Abstract
Noroviruses (NoVs) are the leading cause of gastroenteritis outbreaks in humans worldwide. Since late 2012, a new GII.4 variant Sydney 2012 has caused a significant increase in NoV epidemics in several countries. From November of 2012 to January of 2013, three gastroenteritis outbreaks occurred in two social welfare homes (Outbreaks A and B) and a factory (Outbreak C) in Shenzhen city of China. Feces and swabs were collected for laboratory tests for causative agents. While no bacterial pathogen was identified, all three outbreaks were caused by NoVs with detection rates of 26.2% (16/61) at Outbreak A, 35.2% (38/108) at Outbreak B), and 59.3% (16/27) at Outbreaks C. For Outbreak B, 25 of the 29 symptomatic individuals (86.2%) and 13 of the 79 asymptomatic individuals (16.5%) were found NoV-positive. For Outbreak C, an asymptomatic food handler was NoV-positive. All thirteen NoV sequences from the three outbreaks were classified into genogroup II and genotype 4 (GII.4), which we identified to be the GII.4 Sydney 2012 variant. The genome of two isolates from Outbreaks A and B were recombinant with the opening reading frame (ORF) 1 of GII.4 Osaka 2007 and ORF2 and 3 of the GII.4 New Orleans. Our study indicated that the GII.4 Sydney 2012 variant emerged and caused the outbreaks in China.
Introduction
Noroviruses (NoVs), members of the Norovirus genus in the Caliciviridae family, are recognized as the most common viral cause of gastroenteritis outbreaks throughout the world [1–4], wherein they are responsible for > 90% of non-bacterial outbreaks of gastroenteritis worldwide [5]. In the United States, NoVs are estimated to cause 21-million illnesses each year, which results in 71,000 hospitalizations and 800 deaths [6,7]. In developing countries, NoVs are estimated to claim over 200, 000 deaths annually in children < 5 years of age [5]. Norovirus outbreaks occur frequently in semi-closed institutions, such as hospitals, nursing homes for the elderly, schools, prisons, restaurants, hotels, and cruise ships [8–13]. NoVs transmit via the consumption of contaminated food or water, through person-to-person contact, or by exposure to aerosols from vomitus [14–16]. NoVs are highly contagious owing to their ability to infect at low doses, their high stability in the environment, and by the limited short-term immunity hosts amount [17]. Accordingly, outbreaks of NoVs are extremely difficult to control.
NoVs are non-enveloped RNA viruses that contain a single-stranded, positive-sense, and polyadenylated RNA genome of ~ 7.5 kb in length [18]. The NoV genome consists of three open reading frames (ORFs 1–3). ORF1 encodes a polyprotein that is processed post-translation into six non-structural proteins, including an RNA-dependent RNA polymerase (RdRp) [19]. ORF2 and ORF3 encode the major (capsid protein, VP1) and minor (VP2) structural proteins, respectively [20]. The genomes of NoVs are highly diverse. Each belongs to one of six genogroups (GI–GVI) and each genogroup can be further stratified into one of more than thirty-six genotypes [21–23]. While GI, GII, and GIV NoVs can infect humans, the GII.4 genotype is attributed to causing both outbreaks and many sporadic cases [24].
Since the mid-1990s, global epidemics of NoV gastroenteritis have only been associated with the GII.4 genotype [1,25,13]. New variants tend to emerge from and supersede the then-predominant GII.4 variant every 2–3 years [24]. Several GII.4 variants have been attributed to numerous NoV gastroenteritis pandemics from 1996 to present, including the outbreaks of 95/96 US (1995–96) [25,4], Farmington Hills (2002–03) [13], Hunter (2004–05)[1], 2006b (2006–07) [26,27], New Orleans (2009–10) [28,29], and the most recent variant of Sydney 2012 (2012–13) [30–35]. Other GII.4 variants have caused regional epidemics—namely Henry 2001, Japan 2001, Asia 2003, 2006a, and Abeldoorn 2008 [36,24,37–42].
The recently emerged GII.4 variant, Sydney 2012, was firstly identified in Australia early in 2012, but also lead the increase of acute gastroenteritis outbreaks in the United States, France, Japan, United Kingdom, the Netherlands, New Zealand, and Hong Kong [30–35,43]. Furthermore, the Sydney 2012 variant was also responsible for three gastroenteritis outbreaks in two social welfare homes and a factory in Shenzhen, China, in 2012/13. Herein, we undertake epidemiological and laboratory investigations into these three gastroenteritis outbreaks caused by Sydney 2012 variant.
Materials and Methods
Ethics statement
The present study was approved by the Institutional Review Board of Shenzhen CDC. Written consent was obtained from both the symptomatic and asymptomatic individuals who participated in this study before collecting their stool samples and medical data.
Epidemiological investigation
Cases were defined with ≥ 3 loose stools and /or vomiting in a 24-hour period during the outbreaks. A standardized questionnaire was developed for data collection of demographic data (sex and age), illness onset (symptoms, duration of symptoms) and potential risk factors (water and food consumption, patient contacts). We then collected stool and vomitus from the study set and took environmental swabs of vegetables and the chopping boards, bench surfaces, and cooking utensils used in the preparation of raw and cooked foods for laboratory diagnosis.
