasive methods with acceptable diagnostic accuracy to predict the presence and size of EV.
Several serum and radiological parameters have been put forward for predicting EV, such as serum fibrosis markers, liver stiffness (LS), spleen stiffness (SS), LS-spleen diameter to platelet ratio score [5–7]. Among them, it has been shown that both liver and spleen stiffness were more accurate in identifying EV and the degree of portal hypertension than other non-invasive parameters [8]. LS has been largely accepted to reflect the degree of fibrosis and the presence of EV in CLD. Several studies have revealed that LS measured by elastography may represent a useful non-invasive tool for predicting EV [9,10], notably in combination with other non-invasive parameters [11]. Current European Guidelines recommend to avoid screening EGD in patients with LS< 20kPa and platelet count >150,000 [12]. While the role of LS alone in predicting varices is controversial due to unsatisfactory diagnostic accuracy and lack of consistent results [3]. In the last few years, research emphasis has been placed on SS measurement in predicting EV and clinical significant portal hypertension. Portal hypertension leads to spleen congestion and fibrosis, which is sufficient to increase organ stiffness [13].
Recently, more and more studies have attempted to clarify the utility of SS and LS for EV diagnosis in patients with CLD, but the results have been controversial. Research has shown that SS assessed by elastography was a more effective parameter with high diagnostic accuracy for identifying and grading EV than LS [14,15]. Conversely, other studies have concluded that spleen elastography is not superior to liver elastography in predicting EV for its inconstant accuracy, poor repeatability and highly unreliable measurement [16–18]. In 2014, a meta-analysis summarized the accuracy of SS measurement in predicting EV. It showed that the SS measurement was acceptable, but had limited accuracy for EV diagnosis [19]. However, the diagnostic performance of SS compared with the conventional LS measurement is still uncertain.
In light of the uncertain utility of SS and LS in EV diagnosis, we conducted a systemic review and meta-analysis based on the increasing number of comparative studies. We evaluated the diagnostic performance of SS and LS simultaneously on same individuals in this meta-analysis, and compared the accuracy of the two parameters for predicting and grading EV in CLD.
Materials and Methods
Selection criteria
Studies were included if they met the following inclusion criteria: (1) performed in adults with CLD who did not undergo liver transplantation or transjugular intrahepatic portosystemic shunt (TIPS); (2) reported the performance of SS and LS measurement simultaneously, using the same elastography technique based on ultrasound or magnetic resonance; (3) used EGD as the reference standard for detecting and grading EV; (4) provided necessary data to calculate the true positive, false positive, true negative, and false negative value for both SS and LS on diagnosis of EV; (5) selected an optimum cut-off value to maximize sensitivity and specificity according to the receiver operating characteristic (ROC) or Youden Index. If such data presented in original articles were insufficient, the corresponding author would be contacted by e-mail to provide them. Studies without available relevant data after contacting original authors were excluded.
Search strategy
A systematic search was performed through Pubmed/Medline, Embase, Cochrane Library and Ovid to identify all relevant studies assessing SS and LS simultaneously in EV diagnosis. Relevant studies published prior to 1 May 2016 were searched using the following keywords: spleen stiffness, liver stiffness, elastography, varices. A manual search was also carried out on reference lists of identified articles. All studies were limited to articles with an English abstract.
Study selection and data extraction
Two investigators (X.M. and L.W.) independently screened the search results and reviewed relevant full texts to determine eligibility. Discrepancies were resolved in consultation with a senior reviewer (Q.Z.). For each included study, the following data were extracted: author, country, year of publication, study design, number of patients, age, gender, body mass index (BMI), etiology of CLD, proportion of cirrhosis, Child-Pugh score, prevalence of EV or severe EV, definition of severe EV, measuring techniques, invalid measurement, optimum cut-off value according to ROC curve or Youden Index, sensitivity, specificity and area under ROC curve for SS and LS respectively. We imputed the number of true positive, false positive, false negative and true negative results of SS and LS respectively on EV or severe EV diagnosis in all patients with EGD.
Quality assessment
Risk of bias was assessed separately by two investigators using the revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool [20]. This tool is divided into 4 domains including patient selection, index test, reference standard, flow and timing. Each domain is assessed for risk of bias, and the first 3 domains are assessed for applicability as well. In this meta-analysis, LS and SS measurement were regarded as the index test, and the reference standard referred to EGD.
Data synthesis and analysis
Based on extracted data, the summary sensitivities, specificities, and diagnosis odds ratio (DOR) with corresponding 95% confidence interval (CI) were calculated to evaluate the performance of liver and spleen stiffness measurements for EV and severe EV diagnosis. The DOR comprises a combination of sensitivity and specificity, and it was regarded as a single indicator of diagnostic test accuracy [21]. The summary ROC (SROC) curve was also performed as an alternative global measure of accuracy to avoid the influence of heterogeneity and different cut-off value. All summary parameters were calculated using the bivariate mixed effects models. In addition, using Fagan nomogram, we evaluated the post-test probabilities of EV on assumption of 57% pre-test probability following a positive or negative test result. To provide a clinically meaningful comparison, we conducted the SROC curve for both liver and spleen stiffness measurements simultaneously, and compared their area under SROC curve using Z-test [22]. We also calculated the relative DOR (rDOR) ratios with 95% CI of the two parameters. When 95% CI do not include the unity, the difference of DOR between tests is statistically significant.
Between-study heterogeneity was assessed by computing Higgin’s I2 and chi-square test (P value). An I2 value more than 50% or a P value less than 0.10 was considered substantial heterogeneity. Besides, we used meta-regression analyses according to different study characteristics to investigate sources of heterogeneity. Because there are considerable variations across different techniques for stiffness measurement and different stages of CLD, we also performed subgroup analyses to investigate the influence of such variability on diagnostic performance.
Deek’s funnel plot was used to test the presence of publication bias, in which a regression of diagnostic log odds ratio against 1/sqrt (effective sample size) and weighting by effective sample size was conducted, with a P value less than 0.10 suggesting significant asymmetry [23]. All statistical analyses were performed by STATA 12.0 (College Station, TX) software using MIDAS command. This meta-analysis was based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (S1 PRISMA Checklist).
