Abstract
Aquaporin-4 (AQP4) is a water channel protein that is most highly, but not exclusively, expressed in the central nervous system. In 2005 AQP4 was shown to be the antigenic target of neuromyelitis optica-immunoglobulin G (NMO-IgG, or AQP4-IgG), an antibody found specifically in patients with NMO and in formes frustes of NMO, such as longitudinally extensive transverse myelitis (LETM) or optic neuritis (ON). This discovery facilitated the clinical, pathological, and radiological distinction of NMO and the spectrum of NMO-related disorders from classical multiple sclerosis. In addition to its use as a diagnostic tool, AQP4-IgG predicts a high risk of relapse in patients with a clinically isolated syndrome of either LETM or ON. As disability in NMO is attack-related, early diagnosis and treatment are predicted to have a major effect on long-term disability. Thus, the importance of sensitive and specific assays to detect AQP4-IgG cannot be overstated. Both academic institutions and commercial companies have developed assays to identify AQP4-IgG in patients’ sera or cerebrospinal fluid. Both AQP4 isoforms from different species have been used as the antigenic target in the form of frozen tissue sections in indirect immunofluorescence assays, partially purified protein for fluorescence immunoprecipitation assay, radioimmunoprecipita-tion assay or enzyme-linked immunosorbent assay, or transfected into cells for cell based assays or flow cytometry. We carried out a systematic review of the literature reporting different methodologies used to identify AQP4-IgG, examine whether longitudinal AQP4-IgG titers predict relapses in seropositive patients, and attempt to establish a reasonable timeframe for retesting negative serum samples.
Keywords: antibody, aquaporin-4-immunoglobulin G, multiple sclerosis, neuromyelitis optica, neuromyelitis optica-immunoglobulin G
Introduction
The first known description of a patient with coexisting amaurosis and spinal cord inflammation in the Western literature was by the French anatomist and pathologist, Antoine Portal, in the early 1800s.1,2 Almost a century later, Eugène Devic and his student, Fernand Gault, reported one case and reviewed 16 patients reported in the medical literature that presented with optic neuritis (ON) and myelitis, and coined the term “neuromyélite optique aigue” based on the clinical phenotype.3 However, the most significant discovery in the understanding of this disease occurred in the early 2000s, when Lennon and colleagues at the Mayo Clinic identified an antibody in patients with neuromyelitis optica (NMO) that binds to the water channel called aquaporin-4 (AQP4).4,5 AQP4-immunoglobulin G (IgG) seropositivity was also shown to be associated with a high risk of relapses, of either myelitis or ON.6–9 Disability in this disease is attack-related. Furthermore, treatment with medications effective in multiple sclerosis (MS), with which NMO is often confused, are, in some instances, ineffective or possibly deleterious. Accordingly, early accurate diagnosis is necessary to prevent further relapses and allow for early treatment.10–16 Thus, the importance of accessibility to both sensitive and specific AQP4-IgG testing cannot be overemphasized (Fig. 1).17
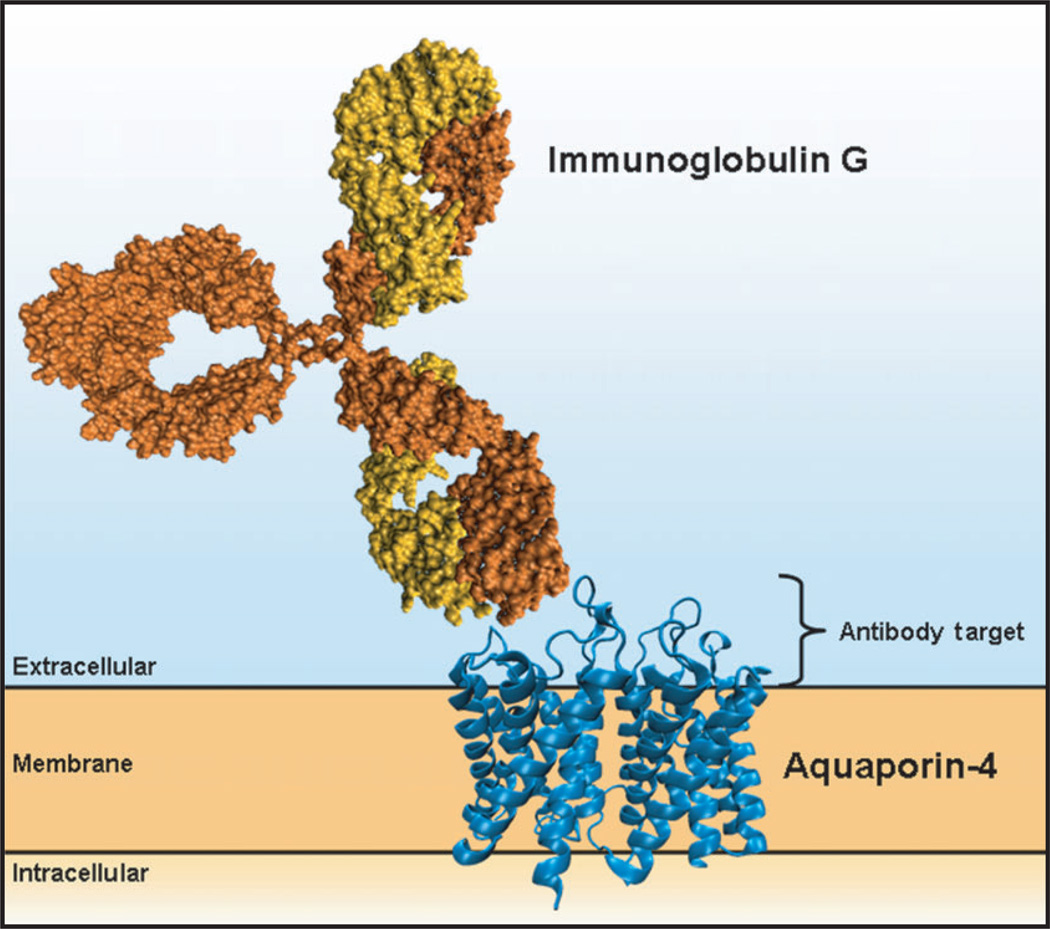
Figure 1.
Assays are developed to detect the interaction of immunoglobulin G with aquaporin-4. Here we show a cartoon depicting an immunoglobulin G molecule (orange) binding to the extracellular 50 amino acids of human aquaporin-4 (blue; PDB3GD8; residues 32–254).
Publication identification and selection
The PubMed database was screened for any articles with combinations of the terms “NMO”, “neuromyelitis optica”, “optic neuritis”, “myelitis” or “Devic’s disease” up to June 2013. In addition, reference lists were checked for relevant publications. A total of 458 publications were identified and screened for relevance. Then 54 publications were appraised under six headings: Study Design, Clinical Evaluation, Patient Evaluation, Assay, Coverage and Statistics (see Table 1). Each publication required at least two patient groups: specifically, NMO and MS that were defined either objectively or by published clinical criteria.17–21 We required the following: (i) AQP4-IgG status could not be included in the clinical criteria for the NMO patient group and they were defined blind to the AQP4-IgG status; (ii) the assay had to be carried out blind to clinical status; (iii) more than 80% of the patients had to be evaluated both clinically and have had the antibody test; and (iv) data presented were sufficient to calculate sensitivity, specificity and measures of diagnostic accuracy.
Table 1.
Evidence-based evaluation of publications assessing neuromyelitis optica-immunoglobulin G or aquaporin-4-immunoglobulin G antibody assays
| Element | Categories | Score |
|---|---|---|
| 1. Study design | Prospective cohort | I |
| Retrospective cohort | II | |
| Case control | III | |
| Case report | IV | |
| 2. Clinical evaluation | Inclusion criteria defined or is objective | I |
| Carried out blind to assay result | I | |
| No criteria/criteria are not clear/not blind to assay result | IV | |
| Assay result included in inclusion criteria or not stated | IV | |
| 3. Patient evaluation | Broad patient groups: NMO(SD)/MS/OND/ AMND/HC | I |
| NMO/MS and a control group (OND/AMND/HC) included | II | |
| NMO/MS included | III | |
| Missing NMO or MS group | IV | |
| 4. Assay | Carried out blind to clinical status | I |
| Not blind | IV | |
| Not stated | IV | |
| 5. Coverage | More than 80% of patients were clinically evaluated and had the diagnostic test | I |
| Otherwise | IV | |
| 6. Statistics | Diagnostic accuracy/statistical precision presented or calculable | I |
| Otherwise | IV | |
| 7. Result* | Lowest individual score from the elements listed above |
All studies scoring I–III are included in the evaluation.
