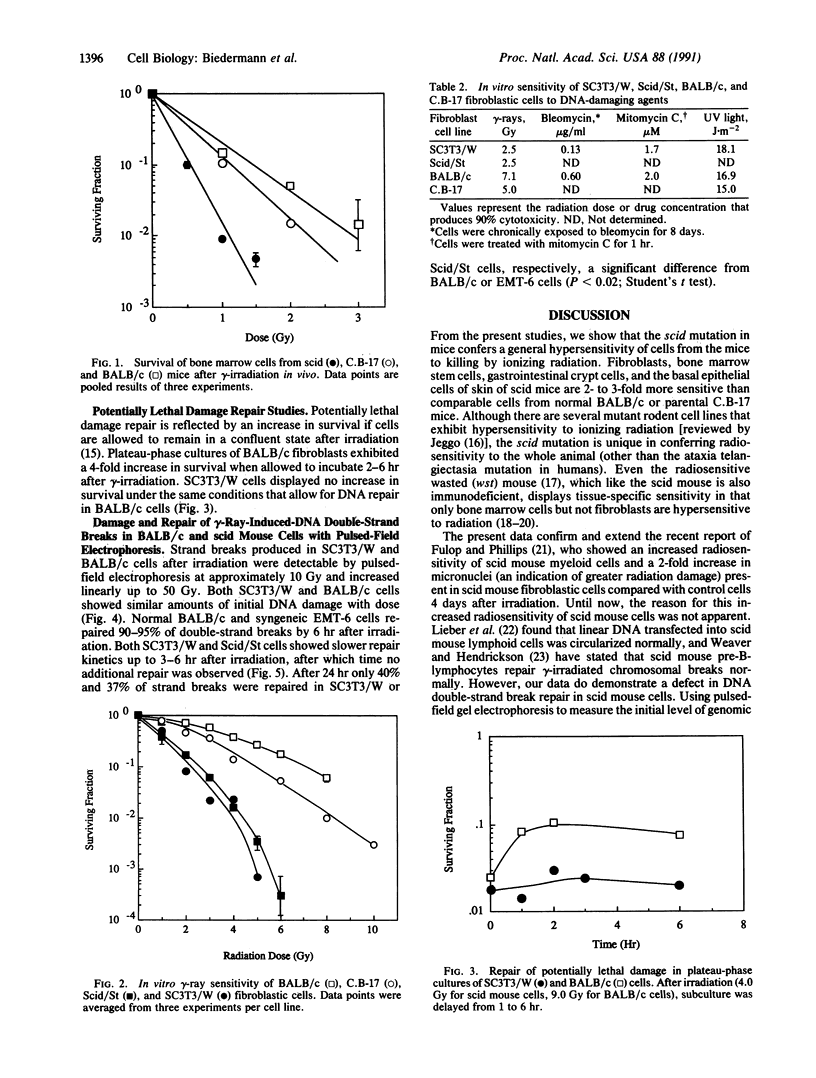
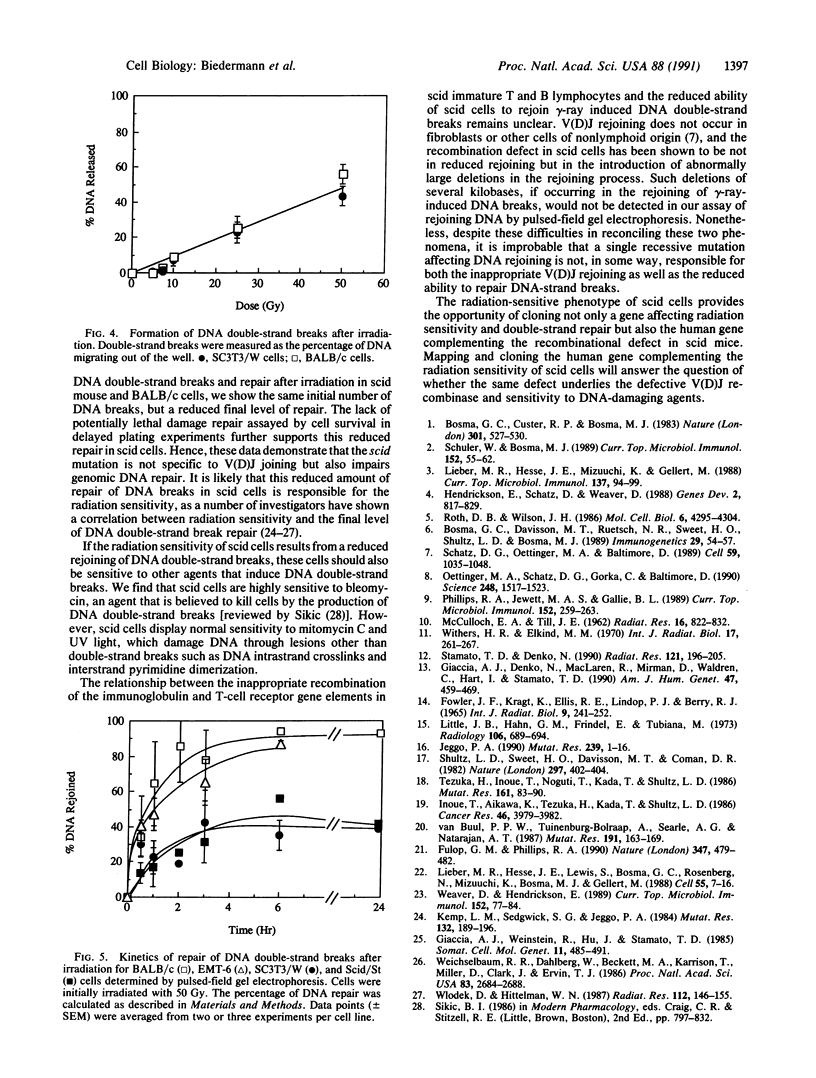
Abstract
C.B-17 severe combined immunodeficient (scid) mice carry the scid mutation and are severely deficient in both T cell- and B cell-mediated immunity, apparently as a result of defective V(D)J joining of the immunoglobulin and T-cell receptor gene elements. In the present studies, we have defined the tissue, cellular, and molecular basis of another characteristic of these mice: their hypersensitivity to ionizing radiation. Bone marrow stem cells, intestinal crypt cells, and epithelial skin cells from scid mice are 2- to 3-fold more sensitive when irradiated in situ than are congenic BALB/c or C.B-17 controls. Two independently isolated embryo fibroblastic scid mouse cell lines display similar hypersensitivities to gamma-rays. In addition, these cell lines are sensitive to cell killing by bleomycin, which also produces DNA strand breaks, but not by the DNA crosslinking agent mitomycin C or UV irradiation. Measurement of the rejoining of gamma-ray-induced DNA double-strand breaks by pulsed-field gel electrophoresis indicates that these animals are defective in this repair system. This suggests that the gamma-ray sensitivity of the scid mouse fibroblasts could be the result of reduced repair of DNA double-strand breaks. Therefore, a common factor may participate in both the repair of DNA double-strand breaks as well as V(D)J rejoining during lymphocyte development. This murine autosomal recessive mutation should prove extremely useful in fundamental studies of radiation-induced DNA damage and repair.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Davisson M. T., Ruetsch N. R., Sweet H. O., Shultz L. D., Bosma M. J. The mouse mutation severe combined immune deficiency (scid) is on chromosome 16. Immunogenetics. 1989;29(1):54–57. doi: 10.1007/BF02341614. [DOI] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Giaccia A. J., Denko N., MacLaren R., Mirman D., Waldren C., Hart I., Stamato T. D. Human chromosome 5 complements the DNA double-strand break-repair deficiency and gamma-ray sensitivity of the XR-1 hamster variant. Am J Hum Genet. 1990 Sep;47(3):459–469. [PMC free article] [PubMed] [Google Scholar]