Results
Search results
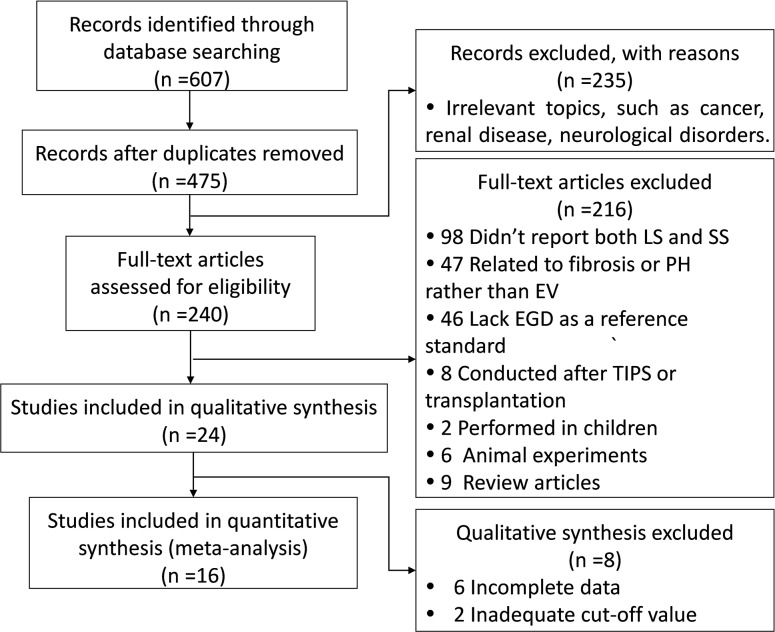
A total of 607 studies were identified based on described search strategies. After removing duplicates and irrelevant articles, 240 studies were screened for further review. 98 studies were excluded because they didn’t report on both liver and spleen stiffness measurement, and 93 studies could not be included for not relevant to EV (n = 47) or lack of EGD (n = 46). 33 studies were excluded for children or animal subjects (n = 8), surgery experience (n = 8), incomplete data (n = 6), inadequate cut-off value (n = 2) or type of reviews (n = 9). Ultimately, a total of 16 studies (14 full-text studies and 2 abstracts) including 1892 patients in whom both SS and LS were measured for EV detection were selected for meta-analysis. 13 of these reported the diagnostic performance of SS and LS in identifying the presence of EV [8,14,18,24–33], while 5 studies were available in severe EV diagnosis through spleen and liver stiffness measurement [27,31,34–36]. The coefficient of agreement between the two investigators was very good. Fig 1 shows the flow diagram of study selection.
Fig 1. Flow diagram showing study identification and selection.
LS, liver stiffness; SS, spleen stiffness; PH, portal hypertension; EV, esophageal varices; EGD, esophagogastroduodenoscopy; TIPS, transjugular intrahepatic portosystemic shunt.
Characteristics of included studies
The main characteristics of studies included in our meta-analysis are summarized in Table 1 and Table 2. In total, 1892 patients (median age, 56.4 years, 68.6% male) were included, of which the overall prevalence of EV and severe EV were 57.4% (10.0%-92.1%), 33.3% (27.1%-60.0%). 6 studies were performed on patients caused by viral hepatitis alone [8,14,18,24–25,27], and 13 studies referred to cirrhosis only [8,18,24,27–36]. As the most commonly used technique, transient elastography (TE) was used in 10 studies for liver and spleen stiffness measurement [8,14,18,24,25,27,29,30,32,36]. Another 4 techniques, acoustic radiation force impulse (ARFI) [26,34], virtual touch tissue quantification (VTTQ) [33], share wave elastography (SWE) and magnetic resonance elastography (MRE) [28,35,31] were used in other included studies. According to the QUADAS-2 scale, overall, studies were felt to be at low risk of bias and had good applicability (S1 Table).
Table 1. Baseline Characteristics of Studies and Patients Included in the Meta-analysis.
| Study, Reference, Year | Country | Technique | Total patients | Mean age | Mean BMI | Gender %male | Cirrhosis (%) | Child score, A/B/C% | Etiology (%viral) |
|---|---|---|---|---|---|---|---|---|---|
| Al-Dahshan et al [24], 2012 | Egypt | TE | 60 | 52.6 | NR | 78.3 | 100 | NR | 100 |
| Alsebaey et al (Ab) [25], 2015 | Egypt | TE | 165 | NR | NR | NR | NR | NR | 100 |
| Attia et al [26], 2015 | Germany | ARFI | 78 | 54 | NR | 79.5 | 86 | 27/59/14 | 15 |
| Bota et al [34], 2012 | Romania | ARFI | 145 | 59.1 | 26.7 | 60 | 100 | 46/43/11 | 50.3 |
| Calvaruso et al [27], 2013 | Italy | TE | 112 | 63.2 | 27 | 69.8 | 100 | 100/0/0 | 100 |
| Calvaruso et al (Ab) [18], 2010 | Italy | TE | 159 | 63 | NR | 71.7 | 100 | NR | 100 |
| Colecchia et al [8], 2012 | Italy | TE | 113 | 54 | 25 | 71 | 100 | 68/32/0 | 100 |
| Elkrief et al [35], 2015 | France | SWE | 79 | 55 | 26 | 78.5 | 100 | 30/25/44 | 45 |
| Fraquelli et al [14], 2014 | Italy | TE | 132 | 52 | 23 | 59.1 | 23 | NR | 100 |
| Grgurevic et al [28], 2015 | Croatia | SWE | 87 | 62.6 | NR | 78.2 | 100 | 51/29/20 | 25.3 |
| Liu et al [29], 2013 | China | TE | 101 | 50.9 | NR | 64.9 | 100 | 76/19/5 | 57.5 |
| Sharma et al [30], 2013 | India | TE | 200 | 49.3 | 24.6 | 88.5 | 100 | 32/57/11 | 29.9 |
| Shin et al [31], 2014 | South Korea | MRE | 139 | 57.3 | NR | 73.4 | 100 | NR | 80.6 |
| Stefanescu et al [32], 2011 | Romania | TE | 137 | 56 | 26.4 | 56.2 | 100 | 65/28/7 | NR |
| Stefanescu et al [36], 2014 | Romania | TE | 90 | 55.7 | 26.7 | 55.6 | 100 | 62/36/1 | 20 |
| Takuma et al [33], 2011 | Japan | VTTQ | 95 | 68.7 | NR | 48.4 | 100 | NR | 76.8 |
ARFI, acoustic radiation force impulse; BMI, body mass index; MRE, magnetic resonance elastography; NR, not reported; SWE, shear wave ultrasound elastography; TE, transient elastography; VTTQ, virtual touch tissue quantification.