RNA extraction and real-time PCR
A 10% stool suspension was prepared by mixing 0.1 g stool with 1.0 mL phosphate-buffered saline (pH 7.2). Viral RNA was extracted from the stool suspensions using the QiaAmp Viral RNA Mini Kit (Qiagen, Hilden, Germany) in accordance with the manufacturer’s protocol. Viral RNA was examined for GI and GII NoVs in a duplex format using the QuantiTect Probe RT-PCR kit (Qiagen, Hilden, Germany) on a 7500 Real-time PCR platform (Applied Biosystem). The final, 25-μL reaction mix consisted of 0.4 μM of each of the four primers (Cog1F, Cog1R, Cog2F, Cog2R)_and 0.2μM of each TaqMan Probe (Ring 1a, Ring1b, Ring 2) [44]. The cycling conditions were configured for a reverse transcription step at 50°C for 30 min, followed by a denaturation step at 95°C for 15 min and 40 cycles of amplification (denaturation at 95°C for 15 s and a combination of annealing and extension at 60°C for 1 min).
Conventional reverse transcription (RT)-PCR
The capsid region used for NoV genotyping was amplified by conventional RT-PCR according to the protocol and primers (CoG2F, G2-SKR, G1-SKF, G1-SKR) reported by Yan et al. [45]. Thirteen stool samples determined to be NoV-positive by real-time PCR were submitted for RT-PCR.
Amplification of full-length NoV genomes
The complete genomes of two of the earliest NoVs—JB031230049 and JB031230054—were amplified. Initially, cDNA was reverse transcribed from viral RNA (9.5 μL) using a modified oligo(dT)20 primer (V3NT20 in Table 1) and a SuperScript III first-strand synthesis system (Invitrogen, Carlsbad, CA). The total, 50-μL reaction mixture contained 5 μL 5× Ex Taq Buffer, 1 μL of each primer (20 pmol), 1 μL dNTP mix (10 mM), 0.5 μL Ex Taq enzyme (Takara, Japan), 5 μL cDNA, and 36.5 μL RNase-free water. CoG2F [45]and V3NT20 [46] primers were used to amplify the fragment from the ORF2 to the 3’ Poly A. The cycling conditions were configured as follows: initial denaturation at 94°C for 3 min, followed by 40 amplification cycles (94°C for 15 s, 62°C for 3 min, and 72°C 30s) and a final extension step at 72°C for 15 min. The ORF1 was divided into five overlapping fragments, which were amplified using the P290 and G2-SKR primers and four pairs of newly designed primer sets (Table 1). The conditions were as follows: initial denaturation at 94°C for 3 min, then 35 cycles (94°C for 45 s, 55°C for 45 s, and 72°C 1 min), and a final extension at 72°C 10 min. The fragment covering the ORF1/ORF2 overlap and amplified by the P290 [47] and G2-SKR [45] primers was also used to examine potential recombination.
Table 1. Primer sequences used for the present genome amplification.
| Primer name | Sequence (5′→3′) | Polarity | Regiona |
|---|---|---|---|
| Sydney2012-1F | GTGAATGAAGATGGCGTCTAAC | + | 1–22 |
| Sydney2012-1R | GGTAAATCCTAGCACCAAACCT | - | 1045–1066 |
| Sydney2012-2F | TGATTGGACCTTCGCAGGCATAG | + | 871–893 |
| Sydney2012-2R | TCTAGCCTCTCATGGAGTAACC | - | 2055–2076 |
| Sydney2012-3F | CATCCATGATGCCCTCAGGT | + | 1660–1679 |
| Sydney2012-3R | GATTTGCTTGATAGGGACTCCG | - | 3148–3169 |
| Sydney2012-4F | GCAACCGAAGAGGACTTCTGTGAAG | + | 2813–2837 |
| Sydney2012-4R | TGAGGAGCCAGTGGGCGATGGAAT | - | 4497–4520 |
| 290 H | GATTACTCCAGGTGGGACTCCAC | + | 4295–4317 |
| 290 I | GATTACTCCAGGTGGGACTCAAC | ||
| 290 J | GATTACTCCAGGTGGGATTCAAC | ||
| 290 K | GATTACTCCAGGTGGGATTCCAC | ||
| G2SKR | CCACCTGCATAACCATTGTACAT | - | 5367–5389 |
| COG2F | CARGARBCNATGTTYAGRTGGATGAG | + | 5003–5029 |
| VN3T20 | GAGTGACCGCGGCCGCT20 | - | Poly A |
a Each sequence number of primer sets region is listed for Sydney 2012 strain (GenBank accession No. JX459908).
DNA Sequencing and phylogenetic analysis
All PCR products were excised from the gel and purified using a QIAquick gel extraction kit (Qiagen, Hilden, Germany). All purified products were sequenced with the primers listed in Table 1 using the Big-Dye terminator cycle sequencing kit and the ABI Prism 310 Genetic Analyzer (Applied Biosystems Inc., Foster City, CA). The resulting NoV sequences were analyzed using CLUSTAL X (Version 1.83) followed by phylogenetic analysis using MEGA version 4.1. The statistical significance of the inferred phylogenies was estimated using a bootstrap analysis of 1,000 pseudoreplicate data sets. SimPlot software (Version 1.3) was used to align and compare sequences to identify potential recombination(s) among known genotypes of NoVs. The nucleotide sequences generated in this study were deposited in the GenBank (KJ995534–KJ995550 for short sequences within the capsid region and KJ955492–KJ955493 for complete genome sequences).
