Abstract
Poor cognitive control is associated with nearly every mental disorder and has been proposed as a transdiagnostic risk factor for psychopathology, including depression and anxiety. What specific mechanisms might cause individuals with poor cognitive control to experience higher levels of psychopathology? The current research tests a new process model linking poor cognitive control to depression and anxiety symptoms via increased dependent stress (i.e., self-generated stressors) and subsequent rumination. This model was supported across two studies in youth during the key period for emergence of internalizing psychopathology. Study 1 provides longitudinal evidence for prospective prediction of change in symptoms. Study 2 confirms this model using well-established executive function tasks in a cross-sectional study. These finding have potential implications for understanding why cognitive control impairments may be broadly associated with psychopathology, and suggest that interventions to prevent stress generation might be effective in preventing negative consequences of poor cognitive control.
Keywords: depression, anxiety, executive function, cognitive control, stress, rumination
Spiraling out of control: Stress generation and subsequent rumination mediate the link between poorer cognitive control and internalizing psychopathology
Cognitive control impairments are now widely acknowledged as an important aspect of psychopathology, and are important contributors to impairments in social, occupational and educational functioning (e.g., McIntyre et al., 2013). While cognitive control has been defined in different ways, these definitions all share in common the idea that cognitive control consists of higher-level cognitive processes, which control and regulate lower-level cognitive processes (e.g. perception, motor responses) to guide behavior towards a goal, especially in non-routine situations (e.g., Miyake & Friedman, 2012). Poor cognitive control is associated with nearly every mental disorder (e.g., for review see Snyder, Hankin, & Miyake, 2015). Therefore, it has recently been proposed that such cognitive control deficits may be a transdiagnostic risk factor for psychopathology (Buckholtz & Meyer-Lindenberg, 2012; Goschke, 2014). However, the specific mechanisms that lead individuals with poor cognitive control to experience higher levels of psychopathology remain largely unknown. The current study tests a new process model linking poor cognitive control to two highly prevalent and impairing forms of psychopathology, depression and anxiety, in youth during the key period for emergence of internalizing psychopathology.
Poor Cognitive Control is Associated with Internalizing Psychopathology
In their lifetime, approximately 21% of individuals will experience a mood disorder and 29% an anxiety disorder (Kessler et al., 2005). Adolescence is a key developmental period for both anxiety and depression. Rates of anxiety increase steadily across this period, reaching adult levels by late adolescence, while rates of depression increase dramatically from middle to late adolescence, when they reach adult levels (e.g., Merikangas et al., 2010). Moreover, many more experience subclinical levels of depression and anxiety symptoms, which can be equally distressing and impairing (e.g., Aalto-Setälä, Marttunen, Tuulio-Henriksson, Poikolainen, & Lönnqvist, 2014; Beesdo, Knappe, & Pine, 2009). In addition, cognitive control continues to develop through adolescence into early adulthood (e.g., for review see Best, Miller, & Jones, 2009).
Depression and anxiety, both at the symptom and syndrome level, frequently co-occur in adolescents (e.g., for review see Cummings, Caporino, & Kendall, 2014), and hierarchical models of psychopathology have consistently demonstrated that depression and anxiety are best conceptualized as nested within a latent internalizing psychopathology dimension (e.g., see Carragher, Krueger, Eaton, & Slade, 2015 for review). While there are important differences between depression and anxiety, they share many risk factors in common, including impaired cognitive control (e.g., for review see Snyder et al., 2015). Thus, better understanding how cognitive control deficits may serve as risk factors for anxiety and depression in youth has important implications for understanding the etiology of these disorders during their key developmental period.
Depression and anxiety are both associated with impaired cognitive control as assessed by executive function (EF) task performance and effortful control (EC) questionnaire measures. EF and EC are two distinct ways of assessing cognitive control that are conceptually very similar and have been defined in nearly identical terms, for example EC has been defined as “the efficiency of executive attention” (Rothbart & Bates, 2006), but have generally been studied by those in different fields (temperament/developmental psychology vs. cognitive psychology) and using different methodologies (e.g., questionnaires vs. tasks). While these ways of assessing cognitive control differ in a number of important respects (see Study 1 Discussion section), there is nonetheless converging evidence across these two types of measures that cognitive control deficits are associated with internalizing psychopathology.
Specifically, individuals with depression and anxiety report more cognitive control difficulties in daily life (e.g., Letkiewicz et al., 2014; Muris & Ollendick, 2005; van Oort, Greaves Lord, Ormel, Verhulst, & Huizink, 2011) and have impaired performance on executive function tasks (e.g., Berggren & Derakshan, 2013; Derakshan & Eysenck, 2009; Owens, Koster, & Derakshan, 2012; Snyder, 2013; Snyder et al., 2010). Moreover, while the vast majority of research has been cross-sectional, there is some emerging evidence that poor cognitive control, as assessed by self-report measures, prospectively predicts increased depression (Letkiewicz et al., 2014) and internalizing symptoms more broadly (Eisenberg, Valiente, & Spinrad, 2009; Oldehinkel, Hartman, Ferdinand, Verhulst, & Ormel, 2007). Thus, there is evidence that cognitive control, as assessed by both executive function task performance and self-report effortful control questionnaires, is an important factor in understanding psychopathology, and may be a risk factor for depression and anxiety. Given the conceptual similarity of cognitive control constructs assessed by executive function task performance and effortful control questionnaires and the empirical evidence that both are related to depression and anxiety, in the current study we assessed both EC and EF to provide converging evidence across multiple measures of cognitive control.
Mediating Mechanisms between Poor Cognitive Control and Internalizing Psychopathology
Why might poor cognitive control be associated broadly with internalizing psychopathology? In other words, what specific mechanisms might lead individuals with poor cognitive control to experience higher levels of internalizing psychopathology? The prominent set of theories to date have posited rumination as the key mediator. Specifically, they have proposed that difficulty preventing the entry of, and/or removing, negative information from working memory (e.g., for review see Gotlib & Joorman, 2010; Koster, De Lissnyder, Derakshan, & De Raedt, 2011), or alternatively a narrow scope of attention (for review see Whitmer & Gotlib, 2013), leads to difficulty disengaging from unwanted negative thoughts, and thus rumination. Consistent with these theories, trait rumination is associated with impaired performance on behavioral tasks that require removing no longer relevant negative information from working memory (e.g., Joormann & Gotlib, 2008; Joormann, Nee, Berman, Jonides, & Gotlib, 2010; Whitmer & Banich, 2007; Zetsche, D’Avanzato, & Joormann, 2012). To our knowledge only one study to date has directly tested the role of rumination as a mediator between poor cognitive control and psychopathology. In a small sample of remitted adult depressed patients, poor cognitive control over emotional information predicted later rumination, which mediated the association between cognitive control and depressive symptoms (measured simultaneously with rumination) (Demeyer, De Lissnyder, Koster, & De Raedt, 2012).
Thus, there is evidence linking specific aspects of cognitive control, namely controlling the content and scope of working memory, to rumination, and some preliminary evidence that rumination may mediate the association between poor cognitive control and depressive symptoms. However, there are a number of limitations to this model. First, the mediating role of rumination has only been directly tested in a single study, which had a very small sample size (n = 23), included only remitted depressed patients who may differ from the general population (Just, Abramson, & Alloy, 2001), and found effects only for an affective, but not a non-affective, cognitive control task (Demeyer et al., 2012). Other studies linking cognitive control to rumination have been almost exclusively cross-sectional. Of those few studies that did investigate associations longitudinally, cognitive control prospectively predicted rumination in one (Zetsche & Joormann, 2011), but not another (Connolly et al., 2014). Second, while specific aspects of cognitive control have been associated with rumination, other aspects of cognitive control have not been consistently associated with rumination (for review see Whitmer & Gotlib, 2013). Indeed, trait ruminators appear to be better at stably maintaining goals in working memory (Altamirano, Miyake, & Whitmer, 2010; Zetsche & Joormann, 2011). However, internalizing psychopathology is associated with broad impairments in cognitive control, including those aspects of cognitive control that appear to be unimpaired or enhanced in ruminators (e.g., for review see Hankin et al., 2015). Thus, while rumination may directly mediate links between specific aspects of cognitive control and psychopathology, it cannot explain why impairments in other aspects of cognitive control are associated with psychopathology. This suggests that some process, in addition to rumination, is likely involved in translating poor cognitive control to increased psychopathology.
Potential Mediating Role for Stress Generation
One plausible, but as yet untested, candidate for an additional mediating process is stress generation. The stress generation model (e.g., Conway, Hammen, & Brennan, 2012; Hammen, 1991) posits that individual difference vulnerabilities related to depression and anxiety, as well as internalizing psychopathology itself, impair functioning and may increase the risk for self-generated (i.e., dependent) stressful life events (e.g., achievement failures, interpersonal conflict). These dependent stressors in turn increase risk for psychopathology, including depression and anxiety (e.g., Grant et al., 2014; Hammen, 2005; Hankin & Abramson, 2001). It is well established that stress generation mediates the link between several risk factors–including negative cognitive style (Hamilton et al., 2013), negative emotionality (Hankin, 2010), rumination and corumination (Hankin, Stone, & Wright, 2010; McLaughlin & Nolen-Hoeksema, 2012) and prior levels of emotional distress (Shapero, Hankin, & Barrocas, 2013)–and internalizing psychopathology in adolescents and young adults.
It has been proposed that poor cognitive control might also lead to stress generation, and thus mediate the link between cognitive control deficits and psychopathology (Williams, Suchy, & Rau, 2009). However, this proposal has never been directly tested, and the potential role for cognitive control in stress generation has received scant attention in the literature. Rather, the literature on stress and cognitive control has largely focused on the effect of early life stress, chronic stress exposure, or acute laboratory stressors on cognitive control (e.g., for review see Lupien, McEwen, Gunnar, & Heim, 2009). However, some indirect evidence suggests that poor cognitive control might lead to experiencing more dependent stressful events. First, correlational studies have found that poor cognitive control is associated with poor functioning across multiple domains, including poor school functioning (e.g., Jacobson, Williford, & Pianta, 2011; Liew, 2012; Martel et al., 2007) and more interpersonal problems (e.g., Liew, 2012; Martel et al., 2007; Ogilvie, Stewart, Chan, & Shum, 2011), which are often considered to be dependent stressors. Second, a recent longitudinal study found that adolescents’ self-reported cognitive control at baseline prospectively predicted daily stressful life events over a two-week follow-up period, such that adolescents with poorer cognitive control reported experiencing more stressors (Galla & Wood, 2014). However, links to rumination and psychopathology were not investigated.