Table 2. Characteristics of the Diagnostic Performance of LS and SS for Predicting EV in 16 Included Studies.
| Study, Reference | Total patients (invalid measures) | No. of EV/SEV | Liver Stiffness | Spleen Stiffness | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CUT-OFF | SEN | SPE | PPV | NPV | LR+ | LR- | CUT-OFF | SEN | SPE | PPV | NPV | LR+ | LR- | |||
| Al-Dahshan et al [24] | 60 (NR) | EV:30 | 17.75 kPa | 0.93 | 0.47 | 0.64 | 0.88 | 1.75 | 0.14 | 50.4 kPa | 0.80 | 0.73 | 0.75 | 0.79 | 3.00 | 0.27 |
| Alsebaey et al (Ab) [25] | 165 (NR) | EV:55 | 20.4 kPa | 0.82 | 0.72 | 0.59 | 0.89 | 2.90 | 0.25 | 43.2 kPa | 0.93 | 0.84 | 0.74 | 0.96 | 5.67 | 0.09 |
| Attia et al [26] | 78 (0) | EV:59 | 2.45 m/s | 0.93 | 0.79 | 0.93 | 0.79 | 4.43 | 0.09 | 2.63 m/s | 0.98 | 0.89 | 0.97 | 0.94 | 9.34 | 0.02 |
| Bota et al [34] | 145 (L2/S3) a | SEV:62 | 2.25 m/s | 0.94 | 0.29 | 0.50 | 0.86 | 1.32 | 0.22 | 2.55 m/s | 0.97 | 0.20 | 0.48 | 0.89 | 1.22 | 0.16 |
| Calvaruso et al [27] | 112 (16) | EV:54 | 17.0 kPa | 0.70 | 0.57 | 0.68 | 0.6 | 1.64 | 0.52 | 50.0 kPa | 0.65 | 0.60 | 0.67 | 0.57 | 1.60 | 0.59 |
| 112 (16) | SEV:26 | 19.0 kPa | 0.73 | 0.54 | 0.37 | 0.84 | 1.60 | 0.50 | 54.0 kPa | 0.81 | 0.70 | 0.5 | 0.91 | 2.69 | 0.27 | |
| Calvaruso et al (Ab) [18] | 159 (15) | EV:80 | 21 kPa | 0.70 | 0.72 | 0.76 | 0.66 | 2.49 | 0.42 | 47 kPa | 0.79 | 0.70 | 0.77 | 0.73 | 2.65 | 0.30 |
| Colecchia et al [8] | 113 (13) | EV:53 | 21.4 kPa | 0.83 | 0.81 | 0.83 | 0.81 | 4.34 | 0.21 | 46 kPa | 0.94 | 0.77 | 0.82 | 0.92 | 4.03 | 0.07 |
| Elkrief et al [35] | 79 (5) | SEV:45/46b | 24.7 kPa | 0.82 | 0.45 | 0.70 | 0.62 | 1.49 | 0.40 | 32.3 kPa | 0.48 | 0.71 | 0.73 | 0.45 | 1.67 | 0.73 |
| Fraquelli et al [14] | 132 (22) | EV:11 | 19 kPa | 0.73 | 0.47 | 0.13 | 0.94 | 1.38 | 0.57 | 65 kPa | 0.91 | 0.80 | 0.33 | 0.99 | 4.50 | 0.11 |
| Grgurevic et al [28] | 87 (0) | EV:54 | 19.7 kPa | 0.83 | 0.67 | 0.80 | 0.71 | 2.50 | 0.25 | 30.3 kPa | 0.80 | 0.76 | 0.84 | 0.69 | 3.28 | 0.27 |
| Liu et al [29] | 101 (0) | EV:93 | 18.0 kPa | 0.91 | 0.63 | 0.97 | 0.38 | 2.44 | 0.14 | 44.5 kPa | 0.88 | 0.63 | 0.96 | 0.31 | 2.35 | 0.19 |
| Sharma et al [30] | 200 (26) | EV:124 | 27.3 kPa | 0.86 | 0.70 | 0.89 | 0.77 | 3.25 | 0.12 | 40.8 kPa | 0.85 | 0.79 | 0.91 | 0.84 | 3.93 | 0.07 |
| Shin et al [31] | 139 (0) | EV:78 | 4.58 kPa | 0.91 | 0.72 | 0.79 | 0.80 | 2.91 | 0.20 | 7.23 kPa | 0.94 | 0.76 | 0.84 | 0.8 | 3.97 | 0.20 |
| 139 (0) | SEV:45 | 4.81 kPa | 0.60 | 0.72 | 0.49 | 0.91 | 2.04 | 0.20 | 7.60 kPa | 0.76 | 0.66 | 0.52 | 0.85 | 2.22 | 0.37 | |
| Stefanescu et al [32] | 137 (NR) | EV:116 | 28 kPa | 0.74 | 0.62 | 0.91 | 0.30 | 1.95 | 0.42 | 46.4 kPa | 0.84 | 0.71 | 0.94 | 0.44 | 2.93 | 0.23 |
| Stefanescu et al [36] | 90 (0) | SEV:47 | 38 kPa | 0.89 | 0.56 | 0.70 | 0.62 | 2.13 | 0.56 | 53 kPa | 0.89 | 0.51 | 0.67 | 0.81 | 1.83 | 0.21 |
| Takuma et al [33] | 95 (0) | EV:40 | 2.33 m/s | 0.75 | 0.58 | 0.61 | 0.72 | 1.78 | 0.43 | 3.43 m/s | 0.88 | 0.96 | 0.95 | 0.90 | 19.69 | 0.13 |
EV, esophageal varices; SEV, severe esophageal varices; SEN, sensitivity; SPE, specificity; PPV, positive predictive value; NPV, negative predictive value; LR+, positive likelihood value; LR-, negative likelihood value; NR, not reported.
a Valid ARFI measurements in the liver in 143/145 patients, and in the spleen in 142/145 patients.
b 45 SEV in patients with valid LS measurement, while 46 SEV in patient with valid SS measurement.
