Abstract
Fetal alcohol spectrum disorders (FASD) are difficult to diagnose since many heavily exposed infants, at risk for intellectual disability, do not exhibit craniofacial dysmorphology or growth deficits. Consequently, there is a need for biomarkers that predict disability. In both animal models and human studies, alcohol exposure during pregnancy resulted in significant alterations in circulating microRNAs (miRNAs) in maternal blood. In the current study, we asked if changes in plasma miRNAs in alcohol-exposed pregnant mothers, either alone or in conjunction with other clinical variables, could predict infant outcomes. Sixty-eight pregnant women at two perinatal care clinics in western Ukraine were recruited into the study. Detailed health and alcohol consumption histories, and 2nd and 3rd trimester blood samples were obtained. Birth cohort infants were assessed by a geneticist and classified as unexposed (UE), heavily prenatally exposed and affected (HEa) or heavily exposed but apparently unaffected (HEua). MiRNAs were assessed in plasma samples using qRT-PCR arrays. ANOVA models identified 11 miRNAs that were all significantly elevated in maternal plasma from the HEa group relative to HEua and UE groups. In a random forest analysis classification model, a combination of high variance miRNAs, smoking history and socioeconomic status classified membership in HEa and UE groups, with a misclassification rate of 13%. The RFA model also classified 17% of the HEua group as UE-like, whereas 83% were HEa-like, at least at one stage of pregnancy. Collectively our data indicate that maternal plasma miRNAs predict infant outcomes, and may be useful to classify difficult-to-diagnose FASD subpopulations.
Introduction
Fetal Alcohol Spectrum Disorders (FASD) are a leading cause of intellectual disability in the US and worldwide. The global prevalence of Fetal Alcohol Syndrome (FAS), the severe end of the FASD continuum, is estimated at ~2.9‰, with regional prevalence estimates ranging up to 55.42‰, and the prevalence for FASD at 22.77‰, with regional highs of up to 113.22‰ [1]. FASD-associated cognitive and behavioral deficits (reviewed in [2]) contribute to the emergence of secondary mental health disabilities [3, 4], and result in significant public health and economic burdens [5].
Despite published prevention guidelines [6], FASD remains difficult to prevent, because women with unplanned pregnancies, ~53% of pregnancies in US women aged 30 years and younger [7], may not recognize their pregnancy status and episodically engage in heavy drinking including binge drinking [8], a pattern that is particularly damaging to fetal development [9]. There is therefore, a significant need to identify affected children early, to facilitate early intervention, and mitigate disabilities that emerge later in life [10]. However, early diagnosis is difficult, because while some heavily exposed infants exhibit distinctive facial dysmorphology, small head-circumference and perinatal growth restriction [11], other heavily exposed children with neurodevelopmental impairment do not exhibit readily identifiable dysmorphic features [12, 13] associated with FASD. Moreover, a documented history of drinking during pregnancy is often difficult to ascertain [14]. Previous studies have identified ethanol metabolites in neonatal meconium [15, 16], placenta [17], and in newborn dried blood spots [18] as biomarkers for fetal alcohol exposure. However, these biomarkers are not specifically predictors of infant health outcomes. Here we assessed microRNAs (miRNAs) that are present in maternal plasma during pregnancy as potential predictors of infant outcomes, following prenatal alcohol exposure.
MiRNAs are small non-protein-coding RNA molecules that repress protein translation. In 2007, we showed that miRNAs were sensitive to alcohol [19] and mediated alcohol effects on fetal neural [19, 20] and cranial development [21] in animal models. MiRNAs play an important role in alcohol addiction [22–26] and neurotoxicity [27], alcohol-associated alterations in intestinal and hepatic integrity [28, 29], inflammation [30], fibrosis [31], and bone remodeling [32]. Thus, miRNAs are not only sensitive to alcohol exposure, but also mediate many alcohol effects.
In 2008, evidence emerged that miRNAs were secreted into plasma [33] and could be used to diagnose disease [34, 35]. Many organs are predicted to secrete miRNAs into circulation [36], however, during pregnancy fetal tissues like the placenta are an additional source of miRNAs in maternal circulation [37]. Consequently, maternal plasma miRNAs have a dual origin and may therefore serve as composite biomarkers for both maternal and fetal health. We found that ethanol exposure in an ovine pregnancy model, altered maternal plasma miRNAs [38]. Recently, exposure during pregnancy in humans has also been shown to alter maternal serum miRNA content [39]. These data collectively show that circulating miRNAs, like ethanol metabolites, may be used as biomarkers for exposure to alcohol exposure during pregnancy. However, the question that arises is, “can circulating miRNAs in the pregnant mother predict infant outcomes?” To address this question, we assessed the relationship between miRNAs in plasma samples obtained from pregnant women who reported varying levels of alcohol consumption, to the presence or absence of FASD characteristics in their offspring.
Materials & Methods
Description of the Cohort
The sample for this study was drawn from a larger group of pregnant women and their infants who were enrolled in a longitudinal cohort study conducted in two regions of Western Ukraine as part of the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD.org) between the years 2006 and 2011. The sites, a prenatal diagnostic center in the Rivne province and a perinatal center in the Khmelnytsky province where women received prenatal care, were members of the Omni-Net Ukraine Birth Defects Prevention Program. Recruitment methods have been described in detail elsewhere [40, 41]. Briefly, pregnant women were screened for alcohol consumption and selected for enrollment if they reported either frequent daily or weekly episodic (binge) drinking in the month around conception or the most recent month of pregnancy. For each enrolled woman who met the frequent or heavy drinking criteria, the next eligible woman who reported infrequent or no alcohol consumption and no binge drinking was also asked to participate. At enrollment, women provided written informed consent, and were interviewed extensively about demographics, pregnancy and health history, tobacco and other drug use. Women were asked about day-by-day alcohol consumption in the week around conception (variables AAD0 and AADD0, Table 1) and the most recent two weeks of pregnancy (variables AADXP and AADDXP, Table 1) using the timeline followback procedure [42]. The timeline follow back procedure generates day-by-day quantity and frequency estimates of past alcohol and drug consumption with high test-retest reliability [43], and has been validated for assessment of past alcohol use in populations of pregnant women [44]. Women were enrolled in the study on average between 17 and 19 weeks of pregnancy (Table 1), and therefore, the AADXP and AADDXP variables recorded alcohol consumption in the previous two weeks, during the second trimester. Women were interviewed again in the third trimester about alcohol consumption. Patient gestational age at enrollement, alcohol consumption estimates and other demographic and clinical characteristics are outlined in Table 1. Blood samples were collected by venipuncture, into EDTA-coated tubes, from mothers at each of the two interview timepoints, and centrifuged. Aspirated plasma samples were frozen, shipped to investigators in the U.S. and stored at -80°C until analysis.
Table 1. Maternal characteristics of the sample, Ukraine, 2006–2011.
| Variable | HEa (N = 22) | HEua (N = 23) | UE (N = 23) | p-value | |
|---|---|---|---|---|---|
| Mother’s age at enrollment (momage) | 26.95 ±6.00 | 24.04 ± 4.40 | 26.30 ± 4.70 | 0.1377a | |
| Gestational age at enrollment | 18.60 ± 4.83 | 19.13 ± 5.90 | 17.94 ± 6.38 | 0.5051a | |
| Recruitment site | Khmelnytsky | 50.0% (11) | 17.4% (04) | 21.7% (05) | 0.0467b |
| Rivne | 50.0% (11) | 82.6% (19) | 78.3% (18) | ||
| Marital status | Married or cohabiting | 72.7% (16) | 91.3% (21) | 100.0% (23) | 0.0093 b |
| Single/separated/divorced | 27.3% (06) | 8.7% (02) | 0.0% (00) | ||
| Education | Less than high school | 13.6% (03) | 8.7% (02) | 0.0% (00) | 0.0246 b |
| High school or equivalent | 54.5% (12) | 60.9% (14) | 30.4% (07) | ||
| Some college or higher | 31.8% (07) | 30.4% (07) | 69.6% (16) | ||
| Socio-economic category (sescat, Hollingshead score) | 55–66 | 9.1% (02) | 8.7% (02) | 17.4% (04) | 0.0315 a |
| 40–54 | 13.6% (03) | 17.4% (04) | 43.5% (10) | ||
| 30–39 | 45.5% (10) | 43.5% (10) | 21.7% (05) | ||
| 20–29 | 4.5% (01) | 26.1% (06) | 17.4% (04) | ||
| 08–19 | 27.3% (06) | 4.3% (01) | 0.0% (00) | ||
| Gravidity | >1 | 54.5% (12) | 34.8% (08) | 56.5% (13) | 0.3032b |
| 1 | 45.5% (10) | 65.2% (15) | 43.5% (10) | ||
| Parity | >0 | 40.9% (09) | 30.4% (07) | 47.8% (11) | 0.4767b |
| 0 | 59.1% (13) | 69.6% (16) | 52.2% (12) | ||
| Body Mass Index | 22.01 ± 4.55 | 22.75 ± 3.98 | 21.18 ± 3.05 | 0.3701a | |
| Smoking status (smokstat) | Current Smoker | 31.8% (07) | 21.7% (05) | 0.0% (00) | 0.0001 b |
| Never Smoked | 22.7% (05) | 21.7% (05) | 95.7% (22) | ||
| Quit after realized pregnant | 36.4% (08) | 34.8% (08) | 0.0% (00) | ||
| Quit Before Pregnancy | 9.1% (02) | 21.7% (05) | 4.3% (01) | ||
| Number of cigarettes per day during pregnancy | 2.50 ±4.34 | 0.87 ±2.26 | 0.00 ±0.00 | 0.0142 a | |
| Multi-Vitamins after enroll | No | 36.4% (08) | 39.1% (09) | 43.5% (10) | 0.2216a |
| Yes | 63.6% (14) | 60.9% (14) | 56.5% (13) | ||
| Multi-vitamins pre-enroll | No | 36.4% (08) | 21.7% (05) | 26.1% (06) | 0.5392a |
| Yes | 63.6% (14) | 78.3% (18) | 73.9% (17) | ||
| Gestational age at 1st blood draw | 19.09 ± 5.18 | 18.30 ± 5.82 | 18.00 ± 4.45 | 0.7241a | |
| Gestational age at 2nd blood draw | 32.95 ± 2.77 | 33.52 ± 2.50 | 32.26 ± 3.15 | 0.2238a | |
| AAD0: Absolute ounces of alcohol per day at time of conception | 0.69 ±0.65 | 0.50 ±0.27 | 0.00 ±0.00 | 0.0001 a | |
| AADD0: Absolute ounces of alcohol per drinking day at time of conception | 1.72 ±1.33 | 1.50 ±1.05 | 0.00 ±0.00 | 0.0001 a | |
| AADXP: absolute ounces of alcohol per day in two weeks prior to enrollment | 0.09 ±0.17 | 0.03 ±0.06 | 0.00 ±0.00 | 0.0003 a | |
| AADDXP: Absolute ounces of alcohol per drinking day in two weeks prior to enrollment | 0.55 ±0.79 | 0.24 ±0.35 | 0.00 ±0.00 | 0.0002 a | |
x ± y represents the mean ± standard deviation, (n) = sample size. P-values in Bold are statistically significant.