AMND, antibody mediated neurologic disease; HC, healthy controls; MS, multiple sclerosis; NMO, neuromyelitis optica; OND, other neurological disease; SD, Spectrum disease.
Published assays
After examination of the literature, 23 publications with one or more assays were selected for data extraction. Six different classes of assay were identified based on the substrate used and the method of detection (see Fig. 2 for a cartoon of these methods). These encompass indirect immunofluorescence (IIF) on various frozen sections from mouse,5,22–31 rat32,33 or non-human primate tissue;29,31,34 immunofluorescence on cells expressing human AQP4 quantified either visually24,27,30,34–38 by fluorescence microscopy (cell-based assay [CBA]) or quantitatively by flow cytometry30,39,40 (FACS); and partially purified AQP4 quantified colorimetrically (enzyme-linked immunosorbent assay [ELISA]23,30,31,38,41), by radioactivity (radioimmunoprecipitation assay [RIPA]42) or by fluorescence (fluorescence immunoprecipitation assay [FIPA]8,24,30,43). If more than one assay was utilized in an individual publication, each assay was evaluated separately. Most studies included patients with monophasic or recurrent ON or transverse myelitis in addition to patients who meet criteria for NMO that requires both ON and myelitis. These patient subgroups have lower percentages of AQP4-IgG seropositivity when compared with NMO patients. Hence, only the data from patients with NMO and MS, defined either objectively or by published criteria, were used to calculate assay sensitivity and specificity.
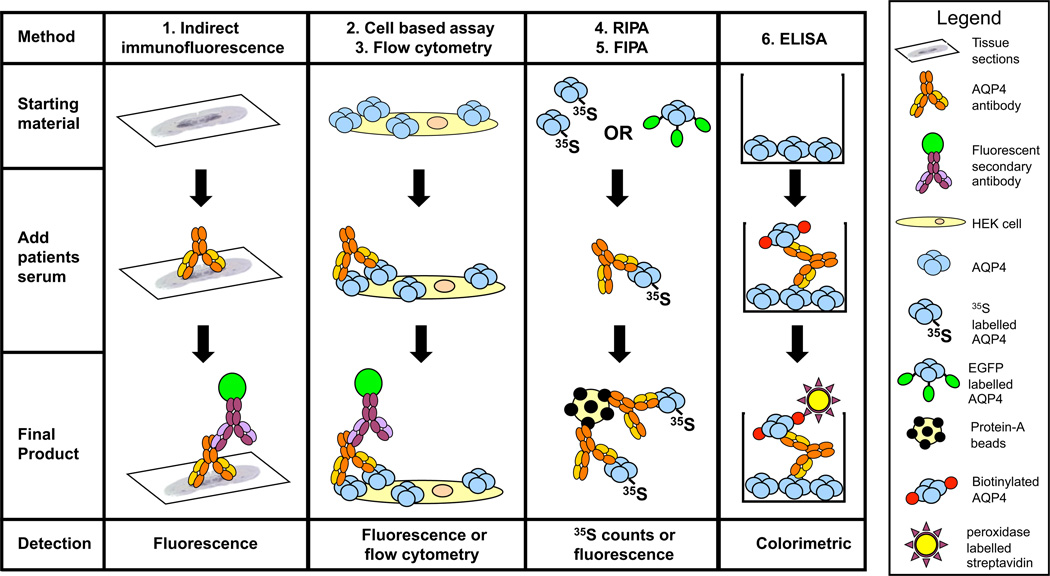
Figure 2.
The six techniques used to detect neuromyelitis optica-immunoglobulin G/aquaporin-4 antibodies (AQP4-IgG). Technique 1, tissue-based indirect immunofluorescence (IIF). Techniques 2 and 3, cell-based assay measured either visually or by flow cytometry (FACS). Techniques 4 and 5, immunoprecipitation measured either by radioimmunoprecipitation assay (RIPA) or fluorescence immunoprecipitation assay (FIPA). Technique 6, enzyme-linked immunosorbent assay (ELISA). The schematics represent an overview of the basic techniques, variations of the techniques shown here have also been published.
Data extraction from the literature
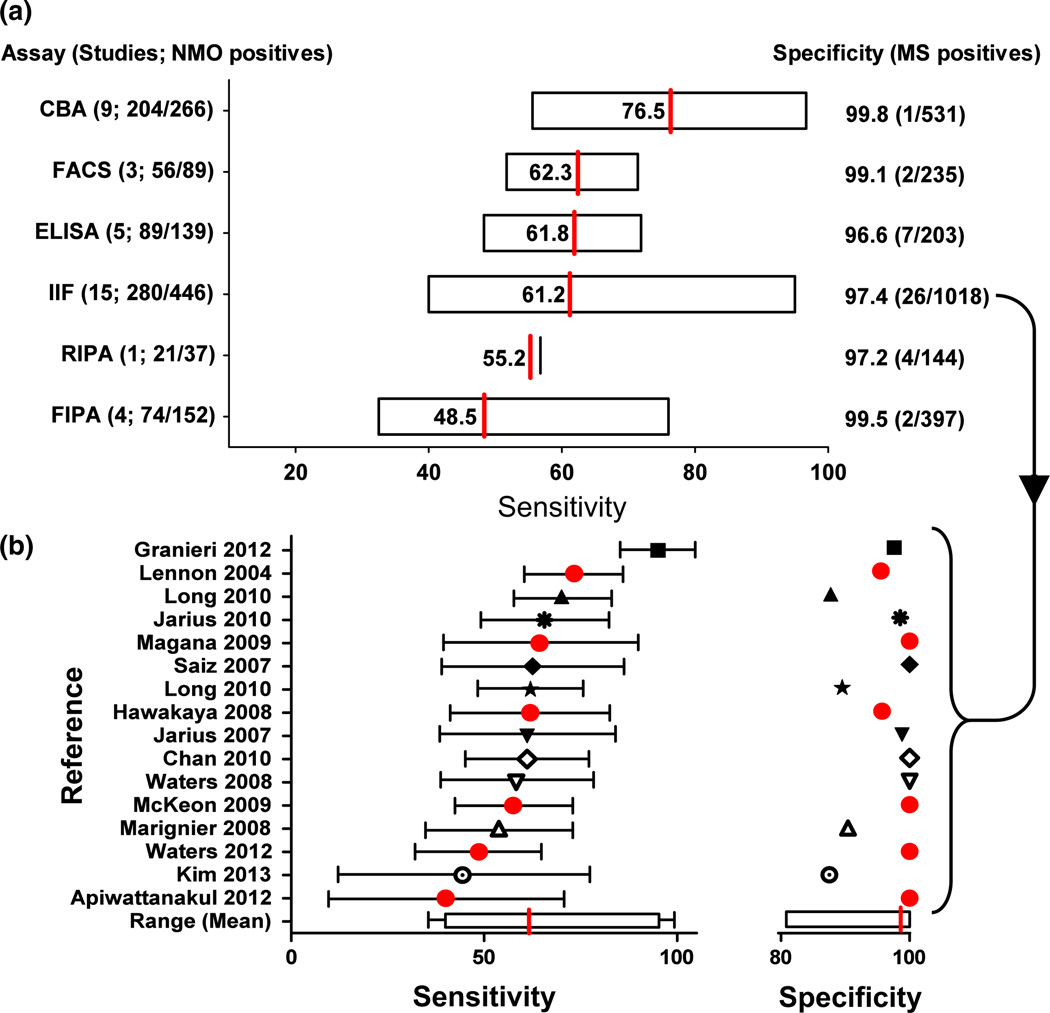
The specificities of the six different assay classes described in Table 2 are based on 144–1018 test results from MS patients reported in the literature. The average specificities of the six assay classes range from 96.6% (7/203 false positives in 5 ELISA studies) to 99.8% (1/531 false positives in 9 CBA studies). However, a caveat in these studies is the possibility of false positive results due to the misclassification of NMO patients as MS.
Table 2.