Given this incomplete, but promising, preliminary evidence, it is possible that deficits in cognitive control increase risk for psychopathology via stress generation. Since impairments in multiple forms of cognitive control may all contribute to stress generation (e.g., via different functional impairments), and stress is a common risk factor across many disorders (e.g., for review see Grant et al., 2014), this model could provide a parsimonious explanation for why broad impairments in cognitive control are associated transdiagnostically with psychopathology.
In this model, while certain specific aspects of cognitive control (e.g., controlling negative information in working memory) might directly predict rumination, the broader effects of cognitive control on rumination may be indirect through stress generation. Specifically, it is well established that stress is a strong predictor of rumination (e.g., Smith & Alloy, 2009). Predominant models of rumination posit that the ruminative cycle is initiated when there is a discrepancy between one’s target and actual status (e.g., Smith & Alloy, 2009). Thus dependent negative life events, such as failing to achieve a goal, are potent triggers of rumination and can lead to long-lasting increases in the tendency to ruminate (Michl, McLaughlin, Shepherd, & Nolen-Hoeksema, 2013). Rumination, in turn, predicts increases in depression and anxiety, likely through multiple mechanisms, including preventing more effective active coping mechanisms (e.g., problem solving) and reinforcing and prolonging negative mood (for review see Smith & Alloy, 2009). Indeed, rumination has been found to mediate the association between stress and internalizing symptoms (Michl et al., 2013). Thus, there is emerging evidence that poor cognitive control may lead to generation of dependent stress, which in turn can serve as a trigger for rumination, which in turn increases risk for internalizing psychopathology. However, this model with multiple mediating mechanisms has never been tested as an integrated whole.
Current Study
The current study therefore tests a new process model linking poor cognitive control to internalizing psychopathology (depression and anxiety symptoms) via generation of more dependent stressful life events and subsequent rumination. We test this model across two studies in youth during the key developmental period for the emergence of internalizing psychopathology, from late childhood through adolescence and early adulthood. Study 1 tests this pathway longitudinally across a three-year study period and provides evidence for prospective prediction. Self-reported cognitive control (EC) predicted prospective change in symptoms of depression and anxiety, and these associations were longitudinally mediated by stress generation (whether dependent stress was assessed by a contextual stress interview or self-report) and rumination. Study 2 addresses the possibility that self-reported cognitive control could be contaminated by negative self-evaluations, rather than reflecting true cognitive control ability. Here we used a battery of well-established executive function (EF) tasks to assess cognitive control in a cross-sectional study. Study 2 also examined the possibility that effects of EF may increase with age, as EF task performance is still developing during adolescence (e.g., Best et al., 2009). Taken together, these two studies use multiple methods and designs to provide conceptual replication of this novel expanded process model, showing that poor cognitive control predicts later increases in internalizing psychopathology transdiagnostically via the mediating mechanisms of stress generation and rumination.
Study 1
Method
Participants
Participants were recruited by letters mailed to families with a child in 3rd, 6th, or 9th grades of public schools in the Denver metropolitan area and were followed for three years as part of a longitudinal study of psychopathology risk (for detail, see Hankin, Young et al, in press). Participants were 360 youth who were 8–16 years old at the time of initial assessment (M=12.06, SD=2.35). Participants were 57.2% female and were ethnically and racially representative of both the regional and U.S. population (15% Hispanic/Latino, 75% white, 9% African American, 3% Asian, 1% American Indian and 12% more than one race). Parents provided informed consent, and youth provided assent. The Institutional Review Board approved all procedures. Youth and parents were reimbursed for participation.
Procedure
Data for Study 1 were collected as part of a three-year longitudinal study in which youth and a parent completed extensive laboratory assessments every 18 months and telephone assessments every 3 months. The current study used data collected during four time periods, as shown in Figures 1 and 2. T1 (the first laboratory visit) assessed baseline depression symptoms (Children’s Depression Inventory, CDI), anxiety symptoms (Manifest Anxiety Scale for Children, MASC), dependent stressors (Adolescent Life Events Questionnaire, ALEQ), rumination (Children’s Response Styles Questionnaire, CRSQ) and effortful control (Early Adolescent Temperament Questionnaire Revised, EATQ-R). At T2, stress was assessed in two different ways to provide converging evidence across methods: (1) ALEQ stress data collected during the 3, 6, 9, 12, and 15 month follow-up assessments, averaged to obtain a robust estimate of stress experienced during the 3–15 month period of the study, and (2) chronic contextual stress interview data collected at the 18 month follow-up and covering the previous 18 months. These measures were analyzed in separate analyses. T3 consists of CRSQ rumination data collected at the 18 month follow-up. Finally, in analyses with depression and anxiety as the outcome variable respectively, T4 consists of CDI depression symptom or MASC anxiety symptom data collected at the 21, 24, 27, 30, 33, and 36 month follow-ups, averaged to obtain robust estimates of depression and anxiety symptoms during the 21–36 month period of the study.
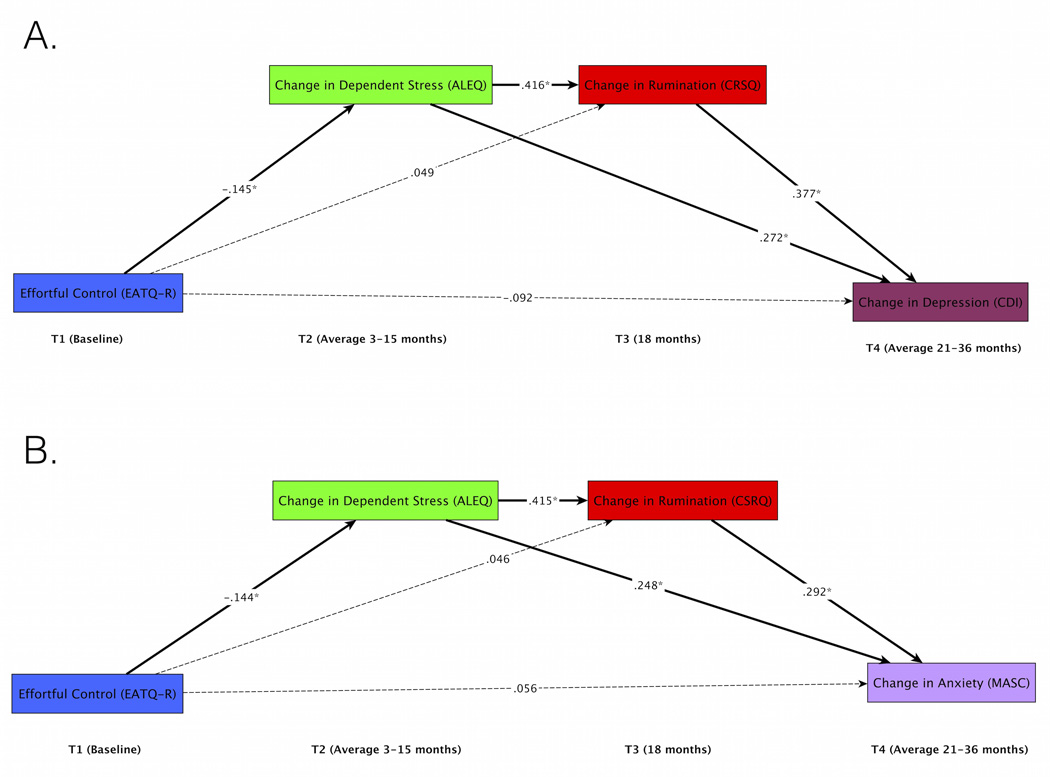
Figure 1.
Path models with ALEQ dependent stress showing key paths. Full models with all paths shown are available online in Supplemental Materials. Effortful control prospectively predicts depression (A) and anxiety (B) via two indirect pathways: (1) EC to stress to rumination to symptoms and (2) EC to stress to symptoms. Bold lines indicate significant indirect paths. Dotted lines indicate non-significant paths. * p<.05
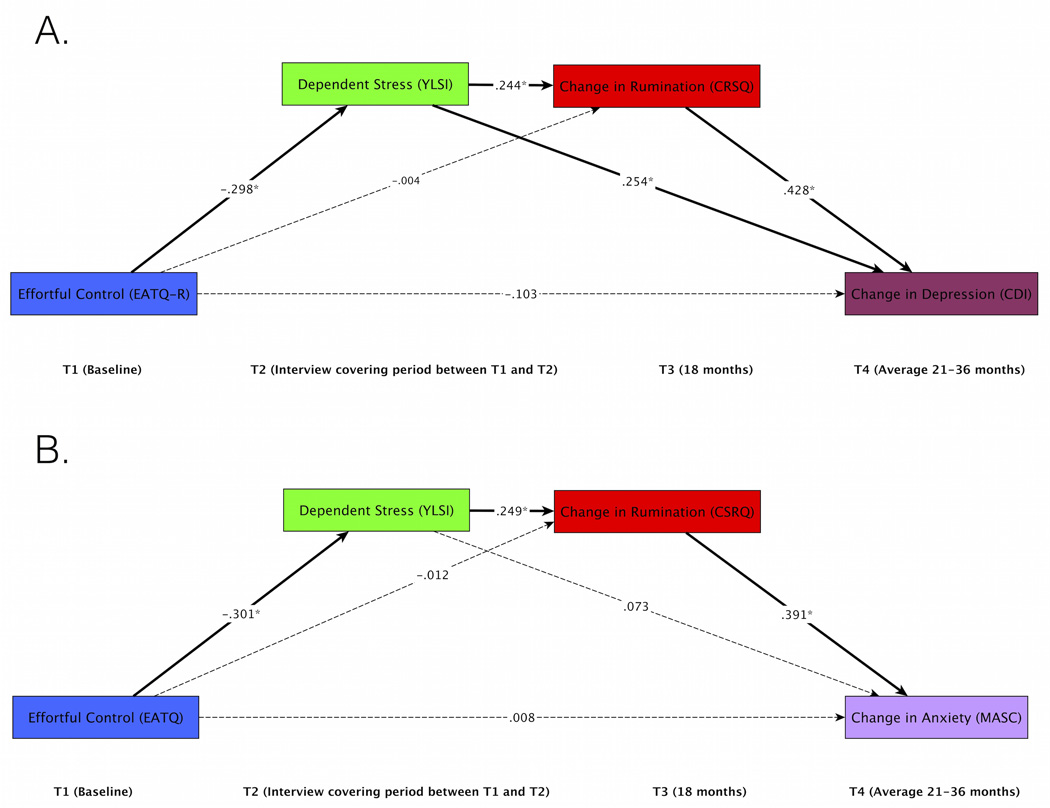
Figure 2.
