Abstract
An extended‐release opioid analgesic (OxyContin, OC) was reformulated with abuse‐deterrent properties to deter abuse. This report examines changes in abuse through oral and nonoral routes, doctor‐shopping, and fatalities in 10 studies 3.5 years after reformulation. Changes in OC abuse from 1 year before to 3 years after OC reformulation were calculated, adjusted for prescription changes. Abuse of OC decreased 48% in national poison center surveillance systems, decreased 32% in a national drug treatment system, and decreased 27% among individuals prescribed OC in claims databases. Doctor‐shopping for OC decreased 50%. Overdose fatalities reported to the manufacturer decreased 65%. Abuse of other opioids without abuse‐deterrent properties decreased 2 years later than OC and with less magnitude, suggesting OC decreases were not due to broader opioid interventions. Consistent with the formulation, decreases were larger for nonoral than oral abuse. Abuse‐deterrent opioids may mitigate abuse and overdose risks among chronic pain patients.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Eight publications have assessed in single studies the impact of opioids with abuse‐deterrent properties on abuse‐related outcomes associated with these opioids. No paper has summarized assessments in 10 studies using a consistent study timeframe.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Did a reformulation of OxyContin with abuse‐deterrent properties result in meaningful reductions in its abuse, misuse, addiction, overdose, and death in the postapproval setting?
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
☑ The results indicate a reduction in abuse and related outcomes of an opioid analgesic after reformulation with abuse‐deterrent properties that was consistent across studies and that occurred earlier and was larger in magnitude than abuse decreases for other opioids.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
☑ According to the US Centers for Disease Control, there is an epidemic of opioid abuse and overdose. Pharmaceutical innovation in the form of robust abuse‐deterrent formulations may contribute to addressing the epidemic as part of a multifaceted intervention approach. The results assess a “proof of concept” of one such formulation in the postapproval setting.
Abuse of opioid analgesics is a serious problem that has increased over the past decade, with accompanying increases in addiction, overdoses, and deaths.1 However, opioid analgesics are a treatment option for moderate to severe pain2 that are prescribed to over 4 and 68 million pain patients annually in the US as extended‐release (ER) and immediate‐release (IR) formulations, respectively (IMS health, unpublished data). Although prescription opioids can be abused through oral ingestion of intact tablets, the frequency of abuse via injecting and snorting routes increases as the duration and severity of abuse increases.3, 4 Anecdotal reports also state that the risk of addiction increases substantially with snorting or injecting.5
Opioid analgesics with abuse‐deterrent properties (OADP) are an approach to decrease abuse and patient medication errors involving breaking tablets, while preserving efficacy for patients.6 The US Food and Drug Administration (FDA) considers OADP development “a high public health priority” and has stated its support for making all opioid analgesics less susceptible to abuse than conventional formulations.7 OADPs are part of the US President's plan for preventing prescription drug abuse that integrates industry innovation, policing, regulation, and education into a comprehensive strategy,8 and also are considered important by Health Canada.9 Innovative approaches to imparting abuse‐deterrent properties (ADPs) to opioids include: 1) adding an opioid antagonist, 2) adding agents that induce unpleasant symptoms with excessive intake, 3) matrix transdermal delivery systems, and 4) incorporating physicochemical barriers intended to confer resistance to physical compromise and active ingredient extraction from tablets, capsules, or patches.10 However, whether OADPs result in meaningful reductions in abuse, misuse, addiction, overdose, and death in the postapproval setting has not been definitively demonstrated.11