Metrics from six classes of aquaporin-4 antibody assays
| NMO Patients | Sensitivity Mean (95% CI)¶ |
Range † | MS Patients | Specificity Mean (95% CI) |
Range | Likelihood Ratios§ |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Assay | Studies | Tested | Pos | Min | Max | Tested | Pos | Min | Max | Accuracy‡ | LR+ | LR− | ||
| CBA | 9 | 266 | 204 | 76.7 (71.6–81.8) | 55.6 | 96.7 | 531 | 1 | 99.8 (99.4–100.2) | 87.5 | 100 | 76.5 | 383.5 | 0.23 |
| FACS | 3 | 89 | 56 | 62.9 (52.9–73.0) | 51.7 | 71.4 | 235 | 2 | 99.1 (98.0–100.3) | 82.4 | 100 | 62.3 | 69.9 | 0.37 |
| ELISA | 5 | 139 | 89 | 64.0 (56.1–72.0) | 48.3 | 71.9 | 203 | 7 | 96.6 (94.0–99.1) | 85.9 | 100 | 61.8 | 18.8 | 0.37 |
| IIF | 15 | 446 | 280 | 62.8 (58.3–67.3) | 40.0 | 95.0 | 1018 | 26 | 97.4 (96.5–98.4) | 87.5 | 100 | 61.2 | 24.2 | 0.38 |
| RIPA | 1 | 37 | 21 | 56.8 (40.8–72.7) | 144 | 4 | 97.2 (94.5–99.9) | 55.2 | 20.3 | 0.44 | ||||
| FIPA | 4 | 152 | 74 | 48.7 (40.7–56.6) | 32.5 | 76.0 | 397 | 2 | 99.5 (98.8–100.2) | 97.4 | 100 | 48.5 | 97.4 | 0.52 |
The lowest and highest mean sensitivity/specificity from individual studies in each method category.
Accuracy = (mean sensitivity × mean specificity)/100.
Likelihood ratio (LR)+ = sensitivity/(1-specificity), LR− = (1-sensitivity)/specificity.
Ninety-five percentage confidence interval for sensitivity (or specificity) = √((1-sensitivity) × sensitivity/number of tests) × 1.96.
CBA, cell-based assay; ELISA, enzyme-linked immunosorbent assay; FACS, flow cytometry; FlPA, fluorescence immunoprecipitation assay; IIF, indirect immunofluorescence; MS, multiple sclerosis; NMO, neuromyelitis optica; RlPA, radioimmunoprecipitation assay.
The high number of samples currently tested worldwide for NMO (e.g. >20 000/year at a single center30) renders it likely that many patients tested to date in a routine clinical setting probably do not have NMO, but MS or another disorder. Therefore, these small differences in specificity between assay classes might be of high clinical relevance. For example, based on a presumed ratio of NMO-to-MS of 1:19 among 20 000 tested samples, an assay with 97% specificity and 70% sensitivity would yield approximately 700 true positive results, but also 570 false positive test results. However, if the assay specificity was 99.8%, the false positive results drop to 38. This example highlights the need for highly specific assays.
There was also an important degree of variation in assay sensitivity between assay classes (see Fig. 3a). The average sensitivities of the six assay classes, based on positivity in clinically-defined NMO patients, ranged between 48.7% and 76.7% with the highest sensitivity obtained with CBA (mean 76.7%, range 55.6–96.7%). The mean sensitivity of the ELISA, FACS and IIF assays were very similar (ELISA mean 64.0%, range 48.3–71.9%; FACS mean 62.9%, range 51.7–71.4%; IIF mean 62.8%, range 40.0–95.0%), and the RIPA and FIPA were the least sensitive of the assays (RIPA mean 56.8%; FIPA mean 48.7%, range 32.5–76.0%). There is a great overlap of sensitivities across different assay methodologies, but special consideration should be given to the CBA, which showed a significantly higher mean accuracy than the other methods: 76.5 versus 48.5– 62.3 (Table 2).
Figure 3.
(a) Comparison of the range of sensitivities (based on neuromyelitis optica [NMO] patients) and accuracy of assays used to identify aquaporin-4 antibodies in serum of NMO patients (accuracy = [sensitivity × specificity] / 100). The specificities, listed on the right hand side, are based on multiple sclerosis (MS) patients. (b) The sensitivity (±95% confidence interval) and specificity of indirect immunofluorescence (IIF) assays published. Assays marked by red dots were carried out at an individual center, suggesting that cohort selection might influence sensitivity. CBA, cell-based assay; ELISA, enzyme-linked immunosorbent assay; FACS, flow cytometry; FIPA, fluorescence immunoprecipitation assay; RIPA, radioimmunoprecipitation assay.
The aforementioned wide range in sensitivities within the same assay class could be explained in part by the low number of NMO patients in each study (average of 31 patients/study). In addition, differences in methodology might also play a role. For example, one study reported a higher sensitivity using rat tissue than non-human primate tissue as a substrate. However, fixation methods differed between the two substrates, leaving the possibility that the superiority of the rat tissue was due to fixation method rather than the tissue species.29 Other variables might impact specificity rather than sensitivity. For example, IIF studies that examined serum diluted at <1:60 were less specific than those that used a serum dilution of 1:60 or greater (91.9% vs 98.5%),5,22–25,29,32–34,37,43 possibly as a result of higher background staining. Further factors that potentially impact on assay accuracy include preparation of the substrate, source tissue, AQP4 isoform, fixation methods, or length of incubation with primary or secondary antibodies.
Of note, the data in six of 15 tissue-based IIF studies were generated from a single laboratory (red dots on Fig. 3b). There is still a large variation in the sensitivities from these six studies (40.0–73.3%). This suggests that the variation in sensitivity might also be due to the cohort selected (genetic backgrounds or use of different inclusion criteria for example), rather than intrinsic issues with assay reproducibility.
Direct comparison in a single study
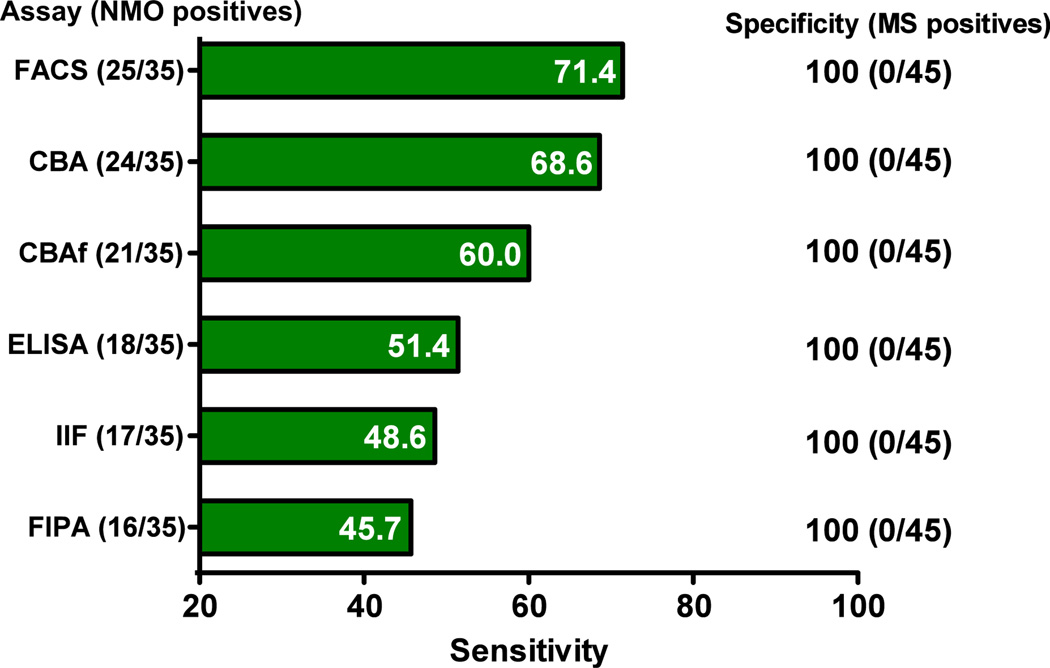
As some of the differences in assay performance observed between studies might be as a result of differences in the patient populations, several studies have directly compared assay performance within the same cohort. Almost all of these studies found individual recombinant assays more sensitive than tissue-based IIF.23,24,27,28,30,31,34,39 One recent study compared six different assays in a blinded fashion using a single series of 146 serum samples (including 35 from patients with NMO and 45 with MS) from three international centers.30 In the context of that small study, all of the assays were 100% specific (see Fig. 4). Assays based on live transiently transfected cells (CBA or FACS) expressing human M23-AQP4 were the most sensitive (68.6–71.4%). These assays were more sensitive than CBA using fixed cells (Euroimmun AG, Lübeck, Germany), two FIPA using enhanced green fluorescent protein-M23-AQP4 or EGFP-M1-AQP4, a commercial ELISA (RSR Ltd, Cardiff, UK) and the commonly used IIF assay using mouse cerebellum tissue sections. These findings suggest that CBA might be the optimal method for detection of AQP4 antibodies at the current time. A recent study by Jiao et al.44 confirms these findings.
