Abstract
Purpose
Strabismus correction surgery is well documented in both the literature and practice with varying levels of success and permanence. Our goal was to characterize longitudinal changes in eye alignment and eye movements following strabismus correction surgery in a monkey model for developmental strabismus.
Methods
We studied two juvenile rhesus monkeys with exotropia previously induced via an optical prism-rearing paradigm in infancy. Eye misalignment was corrected via a resection–recession surgery of the horizontal rectus muscles of one eye. Binocular search coils were used to collect eye movement data during smooth-pursuit, saccades, and fixation tasks before surgical treatment, immediately after surgery, and through 6 months after treatment.
Results
Both animals showed an immediate ∼70% reduction in misalignment as a consequence of surgery that regressed to a 20%–40% improvement by 6 months after treatment. Significant changes were observed in saccade and smooth-pursuit gain of the nonviewing eye after surgery, which also reverted to presurgical values by 6 months. A temporary improvement in fixation stability of the nonviewing eye was observed after surgery; naso-temporal (N/T) asymmetry of monocular smooth-pursuit remained unchanged.
Conclusions
Surgical realignment is followed by plastic changes that often lead to reversal of surgery effects. Immediate improvement in misalignment and changes in eye movement gains are likely a result of contractility changes at the level of the extraocular muscle, whereas longer-term effects are likely a combination of neural and muscle adaptation.
Keywords: strabismus, resection, recession, eye movements, nonhuman primate
Strabismus is a development disorder that affects ∼2%–4% of children worldwide.1–3 Although the exact etiology of strabismus is often unknown, a disruption of binocular vision in infancy likely leads to eye misalignment, a strategy also used to develop animal models for strabismus.4–6 These animal models have been very effective in replicating a variety of strabismus oculomotor features beyond just horizontal misalignment, such as dissociated deviations, latent nystagmus, fixation instability, fixation switch behavior, naso-temporal asymmetry of horizontal smooth-pursuit and optokinetic nystagmus, and disconjugate eye movements.6–16 Studies in animal models have also led to several insights into neural strabismus mechanisms including identifying neural substrates for misalignment, A/V patterns, and saccade disconjugacies. For example, previous studies from our laboratory and others have shown that, in monkeys with a sensory strabismus, an innervational drive from the motor nuclei (oculomotor and abducens nucleus) plays a critical role in setting the state of ocular misalignment on a moment-to-moment basis.17,18
In addition to investigating basic mechanisms for strabismus, the availability of appropriate animal models provides an outstanding opportunity to study treatment strategies for the disorder. One of the most prevalent approaches to correct misalignment is to surgically alter extraocular muscle (EOM) function. Strabismus correction surgery is well documented in the literature and practice with varying levels of permanence.19,20 By various accounts, approximately 40% of the subjects that undergo surgery, develop significant recurrent strabismus, and may have to undergo multiple surgeries.21,22 Although it is not known why surgery sometimes works and sometimes does not, it is possible to speculate that both central (brain) and peripheral (muscle) factors influence the outcome of strabismus correction surgery. Muscle remodeling has been shown to occur on surgical manipulation of EOM in rabbits and in monkey.23–25 For example, sarcomeres are added or removed when eye muscles were detached from the globe and attached to the orbit for a few days.25,26 It is also likely that eye movement properties and visual experience following surgery influences the outcome of surgery via neural mechanisms.27–30 Similarly proprioceptive feedback following surgery could also influence central oculomotor circuits that are innervating the EOM.31,32
Thus, the overall goal of our research is to gain insight into neural and muscle adaptive mechanisms that are triggered following strabismus correction surgery. In this particular study, we examined the longitudinal changes in eye alignment and eye movements that occur in monkey models of strabismus following a typical strabismus correction surgery to provide a framework to interpret future neural investigation.
Methods
Subjects and Rearing Paradigms
Behavioral and neurophysiological data were collected from two exotropic juvenile rhesus (Macaca mulatta) monkeys (∼6 years, ∼9–10 kg). Strabismus was previously induced by optical prism-rearing of infant monkeys.4 During the prism-rearing paradigm, infant monkeys wore a helmet like device that housed a horizontally oriented Fresnel prism in front of one eye and a vertically oriented Fresnel prism (20 prism diopter) in front of the other eye, starting from day 1 after birth to 4 months of age, after which they were allowed unrestricted vision. Disruption of binocular vision due to prism-viewing during this initial period leads to strabismus as it is the critical period for development of eye alignment, stereopsis, and binocular sensitivity.33–35
Surgical Procedures
All procedures were carried out in accordance with National Institutes of Health guidelines and were approved by the University of Houston Institutional Animal Care and Use Committee. Prior to commencement of experiments associated with this study, each juvenile monkey (∼4 years of age) underwent surgical procedures carried out under aseptic conditions under isoflurane anesthesia (1.25%–2.5%). In the first surgery, a head stabilization post was implanted to restrict head movements during the experiments.36 In subsequent procedures, scleral search coils were implanted in both eyes, using the technique of Judge et al.,37 to record binocular eye movements.
After collecting baseline data, each animal underwent an extraocular muscle resection–recession procedure to reduce their angle of misalignment (strabismus correction surgery). Strabismus correction surgery was performed by an expert strabismus surgeon (also one of the study authors) on the left eye only of M1 and right eye only of M2 and consisted of lateral rectus recession (weakening the lateral rectus muscle) and medial rectus resection (strengthening the medial rectus muscle) to improve their strabismus angle. We specifically chose to operate on only one eye, because we wanted to use the fellow eye as a control.
Data Acquisition and Experimental Procedures
The goal of data acquisition and analysis was to perform a longitudinal evaluation of eye alignment and eye movements following strabismus correction surgery. Therefore, binocular eye movement data were collected as the animals performed central and eccentric fixation, saccades (±15° horizontal and vertical target locations), and smooth pursuit (0.3 Hz, ±15°) tasks under monocular left eye or right eye viewing conditions. Alignment data were collected at five time points: before surgical treatment (Pre), the day after surgical treatment (P1d), 1 week after treatment (P1w), 1 month after treatment (P1m), and 6 months after treatment (P6m). Saccade and smooth-pursuit data were analyzed at Pre, P1w, P1m, and P6m because there were not enough experimental trials collected at P1d.