Results
Epidemiological investigation
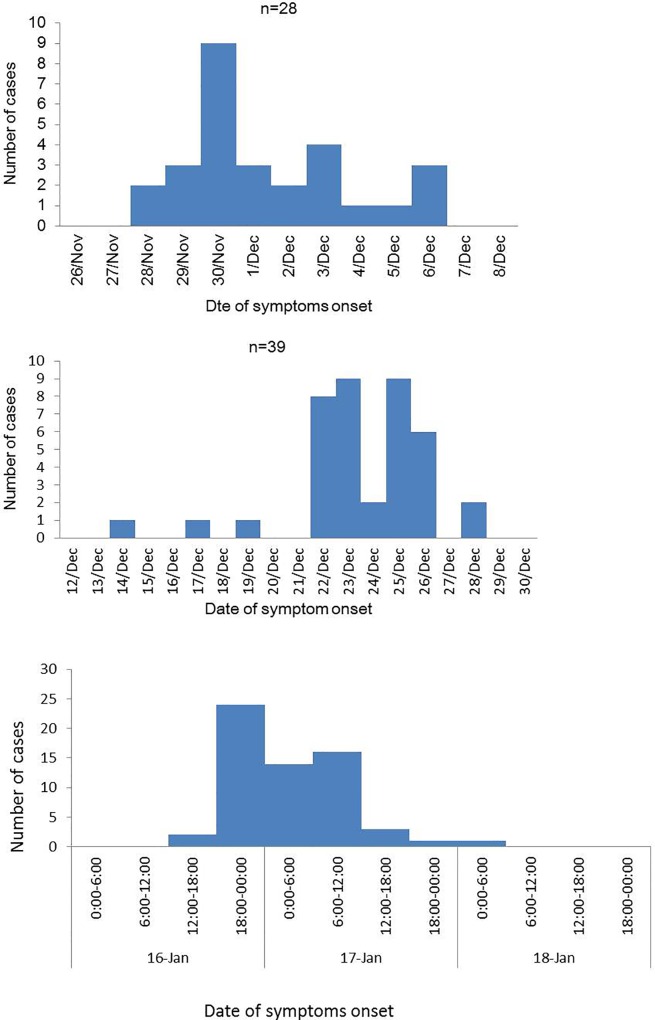
Among the three outbreaks of gastroenteritis in Shenzhen, China, during 2012/13 winter season, two arose in social welfare homes in the Luohu (Outbreak A) and Nanshan (Outbreak B) districts, while the epicenter of Outbreak C was in a factory in the Baoan district. Twenty-eight individuals were sick with acute gastroenteritis in the Outbreak A, including 22 residents and 6 healthcare workers. The index case occurred on November 28, 2012, with the outbreak peaking on November 30 (Fig 1). The most frequent symptoms were diarrhea (71.43%), followed by vomiting (60.71%), nausea (35.71%), abdominal pain (7.14%) and fever (3.57%; Table 2). No hospitalizations resulted from Outbreak A. Of the 28 infected persons, 19 were living on the fourth floor, 6 on the fifth, 2 on the third, and 1 on the sixth. The index case was an 82-year-old female living on the fourth floor.
Fig 1. Date of illness onset associated with NoV Outbreaks A–C during the 2012/13 winter season in Shenzhen, China.
Table 2. Frequency of reported clinical symptoms of gastroenteritis associated with Outbreaks A–C in Shenzhen between November and December 2013.
| Symptom | Numbers of cases (%) | ||
|---|---|---|---|
| Outbreak A | Outbreak B | Outbreak C | |
| Diarrhea | 20 (71.4) | 37 (94.9) | 47 (77.0) |
| Vomiting | 17 (60.7) | 32 (82.1) | 36 (59.0) |
| Nausea | 10 (35.7) | 2 (5.1%) | 46 (75.4) |
| Abdominal pain | 2 (7.1) | 17 (43.6) | 51 (83.6 |
| Fever | 1 (3.6) | 1 (2.6) | 12 (19.7) |
In Outbreak B, 39 individuals were sick with gastroenteritis, including 33 residents, 5 healthcare workers, and 1 staff. The index case occurred on December 14, 2012. Outbreak B peaked between 23 and 25 of December (Fig 1). The most frequent symptoms were diarrhea (94.87%), followed by vomiting (82.05%), nausea (5.13%), abdominal pain (43.59%) and fever (2.56%; Table 2). We analyzed the attack rates among males and females stratified into age groups. Among the 33 sick residents, the attack rates were 18.33% (11/60) for males and 16.30% (22/135) for females, though this difference was not statistical significant (X2 = 0.12, P = 0.73). The ages of the 33 patients spanned from 48 to 92 years of age. The infection rate was highest in those > 80 years of age (54.55%, 18/33), followed by those aged 70–79 (27.27%, 9/33). The index case was an 83-year-old female living on the fifth floor of the healthcare center. She had 6–8 episodes of diarrhea on December 14 during her leave starting on December 8. She returned to the center on December 15 and had no symptoms of diarrhea on December 16. Thereafter, she visited entertainment rooms on the other floors, which culminated with at the highest attack rate on the fifth floor (31.25%; Table 3).
Table 3. Floor distribution of gastroenteritis cases of Outbreak B.
| Floor | Total number | Numbers of cases | Affected |
|---|---|---|---|
| 2th | 42 | 9 | 21.43% |
| 3th | 37 | 9 | 24.32% |
| 4th | 27 | 2 | 7.41% |
| 5th | 32 | 10 | 31.25% |
| 6th | 38 | 1 | 2.63% |
| 7th | 19 | 2 | 10.63% |
Seventy-six individuals were sick with acute gastroenteritis in Outbreak C. The index case occurred on January 16, 2013. Outbreak C peaked between January 16 and 17 (Fig 1). The common symptoms reported were pain (83.6%), nausea (5.13%), diarrhea (77.0%), vomiting (59.0%), and fever (19.7%; Table 2). Among the 414 staff, the attack rates were 15.63% (10/64) in males and 18.86% (66/350) in females—a different that was not statistically significant. The factory was a six-story building. The highest attack rate was 71.43% (5/7) on the sixth floor, followed by 25.32% (46/158) on the third, 16.13% (10/62) on the fourth, 11.83% (11/93) on the second, 4.94% (4/81) on the first floor. There were no cases on the fifth floor. Of the 76 infected, 43 (43/223, attack rate 19.28%) lived in the factory’s dormitory, 2 (2/15, attack rate 13.33%) lived in a dormitory external to the factory, and 31 (31/176, attack rate 17.61%) lived in private accommodation. There was no statistical significance among the attack rates when stratified by the location of dwelling or floors of the factory’s dormitory (Table 4).