a Kruskal-Wallis Rank Sum Test
b Fisher's Exact Test
After delivery, data were collected on gestational age at birth, birth size and sex of the infant. Live-born infants subsequently received a study-related dysmorphological examination that was conducted by a study geneticist (LY or NZZ) with specific training in FASD. Infants were evaluted for the physical features of FASD and for growth using a standard checklist. At approximately 6 and/or 12 months of age, infants were evaluated for neurobehavioral performance by a study psychologist using the Bayley Scales of Infant Development, second edition (BSID-II), and standard scores were obtained on the Mental Developmental Index (MDI) and the Psychomotor Developmental Index (PDI) after adjustment for gestational age at delivery and standardized for infant sex (Table 2).
Table 2. Infant characteristics of the sample, Ukraine, 2006–2011.
| Variable | HEa (N = 22) | HEua (N = 23) | UE (N = 23) | p-value | |
|---|---|---|---|---|---|
| Child's Sex (CSEX) | Male | 40.9% (09) | 43.5% (10) | 69.6% (16) | 0.1098 b |
| Female | 59.1% (13) | 56.5% (13) | 30.4% (07) | ||
| Height < 10th percentile | No | 86.3% (19) | 100.0% (23) | 95.7% (22) | 0.1177 b |
| Yes | 13.6% (03) | 0.0% (00) | 4.3% (01) | ||
| Weight < 10th percentile | No | 86.3% (19) | 95.7% (22) | 91.3% (21) | 0.5129b |
| Yes | 13.6% (03) | 4.3% (01) | 8.7% (02) | ||
| Occipital-Frontal Circumference < 10th percentile | No | 63.6% (14) | 100% (23) | 91.3% (21) | 0.0009 b |
| Yes | 36.4% (08) | 0% (00) | 8.7% (02) | ||
| Smooth Philtrum | No | 77.3% (17) | 100.0% (23) | 100.0% (23) | 0.0025 b |
| Yes | 22.7% (05) | 0.0% (00) | 0.0% (00) | ||
| Thin vermilion border | No | 63.6% (14) | 100.0% (23) | 69.6% (16) | 0.0022 b |
| Yes | 36.4% (08) | 0.0% (00) | 30.4% (07) | ||
| Palpebral Fissure <10th percentile | No | 45.5% (10) | 100.0% (23) | 95.7% (22) | 0.0001 b |
| Yes | 54.5% (12) | 0.0% (00) | 4.3% (01) | ||
| $MDI 6 month | 81.95 ± 13.68 | 96.67±5.1 | 93.96±9.05 | 0.0005 a | |
| #PDI 6 month | 81.14 ± 15.82 | 99.28±9.99 | 96.22±10.56 | 0.0068 a | |
| MDI12 month | 81.10 ± 12.46 | 97.16±10.17 | 96.17±9.55 | 0.0056 a | |
| PDI 12 month | 90.81±16.21 | 106.84±10.38 | 103.17±11.46 | 0.0368 a | |
| Gestational Age at Delivery | 37.94 ± 2.51 | 40.00 ± 1.10 | 40.03 ± 1.07 | 0.0876 a | |
x ± y represents the mean ± standard deviation, (n) = sample size. P-values in Bold are statistically significant
$ Mental Developmental Index
# Psychomotor Development Index
a Kruskal-Wallis Rank Sum Test
b Fisher's Exact Test
Infants were classified as affected (having an FASD) if they were in the moderate to heavily prenatally exposed group, and had at least two characteristic alcohol-related craniofacial features (short palpebral fissures, smooth philtrum and thin vermilion border of the upper lip) and growth deficiency, and/or neurobehavioral impairment defined as scores on the BSID-II that were more than one standard deviation (15 points) below the mean on the MDI and/or PDI adjusted for prematurity (Table 2). Study protocols were approved by Institutional Review Boards at the Lviv National Medical University, Ukraine, and the University of California San Diego and Texas A&M University in the US, and research was conducted according to the principles expressed in the Declaration of Helsinki.
Description of the Sample
The sample for this analysis was selected based on the criteria of mother-infant pairs for whom complete data were available on maternal alcohol exposure, a dysmorphological examination and neurobehavioral testing of the infant, and the availability of maternal blood samples obtained at both enrollment and in the third trimester. The sample selected consisted of three groups. The first group represented moderate to heavily-exposed mothers with an FASD-affected child (HEa, n = 22); the second group represented moderate to heavily-exposed mothers with an apparently unaffected child, i.e., no facial features, normal head circumference and normal neurobehavioral test scores (HEua, n = 23); and low alcohol consuming or unexposed mothers with an unaffected child (UE, n = 23). By group, maternal and infant characteristics were summarized from the maternal interviews and infant examinations. Socioeconomic status (sescat) as measured by the Hollingshead categories was based on maternal and paternal education and occupation and was classified in category 1–5 with 1 being the highest. Pre-pregnancy body mass index (BMI) was calculated based on mother’s height and her self-reported weight prior to conception. Smoking status (smokstat) was classifed as ‘never smoker’, ‘ever smoker but quit before pregnancy’, ‘ever smoker but quit once recognized pregnant’, and ‘continued or current smoker in pregnancy’. Among current smokers, the average number of cigarettes per day at the time of enrollment was captured. Maternal use of multivitamin supplements was collected from interviews and classified as yes/no for vitamin use prior to enrollment, and for vitamin use after enrollment. The amount of alcohol consumed in the week around conception and in the most recent two weeks prior to enrollment was calculated by daily alcohol type and quantity and classified into four summary variables: absolute ounces of alcohol per day at the time of conception (AAD0), absolute ounces of alcohol per drinking day at the time of conception (AADD0), absolute ounces of alcohol per day in the most recent two weeks (AADXP) and absolute ounces of alcohol per drinking day in the most recent two weeks (AADDXP). Maternal demographic data are summarized in Table 1, and infant outcome measurements in Table 2.
MiRNA analysis
Plasma sample quality control analysis and RNA isolation was performed as outlined in Methods A in S1 File. MiRNA profiles were measured using Human miRCURY LNA™ microRNA real-time PCR arrays (V3&4, Exiqon, Denmark), which assess 752 unique miRNAs [38, 45]. This platform has been shown in comparison tests, to detect miRNAs in biofluids with greater sensitivity and specificity than other miRNA detection methods [46]. Methods and control assays for specificity and sample contamination were performed as outlined in Methods B in S1 File.
Placental Lactogen ELISA
Human Placental Lactogen (hPL) concentration in human plasma was quantitatively determined using a commercially available solid phase sandwich-type enzyme-linked immunoassay (hPL micro-ELISA, Leinco Technologies, Inc., St. Louis, Missouri, USA). The chromogenic reaction product was quantified spectrometrically (at 450nm, Tecan Infinite m200 with Magellan v7.2 control software, Tecan, Austria) against a recombinant hPL (0–15 μg/ml) standard curve.
