Abstract
The Western Honeybee is a key pollinator for natural as well as agricultural ecosystems. In the last decade massive honeybee colony losses have been observed worldwide, the result of a complex syndrome triggered by multiple stress factors, with the RNA virus Deformed Wing Virus (DWV) and the mite Varroa destructor playing crucial roles. The mite supports replication of DWV to high titers, which exert an immunosuppressive action and correlate with the onset of the disease.
The aim of this study was to investigate the effect of 1,3–1,6 β-glucan, a natural innate immune system modulator, on honeybee response to low-titer natural and high-titer experimental DWV infection. As the effects exerted by ß-glucans can be remarkably different, depending on the target organism and the dose administered, two parallel experiments were performed, where 1,3–1,6 ß-glucan at a concentration of 0.5% and 2% respectively, was added to the diet of three cohorts of newly emerged honeybees, which were sampled from a Varroa-free apiary and harboured a low endogenous DWV viral titer. Each cohort was subjected to one of the following experimental treatments: no injection, injection of a high-copy number DWV suspension into the haemocel (experimental DWV infection) or injection of PBS into the haemocoel (physical injury). Control bees fed a ß-glucan-free diet were subjected to the same treatments. Viral load, survival rate, haemocyte populations and phenoloxidase activity of each experimental group were measured and compared. The results indicated that oral administration of 0.5% ß-glucan to naturally infected honeybees was associated with a significantly decrease of the number of infected bees and viral load they carried, and with a significant increase of the survival rate, suggesting that this natural immune modulator molecule might contribute to increase honeybee resistance to viral infection.
Introduction
The Western honeybee is the best known and widely managed pollinator species, whose services to agriculture have been estimated at $ 200 billion per year worldwide [1].
Honeybees have recently suffered extensive losses worldwide [2]. This phenomenon is not new, as it has repeatedly been reported in the Northern hemisphere since the second half of the nineteenth century [3]. The intensive growth of the bee-keeping industry and the huge losses observed in the last two decades prompted the scientific community to undertake intensive research to investigate the causes of these recurrent outbreaks. Exposure to pesticides, malnutrition and pathogens, including bacteria, fungi, mites, and RNA viruses have been in turn identified as the main threat to bee health [4–6]. However, there is now a general consensus that colony mortality is the product of multiple factors, acting singly or synergistically [7, 8]. Among them viruses play a prominent role, which was long not appreciated, as most of them do not induce apparent disease [9]. However, in recent years evidence has emerged that the association of these viruses with the mite Varroa destructor is a contributing factor in the collapse of honeybee colonies worldwide [10].
Deformed Wing Virus (DWV) is one of the main viruses associated with honeybee colony losses [11, 12]. DWV can be transmitted vertically and persist in the bee colony as covert infection. Overt infections, characterized by deformities and premature death, leading ultimately to the collapse of the colony, are usually observed in apiaries concomitantly infested by Varroa [13, 14]. The mite supports replication of DWV to high titers, which exert an immunosuppressive action [14], and correlate with the onset of clinical signs [15], consisting of crumpled and/or vestigial wings and a bloated abdomen [11]. The symptomatic bees die soon after emergence. Asymptomatic bees can also be heavily infected, though with lower viral titers. During the first stage of the infection DWV is only detected in the abdomen, at later stages presence of an actively replicating virus is readily detectable in the head [16–18]. Although honeybees, like all insects, lack an acquired immune system, they have a well developed innate immune system, which can generate a wide array of non-specific humoral and cellular responses triggered by the recognition of pathogen-associated molecular patterns (PAMPs) such as bacterial peptidoglycans, fungal ß-glucans and viral dsRNA, by host receptors (PRRs—Pathogen Recognition Receptors) [19]. The humoral response includes antimicrobial peptides (AMPs), reactive intermediates of oxigen or nitrogen and the enzymatic cascades that regulate coagulation or melanization of haemolymph [20, 21]. The cellular immunity is mediated by haemocytes, whose response includes phagocytosis, nodulation and encapsulation [22, 23]. Nodulation and encapsulation depend on melanization (synthesis and deposition of melanin around the pathogen) [24, 25]. Activation of melanogenesis in turn depends on several intermediate products and enzymes, the most important being phenoloxidase (PO) [26]. Phenoloxidase is expressed as an inactive zymogen (proPO), converted to active PO by proteases [27]. Activation of proPO, as well as other concomitant immune reaction, are triggered by the presence of immuno modulators of microbial origin, such as 1,3 ß-glucans, lipopolysaccharides, and peptidoglycans [28]. ß-glucans are a heterogeneous group of glucose homopolymers found in fungi, plants, algae and some bacteria, which exert a strong immune-stimulatory activity in a wide variety of vertebrate and invertebrate species [29]. To our knowledge, the effects of ß-glucans on honeybee resistance to viral infections have not been analysed so far. As they are non-toxic, non-polluting, highly stable, and biodegradable, they might possibly represent a safer alternative to synthetic pharmacological immune modulators [29–31]. Investigation of the immune response to ß-glucans in insects, particularly in Drosophila, has highlighted the antimicrobial role of Toll and Imd signaling pathways [32]. Antiviral defense in insects is achieved mainly via RNA interference (RNAi); however recent data suggest that Toll and Imd pathways also contribute to defense against viral pathogens [33, 34]. Insects may not completely rely on RNAi to fight against RNA viruses because these viruses have developed ways of suppressing RNAi responses at different stages of RNAi pathway, as observed in honeybees infected with DWV [35]. These findings prompted us to investigate whether the administration of 1,3–1,6 ß-glucan would modify honeybee resistance to endogenous and/or experimental viral infection. The response triggered by ß-glucans can be remarkably different depending on the target organism and the dose administered [29, 36]. To this purpose two parallel experiments were performed, in which 1,3–1,6 ß-glucan, at a concentration of 0.5 and 2% respectively, was added to the diet of three cohorts of newly emerged honeybees, which were sampled from a Varroa-free apiary and harboured a low endogenous DWV viral titer. Each cohort was subjected to one of the following experimental treatments: no injection, injection of a high-copy number DWV suspension into the haemocel (experimental DWV infection) and PBS injection into the haemocoel (physical injury). Control bees fed a ß-glucan-free diet were subjected to the same treatments. Viral load, survival rate, haemocyte populations and phenoloxidase activity of honeybees fed in presence or absence of β-glucan were measured and compared.