Table 4. Floor distribution of gastroenteritis cases of Outbreak C.
| Floor | Total number | Numbers of cases | Attack rate |
|---|---|---|---|
| 1th | 40 | 6 | 15.00% |
| 2th | 48 | 8 | 16.67% |
| 3th | 51 | 10 | 19.60% |
| 4th | 52 | 12 | 23.08% |
| 5th | 32 | 7 | 21.88% |
Virological investigation
All samples from the three outbreaks were negative for E. coli, Salmonella, Shigella, Campylobacter, Y. enterocolittica, rotavirus, adenovirus, and astrovirus. For Outbreak A, 14 of the 59 swabs and stool samples collected were NoV-positive, while all vomitus samples and environmental swabs were negative for NoV. For Outbreak B, 25 of the 29 symptomatic individuals (25/29, 86.2%) and 13 of the 79 asymptomatic individuals (13/79, 16.5%) were NoV-positive. For Outbreak C, 16 of the 27 stool swabs from symptomatic individuals (16/27, 59.26%) were NoV-positive; 18 environmental swabs and 2 food samples were negative for NoV; while one of the 7 asymptomatic food handlers was NoV-positive.
Phylogenetic analysis
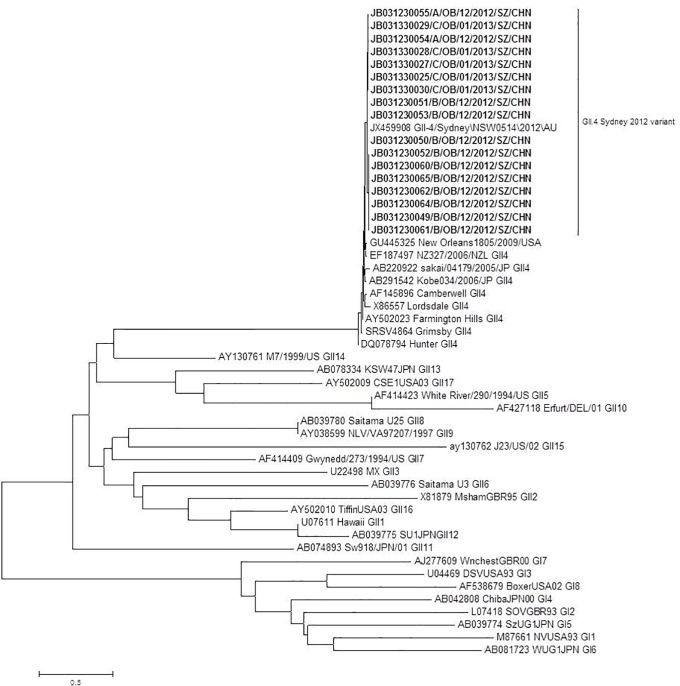
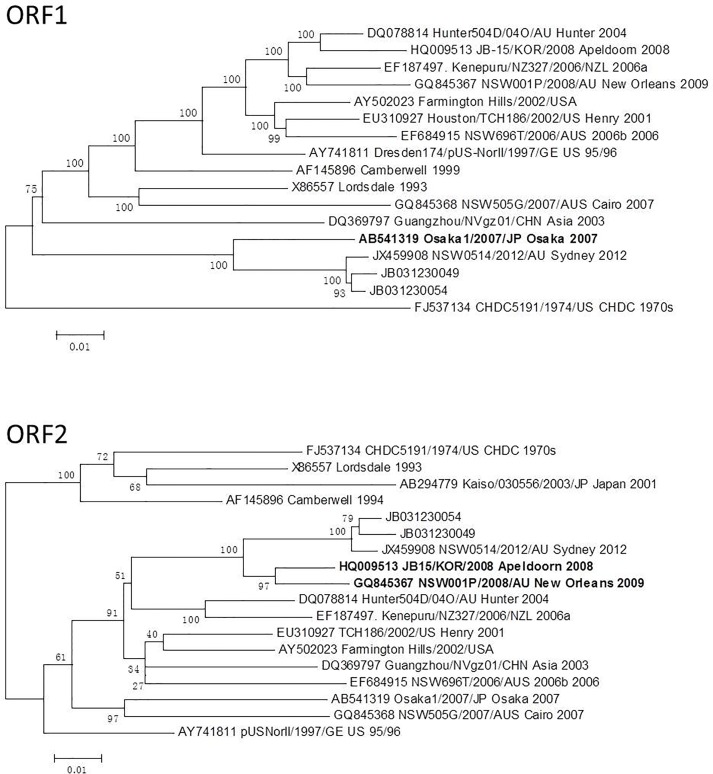
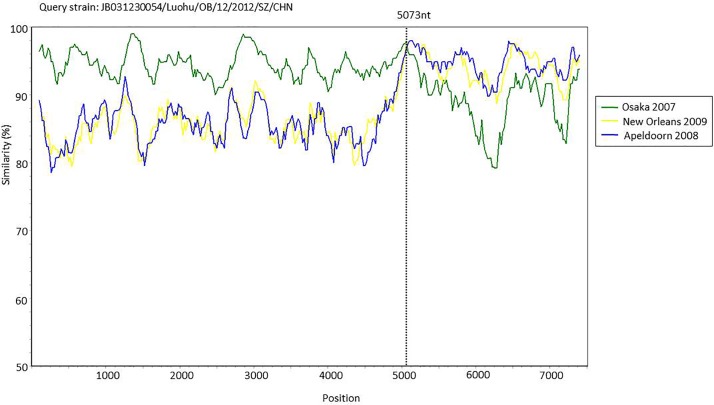
Thirteen sequences from the three outbreaks were obtained and analyzed, including two from Outbreak A, six from Outbreak B, and five from Outbreak C. Phylogenetic analysis using a collection of reference sequences representing a variety of NoV genotypes showed that all thirteen of the sequences were classified into the genotype GII.4 and closely resembled the GII.4 Sydney 2012 variant (Genbank accession No. JX459908; Fig 2). To examine the potential recombination of the detected NoVs, the complete genome sequences of two isolates, JB031230054 (Outbreak A) and JB031230049 (Outbreak B) were amplified and sequenced. While both isolates clustered with the Sydney 2012 reference strain in three ORFs, their ORF1 clustered with the Osaka 2007 strain (GII.4 variant of 2007), while their ORFs 2&3 were clustered with the variants of Apeldoorn 2008 (GII.4 variant of 2008) and New Orleans (GII.4 variant of 2009), thereby suggesting a recombination event occurred during the overlap of ORF1/ORF2 from both isolates (Fig 3). To eliminate the possibility that the polymerase and capsid encoding genes were from two separate NoVs that co-infected the same patients, a fragment of ~1100 nucleotides in length, spanning both ORF1 and ORF2, was amplified from a stool sample. Simplot analyses of the junction regions between ORF1 and ORF2 confirmed the presence of recombinant strains and indicated a potential cross-over site where the recombination between ORF1 and ORF2 is likely to have occurred (Fig 4).