Path models with Youth Life Stress Interview chronic dependent stress showing key paths. Full models with all paths shown are available online in Supplemental Materials. Effortful control prospectively predicts depression (A) and anxiety (B) via two indirect pathways: (1) EC to stress to rumination to symptoms and (2) EC to stress to symptoms. Bold lines indicate significant indirect paths. Dotted lines indicate non-significant paths. * p<.05
Measures
All measures have established good reliability and validity, as detailed in Supplemental Materials.
Children’s Depression Inventory
(CDI, Kovacs, 1983). The CDI is a widely-used self-report measure of depressive symptoms in children and adolescents (e.g., “I am sad all the time,” “I feel like crying every day.”). The current study used CDI data collected at baseline and an average of data collected at the 21–36 month follow-ups; internal consistency was good (α > .82 for all time points).
Multidimensional Anxiety Scale for Children
(MASC, March, Parker, Sullivan, Stallings, & Conners, 1997). The MASC is a widely used self-report measure of anxious symptoms in children and adolescents, including social anxiety, separation/panic, physical symptoms, and harm avoidance (e.g., “I get shaky or jittery”, “I worry about what other people think of me”). The current study used MASC data collected at baseline and an average of data collected at the 21–36 month follow-ups; internal consistency was good (α > .87 for all time points).
Early Adolescent Temperament Questionnaire Revised
(EATQ-R, Ellis & Rothbart, 2001): The EATQ-R is a commonly used self-report measure of temperament in children and adolescents. Only the effortful control (EC) scale were analyzed in this study; it includes (i) attentional control (capacity to focus and shift attention appropriately, e.g., “It is easy for me to really concentrate on homework problems.”), (ii) inhibitory control (capacity to suppress inappropriate responses and plan future action, e.g., “When someone tells me to stop doing something, it is easy for me to stop.”), and (iii) activation control (ability to perform an action when there is a strong tendency to avoid it, e.g., “If I have a hard assignment to do I get started right away.”). Higher scores indicate better cognitive control. The current study used EATQ-R data collected at baseline; internal consistency was good (α =.87).
Children’s Response Styles Questionnaire
(CRSQ, Abela, Rochon, & Vanderbilt, 2000): The CRSQ was used to assess rumination. Only the Rumination subscale was used, which assesses how frequently youth respond to a sad mood with the rumination response style (e.g., “When I am sad, I think about a recent situation wishing it had gone better, “When I am sad, I think about all my faults, failures and mistakes”). The current study used CRSQ data collected at baseline and the 18 month follow-up; internal consistency was good (α > .84 for both time points).
Adolescent Life Events Questionnaire-Revised
(ALEQ, Hankin & Abramson, 2002): The ALEQ self- report instrument assessing a broad range of negative life events typically experienced by youth, occurring in the past three months. For all items, participants rated how often such events occurred from 1 (never) to 5 (always). For the current study, we included only items that were dependent (i.e., at least in part dependent on the actions of the individual), including interpersonal and achievement stressors (e.g., “Fighting with or problems with a friend”, “Got a bad grade on an exam, project or paper in class.”) (e.g., Technow, Hazel, Abela, & Hankin, 2015). Items were coded for dependence by two independent coders and initial agreement was high (κ= .86); coding of the two discrepant items was resolved by the first author.
Youth Life Stress Interview Chronic Stress
(YLSI, Rudolph & Flynn, 2007). The YLSI is a reliable, valid, semi-structured contextual stress interview used to assess youths’ ongoing chronic stress level in multiple domains. Contextual stress interview methods such as the YLSI are considered the gold-standard approach to assess stressful life events (e.g., Hammen, 2005). For this study, we included only domains that were dependent (i.e., at least in part dependent on the actions of the individual): academic problems, behavioral problems at school, problems related to extracurricular activities, and interpersonal problems with peers, parents and romantic partners. Information on each domain gathered during the interview was presented to a team of three or more blind raters, who came to an agreed upon severity score on a scale from 1 (little/no stress) to 5 (severe stress). Specifically, coding for the YLSI is based on manualized objective ratings for each stressor type, with no consideration of the participant’s subjective response to the stressor. Ratings were averaged across domains to compute a total dependent chronic stress severity score.
Data Analysis
Path models were run in Mplus (Muthén & Muthén, 2012) to test the following hypotheses: (1) Poorer self-reported cognitive control (T1 EATQ-R EC) predicts later internalizing psychopathology (T4 CDI depression and MASC anxiety, (2) this effect is mediated by increased dependent stress (T2 ALEQ or YLSI) and subsequent rumination (T3 CRSQ). Separate models were tested for depression and anxiety, and for the two measures of dependent stress: ALEQ scores and the Youth Life Stress Interview (YLSI). Because there are well-established age and gender differences in depression and anxiety symptoms (e.g., Hankin & Abramson, 2001), age and gender were controlled for in all paths of each model.
Results
Descriptive statistics are reported in Supplemental Materials (Table S1). At T2, average ALEQ scores from the 3–15 month follow-ups were calculated: participants had data from 4.59 out of a possible 5 time points on average (SD=0.83). At T4, average CDI and MASC scores from the 21–36 month follow-ups were calculated: on average participants had data on the CDI from 5.47 out of 6 time points (SD=0.94) and from the MASC from 5.44 out of 6 time points (SD=0.94).
Total Effects Models
Controlling for age and gender, T1 EC significantly predicted T4 depression (β=−.310, t(291)=−5.60, p<.001) and anxiety (β=−.175, t(291)=−3.04, p=.003), such that lower levels of EC at baseline were associated with higher levels of symptoms of depression and anxiety averaged from 21–36 months.
Longitudinal Mediation Path Models
To provide converging evidence, separate mediation models were tested for two measures of dependent stress: ALEQ scores and the Youth Life Stress Interview (YLSI). Standardized path coefficients and significance levels for the key pathways of interest are shown in Figures 1 and 2, and full model figures and tables are reported in Supplemental Materials (Tables S3–S6, Figures S1–S2).
ALEQ
Models tested the effects of effortful control at T1 on T4 depression (Figure 1a) and anxiety (Figure 1b), with ALEQ stress at T2 and rumination at T3 as mediators, and controlling for age and gender on all paths and ALEQ stress, CRSQ rumination, and internalizing psychopathology (CDI depression or MASC anxiety in their respective models) at baseline. In both models, EC at T1 significantly predicted ALEQ dependent stress at T2, controlling for ALEQ dependent stress at T1, such that lower EC predicted more dependent stressful life events. Dependent stress at T2 in turn significantly predicted an increase in rumination at T3, controlling for rumination at T1. Both dependent stress at T2 and rumination at T3 significantly predicted an increase in depression and anxiety at T4, controlling for depression and anxiety at T1, in their respective models. Critically, for both the depression and anxiety models, there were significant indirect paths from EC to dependent stress to rumination to depression/anxiety (p=.024 depression, p=.026 anxiety). There was also a significant indirect path from EC to dependent stress to depression/anxiety (p=.013 depression, p=.020 anxiety). The indirect path from EC to rumination to depression/anxiety was not significant in either model (p=.5 depression, p=.5 anxiety). The direct effect of EC on depression remained marginal (p=.087), while the direct effect of EC on anxiety was not significant in the mediation model (p=.5).
YLSI
Models tested the effects of effortful control at T1 on T4 depression (Figure 2a) and anxiety (Figure 2b), with YLSI stress at T2 and rumination at T3 as mediators, and controlling for age, gender and baseline rumination and depression/anxiety. In both models, EC at T1 significantly predicted YLSI chronic dependent stress at T2, such that lower EC predicted higher levels of stress. Dependent stress at T2 in turn predicted higher levels of rumination at T3, controlling for rumination at baseline. Rumination at T3 significantly predicted higher depression and anxiety at T4, controlling for depression and anxiety at T1, in their respective models. Chronic dependent stress at T2 also directly predicted T4 depression, but this effect did not reach significance for not anxiety. Critically, for both the depression and anxiety models, there was a significant indirect paths from EC to dependent stress to rumination to depression/anxiety (p=.005 depression, p=.005 anxiety). There was also a significant indirect path from EC to dependent stress to depression (p=.002) but not anxiety (p=.216). The indirect path from EC to rumination to depression/anxiety was not significant in either model (p=.9 depression, p=.9 anxiety). The direct effect of EC on depression remained marginal (p=.062), while the direct effect of EC on anxiety was not significant in the mediation model (p=.9).
Study 1 Discussion
Study 1 supported the hypothesized multiple-mediating mechanisms process model. Self-reported cognitive control prospectively predicted anxiety and depression symptoms, and this effect was mediated by dependent stress and subsequent rumination. Critically, these results replicated across both self-report (ALEQ) and interview based (YLSI) measures of stress. These results are consistent with the hypothesis that poor cognitive control can lead to stress generation, which in turn can lead to increases in rumination as individuals dwell on these stressful life events. In contrast, rumination did not directly mediate the link between poor cognitive control and internalizing psychopathology, challenging models that posited a key role for rumination in directly mediating associations between cognitive control and internalizing psychopathology.