Figure 4.
Direct comparison of six assays in a single blinded study using the same patient cohort. CBA, cell-based assay; CBAf, fixed cell-based assay; ELISA, enzyme-linked immunosorbent assay; FACS, flow cytometry; FIPA, fluorescence immunoprecipitation assay; IIF, indirect immunofluorescence; MS, multiple sclerosis; RIPA, radioimmunoprecipitation assay.30
Likelihood ratios
One way to assess the clinical usefulness of diagnostic assays is a calculation of an assay’s positive and negative likelihood ratios (LR). LR are considered to be less dependent on prevalence than predictive values. Per convention, tests yielding positive LR (LR+) >10 or negative LR (LR−) <0.1 are considered clinically useful. The LR+ indicates the factor by which a positive test results increases the pretest odds of having the respective disease. The CBA outperformed all other assays as measured by LR+ (CBA 383.5; other assays 18.8–97.4). The LR− indicates the factor by which a negative result reduces the pretest odds of having the respective disease. However, none of the assay classes analyzed for the present review yielded negative LR (LR-) <0.1 (CBA 0.23; other assays 0.37–0.52; see Table 2).
Although the data on sensitivity and specificity and LR presented here suggest that the CBA are the most accurate and should be used diagnostically, it should be kept in mind that these studies are based on a limited number of individuals, an ever present constraint on research in rare diseases. Expanded numbers and ranges of patients would lead to more precise estimates in different clinical situations.
Methodological factors that influence assays
Numerous methodological factors influence assay sensitivity and/or specificity, including, among others, the type and species of tissue sections used for IIF, transfection methods, choice of AQP4 species and isoforms, and the use of tagged versus untagged AQP4 in cell-based and protein-based assays.
Tissue-based assays (IIF)
Fresh frozen cerebellum tissue sections, generally 4–10 µm thick, from mice, rats or non-human primates, are typically used as the substrate in IIF. The analyses of IIF can be augmented by the addition of midbrain, spinal cord, stomach and/or kidney tissue sections, though an increase in sensitivity or specificity by the use of such composite substrates has not been formally shown. Antibody binding is frequently detected by fluorescent secondary antibodies. This methodology is widely used because of the ease of substrate acquisition, the presentation of both AQP4 isoforms in native tissue and because this was the method used in the discovery of AQP4-IgG.5 IIF has the advantage of being able to detect coexisting antibodies as evidenced by different staining patterns in a single assay. However, once the target antigen is known, having a wide range of different cell types with extracellular, cytosolic and nuclear antigens available for antibody binding has potential for false-positive results when the assay is used diagnostically. Considerable effort must be spent in reducing nonspecific staining. For this purpose, the tissue is blocked with 10% normal goat serum in most studies, but others have used sucrose or a gelatin-triton solution. The tested serum can be preadsorbed against bovine serum albumin and guinea pig liver powder in an attempt to remove antibodies that bind non-neuronal targets, which takes several hours and might not be fully successful. Furthermore, to reduce background staining, sera must be significantly diluted (1:60–1:120), which could result in false negative results when low titer samples are tested. This is evident in the direct comparison of AQP4-IgG assays where IIF was particularly poor at detecting AQP4-IgG in serum samples with low antibody titers, as measured by flow cytometry (Fig. 4).30
Reducing substrate complexity (ELISA, RIPA and FIPA)
Several studies have used partially purified AQP4 that has been overexpressed in insect, yeast or mammalian systems. Although one expects that such approaches, by increasing the amount and accessibility of the antigen, and by reducing substrate complexity, would increase assay specificity, all protein-based assays (ELISA, RIPA and FIPA) developed so far showed a lower mean sensitivity and specificity when directly compared with CBA (Table 2). This could in part be because of the variability in the recovery of conformationally intact protein, the level of purity, the availability of AQP4 cytosolic determinants for irrelevant antibody binding and the lack of AQP4 higher order arrays (see below). Furthermore, these processes are time-consuming and yields of purified protein can vary greatly depending on the expression system used.
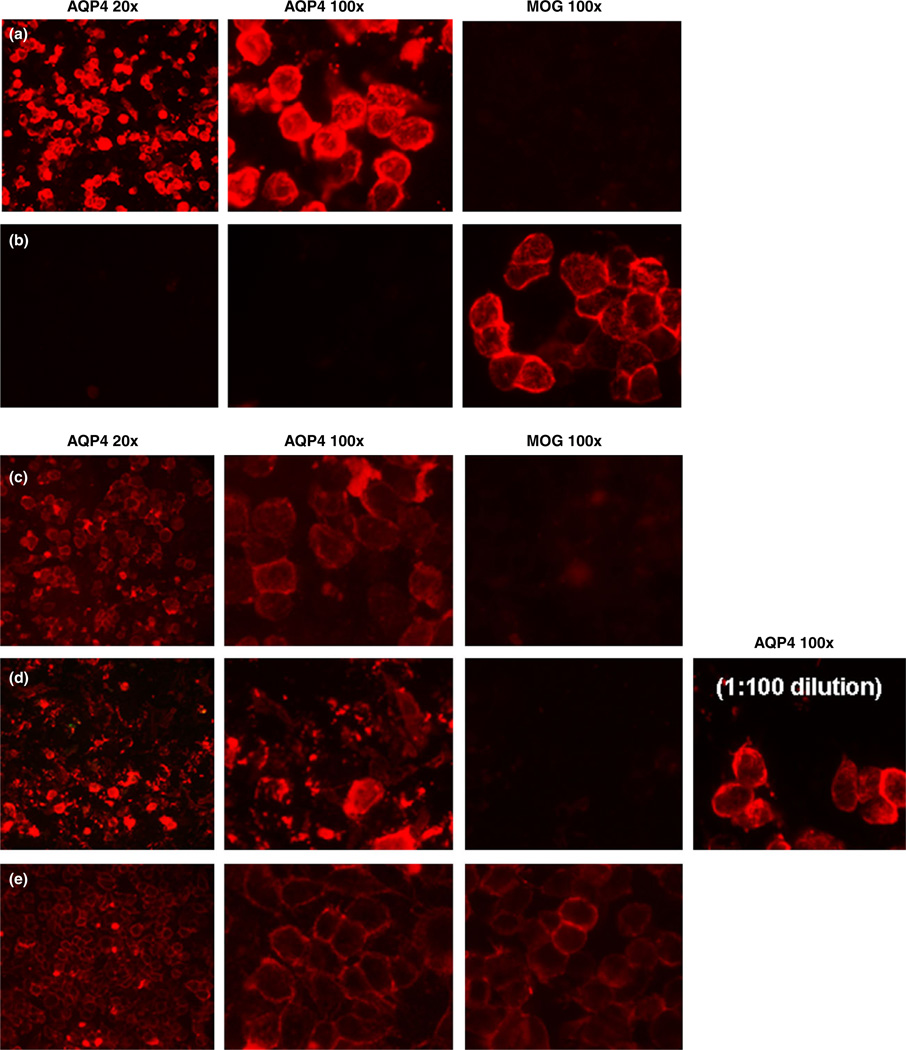
The most common method used to generate substrate for CBA is transfection of human embryonic kidney cells with a plasmid encoding human AQP4. It is an advantage of CBAs that they use natively folded, membrane-expressed AQP4 that only expose the extracellular domains of AQP4 to patient serum. Typically, the result is clear. AQP4-IgG bind to AQP4 transfected cells and not to mock-transfected cells or cells expressing a control antigen (e.g. myelin oligodendrocyte glycoprotein [MOG]; Fig. 5a). Patient serum with MOG-IgG do the opposite, binding to MOG transfected cells, but not to AQP4 transfected cells (Fig. 5b). However, there are instances where low titers of AQP4-IgG are difficult to detect (Fig. 5c). Furthermore, in live cell systems, high titer sera lyse AQP4-expressing cells, giving a “dirty” but negative appearance (see Fig. 5d). In this instance, the cells transfected with the control antigen remain “clean”; and when these sera are diluted to 1:100, excellent live cell staining is seen.