ER opioids are designed to provide pain relief over an extended time by gradual delivery of their active ingredient. Most, but not all, strengths of ER opioids contain more opioid than IR dosage forms. Abusers manipulate ER opioids to break down the extended‐release properties and render the active ingredient available for an immediate effect.12 OxyContin (OC) is an ER oxycodone tablet.13 In its original form (original OC), it was widely misused and abused particularly through nonoral routes such as snorting and injecting following crushing or dissolving tablets.14 Prior to reformulation, original OC was abused in a national surveillance sample of people assessed at drug treatment centers as much by snorting it (53% of people) as orally (55% of people), and 36% reported injecting it (note: people could endorse more than one route).15 In response to concerns about its misuse and abuse, OC tablets were reformulated (reformulated OC) with physicochemical barriers to deter breaking, crushing, or dissolving, and making it harder to extract oxycodone.16 OC was reformulated with a polyethylene oxide matrix that hardens tablets and resists syringe aspiration and subsequent injection. Even after mechanical manipulation, it retains some controlled‐release properties. In human abuse potential studies, recreational users reported reduced liking of reformulated OC in comparison to the original formulation.17 On August 9, 2010, the manufacturer stopped shipping original OC and started exclusively shipping reformulated OC with no notification to the general public or prescribers, and no increment in price. The FDA approved new labeling for OC that described its ADPs, demonstrated by rigorous in vitro and clinical human abuse potential trials.13, 16, 17 The FDA required postmarketing epidemiological studies to evaluate changes “in the community” in abuse, misuse, overdose, and death, and required that the proposed study program be reviewed at an Advisory Committee meeting held in October 2010.18
Three questions have been debated in the literature about the effect of OC with abuse‐deterrent properties: 1) Did it result in lower rates of abuse of OC?15, 19 2) Did it result in a net decrease in abuse of prescription opioids?20, 21 and 3) Did it result in an increase in heroin use?22, 23, 24, 25, 26 This article focuses on the first question regarding if the introduction of OC resulted in lower rates of abuse of OC and presents results from 10 studies conducted as part of the FDA‐required postmarketing program. All data and SAS codes from these studies were submitted to the FDA.
RESULTS
After August 2010, OC dispensing rapidly transitioned from original OC to reformulated OC (Figure 1 a). One, 2, and 3 years after reformulation, 97%, 99%, and 100% of dispensed OC was reformulated OC, respectively. Prescriptions for overall OC decreased 13% from the 1 year before to 3 years after reformulation. A small proportion of prescriptions for Schedule II opioid analgesic pills were for OC (Figure 1 b), and the proportion decreased from 3.6% in the preformulation period to 2.9% in postreformulation periods.
Figure 1.
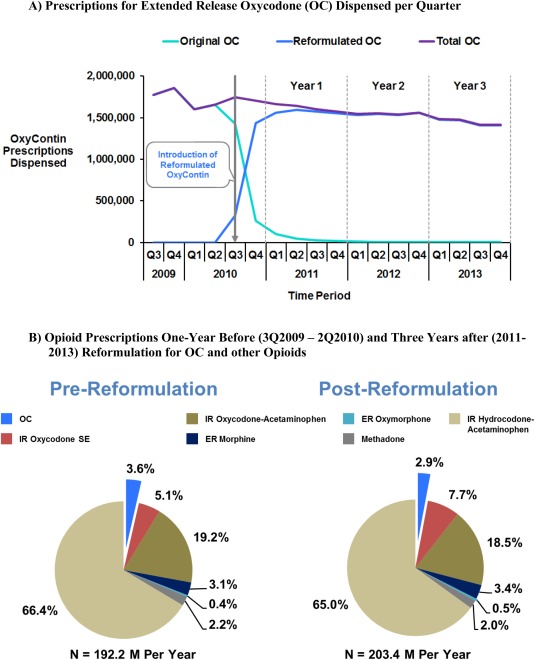
Prescriptions for opioid analgesics in the US.
Comparing the 1 year before to the 3 years after the introduction of reformulated OC, there were reductions across all outcomes, including rates of abuse, misuse, overdose, death, and drug diversion for OC (Figure 2 a and Table 2). Descriptive analyses of trends showed earlier decreases in abuse, diversion, and misuse for OC as compared to other Schedule II opioids or immediate‐release oxycodone (Figure 3), and the OC decreases occurred in close temporal proximity to introduction of reformulated OC. These trend analyses used prescription‐adjusted rates.