One potential limitation of Study 1 is that it used EC measures of cognitive control, rather than assessing EF task performance. Despite similarities in how these constructs are defined, relatively few studies have assessed the relation between EC and EF, especially beyond early-middle childhood (2–10 years), and they reported mixed results, with generally weak to modest correlations between EF task performance and EC questionnaires (for review, see Toplak et al., 2012). Thus, EC questionnaires and EF task performance should not be assumed to be measuring precisely the same constructs. Questionnaire-based measures ask about behavior in complex real-world situations (e.g., completing tasks on time, staying organized). This has advantages in terms of ecological validity, and some have argued in favor of using questionnaires rather than EF tasks (e.g., Barkley and Fischer, 2011). However, questionnaire measures pose interpretational problems in that these real-world behaviors involve multiple executive and non-executive processes, and can also be heavily influenced by contextual factors (e.g., having the motivation and opportunity to complete homework on time).
In addition, EC questionnaire responses could potentially be influenced by self-report biases such as negativity bias, rather than reflecting only cognitive control ability. Thus, Study 2 tested whether the effects found in Study 1 for self-reported cognitive control extended to more specific and unbiased measures of cognitive control by using behavioral EF tasks, providing a conceptual replication of Study 1.
Study 2
Study 2 assessed cognitive control using a battery of well-established EF tasks assessing shifting, updating, inhibition and working memory, to form a composite measure capturing the unitary aspect of EF shared across tasks, i.e., Common EF (e.g., Miyake and Friedman, 2012). Specifically, different components of EF correlate with one another, thus tapping some common underlying ability, posited to be the ability to actively maintain task goals and use this information to provide top–down support for task-relevant responses (e.g., Friedman et al., 2008; Miyake and Friedman, 2012). Recent evidence suggests that common EF, rather than specific EF components (e.g., shifting, updating) may be the primary predictor of psychopathology (e.g., Snyder et al., 2015; Young et al., 2009).
Thus, we selected tasks spanning different specific EF components and aggregated them to form a composite measure of common EF ability that is a more accurate and reliable measure of EF than single tasks, because the non-executive task requirements specific to each task (e.g.. color processing and articulation speed in the Stroop task, visuospatial processing in the spatial span task) have less influence (e.g., Miyake et al., 2000; Snyder et al., 2015). The measures used in the current study are identical or similar to those used in previous studies of common EF (e.g., Friedman et al., 2008), and a confirmatory factor analysis of Study 2 data demonstrated that all EF tasks loaded significantly onto a common factor, with adequate model fit, justifying creation of a composite score.
Method
Participants
Participants were recruited after the original three-year study to participate in an additional sub-study with a planned sample size of 150. Participants were a sub-sample of 148 participants from Study 1 (original cohorts: 52 3rd grade, 55 6th grade, 41 9th grade), who were 11–20 years old at the time of testing (M=16.29, SD=2.46) and 56.8% female. Seven additional participants were excluded due to parent or self-reported reading disability, which precluded valid assessment of EF given that most measures required reading. For participants younger than 18, parents gave informed consent and participants gave informed assent. Participants 18 and older gave informed consent. The Institutional Review Board approved all procedures. Youth and parents were reimbursed for participation.
Procedure
Participants completed all measures as part of a single three-hour laboratory visit. Participants were tested individually by trained research assistants in a quiet room. All participants completed measures in the same order to optimize the design for examination of individual differences.
Measures
Participants completed five tasks to assess EF: Stroop, Category-switch, Keep Track working-memory updating, spatial span forward and spatial span backward. To control for potential effects of overall psychomotor speed and IQ that could influence EF task performance, participants also completed a choice reaction time task to assess psychomotor speed and the WASI-II to assess IQ. After completing all tasks, participants completed questionnaires assessing depression, anxiety, stress, and rumination. See Supplemental Materials for detailed descriptions of each task.
Stroop (adapted from Friedman et al., 2008)
Participants named words and strings of asterisks in red, green, and blue type using a voice-activated microphone. RTs for neutral trials (asterisks) are compared to incongruent trials (e.g., red written in blue type) to calculate Stroop interference (Incongruent – Neutral RT), for correct trials only. Reaction times identified as within-subject outliers by the Wilcox–Kessleman trimming procedure (Wilcox & Keselman, 2003) were also removed before averaging (average of 7.4% of trials trimmed).
Category Switch (Reineberg, Andrews-Hanna, Depue, Friedman, & Banich, 2015 adapted from Friedman et al., 2008)
Based on a shape cue, participants pressed buttons to classify words as either as living/non-living or smaller/larger than a soccer ball, with an equal number of trials in which the task switched vs. repeated. The dependent measure is the switch cost (switch – repeat trial RT) for correct trials only. Reaction times identified as within-subject outliers by the Wilcox–Kessleman trimming procedure (Wilcox & Keselman, 2003) were also removed before averaging (average of 9.89% of trials trimmed).
Keep Track Working Memory Updating (Reineberg et al., 2015 adapted from Friedman et al., 2008)
On each trial, participants were presented with a stream of words and instructed to keep track of the last presented word from 2–5 target categories (out of six possible categories), and reported those words to the experimenter at the end of each trial. The dependent measure is the proportion of correctly recalled words across all trials.
Spatial Span Forward and Backward (ADHD/LD Cognition Lab at The Hospital for Sick Children)
Boxes on the screen changed color one at a time. After each sequence, participants clicked on the boxes in the same order (forward span) or opposite order (backward span) (performed in that order). Participants completed two trials at each span level, until they got both trials at a span level wrong, ending the task. For both forward and backward span, the dependent measure is the total number of boxes clicked in the correct order (i.e., partial credit load scoring, Conway et al., 2005), a more sensitive measure than all-or-nothing span scoring.
Choice RT
Participants pressed buttons with their left and right hands as fast as possible when presented with left or right pointing triangles, with 60 trials. The dependent variable is mean RT.
WASI-II
Participants completed the two-subtest form of the Wechsler Abbreviated Scale of Intelligence Second Edition (WASI-II, Pearson), consisting of the Vocabulary and Matrix Reasoning subtests. The dependent variable is IQ, as calculated based on the WASI-II manual.
Questionnaires
As in Study 1, participants completed the CDI to assess depression symptoms, the MASC to assess anxiety symptoms, the CRSQ to assess rumination, and the ALEQ to assess frequency of dependent stressors. Participants also completed the Penn State Worry Questionnaire for Children (PSWQ-C, Chorpita, Tracey, Brown, Collica, & Barlow, 1997), a commonly used self-report measure of worry (e.g., “Many things make me worry,” “Once I start worrying I can’t stop.”), as an additional measure of anxiety. Internal consistency in the current sample was good (α = .92). PSWQ-C and MASC scores were combined to form an anxiety composite score.
Data Analysis
Path models were run in Mplus (Muthén & Muthén, 2012) to test the following hypotheses: (1) Poor cognitive control task performance (EF composite) predicts internalizing psychopathology (CDI depression and MASC/PSWQ anxiety), (2) this effect is mediated by increased dependent stress (ALEQ) and subsequent rumination (CRSQ), and (3) these relationships change with age from early adolescence to young adulthood. Separate models were tested for depression and anxiety. As in Study 1, age and gender were controlled for in all paths of each model. In addition, to control for potential confounding effects of overall psychomotor speed and IQ, choice RT and WASI IQ were controlled for in all paths of each model.
Results
EF composite scores where calculated as the z-score average across tasks, after reversing signs as appropriate such that higher scores correspond to better performance for all tasks. Scores on individual tasks >3 SD from the mean were excluded prior to calculating the composite scores, leading to exclusion of the Stroop, Category Switch and Keep Track tasks for two participants each. In addition, the following data were missing: one participant each for Stroop (computer error), Keep Track (fire alarm), forward spatial span (computer error) and WASI-II IQ (experimenter error), and two participants for backward spatial span (computer error). No participant was missing data or had data excluded on more than one task. Descriptive statistics are reported in Supplemental Materials (Table S7).
Total Effects Models
All analyses are controlling for IQ, choice RT and gender. Poorer EF was marginally associated with higher depression, controlling for age (β=−.174, t(139)=−1.94, p=.055). Including age as a moderator, rather than covariate, there was a significant EF x age interaction, such that the effect of EF on depression increased with age (β=−.235, t(138)=−2.89, p=.004). For anxiety, there was a significant EF x age interaction, such that the effect of EF on anxiety increased with age (β=−.168, t(138)=−2.07, p=.041). However, there was no significant main effect of EF on anxiety controlling for age (β=−.046, t(139)=−0.52, p=.6). Thus, moderated mediation models, with age as a moderator, were tested.
Moderated mediation models
The moderated mediation models included EF as the predictor, ALEQ dependent stressors and rumination as the mediators, and CDI depression symptoms or MASC/PSWQ anxiety symptoms as the outcome variable, with age moderating all effects of EF (Figure 5a, 5b). Gender, IQ and choice RT were entered as covariates on all paths. Standardized path coefficients and significance levels are shown in Figure 3, and full model tables are reported in Supplemental Materials (Tables S9–S10).