Materials and Methods
Honeybees and experimental design
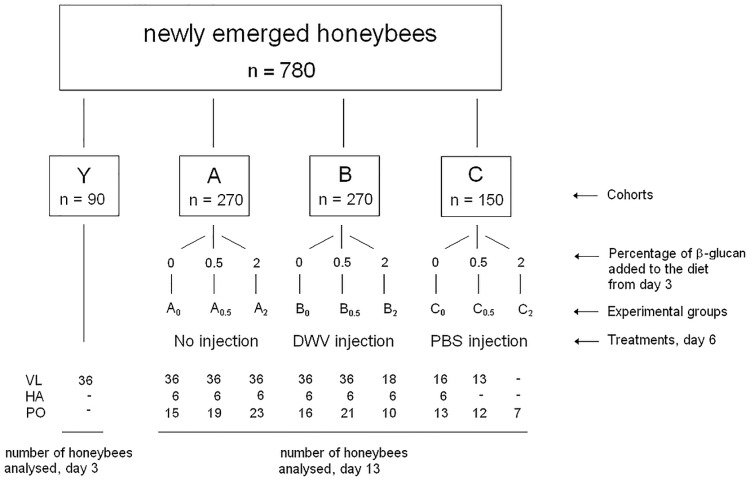
In May 2015, newly emerged honeybees (Apis mellifera ligustica L.) were transferred from a Varroa-free hive located in Gorgona Island (43°26′N 9°54′E) [37] to the Department of Veterinary Science of Pisa University (Italy). Samplings were performed to generate three replicates of the experiment, with a total of 780 newly emerged honeybees used in the study. Bees were divided in four experimental cohorts: ‘Y’ (n = 90), ‘A’ (n = 270), ‘B’ (n = 270) and ‘C’ (n = 150). Cohort A was not subjected to any injection. Cohorts B was injected with a viral suspension obtained from DWV infected, symptomatic bees; cohort C was injected with phosphate-bufferd saline (PBS). Each cohort was further divided in three groups named ‘0’, ‘0.5’ and ‘2’ (Fig 1).
Fig 1. Experimental design diagram.
The number of individual honeybees constituting each cohort and group, and the number of honeybees analysed for each group are shown. (VL: Viral load; HA: Haemocyte analysis; PO: Phenoloxidase activity).
Bees belonging to groups Y, A0, B0 and C0 were fed a commercial sugar solution consisting of 19% glucose, 35% fructose, 12% disaccharides, 6% trisaccharides and 6% polysaccharides (Fruttosweet 45, A.D.E.A.). Groups A0.5, B0.5, C0.5 and A2, B2, C2 were fed from day 3 the same sugar solution containing 0.5% and 2% (w/w) 1,3–1,6 β-glucan, respectively. MacroGard (Biorigin, Brazil) was used as a source of 1,3–1,6 ß-glucans. Twenty five small cages with a maximun of 30 (mean 27.2; median 30; range 10–30) honeybees per cage were kept at 28°C for 13 days, with the exception of the bees belonging to cohort Y, which were sacrificed at day 3 to ascertain their DWV status. Experimental cages were kept at 28°C since in preliminary trials we observed that setting the temperature at 34°C generated a high level of moisture, detrimental to bee survival. Setting the temperature at 28°C was a good compromise to avoid any moisture effect without affecting the well-being of bees. Dead individuals were counted and removed twice per day.
Preparation of a viral suspension from DWV infected, symptomatic bees
Four DWV-infected honeybees were collected from a Varroa mite infested apiary kept at the Department of Veterinary Science of Pisa University in San Piero a Grado and processed as previously described [18]. Briefly, the bees were deprived of the poison sac, pooled, homogenized with TissueLyser II (Qiagen, Hilden, Germany) for 3 min at 25 MHz in presence of 1 ml of phosphate-buffered saline (PBS) pH7,2 and centrifuged at 14000 rpm for 5 min. The supernatant was sterilized by filtration through a 0.22 μm filter and stored at 4°C in the dark. The suspension was shown by qRT-PCR to contain 5x108 copies of DWV/μl. A dilution containing 4x104 DWV copies per microliter was prepared and used for the injection experiment.
Injection assay
Bees of cohorts B and C were anaesthetized by CO2 inhalation and injected at day 6 into the thorax haemolymph with 2.5 μl of a diluted viral suspension, containing 1x105 DWV copies and with PBS pH7.2, respectively. Bees belonging to cohort A were also anesthetized by CO2 inhalation to be exposed to the same experimental condition as those of cohorts B and C.