Figure 2.
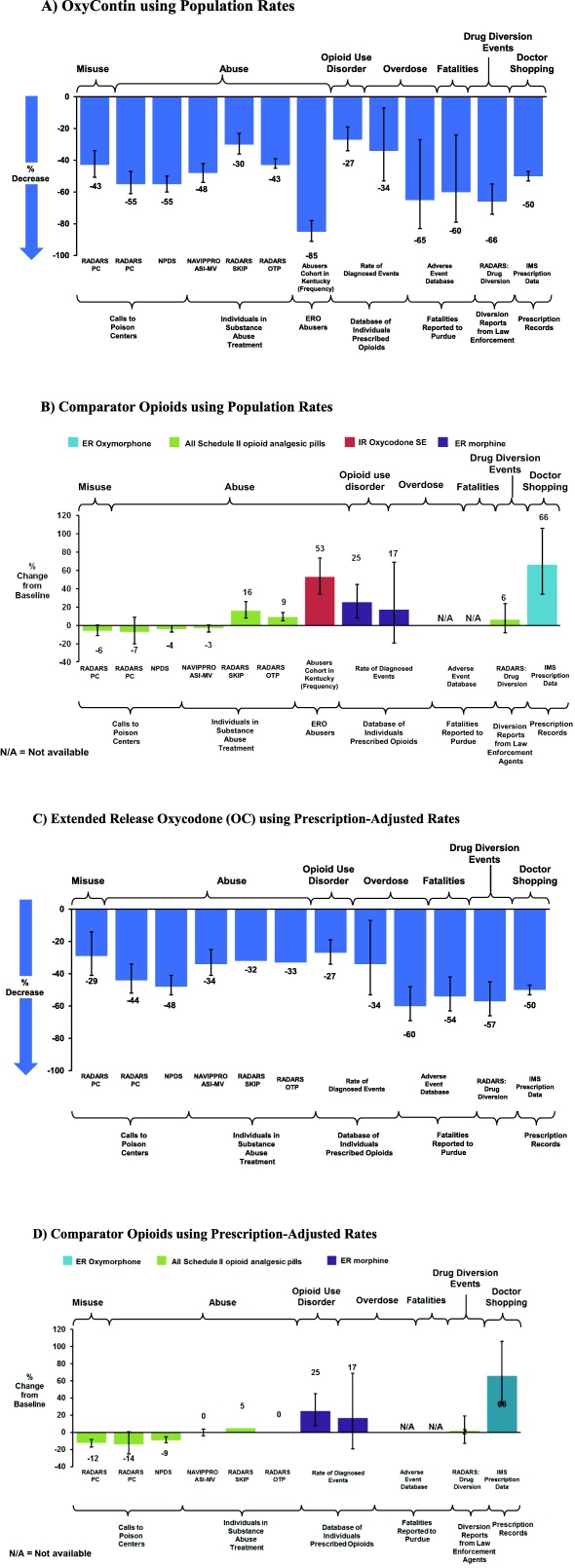
Changes in abuse, misuse, overdose, death, and diversion from 1 year before to 3 years after the introduction of reformulated OC.
Table 2.