Statistical modeling
Cycle Thresholds (CTs) were determined using SDS2.4 (ABI/Life Technologies). The CT for all un-amplified (unexpressed) miRNAs was set to a value of 50. ΔCT for each miRNA in each sample was calculated as the CTmiRNA_x—CTAverage_Expressed_miRNAs, i.e., normalized to the average CT value for all of the expressed miRNAs in that sample. All miRNA identities in V3 and V4 Exiqon qPCR array platforms were cross-referenced to their unique mature sequence accession number assigned in miRBase (MIMAT, www.mirbase.org, [47]), before data were aggregated for statistical analyses. All subsequent data analyses were linked to MIMAT accession numbers. Data were analyzed using SPSS (V20, IBM, Armonk, New York), Gensifter® analysis edition (GSEA, Geospiza/PerkinElmer, Seattle, WA) and R (V3.2.2, R Foundation for Statistical Computing, Vienna, Austria). Data were subjected to Analysis of Variance (ANOVA) or T-tests, with Benjamini and Hochberg false discovery rate (FDR) correction for multiple comparisons (α = 0.05 or 0.1). Random forest analysis (RFA, [48]), a non-parametric tree-based ensemble method of classification was implemented in the R ‘randomForest’ package (V4.6–10) as a means to predict group membership of maternal samples based on a combination of miRNAs and demographic variables (see Methods C in S1 File). As a prescreening measure, we included in the RFA model, the 5% (37 out of 752) of sampled miRNAs with the highest variance (while blinded to the class labels) in order to focus in on those miRNAs with potentially the most predictively useful differences. In order to assess the prediction performance of the RFA models, we used out-of-bag (OOB) sampling to compute the misclassification rate [49]. Candidate miRNAs derived from ANOVA (11 miRNAs that exceeded FDR-corrected P < 0.05) and from random forest analysis (top 5 predictive miRNAs) were subject to pathway overrepresentation analysis using the Ingenuity Pathway Analysis® software suite (IPA, QIAGEN, see Methods D in S1 File).
Results
Cohort characteristics
Characteristics of the mothers and infants in each of the three groups in the sample are shown in Tables 1 and 2. Notably, maternal age, pre-pregnancy body mass index (BMI), gestational age at enrollment, gestational ages at blood draws, and the frequency of multivitamin use both before and after enrollment, were similar across all three groups. There were some differences by group in the distribution of women by site, and as expected, socioeconomic status and maternal education levels were lower, and current smoking was higher in the moderate to heavy alcohol-exposed groups compared to the low or unexposed group. Importantly, while alcohol consumption did significantly differ across groups (Table 1, Kruskal-Wallis Rank Sum Test, all P’s < 0.0003 for AAD0, AADD0, AADXP and AADDXP), post-hoc analysis showed that the HEa and HEua groups were not different from each other with respect to prenatal alcohol exposure (Mann-Whitney U, AAD0, P = 0.21; AADD0, P = 0.54; AADXP, P = 0.16; AADDXP, P = 0.093, data not shown). In addition, patterns of alcohol consumption among women who smoked did not differ between the HEa and HEua groups (data not shown). In terms of outcomes, gestational age at delivery was slightly lower in the HEa group compared to HEua and UE groups, but overall, the differences were not statistically significant, and Bayley Scales of Infant Development scores, as defined for group membership, were on average lower in the HEa group than the two unaffected groups (Table 2).
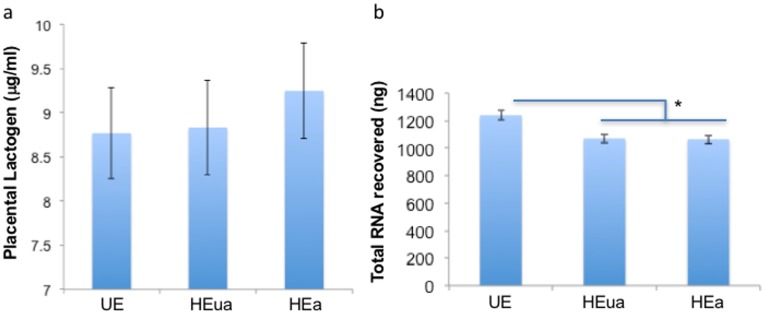
All samples passed quality control analyses and showed no evidence for erythrocyte miRNA contamination (S2 File and S1 Fig). We also found no effect of exposure group on hPL levels (ANOVA, F(2,65) = 0.24, P = 0.79, Fig 1a), asessed near the end of the 3rd trimester. hPL is a sensitive measure of placental health [50], and consequently placental damage is unlikely to account for altered maternal miRNA profiles.
Fig 1. Placental Lactogen (hPL) and total RNA content in maternal plasma samples.
(a) Plasma placental lactogen content in late pregnancy was not significantly different among HEa, HEua and UE groups. (b) Analysis of total plasma RNA content indicated that there was a statistically significant, ~15% decrease in total RNA recovery in the alcohol exposure groups (HEa and HEua) compared to UE controls.
There was however, a significant effect of exposure condition on the quantity of recovered plasma total RNA (ANOVA, F(2,124) = 9.86, P = 0.0001). On average 15% more total RNA was isolated from an equi-volume of plasma obtained from control mothers (UE), compared to alcohol-consuming mothers who subsequently gave birth to both affected (HEa) and unaffected (HEua) infants (all post-hoc t-test P-values < 0.05, Fig 1b). However, there was no significant effect of recruitment site (ANOVA, P = 0.41) and a marginal, though non-significant difference due to pregnancy stage (ANOVA, P = 0.051).
Assessment of group differences in miRNA expression
We observed no group differences in total numbers of expressed miRNAs as a function of recrutiment site (P = 0.74), exposure group (P = 0.99) or pregnancy stage (P = 0.67) by ANOVA. We also did not observe significant differences in the average expression level of expressed miRNAs as a function of exposure group (P = 0.53) or pregnancy stage (P = 0.45), though there was a marginal, non-statistically-significant effect of recruitment site (ANOVA, F(1,124) = 3.513, P = 0.063), on average miRNA expression. Further analysis indicated that a single miRNA, miR-29b, was significantly increased by ~200-fold in maternal samples obtained at mid-pregnancy from Khmelnytsky compared to Rivne (FDR-adjusted t-test, P = 0.047). However, no miRNAs were significantly altered as a function of recruitment site at the end of pregnancy.
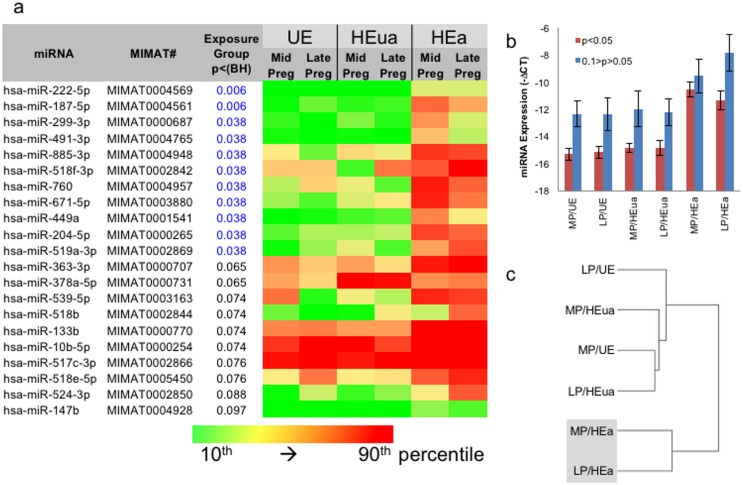
In a 2-way ANOVA assessing the main effects of pregnancy stage and exposure group, and using a 2-ΔCT threshold for between-group changes as a cutoff, to eliminate smaller and perhaps non-biologically relevant group differences, we identified 11 miRNAs that exceeded a FDR of α = 0.05 and a total of 21 that exceeded α = 0.1 for the main effect of exposure condition (Fig 2a). Interestingly, a majority of significantly altered miRNAs were increased in plasma samples from women in the HEa group at both mid- and late-pregnancy compared to both HEua and UE groups (i.e., HEa>(HEua ≅ UE)). This effect was stronger for miRNAs that exceeded the FDR-corrected criterion of P < 0.05 compared to P < 0.1 (Fig 2b). Cluster analysis of miRNAs that exceeded the FDR-corrected criterion of P < 0.1 emphasized our finding that these miRNAs discriminated between the HEa group and both other groups, and that the UE control group was similar to the HEua group (Fig 2c). No miRNAs exceeded the FDR threshold of α = 0.1 for the effect of pregnancy stage.
Fig 2. ANOVA model identifies maternal plasma miRNAs elevated specifically in the HEa group.
(a) List of miRNAs that pass the FDR-corrected criterion of P < 0.05. Color scale ranges from 10th (green) to 90th (red) percentile of expression. miRNA expression in the HEa group at both mid and late pregnancy was generally elevated compared to expression in all other groups (for additional detail, see S2 Fig). (b) Average expression of miRNAs that exceed P < 0.05 and P < 0.1 BH-corrected criteria. (c) Cluster analysis (with Euclidean distance and average linkage) of miRNAs that exceed the BH-corrected P < 0.1 criterion indicates that HEua and UE groups cluster together and are different from HEa groups at mid and late pregnancy. MP, mid-pregnancy; LP, late pregnancy; MIMAT#######, miRNA unique ID as in mirbase.org.