- Giaccia A., Weinstein R., Hu J., Stamato T. D. Cell cycle-dependent repair of double-strand DNA breaks in a gamma-ray-sensitive Chinese hamster cell. Somat Cell Mol Genet. 1985 Sep;11(5):485–491. doi: 10.1007/BF01534842. [DOI] [PubMed] [Google Scholar]
- Hendrickson E. A., Schatz D. G., Weaver D. T. The scid gene encodes a trans-acting factor that mediates the rejoining event of Ig gene rearrangement. Genes Dev. 1988 Jul;2(7):817–829. doi: 10.1101/gad.2.7.817. [DOI] [PubMed] [Google Scholar]
- Inoue T., Aikawa K., Tezuka H., Kada T., Shultz L. D. Effect of DNA-damaging agents on isolated spleen cells and lung fibroblasts from the mouse mutant "wasted," a putative animal model for ataxia-telangiectasia. Cancer Res. 1986 Aug;46(8):3979–3982. [PubMed] [Google Scholar]
- Jeggo P. A. Studies on mammalian mutants defective in rejoining double-strand breaks in DNA. Mutat Res. 1990 Jul;239(1):1–16. doi: 10.1016/0165-1110(90)90028-a. [DOI] [PubMed] [Google Scholar]
- Kemp L. M., Sedgwick S. G., Jeggo P. A. X-ray sensitive mutants of Chinese hamster ovary cells defective in double-strand break rejoining. Mutat Res. 1984 Nov-Dec;132(5-6):189–196. doi: 10.1016/0167-8817(84)90037-3. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Studies of V(D)J recombination with extrachromosomal substrates. Curr Top Microbiol Immunol. 1988;137:94–99. doi: 10.1007/978-3-642-50059-6_15. [DOI] [PubMed] [Google Scholar]
- Little J. B., Hahn G. M., Frindel E., Tubiana M. Repair of potentially lethal radiation damage in vitro and in vivo. Radiology. 1973 Mar;106(3):689–694. doi: 10.1148/106.3.689. [DOI] [PubMed] [Google Scholar]
- McCulloch E. A., Till J. E. The sensitivity of cells from normal mouse bone marrow to gamma radiation in vitro and in vivo. Radiat Res. 1962 Jun;16:822–832. [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Phillips R. A., Jewett M. A., Gallie B. L. Growth of human tumors in immune-deficient scid mice and nude mice. Curr Top Microbiol Immunol. 1989;152:259–263. doi: 10.1007/978-3-642-74974-2_31. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schuler W., Bosma M. J. Nature of the scid defect: a defective VDJ recombinase system. Curr Top Microbiol Immunol. 1989;152:55–62. doi: 10.1007/978-3-642-74974-2_8. [DOI] [PubMed] [Google Scholar]