Diagnostic accuracy of liver stiffness for the prediction of esophageal varices
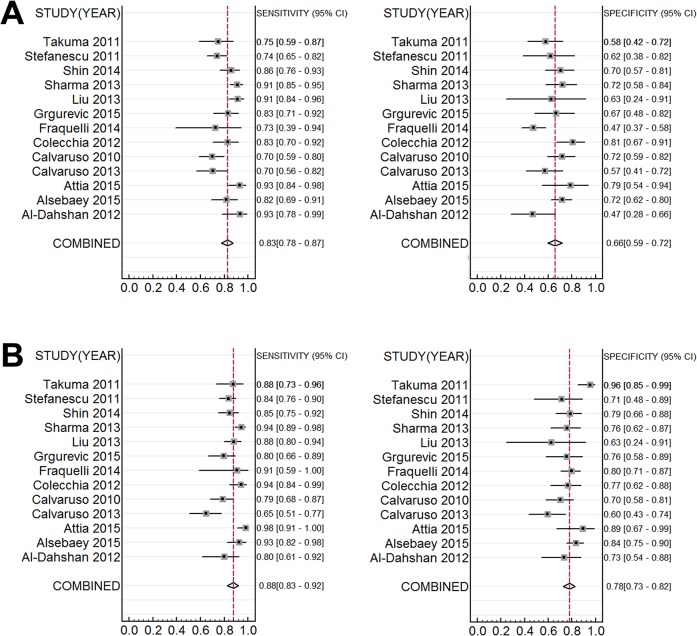
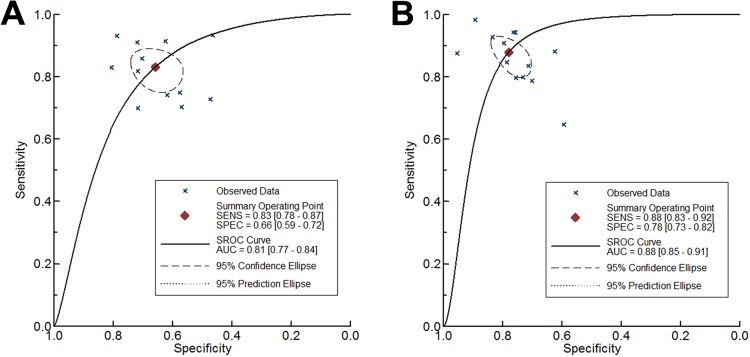
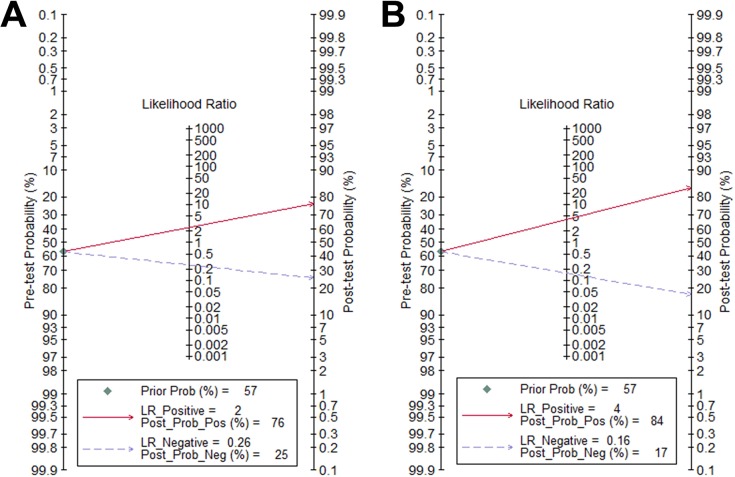
The diagnostic accuracy of liver and spleen stiffness measurement for prediction of the presence of EV was evaluated in 13 studies [8,14,18,24–27,28–33]. For LS measurement, the summary sensitivity was 0.83 (95% CI: 0.78–0.87), the summary specificity was 0.66 (95% CI: 0.60–0.72) (Fig 2A), the summary positive likelihood ratio (LR+) was 2.44 (95% CI: 1.99–2.99), the summary negative likelihood ratio (LR-) was 0.26 (95% CI: 0.19–0.35), the summary DOR was 9.54 (95% CI: 5.85–15.56), and the area under SROC curve was 0.81 (95% CI: 0.77–0.84) (Fig 3A). When the pre-test probability of EV was 57%, according to the Fagan plot analysis, LS was able to increase the post-probability to 76% following a positive result and lower the probability of to 25% with a negative measurement (Fig 4A).
Fig 2. Forest plot of individual study evaluates of sensitivity and specificity for any esophageal varices diagnosis.
The base vertical imaginary line indicates the combined effects. (A) Accuracy of liver stiffness measurement for estimating the presence of esophageal varices. (B) Accuracy of spleen stiffness for detecting the presence of any esophageal varices in chronic liver disease.
Fig 3. Summary receiver operating characteristic (SROC) curve of sensitivity versus specificity.
(A) SROC curve of liver stiffness for prediction of any esophageal varices. (B) SROC curve of spleen stiffness for detecting the presence of esophageal varices.
Fig 4. Fagan plot analysis to evaluating the clinical utility of liver and spleen stiffness for diagnosis of esophageal varices.
(A) For LS measurements, with a pre-test probability of EV of 57%, the post-test probability of EV, given negative and positive results, were 25% and 76%. (B) For SS measurements, with a pre-test probability of EV of 57%, the post-test probability of EV, given negative and positive results, were 17% and 84%.
Minor heterogeneity has been observed between studies on LS measurement, with I2 = 41.76%, P = 0.09. There was not significant threshold effect between studies, with the Spearman correlation coefficient = -0.17, P = 0.58. According to meta-regression analyses, basic characteristics of patients could explain the source of heterogeneity. Studies with the mean age less than 55 years old showed higher diagnostic accuracy compared to those performed in older patients. Research involving more male participants (over 70%) also improved the diagnostic performance of LS (P<0.01). The accuracy of LS was not affected by technique for measurement, location, quality of study, proportion of cirrhosis, etiology of disease, sample size (P>0.05) (S2 Table). Funnel plot asymmetry test demonstrated that there was no evidence of publication bias between studies (P = 0.68).