Figure 5.
Cell-based assay. (a) Aquaporin-4 (AQP4)-positive result. Serum-binding AQP4 is detected in human embryonic kidney cells expressing AQP4 (in red). No binding is seen in myelin oligodendrocyte glycoprotein (MOG)-expressing cells. (b) MOG-positive result. Serum binding is detected in MOG-expressing cells. No binding is seen in AQP4-expressing cells. (c) Weak positive result. Weak red fluorescence showing low levels of AQP4 antibodies binding to AQP4-expressing cells, but not to MOG cells. (d) Strong positive sample. Kills AQP4 transfected cells and looks like a dirty background. No background is seen in MOG-transfected cells, and when the serum is diluted to 1:100 strong binding to whole cells is seen. (e) Nonspecific binding to HEK cells shows weak binding to both AQP4 and MOG-transfected HEK cells.
Rarely, sera have antibodies that bind to non-AQP4 antigens on target cells (Fig. 5e). However, if such sera also possess AQP4-IgG, it might be recognizable by brighter staining of some cells, as would be expected in a transient transfection system. Alter-natively, these sera can be preadsorbed against untransfected target cells and then retested in the routine manner. Another option is to directly label AQP4 with an intrinsically fluorescent protein to highlight only those cells that express AQP4. This method allows for additional specificity through colocalization of the fluorescent signals, and permits additional quantification by normalizing for transfection efficiency. However, as described later, tagging AQP4 with a large fluorescent protein might alter conformation of the target antigen. Flow cytometry, a quantitative technique, eliminates many of the problems arising from the visual scoring system when interpreting CBA.
AQP4 isoforms
There are two major isoforms of human AQP4: the full-length 323 amino acid M1 isoform and the shorter 301 amino acid M23 isoform. They differ by 22 N-terminal cytosolic amino acids, which although not a target of AQP4-IgG, influence the quaternary structure of these proteins on the cell surface.45 Both isoforms are assembled into homo- and heteromeric AQP4 tetramers; only the M23 isoform forms large orthogonal arrays of particles (OAP). The M1 isoform is found on the outer aspects of these OAP, and limits their size.46
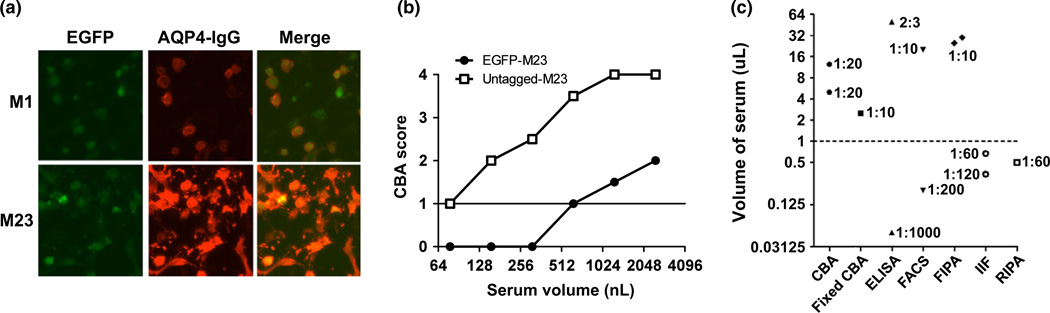
Both AQP4 isoforms have been used in a variety of assays: FIPA, ELISA, CBA and FACS. Kim et al.41 tested both isoforms in parallel during the development of their in-house ELISA. Although the data are not presented in full, the authors did not find any differences between isoforms in the detection rate among definite or high-risk NMO patients. The authors chose to use the M23 isoform, because it increased the signal-to-noise ratio. Waters et al. found no difference in sensitivity when comparing the human M1 or M23 isoforms by commercial CBA (Euroimmun AG, Lübeck, Germany) with formaldehyde-fixed cells (unpublished data). Similar to Kim et al., an increase in signal using the M23 was evident (see Fig. 6a), but this advantage of M23 was offset by a small increase in background.
Figure 6.
(a) The M23-aquaporin-4 (AQP4) isoform gives a brighter signal than the M1 isoform by cell-based assay (CBA). (b) N-terminal enhanced green fluorescent protein (EGFP)-tagged M23 AQP4 assay is less sensitive than untagged M23 AQP4 assay, by end-point titration. (c) There is a wide variation in the serum volume and concentrations used in assays to detect AQP4-IgG. ELISA, enzyme-linked immunosorbent assay; FACS, flow cytometry; FIPA, fluorescence immunoprecipitation assay; IIF, indirect immunofluorescence; RIPA, radioimmunoprecipitation assay.
In contrast, Mader et al.36 noted a significant difference in sensitivity using M1 and M23 human isoforms in live human embryonic kidney (HEK) cells. They observed a different staining pattern in the two assays. Antibody binding to the M1 isoform showed much smaller punctate staining compared with the M23 isoform, supporting previous freeze fracture data showing that the M23 isoform forms OAP in HEK cells.47 Although the majority of sera were identified using the M1 isoform (70% sensitivity, 100% specificity), the M23 isoform was more sensitive (96.7% sensitive) without loss of specificity. All of the patients positive by the M1 assay were also positive by the M23 assay. In addition, using NMO patient sera and recombinant AQP4-specific monoclonal antibodies, Crane et al.48 showed that AQP4-IgG, monoclonal antibodies or Fab fragments made from the monoclonal antibodies show consistently greater affinity for M23 than M1 because of OAP assembly, again suggesting that the choice of AQP4 isoform, and therefore OAP assembly, can directly influence assay sensitivity.
Fixation
Waters et al.30 showed that there was a slight reduction in sensitivity when comparing human M23 AQP4 as a substrate in commercial formaldehydefixed HEK cells versus similarly transfected live HEK cells in a small cohort of patients (60% vs 69% sensitive; both 100% specificity). All sera that were not identified by the formaldehyde-fixed assay, but were positive in the live CBA, were low scoring on the live CBA. The different results using fixed and live cells suggest that fixation might alter some AQP4-IgG epitopes or the organization of AQP4 OAP; but other factors, such as differences in transfection rates, detecting antibodies or incubation times, could also explain these findings.
Fluorescent tags
Fluorescent tags or intrinsically fluorescent proteins have been covalently attached to AQP4 for FIPA and CBA. These tags can be inserted anywhere in a protein using basic molecular biological techniques, but the N- or C-termini are the easiest and most often used sites of attachment. These tags are used in FIPA to standardize antigen input and to quantify specific antibody precipitated. This assay also provides a useful method to follow patient titers longitudinally.30,49 Apiwattanakul et al.50 used FIPA as a complementary assay to IIF in one study. They identified 80/4943 patient sera that were negative by IIF, but positive by FIPA, precipitating GFP-AQP4. Of these 80 samples, four also bound to an extract containing GFP alone. These sera became negative when the GFP score was subtracted from the AQP4-GFP score, suggesting antibodies in some patients sera can immunoprecipitate GFP and yield false positive results; the clinical diagnosis of the four patients that bound GFP alone was not NMO, but neuroretinitis, MS, neurosarcoidosis and paraneoplastic optic neuropathy.
In CBAs, based on transiently transfected cells, fluorescently tagged AQP4 could be used to show colocalization of patient serum binding only to cells that express AQP4. However, care must be taken when tagging AQP4 with GFP, as the structure of the antibody target could be changed by the addition of the 27 kD GFP protein; nevertheless, four publications from two centers reported that N-terminal GFP-tagged AQP4 in solution was detected with sensitivities of up to 76.0%.24,26,30,50,51 However, unlike the CBA, there was no difference in sensitivity between the N-terminal tagged M1 and M23 isoforms. The lack of difference between isoforms is as a result of the disruption of OAP formation by the N-terminal-fused GFP tag. This can be evident in the end-point titration of patient serum on HEK cells that express either untagged M23 AQP4 or N-terminally GFP tagged M23 AQP4. A single patient sample shown in Fig. 6b requires eightfold more serum on the tagged CBA to give a positive result (i.e. a score of 1 or more) than on the untagged assay.