Binocular data were collected using the magnetic search coil technique (Primelec Industries, Regensdorf, Switzerland). Eye coils were calibrated at the beginning of each experiment by rewarding the animal with small amounts of juice as they looked at a series of targets projected along the horizontal or vertical meridian on a screen at distance of 57 cm. Calibration of each eye was performed independently during monocular viewing forced by occluding one or the other eye using liquid crystal shutter goggles (Citizen Fine Devices, Nagano, Japan) that were under computer control. Visual stimuli were generated using the BITS# visual stimulus generator (Cambridge Research Systems, Cambridge, United Kingdom) and Psychtoolbox 338 operated under computer control and presented using a DepthQ projector running at a 120-Hz frame rate (Lightspeed Design, Inc., Bellevue, WA, USA). The target and binocular eye position signals were passed through anti-aliasing filters with a cutoff of 400 Hz before digitization at 2.79 kHz with 12-bit precision (AlphaLab SNR system; Alpha-Omega Engineering, Nazareth, Israel). All eye movement data were additionally calibrated offline and filtered using a software finite impulse response (FIR) low-pass filter with a cutoff of 80 Hz prior to further analysis.
Data Analysis
Analysis of eye movement data was partially automated using custom scripts written in MATLAB (Mathworks, Natick, MA, USA). For saccade data, the computer displayed target position, binocular eye position, eye velocity, and eye acceleration traces of a single saccade trial. Velocity and acceleration signals were generated by digital differentiation of the position signal using a central difference algorithm. The investigator viewed the traces and decided whether the saccade trial was to be accepted or rejected. Trials that were rejected were usually those in which the animal was not fixating prior to the target step, the saccade did not appear to be directed toward the target, or the saccade did not occur within 400 ms of the target step. Once a decision to accept the trial was made, mean and SD of control eye acceleration prior to the saccade were calculated over a 70-ms fixation period. Saccade onset was automatically determined by the software as the first time point at which eye acceleration was greater than 5 SDs away from the control eye acceleration, and saccade offset was determined as the last time point at which eye deceleration was less than 5 SDs away from the same mean eye acceleration. Although detection of saccade onset and offset was automated, the investigator visually examined the velocity and acceleration traces of every saccade and had the option of either accepting or changing the computer selection, although this was not typical. Once the saccade was identified, saccade metric parameters (amplitude and peak velocity) for each eye were calculated. Main sequence plots of horizontal saccade peak velocity versus horizontal saccade amplitude were fitted with the following exponential rise to maximum equation:
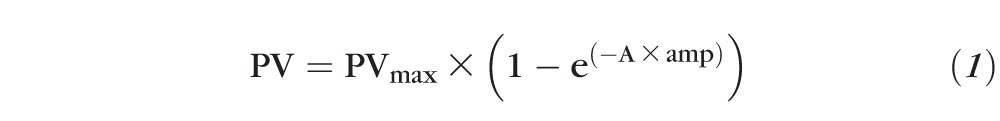 |
where amp = horizontal saccade amplitude, PV = horizontal saccade peak velocity, A = constant determining exponential rise, and PVmax = asymptotic peak velocity.
For sinusoidal smooth-pursuit analysis, the eye movement data were first desaccaded by a custom MATLAB program. Then smooth-pursuit gain (ratio of peak eye velocity to peak target velocity) was calculated separately for nasalward and temporalward movements. To assess fixation stability, ∼10 seconds of eye movement data from both the eyes were analyzed as the monkey maintained monocular fixation at a stationary straight-ahead target. Trials were checked manually offline to make sure the animal was fixating the target. Fixation stability was quantified by calculating the bivariate contour ellipse area (BCEA), a metric that quantifies the area of the region over which eye positions are dispersed during attempted fixation. Therefore, a smaller value for BCEA is indicative of greater fixation stability. The BCEA encompassing 68.2% of fixation points was calculated using the following equation39:
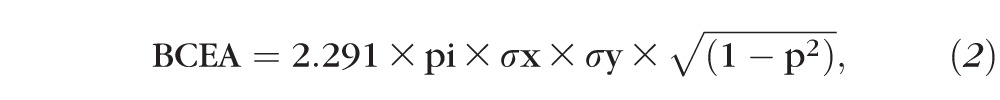 ,
,
|
where σx = SD of horizontal eye position, σy = SD of vertical eye position, 2.291 is the χ2 value (2 df) corresponding to a probability of 0.68, and p is the Pearson product moment correlation coefficient of horizontal and vertical eye positions.
For statistical analysis, the Kruskal-Wallis 1-way ANOVA on ranks was performed, and if significant, post hoc testing was performed using Dunn's method.
Results
Longitudinal Changes in Eye Alignment
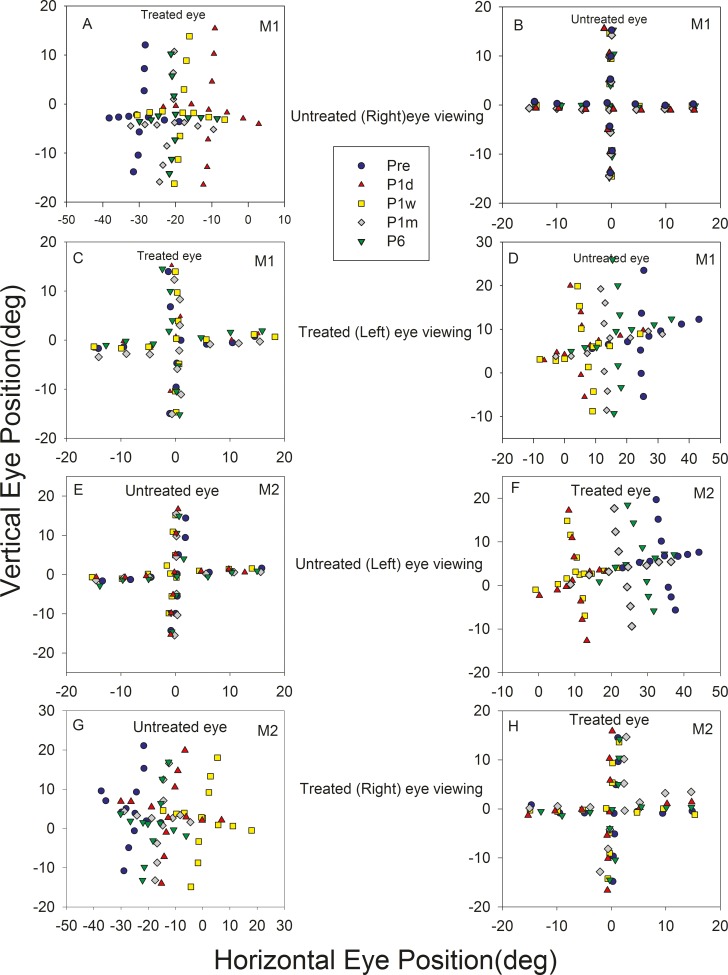
Prior to treatment, both monkeys had an exotropia whose magnitude depended on the eye of fixation, a likely indicator for dissociated horizontal deviation. M1 had an exotropia of ∼27° under left eye viewing (LEV) conditions and ∼30° under right eye viewing (REV) conditions. M2 had an exotropia of ∼37° under LEV conditions and ∼30° during REV. Immediately after surgery (P1d), exotropia in M1 reduced to ∼10° (LEV) and ∼11° (REV) and exotropia in M2 reduced to ∼9° (LEV) and ∼12° (REV). The initial decrease in strabismus angle after the surgery was followed by a gradual reversal toward presurgical exotropia by the end of 6 months. Figure 1 is a Hess plot showing alignment at different time points as the animals fixated a series of targets along the horizontal and vertical meridian. By 6 months after the correction surgery, M1 showed an exotropia of ∼20° during either eye viewing and M2 showed an exotropia of ∼30° (LEV) and ∼20° (REV). Thus, it appeared that both animals reverted to ∼60%–80% of their original strabismus angle within 6 months. Alignment was also examined at 10 months, and the strabismus angle for M1 was still ∼20° but had changed slightly in M2 to ∼24° (LEV) and ∼18° (REV).