Honeybee sample processing
All bees were anesthetized by CO2 inhalation before processing. Bees belonging to cohorts Y, A, B and C used for the analysis of the viral load were sacrificed, individual heads and abdomens were dissected immediately, soaked into RNAlater solution (Qiagen) and stored at -80°C until processed. Haemolymph was collected from 42 honeybees belonging to cohort A, B and C. Bees of cohorts A, B and C used for the phenoloxidase assay were stored at -20°C until processed.
Quantification of DWV viral load
Total RNA extraction and DWV absolute quantification were performed as previously described [18]. Briefly, individual heads and abdomens were separately homogenized using a TissueLyser II (Qiagen), total RNA was extracted with RNeasy mini Kit (Qiagen), eluted in 30 μl RNase-free water and quantified with RiboGreen RNA Quantitation Kit (Invitrogen, Carlsbad, CA, USA). Ten microliters of each RNA were used as template to determine the viral load by RT-qPCR. Samples with RT-qPCR amplification signal were considered positive and those without signal negative. Results were expressed as viral copy number per microgram of RNA.
Survival analysis
To determine whether oral administration of 1,3–1,6 ß-glucan might affect the bee life span, survival rate was calculated as the percentage of the individuals of each experimental group which survived until day 13.
Haemocyte analysis
Haemolymph of 42 honeybees belonging to the cohorts A, B and C was analysed. One microliter of haemolymph was collected using a glass capillary introduced in the sinus dorsalis, between the third and fourth tergite [38]. The slides were immediately prepared and stained with Diff Quik®. Slides with less than 100 cells were considered inadequate for analysis. Haemocyte subtype analysis for each individual honeybee was done by optic microscopy (magnification 40X and 100X) on 10 high power fields. Based on morphology, the haemocytes were classified as prohaemocytes, plasmatocytes, granulocytes and oenocytoids [38, 39].
Phenoloxidase activity
To determine the phenoloxidase (PO) activity, each head was soaked in 200μl of 50mM PBS pH7.4, 1% Triton X-100 at -20°C for 20 min, homogenized, centrifuged at 4000 rpm at 4°C for 15 min and the supernatant collected. The pellet was resuspended in 200μl of 50mM PBS pH7,4 and centrifuged as above. The supernatants were combined and protein concentration was measured by Qubit fluorimeter (Invitrogen). The enzymatic activity was determined according to Alaux et al. [40] using thirty μg of total protein. Results were expressed as mUE/min/mg of tissue.
Statistical analysis
Statistical analysis was performed using SAS Institute, 2008 (JMP Statistics and Graphics Guide. SAS Institute Inc., Cary, NC, USA). The estimated survival rate was analysed by Wilcokson rank test using the product-limit (Kaplan-Meier) method for more factors of right-censored data. When factors significantly differed from homogeneous distribution, paired tests were performed on pre-ordered means [41]. PO analysis was performed by the following criteria: data residues obtained by preliminary ANOVA for more factors (treatments, doses and interaction treatment x doses) were tested for normal distribution. Since their distribution differed significantly from the normal distribution, these data were analysed by non parametric Kruskal-Wallis Test. The same analysis was done for viral load and haemocyte counts [42]. When significant differences were found between factors, means were ordered and differences were again tested with non parametric Kruskal-Wallis Test.
Results
DWV viral load
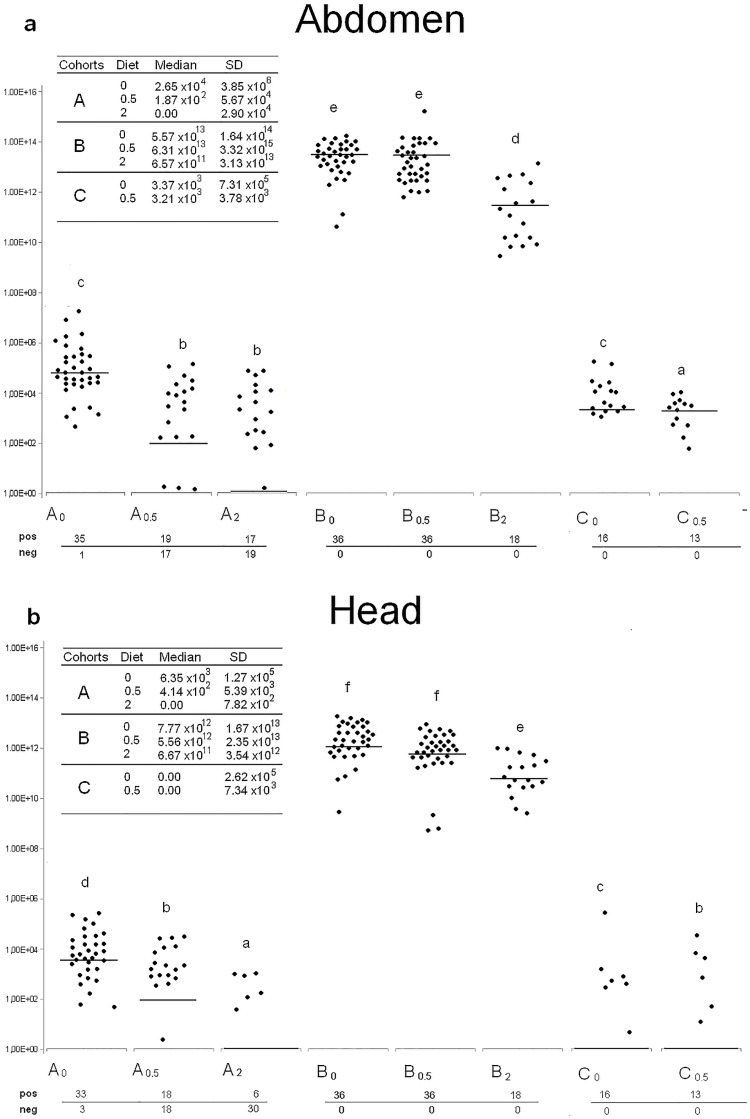
Abdomens and heads of 36 honeybees belonging to cohort Y were analysed at day 3 by qRT-PCR to ascertain DWV presence and load in the population selected for the study: 20 abdomens and 7 heads harboured the viral genome, with DWV copy numbers below 3 x102 and 5x101respectively. At day 13, the analysis of the viral load in the experimental cohorts (Table 1a) revealed significantly different values in the abdomens as well as in the heads (p<0.01). ß-glucan dosage (Table 1b) significantly affected viral load values in abdomens (p<0.01), whereas viral loads in the heads were only significantly reduced by 2% ß-glucan. With regard to the effect of ß-glucan dosage, group A0 abdomens were all positive except one, with viral loads ranging from 103 to 107. Notably, only 19 A0.5 and 17 A2 abdomens carried the virus, with lower viral loads (p<0.01), not exceeding 1 x105 (Fig 2a). A similar trend was observed for the heads, with 33 positives in A0 group, 18 in A0.5 and only 6 in A2, with viral loads up to 5 x105, 2 x104 and 3 x103 respectively (Fig 2b).