Changes in outcomes after introduction of reformulated OC using population−adjusted rates
| Study | Metric | OxyContina | Comparator opioid group | Difference between % changes | |||
|---|---|---|---|---|---|---|---|
| Outcome measureb | % Changea (95%CI) | P‐value | Comparator | % Change (95%CI)a | P‐value | P‐value | |
| RADARS−Poison Centers |
Abuse (Any route) |
−55 (−61,−47) | <0.001 | Schedule II opioid pillsb | −7 (−20, 9) | 0.374 | <0.001 |
| Oral | −52 (−64, −36) | <0.001 | −15 (−32, 7) | 0.164 | 0.115 | ||
| Nonoral | −74 (−79,−68) | <0.001 | 3 (−26, 43) | 0.874 | <0.001 | ||
| NPDS |
Abuse (Any route) |
−55 (−60,−50) | <0.0001 | Schedule II opioid pillsb | −4 (−7, 0) | 0.0349 | <0.0001 |
| Oral | −54 (−60, −48) | <0.0001 | −8 (−11, −4) | <.0001 | <.00001 | ||
| Nonoral | −63 (−70,−54) | <0.0001 | 35 (24, 50) | <.0001 | <0.0001 | ||
| NAVIPPRO Drug Treatment |
Abuse (Any route) |
−48 (−54, −42) | <0.0001 | Schedule II opioid pillsb | −3(−7,0.4) | 0.0809 | <0.0001 |
| Oral | −24 (−33, −14) | <.0001 | −9(−13, −6) | <0.0001 | 0.0042 | ||
| Nonoral | −69 (−73, −64) | <0.0001 | 12(1, 23) | 0.0305 | <0.0001 | ||
| RADARS Drug Treatment | Any Abuse (SKIP) | −30 (−36, −23) | <0.001 | Schedule II opioid pillsb | 16 (8, 26) | <0.001 | <0.001 |
| Any Abuse (OTP) | −43 (−45, −39) | <0.001 | 9 (5, 14) | <0.001 | <0.001 | ||
| Kentucky Survey | Frequency Abuse/Any | −85 (−91, −78) | <0.0001 | IR oxycodone | 53 (34, 74) | <.0001 | <0.0001 |
| Frequency Abuse/Injection | −99.9 (−100, −99) | <0.0001 | 83 (46, 130) | <.0001 | <0.0001 | ||
|
Frequency Abuse/Snorting |
−96 (−99, −90) | <0.0001 | 38 (16, 66) | 0.0004 | <0.0001 | ||
| Adverse Event Reports to the Sponsor |
Fatality Reports Year Three |
−60 (−79, −24) | <0.0001 | Nonfatal AEs for OxyContin (total) | NA | NA | NA |
|
Overdose Fatality Reports Year Three |
−65 (−83, −27) | <0.0001 | NA | NA | NA | ||
| RADARS Drug Diversion |
Drug Diversion |
−66 (−74, −55) | <0.001 | Schedule II opioid pillsb | 6 (−8, 24) | 0.418 | <0.001 |
| Insured Population (MarketScan) | Overdose | −34 (−53, −7) | 0.0189 | ER Morphine | 17 (−19, 69) | 0.4059 | 0.0272 |
| Use Disorder | −27 (−34, −19) | <0.0001 | 25 (8, 45) | 0.0027 | <0.0001 | ||
OC consists of postreformulation of reformulated OC in studies that differentiate between the two formulations, and of both original and reformulated OC in RADARS Drug Treatment and Drug Diversion, Adverse Event Report, and MarketScan studies.
Consists of all other (non−OxyContin) Schedule II opioid analgesic tablets and capsules with the active agents of hydrocodone, hydromorphone, morphine, oxymorphone, and immediate–release oxycodone products. Methadone was excluded since it is used for both analgesia and opioid dependence, as were transdermal patches.
RADARS, Researched Abuse, Diversion and Addiction−Related Surveillance System; NPDS, National Poison Data System; NAVIPPRO, National Addictions Vigilance Intervention and Prevention Program; SKIP, Survey of Key Informants' Patients; OTP = Opioid Treatment Program.
Figure 3.