Fig 2. Phylogenetic tree based on the nucleotide sequences encoding the partial capsid (281 bp) associated with NoV outbreaks.
Strains from the three studied outbreaks are denoted in bold. Phylogenetic analysis was performed using the neighbor-joining method (distance calculated by Kimura-2-parameter correction and pairwise deletion). Results were validated with 1,000 bootstrap pseudoreplicates.
Fig 3. Changes in the phylogenetic locations of ORF1 and ORF2.
JB031230054 strain from Outbreak A; JB031230049 strain from Outbreak B.
Fig 4. Simplot analysis for of putative, recombinant JB031230054 strain.
Comparisons of genetic similarity between the strain JB031230054 and possible parental strains was made using SimPlot. The Y-axis represents the percentage of the nucleotide sequence similarity between the strain JB031230054 and other strains used for comparison. The X axis shows the relative nucleotide position along the full-length genome. The breakpoint positions are marked with dashed lines. A window size of 200 nucleotides with an increment of 20 was used.
Discussion
The present study describes three outbreaks of NoV that occurred in Shenzhen, China, during the epidemic season of 2012/13 caused by the new GII.4 variant Sydney 2012. During the past decade, new GII.4 variants have emerged and replaced previously predominant GII.4 strain every 2–3 years. Several global epidemics caused by different GII.4 variants in the winter season have been reported, including those that arose during the winter season of 1995/96 (caused by the 95/96 US variant), 2002/03 (caused by the Farmington Hills variant), 2004/06 (caused by the Hunter variant), 2006/07 (caused by the 2006b variant), and 2008/09 (caused by the New Orleans variant) [1,26,4,28,13]. The Sydney 2012 variant was first identified in Australia in March 2012, which had become global by late 2012 [30–35]. In the United States, the GII.4 Sydney variant caused a total of 141 (53%) of the 266 NoV outbreaks recorded from September to December of 2012 [48]. In Denmark, GII.4 Sydney accounted for 46 of the 106 (43%) NoV-positive samples typed from both surveillance and outbreaks from October to December of 2012 [49]. An increase in epidemics associated with the emergence of GII.4 Sydney was also reported in the UK, France, Japan, Australia, New Zealand by late 2012 [30–35,43].
Herein, we reported on three outbreaks caused by the same GII.4 Sydney NoV during the season of 2012/13 in Shenzhen, China. According to the outbreak data from several provincial CDCs in China, the GII.4 Sydney variant was responsible for 12 of the 13 other NoV outbreaks in the Guangdong and Jiangsu provinces in China between October of 2012 and March of 2013 (data not shown). These data suggested that the GII.4 Sydney strain has already caused an increase in NoV activities in China. The first case of GII.4 Sydney in China was reported in August under the national sporadic surveillance of NoV (data not shown).
NoV-associated gastroenteritis outbreaks are common in semi-closed settings, including hospitals, cruise ships, military camps, schools, and elderly care homes [8–13]. Over a quarter (27%) of NoV outbreaks, for example, are epicentered about healthcare settings [50], while long-term care facilities (elderly care homes, etc.) account for 50% of these NoV outbreaks occurring in a healthcare setting [50]. In our study, two of the three NoV outbreaks occurred in two social welfare centers that were funded by the local government as long-term care facilities for the elderly, while the remaining outbreak occurred in a factory that was a semi-close setting where many employees dwelled in communal dormitories.
A NoV outbreak is declared in accordance with Kaplan’s criteria, wherein (1) > 50% of affected persons must be afflicted with vomiting, (2) the mean or median incubation period is 24–48 hr, (3) that the mean or median illness duration is 12–60 hr, and (4) that no bacterial pathogens are isolated from stool cultures [51]. The epidemiological investigation in this study revealed that the vomiting rates of all three outbreaks were greater than 50% (60.7% for Outbreak A, 82.1% for Outbreak B, and 59.0% for Outbreak C). Asymptomatic infections are estimated to occur in about one-third of all studies investigating outbreaks involving volunteers. We detected 16% (13 out of 79) of asymptomatic persons who were NoV-positive in Outbreak C, including a food handler who could have been the source of this outbreak. Ozawa et al. reported that the prevalence of NoV detection in food handlers was 19% in different food-catering settings in Japan and that 73% of symptomatic and 7% of asymptomatic food handlers were positive for the NoV [52].