In contrast, Mader et al. found no difference in sensitivity by CBA, when the C-terminus of M23 AQP4 was tagged. A similar punctate binding pattern of AQP4-IgG was observed to cells expressing C-terminal tagged or native M23 isoform.36 Binding sensitivity was also unchanged. Therefore, multiple experiments suggest that N-terminally-tagged AQP4 should not be used in diagnostic assays. Although C-terminal tags do not impact on the sensitivity of CBA, and can be useful to see colocalization of antigen and antibody binding, some fluorescent tags are more cytotoxic than others and might influence the assay.
Serum volume
The more serum that is used in an assay, the greater the chance of identifying antibodies, particularly in sera with low titers of AQP4 antibodies. However, the higher the concentration of serum used, the greater the risk of strong background staining or false positive results. The volume and concentration of serum that can be used is dependent on the substrate and detection methods. A wide range of serum volume is used in AQP4 assays: 50 nL to 50 lL, meaning that some assays begin with 1000-fold less antibody available for detection than others. CBAs generally use 1:10–1:20 dilutions (2– 12.5 lL serum; Fig. 6c), with <1 µL used in assays that have background issues. Technical differences within individual assay classes can help to explain some of this variation; for example, stable versus transient transfection systems, or differences in detection methods; for example, the use of secondary antibodies in some ELISAs to identify AQP4-IgG versus labeled target antigen as the secondary probe in others.
Publishing an assay to detect antibodies
Future studies evaluating the performance of AQP4-IgG assays should ideally meet the following criteria:
Inclusion of several patient groups, most importantly the disease group and groups that pose the greatest diagnostic challenge. Other antibody-mediated disease control groups and healthy controls should also be included.
Patient stratification is based on published diagnostic criteria or on criteria clearly defined in the study protocol, and is carried out blind to assay result.
More than 80% of patients should be clinically evaluated at a center with expertise in the differential diagnosis of NMO from other clinical mimics.
Assay must be carried out blind to clinical information.
Data on assay precision (inter-run and intra-run reproducibility) should be given.
Data on sensitivity, specificity and likelihood ratios are given or are calculable, and confidence intervals are given for all data on assay performance.
As many NMO patients have been misdiagnosed as MS,52 detailed clinical and magnetic resonance imaging (MRI) information on AQP4-IgG-positive control subjects should be given.
Testing cerebrospinal fluid for AQP4-IgG
Routine diagnostic testing for AQP4-IgG is carried out on serum samples. An audit of paired serum/ cerebrospinal fluid (CSF) samples sent for AQP4-IgG testing to the Mayo Clinic showed that 88% (107/ 122) of seropositive patients were also positive in the CSF.53 An additional six patients tested positive only in the CSF. The authors stated that these CSF results led to the unambiguous distinction of NMO from MS in these cases. However, the IIF assay is relatively prone to false negative serum results; CSF mostly shows less background staining. Two additional studies found a greater discrepancy between serum and CSF using cell-based assays.35,54 Just 40% and 68%, respectively, of AQP4-IgG seropositive patients whose paired CSF was also evaluated had positive results in CSF in those two studies. Patients whose serum end-point titer was 1:250 or less were invariably negative for AQP4-IgG in the CSF, and none of the 14 seronegative NMO spectrum disease patients tested in one of these studies was positive in the CSF.54 These data suggest that serum testing is more sensitive than CSF testing, and no significant improvement in assay sensitivity is gained if CSF testing is carried out in addition to serum testing when sensitive methods are used.
Prediction of a relapse
AQP4-IgG positive serology predicts a high risk of relapse in patients with a clinically isolated syndrome (ON or longitudinally extensive transverse myelitis [LETM]).6–8 However, the value of longitudinal AQP4-IgG titer measurements in individuals to predict further relapses and as a surrogate of treatment efficacy is unclear. Serum antibody levels are higher in relapse than in remission both within cohorts and in individuals suggesting longitudinal AQP4-IgG titers might be useful.35,41,49,54,55 Two studies showed relapses in individuals at different titers, showing that there is not a simple relationship between antibody titers and clinical activity, eliminating the possibility of a specific cut-off that predicts a relapse.41,49 In addition, although most of the relapses are preceded by a variable increase of AQP4-IgG titers, there are examples of relapses occurring in patients with decreasing AQP4-IgG titers.41 All of the studies investigating AQP4-IgG levels were retrospective, and accordingly, there was considerable variation in the number of samples per patient and the timing from last sampling to relapse. No clear antibody baseline level was achieved in these patients, and there was no indication of the error or variation associated with an antibody titer at any individual time-point. Therefore, serum titers could prove useful in predicting relapse or monitoring treatment, but considerably more study will be necessary before this is possible. Prospective studies of AQP4 antibody titers in future clinical treatment trials will likely prove critical in addressing these issues. Even if it is not possible to compare relapse risk across patients, it might be possible that within a given patient a change in titer could be associated with increased individual risk.
Who to test
We recommend testing for all patients with acute transverse myelitis associated with a LETM lesion on spinal cord MRI, regardless of brain MRI abnormalities, because this lesion pattern is so strongly associated with NMO. Testing is also reasonable in cases of “idiopathic” acute transverse myelitis that lack other features of MS, especially those with normal brain MRI scans, and even if the cord lesion is less than the three vertebral segments required to meet LETM criteria. The yield of AQP4-IgG is very low for isolated ON; therefore, we do not advocate routine testing in that scenario. However, AQP4-IgG should be considered in cases of severe ON with poor visual recovery, bilateral simultaneous acute optic neuritis, or detection of a long segment of optic nerve or chiasmatic signal abnormality on T2- or T1-gadolinium orbital MRI sequences. We also recommend testing for patients with intractable nausea, vomiting or hiccups in whom a gastrointestinal cause is not detected, or in whom a dorsal medullary (area postrema) MRI lesion is found. Testing should also be considered for patients with diencephalic clinical syndromes and lesions without other cause, and in patients with cryptogenic leukoencephalopathy. Routine AQP4-IgG testing is not recommended for patients in whom all clinical symptoms, signs, and MRI and CSF findings are typical for MS, because the possibility of a false positive result is much more likely than in the aforementioned scenarios. However, because individual features might overlap the two diseases, detection of any characteristic more typical of NMO than MS should prompt AQP4-IgG testing.
Time to retesting
If a patient has a negative antibody result, but there is significant clinical suspicion of NMO, is retesting appropriate and at what interval? There is minimal published data to help answer these questions. Leite et al.56 described the coincidence of NMO and myasthenia gravis in 16 patients. They presented two graphs that showed AQP4-IgG titers increasing over 10 years in two patients that were not receiving immunotherapy and before the clinical onset of NMO. A second study from Japan showed low titers in a blood sample from a person who 10 years later developed NMO.57 Assuming a linear increase over time, this would correspond to an increase of 0.3– 0.8 doubling dilutions/year. The data from these few individuals suggest a slow increase in serum antibody titer over time before clinical expression of the disease. This suggests at least a 3–6-month interval before retesting is required to see a significant difference in assay result.
The risk of false negative test results is highest during remission and in patients treated with immunosuppressants or after plasma exchange.24,30,41,49,57 Therefore, retesting is particularly recommended during acute attacks and during treatment-free intervals. However, fluctuations in AQP4-IgG titers have also been observed during remission and even under immunosuppressive treatment. Accordingly, retesting might also be justified in disease-free intervals and during treatment (e.g. perhaps after an interval of 3–6 months, or earlier in cases where new symptoms occur). Furthermore, as the sensitivity of AQP4-IgG assays varies greatly, it should be noted which assay was used, and retesting carried out using the most sensitive assay. However, as repeat testing in genuinely seronegative patients increases the risk of false positive results, “sero-conversion” in previously seronegative patients should ideally be confirmed by testing of a follow-up sample.
Conclusions
There is important variation in both sensitivity and specificity between AQP4-IgG assays. It is difficult to assess assay specificity considering the small number of control patients in published studies, but based on results from MS patients, which poses the greatest diagnostic dilemma, CBA are the most specific assay. CBA are currently the most sensitive assays.
In the first event of LETM or ON, a positive AQP4-IgG result predicts a high likelihood of relapse, which can help stratify patient treatment. However, further research on following longitudinal AQP4-IgG titers in seropositive NMO patients is required before AQP4 titers can be used to guide therapeutic decisions, such as discontinuation or escalation of therapy. The published data are limited by their retrospective nature, and limited and non-systematic sampling in follow up.