Figure 1.
Longitudinal change in eye misalignment following strabismus treatment in M1 (A–D) and M2 (E–H). Plots show eye positions of the viewing eye (B, C, E, H) and the nonviewing eye (A, D, F, G) during monocular viewing of a series of targets along the horizontal or vertical meridian. A, B (M1) and E, F (M2) show data collected when the animal viewed with their untreated eye (treated eye under cover) and C, D (M1) and G, H (M2) show data collected when the animals viewed with their treated eye. Each plot shows data collected at five time points: before surgery (pre; blue circle), 1 day after surgery (P1d; red triangle), 1 week after surgery (P1w; yellow square), 1 month after surgery (P1m; gray diamond), and 6 months after surgery (P6; green inverted triangle). Pairs of plots on each row show that the viewing eye is on target while the covered eye is abducted. The angle of misalignment is reduced on P1d and gradually reverses by P6. Rightward and upward eye positions are positive and leftward and downward eye positions are negative.
Longitudinal Changes in Saccade Parameters
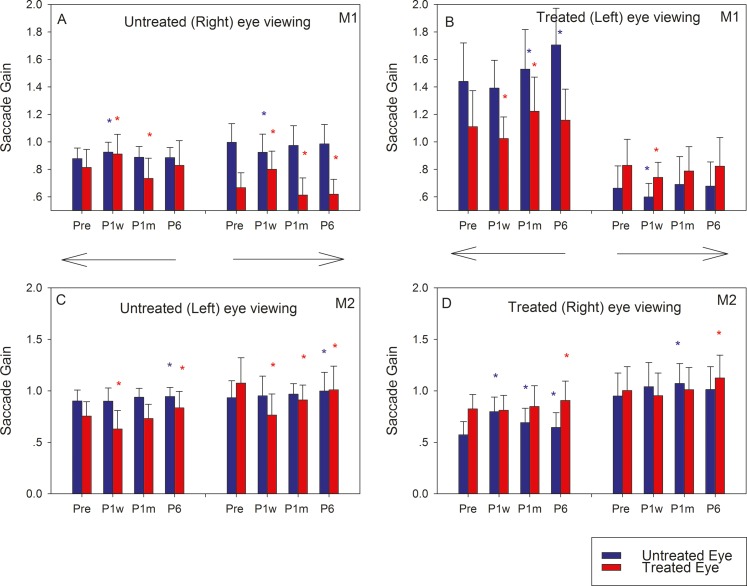
We analyzed 4021 saccades at four time points (Pre [1449 saccades], P1w [648 saccades], P1m [1103 saccades], and P6m [821 saccades]). Because the numbers of saccadic trials acquired on any single day were relatively low, data from multiple days near the indicated time point were pooled for analysis. Average saccade gains (ratio of saccade amplitude to target amplitude) of the two animals are summarized in Figure 2. In general, saccade gain of the viewing eye, irrespective of whether the animal viewed with the treated or untreated eye, did not show significant longitudinal changes (blue bars in 2A, 2C; red bars in 2B, 2D), as might be expected if the goal of the viewing eye is to saccade onto the target. Longitudinal changes in the nonviewing eye were more striking, although slightly different in the two monkeys. When M1 viewed with the untreated eye (Fig. 2A), the treated eye (red bars) showed a significant increase in saccade gain after surgery that decreased to presurgery values by P6. On the other hand, when M2 viewed with the untreated eye (Fig. 2C), there was an immediate drop in saccade gain in the treated eye (red bars) following surgery that reverted to presurgical values by P1m–P6. These findings were generally true for both rightward and leftward saccades. Note that when viewing with the untreated eye, any changes in treated eye saccade gains can be attributed to adaptive muscle or neural changes. Complementary findings were observed when the animals viewed with their treated eye (Figs. 2B, 2D). Thus, in M1 (Fig. 2B), the untreated eye saccade gain (blue bars) decreased or remained the same at P1w and thereafter increased by P6 (greater than Pre values for leftward saccades). In M2 (Fig. 2D), the untreated eye saccade gain increased at P1w and thereafter decreased by P6 for leftward saccades only. Note that when viewing with the treated eye, any changes in untreated eye saccade gains are likely driven by a combination of Hering's law based neural mechanisms and adaptive untreated eye muscle or neural changes triggered by the surgical intervention of the treated muscle.
Figure 2.
Longitudinal change in horizontal saccade gains in M1 (A, B) and M2 (C, D) following strabismus treatment. Bars in each plot show means and SDs of saccade gains of the untreated eye (blue) and treated eye (red) at four time points (Pre, P1w, P1m, and P6). Leftward and rightward saccades were analyzed separately and are represented by the directional arrows. The asterisks represent statistically significant difference compared with presurgical values (blue, untreated eye; red, treated eye).