The evolution of GII.4 NoV variants is potentially driven by several factors [53]. In a similar manner to the influenza A virus, a new GII.4 variant tends to emerge every 2–3 year through antigenic drift. Furthermore, recombination is likely to be an important factor for the emergence of the GII.4 variants in a resemblance to the reassortment of genetic material by the influenza virus [54]. Intra-genotype recombination has frequently been reported in some GII.4 variants [41,55]. In this study, two genomes of the GII.4 Sydney 2012 variant from two outbreaks were analyzed for recombination. We found that ORF1 was derived from an Osaka 2007 virus, while the ORFs 2&3 were from the viruses responsible for Apeldoorn 2008 and New Orleans 2009 (Fig 3).
In conclusion, the emerging GII.4 Sydney 2012 variant caused three gastroenteritis outbreaks in the winter season of 2012/13 in Shenzhen in China. These outbreaks occurred during the same time frame when the GII.4 Sydney 2012 variant caused global epidemics. Two outbreaks occurred in social welfare centers for the elderly and were most likely due to an asymptomatic food handler. Our study highlights first the susceptibility and vulnerability of the elderly in the closed environment of a nursing home setting and secondly the need to improve sanitation practices by food handlers.
Acknowledgments
We thank the local CDCs in Shenzhen for collecting the specimens used in this study.
Data Availability
The nucleotide sequences generated in this study were deposited in GenBank under accession numbers KJ995534- KJ995550 (short sequences in capsid region) and KJ955492-KJ955493 (genome sequences). All sequnce files are available from the GenBank database under accession numbers KJ995534- KJ995550 (short sequences in capsid region) and KJ955492-KJ955493 (genome sequences).
Funding Statement
The research was supported by The Key Project of Science and Technology (Grant No. 2012ZX10004215) funded by the Ministry of Science and Technology of China and the Health Industry Research (Grant No. 201302004) funded by National Health and Family Planning Commission of China to Miao Jin. The study was also supported by the National Natural Science foundation of China (Grant No. 81470856) to Yuanping Zhou. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bull RA, Tu ET, McIver CJ, Rawlinson WD, White PA. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol. 2006; 44: 327–333. 10.1128/JCM.44.2.327-333.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston CP, Qiu H, Ticehurst JR, Dickson C, Rosenbaum P, Lawson P, et al. Outbreak management and implications of a nosocomial norovirus outbreak. Clin Infect Dis. 2007; 45: 534–540. 10.1086/520666 [DOI] [PubMed] [Google Scholar]
- 3.Lopman BA, Reacher MH, Vipond IB, Sarangi J, Brown DW. Clinical manifestation of norovirus gastroenteritis in health care settings. Clin Infect Dis. 2004; 39: 318–324. 10.1086/421948 [DOI] [PubMed] [Google Scholar]
- 4.Noel JS, Fankhauser RL, Ando T, Monroe SS, Glass RI. Identification of a distinct common strain of "Norwalk-like viruses" having a global distribution. J Infect Dis. 1999; 179: 1334–1344. 10.1086/314783 [DOI] [PubMed] [Google Scholar]
- 5.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg Infect Dis. 2008; 14: 1224–1231. 10.3201/eid1408.071114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis. 2011; 17: 7–15. 10.3201/eid1701.P11101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopman BA, Hall AJ, Curns AT, Parashar UD. Increasing rates of gastroenteritis hospital discharges in US adults and the contribution of norovirus, 1996–2007. Clin Infect Dis. 2011; 52: 466–474. 10.1093/cid/ciq163 [DOI] [PubMed] [Google Scholar]
- 8.Drinka PJ. Norovirus outbreaks in nursing homes. J Am Geriatr Soc. 2005; 53: 1839–1840. 10.1111/j.1532-5415.2005.53528_6.x [DOI] [PubMed] [Google Scholar]
- 9.Khanna N, Goldenberger D, Graber P, Battegay M, Widmer AF. Gastroenteritis outbreak with norovirus in a Swiss university hospital with a newly identified virus strain. J Hosp Infect. 2003; 55: 131–136. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S, Morishita T, Yamashita T, Sakae K, Nishio O, Miyake T, et al. A large outbreak of gastroenteritis associated with a small round structured virus among schoolchildren and teachers in Japan. Epidemiol Infect. 1991; 107: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koopmans M, Vinje J, de Wit M, Leenen I, van der Poel W, van Duynhoven Y. Molecular epidemiology of human enteric caliciviruses in The Netherlands. J Infect Dis. 2000; 181 Suppl 2: S262–269. [DOI] [PubMed] [Google Scholar]
- 12.Marshall JA, Yuen LK, Catton MG, Gunesekere IC, Wright PJ, Bettelheim KA, et al. Multiple outbreaks of Norwalk-like virus gastro-enteritis associated with a Mediterranean-style restaurant. J Med Microbiol. 2001; 50: 143–151. 10.1099/0022-1317-50-2-143 [DOI] [PubMed] [Google Scholar]
- 13.Widdowson MA, Cramer EH, Hadley L, Bresee JS, Beard RS, Bulens SN, et al. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus—United States, 2002. J Infect Dis. 2004; 190: 27–36. 10.1086/420888 [DOI] [PubMed] [Google Scholar]