Random Forest Analysis and prediction of HEua group membership
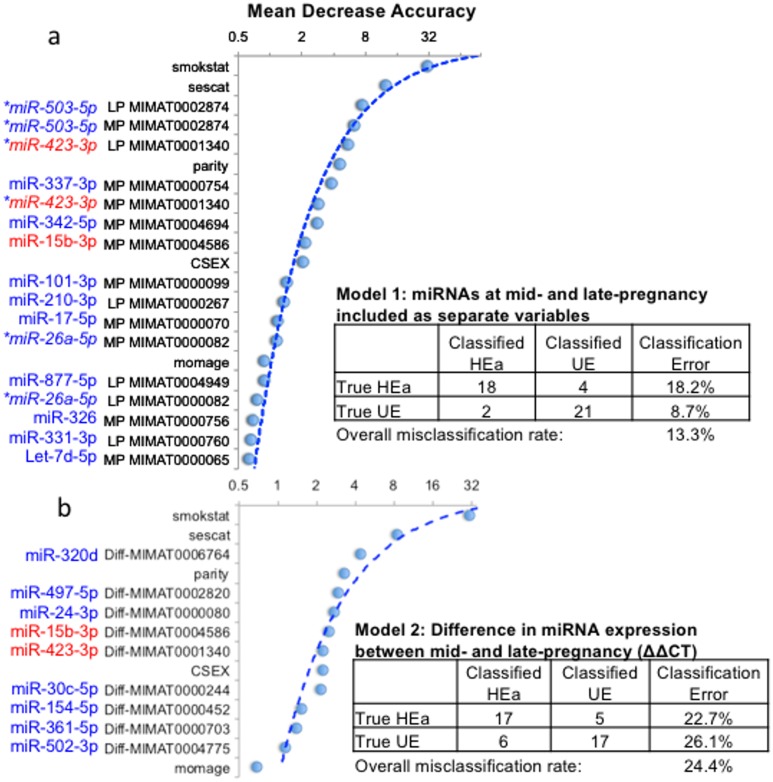
The above analyses collectively show that plasma miRNA profiles can predict infant outcomes, i.e., the presence of dysmorphia and/or neurobehavioral impairment in infancy due to prenatal alcohol exposure. However, the group of prenatally exposed infants with dysmorphic features and/or neurobehavioral impairment measurable in early life are relatively easy to identify and diagnose, while it is significantly more difficult to identify the class of heavily prenatally-exposed infants who do not exhibit obvious dysmorphia or early evidence for developmental delay. To determine the extent to which members of the HEua group could be classified as more like the HEa or UE control group, we first selected the 37 miRNAs (5% of the 752 sampled miRNAs) exhibiting the highest variance irrespective of group membership. Prelimnary analyses, indicated that the 5% criteria represented the best balance between miRNA number and OOB mis-classification rate (S3 Fig). We addiitonally included demographic, clinical and other pregnancy-associated variables (maternal age, maternal multivitamin use before and after enrollment, maternal smoking status (categorical), socioeconomic status category, gravidity, parity, and infant sex) to the random forest model to predict group membership in a comparison of the HEa and UE control group.
Our analyses show that the HEa and UE groups can be classified into their respective groups with an overall OOB mis-classification rate (proportion of misclassified observations) of 13.3% (i.e., 6 out of 45 samples missclassified, Model 1, Fig 3a and S4 Fig). The classification model more accurately assigned membership of UE samples to the UE group, with a classification error rate of 8.7%, whereas the error rate for the HEa group was 18.2% (2 out of 23 and 4 out of 22 samples respectively, Fig 3a). Demographic variables including a history of smoking and socioeconomic status contributed heavily to the accuracy of classification. However, miRNAs, including hsa-miR-503-5p (MIMAT0002874) and hsa-miR-423-3p (MIMAT0001340) which were predictors at mid-pregnancy as well as in late pregnancy, constituted 7 out of the top 10 predictors of class membership. We next asked if a change in miRNA expression from mid- to late-pregnancy (ΔΔCT) could perform as a better classifier of class membership. However, the OOB mis-classification rate for the UE vs HEa comparison increased to 24.4% (Model 2, Fig 3b and S4 Fig), largely due to an increased mis-classification rate (26.1%) in the UE group. We earlier observed that we recovered ~15% less total RNA in HEa and HEua groups compared to UE group (Fig 1b). Total circulating RNA is heterogenous in composition and includes a number of classes of small [51]) and large RNAs [52]. Moreover, the ANOVA model identified several miRNAs that were selectively increased in the HEa group (Fig 2) but not HEua group, suggesting that this variable, total RNA, may contain additional information for class prediction, not contained in the miRNA analyses. Therefore total RNA was included in a secondary analysis. However, inclusion of the total RNA variable resulted in an overall misclassification rate of 17.78% for model 1 and to 24.44% for model 2 (S5 Fig, panels a and b), indicating that addition of this variable did not improve classification.
Fig 3. Random Forest Analysis (RFA) classifies HEa and UE maternal samples into distinct groups.
(a) RFA analysis comparing HEa and UE groups at mid (MP) and late (LP) pregnancy resulted in an overall classification error rate of 13% (18.2% for the HEa group and 8.7% for the UE group). miRNAs constituted 7 out of the top 10 variables that contributed to classification accuracy. Graph depicts Mean Decrease Accuracy (the effect of permuting a variable on prediction after training) on the X-axis and contributory variables in order of decreasing importance on the Y-axis. Astrisks indicate miRNA variables that contributed to prediction accuracy at mid- and late-pregnancy. (b) RFA analysis with difference in miRNA expression (ΔΔCT) between mid and late pregnancy. The overall misclassification rate increased to 24.4%. However, a plot of ‘Mean Decrease Accuracy’ (Y-axis) against variables (X-axis) showed that miRNAs constituted 6 out of the top 10 predictive variables. miRNAs in red text represent variables present in both model 1 and 2. For additional details, see S4 Fig. Smokstat, sescat, parity and momage are as defined in Table 1, CSEX is as defined in Table 2.
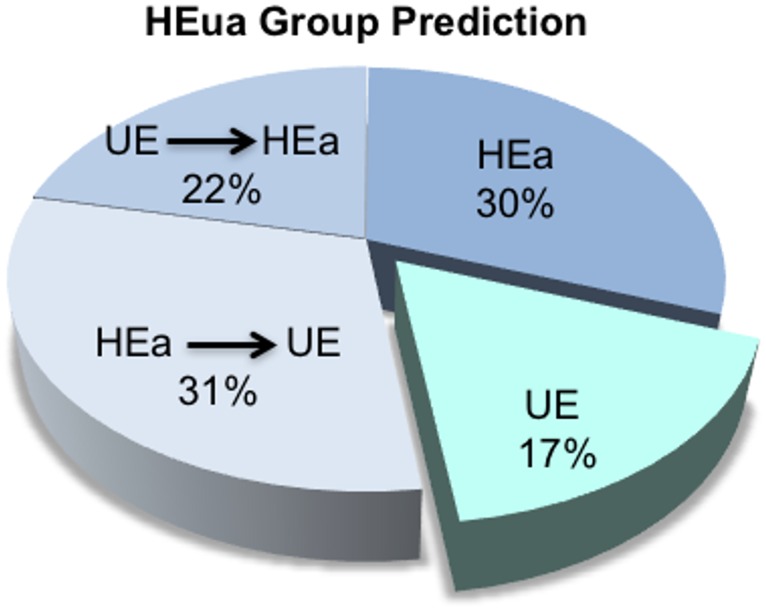
We next asked whether the identified predictive variables could be used to assign members of the HEua group to HEa and UE groups. Based on the RFA model, 17% of the HEua group could be classified completely as the UE group throughout pregnancy, while 83% were more like the HEa group at some time during pregnancy. Among the HEa-like group, a majority remained stably classified as HEa-like throughout pregnancy or were identified as more HEa-like by the end of pregnancy, though a smaller sub-population of the HEa-like group moved towards the UE-like classification by the end of pregnancy (Fig 4). Including total RNA in the prediction model, 78% of the HEua group were classified as HEa-like at some time during pregnancy and 22% were classified as UE-like (S5 Fig, panel c). The total RNA-containing model resulted in increased homogeneity in the portion of the HEua group that was classified as HEa-like. In this prediction model, only 4% of the HEua group who were classified as HEa-like at mid pregnancy moved towards the UE-like classification by the end of pregnancy. Collectively these data indicate that, whereas an analysis of variance strategy emphasised the similarity of the HEua group to the UE group, the random forest classification model preferentially associated the HEua group with the HEa group, i.e., (HEa ≅ HEua)≠UE.
Fig 4. Classification of maternal samples from the HEua group.