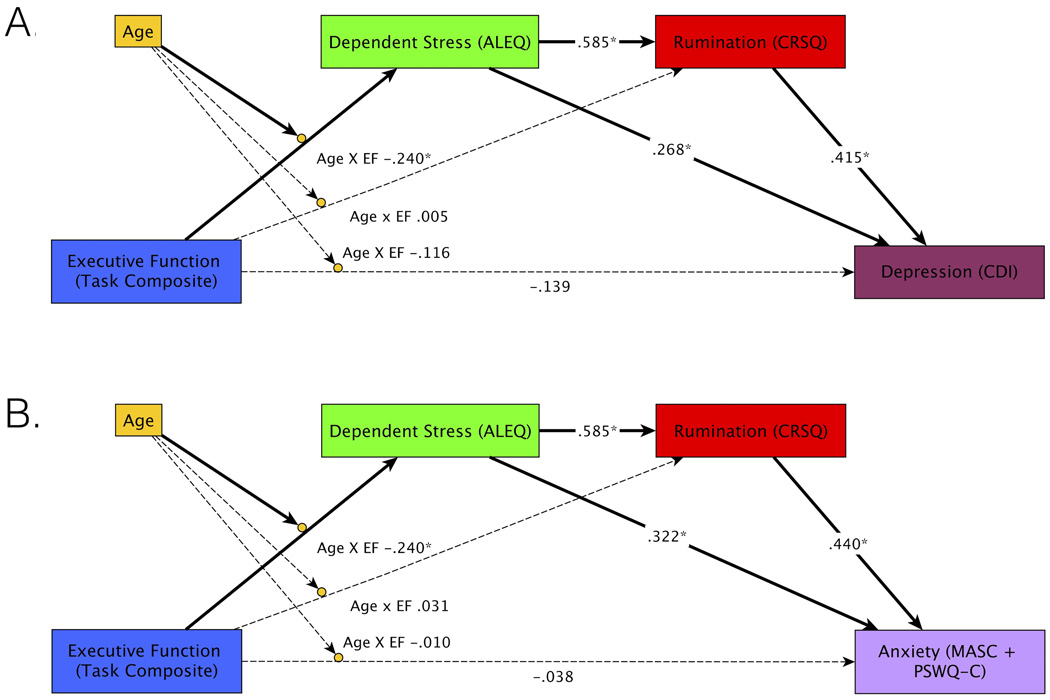
Figure 3.
Study 2 path models. Executive function task performance predicts depression (A) and anxiety (B) via two indirect pathways: (1) EF to stress to rumination to symptoms and (2) EF to stress to symptoms. Bold lines indicate significant indirect paths. Dotted lines indicate non-significant paths.* p<.05
In both the depression and anxiety model, there was a significant age x EF interaction predicting dependent stress, such that participants with poorer EF reported experiencing more dependent stressors, and this effect increased with age. In both models, there was a significant effect of dependent stress on rumination, such that participants reporting higher levels of dependent stress reported higher levels of rumination. In their respective models, there were significant effects of both dependent stress and rumination on depression and anxiety, such that participants who reported more dependent stressors and higher levels of rumination reported higher levels of depression and anxiety symptoms. Critically, for both the depression and anxiety models, there were significant indirect paths from EF to dependent stress to rumination to depression/anxiety (p=.016 depression, p=.013 anxiety). There were also significant indirect paths from EF to dependent stress to depression/anxiety (p=.031 depression, p=.013 anxiety). The indirect path from EF to rumination to depression/anxiety was not significant in either model (p=.9 depression, p=.9 anxiety). The direct effects of EF x Age on depression (p=.2), and anxiety (p=.7) were not significant in the mediation model.
General Discussion
Poor cognitive control is associated with nearly every mental disorder and has been proposed as transdiagnostic risk factor for psychopathology, including the internalizing psychopathology dimensions of depression and anxiety. What specific mechanisms might cause individuals with poor cognitive control to experience higher levels of internalizing psychopathology? Across two studies, we found support for our hypothesized process model linking poorer cognitive control to depression and anxiety symptoms in youth via generation of more dependent stressful life events and subsequent rumination. Thus, across both studies there was strong and complementary evidence in favor of the proposed role of poor cognitive control in stress generation, with stress in turn triggering rumination, which is a potent immediate risk factor for both depression and anxiety (e.g., Michl et al., 2013). These studies for the first time put together multiple links in a pathway, some of which had been previously investigated in isolation but which had never been jointly tested before (Williams et al., 2009).
Study 1 found that self-reported cognitive control prospectively predicted both self-report and interview based measures of dependent stress over time. Consistent with the hypothesis that poor cognitive control leads to stress generation, individuals with poorer self-reported cognitive control experienced more dependent stress over 18 months. Dependent stress in turn prospectively predicted increases in rumination, which in turn predicted increases in depression and anxiety symptoms over 18 months. Thus, Study 1 provided a strong test of longitudinal mediation, demonstrating that the proposed process pathway predicts change in symptoms over time, controlling for baseline levels of symptoms.
Study 1 used a self-report measure of cognitive control, so it was critical to conceptually replicate these findings and address the possibility that these effects might solely reflect self-report negativity bias (i.e., that individuals who view themselves negatively might report more problems on all questionnaires), or factors other than cognitive control affecting self-reported real-world behaviors (e.g., motivation, environmental effects). Study 2 addressed this possibility by using executive function task performance to objectively assess cognitive control ability. As in Study 1, there was a significant pathway from poorer cognitive control (as assessed with EF task performance) to dependent stress, to rumination, to depression and anxiety symptoms. In addition, Study 2 suggests that the link between EF task performance and stress increases with age from early adolescence to young adulthood, a topic we return to in Future Directions. While Study 2 was cross-sectional, these findings conceptually replicate the longitudinal findings from Study 1 using different methods and timeframes.
Thus, despite potential differences in the constructs assessed by self-report questionnaire versus EF task measures of cognitive control, in this case these different ways of conceptualizing and measuring cognitive control yielded strikingly convergent evidence for the same risk pathways. Nonetheless, it is important to keep in mind the possibility that different measures of cognitive control could potentially participate in the same pathways through different specific mechanisms. Future longitudinal research with EF task measures will thus be important for clarifying the mechanisms by which different aspects of cognitive control contribute to psychopathology risk over time.
In most models, there were also effects of dependent stress directly on internalizing symptoms, independent of the significant indirect pathway through rumination. This finding is consistent with evidence that stress increases depression and anxiety via multiple mechanisms in addition to rumination, including reduced adaptive coping, self-esteem and self-efficacy (e.g., see Grant et al., 2014 for review), stress physiology mechanisms (e.g., Doom & Gunnar, 2013), sleep problems (e.g., see Van Reeth et al., 2000 for review), and inflammatory pathways (e.g., see Slavich & Irwin, 2014 for review). Thus, stress generation related to poor cognitive control may increase risk for internalizing psychopathology via multiple final mediating mechanisms. The current research has implications for models of psychopathology risk and for interventions, and suggests promising directions for future research.
Mediating Role of Stress
Mediation by stress generation could potentially explain why cognitive control impairments are so broadly associated with psychopathology (Buckholtz & Meyer-Lindenberg, 2012; Goschke, 2014; Snyder et al., 2015), since stress is a transdiagnostic risk factor (e.g., Grant et al., 2014). Furthermore, the existence of common risk pathways for anxiety and depression, such as those identified in the current study, may be one source of comorbidity between them (e.g., Cummings et al., 2014).
Future research is needed to better understand the specific mechanisms involved in this mediation pathway, at multiple levels of analysis. There may be different mechanisms involved for different aspects of cognitive control and different types of stressors. For example, it is possible that poor goal management and planning might lead to generation of achievement stressors (e.g., failing an exam because of failure to plan ahead to study or stay focused while studying). Interpersonal stressors might be related to other types of cognitive control problems, such as poor ability to stop automatic but counterproductive social behaviors (e.g., yelling at a parent or peer during an argument), or poor affective theory of mind, which is related to cognitive control in adolescents (Vetter, Altgassen, Phillips, Mahy, & Kliegel, 2013).
Future research is also need to explore additional mechanisms by which cognitive control could affect stress. Specifically, the current research demonstrates that individuals with poor cognitive control experience more dependent stressors. However, some evidence indicates cognitive control may also be associated with different responses to stress. For example, recent studies have found that individuals with poor executive function respond to acute laboratory social stressors with a greater physiological stress response (cortisol and skin conductance) and greater negative affect (Hendrawan, Yamakawa, Kimura, Murakami, & Ohira, 2012), and respond to daily stressors with greater negative affect and reduced problem solving to cope with stress (Compton et al., 2011). Likewise, lower self-reported cognitive control predicts a greater cortisol response and increased subjective arousal and unpleasantness (Oldehinkel, Hartman, Nederhof, Riese, & Ormel, 2011). In addition, prefrontal mechanisms are critical for the perception of stress controllability, reducing the physiological stress response when stressors are perceived as controllable (e.g., Varela, Wang, Christianson, Maier, & Cooper, 2012). This raises the possibility that poor cognitive control may be associated with increased stress responses via lower perceived stress controllability.
It is also possible that once a stressor has occurred, additional attentional control mechanisms play a role in reactivity to the stressor, further mediating the link between poor cognitive control and psychopathology. For example, attention biases towards negative aspects of a stressful situation or difficulty disengaging attention from stressors may lead to a greater psychological and/or physiological stress response, increasing risk for psychopathology. In addition, rumination itself may prolong or re-activate physiological stress responses (e.g., Brosschot, 2010), which may help mediate the link between rumination and psychopathology. Thus, it seems likely that cognitive control may affect both the generation of stressful life events and the physiological and psychological reactivity to those events.
In addition, stress and cognitive control may bi-directionally affect one another. Specifically, there is evidence that laboratory stressors can impair cognitive control, especially when they are perceived as severe and uncontrollable (Henderson, Snyder, Gupta, & Banich, 2012), and that poor post-stress cognitive control is associated with depression symptoms in individuals who tend to ruminate (Quinn & Joormann, 2015). While a full exploration of such transactional effects is beyond the scope of the current paper, we tested a reversed model for Study 1, in which symptoms at T1 where used to predict effortful control at T4, via stress generation and rumination (Supplemental Materials Tables S11–S12). For depression, there was a significant indirect pathway from depression to increased dependent stress to decreased effortful control (the pathway through rumination did not reach significance; Table S11). Thus, for depression this pathway is likely bidirectional, potentially leading to a positive feedback loop of increasing depression and decreasing cognitive control over time. Support was not found for this reversed pathway for anxiety (Table S12), consistent with previous evidence that depressive symptoms play a stronger role in stress generation than anxious symptoms (e.g., Connolly et al., 2010; but see Shapero et al., 2013 for independent effects of depression and anxiety symptoms on stress generation). Investigating such transactional pathways and how they may differ between depression and anxiety is a promising direction for future research.