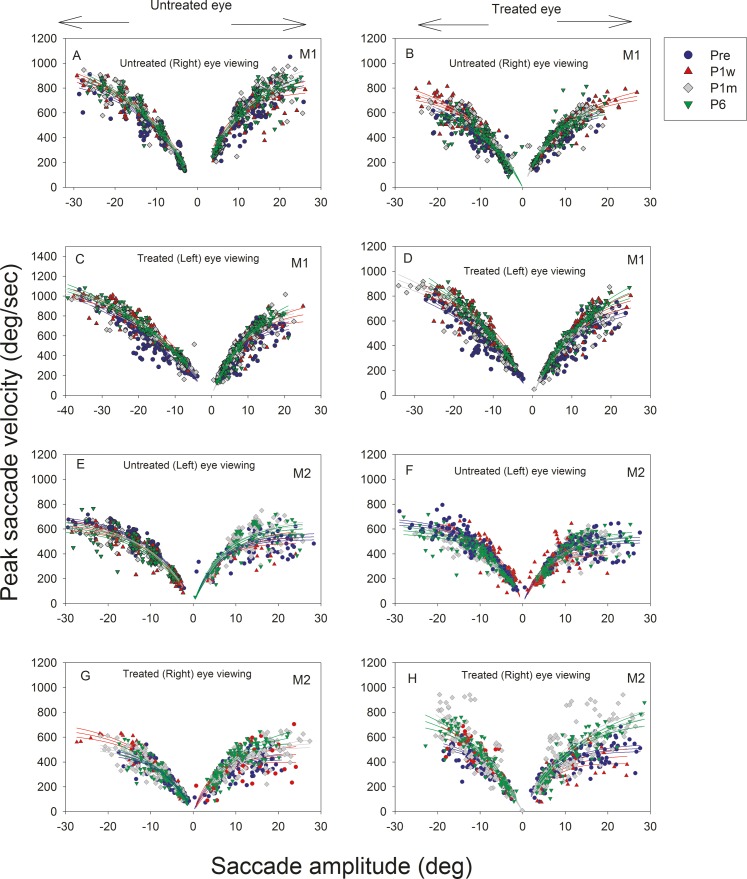
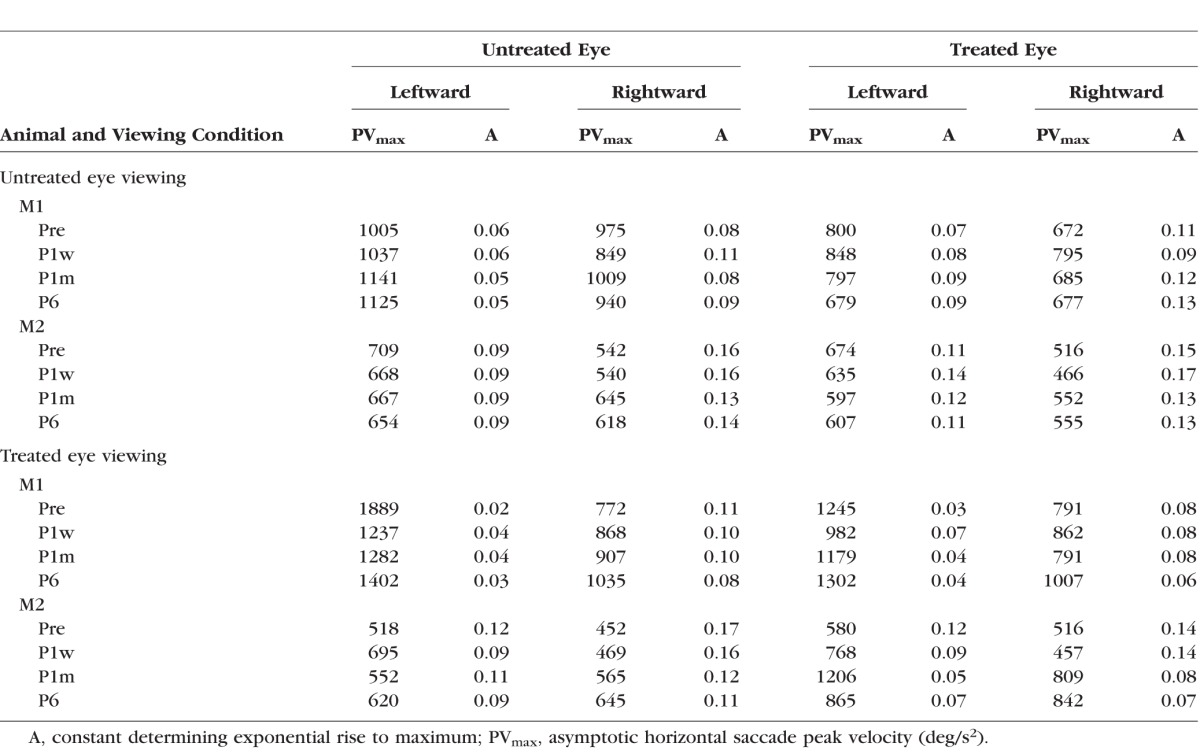
Main sequence plots are shown in Figure 3 along with the mean and 95% upper and lower confidence limits. Significant differences were visually identified as those where 95% confidence limits do not overlap. Longitudinal changes in saccade peak velocity were relatively small and difficult to identify because of the large variability in saccade velocities. There was a slight but significant increase in saccade velocity in both the treated and untreated eyes as animal M1 viewed with the treated eye (Figs. 3C, 3D) as P6 velocities are higher than they were before surgery. A similar, albeit greater magnitude, increase in peak saccade velocity was observed in both the treated and untreated eyes as animal M2 viewed with the treated eye (more so for rightward than leftward saccades; Figs. 3G, 3H). Estimated fit parameters for PVmax and A from Equation 1 for each of the eyes under the different viewing conditions and at the different time points are provided in the Table.
Figure 3.
Longitudinal changes in horizontal saccade dynamics following strabismus treatment. Main sequence plots (M1, A–D; M2, E–H) showing horizontal saccade amplitude on the x-axis and horizontal saccade peak velocity on the y-axis. Negative saccade amplitudes indicate leftward saccades and positive amplitudes indicate rightward saccades. Left column panels (A, C, E, G) show data from the untreated eye and right column panels (B, D, F, G) show data from the treated eye. Each data point represents a single saccade, and lines represent exponential rise-to-maximum fits to the main sequence data. For these plots, viewing and nonviewing eyes' data were collected when the animals viewed monocularly with either treated eye or untreated eye. Data points and curve fits are overlaid for saccade data collected at Pre (blue circle), P1w (red triangle), P1m (gray diamond), and P6 (green inverted triangle).
Table.