- Shultz L. D., Sweet H. O., Davisson M. T., Coman D. R. 'Wasted', a new mutant of the mouse with abnormalities characteristic to ataxia telangiectasia. Nature. 1982 Jun 3;297(5865):402–404. doi: 10.1038/297402a0. [DOI] [PubMed] [Google Scholar]
- Stamato T. D., Denko N. Asymmetric field inversion gel electrophoresis: a new method for detecting DNA double-strand breaks in mammalian cells. Radiat Res. 1990 Feb;121(2):196–205. [PubMed] [Google Scholar]
- Tezuka H., Inoue T., Noguti T., Kada T., Shultz L. D. Evaluation of the mouse mutant "wasted" as an animal model for ataxia telangiectasia. I. Age-dependent and tissue-specific effects. Mutat Res. 1986 Jun;161(1):83–90. doi: 10.1016/0027-5107(86)90102-8. [DOI] [PubMed] [Google Scholar]
- Weaver D., Hendrickson E. The scid mutation disrupts gene rearrangement at the rejoining of coding strands. Curr Top Microbiol Immunol. 1989;152:77–84. doi: 10.1007/978-3-642-74974-2_11. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R., Dahlberg W., Beckett M., Karrison T., Miller D., Clark J., Ervin T. J. Radiation-resistant and repair-proficient human tumor cells may be associated with radiotherapy failure in head- and neck-cancer patients. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2684–2688. doi: 10.1073/pnas.83.8.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers H. R., Elkind M. M. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1970;17(3):261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- Wlodek D., Hittelman W. N. The repair of double-strand DNA breaks correlates with radiosensitivity of L5178Y-S and L5178Y-R cells. Radiat Res. 1987 Oct;112(1):146–155. [PubMed] [Google Scholar]
- van Buul P. P., Tuinenburg-Bolraap A., Searle A. G., Natarajan A. T. A search for radiosensitive mouse mutants by use of the micronucleus technique. Mutat Res. 1987 Jul-Aug;191(3-4):163–169. doi: 10.1016/0165-7992(87)90148-5. [DOI] [PubMed] [Google Scholar]