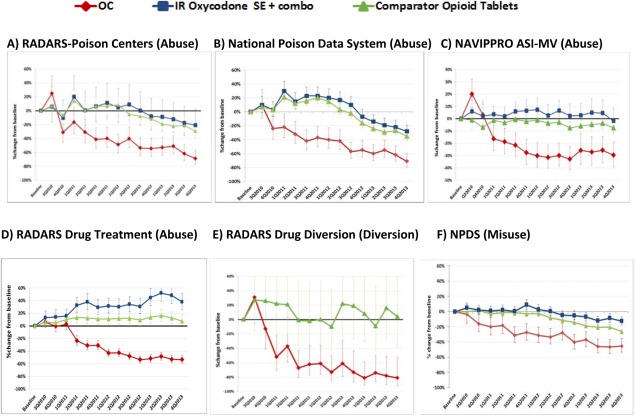
Trends in abuse, diversion, and misuse for extended release oxycodone (OC) and comparator opioids.
Overdose and poisoning
Rates of opioid overdose/poisoning diagnoses decreased 34%, from 0.42 per 100 person‐years of opioid use in the year before reformulation (51 cases among 85,978 people prescribed OC) to 0.28 per 100 person‐years of opioid use after (30 cases among 87,935 people prescribed OC on average per year), while that for the four comparator opioids remained stable or unchanged (Figure 4 a). The absolute rate of opioid overdose diagnoses were lower among patients dispensed OC than for the four comparator opioids: 0.28 per 100 person‐years of opioid use in people prescribed OC, compared to 0.51 for ER morphine, 0.49 for ER oxymorphone, 0.40 for IR oxycodone single entity, and 0.68 per 100 person‐years for IR hydromorphone single entity during the intervention period (Figure 4 a).
Figure 4.
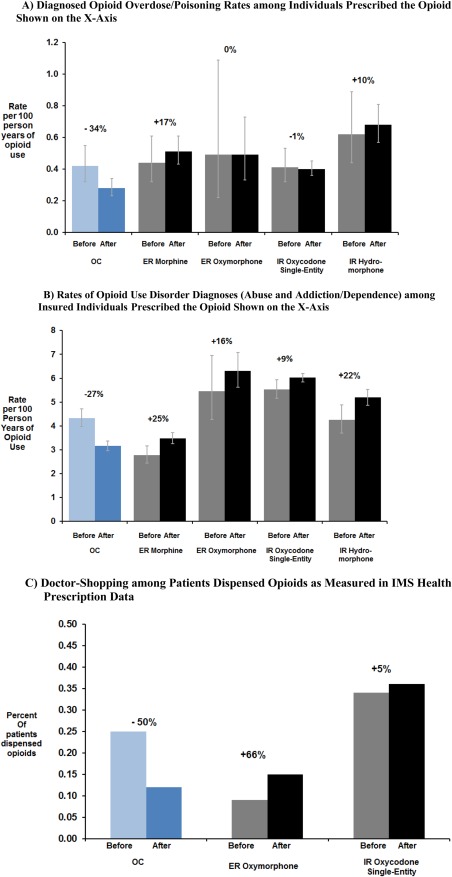
Changes from before to after reformulation of extended‐release oxycodone (OC).
Abuse
The magnitude of reductions in rates of abuse of OC ranged from an 85% decrease among the cohort of OC abusers in Kentucky to a 30% decrease in substance abuse treatment in the RADARS SKIP Program. Rates of abuse of OC decreased 55% in the RADARS‐PC Study using population‐adjusted rates (Figure 2 a) and decreased 44% using prescription‐adjusted rates (Figure 2 c), while abuse of all other Schedule II opioid pills decreased 7% using population‐adjusted rates (Figure 2 b) and decreased 14% using prescription‐adjusted rates (Figure 2 d) in the same period. In the National Survey of Drug Use and Health27 past year initiation of nonmedical use of OC abuse decreased by 18%, 37%, 26%, and 49% in 2011, 2012, 2013, and 2014, relative to 2009, respectively.
Rates of diagnosed opioid use disorder among individuals dispensed OC decreased 27%, from 4.3 to 3.2 per 100 person‐years of OC use (Figure 4 b). In contrast, rates of diagnosed opioid use disorder for two ER and two IR comparator opioids increased during the same time. Absolute rates of opioid use disorder diagnoses were lower for OC after its reformulation than that for comparator opioids: 3.1 per 100 person‐years of opioid use in people prescribed OC, compared to 3.6 for ER morphine, 6.5 for ER oxymorphone, 6.4 for IR single entity oxycodone, and 5.5 per 100 person‐years for IR hydromorphone during the intervention period.