- 14.Webby RJ, Carville KS, Kirk MD, Greening G, Ratcliff RM, Crerar SK, et al. Internationally distributed frozen oyster meat causing multiple outbreaks of norovirus infection in Australia. Clin Infect Dis. 2007; 44: 1026–1031. 10.1086/512807 [DOI] [PubMed] [Google Scholar]
- 15.O'Reilly CE, Bowen AB, Perez NE, Sarisky JP, Shepherd CA, Miller MD, et al. A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clin Infect Dis. 2007; 44: 506–512. 10.1086/511043 [DOI] [PubMed] [Google Scholar]
- 16.Marks PJ, Vipond IB, Regan FM, Wedgwood K, Fey RE, Caul EO. A school outbreak of Norwalk-like virus: evidence for airborne transmission. Epidemiol Infect. 2003; 131: 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutson AM, Atmar RL, Estes MK. Norovirus disease: changing epidemiology and host susceptibility factors. Trends Microbiol. 2004; 12: 279–287. 10.1016/j.tim.2004.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green KY, Chanock RM, Kapiakan AZ. Human caliciviruses Fields virology 4th ed 2001; Philadelphia:Lippincoott Williams&Wilkins.: 841–874. [Google Scholar]
- 19.Belliot G, Sosnovtsev SV, Mitra T, Hammer C, Garfield M, Green KY. In vitro proteolytic processing of the MD145 norovirus ORF1 nonstructural polyprotein yields stable precursors and products similar to those detected in calicivirus-infected cells. J Virol. 2003; 77: 10957–10974. 10.1128/JVI.77.20.10957-10974.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertolotti-Ciarlet A, Crawford SE, Hutson AM, Estes MK. The 3' end of Norwalk virus mRNA contains determinants that regulate the expression and stability of the viral capsid protein VP1: a novel function for the VP2 protein. J Virol. 2003; 77: 11603–11615. 10.1128/JVI.77.21.11603-11615.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006; 346: 312–323. 10.1016/j.virol.2005.11.015 [DOI] [PubMed] [Google Scholar]
- 22.Mesquita JR, Barclay L, Nascimento MS, Vinje J. Novel norovirus in dogs with diarrhea. Emerg Infect Dis. 2010; 16: 980–982. 10.3201/eid1606.091861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroneman A, Vennema H, Deforche K, v d Avoort H, Penaranda S, Oberste MS, et al. An automated genotyping tool for enteroviruses and noroviruses. J Clin Virol. 2011; 51: 121–125. 10.1016/j.jcv.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 24.Siebenga JJ, Vennema H, Zheng DP, Vinje J, Lee BE, Pang XL, et al. Norovirus illness is a global problem: emergence and spread of norovirus GII.4 variants, 2001–2007. J Infect Dis. 2009; 200: 802–812. 10.1086/605127 [DOI] [PubMed] [Google Scholar]
- 25.White PA, Hansman GS, Li A, Dable J, Isaacs M, Ferson M, et al. Norwalk-like virus 95/96-US strain is a major cause of gastroenteritis outbreaks in Australia. J Med Virol. 2002; 68: 113–118. 10.1002/jmv.10177 [DOI] [PubMed] [Google Scholar]
- 26.Eden JS, Bull RA, Tu E, McIver CJ, Lyon MJ, Marshall JA, et al. Norovirus GII.4 variant 2006b caused epidemics of acute gastroenteritis in Australia during 2007 and 2008. J Clin Virol. 2010; 49: 265–271. 10.1016/j.jcv.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 27.Tu ET, Bull RA, Greening GE, Hewitt J, Lyon MJ, Marshall JA, et al. Epidemics of gastroenteritis during 2006 were associated with the spread of norovirus GII.4 variants 2006a and 2006b. Clin Infect Dis. 2008; 46: 413–420. 10.1086/525259 [DOI] [PubMed] [Google Scholar]
- 28.Vega E, Barclay L, Gregoricus N, Williams K, Lee D, Vinje J. Novel surveillance network for norovirus gastroenteritis outbreaks, United States. Emerg Infect Dis. 2011; 17: 1389–1395. 10.3201/eid1708.101837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tra My PV, Lam HM, Thompson CN, Phuc HL, Tuyet PT, Vinh H, et al. The dynamics of GII.4 Norovirus in Ho Chi Minh City, Vietnam. Infect Genet Evol. 2013; 18: 335–343. 10.1016/j.meegid.2013.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allen DJ, Adams NL, Aladin F, Harris JP, Brown DW. Emergence of the GII-4 Norovirus Sydney2012 strain in England, winter 2012–2013. PLoS One. 2014; 9: e88978 10.1371/journal.pone.0088978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thongprachum A, Chan-It W, Khamrin P, Saparpakorn P, Okitsu S, Takanashi S, et al. Molecular epidemiology of norovirus associated with gastroenteritis and emergence of norovirus GII.4 variant 2012 in Japanese pediatric patients. Infect Genet Evol. 2014; 23: 65–73. 10.1016/j.meegid.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 32.Leshem E, Wikswo M, Barclay L, Brandt E, Storm W, Salehi E, et al. Effects and clinical significance of GII.4 Sydney norovirus, United States, 2012–2013. Emerg Infect Dis. 2013; 19: 1231–1238. 10.3201/eid1908.130458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett S, MacLean A, Miller RS, Aitken C, Gunson RN. Increased norovirus activity in Scotland in 2012 is associated with the emergence of a new norovirus GII.4 variant. Euro Surveill. 2013; 18: [PubMed] [Google Scholar]
- 34.Fonager J, Barzinci S, Fischer TK. Emergence of a new recombinant Sydney 2012 norovirus variant in Denmark, 26 December 2012 to 22 March 2013. Euro Surveill. 2013; 18: [DOI] [PubMed] [Google Scholar]
- 35.van Beek J, Ambert-Balay K, Botteldoorn N, Eden JS, Fonager J, Hewitt J, et al. Indications for worldwide increased norovirus activity associated with emergence of a new variant of genotype II.4, late 2012. Euro Surveill. 2013; 18: 8–9. [PubMed] [Google Scholar]