Fit Parameters for Main Sequence Plots Derived From Equation 1
Longitudinal Changes in Smooth-Pursuit Behavior
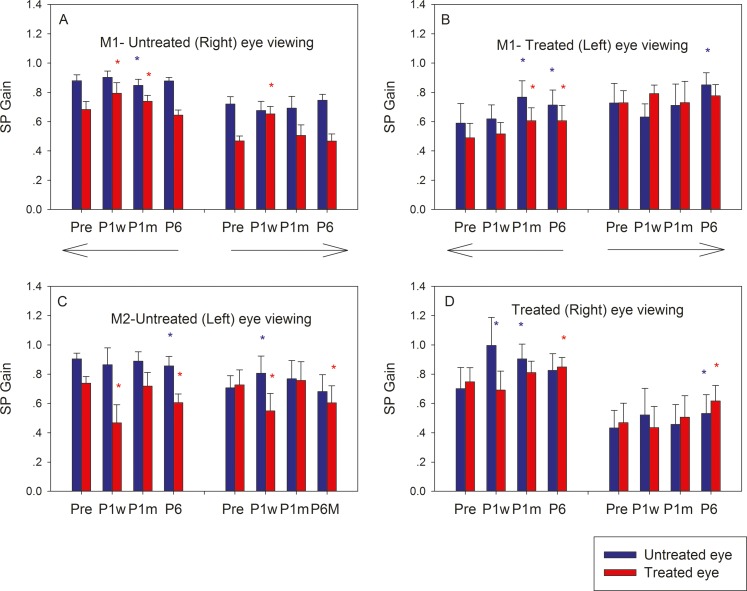
Summary of leftward and rightward smooth-pursuit gains obtained from 15 to 30 cycles of smooth pursuit at each time point for each monkey are shown in Figure 4 along with results of statistical comparison to presurgical smooth-pursuit gains. Longitudinal trends in smooth-pursuit gains reflected the trends observed for saccadic eye movement gains. Gains of viewing eye remained more or less constant following surgery (blue bars in Figs. 4A, 4C; red bars in Figs. 4B, 4D). When M1 viewed with the untreated eye (Fig. 4A), there was a significant increase in gain in the treated eye (red bars) for both rightward and leftward pursuit that was mostly reversed by P6. On the other hand, when M2 viewed with the untreated eye (Fig. 4C), there was a significant decrease in gain in the treated eye that mostly reversed by P6. Smooth-pursuit gains calculated when the animals viewed with their treated eyes (Figs. 4B, 4D) also followed trends observed during saccade analysis.
Figure 4.
Longitudinal change in horizontal smooth-pursuit gains in M1 (A, B) and M2 (C, D) following strabismus treatment. Bars in each plot show mean smooth-pursuit gains and SDs of the untreated eye (blue) and treated eye (red) at four time points (Pre, P1w, P1m, and P6). Leftward and rightward smooth-pursuit was analyzed separately and are represented by the directional arrows. The asterisks represent statistically significant difference compared with presurgical values (blue, untreated eye; red, treated eye).
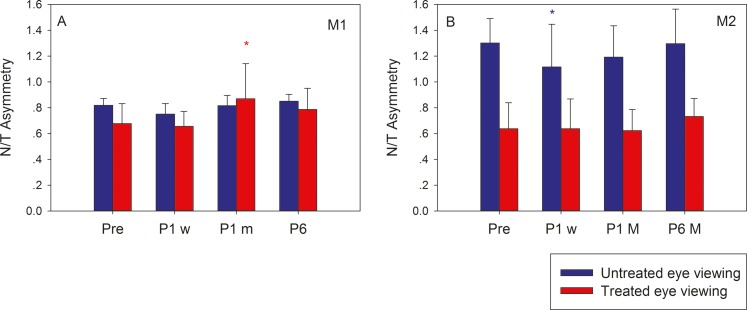
Strabismic humans and monkeys often exhibit increased monocular viewing smooth-pursuit tracking gains for nasalward target motion compared with temporalward motion (naso-temporal asymmetry). Both the monkeys in the current study displayed a naso-temporal (N/T) asymmetry prior to surgery (except M2 during left eye viewing; Fig. 5B, blue bars), and we wondered whether surgical manipulation of EOM resulted in any modification of this feature of strabismus. A naso-temporal asymmetry index was calculated as the ratio of viewing eye temporalward smooth-pursuit gain to viewing eye nasalward smooth-pursuit gain for both treated and untreated eyes viewing conditions. In M1, the naso-temporal asymmetry persisted mostly unchanged following surgery during either untreated or treated eye viewing. In M2 also, N/T asymmetry was unchanged during treated (right) eye viewing. During untreated (left) eye viewing, there was a slight improvement in the index (reduced asymmetry) that reversed by P1m.
Figure 5.
Longitudinal change in naso-temporal asymmetry following strabismus treatment. Bars in each plot show a naso-temporal asymmetry index calculated as the ratio of the viewing eye temporalward and nasalward smooth-pursuit gain for M1 (A) and M2 (B) at four time points (Pre, P1w, P1m, P6). N/T asymmetry index during untreated eye viewing is shown in blue and during treated eye viewing is shown in red.
Longitudinal Changes in Fixation Stability
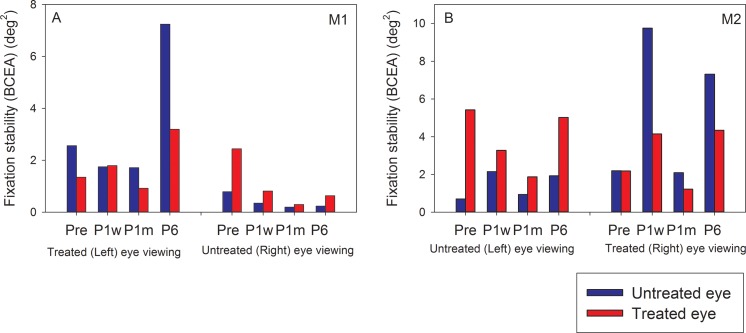
Fixation stability was assessed by calculating the BCEA metric for each eye during monocular viewing (Fig. 6). In both monkeys, when the untreated eye was viewing, fixation stability of the nonviewing treated eye (red bars) improved (lower BCEA) after surgery but regressed by P6 in M2 only. These data were not amenable for statistical testing because each bar represents a single data point for the fixation stability metric. Fixation stability of the viewing untreated eye (blue bars) did not show consistent longitudinal effects. When the treated eye was viewing, fixation stability of nonviewing untreated eye (blue bars) showed postsurgical improvement that regressed by P6 in M1 but no consistent longitudinal effects in M2. Fixation stability of the viewing treated eye also did not show consistent longitudinal effects. Fixation stability of the nonviewing eye was worse at all time points for both the monkeys, consistent with previous studies of fixation stability in strabismic monkeys.8
Figure 6.
Longitudinal change in fixation stability (M1, A; M2, B) following strabismus treatment. Bars in each plot show BCEA values of the treated eye (red) and untreated eye (blue) during either eye viewing at the four time points (Pre, P1w, P1m, P6m).