There is little evidence to guide time to retest seronegative patients that have high clinical suspicion of NMO. The risk of false negative test results is highest during remission and in patients treated with immunosuppression therapy. Hence, retesting is particularly recommended during acute attacks and during treatment-free intervals, possibly 3– 6 months after a previous test and with a more sensitive method.
Acknowledgments
This review was carried out under the auspices of the International Panel on NMO Diagnosis, funded by the Guthy-Jackson Charitable Foundation for NMO Research of which all authors are members. PJW is supported by the Oxford NIHR Biomedical Research Center and the NHS National Specialised Commissioning Group for Neuromyelitis Optica. SJP is supported by Mayo Clinic Foundation, the Guthy-Jackson Charitable Foundation and the National Institutes of Health (NS065829). JLB was supported by grants from the Guthy-Jackson Charitable Foundation and the National Institutes of Health (R01EY022936). SJ was supported by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and received a travel grant from the Guthy Jackson Charitable Foundation. BGW has received research support from the Guthy-Jackson Charitable Foundation. DMW was supported by the National Multiple Sclerosis Society (US) and the Guthy-Jackson Charitable Foundation. PJW acknowledges Dr Mark Woodhall for the images in Fig. 5.
Disclosures
Dr Waters and the Nuffield Department of Clinical Neurosciences in Oxford receive royalties and payments for antibody assays. Dr Waters is a named coinventor on application WO/2010/046716 entitled “Neurological Autoimmune Disorders,” and has received a speaker honorarium from Biogen Idec Japan. Dr Pittock is a named inventor on patents (#12/678,350 filed 2010 and #12/573,942 filed 2008) that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; and receives research support from Alexion Pharmaceuticals, Inc., the Guthy-Jackson Charitable Foundation, and the National Institutes of Health (NS065829). Dr Pittock has provided consultation to Alexion Pharmaceuticals, but has received no personal fees or personal compensation for these consulting activities. All compensation for consulting activities is paid directly to the Mayo Clinic. Dr Bennett serves as a consultant for Novartis Pharmaceuticals, Alnaylam Pharmaceuticals, MedImmune, Chugai Pharmaceuticals, EMD Serono, Abbott Pharmaceuticals, Genentech, Genzyme and Questcor Pharmaceuticals; receives license royalties for a patent re Compositions and Methods for the Treatment of Neuromyelitis Optica; and serves on the editorial boards of the Multiple Sclerosis Journal and Journal of Neuroophthalmology. SJ was supported by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and received a travel grant from the Guthy Jackson Charitable Foundation. Dr Weinshenker serves on data safety monitoring boards for Novartis and Biogen Idec; has received payment for consultation from Elan Pharmaceuticals, GlaxoSmithKline, Chugai Pharmaceuticals, Ono Pharmaceuticals and Asahi Kasei Kuraray Medical Co, Ltd.; serves on the editorial boards of the Canadian Journal of Neurological Sciences, the Turkish Journal of Neurology; and receives license royalties from RSR Ltd. for a patent re AQP4-associated antibodies for diagnosis of neuromyelitis optica. Dr Wingerchuk has received research support from Genentech, Genzyme, Alexion and CaridianBCT.
References
- 1.Jarius S, Wildemann B. The history of neuromyelitis optica. J Neuroinflammation. 2013;10:8. doi: 10.1186/1742-2094-10-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jarius S, Wildemann B. The case of the Marquis de Causan (1804): an early account of visual loss associated with spinal cord inflammation. J Neurol. 2012;259:1354–1347. doi: 10.1007/s00415-011-6355-8. [DOI] [PubMed] [Google Scholar]
- 3.Gault F. De la neuromyélite optique aiguë. Faculté de Médecine et de Pharmacie. 1894 [Google Scholar]
- 4.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202:473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet. 2004;364:2106–2112. doi: 10.1016/S0140-6736(04)17551-X. [DOI] [PubMed] [Google Scholar]
- 6.Matiello M, Lennon VA, Jacob A, et al. NMO-IgG predicts the outcome of recurrent optic neuritis. Neurology. 2008;70:2197–2200. doi: 10.1212/01.wnl.0000303817.82134.da. [DOI] [PubMed] [Google Scholar]
- 7.Weinshenker BG, Wingerchuk DM, Vukusic S, et al. Neuromyelitis optica IgG predicts relapse after longitudinally extensive transverse myelitis. Ann Neurol. 2006;59:566–569. doi: 10.1002/ana.20770. [DOI] [PubMed] [Google Scholar]
- 8.Jarius S, Frederikson J, Waters P, et al. Frequency and prognostic impact of antibodies to aquaporin-4 in patients with optic neuritis. J Neurol Sci. 2010;298:158–162. doi: 10.1016/j.jns.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Jarius S, Ruprecht K, Wildemann B, et al. Contrasting disease patterns in seropositive and seronegative neuromyelitis optica: a multicentre study of 175 patients. J Neuroinflammation. 2012;9:14. doi: 10.1186/1742-2094-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu Y, Yokoyama K, Misu T, et al. Development of extensive brain lesions following interferon beta therapy in relapsing neuromyelitis optica and longitudinally extensive myelitis. J Neurol. 2008;255:305–307. doi: 10.1007/s00415-007-0730-5. [DOI] [PubMed] [Google Scholar]
- 11.Palace J, Leite MI, Nairne A, Vincent A. Interferon Beta treatment in neuromyelitis optica: increase in relapses and aquaporin 4 antibody titers. Arch Neurol. 2010;67:1016–1017. doi: 10.1001/archneurol.2010.188. [DOI] [PubMed] [Google Scholar]
- 12.Harrer A, Tumani H, Niendorf S, et al. Cerebrospinal fluid parameters of B cell-related activity in patients with active disease during natalizumab therapy. Mult Scler. 2013;19:1209–1212. doi: 10.1177/1352458512463483. [DOI] [PubMed] [Google Scholar]
- 13.Jurynczyk M, Zaleski K, Selmaj K. Natalizumab and the development of extensive brain lesions in neuromyelitis optica. J Neurol. 2013;260:1919–1921. doi: 10.1007/s00415-013-6965-4. [DOI] [PubMed] [Google Scholar]
- 14.Jacob A, Hutchinson M, Elsone L, et al. Does natalizumab therapy worsen neuromyelitis optica? Neurology. 2012;79:1065–1066. doi: 10.1212/WNL.0b013e31826845fe. [DOI] [PubMed] [Google Scholar]
- 15.Kleiter I, Hellwig K, Berthele A, et al. Failure of natalizumab to prevent relapses in neuromyelitis optica. Arch Neurol. 2012;69:239–245. doi: 10.1001/archneurol.2011.216. [DOI] [PubMed] [Google Scholar]
- 16.Barnett MH, Prineas JW, Buckland ME, Parratt JD, Pollard JD. Massive astrocyte destruction in neuromyelitis optica despite natalizumab therapy. Mult Scler. 2012;18:108–112. doi: 10.1177/1352458511421185. [DOI] [PubMed] [Google Scholar]
- 17.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 18.Wingerchuk DM, Hogancamp WF, O’Brien PC, Weinshenker BG. The clinical course of neuromyelitis optica (Devic’s syndrome) Neurology. 1999;53:1107–1114. doi: 10.1212/wnl.53.5.1107. [DOI] [PubMed] [Google Scholar]
- 19.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 20.Polman CH, Reingold SC, Edan G, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 21.Poser CM, Paty DW, Scheinberg L, et al. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983;13:227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- 22.Jarius S, Franciotta D, Bergamaschi R, et al. NMO-IgG in the diagnosis of neuromyelitis optica. Neurology. 2007;68:1076–1077. doi: 10.1212/01.wnl.0000256822.01222.bd. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa S, Mori M, Okuta A, et al. Neuromyelitis optica and anti-aquaporin-4 antibodies measured by an enzyme-linked immunosorbent assay. J Neuroimmunol. 2008;196:181–1817. doi: 10.1016/j.jneuroim.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Waters P, Jarius S, Littleton E, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol. 2008;65:913–919. doi: 10.1001/archneur.65.7.913. [DOI] [PubMed] [Google Scholar]