Table 1. Viral load analysis.
| A | Cohort A | Cohort B | Cohort C |
| Abdomen | 1,4 x103 (2.2 x106) a | 3,9 x1013 (2.1 x1015) b | 5,3 x103 (5.2 x105) c |
| Head | 4,1 x102 (7.7x104) a | 4,8 x1012 (1.9 x1013) b | 3,1 x101 (1.8 x105) c |
| B | no ß-glucan | 0.5% ß-glucan | 2% ß-glucan |
| Abdomen | 2.7 x105 (1.2 x1014) a | 1.7 x104 (2.2 x1015) b | 9.2 x103 (1.8 x1013) c |
| Head | 3.8 x104 (1.2 x1013) a | 4,4 x103 (1.7 x1013) a | 0.0 (2.1 x1012) b |
(A) Viral load values of abdomens and heads are shown for cohort A, B and C. (B) Viral load values detected in honeybees fed no-ß-glucan, 0.5% and 2% ß-glucan. Median and standard deviation (within brackets) are reported. Different letters indicate statistically significant differences.
Fig 2. DWV viral load.
Viral load values of individual abdomens (a) and heads (b) are shown for cohort A, B and C respectively. Median and standard deviation values are shown in the box. Black dots (●) represent individual DWV viral load values per μg of RNA. Horizontal lines indicate the median. Different letters on top of boxplot indicate statistically significant differences among groups (p<0.01). Number of positive and negative samples within each experimental group are shown.
Abdomens and heads of all honeybees belonging to cohort B, experimentally infected by injection of 1x 105 viral particles, harboured the virus with most viral loads ranging from 3.5x 1010 to 1.4x 1014 and from 2.5x 109 to 8.3x 1013, respectively. These values were about 10 orders of magnitude higher than those of group A (p<0.01). Abdomen and head viral load values of B2 were significantly lower than the other B groups.
Cohort C results were similar to cohort A (Fig 2a and 2b); however, due to the low number of honeybees available for group C2, statistical analysis was not performed.
Survival analysis
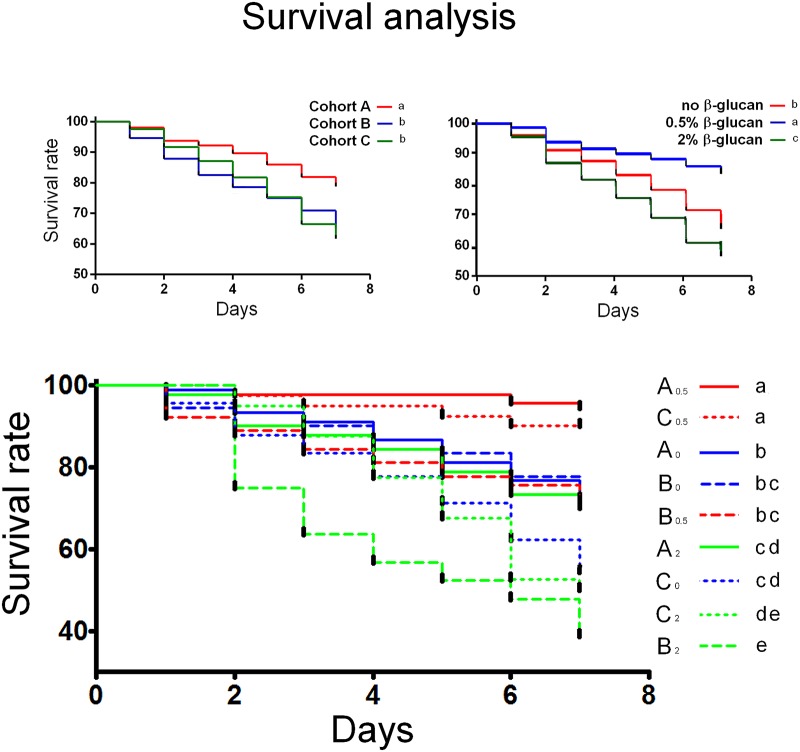
Cohort A had the highest survival rate (p<0.01); no differences were observed between cohorts B and C (Fig 3a). With regard to the effect of ß-glucan dosage the highest and lowest survival rates were associated with 0.5% and 2% ß-glucan, respectively (Fig 3b; p<0.05). Both naturally infected groups fed 0.5% ß-glucan (A0,5 and C0,5) had the highest survival rate among all groups and cohorts whereas the administration of 0.5% ß-glucan did not increase the survival rate of the experimentally infected bees (B0,5) with respect to the groups fed no- ß-glucan. The administration of 2% ß-glucan significantly reduced the survival rate within cohorts A and B, with groups B2 and C2 showing the lowest survival rate among all groups and cohorts (Fig 3c).