Mediating Role of Rumination
Contemporary theories posit that poor cognitive control directly leads to increased rumination (Gotlib & Joorman, 2010; Koster et al., 2011; Whitmer & Gotlib, 2013). Our findings challenge and suggest a modification to these models, in that rumination did not directly mediate the link between cognitive control and psychopathology in the current studies. Instead cognitive control has indirect effects on rumination via dependent stress generation. Importantly, previous studies of cognitive control and rumination, including the only previous mediation analysis (Demeyer et al., 2012), have primarily found links between rumination and specific aspects of cognitive control over negative information. Other types of cognitive control are unimpaired or even enhanced in ruminators (for review see Whitmer & Gotlib, 2013).
Thus, it is possible that rumination directly mediates links between cognitive control over affective information, or specific cognitive control processes (e.g., controlling the affective contents and scope of working memory) and psychopathology, while broad impairments in non-affective “cold” cognitive control are associated with rumination only indirectly through stress generation. Future research on this distinction could potentially help to explain the mixed evidence for cognitive control-rumination links in the literature, and elucidate the multiple mechanisms by which poor cognitive control could confer risk for psychopathology. In addition, future research would benefit from examining the role of different specific forms of rumination (e.g., brooding vs. reflection) as well as other forms of repetitive negative thinking (e.g., worry).
Translational Implications
The current research has implications for interventions aimed at treating or preventing depression and anxiety. It is important to note that the current studies took a dimensional approach, assessing anxiety and depression symptoms in a general community sample. Thus, these findings will need to be confirmed in clinical samples prior to definitive efforts to translate them to practice in clinical settings. Nonetheless, better understanding the mediating mechanisms between poor cognitive control and internalizing psychopathology has the potential to inform new targets for intervention.
There has been a great deal of interest in cognitive control training as a potential prevention or treatment strategy. However, thus far there is little evidence that directly training executive function (i.e., targeting the weakness rather than compensatory strategies) effectively generalizes to real-world function or improves clinical symptoms (for review see Rabipour & Raz, 2012), although there is some evidence of that cognitive control training can transfer to other cognitive control tasks in individuals with high depressive symptoms, suggesting further transfer may be possible (Owens, Koster, & Derakshan, 2013). The current research suggests that interventions aimed at disrupting the link between poor cognitive control and stress generation might be a more promising approach. That is, training on compensatory strategies and providing adolescents with additional supports (e.g., for time management, organization and planning when doing school work) could mitigate the effects of poor cognitive control to reduce stress (Cicerone, Levin, Malec, & Stuss, 2006). There is little research on the effectiveness of such compensatory strategies in individuals with depression or anxiety disorders, although it is intriguingly suggestive that some therapies such as behavioral activation (e.g., Dimidjian, Barrera, Martell, Muñoz, & Lewinsohn, 2011) incorporate compensatory strategies (e.g., cues to engage in a desired activity).
Future research is needed to determine if the effectiveness of these approaches, as well as other interventions which have been shown to affect prefrontal function (e.g., mindfulness; Tang, Holzel, & Posner, 2015; exercise; Hillman, Erikson, & Kramer, 2008) are mediated by reductions in stress generation, and whether they might be effective in preventing internalizing psychopathology in at risk youth. In addition, future translational research will need to take developmental trajectories into account. For example, some youth may have a cognitive developmental trajectory that is delayed but eventually catches up to their peers, while others remain impaired (e.g., Luu, Vohr, Allan, Schneider, & Ment, 2011). Thus, some youth may only need compensatory strategies to bridge the developmental gap until their cognitive control matures, while others may need to continue using such strategies to compensate for poor cognitive control.
Key Future Directions
The findings of the current studies suggest a number of directions for future research, including those we have briefly discussed above. Here, we highlight two key directions we see as particularly critical to advancing this area of study.
Integrating modern models of cognitive control and psychopathology
First, integrating recent developments in how the structure of both psychopathology and cognitive control are conceptualized and modeled has the potential to greatly clarify the specificity and generality of the links between them (e.g., Snyder et al., 2015). Dimensional models of psychopathology, including both a general psychopathology factor (i.e., p factor) that spans common psychopathologies in addition to specific internalizing and externalizing latent factors, have recently gained prominence (e.g., Caspi et al., 2014; Laceulle, Vollebergh, & Ormel, 2015; Lahey et al., 2012). Like psychopathology, structural models of cognitive control (e.g., unity/diversity model; Friedman et al., 2008; Miyake and Friedman, 2012) highlight that there is a general common EF, posited to be the ability to actively maintain task goals and use this information to provide top–down support for task-relevant responses, and specific EF abilities.
Broad impairment across most EF tasks is associated with both internalizing and externalizing psychopathology (e.g., see Snyder et al., 2015 for review), consistent with the notion that common EF is associated with common psychopathology (i.e., the p factor). This suggests the hypothesis that the pathways demonstrated in the current studies may reflect associations between common EF and the p factor. Indeed, preliminary evidence suggests that the p factor is associated with poorer performance on some EF tasks, and self-reported cognitive and self-control problems (Caspi et al., 2014). However, no study has investigated risk pathways in conjunction with p factor models of psychopathology or latent dimensional models of EF.
The current studies show that the same risk pathways link poor performance on a composite measure of EF tasks to both depressive and anxiety symptoms. This pattern could be consistent with an association between EF and either unique latent internalizing psychopathology or to the p factor, or possibly to both. However, the current research cannot differentiate these important hypotheses. Future research is needed to directly test the hypothesis that common EF may be a liability factor for common psychopathology, by using latent bifactor models of both EF (e.g., Miyake and Friedman, 2012) and psychopathology (e.g., Caspi et al., 2014). It is also possible that individuals with psychopathology have processing-specific impairments in specific aspects of EF (e.g., shifting, working memory updating, e.g., Friedman et al., 2008) in addition to deficits in common EF. These deficits could increase risk for psychopathology via either shared or distinct mediating mechanisms. Such future inquiry can clarify the nature of risk pathways between EF impairments and particular forms of psychopathology, and accelerate progress in understanding how EF impairments contribute to comorbidity across disorders.
Understanding how risk pathways unfold and change across development
Both cognitive control and psychopathology change greatly across adolescence and into young adulthood. Thus, it is likely that risk pathways between cognitive control and psychopathology change with development. Indeed, in Study 2, the relationship between poor EF and stress generation increased with age. This may have occurred because adult caretakers may partly compensate for younger adolescents’ poor EF (e.g., reminders to complete homework), and thus prevent poor EF from being translated into behaviors that lead to stress (e.g., bad grades) among younger aged youth. Future research is needed to investigate this possibility and disentangle maturational from environmental support explanations for these age effects.
Conclusion
The current research proposed and found support for a novel process model linking poor cognitive control to internalizing psychopathology (depression and anxiety symptoms) in youth via generation of more dependent stressful life events and subsequent rumination. While it is possible that rumination directly mediates links between specific cognitive control processes (e.g., controlling negative information in working memory) and psychopathology, the current research suggests that broad impairments in cognitive control are associated with rumination only indirectly through stress generation. Given that stress is a transdiagnostic risk factor for psychopathology (e.g., Grant et al., 2014), the role of poor cognitive control in stress generation could potentially explain why cognitive control impairments are so broadly associated with psychopathology (Buckholtz & Meyer-Lindenberg, 2012; Goschke, 2014; Hankin et al., 2015). This research is an important step in understanding the developmental pathways leading to depression and anxiety in youth, and raises the possibility that interventions aimed at disrupting the link between poor cognitive control and stress could be powerful tools for preventing the development of internalizing psychopathology.
Supplementary Material
Acknowledgments
This research and preparation of this manuscript was supported by grants from the National Institute of Mental of Health (R01MH077195 to B.L.H, R21MH102210 to B.L.H. and H.R.S., and F32MH098481 & T32MH15442 to H.R.S), the University of Colorado Denver Developmental Psychobiology Endowment Fund (to B.L.H. and H.R.S), and a University of Denver PROF award (to B.L.H). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. We thank the many members of the GEM Lab for data collection and helpful discussion, and Naomi Friedman and Akira Miyake for sharing tasks used in Study 2.
Footnotes
Author Contributions
B.L.H designed Study 1 and H.R.S and B.L.H designed Study 2. B.L.H supervised data collection for Study 1 and B.L.H and H.R.S. supervised data collection for Study 2. H.R.S. performed the analyses and drafted the paper. B.L.H provided critical revisions. Both authors approved the final version of the paper for submission.