- 25.Magana SM, Pittock SJ, Lennon VA, Keegan BM, Weinshenker BG, Lucchinetti CF. Neuromyelitis optica IgG serostatus in fulminant central nervous system inflammatory demyelinating disease. Arch Neurol. 2009;66:964–966. doi: 10.1001/archneurol.2009.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKeon A, Fryer JP, Apiwattanakul M, et al. Diagnosis of neuromyelitis spectrum disorders: comparative sensitivities and specificities of immunohistochemical and immunoprecipitation assays. Arch Neurol. 2009;66:1134–1138. doi: 10.1001/archneurol.2009.178. [DOI] [PubMed] [Google Scholar]
- 27.Jarius S, Probst C, Borowski K, et al. Standardized method for the detection of antibodies to aquaporin-4 based on a highly sensitive immunofluorescence assay employing recombinant target antigen. J Neurol Sci. 2010;291:52–56. doi: 10.1016/j.jns.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Apiwattanakul M, Asawavichienjinda T, Pulkes T, et al. Diagnostic utility of NMO/AQP4-IgG in evaluating CNS inflammatory disease in Thai patients. J Neurol Sci. 2012;320:118–120. doi: 10.1016/j.jns.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long Y, Hu X, Peng F, et al. Neuromyelitis optica immunoglobulin g in chinese patients detected by immunofluorescence assay on a monkey brain substrate. Neuro Immuno Modulation. 2012;19:20–24. doi: 10.1159/000326779. [DOI] [PubMed] [Google Scholar]
- 30.Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78:665–671. doi: 10.1212/WNL.0b013e318248dec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim YJ, Jung SW, Kim Y, Park YJ, Han K, Oh EJ. Detection of anti-aquaporin-4 antibodies in neuromyelitis optica: comparison of tissue-based and cell-based indirect immunofluorescence assays and ELISA. J Clin Lab Anal. 2013;26:184–189. doi: 10.1002/jcla.21508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saiz A, Zuliani L, Blanco Y, Tavolato B, Giometto B, Graus F. Revised diagnostic criteria for neuromyelitis optica (NMO) Application in a series of suspected patients. J Neurol. 2007;254:1233–1237. doi: 10.1007/s00415-007-0509-8. [DOI] [PubMed] [Google Scholar]
- 33.Marignier R, De Seze J, Vukusic S, et al. NMO-IgG and Devic’s neuromyelitis optica: a French experience. Mult Scler. 2008;14:440–445. doi: 10.1177/1352458507084595. [DOI] [PubMed] [Google Scholar]
- 34.Chan KH, Kwan JS, Ho PW, Ho JW, Chu AC, Ramsden DB. Aquaporin-4 autoantibodies in neuromyelitis optica spectrum disorders: comparison between tissue-based and cell-based indirect immunofluorescence assays. J Neuroinflammation. 2010;7:50. doi: 10.1186/1742-2094-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain. 2007;130(Pt 5):1235–1243. doi: 10.1093/brain/awm062. [DOI] [PubMed] [Google Scholar]
- 36.Mader S, Lutterotti A, Di Pauli F, et al. Patterns of antibody binding to aquaporin-4 isoforms in neuromyelitis optica. PLoS ONE. 2010;5:e10455. doi: 10.1371/journal.pone.0010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granieri L, Marnetto F, Valentino P, et al. Evaluation of a multiparametric immunofluorescence assay for standardization of neuromyelitis optica serology. PLoS ONE. 2012;7:e38896. doi: 10.1371/journal.pone.0038896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Isobe N, Yonekawa T, Matsushita T, et al. Quantitative assays for anti-aquaporin-4 antibody with subclass analysis in neuromyelitis optica. Mult Scler. 2012;18:1541–1551. doi: 10.1177/1352458512443917. [DOI] [PubMed] [Google Scholar]
- 39.Kalluri SR, Illes Z, Srivastava R, et al. Quantification and functional characterization of antibodies to native aquaporin 4 in neuromyelitis optica. Arch Neurol. 2010;67:1201–1208. doi: 10.1001/archneurol.2010.269. [DOI] [PubMed] [Google Scholar]
- 40.Ketelslegers IA, Modderman PW, Vennegoor A, Killestein J, Hamann D, Hintzen RQ. Antibodies against aquaporin-4 in neuromyelitis optica: distinction between recurrent and monophasic patients. Mult Scler. 2011;12:1527–1530. doi: 10.1177/1352458511412995. [DOI] [PubMed] [Google Scholar]
- 41.Kim W, Lee JE, Li XF, et al. Quantitative measurement of anti-aquaporin-4 antibodies by enzyme-linked immunosorbent assay using purified recombinant human aquaporin-4. Mult Scler. 2012;5:578–586. doi: 10.1177/1352458511424590. [DOI] [PubMed] [Google Scholar]
- 42.Paul F, Jarius S, Aktas O, et al. Antibody to aquaporin 4 in the diagnosis of neuromyelitis optica. PLoS Med. 2007;4:e133. doi: 10.1371/journal.pmed.0040133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKeon A, Pittock SJ. Neuromyelitis optica and the evolving spectrum of water channel autoimmunity: a new direction. Eur J Neurol. 2009;16:433–435. doi: 10.1111/j.1468-1331.2008.02385.x. [DOI] [PubMed] [Google Scholar]
- 44.Jiao Y, Fryer JP, Lennon VA, et al. Updated estimate of AQP4-IgG serostatus and disability outcome in neuromyelitis optica. Neurology. 2013;81:1197–1204. doi: 10.1212/WNL.0b013e3182a6cb5c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rossi A, Baumgart F, van Hoek AN, Verkman AS. Post-Golgi supramolecular assembly of aquaporin-4 in orthogonal arrays. Traffic. 2012;13:43–53. doi: 10.1111/j.1600-0854.2011.01299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rash JE, Davidson KG, Yasumura T, Furman CS. Freeze-fracture and immunogold analysis of aquaporin-4 (AQP4) square arrays, with models of AQP4 lattice assembly. Neuroscience. 2004;129:915–934. doi: 10.1016/j.neuroscience.2004.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furman CS, Gorelick-Feldman DA, Davidson KG, et al. Aquaporin-4 square array assembly: opposing actions of M1 and M23 isoforms. Proc Natl Acad Sci USA. 2003;100:13609–13614. doi: 10.1073/pnas.2235843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crane JM, Lam C, Rossi A, Gupta T, Bennett JL, Verkman AS. Binding affinity and specificity of neuromyelitis optica autoantibodies to aquaporin-4 M1/M23 isoforms and orthogonal arrays. J Biol Chem. 2011;286:16516–16524. doi: 10.1074/jbc.M111.227298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarius S, Aboul-Enein F, Waters P, et al. Antibody to aquaporin-4 in the long-term course of neuromyelitis optica. Brain. 2008;131(Pt 11):3072–3080. doi: 10.1093/brain/awn240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Apiwattanakul M, McKeon A, Pittock SJ, Kryzer TJ, Lennon VA. Eliminating false-positive results in serum tests for neuromuscular autoimmunity. Muscle Nerve. 2010;41:702–704. doi: 10.1002/mus.21653. [DOI] [PubMed] [Google Scholar]
- 51.Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012;69:1176–1180. doi: 10.1001/archneurol.2012.314. [DOI] [PubMed] [Google Scholar]
- 52.McKeon A, Pittock SJ, Lennon VA. CSF complements serum for evaluating paraneoplastic antibodies and NMO-IgG. Neurology. 2011;76:1108–1110. doi: 10.1212/WNL.0b013e318211c379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarius S, Franciotta D, Paul F, et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J Neuroinflammation. 2010;7:52. doi: 10.1186/1742-2094-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jarius S, Franciotta D, Paul F, et al. Testing for antibodies to human aquaporin-4 by ELISA: sensitivity, specificity, and direct comparison with immunohistochemistry. J Neurol Sci. 2012;320:32–37. doi: 10.1016/j.jns.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 55.Leite MI, Coutinho E, Lana-Peixoto M, et al. Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. Neurology. 2012;78:1601–1607. doi: 10.1212/WNL.0b013e31825644ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishiyama S, Ito T, Misu T, et al. A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset. Neurology. 2009;72:1960–1961. doi: 10.1212/WNL.0b013e3181a82621. [DOI] [PubMed] [Google Scholar]
- 57.Nagaishi A, Takagi M, Umemura A, et al. Clinical features of neuromyelitis optica in a large Japanese cohort: comparison between phenotypes. J Neurol Neurosurg Psychiatry. 2011;82:1360–1364. doi: 10.1136/jnnp-2011-300403. [DOI] [PubMed] [Google Scholar]