Pie chart shows classification of the HEua group as either like HEa or HEua groups. 17% of pregnant mothers assigned to the HEua group were classified as UE-like throughout pregnancy. 52% were either like the HEa (30%) throughout pregnancy or moved from being UE-like at mid pregnancy to HEa like (22%) by the end of pregnancy. However, 31% of HEua mothers were HEa-like at mid pregnancy, but more UE-like by the end of pregnancy.
Predictions of function
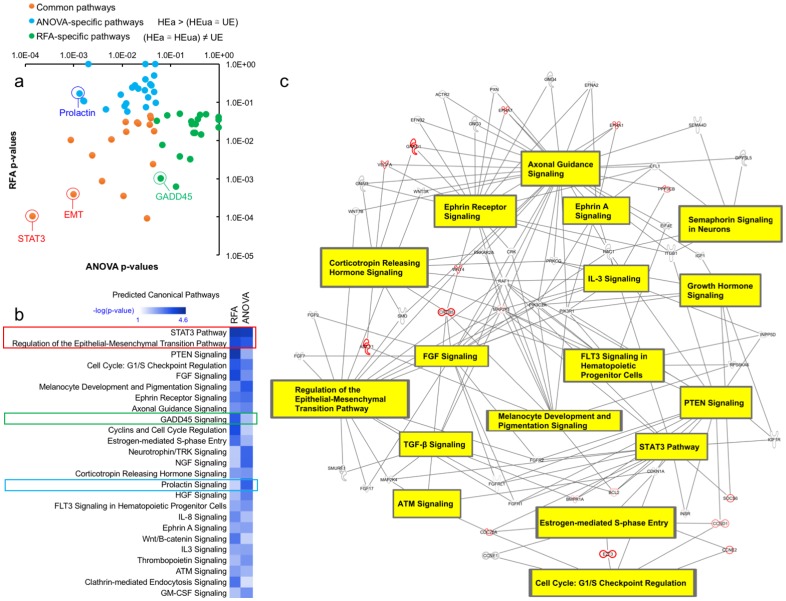
Plasma miRNAs may function as endocrine factors to influence recipient cells and tissues [53]. To assess potential endocrine functions, eleven miRNAs that exceeded the FDR-corrected ANOVA criterion of P < 0.05 (HEa>(HEua ≅ UE)), and the five unique miRNAs among the top 10 contributory variables by RFA ((HEa ≅ HEua)≠UE) were subject to pathway overrepresentation analysis. IPA models showed that despite non-overlapping miRNA content, both groups are predicted to influence common downstream pathways related to fetal and placental growth as well as distinct downstream pathways (Fig 5a and 5b). For example, the Stat3 and Ephrin A pathways and epithelial-mesenchymal transition (EMT) were predicted to be common targets, whereas prolactin signaling was specifically associated with the ANOVA model (HEa>(HEua ≅ UE) and the GADD45 pathway with the RFA model ((HEa ≅ HEua)≠UE). Further analysis of the common predicted pathways indicate that Ephrin, Stat3 and EMT pathways are core members of a highly interconnected and potentially coordinately regulated signaling network (Fig 5c) that is critical for placental and fetal growth and maturation.
Fig 5. Pathway overrepresentation analysis to assess functions of predictive plasma miRNAs.
Pathway overrepresentation analysis was performed using IPA software on targets of the eleven miRNAs that exceeded the FDR-corrected ANOVA criterion of P<0.05 (HEa>(HEua ≅ UE), and the five unique miRNAs among the top 10 contributory variables by random forest analysis ((HEa ≅ HEua)≠UE). (a) The -log10 p-values of significantly enriched pathways (P < 0.05) for both the ANOVA model and the RFA model were plotted against each other with transformed significance values for pathways exclusively enriched among the ANOVA model depicted in blue, the RFA model in green, and pathways enriched in both the RFA and ANOVA models in red. (b) A heat map was constructed of the top 25 significantly enriched pathways among the RFA and ANOVA models. (c) The 17 pathways enriched among both the RFA and ANOVA model share a high degree of interconnectedness. Proteins outlined in red indicate overlapping targeting by miRNAs in both the RFA and ANOVA models.
Discussion
FASD is a significant global health problem, but is difficult to prevent due to the combined prevalence of unplanned pregnancies and patterns of heavy alcohol consumption in women of childbearing age. The average gestational age at enrollment in our study was 18–19 weeks and many women reported alcohol use in the previous month, well into the critical period for neurogenesis and the addition of new neurons to the developing fetal brain [54]. An additional complication is that many prenatally exposed infants do not exhibit craniofacial dysmorphology or growth deficits that facilitate diagnosis. Thus in the current study population, equally heavily exposed mothers gave birth to both affected and apparently unaffected infants, i.e., HEa and HEua groups. Nevertheless, HEua offspring may exhibit later neurodevelopmental deficits that are not readily identifiable in infancy [12, 13]. Previous studies showed that circulating miRNAs in the pregnant mother are promising biomarkers for alcohol exposure in animal models [38] and in human populations [39]. We report here that plasma miRNAs in the pregnant mother may also be useful as a means to predict infant outcomes due to prenatal alcohol exposure.
The ANOVA model, which favors minimal within-group variance relative to systematic variance, selected miRNAs that were generally induced in the HEa group in both mid- and late-pregnancy, compared to HEua and UE groups, while minimizing differences between the latter two groups (HEa>(HEua ≅ UE). These miRNAs represent a maternal signature for risk in the HEa group, i.e., for giving birth to an affected infant. Moreover, this miRNA signature for prenatal ethanol effect could be observed as early as the second trimester of pregnancy and the detection of such a signature may facilitate early intervention to promote maternal-fetal health. It is likely that at least some miRNAs identified in the ANOVA model may be general indicators of risk for adverse pregnancy outcomes. For example, miR-222-5p (MIMAT0004569) which was elevated in the HEa group, is also reportedly elevated in placentas from women diagnosed with severe preeclampsia [55], which, like prenatal alcohol exposure, is an important cause of fetal intrauterine growth restriction (IUGR) in human populations [56]. Similarly, elevation of miR-299-3p (MIMAT0000687), as observed in the HEa group, has been shown to result in senescence of umbilical vein endothelial cells [57] which may also impair fetal growth. Research with animal models convincingly shows that prenatal alcohol compromises placental growth and function [58], and that these deficits correlate with fetal growth restriction [59], a characteristic of the HEa group. Therefore, one explanatory hypothesis is that the ANOVA model identified plasma miRNA in the HEa group that are biomarkers for functional insufficiency of the placenta and associated structures, which in turn increase the risk for adverse infant outcomes.
The ANOVA model did not help identify alcohol-exposed pregnancies that did not result in immediately obvious adverse infant outcomes. To better classify the HEua group, we adopted the RFA classification model. High variance miRNAs (selected without attention to the source of variance) along with a history of cigarette smoking and socioeconomic status, achieved a promising classification error rate of ~13% when comparing the HEa and UE groups. MiRNAs like miR-503-5p (MIMAT0002874) and miR-423-3p (MIMAT0001340) were stable contributors to prediction accuracy in both mid- and late pregnancy, whereas miR-337-3p (MIMAT0000754) contributed to prediction accuracy in late pregnancy. Importantly, using this model, we were able to segregate the HEua group into two sub-populations. The majority sub-population (83%) was more like the HEa group either throughout, or at one stage of pregnancy, while a smaller sub-population (17%) was more like the UE group consistently throughout pregnancy. Interestingly, a sub-population of the group classified as HEa-like at mid-pregnancy were predicted to be more like the controls by the end of pregnancy. This shift in classification suggests that currently unknown genetic or environmental resiliency factors may protect some pregnancies against adverse outcomes. It will be important to track neurocognitive performance in subcategories of the HEua group, as these children grow older, to determine whether our predictive models do indeed categorize future risk. These assessments are currently being performed.
While miRNAs contributed to the overall accuracy of group classification, two important variables, smoking history and socioeconomic status contributed significantly to the accuracy of classification of HEa and UE groups. The relationship between high variance miRNAs and smoking history or socioeconomic status is unknown at this time, but clearly, more research is needed to understand why these factors collectively contribute to predicting outcomes. Ongoing smoking during pregnancy may be a proxy measure for ongoing heavy alcohol consumption. However, cigarette smoking is also a well-established causal factor for fetal growth restriction [60, 61] and therefore, concurrent smoking may increase the severity of effects due to prenatal alcohol exposure. We also previously reported that both alcohol and nicotine target common miRNAs [20, 62]. Therefore, the predictive strength of maternal plasma miRNAs for infant outcomes may in part be determined by the history of smoking. Since 5 out of 7 members (71%) of the HEua group who were classified as HEa-like in the second trimester but as UE-like in the third trimester reported quitting smoking upon realization of pregnancy, smoking cessation programs during pregnancy may independently mitigate harm due to prenatal alcohol exposure.