References
- Aalto-Setälä T, Marttunen M, Tuulio-Henriksson A, Poikolainen K, Lönnqvist J. Depressive symptoms in adolescence as predictors of early adulthood depressive disorders and maladjustment. American Journal of Psychiatry. 2014;159:1235–1237. doi: 10.1176/appi.ajp.159.7.1235. http://doi.org/10.1176/appi.ajp.159.7.1235. [DOI] [PubMed] [Google Scholar]
- Abela JRZ, Rochon A, Vanderbilt E. The Children’s Response Styles Questionnaire. Unpublished Questionnaire. 2000 [Google Scholar]
- Altamirano LJ, Miyake A, Whitmer AJ. When mental inflexibility facilitates executive control: Beneficial side effects of ruminative tendencies on goal maintenance. Psychological Science. 2010;21:1377–1382. doi: 10.1177/0956797610381505. http://doi.org/10.1177/0956797610381505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Fischer M. Predicting impairment in major life activities and occupational functioning in hyperactive children as adults: Self-reported executive function (EF) deficits versus EF tests. Developmental Neuropsychology. 2011;36:137–161. doi: 10.1080/87565641.2010.549877. http://doi.org/10.1080/87565641.2010.549877. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. The Psychiatric Clinics of North America. 2009;32:483–524. doi: 10.1016/j.psc.2009.06.002. http://doi.org/10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggren N, Derakshan N. Attentional control deficits in trait anxiety: Why you see them and why you don’t. Biological Psychology. 2013;92:440–446. doi: 10.1016/j.biopsycho.2012.03.007. http://doi.org/10.1016/j.biopsycho.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Best JR, Miller PH, Jones LL. Executive functions after age 5: Changes and correlates. Developmental Review. 2009;29:180–200. doi: 10.1016/j.dr.2009.05.002. http://doi.org/10.1016/j.dr.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosschot JF. Markers of chronic stress: Prolonged physiological activation and (un)conscious perseverative cognition. Neuroscience and Biobehavioral Reviews. 2010;35:46–50. doi: 10.1016/j.neubiorev.2010.01.004. http://doi.org/10.1016/j.neubiorev.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Buckholtz JW, Meyer-Lindenberg A. Psychopathology and the human connectome: Toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. http://doi.org/10.1016/j.neuron.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Carragher N, Krueger RF, Eaton NR, Slade T. Disorders without borders: Current and future directions in the meta-structure of mental disorders. Social Psychiatry and Psychiatric Epidemiology. 2015;50:339–350. doi: 10.1007/s00127-014-1004-z. http://doi.org/10.1007/s00127-014-1004-z. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Moffitt TE. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2:119–137. doi: 10.1177/2167702613497473. http://doi.org/10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chorpita BF, Tracey SA, Brown TA, Collica TJ, Barlow DH. Assessment of worry in children and adolescents: An adaptation of the Penn State Worry Questionnaire. Behavioral Research and Therapy. 1997;35:569–581. doi: 10.1016/s0005-7967(96)00116-7. http://doi.org/10.1016/S0005-7967(96)00116-7. [DOI] [PubMed] [Google Scholar]
- Cicerone K, Levin H, Malec J, Stuss D. Cognitive rehabilitation interventions for executive function: moving from bench to bedside in patients with traumatic brain injury. Journal of Cognitive Neuroscience. 2006;18:1212–1222. doi: 10.1162/jocn.2006.18.7.1212. http://doi.org/10.1162/jocn.2006.18.7.1212. [DOI] [PubMed] [Google Scholar]
- Compton RJ, Arnstein D, Freedman G, Dainer-Best J, Liss A, Robinson MD. Neural and behavioral measures of error-related cognitive control predict daily coping with stress. Emotion. 2011;11:379–390. doi: 10.1037/a0021776. http://doi.org/10.1037/a0021776. [DOI] [PubMed] [Google Scholar]
- Connolly NP, Eberhart NK, Hammen CL, Brennan PA. Specificity of stress generation: A Comparison of adolescents with depressive, anxiety, and comorbid diagnoses. International Journal of Cognitive Therapy. 2010;3:368–379. doi: 10.1521/ijct.2010.3.4.368. http://doi.org/10.1521/ijct.2010.3.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly SL, Wagner CA, Shapero BG, Pendergast LL, Abramson LY, Alloy LB. Rumination prospectively predicts executive functioning impairments in adolescents. Journal of Behavior Therapy and Experimental Psychiatry. 2014;45:46–56. doi: 10.1016/j.jbtep.2013.07.009. http://doi.org/10.1016/j.jbtep.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Bunting MF, Hambrick DZ, Wilhelm O, Engle RW. Working memory span tasks: A methodological review and user’s guide. Psychonomic Bulletin & Review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- Conway CC, Hammen C, Brennan PA. Expanding stress generation theory: Test of a transdiagnostic model. Journal of Abnormal Psychology. 2012;121:754–766. doi: 10.1037/a0027457. http://doi.org/10.1037/a0027457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin. 2014;140:816–845. doi: 10.1037/a0034733. http://doi.org/10.1037/a0034733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeyer I, De Lissnyder E, Koster EHW, De Raedt R. Rumination mediates the relationship between impaired cognitive control for emotional information and depressive symptoms: A prospective study in remitted depressed adults. 2012;50:292–297. doi: 10.1016/j.brat.2012.02.012. http://doi.org/10.1016/j.brat.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Eysenck M. Anxiety, processing efficiency, and cognitive performance: New developments from attentional control theory. European Psychologist. 2009;14:168–176. [Google Scholar]
- Dimidjian S, Barrera M, Martell C, Muñoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annual Review of Clinical Psychology. 2011;7:1–38. doi: 10.1146/annurev-clinpsy-032210-104535. http://doi.org/10.1146/annurev-clinpsy-032210-104535. [DOI] [PubMed] [Google Scholar]
- Doom JR, Gunnar MR. Stress physiology and developmental psychopathology: Past, present, and future. Development and Psychopathology. 2013;25:1359–1373. doi: 10.1017/S0954579413000667. http://doi.org/10.1017/S0954579413000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Spinrad TL, Liew J, Zhou Q, Losoya SH, Cumberland A. Longitudinal relations of children’s effortful control, impulsivity, and negative emotionality to their externalizing, internalizing, and co-occurring behavior problems. Developmental Psychology. 2009;45:988–1008. doi: 10.1037/a0016213. http://doi.org/10.1037/a0016213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LK, Rothbart MK. Revision of the early adolescent temperament questionnaire; Poster Presented at the 2001 Biennial Meeting of the Society for Research in Child Development; Minneapolis, MN. 2001. [Google Scholar]
- Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. Journal of Experimental Psychology: General. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. http://doi.org/10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galla BM, Wood JJ. Trait self-control predicts adolescents’ exposure and reactivity to daily stressful events. Journal of Personality. 2015;83:69–83. doi: 10.1111/jopy.12083. http://doi.org/10.1111/jopy.12083. [DOI] [PubMed] [Google Scholar]
- Goschke T. Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: Advances, gaps, and needs in current research. International Journal of Methods in Psychiatric Research. 2014;23:41–57. doi: 10.1002/mpr.1410. http://doi.org/10.1002/mpr.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. http://doi.org/10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KE, McMahon SD, Carter JS, Carleton RA, Adam EK, Chen E. Handbook of Developmental Psychopathology. Boston, MA: Springer US; 2014. The influence of stressors on the development of psychopathology; pp. 205–223. http://doi.org/10.1007/978-1-4614-9608-3_11. [Google Scholar]
- Hamilton JL, Stange JP, Shapero BG, Connolly SL, Abramson LY, Alloy LB. Cognitive vulnerabilities as predictors of stress generation in early adolescence: Pathway to depressive symptoms. Journal of Abnormal Child Psychology. 2013;41:1027–1039. doi: 10.1007/s10802-013-9742-z. http://doi.org/10.1007/s10802-013-9742-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Generation of stress in the course of unipolar depression. Journal of Abnormal Psychology. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. http://doi.org/10.1037/0021-843X.100.4.555. [DOI] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annual Review of Clinical Psychology. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. http://doi.org/10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Hankin BL. Personality and depressive symptoms: Stress generation and cognitive vulnerabilities to depression in a prospective daily diary study. Journal of Social and Clinical Psychology. 2010;29:369–401. doi: 10.1521/jscp.2010.29.4.369. http://doi.org/10.1521/jscp.2010.29.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. http://doi.org/10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY. Measuring cognitive vulnerability to depression in adolescence: Reliability, validity, and gender differences. Journal of Clinical Child & Adolescent Psychology. 2002;31:491–504. doi: 10.1207/S15374424JCCP3104_8. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Young JF, Abela JRZ, Smolen A, Jenness JL, Gulley LD, et al. Depression from childhood into late adolescence: Influence of gender, development, genetic susceptibility, and peer stress. Journal of Abnormal Psychology. doi: 10.1037/abn0000089. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson RK, Snyder HR, Gupta T, Banich MT. When does stress help or harm? The effects of stress controllability and subjective stress response on Stroop performance. Frontiers in Psychology. 2012;3:179. doi: 10.3389/fpsyg.2012.00179. http://doi.org/10.3389/fpsyg.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrawan D, Yamakawa K, Kimura M, Murakami H, Ohira H. Executive functioning performance predicts subjective and physiological acute stress reactivity: Preliminary results. International Journal of Psychophysiology. 2012;84:277–283. doi: 10.1016/j.ijpsycho.2012.03.006. http://doi.org/10.1016/j.ijpsycho.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9:58–65. doi: 10.1038/nrn2298. http://doi.org/10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- Jacobson LA, Williford AP, Pianta RC. The role of executive function in children’s competent adjustment to middle school. Child Neuropsychology. 2011;17:255–280. doi: 10.1080/09297049.2010.535654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117:182–192. doi: 10.1037/0021-843X.117.1.182. http://doi.org/10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Joormann J, Nee DE, Berman MG, Jonides J, Gotlib IH. Interference resolution in major depression. Cognitive Affective & Behavioral Neuroscience. 2010;10:21–33. doi: 10.3758/CABN.10.1.21. http://doi.org/10.3758/CABN.10.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just N, Abramson LY, Alloy LB. Remitted depression studies as tests of the cognitive vulnerability hypotheses of depression onset. Clinical Psychology Review. 2001;21:63–83. doi: 10.1016/s0272-7358(99)00035-5. http://doi.org/10.1016/S0272-7358(99)00035-5. [DOI] [PubMed] [Google Scholar]
- Kessler R, Berglund P, Demler O, Jin R, Merikangas K, Walters E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. http://doi.org/10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review. 2011;31:138–145. doi: 10.1016/j.cpr.2010.08.005. http://doi.org/10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Kovacs M. The Children’s Depression Inventory. 1983 [Google Scholar]