Misuse
Rates of patient misuse of OC decreased 43% in the RADARS‐PC Study using population‐adjusted rates (Figure 2 a) and decreased 29% using prescription‐adjusted rates (Figure 2 c), while patient misuse of all other Schedule II opioid pills decreased 6% using population‐adjusted rates (Figure 2 b) and decreased 12% using prescription‐adjusted rates (Figure 2 d) in the same period.
Diversion and doctor‐shopping
OC diversion events reported by law enforcement officials decreased 66% in the RADARS Drug Diversion Study. These effects were specific to reformulated OC and no similar decreases occurred for other opioid analgesics, as shown in Figure 2 b. The proportion of individuals dispensed OC who met a threshold of doctor‐shopping (overlapping prescriptions from ≥2 doctors and ≥3 pharmacies in 6 months) decreased 50%, from 0.25% to 0.12% of people (Figure 4 c). Doctor shopping increased 66% for ER oxymorphone and 5% for IR single‐entity oxycodone during the same period.
When adjusted for prescription numbers (Figure 2 c), the reductions in rates of misuse, abuse, opioid use disorder, overdose, death, and drug diversion for reformulated OC persisted. Prescription‐adjusted rates for OC had similar, but slightly smaller, decreases from baseline in abuse and other outcomes as compared to population rates. The effects were specific to reformulated OC and not to other opioid analgesics, as shown in Figure 2 d. In people prescribed other opioids, no decreases occurred on average during the postintervention period.
Mortality and fatal overdose
Pharmaceutical companies are required to report product‐related adverse events they receive to the FDA. Reports of deaths involving OC spontaneously reported to the manufacturer and containing date of death decreased 60% and overdose fatalities decreased 65%. By the third year after reformulation (2013), death and overdose death reported to the manufacturer decreased 80% and 85%, respectively. Reports to the company of nonfatal adverse events involving OC (e.g., constipation, nausea) did not decrease after introduction of reformulated OC, suggesting that there was no generally decreased reporting of adverse events associated with OC.
Summary of findings
Table 2 displays abuse‐related rate changes for OC and comparators pre‐ and postreformulation. Among the cohort of prescription opioid abusers in rural Kentucky, OC abuse decreased 85% postreformulation, with injecting of OC decreasing by 99% from an average of 8.6 days/month to 0.01 days/month (i.e., one of 189 individuals reporting injecting on 1 day).42 In all these studies' outcomes, OC decreases were greater than those for comparator opioids. Decreases in abuse rates occurred for both nonoral and oral abuse in the RADARS‐PC, NPDS, and NAVIPPRO Drug Treatment Studies, although nonoral abuse decreased more than oral abuse.
Temporal trends in five national surveillance systems (Figure 3) indicate OC abuse, diversion, and misuse began to decrease within one‐ to three‐quarters after reformulated OC introduction and continued to decrease throughout the study period. For comparator opioids, abuse and diversion rates remained unchanged or increased until 2Q2012, and then began to decrease in poison center studies (RADARS‐PC and NPDS), but not drug treatment center (NAVIPPRO ASI‐MV) or drug diversion (RADARS) studies. Nonpatient accidental exposures for OC also decreased significantly more than for comparator opioids (data not shown). The majority (60%) of accidental exposures to OC occurred among children 12–30 months old; accidental exposures to OC in this age group decreased 55% after reformulation of OC.