Socioeconomic status, the second important contributory classification variable is strongly associated with health status and is thought to encompass a variety of physical and psychosocial stressors [63] that are likely to adversely influence fetal growth and development during pregnancy [64], and amplify deficits due to prenatal alcohol exposure [65]. Moreover, stress has also been show to alter circulating miRNA expression profiles [66], and consequently, socioeconomic status may also contribute to the alterations in miRNA expression observed in the current study. It is encouraging to note that interventions such as social enrichment, which may be expected to mitigate effects of socioeconomic status, have recently been shown to reverse effects of prenatal ethanol exposure on miRNA expression profiles [67]. Both socioeconomic status and current smoking have been identified as risk factors for FASD in other populations as well [68]. As with smoking cessation, strategies to minimize the effects of socioeconomic status may also minimize the effects of prenatal alcohol exposure.
At this time, we do not know which tissues and cell-types contribute circulating miRNAs that discriminate between the HEa and UE groups or permit classification of HEua group members. Plasma miRNAs are likely to represent a composite of cellular secretory activity and cell death mechanisms (reviewed in [69]), and while currently a controversial concept, these miRNAs may also constitute a novel class of endocrine factors that regulate protein translation in recipient cells [70–72]. The fetus may also contribute functional miRNAs to maternal circulation. For example, fetal cell-free RNA molecules appear in maternal plasma early during pregnancy and are maintained throughout pregnancy [73, 74], and trophoblast-secreted miRNAs have been shown to influence target gene expression in maternal immune cells [75]. Irrespective of their tissue source, the identified miRNAs are predicted to control important biological processes associated with fetal growth. For example, though third trimester placental lactogen levels were not altered in our study, we identified downstream prolactin signaling, which regulates angiogenesis [76] and maternal insulin metabolism [77] during pregnancy, as a candidate HEa group-specific miRNA-targeted pathway. In contrast, the Growth Arrest and DNA Damage-inducible 45 (GADD45) pathway, a pro-apoptotic pathway that is elevated in response to environmental stress [78] was preferentially identified as a candidate pathway in RFA model which emphasized commonalties between HEa and HEua groups. Despite non-overlapping miRNA content, both HEa>(HEua ≅ UE) and (HEa ≅ HEua)≠UE class miRNAs are nevertheless predicted to target a highly interconnected set of cytokine and growth factor pathways including Stat3 and Ephrin signaling pathways that converge on the epithelial-to-mesenchymal transition (EMT) process. The growth and maturation of the placenta is understood to be an EMT-like process [79] and placental angiogenesis and trophoblast invasion is mediated by activation of Stat3 [80] and ephrin [81] pathways. Collectively, our predictive models suggest that HEa>(HEua ≅ UE) and (HEa ≅ HEua)≠UE class maternal miRNAs are likely to both serve as biomarkers and functional mediators for important fetal developmental pathways.
In this pilot study, we present evidence that plasma miRNA profiles in the pregnant mother do predict alcohol-related infant health outcomes and help classify prenatal alcohol-exposed infants who are at risk for intellectual disability. Importantly, demographic variables like smoking history and socioeconomic status contribute significantly to the accuracy of risk assessment, but may also be modifiable causal factors and targets for perinatal intervention. However, these data should be viewed as preliminary and with caution. It will be important to assess whether maternal miRNA profiles discriminate between FASD and, in some respects phenotypically similar syndromes, including Williams and 22q11 deletion syndromes that have a clear genetic etiology, but also result in intellectual disability. The role of geography, ethnicity and other factors in the biofluid miRNA response to alcohol exposure during pregnancy also require further investigation. In this context, a recent study on the effects of alcohol consumption on serum (rather than plasma) miRNAs in a population of pregnant women in New Mexico [39] identified a markedly different group of alcohol-responsive miRNAs. The intent of that study was to assess miRNAs as biomarkers of exposure rather than outcome, and assessment platforms (microarray hybridization vs. qRT-PCR), biofluid source (serum vs. plasma), geography and population ethnicity all differed from the current study, and likely contributed to differences in outcomes. However, since platelets release miRNAs during the coagulation cascade [70], serum and plasma differ in miRNA content even when obtained at the same time, from the same patient [82]. While other intervening variables cannot be discounted, and require further investigation, it is possible that the New Mexico study uncovered an important effect of maternal alcohol exposure on acute hemostasis, whereas our study uncovered a more persistent, stable miRNA response to the effects of alcohol exposure. However, the results of both studies also leave open the possibility that the miRNAs that are significantly altered by alcohol exposure in the pregnant woman may not be the same as those which predict infant outcomes. Finally, several studies have shown that secreted miRNAs are biologically functional [70–72]. Therefore, maternal circulating miRNAs may also be targeted in future for therapeutic intervention, to promote fetal growth and development, especially in pregnancies that are predicted to result in adverse infant outcomes.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Acknowledgments
We are grateful to the OMNI-Net Birth Defects Prevention Program and to the women and their children who participated in this study. We also acknowledge Dr. Edward Riley, San Diego State University (eriley@mail.sdsu.edu) and the Collaborative Initiative on Fetal Alcohol Spectrum Disorders (CIFASD.org) for intellectual and administrative support.
Data Availability
Some restrictions to data will apply. All data will be held in a central repository at CIFASD.org, and is available pending IRB review to researchers who meet the criteria for access to confidential data (http://cifasd.org/data-sharing/).
Funding Statement
This research was supported by NIH-U01AA014835 and the NIH Office of Dietary Supplements to CDC, and by R01AA013440 and a pilot grant subcontract from CIFASD.org (from NIH-U24AA014811) to RCM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Roozen S, Peters GJ, Kok G, Townend D, Nijhuis J, Curfs L. Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcoholism, clinical and experimental research. 2016;40(1):18–32. Epub 2016/01/05. 10.1111/acer.12939 . [DOI] [PubMed] [Google Scholar]
- 2.Williams JF, Smith VC, Levy S, Ammerman SD, Gonzalez PK, Ryan SA, et al. Fetal Alcohol Spectrum Disorders. Pediatrics. 2015;136(5):e1395–e406. Epub 2015/10/21. 10.1542/peds.2015-3113 . [DOI] [PubMed] [Google Scholar]
- 3.O'Connor MJ, Paley B. Psychiatric conditions associated with prenatal alcohol exposure. Developmental disabilities research reviews. 2009;15(3):225–34. Epub 2009/09/05. 10.1002/ddrr.74 . [DOI] [PubMed] [Google Scholar]
- 4.Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60(4):377–85. Epub 2003/04/16. 10.1001/archpsyc.60.4.377 . [DOI] [PubMed] [Google Scholar]
- 5.Popova S, Lange S, Burd L, Rehm J. Health care burden and cost associated with fetal alcohol syndrome: based on official Canadian data. PloS one. 2012;7(8):e43024 Epub 2012/08/18. 10.1371/journal.pone.0043024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ACOG. Committee opinion no. 496: At-risk drinking and alcohol dependence: obstetric and gynecologic implications. Obstetrics and gynecology. 2011;118(2 Pt 1):383–8. Epub 2011/07/22. 10.1097/AOG.0b013e31822c9906 . [DOI] [PubMed] [Google Scholar]
- 7.Finer LB, Zolna MR. Declines in Unintended Pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374(9):843–52. Epub 2016/03/11. 10.1056/NEJMsa1506575 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May PA, Gossage JP, White-Country M, Goodhart K, Decoteau S, Trujillo PM, et al. Alcohol consumption and other maternal risk factors for fetal alcohol syndrome among three distinct samples of women before, during, and after pregnancy: the risk is relative. American journal of medical genetics Part C, Seminars in medical genetics. 2004;127C(1):10–20. Epub 2004/04/20. 10.1002/ajmg.c.30011 . [DOI] [PubMed] [Google Scholar]
- 9.Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14(1):107–18. . [DOI] [PubMed] [Google Scholar]