Separate analysis specific to TE technique (n = 9) was conducted to demonstrate the optimism range of cut-off value. In terms of DOR value, there were not significant differences between studies with the cut-off value lower than 21 kPa (n = 5, DOR = 7.21) and the others (n = 4, DOR = 11.523), P = 0.09.
Diagnostic accuracy of spleen stiffness for the prediction of esophageal varices
For SS measurement, the summary sensitivity was 0.88 (95% CI: 0.83–0.92), the summary specificity was 0.78 (95% CI: 0.73–0.83) (Fig 2B), the summary LR+ was 4.00 (95% CI: 3.11–5.15), the summary LR- was 0.16 (95% CI: 0.10–0.23), the summary DOR was 25.73 (95% CI: 13.74–48.19), and the area under SROC curve was 0.88 (95% CI: 0.85–0.91) (Fig 3B). According to the Fagan plot analysis, when there was 57% pre-test probability of EV, a negative SS measurement could decrease the post-probability to as low as 17%, while a positive result indicated 84% probability of having EV (Fig 4B).
There was not significant heterogeneity in the analysis of SS for the prediction of EV (P = 0.13, I2 = 27.76%). Threshold effect was not observed for SS analysis (P = 0.02). Funnel plot asymmetry test demonstrated that there was no evidence of publication bias for SS in EV diagnosis (P = 0.92).
Separate analysis specific to TE technique (n = 9) was conducted to demonstrate the optimism range of cut-off value. For studies with the cut-off values lower than 47 kPa, the DOR value of SS in predicting EV was 34.92, which is significantly higher than other studies with the cut-off value≥47 kPa, P<0.05.
Spleen stiffness is superior to liver stiffness for the prediction of esophageal varices in patients with chronic liver disease
Our results indicated that SS predicted the presence of EV better than LS, on both sensitivity and specificity. The area under SROC curve of SS for diagnosis of EV was 0.88 (95% CI: 0.85–0.91), while the LS had a value of 0.81 (95% CI: 0.77–0.84) (Fig 3). There was significant difference between the two SROC values according to Z-test (Z = 3.74, P<0.01). The summary DOR of SS (DOR = 25.73) was higher than that of LS (DOR = 9.54), and the difference was statistical significant (rDOR = 2.48, 95% CI: 1.10–5.60, P = 0.03). Because the technique for measurement varies between included studies, a certain cut-off value could not be concluded accurately. To decrease the influence of different diagnostic thresholds, all included studies defined the optimum cut-off value according to the ROC curve or Youden index to maximize the sensitivity and specificity. At corresponding cut-off value, the summary sensitivity of SS and LS for detecting the presence of EV were 0.88 and 0.83 respectively (Z = 1.13, P = 0.26), whereas the specificity of SS was significantly higher than that of LS with the value of 0.78 and 0.66 (Z = 2.35, P = 0.02). A Z-test based on the joint model of sensitivity and specificity demonstrated that the diagnostic accuracy of SS and LS differed significantly for prediction of EV (P = 0.03). Table 3 summarized the pooled accuracy and the comparison of LS and SS measurement.
Table 3. Comparison of LS and SS for the prediction of EV and severe EV.
| Statistical parameters | Prediction of any EV (13 studies) | Prediction of severe EV (5 studies) | ||||
|---|---|---|---|---|---|---|
| LS | SS | Comparison | LS | SS | Comparison | |
| Sensitivity (95%CI) | 0.83 (0.78–0.87) | 0.88 (0.83–0.92) | P = 0.26 | 0.82 (0.69–0.91) | 0.83 (0.61–0.94) | P = 0.99 |
| Specificity (95%CI) | 0.66 (0.60–0.72) | 0.78 (0.73–0.83) | P = 0.02 * | 0.52 (0.39–0.65) | 0.57 (0.37–0.75) | P = 0.75 |
| Area under SROC curve (95% CI) | 0.81 (0.77–0.84) | 0.88 (0.85–0.91) | P<0.01 ** | 0.72 (0.68–0.76) | 0.75 (0.71–0.79) | P = 0.32 |
| Diagnostic odds ratio (95% CI) | 9.54 (5.85–15.56) | 25.73 (13.74–48.19) | rDOR = 2.48 (95%CI:1.10–5.60)P = 0.03* | 4.98 (3.13–7.94) | 6.47 (3.63–11.54) | rDOR = 1.31 (95%CI:0.60–2.89)P = 0.64 |
Z-test was used to compare the SROC and DOR value between LS and SS. CI, confidence interval; LS, liver stiffness; SS, spleen stiffness; SROC, summary receiver operating characteristic; rDOR, relative diagnostic odds ratio.
*P<0.05
**P<0.01
Sensitivity Analysis
For EV identification, on restricting analysis to 9 studies performed with TE alone, the pooled sensitivity and specificity of LS were 0.83 (95%CI: 0.75–0.88) and 0.65 (95% CI: 0.56–0.72), while SS has the sensitivity and specificity of 0.87 (95% CI: 0.81–0.92) and 0.75 (95% CI: 0.69–0.80). The DOR value of SS (20.59) is still higher than LS measurement (8.61). For both LS and SS, sensitivity analysis after excluding non-cirrhosis (not included cirrhosis patients only), non-viral etiology (not caused by viral hepatitis only) and low quality studies (with high risk of bias according to QUADAS-2), did not significantly alter the primary results.
Diagnostic accuracy of liver and spleen stiffness for the prediction of severe esophageal varices
5 studies including 567 cirrhosis patients provided sufficient data to assess the diagnostic performance of spleen and liver stiffness for identifying severe EV. Severe EV were defined as grade 2 or grade 3 varices in 3 included studies, while they were regarded as grade 2 or 3, or varices with the red color sign in the other study.
For LS measurement, the summary sensitivity was 0.82 (95% CI: 0.69–0.91), the summary specificity was 0.52 (95% CI: 0.39–0.65) (S1A Fig), the summary DOR was 4.98 (95% CI: 3.13–7.94), and the area under SROC curve was 0.72 (95% CI: 0.68–0.76). For SS measurement, the summary sensitivity and specificity for detecting severe EV was 0.83 (95% CI: 0.61–0.94) and 0.57 (95% CI: 0.37–0.75) respectively (S1B Fig), the summary DOR was 6.47 (95% CI: 3.63–11.54), and the area under SROC curve was 0.75 (95% CI: 0.71–0.79). The DOR of SS and LS did not differ significantly for detecting severe EV (rDOR = 1.31, 95% CI: 0.60–2.89).