Fig 3. Survival analysis.
(a) Survival rate of cohorts A, B and C. (b) ß-glucan dosage effect on survival rate. (c) Survival rate of individual groups; groups are shown on the right side of the figure, according to decreasing survival rate. Colours indicate ß-glucan dosage: blue = no ß-glucan; red = 0.5%; green = 2%. Continuous line = cohort A; dashed line = cohort B; dotted line = cohort C. Different lower case letters indicate statistical differences (p<0.05).
Haemocyte analysis
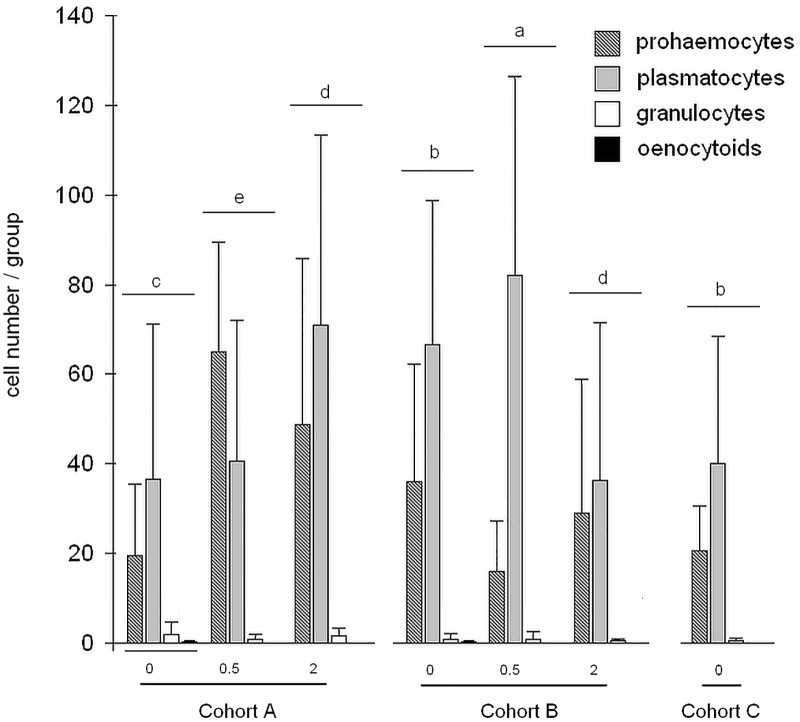
Four haemocyte types were observed in each group: prohaemocytes, plasmatocytes granulocytes and oenocytoids. The cell profile of cohort A differed significantly from cohorts B and C (p<0.01) Statistical analysis of the effects of ß-glucan dosage indicated significant differences (p<0.01). The interaction between treatments and ß-glucan dosage was statistically significant (p<0.01). The analysis of cellular patterns (Fig 4) indicated significant differences among groups (p<0.05). The investigation of individual cell types, showed that only prohaemocytes differed significantly. Cohort A honeybees, in particular A0.5 and A2 groups, showed the highest prohaemocyte counts among all cohorts and groups (p<0.05).
Fig 4. Histogram of cell profile per group.
Bar graph indicates number of haemocyte subtypes / groups. Different letters on top of boxplot indicate statistically significant differences among groups (p<0.05).
Phenoloxidase activity
The PO activity of cohorts A, B and C did not differ significantly. On the other hand, the ß-glucan dosage affected the PO activity (46.6 ± 22.3; 51.5 ± 27.3; 37.9 ± 14.8 mUE/min/mg in groups fed 0, 0.5 and 2% ß-glucan respectively, p<0.01). The PO activity within each cohort was also affected by ß-glucan dosage (p<0.01). In particular, among all groups analysed, the PO values were comprised between 56.2 ± 40.1 and 36.9 ± 13.4 mUE/min/mg, with both values detected in cohort A (A0.5 and A2 respectively). The same trend was observed in the other experimental cohorts where honeybees fed 2% ß-glucan showed lower PO activity than those fed 0.5% ß-glucan.