- 10.Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neuroscience and biobehavioral reviews. 2010;34(6):791–807. Epub 2009/06/24. 10.1016/j.neubiorev.2009.06.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diwadkar VA, Meintjes EM, Goradia D, Dodge NC, Warton C, Molteno CD, et al. Differences in cortico-striatal-cerebellar activation during working memory in syndromal and nonsyndromal children with prenatal alcohol exposure. Human brain mapping. 2013;34(8):1931–45. Epub 2012/03/28. 10.1002/hbm.22042 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson SW, Stanton ME, Molteno CD, Burden MJ, Fuller DS, Hoyme HE, et al. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol Clin Exp Res. 2008;32(2):365–72. Epub 2007/12/29. 10.1111/j.1530-0277.2007.00585.x . [DOI] [PubMed] [Google Scholar]
- 14.May PA, Blankenship J, Marais AS, Gossage JP, Kalberg WO, Barnard R, et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South african population-based study. Alcohol Clin Exp Res. 2013;37(5):818–30. Epub 2012/12/18. 10.1111/acer.12033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson J, Kirchner HL, Xue W, Minnes S, Singer LT, Bearer CF. Fatty acid ethyl esters in meconium are associated with poorer neurodevelopmental outcomes to two years of age. J Pediatr. 2008;152(6):788–92. 10.1016/j.jpeds.2007.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bearer CF, Santiago LM, O'Riordan MA, Buck K, Lee SC, Singer LT. Fatty Acid ethyl esters: quantitative biomarkers for maternal alcohol consumption. The Journal of pediatrics. 2005;146(6):824–30. Epub 2005/06/24. 10.1016/j.jpeds.2005.01.048 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matlow JN, Aleksa K, Lubetsky A, Koren G. The detection and quantification of ethyl glucuronide in placental tissue and placental perfusate by headspace solid-phase microextraction coupled with gas chromatography-mass spectrometry. Journal of population therapeutics and clinical pharmacology = Journal de la therapeutique des populations et de la pharamcologie clinique. 2012;19(3):e473–82. Epub 2012/11/06. . [PubMed] [Google Scholar]
- 18.Bakhireva LN, Savich RD, Raisch DW, Cano S, Annett RD, Leeman L, et al. The feasibility and cost of neonatal screening for prenatal alcohol exposure by measuring phosphatidylethanol in dried blood spots. Alcohol Clin Exp Res. 2013;37(6):1008–15. Epub 2013/02/21. 10.1111/acer.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27(32):8546–57. Epub 2007/08/10. 27/32/8546 [pii] 10.1523/JNEUROSCI.1269-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai PC, Bake S, Balaraman S, Rawlings J, Holgate RR, Dubois D, et al. MiR-153 targets the nuclear factor-1 family and protects against teratogenic effects of ethanol exposure in fetal neural stem cells. Biology open. 2014;3(8):741–58. Epub 2014/07/27. 10.1242/bio.20147765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pappalardo-Carter DL, Balaraman S, Sathyan P, Carter ES, Chen WJ, Miranda RC. Suppression and epigenetic regulation of MiR-9 contributes to ethanol teratology: evidence from zebrafish and murine fetal neural stem cell models. Alcohol Clin Exp Res. 2013;37(10):1657–67. Epub 2013/06/27. 10.1111/acer.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59(2):274–87. 10.1016/j.neuron.2008.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewohl JM, Nunez YO, Dodd PR, Tiwari GR, Harris RA, Mayfield RD. Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin Exp Res. 2011;35(11):1928–37. Epub 2011/06/10. 10.1111/j.1530-0277.2011.01544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tapocik JD, Solomon M, Flanigan M, Meinhardt M, Barbier E, Schank JR, et al. Coordinated dysregulation of mRNAs and microRNAs in the rat medial prefrontal cortex following a history of alcohol dependence. The pharmacogenomics journal. 2013;13(3):286–96. Epub 2012/05/23. 10.1038/tpj.2012.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo Y, Chen Y, Carreon S, Qiang M. Chronic intermittent ethanol exposure and its removal induce a different miRNA expression pattern in primary cortical neuronal cultures. Alcohol Clin Exp Res. 2012;36(6):1058–66. Epub 2011/12/07. 10.1111/j.1530-0277.2011.01689.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steenwyk G, Janeczek P, Lewohl JM. Differential Effects of Chronic and Chronic-Intermittent Ethanol Treatment and Its Withdrawal on the Expression of miRNAs. Brain Sci. 2013;3(2):744–56. 10.3390/brainsci3020744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav S, Pandey A, Shukla A, Talwelkar SS, Kumar A, Pant AB, et al. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J Biol Chem. 2011;286(43):37347–57. Epub 2011/09/01. 10.1074/jbc.M111.235531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, et al. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32(2):355–64. 10.1111/j.1530-0277.2007.00584.x [DOI] [PubMed] [Google Scholar]
- 29.Dolganiuc A, Petrasek J, Kodys K, Catalano D, Mandrekar P, Velayudham A, et al. MicroRNA expression profile in Lieber-DeCarli diet-induced alcoholic and methionine choline deficient diet-induced nonalcoholic steatohepatitis models in mice. Alcohol Clin Exp Res. 2009;33(10):1704–10. Epub 2009/07/04. 10.1111/j.1530-0277.2009.01007.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bala S, Szabo G. MicroRNA Signature in Alcoholic Liver Disease. International journal of hepatology. 2012;2012:498232 Epub 2012/04/21. 10.1155/2012/498232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng F, Glaser SS, Francis H, Yang F, Han Y, Stokes A, et al. Epigenetic regulation of miR-34a expression in alcoholic liver injury. Am J Pathol. 2012;181(3):804–17. Epub 2012/07/31. 10.1016/j.ajpath.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sampson HW, Chaput CD, Brannen J, Probe RA, Guleria RS, Pan J, et al. Alcohol induced epigenetic perturbations during the inflammatory stage of fracture healing. Exp Biol Med (Maywood). 2011;236(12):1389–401. Epub 2011/11/17. 10.1258/ebm.2011.011207 . [DOI] [PubMed] [Google Scholar]
- 33.Hunter MP, Ismail N, Zhang X, Aguda BD, Lee EJ, Yu L, et al. Detection of microRNA expression in human peripheral blood microvesicles. PLoS ONE. 2008;3(11):e3694 Epub 2008/11/13. 10.1371/journal.pone.0003694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go AT, Visser A, van Dijk M, Mulders MA, Eijk P, Ylstra B, et al. A novel method to identify syncytiotrophoblast-derived RNA products representative of trisomy 21 placental RNA in maternal plasma. Methods in molecular biology (Clifton, NJ. 2008;444:291–302. 10.1007/978-1-59745-066-9_23 [DOI] [PubMed] [Google Scholar]
- 35.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105(30):10513–8. 10.1073/pnas.0804549105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou M, Hara H, Dai Y, Mou L, Cooper DK, Wu C, et al. Circulating Organ-Specific MicroRNAs Serve as Biomarkers in Organ-Specific Diseases: Implications for Organ Allo- and Xeno-Transplantation. Int J Mol Sci. 2016;17(8). 10.3390/ijms17081232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Visone R, Russo L, Pallante P, De Martino I, Ferraro A, Leone V, et al. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer. 2007;14(3):791–8. 10.1677/ERC-07-0129 . [DOI] [PubMed] [Google Scholar]
- 38.Balaraman S, Lunde ER, Sawant O, Cudd TA, Washburn SE, Miranda RC. Maternal and neonatal plasma microRNA biomarkers for fetal alcohol exposure in an ovine model. Alcohol Clin Exp Res. 2014;38(5):1390–400. Epub 2014/03/05. 10.1111/acer.12378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardiner AS, Gutierrez HL, Luo L, Davies S, Savage DD, Bakhireva LN, et al. Alcohol Use During Pregnancy is Associated with Specific Alterations in MicroRNA Levels in Maternal Serum. Alcohol Clin Exp Res. 2016;40(4):826–37. Epub 2016/04/04. 10.1111/acer.13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chambers CD, Yevtushok L, Zymak-Zakutnya N, Korzhynskyy Y, Ostapchuk L, Akhmedzhanova D, et al. Prevalence and predictors of maternal alcohol consumption in 2 regions of Ukraine. Alcohol Clin Exp Res. 2014;38(4):1012–9. Epub 2014/05/17. 10.1111/acer.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coles CD, Kable JA, Keen CL, Jones KL, Wertelecki W, Granovska IV, et al. Dose and Timing of Prenatal Alcohol Exposure and Maternal Nutritional Supplements: Developmental Effects on 6-Month-Old Infants. Maternal and child health journal. 2015;19(12):2605–14. Epub 2015/07/15. 10.1007/s10995-015-1779-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sobell LC, Sobell MB. Timeline Follow-Back In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa, NJ: Humana Press; 1992. p. 41–72. [Google Scholar]
- 43.Sobell LC, Sobell MB, Leo GI, Cancilla A. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83(4):393–402. . [DOI] [PubMed] [Google Scholar]
- 44.Jacobson SW, Chiodo LM, Sokol RJ, Jacobson JL. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics. 2002;109(5):815–25. Epub 2002/05/03. . [DOI] [PubMed] [Google Scholar]
- 45.Selvamani A, Williams MH, Miranda RC, Sohrabji F. Circulating miRNA profiles provide a biomarker for severity of stroke outcomes associated with age and sex in a rat model. Clin Sci (Lond). 2014;127(2):77–89. Epub 2014/01/17. 10.1042/CS20130565 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mestdagh P, Hartmann N, Baeriswyl L, Andreasen D, Bernard N, Chen C, et al. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (miRQC) study. Nature methods. 2014;11(8):809–15. 10.1038/nmeth.3014 [DOI] [PubMed] [Google Scholar]