Discussion
To our knowledge, this study is the first attempt at investigating longitudinal changes in eye alignment and eye movements following strabismus treatment surgery in either humans or animal models. In addition to providing a detailed account of time course of change in alignment and movements, these data will provide a basis for examination of neural and muscle plasticity that is likely to occur as a consequence of correction surgery. Our main findings in this study were that 1) eye misalignment reduced as a consequence of resect–recess surgery, but regressed to close to presurgical values by 6 months after surgery; 2) patterns of longitudinal changes in saccadic and smooth-pursuit eye movements also showed immediate postsurgical changes that reversed by 6 months after treatment, although naso-temporal asymmetry of smooth-pursuit tracking was unchanged; 3) there were only small changes in fixation stability; and 4) the overall similarity in response to human strabismus surgical treatment validates our monkey model for strabismus. Below we discuss each of our findings in the context of longitudinal adaptive changes occurring in strabismus.
Eye Alignment
Compared with dynamic changes in eye movements that were generally small but significant (discussed later), longitudinal changes in eye alignment were clear and consistent in the two monkeys in our study. Both the exotropic monkeys in the study responded to treatment in that exotropia was reduced significantly to ∼30% of presurgical misalignment immediately after surgery as might be expected following a resect–recess procedure in a human patient. At P1d (day after surgery), the reduction in misalignment observed when viewing with the untreated eye is possibly only a consequence of the change in contractility of the treated EOM as the brain may not have had sufficient time to adapt. In other words, the neural signals to the EOM of viewing (untreated) and nonviewing (treated) eyes are expected to be the same as in the presurgical condition. When the monkey is forced to view with the treated eye, the brain must alter the neural signals to the treated muscles to compensate for their altered contractility to allow fixation of the target, for example, increase in neuronal drive of recessed lateral rectus from ipsilateral abducens nucleus. The anatomical connection between abducens nucleus and contralateral oculomotor nucleus (underpinnings of Hering's law) leads to increase in neuronal drive to the medial rectus muscle of the contralateral (in this case untreated) eye. Note that the change in neural drives to the treated and untreated eye EOM when the monkey is forced to view with the treated eye is not a long-term adaptive response per se as the brain is only momentarily adjusting signals to enable target acquisition and fixation.
Following the initial reduction in strabismus angle, the angle of misalignment reverted to ∼60%–80% of presurgical value by 6 months after treatment, again similar to outcomes of strabismus correction surgery in a proportion of human subjects whose initial procedure resulted in undercorrection.22 The lack of persistence of the initial improvement in strabismus angle may be because improvement in alignment in the two study animals was beyond the range at which central binocular factors would be expected to be able to maintain the initial improvement in postsurgical ocular alignment. Therefore, remodeling at the level of the EOM23,24 along with central neural adaptation could act to “fight” the intent of surgery (Pullela M, et al. IOVS 2015;ARVO E-Abstract 5221) (Agaoglu MN, et al. IOVS 2015;ARVO E-Abstract 5222). Additional studies directly examining neural drives and muscle contractility are needed to understand the relative plastic changes due to these two factors. These studies are underway in our laboratory. Another point to note is that the patterns of alignment change following treatment, which are similar to human strabismus response to treatment, also help to validate the monkey model as suitable for investigating strabismus mechanisms.
Saccadic and Smooth-Pursuit Eye Movements
There were significant small magnitude changes in saccadic and smooth-pursuit gains following treatment, although it was different in the two monkeys (Figs. 2, 4). Thus, saccade and smooth-pursuit gain for M1 showed an increase after surgery, whereas saccade and smooth-pursuit gains for M2 showed a decrease after surgery, although both animals regained presurgical gain values by P6. Analysis of performance of saccades in human subjects after undergoing strabismus surgery showed an improvement after surgery that was proposed as due to improvement in binocular coordination because eye misalignment is corrected.29 Because full alignment was not achieved in our monkeys even on P1d, we suggest that the observed changes in saccade and smooth-pursuit gain in our monkeys are more likely to be driven by brain and muscle adaptation that is unrelated to binocular coordination. It is unclear why eye movement gains increased after surgery in M1 but decreased after surgery in M2. We suggest that this likely represents variability that might be observed in humans as well and could represent differences in relative adaptation (neural or muscle related) of the recessed versus the resected muscle. Confirmation of this hypothesis would come from future studies that measure neuronal drive to the treated and untreated muscles.
Longitudinal changes in saccade peak velocities were unremarkable. The most likely explanation is that saccade velocities are inherently too variable to show significant changes following surgical treatment. We also did not find any consistent changes in N/T asymmetry during monocular smooth-pursuit. Because N/T asymmetry is fundamentally due to developmental loss of binocular inputs from sensory areas such as middle temporal/middle superior temporal (MT/MST) to brainstem areas such as the nucleus of the optic tract (NOT), any longitudinal changes in N/T asymmetry would have been, in fact, surprising.14,15,40,41
Fixation Stability
Fixation stability or lack thereof in strabismic animals and humans is usually influenced by presence of visual problems such as amblyopia42–46 and oculomotor instabilities such as nystagmus and excessive drifts. In addition, fixation stability may be influenced by several visual factors such as target size, shape, contrast, and luminance.8 We measured fixation stability in these animals at the different time points and found a temporary improvement in fixation stability of the treated eye when the untreated eye was viewing but no consistent changes when the treated eye was viewing. Dell'Osso et al. suggested that tenotomy surgery reduces nystagmus intensity47,48 in humans with infantile nystagmus syndrome due to manipulation of proprioceptive receptors in the EOM. It is possible that similar mechanisms are at play following the strabismus correction surgery that we performed, although another study that attempted tenotomy surgery in monkeys with infantile nystagmus showed inconsistent results.49
Implications for Plasticity Mechanisms
Our data suggest that surgical treatment of strabismus is followed by an adaptive response that evolves and reaches a steady state within the first 6 months after treatment. Because adaptation can either oppose or facilitate the outcome of treatment strategies, the fundamental challenge to improving treatment methods is to identify and devise methods that will induce adaptation that promotes desired treatment effects while avoiding adaptation that may oppose or reverse desired treatment effects. As suggested earlier, both central neural adaption and peripheral muscle remodeling are likely driving the final state of alignment and ultimately determining the success or failure of the surgical treatment. Immediate (P1d) changes in alignment and eye movements are likely driven by changes in muscle contractility alone, but later changes (P1m and P6m) are likely due to a combination of neural plasticity and peripheral remodeling.23,24 Behavioral studies alone cannot readily differentiate between these mechanisms, but they are critical to develop a framework to conduct and interpret neural recording studies or studies that evaluate muscle function. Such neuronal studies are underway in our laboratory in the same animals as in this study.