Significant heterogeneity was observed in the analysis of severe EV. Because of the limited number of included studies, meta-regression could not be used to explore the factors inducing heterogeneity. Funnel plot asymmetry test demonstrated that there was no publication bias for LS and SS in detecting severe EV, with P = 0.15 and 0.55.
Discussion
LS and SS are two non-invasive parameters receiving the most attention for identifying patients suffered from EV, but the diagnostic value of these two predictors is still controversial. In this meta-analysis, we evaluated the performance of LS and SS simultaneously for detecting EV and severe EV in patients with CLD, and compared their diagnostic accuracy. Our results indicated that SS was superior to LS for predicting the presence of EV in patients with CLD, while the diagnostic accuracy of both LS and SS were limited in predicting severe EV.
During the progression of liver cirrhosis and portal hypertension, passive congestion and tissue hyperplasia characterized by a combination of angiogenesis and fibrogenesis frequently occur in the spleen [37]. All these changes result in increased SS, which is closely related to portal hypertension and reflects the extra-hepatic hemodynamic changes. When it comes to LS, although it appears to be a reliable surrogate for liver biopsy in identifying mild or advanced fibrosis, the pathophysiological basis for its correlation with portal hypertension remains poorly defined [38]. It is clear that LS only reflects the increased intra-hepatic vascular resistance, but not the hyperdynamic circulation and the opening of portal-systemic shunts [38]. For this reason, SS predicts the formation of EV caused by splanchnic hemodynamics changes better than LS [8], which is consistent with our results.
Combination of different non-invasive markers is also an important and valid approach to exclude EV in clinical practice. It is considered that the combination of LS value with other spleen-related parameters results in an increased diagnostic accuracy [8]. This phenomenon indicates that the association of LS and parameters reflecting the extra-hepatic hemodynamic could be a valuable tool with better diagnostic accuracy for the prediction of EV. Studies have shown that combining the LS and SS measurements further increased the diagnostic accuracy of EV [30,32]. Hence, it is possible to construct a combinative model with satisfactory accuracy for predicting EV based on the SS measurement.
Several techniques were enrolled in our studies for liver and spleen stiffness measurement. As the most widely used method for organ stiffness assessment, TE is available in many clinical centers, although it requires a dedicated Fibroscan device [39]. It should be mentioned that the reliable measurements by TE is quite low in obese cases and patients with ascites. We observed that these kinds of cases were tend to be avoided in most original studies involved in this meta-analysis. In contrast, ARFI and SWE are two novel, popular, ultrasound technique based technologies, which could be used in the existence of ascites. However, there is limited validation of these two techniques and the measures of quality are not well defined [19]. In this meta-analysis, we observed that there was no significant heterogeneity between different techniques, and the threshold effect was not obvious. Thus, the diagnostic performance of all these techniques were comparable. Moreover, we excluded studies with a different threshold standard. All studies included in our meta-analysis determined its own cut-off value following the accordant standard, which minimizes the influence of different techniques and cut-off values and ensures the comparability of the studies.
For severe EV, our results indicated that both liver and spleen stiffness measurement showed limited diagnostic accuracy. From the current studies, LS is considered not to correlate with the grades of EV [40,41], whereas the SS measurement may be possible to identify severe EV, but the accuracy is not high [42]. Certainly, additional studies are needed to verify the diagnostic performance of LS and SS in predicting severe EV.
Singh et al summarized the accuracy of SS measurement as a new predictor in detection of EV [19]. Extending upon previous studies, we compared the diagnostic value of this new proposed parameter with the conventional LS measurement in the prediction of EV. We concluded that SS is significantly superior to LS in EV diagnosis, which is helpful in clinical practice. Besides, with the development of elastography techniques, more recent studies (especially in last two years) were involved in this meta-analysis, which keeps our study novel and timely. Thus, only 5 studies included in our meta-analysis were involved in the previous publication.
The strengths of our study were the comprehensive and simultaneous assessment of the diagnostic value of LS and SS for the prediction of EV, and provided an authentic comparison of the two useful parameters. All comparative studies included in our meta-analysis provided sufficient data for both LS and SS simultaneously, which was able to decrease the risk of bias from patient spectrum, disease prevalence and inter-observer variability. Furthermore, a Z-test was used to compare the SROC value of LS and SS for predicting the presence of EV, and the rDOR was also conducted to compare the diagnostic accuracy based on the DOR value, which confirms the reliability of our study.
The limitations of our meta-analysis should be taken into consideration. First, only 5 studies described the performance of SS and LS for severe EV diagnosis, which limited the conduction of meta-regression and subgroup analysis for explaining the heterogeneity. More research is also needed to validate our summary results of LS and SS in identifying severe EV. Second, minor heterogeneity existed in the analysis of LS for prediction of EV in our meta-analysis. Although the heterogeneity is acceptable and could be explained by characteristics of involved patients, it also affected the reliability of our results. Third, the range of detection and units are completely different regarding variety of included techniques, which limited their comparisons. Because all studies involved in this analysis have to report the performance of LS and SS simultaneously, the included number of some clinical frequently-used techniques, such as ARFI, SWE, was too small to be analyzed separately. For this reason, we could not obtain the optimism cut-off range of each technique. In this meta-analysis, only separate analysis specific to TE was provided. Therefore, our summary conclusion that SS is superior to LS for predicting the presence of EV also needs to be validated under specific techniques respectively based on more original studies in future.
In conclusion, our meta-analysis demonstrated that SS is superior to LS for predicting the presence of EV in patients with CLD. Although the accuracy of the two parameters in identifying severe EV is not high, they still could be considered as a choice for screening EV in newly diagnosed cirrhosis. Combination of LS and LS may improve the diagnostic accuracy, and it is also possible to construct a novel combinative model with higher accuracy in predicting EV. Simple, low-cost and more accurate non-invasive models are needed in future as surrogates of endoscopy for EV detection.
Supporting Information
(DOC)
(XLS)
(A) Accuracy of liver stiffness measurement for estimating severe esophageal varices. (B) Accuracy of spleen stiffness for detecting severe esophageal varices in chronic liver disease.
(TIF)
(DOC)
(DOC)
Acknowledgments
We thank all included physicians contributing data to this meta-analysis.