- 47.Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36(Database issue):D154–8. Epub 2007/11/10. gkm952 [pii] 10.1093/nar/gkm952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breiman L. Random Forests. Machine Learning. 2001;45(1):5–32. 10.1023/a:1010933404324 [DOI] [Google Scholar]
- 49.James J, Witten D, Hastie T, Tibshirani R. An Introduction to Statistical Learning with Applications in R. DeVeaux R, Fienberg SE, editors. New York: Springer-Verlag; 2015. [Google Scholar]
- 50.Dutton PJ, Warrander LK, Roberts SA, Bernatavicius G, Byrd LM, Gaze D, et al. Predictors of poor perinatal outcome following maternal perception of reduced fetal movements—a prospective cohort study. PLoS ONE. 2012;7(7):e39784 Epub 2012/07/19. 10.1371/journal.pone.0039784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dhahbi JM, Spindler SR, Atamna H, Boffelli D, Mote P, Martin DI. 5'-YRNA fragments derived by processing of transcripts from specific YRNA genes and pseudogenes are abundant in human serum and plasma. Physiological genomics. 2013;45(21):990–8. 10.1152/physiolgenomics.00129.2013 . [DOI] [PubMed] [Google Scholar]
- 52.Tsui NB, Ng EK, Lo YM. Molecular analysis of circulating RNA in plasma. Methods Mol Biol. 2006;336:123–34. 10.1385/1-59745-074-X:123 . [DOI] [PubMed] [Google Scholar]
- 53.Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, et al. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–15. Epub 2014/04/17. 10.1016/j.ccr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nature reviews Neuroscience. 2008;9(2):110–22. Epub 2008/01/23. 10.1038/nrn2252 . [DOI] [PubMed] [Google Scholar]
- 55.Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin Chem Lab Med. 2009;47(8):923–9. Epub 2009/08/01. 10.1515/CCLM.2009.228 . [DOI] [PubMed] [Google Scholar]
- 56.Srinivas SK, Edlow AG, Neff PM, Sammel MD, Andrela CM, Elovitz MA. Rethinking IUGR in preeclampsia: dependent or independent of maternal hypertension? J Perinatol. 2009;29(10):680–4. 10.1038/jp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jong HL, Mustafa MR, Vanhoutte PM, AbuBakar S, Wong PF. MicroRNA 299-3p modulates replicative senescence in endothelial cells. Physiological genomics. 2013;45(7):256–67. Epub 2013/01/31. 10.1152/physiolgenomics.00071.2012 . [DOI] [PubMed] [Google Scholar]
- 58.Gundogan F, Elwood G, Mark P, Feijoo A, Longato L, Tong M, et al. Ethanol-induced oxidative stress and mitochondrial dysfunction in rat placenta: relevance to pregnancy loss. Alcohol Clin Exp Res. 2010;34(3):415–23. Epub 2009/12/24. 10.1111/j.1530-0277.2009.01106.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gundogan F, Gilligan J, Qi W, Chen E, Naram R, de la Monte SM. Dose effect of gestational ethanol exposure on placentation and fetal growth. Placenta. 2015;36(5):523–30. Epub 2015/03/10. 10.1016/j.placenta.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy JF, Mulcahy R. The effect of age, parity, and cigarette smoking on baby weight. Am J Obstet Gynecol. 1971;111(1):22–5. Epub 1971/09/01. 10.1016/0002-9378(71)90920-3 . [DOI] [PubMed] [Google Scholar]
- 61.Russell CS, Taylor R, Law CE. Smoking in pregnancy, maternal blood pressure, pregnancy outcome, baby weight and growth, and other related factors. A prospective study. Br J Prev Soc Med. 1968;22(3):119–26. Epub 1968/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balaraman S, Winzer-Serhan UH, Miranda RC. Opposing actions of ethanol and nicotine on microRNAs are mediated by nicotinic acetylcholine receptors in fetal cerebral cortical-derived neural progenitor cells. Alcohol Clin Exp Res. 2012;36(10):1669–77. Epub 2012/03/31. 10.1111/j.1530-0277.2012.01793.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–52. Epub 2005/04/30. 10.1126/science.1106477 . [DOI] [PubMed] [Google Scholar]
- 64.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59(3):279–89. Epub 2010/07/02. 10.1016/j.yhbeh.2010.06.007 . [DOI] [PubMed] [Google Scholar]
- 65.Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27(1–2):285–98. Epub 2001/12/26. . [DOI] [PubMed] [Google Scholar]
- 66.Beninson LA, Brown PN, Loughridge AB, Saludes JP, Maslanik T, Hills AK, et al. Acute stressor exposure modifies plasma exosome-associated heat shock protein 72 (Hsp72) and microRNA (miR-142-5p and miR-203). PLoS One. 2014;9(9):e108748 Epub 2014/09/27. 10.1371/journal.pone.0108748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ignacio C, Mooney SM, Middleton FA. Effects of Acute Prenatal Exposure to Ethanol on microRNA Expression are Ameliorated by Social Enrichment. Frontiers in pediatrics. 2014;2:103 Epub 2014/10/14. 10.3389/fped.2014.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.May PA, Gossage JP, Brooke LE, Snell CL, Marais AS, Hendricks LS, et al. Maternal risk factors for fetal alcohol syndrome in the Western cape province of South Africa: a population-based study. Am J Public Health. 2005;95(7):1190–9. Epub 2005/06/04. 10.2105/AJPH.2003.037093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turchinovich A, Samatov TR, Tonevitsky AG, Burwinkel B. Circulating miRNAs: cell-cell communication function? Frontiers in genetics. 2013;4:119 Epub 2013/07/05. 10.3389/fgene.2013.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laffont B, Corduan A, Ple H, Duchez AC, Cloutier N, Boilard E, et al. Activated platelets can deliver mRNA regulatory Ago2*microRNA complexes to endothelial cells via microparticles. Blood. 2013;122(2):253–61. Epub 2013/05/09. 10.1182/blood-2013-03-492801 . [DOI] [PubMed] [Google Scholar]
- 71.Gidlof O, van der Brug M, Ohman J, Gilje P, Olde B, Wahlestedt C, et al. Platelets activated during myocardial infarction release functional miRNA, which can be taken up by endothelial cells and regulate ICAM1 expression. Blood. 2013;121(19):3908–17, S1–26. Epub 2013/03/16. 10.1182/blood-2012-10-461798 . [DOI] [PubMed] [Google Scholar]
- 72.Zhang Y, Liu D, Chen X, Li J, Li L, Bian Z, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39(1):133–44. Epub 2010/07/07. 10.1016/j.molcel.2010.06.010 . [DOI] [PubMed] [Google Scholar]
- 73.Chiu RW, Lui WB, Cheung MC, Kumta N, Farina A, Banzola I, et al. Time profile of appearance and disappearance of circulating placenta-derived mRNA in maternal plasma. Clin Chem. 2006;52(2):313–6. Epub 2006/02/02. 10.1373/clinchem.2005.059691 . [DOI] [PubMed] [Google Scholar]
- 74.Poon LL, Leung TN, Lau TK, Lo YM. Presence of fetal RNA in maternal plasma. Clin Chem. 2000;46(11):1832–4. Epub 2000/11/09. . [PubMed] [Google Scholar]
- 75.Kambe S, Yoshitake H, Yuge K, Ishida Y, Ali MM, Takizawa T, et al. Human exosomal placenta-associated miR-517a-3p modulates the expression of PRKG1 mRNA in Jurkat cells. Biology of reproduction. 2014;91(5):129 Epub 2014/10/03. 10.1095/biolreprod.114.121616 . [DOI] [PubMed] [Google Scholar]
- 76.Corbacho AM, Martinez De La Escalera G, Clapp C. Roles of prolactin and related members of the prolactin/growth hormone/placental lactogen family in angiogenesis. The Journal of endocrinology. 2002;173(2):219–38. Epub 2002/05/16. . [DOI] [PubMed] [Google Scholar]
- 77.Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, et al. Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132(2):879–87. Epub 1993/02/01. 10.1210/endo.132.2.8425500 . [DOI] [PubMed] [Google Scholar]
- 78.Lucas A, Mialet-Perez J, Daviaud D, Parini A, Marber MS, Sicard P. Gadd45gamma regulates cardiomyocyte death and post-myocardial infarction left ventricular remodelling. Cardiovascular research. 2015;108(2):254–67. Epub 2015/09/16. 10.1093/cvr/cvv219 . [DOI] [PubMed] [Google Scholar]
- 79.Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta—epithelial-mesenchymal transition (EMT) and placental development. Placenta. 2010;31(9):747–55. Epub 2010/07/28. 10.1016/j.placenta.2010.06.017 . [DOI] [PubMed] [Google Scholar]
- 80.Chen CY, Liu SH, Chen CY, Chen PC, Chen CP. Human placenta-derived multipotent mesenchymal stromal cells involved in placental angiogenesis via the PDGF-BB and STAT3 pathways. Biology of reproduction. 2015;93(4):103 Epub 2015/09/12. 10.1095/biolreprod.115.131250 . [DOI] [PubMed] [Google Scholar]
- 81.Goldman-Wohl D, Greenfield C, Haimov-Kochman R, Ariel I, Anteby EY, Hochner-Celnikier D, et al. Eph and ephrin expression in normal placental development and preeclampsia. Placenta. 2004;25(7):623–30. Epub 2004/06/15. 10.1016/j.placenta.2004.01.016 . [DOI] [PubMed] [Google Scholar]
- 82.Wang K, Yuan Y, Cho JH, McClarty S, Baxter D, Galas DJ. Comparing the MicroRNA spectrum between serum and plasma. PLoS One. 2012;7(7):e41561 Epub 2012/08/04. 10.1371/journal.pone.0041561 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
Some restrictions to data will apply. All data will be held in a central repository at CIFASD.org, and is available pending IRB review to researchers who meet the criteria for access to confidential data (http://cifasd.org/data-sharing/).