Discussion
The pathogen-associated decline of pollinator species is a serious threat for agricultural as well as natural ecosystems that may have profound economic and environmental consequences worldwide. The aim of this work was to investigate whether the oral administration of 1,3–1,6 ß-glucan, a natural immune-modulator, could contribute to the response of honeybees to low-titer natural and high-titer experimental DWV infection. Viral load, survival rate, haemocyte types and phenoloxidase activity were evaluated and compared between 1,3–1,6 ß-glucan fed and unfed honeybees. As the experimental infection involved injection of virus into the haemolymph, a cohort of honeybees was injected with saline solution only, in order to ascertain whether the injection (physical injury) itself might affect some of the parameters under study. The immune status of the honeybees depends on age [43] and behavioural role, as demonstrated by Bull et al. [44] who compared survival rate and expression of immune genes in forager and house honeybees experimentally infected with a pathogenic fungus. Forager bees survived significantly longer than the younger house honeybees, their greater resistance being associated with the developmentally regulated increased expression of genes that control the production of antimicrobial proteins [44]. All honeybees used in this study were of the same age, as we collected newly emerged bees from brood frames removed from an apiary maintained on the Gorgona Island, where the disappearance of the Varroa mite recorded during the last three years was associated with low DWV viral loads [37]. Consistent with these findings, at day 3, before ß-glucan was administered to cohorts A, B and C, twenty out of thirty six honeybees belonging to cohort Y exhibited barely detectable numbers of viral genome in the abdomen and only seven in the head. When, at day 13, we analysed the number of DWV-positive A0 abdomens and heads and the respective viral loads, it was clear that the endogenous infection had progressed during the course of the experiment, since the number of DWV-positive abdomens and heads as well as the viral load values were significantly higher than the corresponding values of cohort Y. Interestingly, day 13 analysis of ß-glucan-fed A0.5 and A2 groups clearly revealed that the numbers of DWV-positive abdomens and heads and the respective viral loads were significantly lower than the A0 corresponding values. Although we could not analyse each individual bee during the course of the experiment, we can hypothesize that ß-glucan restrained the viral replication in bees negative to the RT-qPCR assay as well as in bees positive at day 3. As all bees belonging to cohort B were injected with a high-copy-number viral suspension, they were clearly all positive and therefore only the effect of ß-glucan on the viral load was analysed, revealing a trend similar to the one observed in cohort A, although only group B2 significantly differed from B0 and B0.5. All cohort B viral load values were approximately 10 orders of magnitude higher than those of cohort Y and 7 orders of magnitude higher than those of cohort A. All heads were positive to DWV indicating that the injected DWV suspension contained productive viral particles. The number of viral particles injected in the honeybees most likely exceeded the ability of β-glucan to effectively restrain the viral replication. We cannot exclude the possibility that the suspension used for the injection might contain other bee viruses considering that most of them are asymptomatic. However, it may be assumed that the immune response enhancement triggered by the ß-glucan stimulation might not be restricted to DWV, contributing to the response to other viral bee pathogens as well. Cohort C results were similar to cohort A, however only C0 and C0.5 were analysed, as the number of C2 honeybees available for viral load analysis was too low to allow a statistical analysis to be performed.
The survival rate of cohort A was significantly different from DWV and PBS injected cohorts B and C. It has been reported that the effects exerted by ß-glucans can differ dramatically, depending on the target organism and the dose administered [29]. Consistent with these observations, 0.5% ß-glucan significantly increased the survival rate with respect to a ß-glucan-free diet, while administration of 2% ß-glucan was associated with a significantly lower survival. With regard to the analysis of individual groups, we observed that 0.5% ß-glucan was associated with a significantly higher survival of naturally infected A0.5 and C0.5 groups. The survival rate of A2 group was the lowest within the cohort while C2 group survival was unaltered with respect to C0. Cohort B survival response to ß-glucan was remarkably different, in that 0.5% ß-glucan did not affect the survival rate, while administration of 2% ß-glucan, even though it restrained the viral replication, appeared to be detrimental to the survival rate of B2 honeybees, the lowest one among all cohorts and groups. Interestingly, this result did not seem to be caused exclusively by the extremely high viral load harboured by all B honeybees, as groups B0 and B0.5 had the same survival rate as groups A0 and A2 whose viral loads were orders of magnitude lower. It can be speculated that the immune pathways’ activation triggered by 2% ß-glucan in presence of high viral load might adversely affect the host, as observed by Fronte et al. (personal communication). In agreement with previous reports [39, 45], plasmatocytes were the most represented haemocyte subtype in our study population, except in group A0.5. This finding may support what previously reported by Sabcaliu et al. [38] that described an increase of plasmatocytes in haemolymph of honeybees under stressful conditions. Prohaemocytes were the most represented haemocyte subtype in group A0.5 suggesting that 0.5% ß-glucan may exert an immunostimulatory effect in naturally infected honeybees. Szymas and Jedruszuk [46] described a granulocyte decrease in bees fed a pollen substitute containing ß-glucan and this may explain the low percentage of granulocytes in our study. No significant variation in the haemocyte subpopulations was found in ß-glucan-fed cohort B bees.
The PO activation pathways are complex and affected by many factors [28, 47]. It is noteworthy that the highest PO activity was observed in the A0,5 group, which also had the highest survival rate, one of the lowest numbers of DWV-infected bees, the lowest viral load values and the highest number of prohaemocytes. On the contrary, the lowest PO activity was observed in B2 group which had the lowest survival rate and one of the highest viral load values. Although we did not find any interaction between DWV and ß-glucan administration in relation to the innate immune response (PO activity) the overall results indicate an effect of those on honeybee survival rate.
Conclusions
In conclusion our findings suggest that the oral administration of 0.5% ß-glucan to newly emerged honeybees harbouring a low-titer natural DWV infection might contribute to increase honeybee resistance, by restraining viral replication, increasing the number of prohaemocytes and the phenoloxidase activity, ultimately prolonging the honeybee lifespan.
Acknowledgments
We thank Raffaele Cirone, president of FAI (Italian Beekeepers’ Association) for making the Gorgona apiary honeybees available for our investigation, Roberto Papucci who set up the Gorgona apiary and Matteo Giusti for collection of bees.
Data Availability
All relevant data are within the paper.