Abbreviations
- ARFI
acoustic radiation force impulse
- CI
confidence interval
- CLD
chronic liver disease
- DOR
diagnostic odds ratio
- EGD
esophagogastroduodenoscopy
- EV
esophageal varices
- LR+
positive likelihood ratio
- LR-
negative likelihood ratio
- LS
liver stiffness
- MRE
magnetic resonance elastography
- NPV
negative predictive ratio
- PPV
positive predictive ratio
- rDOR
relative diagnostic odds ratio
- ROC
receiver operator curve
- SROC
summary receiver operator curve
- SS
spleen stiffness
- SWE
share wave elastography
- TE
transient elastography
- VTTQ
virtual touch tissue quantification.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from National Natural Science Foundation of China (Grant No.81370554). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rosołowski M, Hartleb M, Marek T, Milewski J, Linke K, Wallner G, et al. Therapeutic and prophylactic management of bleeding from oesophageal and gastric varices—recommendations of the Working Group of the National Consultant for Gastroenterology. Prz Gastroenterol 2014;9:63–68. 10.5114/pg.2014.42497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol 2007;102:2086–2102. 10.1111/j.1572-0241.2007.01481.x [DOI] [PubMed] [Google Scholar]
- 3.Tripathi D, Stanley AJ, Hayes PC, Patch D, Millson C, Mehrzad H, et al. U.K. guidelines on the management of variceal haemorrhage in cirrhotic patients. Gut 2015;64:1680–1704. 10.1136/gutjnl-2015-309262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berzigotti A, Bosch J, Boyer TD. Use of noninvasive markers of portal hypertension and timing of screening endoscopy for gastroesophageal varices in patients with chronic liver disease. Hepatology 2014;59:729–731. 10.1002/hep.26652 [DOI] [PubMed] [Google Scholar]
- 5.de Franchis R, Dell'Era A. Invasive and noninvasive methods to diagnose portal hypertension and esophageal varices. Clin Liver Dis 2014;18:293–302. 10.1016/j.cld.2013.12.002 [DOI] [PubMed] [Google Scholar]
- 6.Deng H, Qi X, Guo X. Diagnostic Accuracy of APRI, AAR, FIB-4, FI, King, Lok, Forns, and FibroIndex Scores in Predicting the Presence of Esophageal Varices in Liver Cirrhosis: A Systematic Review and Meta-Analysis. Medicine (Baltimore) 2015;94:e1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim BK, Han KH, Park JY, Ahn SH, Kim JK, Paik YH, et al. A liver stiffness measurement-based, noninvasive prediction model for high-risk esophageal varices in B-viral liver cirrhosis. Am J Gastroenterol 2010;105:1382–1390. 10.1038/ajg.2009.750 [DOI] [PubMed] [Google Scholar]
- 8.Colecchia A, Montrone L, Scaioli E, Bacchi-Reggiani ML, Colli A, Casazza G, et al. Measurement of spleen stiffness to evaluate portal hypertension and the presence of esophageal varices in patients with HCV-related cirrhosis. Gastroenterology 2012;143:646–654. 10.1053/j.gastro.2012.05.035 [DOI] [PubMed] [Google Scholar]
- 9.Kazemi F, Kettaneh A, N'kontchou G, Pinto E, Ganne-Carrie N, Trinchet JC, et al. Liver stiffness measurement selects patients with cirrhosis at risk of bearing large oesophageal varices. J Hepatol 2006;45:230–235. 10.1016/j.jhep.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 10.Shi KQ, Fan YC, Pan ZZ, Lin XF, Liu WY, Chen YP, et al. Transient elastography: a meta-analysis of diagnostic accuracy in evaluation of portal hypertension in chronic liver disease. Liver Int 2013;33:62–71. 10.1111/liv.12003 [DOI] [PubMed] [Google Scholar]
- 11.Berzigotti A, Seijo S, Arena U, Abraldes JG, Vizzutti F, García-Pagán JC, et al. Elastography, spleen size, and platelet count identify portal hypertension in patients with compensated cirrhosis. Gastroenterology 2013;144:102–111. 10.1053/j.gastro.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 12.de Franchis R. Expanding consensus in portal hypertension. Report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol 2015;65:743–752. [DOI] [PubMed] [Google Scholar]
- 13.Abraldes JG, Reverter E, Berzigotti A. Spleen stiffness: toward a noninvasive portal sphygmomanometer? Hepatology 2013;57:1278–1280. 10.1002/hep.26239 [DOI] [PubMed] [Google Scholar]
- 14.Fraquelli M, Giunta M, Pozzi R, Rigamonti C, Della Valle S, Massironi S, et al. Feasibility and reproducibility of spleen transient elastography and its role in combination with liver transient elastography for predicting the severity of chronic viral hepatitis. J Viral Hepat 2014;21:90–98. 10.1111/jvh.12119 [DOI] [PubMed] [Google Scholar]
- 15.Takuma Y, Nouso K, Morimoto Y, Tomokuni J, Sahara A, Toshikuni N, et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology 2013;144:92–101. 10.1053/j.gastro.2012.09.049 [DOI] [PubMed] [Google Scholar]
- 16.Zykus R, Jonaitis L, Petrenkienė V, Pranculis A, Kupčinskas L. Liver and spleen transient elastography predicts portal hypertension in patients with chronic liver disease: a prospective cohort study. BMC Gastroenterol 2015;15:183 10.1186/s12876-015-0414-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Procopet B, Berzigotti A, Abraldes JG, Turon F, Hernandez-Gea V, García-Pagán JC, et al. Real-time shear-wave elastography: applicability, reliability and accuracy for clinically significant portal hypertension. J Hepatol 2015;62:1068–1075. 10.1016/j.jhep.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 18.Calvaruso V, Di Marco V, Bronte F, Licata G, Simone F, Butera G, et al. Spleen stiffness correlates with portal hypertension and increases the accuracy of detection of esophageal varices in HCV cirrhosis [Abstract]. J Hepatol 2010;52:S159–S160. [Google Scholar]
- 19.Singh S, Eaton JE, Murad MH, Tanaka H, Iijima H, Talwalkar JA. Accuracy of spleen stiffness measurement in detection of esophageal varices in patients with chronic liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:935–945. 10.1016/j.cgh.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 20.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 21.Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129–1135. [DOI] [PubMed] [Google Scholar]