Limitations of Study
Major portions of the strabismus treatment literature are accounts of outcomes following different types of surgical procedures. The strength of these studies is the relatively large number and large variety (e.g., esotropes versus exotropes and patients with residual binocularity versus patients with no binocularity) of patients that are evaluated. Due to several (mostly practical) reasons, monkey studies cannot hope to replicate the large n of human studies. However, the strength of our monkey investigation lies in the ability to perform careful and controlled longitudinal evaluation in the same animals that is not possible in human clinical investigation. Moreover, these behavioral studies provide a framework to perform a neurophysiologic investigation that is not possible in humans and is critical to extend our understanding of strabismus mechanisms including plasticity.
It is possible that outcomes in the current study might have been different (i.e., monkeys' exotropia does not revert) if we had chosen to treat both eyes and attempted to more closely align the eyes, given the general notion that surgical realignment in the vicinity of 5–10 PD helps facilitate binocularity. However, on the other hand, both the animals in this study were reared with prisms from the first day of birth, likely preventing the development of even coarse stereopsis.50 Also, we purposely did not attempt a second procedure on the fellow eye because we wanted to be able to use the fellow eye as a control and thereby ascertain the nature and time course of plasticity that follows surgical treatment. Using the fellow eye as an untreated control is also quite critical when evaluating neural responses that are driving the extraocular muscles.
Acknowledgments
The authors thank Mehmet Agaoglu for help with development of data analysis programs and Hui Meng and Ernest Baskin for technical assistance with the animals.
This work was supported by National Institutes of Health Grant R01-EY022723 and University of Houston, College of Optometry (UHCO) Core Grant P30 EY07551.
Disclosure: M. Pullela, None; B.A. Degler, None; D.K. Coats, None; V.E. Das, None
References
- 1. Lorenz B. Genetics of isolated and syndromic strabismus: facts and perspectives. Strabismus. 2002; 10: 147– 156. [DOI] [PubMed] [Google Scholar]
- 2. Govindan M,, Mohney BG,, Diehl NN,, Burke JP. Incidence and types of childhood exotropia: a population-based study. Ophthalmology. 2005; 112: 104– 108. [DOI] [PubMed] [Google Scholar]
- 3. Greenberg AE,, Mohney BG,, Diehl NN,, Burke JP. Incidence and types of childhood esotropia: a population-based study. Ophthalmology. 2007; 114: 170– 174. [DOI] [PubMed] [Google Scholar]
- 4. Crawford ML,, von Noorden GK. Optically induced concomitant strabismus in monkeys. Invest Ophthalmol Vis Sci. 1980; 19: 1105– 1109. [PubMed] [Google Scholar]
- 5. Tusa RJ,, Mustari MJ,, Das VE,, Boothe RG. Animal models for visual deprivation-induced strabismus and nystagmus. Ann New York Acad Sci. 2002; 956: 346– 360. [DOI] [PubMed] [Google Scholar]
- 6. Economides JR,, Adams DL,, Jocson CM,, Horton JC. Ocular motor behavior in macaques with surgical exotropia. J Neurophysiol. 2007; 98: 3411– 3422. [DOI] [PubMed] [Google Scholar]
- 7. Das VE. Alternating fixation and saccade behavior in nonhuman primates with alternating occlusion-induced exotropia. Invest Ophthalmol Vis Sci. 2009; 50: 3703– 3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pirdankar OH,, Das VE. Influence of target parameters on fixation stability in normal and strabismic monkeys. Invest Ophthalmol Vis Sci. 2016; 57: 1087– 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Das VE,, Fu LN,, Mustari MJ,, Tusa RJ. Incomitance in monkeys with strabismus. Strabismus. 2005; 13: 33– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fu L,, Tusa RJ,, Mustari MJ,, Das VE. Horizontal saccade disconjugacy in strabismic monkeys. Invest Ophthalmol Vis Sci. 2007; 48: 3107– 3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Agaoglu MN,, LeSage SK,, Joshi AC,, Das VE. Spatial patterns of fixation-switch behavior in strabismic monkeys. Invest Ophthalmol Vis Sci. 2014; 55: 1259– 1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Walton MM,, Ono S,, Mustari M. Vertical and oblique saccade disconjugacy in strabismus. Invest Ophthalmol Vis Sci. 2014; 55: 275– 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghasia F,, Tychsen L. Horizontal and vertical optokinetic eye movements in macaque monkeys with infantile strabismus: directional bias and crosstalk. Invest Ophthalmol Vis Sci. 2014; 55: 265– 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tychsen L. Causing and curing infantile esotropia in primates: the role of decorrelated binocular input (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007; 105: 564– 593. [PMC free article] [PubMed] [Google Scholar]
- 15. Tychsen L,, Richards M,, Wong A,, et al. Spectrum of infantile esotropia in primates: behavior, brains and orbits. J AAPOS. 2008; 12: 375– 380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Economides JR,, Adams DL,, Horton JC. Perception via the deviated eye in strabismus. J Neurosci. 2012; 32: 10286– 10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Joshi AC,, Das VE. Responses of medial rectus motoneurons in monkeys with strabismus. Invest Ophthalmol Vis Sci. 2011; 52: 6697– 6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Walton MM,, Mustari MJ,, Willoughby CL,, McLoon LK. Abnormal activity of neurons in abducens nucleus of strabismic monkeys. Invest Ophthalmol Vis Sci. 2015; 56: 10– 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joyce KE,, Beyer F,, Thomson RG,, Clarke MP. A systematic review of the effectiveness of treatments in altering the natural history of intermittent exotropia. Br J Ophthalmol. 2015; 99: 440– 450. [DOI] [PubMed] [Google Scholar]
- 20. von Noorden GK,, Campos EC. Binocular Vision and Ocular Motility: Theory and Management of Strabismus. 6th ed. St. Louis, MO: Mosby; 2002. [Google Scholar]