- Laceulle OM, Vollebergh WAM, Ormel J. The Structure of psychopathology in adolescence: Replication of a general psychopathology factor in the TRAILS Study. Clinical Psychological Science. 2015;3:850–860. http://doi.org/10.1177/2167702614560750. [Google Scholar]
- Lahey BB, Applegate B, Hakes JK, Zald DH, Hariri AR, Rathouz PJ. Is there a general factor of prevalent psychopathology during adulthood? Journal of Abnormal Psychology. 2012;121:971–977. doi: 10.1037/a0028355. http://doi.org/10.1037/a0028355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letkiewicz AM, Miller GA, Crocker LD, Warren SL, Infantolino ZP, Mimnaugh KJ, Heller W. Executive function deficits in daily life prospectively predict increases in depressive symptoms. Cognitive Therapy and Research. 2014;38:612–620. doi: 10.1007/s10608-014-9629-5. http://doi.org/10.1007/s10608-014-9629-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew J. Effortful control, executive functions, and education: Bringing self-regulatory and social-emotional competencies to the table. Child Development Perspectives. 2012;6:105–111. [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. http://doi.org/10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Luu TM, Vohr BR, Allan W, Schneider KC, Ment LR. Evidence for catch-up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics. 2011;128:313–322. doi: 10.1542/peds.2010-2655. http://doi.org/10.1542/peds.2010-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK. The Multidimensional Anxiety Scale for Children (MASC): Factor structure, reliability, and validity. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:554–565. doi: 10.1097/00004583-199704000-00019. http://doi.org/10.1097/00004583-199704000-00019. [DOI] [PubMed] [Google Scholar]
- Martel MM, Nigg JT, Wong MM, Fitzgerald HE, Jester JM, Zucker RA. Childhood and adolescent resiliency, regulation, and executive functioning in relation to adolescent problems and competence in a high-risk sample. Development and Psychopathology. 2007;19:541–563. doi: 10.1017/S0954579407070265. http://doi.org/10.1017/S0954579407070265. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Cha DS, Soczynska JK, Woldeyohannes HO, Gallaugher LA, Anusha B. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depression and Anxiety. 2013;30:515–527. doi: 10.1002/da.22063. http://doi.org/10.1002/da.22063. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Nolen-Hoeksema S. Interpersonal stress generation as a mechanism linking rumination to internalizing symptoms in early adolescents. Journal of Clinical Child & Adolescent Psychology. 2012;41:584–597. doi: 10.1080/15374416.2012.704840. http://doi.org/10.1080/15374416.2012.704840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. http://doi.org/10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michl LC, McLaughlin KA, Shepherd K, Nolen-Hoeksema S. Rumination as a mechanism linking stressful life events to symptoms of depression and anxiety: Longitudinal evidence in early adolescents and adults. Journal of Abnormal Psychology. 2013;122:339–352. doi: 10.1037/a0031994. http://doi.org/10.1037/a0031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP. The nature and organization of individual differences in executive functions: Four general conclusions. Current Directions in Psychological Science. 2012;21:8–14. doi: 10.1177/0963721411429458. http://doi.org/10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Ollendick TH. The role of temperament in the etiology of child psychopathology. Clinical Child and Family Psychology Review. 2005;8:271–289. doi: 10.1007/s10567-005-8809-y. http://doi.org/10.1007/s10567-005-8809-y. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- Ogilvie JM, Stewart AL, Chan RCK, Shum DHK. Neuropsychological measures of executive function and antisocial behavior: A meta-analysis. Criminology. 2011;49:1063–1107. http://doi.org/10.1111/j.1745-9125.2011.00252.x. [Google Scholar]
- Oldehinkel AJ, Hartman CA, Ferdinand RF, Verhulst FC, Ormel J. Effortful control as modifier of the association between negative emotionality and adolescents’ mental health problems. Development and Psychopathology. 2007;19:523–539. doi: 10.1017/S0954579407070253. http://doi.org/10.1017/S0954579407070253. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Hartman CA, Nederhof E, Riese H, Ormel J. Effortful control as predictor of adolescents’ psychological and physiological responses to a social stress test: The Tracking Adolescents’ Individual Lives Survey. Development and Psychopathology. 2011;23:679–688. doi: 10.1017/S0954579411000095. http://doi.org/10.1017/S0954579411000095. [DOI] [PubMed] [Google Scholar]
- Owens M, Koster EHW, Derakshan N. Impaired filtering of irrelevant information in dysphoria: an ERP study. Social Cognitive and Affective Neuroscience. 2012;7:752–763. doi: 10.1093/scan/nsr050. http://doi.org/10.1093/scan/nsr050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M, Koster EHW, Derakshan N. Improving attention control in dysphoria through cognitive training: Transfer effects on working memory capacity and filtering efficiency. Psychophysiology. 2013;50:297–307. doi: 10.1111/psyp.12010. http://doi.org/10.1111/psyp.12010. [DOI] [PubMed] [Google Scholar]
- Quinn ME, Joormann J. Stress-induced changes in executive control are associated with depression symptoms: Examining the role of rumination. Clinical Psychological Science. 2015 Advance online publication. http://doi.org/10.1177/2167702614563930. [Google Scholar]
- Rabipour S, Raz A. Training the brain: Fact and fad in cognitive and behavioral remediation. Brain and Cognition. 2012;79:159–179. doi: 10.1016/j.bandc.2012.02.006. http://doi.org/10.1016/j.bandc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Reineberg AE, Andrews-Hanna JR, Depue BE, Friedman NP, Banich MT. Resting-state networks predict individual differences in common and specific aspects of executive function. NeuroImage. 2015;104:69–78. doi: 10.1016/j.neuroimage.2014.09.045. http://doi.org/10.1016/j.neuroimage.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK, Bates EA. Temperament. In: Damon W, Eisenberg N, Lerner RM, editors. Handbook of Child Psychology, Social, Emotional, and Personality Development. 6. Vol. 3. Hoboken, NJ: Wiley; 2006. p. 1232. [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: Influence of gender and pubertal status. Development and Psychopathology. 2007;19:497–521. doi: 10.1017/S0954579407070241. http://doi.org/10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapero BG, Hankin BL, Barrocas AL. Stress generation and exposure in a multi-wave study of adolescents: Transactional processes and sex differences. Journal of Social and Clinical Psychology. 2013;32:989–1012. doi: 10.1521/jscp.2013.32.9.989. http://doi.org/10.1521/jscp.2013.32.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavich GM, Irwin MR. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin. 2014;140:774–815. doi: 10.1037/a0035302. http://doi.org/10.1037/a0035302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JM, Alloy LB. A roadmap to rumination: A review of the definition, assessment, and conceptualization of this multifaceted construct. Clinical Psychology Review. 2009;29:116–128. doi: 10.1016/j.cpr.2008.10.003. http://doi.org/10.1016/j.cpr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin. 2013;139:81–132. doi: 10.1037/a0028727. http://doi.org/10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Hutchison N, Nyhus E, Curran T, Banich MT, O’Reilly RC, Munakata Y. Neural inhibition enables selection during language processing. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16483–16488. doi: 10.1073/pnas.1002291107. http://doi.org/10.1073/pnas.1002291107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: Bridging the gap between clinical and cognitive approaches. Frontiers in Psychology. 2015;6:1–24. doi: 10.3389/fpsyg.2015.00328. http://doi.org/10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y-Y, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. Nature Reviews Neuroscience. 2015;16:213–225. doi: 10.1038/nrn3916. http://doi.org/10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- Technow JR, Hazel NA, Abela JRZ, Hankin BL. Stress sensitivity interacts with depression history to predict depressive symptoms among youth: Prospective changes following first depression onset. Journal of Abnormal Child Psychology. 2015;43:489–501. doi: 10.1007/s10802-014-9922-5. http://doi.org/10.1007/s10802-014-9922-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, West RF, Stanovich KE. Practitioner review: Do performance-based measures and ratings of executive function assess the same construct? Journal of Child Psychology and Psychiatry. 2012;54:131–143. doi: 10.1111/jcpp.12001. http://doi.org/10.1111/jcpp.12001. [DOI] [PubMed] [Google Scholar]
- van Oort F, Greaves Lord K, Ormel J, Verhulst FC, Huizink AC. Risk indicators of anxiety throughout adolescence: The TRAILS study. Depression and Anxiety. 2011;28:485–494. doi: 10.1002/da.20818. http://doi.org/10.1002/da.20818. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Weibel L, Spiegel K, Leproult R, Dugovic C, Maccari S. Interactions between stress and sleep: From basic research to clinical situations. Sleep Medicine Reviews. 2000;4(2):201–219. http://doi.org/10.1053/smrv.1999.0097. [Google Scholar]
- Varela JA, Wang J, Christianson JP, Maier SF, Cooper DC. Control over stress, but not stress per se increases prefrontal cortical pyramidal neuron excitability. Journal of Neuroscience. 2012;32:12848–12853. doi: 10.1523/JNEUROSCI.2669-12.2012. http://doi.org/10.1523/JNEUROSCI.2669-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter NC, Altgassen M, Phillips L, Mahy CEV, Kliegel M. Development of affective theory of mind across adolescence: Disentangling the role of executive functions. Developmental Neuropsychology. 2013;38:114–125. doi: 10.1080/87565641.2012.733786. http://doi.org/10.1080/87565641.2012.733786. [DOI] [PubMed] [Google Scholar]
- Whitmer AJ, Banich MT. Inhibition versus switching deficits in different forms of rumination. Psychological Science. 2007;18:546–553. doi: 10.1111/j.1467-9280.2007.01936.x. http://doi.org/10.1111/j.1467-9280.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- Whitmer AJ, Gotlib IH. An attentional scope model of rumination. Psychological Bulletin. 2013;139:1036–1061. doi: 10.1037/a0030923. http://doi.org/10.1037/a0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox RR, Keselman HJ. Modern robust data analysis methods: Measures of central tendency. Psychological Methods. 2003;8:254–274. doi: 10.1037/1082-989X.8.3.254. http://doi.org/10.1037/1082-989X.8.3.254. [DOI] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: Implications for stress regulation. Annals of Behavioral Medicine. 2009;37:126–140. doi: 10.1007/s12160-009-9100-0. http://doi.org/10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: Liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. Journal of Abnormal Psychology. 2009;118:117–130. doi: 10.1037/a0014657. http://doi.org/10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche U, Joormann J. Components of interference control predict depressive symptoms and rumination cross-sectionally and at six months follow-up. Journal of Behavior Therapy and Experimental Psychiatry. 2011;42:65–73. doi: 10.1016/j.jbtep.2010.06.001. http://doi.org/10.1016/j.jbtep.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Zetsche U, D’Avanzato C, Joormann J. Depression and rumination: Relation to components of inhibition. Cognition & Emotion. 2012;26:758–767. doi: 10.1080/02699931.2011.613919. http://doi.org/10.1080/02699931.2011.613919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.