- 22.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148:839–843. 10.1148/radiology.148.3.6878708 [DOI] [PubMed] [Google Scholar]
- 23.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–893. 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 24.Al-Dahshan M. Clinical application of transient elastography in prediction of portal hypertension related complication in patients with chronic liver diseases. J Egypt Soc Parasitol 2012;42:79–88. [DOI] [PubMed] [Google Scholar]
- 25.Alsebaey A, Elmazaly MA, Elsabaawy MM, Tharwa E-SS, Badran HM, Ehsan N, et al. Evaluation of liver and spleen transient elastography in the diagnosis of esophageal varices [Abstract]. Hepatology 2015;62:SUPPL1(1126A). [Google Scholar]
- 26.Attia D, Schoenemeier B, Rodt T, Negm AA, Lenzen H, Lankisch TO, et al. Evaluation of liver and spleen stiffness with acoustic radiation force impulse quantification elastography for diagnosing clinically significant portal hypertension. Ultraschall Med 2015;36:603–610. 10.1055/s-0041-107971 [DOI] [PubMed] [Google Scholar]
- 27.Calvaruso V, Bronte F, Conte E, Simone F, Craxì A, Di Marco V. Modified spleen stiffness measurement by transient elastography is associated with presence of large oesophageal varices in patients with compensated hepatitis C virus cirrhosis. J Viral Hepat 2013;20:867–874. 10.1111/jvh.12114 [DOI] [PubMed] [Google Scholar]
- 28.Grgurević I, Bokun T, Mustapić S, Trkulja V, Heinzl R, Banić M, et al. Real-time two-dimensional shear wave ultrasound elastography of the liver is a reliable predictor of clinical outcomes and the presence of esophageal varices in patients with compensated liver cirrhosis. Croatian Medical Journal 2015;56:470–481. 10.3325/cmj.2015.56.470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu F, Li TH, Han T, Xiang HL, Zhang HS. Non-invasive assessment of portal hypertension in patients with liver cirrhosis using FibroScan transient elastography. Zhonghua Gan Zang Bing Za Zhi 2013;21:840–844. 10.3760/cma.j.issn.1007-3418.2013.11.010 [DOI] [PubMed] [Google Scholar]
- 30.Sharma P, Kirnake V, Tyagi P, Bansal N, Singla V, Kumar A, et al. Spleen stiffness in patients with cirrhosis in predicting esophageal varices. Am J Gastroenterol 2013;108:1101–1107. 10.1038/ajg.2013.119 [DOI] [PubMed] [Google Scholar]
- 31.Shin SU, Lee JM, Yu MH, Yoon JH, Han JK, Choi BI, et al. Prediction of esophageal varices in patients with cirrhosis: usefulness of three-dimensional MR elastography with echo-planar imaging technique. Radiology 2014;272:143–153. 10.1148/radiol.14130916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefanescu H, Grigorescu M, Lupsor M, Procopet B, Maniu A, Badea R. Spleen stiffness measurement using Fibroscan for the noninvasive assessment of esophageal varices in liver cirrhosis patients. J Gastroenterol Hepatol 2011;26:164–170. 10.1111/j.1440-1746.2010.06325.x [DOI] [PubMed] [Google Scholar]
- 33.Takuma Y, Morimoto Y, Tomokuni J, Toshikuni N, Takabatake H, Shimomura H, et al. Comparison of accuracy for the prediction of esophageal varices obtained by liver and spleen stiffness measurements via virtual touch tissue quantification. Acta Hepatologica Japonica 2011;52:258–259. [Google Scholar]
- 34.Bota S, Sporea I, Sirli R, Focsa M, Popescu A, Danila M, et al. Can ARFI elastography predict the presence of significant esophageal varices in newly diagnosed cirrhotic patients? Ann Hepatol 2012;11:519–525. [PubMed] [Google Scholar]
- 35.Elkrief L, Rautou PE, Ronot M, Lambert S, Dioguardi Burgio M, Francoz C, et al. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology 2015;275:589–598. 10.1148/radiol.14141210 [DOI] [PubMed] [Google Scholar]
- 36.Stefanescu H, Radu C, Procopet B, Lupsor-Platon M, Habic A, Tantau M, et al. Non-invasive menage a trois for the prediction of high-risk varices: stepwise algorithm using lok score, liver and spleen stiffness. Liver Int 2015;35:317–325. 10.1111/liv.12687 [DOI] [PubMed] [Google Scholar]
- 37.Bolognesi M, Merkel C, Sacerdoti D, Nava V, Gatta A. Role of spleen enlargement in cirrhosis with portal hypertension. Dig Liver Dis 2002;34:144–150. [DOI] [PubMed] [Google Scholar]
- 38.Lim JK, Groszmann RJ. Transient elastography for diagnosis of portal hypertension in liver cirrhosis: is there still a role for hepatic venous pressure gradient measurement? Hepatology 2007;45:1087–1090. 10.1002/hep.21731 [DOI] [PubMed] [Google Scholar]
- 39.Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina MA, et al. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int 2013;33:1138–1147. 10.1111/liv.12240 [DOI] [PubMed] [Google Scholar]
- 40.Elkrief L, Rautou PE, Ronot M, Lambert S, Dioguardi Burgio M, Francoz C, et al. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology 2015;275:589–598. 10.1148/radiol.14141210 [DOI] [PubMed] [Google Scholar]
- 41.Ye XP, Ran HT, Cheng J, Zhu YF, Zhang DZ, Zhang P, et al. Liver and spleen stiffness measured by acoustic radiation force impulse elastography for noninvasive assessment of liver fibrosis and esophageal varices in patients with chronic hepatitis B. J Ultrasound Med 2012;31:1245–253. [DOI] [PubMed] [Google Scholar]
- 42.Morisaka H, Motosugi U, Ichikawa S, Sano K, Ichikawa T, Enomoto N. Association of splenic MR elastographic findings with gastroesophageal varices in patients with chronic liver disease. J Magn Reson Imaging 2015;41:117–124. 10.1002/jmri.24505 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(XLS)
(A) Accuracy of liver stiffness measurement for estimating severe esophageal varices. (B) Accuracy of spleen stiffness for detecting severe esophageal varices in chronic liver disease.
(TIF)
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.