- 21. Kampanartsanyakorn S,, Surachatkumtonekul T,, Dulayajinda D,, Jumroendararasmee M,, Tongsae S. The outcomes of horizontal strabismus surgery and influencing factors of the surgical success. J Med Assoc Thai. 2005; 88 (suppl 9): S94– S99. [PubMed] [Google Scholar]
- 22. Pineles SL,, Ela-Dalman N,, Zvansky AG,, Yu F,, Rosenbaum AL. Long-term results of the surgical management of intermittent exotropia. J AAPOS. 2010; 14: 298– 304. [DOI] [PubMed] [Google Scholar]
- 23. Christiansen SP,, McLoon LK. The effect of resection on satellite cell activity in rabbit extraocular muscle. Invest Ophthalmol Vis Sci. 2006; 47: 605– 613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Christiansen SP,, Antunes-Foschini RS,, McLoon LK. Effects of recession versus tenotomy surgery without recession in adult rabbit extraocular muscle. Invest Ophthalmol Vis Sci. 2010; 51: 5646– 5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scott AB. Change of eye muscle sarcomeres according to eye position. J Pediatr Ophthalmol Strabismus. 1994; 31: 85– 88. [DOI] [PubMed] [Google Scholar]
- 26. Guyton DL. The 10th Bielschowsky Lecture. Changes in strabismus over time: the roles of vergence tonus and muscle length adaptation. Binocul Vis Strabismus Q. 2006; 21: 81– 92. [PubMed] [Google Scholar]
- 27. Optican LM,, Zee DS,, Chu FC. Adaptive response to ocular muscle weakness in human pursuit and saccadic eye movements. J Neurophysiol. 1985; 54: 110– 122. [DOI] [PubMed] [Google Scholar]
- 28. Scudder CA,, Batourina EY,, Tunder GS. Comparison of two methods of producing adaptation of saccade size and implications for the site of plasticity. J Neurophysiol. 1998; 79: 704– 715. [DOI] [PubMed] [Google Scholar]
- 29. Bucci MP,, Bremond-Gignac D,, Kapoula Z. Speed and accuracy of saccades, vergence and combined eye movements in subjects with strabismus before and after eye surgery. Vision Res. 2009; 49: 460– 469. [DOI] [PubMed] [Google Scholar]
- 30. Bucci MP,, Kapoula Z,, Yang Q,, Roussat B,, Bremond-Gignac D. Binocular coordination of saccades in children with strabismus before and after surgery. Invest Ophthalmol Vis Sci. 2002; 43: 1040– 1047. [PubMed] [Google Scholar]
- 31. Lewis RF,, Zee DS,, Gaymard BM,, Guthrie BL. Extraocular muscle proprioception functions in the control of ocular alignment and eye movement conjugacy. J Neurophysiol. 1994; 72: 1028– 1031. [DOI] [PubMed] [Google Scholar]
- 32. Quaia C,, Shan X,, Tian J,, et al. Acute superior oblique palsy in the monkey: effects of viewing conditions on ocular alignment and modelling of the ocular motor plant. Prog Brain Res. 2008; 171: 47– 52. [DOI] [PubMed] [Google Scholar]
- 33. Smith EL, III,, Bennett MJ,, Harwerth RS,, Crawford ML. Binocularity in kittens reared with optically induced squint. Science. 1979; 204: 875– 877. [DOI] [PubMed] [Google Scholar]
- 34. Boothe RG,, Dobson V,, Teller DY. Postnatal development of vision in human and nonhuman primates. Annu Rev Neurosci. 1985; 8: 495– 545. [DOI] [PubMed] [Google Scholar]
- 35. Kiorpes L. Visual development in primates: neural mechanisms and critical periods. Dev Neurobiol. 2015; 75: 1080– 1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Adams DL,, Economides JR,, Jocson CM,, Horton JC. A biocompatible titanium headpost for stabilizing behaving monkeys. J Neurophysiol. 2007; 98: 993– 1001. [DOI] [PubMed] [Google Scholar]
- 37. Judge SJ,, Richmond BJ,, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res. 1980; 20: 535– 538. [DOI] [PubMed] [Google Scholar]
- 38. Brainard DH. The psychophysics toolbox. Spat Vis. 1997; 10: 433– 436. [PubMed] [Google Scholar]
- 39. Timberlake GT,, Sharma MK,, Grose SA,, Gobert DV,, Gauch JM,, Maino JH. Retinal location of the preferred retinal locus relative to the fovea in scanning laser ophthalmoscope images. Optom Vis Sci. 2005; 82: 177– 185. [DOI] [PubMed] [Google Scholar]
- 40. Mustari MJ,, Ono S. Neural mechanisms for smooth pursuit in strabismus. Ann N Y Acad Sci. 2011; 1233: 187– 193. [DOI] [PubMed] [Google Scholar]
- 41. Mustari MJ,, Ono S,, Vitorello KC. How disturbed visual processing early in life leads to disorders of gaze-holding and smooth pursuit. Prog Brain Res. 2008; 171: 487– 495. [DOI] [PubMed] [Google Scholar]
- 42. Ciuffreda KJ,, Kenyon RV,, Stark L. Fixational eye movements in amblyopia and strabismus. J Am Optom Assoc. 1979; 50: 1251– 1258. [PubMed] [Google Scholar]
- 43. Subramanian V,, Jost RM,, Birch EE. A quantitative study of fixation stability in amblyopia. Invest Ophthalmol Vis Sci. 2013; 54: 1998– 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schor C,, Hallmark W. Slow control of eye position in strabismic amblyopia. Invest Ophthalmol Vis Sci. 1978; 17: 577– 581. [PubMed] [Google Scholar]
- 45. Weiss AH,, Kelly JP,, Phillips JO. Infantile nystagmus and abnormalities of conjugate eye movements in Down syndrome. Invest Ophthalmol Vis Sci. 2016; 57: 1301– 1309. [DOI] [PubMed] [Google Scholar]
- 46. Chung ST,, Kumar G,, Li RW,, Levi DM. Characteristics of fixational eye movements in amblyopia: limitations on fixation stability and acuity? Vision Res. 2015; 114: 87– 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dell'Osso LF,, Orge FH,, Jacobs JB. Effects of augmented tenotomy and reattachment in the infantile nystagmus syndrome. Digit J Ophthalmol. 2016; 22: 12– 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang ZI,, Dell'Osso LF. Tenotomy procedure alleviates the “slow to see” phenomenon in infantile nystagmus syndrome: model prediction and patient data. Vision Res. 2008; 48: 1409– 1419. [DOI] [PubMed] [Google Scholar]
- 49. Wong AM,, Tychsen L. Effects of extraocular muscle tenotomy on congenital nystagmus in macaque monkeys. J AAPOS. 2002; 6: 100– 107. [DOI] [PubMed] [Google Scholar]
- 50. O'Dell C,, Boothe RG. The development of stereoacuity in infant rhesus monkeys. Vision Res. 1997; 37: 2675– 2684. [DOI] [PubMed] [Google Scholar]