Funding Statement
This research was supported by a grant from the University of Pisa (PROGETTI DI RICERCA DI ATENEO PRA 2015 n. 0053). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gallai N, Salles JM, Settele J, Vaissière BE. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ. Elsevier B.V.; 2009;68: 810–821. 10.1016/j.ecolecon.2008.06.014 [DOI] [Google Scholar]
- 2.vanEngelsdorp D, Meixner MD. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol. Elsevier Inc.; 2010;103: S80–S95. 10.1016/j.jip.2009.06.011 [DOI] [PubMed] [Google Scholar]
- 3.Underwood RM, VanEngelsdorp D. Colony Collapse Disorder: Have We Seen This Before? Bee Cult. 2007;35: 13–18. [Google Scholar]
- 4.Vanbergen AJ, Initiative the IP. Threats to an ecosystem service: pressures on pollinators. Front Ecol Environ. Ecological Society of America; 2013;11: 251–259. 10.1890/120126 [DOI] [Google Scholar]
- 5.Hudson LN, Newbold T, Contu S, Hill SLL, Lysenko I, De Palma A, et al. The PREDICTS database: a global database of how local terrestrial biodiversity responds to human impacts. Ecol Evol. 2014;4: 4701–4735. 10.1002/ece3.1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Potts SG, Biesmeijer JC, Bommarco R, Felicioli A, Fischer M, Jokinen P, et al. Developing European conservation and mitigation tools for pollination services: approaches of the STEP (Status and Trends of European Pollinators) project. J Apic Res. 50: 152–164. 10.3896/IBRA.1.50.2.07 [DOI] [Google Scholar]
- 7.Ratnieks FLW, Carreck NL. Carity on honey bee collapse? Science 2010;327: 152–153. 10.1126/science.1185563 [DOI] [PubMed] [Google Scholar]
- 8.Nazzi F, Pennacchio F. Disentangling multiple interactions in the hive ecosystem. Trends Parasitol. Elsevier Ltd; 2014;30: 556–561. 10.1016/j.pt.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 9.Genersch E, Aubert M. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet Res. 2010;41 10.1051/vetres/2010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin SJ, Highfield AC, Brettell L, Villalobos EM, Budge GE, Powell M, et al. Global Honey Bee Viral Landscape Altered by a Parasitic Mite. Science (80-). 2012;336: 1304–1306. 10.1126/science.1220941 [DOI] [PubMed] [Google Scholar]
- 11.de Miranda JR, Genersch E. Deformed wing virus. J Invertebr Pathol. 2010;103 10.1016/j.jip.2009.06.012 [DOI] [PubMed] [Google Scholar]
- 12.Francis RM, Nielsen SL, Kryger P. Varroa-Virus Interaction in Collapsing Honey Bee Colonies. PLoS One. 2013;8 10.1371/journal.pone.0057540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dainat B, Evans JD, Chen YP, Gauthier L, Neumanna P. Dead or alive: Deformed wing virus and varroa destructor reduce the life span of winter honeybees. Appl Environ Microbiol. 2012;78: 981–987. 10.1128/AEM.06537-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nazzi F, Brown SP, Annoscia D, Del Piccolo F, Di Prisco G, Varricchio P, et al. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012;8 10.1371/journal.ppat.1002735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gisder S, Aumeier P, Genersch E. Deformed wing virus: Replication and viral load in mites (Varroa destructor). J Gen Virol. 2009;90: 463–467. 10.1099/vir.0.005579-0 [DOI] [PubMed] [Google Scholar]
- 16.Zioni N, Soroker V, Chejanovsky N. Replication of Varroa destructor virus 1 (VDV-1) and a Varroa destructor virus 1–deformed wing virus recombinant (VDV-1–DWV) in the head of the honey bee. Virology. 2011;417: 106–112. 10.1016/j.virol.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 17.Möckel N, Gisder S, Genersch E. Horizontal transmission of deformed wing virus: pathological consequences in adult bees (Apis mellifera) depend on the transmission route. J Gen Virol. 2011; 92, 370–37710. [DOI] [PubMed] [Google Scholar]
- 18.Mazzei M, Carrozza ML, Luisi E, Forzan M, Giusti M, Sagona S, et al. Infectivity of DWV associated to flower pollen: Experimental evidence of a horizontal transmission route. PLoS One. 2014;9: 1–16. 10.1371/journal.pone.0113448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans JD, Aronstein K, Chen YP, Hetru C, Imler JL, Jiang H, et al. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol Biol. 2006;15: 645–656. 10.1111/j.1365-2583.2006.00682.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brutscher LM, Daughenbaugh KF, Flenniken ML. Antiviral defense mechanisms in honey bees. Curr Opin Insect Sci. 2015;2: 1–12. 10.1016/j.cois.2015.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hultmark D. Drosophila immunity: paths and patterns. Curr Opin Immunol. 2003;15: 12–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/12495727 [DOI] [PubMed] [Google Scholar]
- 22.Nappi AJ, Christensen BM. Melanogenesis and associated cytotoxic reactions: applications to insect innate immunity. Insect Biochem Mol Biol. 2005;35: 443–59. 10.1016/j.ibmb.2005.01.014 [DOI] [PubMed] [Google Scholar]
- 23.Boman HG, Faye I, Hofsten P v., Kockum K, Lee J-Y, Xanthopoulos KG, et al. Antibacterial Immune Proteins in Insects—A Review of Some Current Perspectives. Springer; Berlin Heidelberg; 1986. pp. 63–73. 10.1007/978-3-642-70768-1_6 [DOI] [Google Scholar]