- 36.Siebenga JJ, Lemey P, Kosakovsky Pond SL, Rambaut A, Vennema H, Koopmans M. Phylodynamic reconstruction reveals norovirus GII.4 epidemic expansions and their molecular determinants. PLoS Pathog. 2010; 6: e1000884 10.1371/journal.ppat.1000884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belliot G, Kamel AH, Estienney M, Ambert-Balay K, Pothier P. Evidence of emergence of new GGII.4 norovirus variants from gastroenteritis outbreak survey in France during the 2007-to-2008 and 2008-to-2009 winter seasons. J Clin Microbiol. 2010; 48: 994–998. 10.1128/JCM.01888-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han TH, Kim CH, Chung JY, Park SH, Hwang ES. Emergence of norovirus GII-4/2008 variant and recombinant strains in Seoul, Korea. Arch Virol. 2011; 156: 323–329. 10.1007/s00705-010-0844-4 [DOI] [PubMed] [Google Scholar]
- 39.Mans J, de Villiers JC, du Plessis NM, Avenant T, Taylor MB. Emerging norovirus GII.4 2008 variant detected in hospitalised paediatric patients in South Africa. J Clin Virol. 2010; 49: 258–264. 10.1016/j.jcv.2010.08.011 [DOI] [PubMed] [Google Scholar]
- 40.Mattison K, Sebunya TK, Shukla A, Noliwe LN, Bidawid S. Molecular detection and characterization of noroviruses from children in Botswana. J Med Virol. 2010; 82: 321–324. 10.1002/jmv.21682 [DOI] [PubMed] [Google Scholar]
- 41.Motomura K, Yokoyama M, Ode H, Nakamura H, Mori H, Kanda T, et al. Divergent evolution of norovirus GII/4 by genome recombination from May 2006 to February 2009 in Japan. J Virol. 2010; 84: 8085–8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pang XL, Preiksaitis JK, Wong S, Li V, Lee BE. Influence of novel norovirus GII.4 variants on gastroenteritis outbreak dynamics in Alberta and the Northern Territories, Canada between 2000 and 2008. PLoS One. 2010; 5: e11599 10.1371/journal.pone.0011599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan MC, Leung TF, Kwok AK, Lee N, Chan PK. Characteristics of Patients Infected with Norovirus GII.4 Sydney 2012, Hong Kong, China. Emerg Infect Dis. 2014; 20: 664–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kageyama T, Kojima S, Shinohara M, Uchida K, Fukushi S, Hoshino FB, et al. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J Clin Microbiol. 2003; 41: 1548–1557. 10.1128/JCM.41.4.1548-1557.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan H, Yagyu F, Okitsu S, Nishio O, Ushijima H. Detection of norovirus (GI, GII), Sapovirus and astrovirus in fecal samples using reverse transcription single-round multiplex PCR. J Virol Methods. 2003; 114: 37–44. [DOI] [PubMed] [Google Scholar]
- 46.Wang QH, Han MG, Cheetham S, Souza M, Funk JA, Saif LJ. Porcine noroviruses related to human noroviruses. Emerg Infect Dis. 2005; 11: 1874–1881. 10.3201/eid1112.050485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang X, Huang PW, Zhong WM, Farkas T, Cubitt DW, Matson DO. Design and evaluation of a primer pair that detects both Norwalk- and Sapporo-like caliciviruses by RT-PCR. J Virol Methods. 1999; 83: 145–154. [DOI] [PubMed] [Google Scholar]
- 48.Emergence of new norovirus strain GII.4 Sydney—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013; 62: 55 [PMC free article] [PubMed] [Google Scholar]
- 49.Fonager J, Hindbaek LS, Fischer TK. Rapid emergence and antigenic diversification of the norovirus 2012 Sydney variant in Denmark, October to December, 2012. Euro Surveill. 2013; 18: [PubMed] [Google Scholar]
- 50.Matthews JE, Dickey BW, Miller RD, Felzer JR, Dawson BP, Lee AS, et al. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol Infect. 2012; 140: 1161–1172. 10.1017/S0950268812000234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaplan JE, Gary GW, Baron RC, Singh N, Schonberger LB, Feldman R, et al. Epidemiology of Norwalk gastroenteritis and the role of Norwalk virus in outbreaks of acute nonbacterial gastroenteritis. Ann Intern Med. 1982; 96: 756–761. [DOI] [PubMed] [Google Scholar]
- 52.Ozawa K, Oka T, Takeda N, Hansman GS. Norovirus infections in symptomatic and asymptomatic food handlers in Japan. J Clin Microbiol. 2007; 45: 3996–4005. 10.1128/JCM.01516-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bull RA, White PA. Mechanisms of GII.4 norovirus evolution. Trends Microbiol. 2011; 19: 233–240. 10.1016/j.tim.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 54.Rambaut A, Pybus OG, Nelson MI, Viboud C, Taubenberger JK, Holmes EC. The genomic and epidemiological dynamics of human influenza A virus. Nature. 2008; 453: 615–619. 10.1038/nature06945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eden JS, Tanaka MM, Boni MF, Rawlinson WD, White PA. Recombination within the pandemic norovirus GII.4 lineage. J Virol. 2013; 87: 6270.–. 10.1128/JVI.03464-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The nucleotide sequences generated in this study were deposited in GenBank under accession numbers KJ995534- KJ995550 (short sequences in capsid region) and KJ955492-KJ955493 (genome sequences). All sequnce files are available from the GenBank database under accession numbers KJ995534- KJ995550 (short sequences in capsid region) and KJ955492-KJ955493 (genome sequences).