- 24.Shiao SH, Higgs S, Adelman Z, Christensen BM, Liu SH, Chen CC. Effect of prophenoloxidase expression knockout on the melanization of microfilariae in the mosquito Armigeres subalbatus. Insect Mol Biol. 2001;10: 315–321. 10.1046/j.0962-1075.2001.00268.x [DOI] [PubMed] [Google Scholar]
- 25.Lourenço AP, Zufelato MS, Gentile Bitondi MM, Paulino Simões ZL. Molecular characterization of a cDNA encoding prophenoloxidase and its expression in Apis mellifera. Insect Biochem Mol Biol. 2005;35: 541–552. 10.1016/j.ibmb.2005.01.013 [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Santoyo I, Cordoba-Aguilar A. Phenoloxidase: A key component of the insect immune system. Entomol Exp Appl. 2012;142: 1–16. 10.1111/j.1570-7458.2011.01187.x [DOI] [Google Scholar]
- 27.Cerenius L, Lee BL, Söderhäll K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008;29: 263–271. 10.1016/j.it.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 28.Cerenius L, Söderhäll K. The prophenoloxidase-activating system in invertebrates. Immunol Rev. 2004;198: 116–26. Available:http://www.ncbi.nlm.nih.gov/pubmed/15199959 [DOI] [PubMed] [Google Scholar]
- 29.Soltanian S, Stuyven E, Cox E, Sorgeloos P, Bossier P. Beta-glucans as immunostimulant in vertebrates and invertebrates. Crit Rev Microbiol. 2009;35: 109–138. 10.1080/10408410902753746 [DOI] [PubMed] [Google Scholar]
- 30.Pathak K, Zaman K. Current Pharmaceutical & Clinical. 2014;4: 71–75. [Google Scholar]
- 31.Patil US, Jaydeokar A V., Bandawane DD. Immunomodulators: A pharmacological review. Int J Pharm Pharm Sci. 2012;4: 30–36. [Google Scholar]
- 32.Hedengren M, Asling B, Dushay MS, Ando I, Ekengren S, Wihlborg M, et al. Relish, a central factor in the control of humoral but not cellular immunity in Drosophila. Mol Cell. 1999;4: 827–37. Available: http://www.ncbi.nlm.nih.gov/pubmed/10619029 [DOI] [PubMed] [Google Scholar]
- 33.Kingsolver MB, Huang Z, Hardy RW. Insect antiviral innate immunity: pathways, effectors, and connections. J Mol Biol. 2013;425: 4921–36. 10.1016/j.jmb.2013.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merkling SH, van Rij RP. Beyond RNAi: Antiviral defense strategies in Drosophila and mosquito. J Insect Physiol. 2013;59: 159–170. 10.1016/j.jinsphys.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 35.Ryabov EV, Wood GR, Fannon JM, Moore JD, Bull JC, Chandler D, et al. A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees (Apis mellifera) Prevails after Varroa destructor-Mediated or In Vitro Transmission. PLoS Pathog. 2014;10 10.1371/journal.ppat.1004230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guselle NJ, Markham RJF, Speare DJ. Timing of intraperitoneal administration of β-1,3/1,6 glucan to rainbow trout, Oncorhynchus mykiss(Walbaum), affects protection against the microsporidian Loma salmonae, J Fish Diseases, 2007;30(2):111–116 [DOI] [PubMed] [Google Scholar]
- 37.Giusti M, Papucci R, Mazzei M, Cirone R, Pinzauti M, Felicioli A. Scientific note: varroa mite eradication, the strange case of Gorgona Island. Apidologie. 2015; 2–4 10.1007/s13592-015-0417-3 [DOI] [Google Scholar]
- 38.Sabcaliu A, Pavel C, Savu V, Cauia ER. I. 2010. Biochemical and cytological Investigations n haemolymph of Apis Mellifera Carpthc Bee in stressful conditions. Bull UASVM, 2010. 67(1–2), 313–320 [Google Scholar]
- 39.El-Mohandes SS, Nafea EA, Fawzy AM. Effect of different feeding diets on the haemolymph of the newly emerged honeybee workers Apis mellifera L. Egypt. Acad. J. Biol. Sci., 2010. 3: 213–220 [Google Scholar]
- 40.Ducloz F, Crauser D, Le Conte Y. Diet effects on honeybee immunocompetence. Biol Lett., 2010. 23; 6(4): 562–565. 10.1098/rsbl.2009.0986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.SAS Institute, 2008. JMP Statistics and Graphics Guide. SAS Institute Inc, Cary, NC, USA [Google Scholar]
- 42.Theodorsson-Norheim E. Kruskal-Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Comput Methods Programs Biomed. 1986;23: 57–62. 10.1016/0169-2607(86)90081-7 [DOI] [PubMed] [Google Scholar]
- 43.Schmid MR, Brockmann A, Pirk CWW, Stanley DW, Tautz J. Adult honeybees (Apis mellifera L.) abandon hemocytic, but not phenoloxidase-based immunity. J Insect Physiol. 2008;54: 439–444. 10.1016/j.jinsphys.2007.11.002 [DOI] [PubMed] [Google Scholar]
- 44.Bull JC, Ryabov EV, Prince G, Mead A, Zhang C, Baxter LA, et al. A strong immune response in young adult honeybees masks their increased susceptibility to infection compared to older bees. PLoS Pathog. 2012;8: e1003083 10.1371/journal.ppat.1003083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Wettere A, Lewbart GA. Cytologic diagnosis of diseases of invertebrates. Vet Clin North Am Exot Anim Pract. 2007;10: 235–54, vii–viii. 10.1016/j.cvex.2006.11.003 [DOI] [PubMed] [Google Scholar]
- 46.Szymas B, Jedruszuk A. The influence of different diets on haemocytes of adult worker honey bees, Apis mellifera. Apidologie. 2003;34: 97–102. [Google Scholar]
- 47.Schmid-Hempel P. Evolutionary ecology of insect immune defenses. Annu Rev Entomol. 2005;50: 529–551. 10.1146/annurev.ento.50.071803.130420 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.