DISCUSSION
Across the 10 studies, there were consistent and significant decreases in all of the abuse‐related outcomes, including reported mortality, overdose/poisoning, abuse, diagnosed opioid use disorder, and doctor‐shopping associated with reformulated OC with abuse‐deterrent properties. In addition, drug diversion and doctor‐shopping rates of OC decreased. The overall pattern of results is consistent with those reported in other publications on this topic,20, 24, 25, 26, 28 and are consistent with papers describing individual study results from the 10 studies.18, 19, 22, 42
During the observation period, there were national and regional programs whose aim was to decrease prescription opioid abuse, e.g., prescription drug monitoring program inception or increased use, Florida's “pill mill” legislation, classwide ER Opioid REMS, drug take‐back programs, increased law enforcement efforts, and drug utilization reviews by managed care organizations.29, 30, 31 These programs were general opioid interventions that were not targeted specifically at OC. The timing of OC abuse decreases occurred shortly after the introduction of reformulated OC, while decreases for other opioid analgesics began ∼2 years after introduction of reformulated OC. The earlier timing of the decreases for OC vs. other Schedule II opioids and other oxycodone tablets shown in Figure 3 suggest the reductions in OC abuse were not due to general interventions. Many interventions began after the decrease in OC abuse, e.g., the REMS began in July 2012 and Florida opioid deaths decreased substantially after July 2011.29, 30, 32 State prescription drug monitoring programs were generally voluntary and intermittently used when OC was reformulated, and published evaluations around the time of reformulated OC introduction reported either slowing trends or no effects, e.g., no change in poison center exposures or drug overdose fatalities occurred between 2010 and 2013.33, 34 In addition to the timing, the magnitude of the OC abuse decreases, ranging from 27% to 65%, observed consistently in independent studies by the end of 2011, was greater than that seen for other opioids during the same time period.
Consistent with the physicochemical mechanisms of abuse deterrence, the greatest decreases in OC abuse were by nonoral routes involving injecting and snorting, which typically lead to more serious health outcomes than oral abuse,4 such as transmission of infections through the sharing of needles.35 However, rates of oral abuse of OC also decreased significantly after reformulation. The decrease in oral abuse could be due to a negative “halo” effect resulting from more difficulty injecting/snorting tablets, decreased availability on the black market due to less overall desirability, deterrence of breaking and chewing for oral immediate‐release ingestion, or a combination of all three. After introduction of reformulated OC, the rates of opioid use disorder and overdose/poisoning were lower for OC than other ER or IR single‐entity opioids, with high rates among people dispensed IR opioids.
Two conducted studies were not included in this summary: 1) a published Internet monitoring study44 showing that recipes to abuse reformulated OC posted on Websites on frequented by drug abusers were rarely reported as being effectively used to inject or snort reformulated OC, and recipe posts decreased over time; 2) a Kaiser Permanente study of overdose that was limited by the 70% decrease in OC prescribing in the health plan during the study, along with other brand medicines.
This study program assessed the effects of reformulation of OC with abuse‐deterrent properties on OC abuse, and not the effects on overall opioid or heroin abuse that have been assessed elsewhere. Compton et al.22 noted that heroin increases preceded changes in prescription‐opioid policies, including reformulation of OC,24 and there is no consistent evidence that opioid policies led to increases in heroin deaths, although data are relatively sparse. LaRochelle et al.20 reported that the increasing trend of opioid overdoses flattened after OC was reformulated, which coincided with heroin overdoses increases, but the absolute number of decreased prescription opioid overdoses was greater than the increase for heroin overdoses. Cicero and Ellis26 reported an increase in heroin abuse associated with the introduction of OC in the RADARS Survey of Key Informants (SKIP) program, which is one of the studies within this 10‐study program; however, Dart et al.25 pointed out that this was not seen consistently in all surveillance programs in the RADARS System.24 Other factors to consider are that OC represents less 3% of opioid prescriptions in the US (Figure 1), and the rate of prescribing of ER oxycodone makes up 2.3% of the total opioid prescribing rate in eight states36; thus, OC reformulation alone is unlikely to be a major driver of total opioid abuse. For example, in the NPDS study the decrease in the number of OC abuse cases reported to poison centers was small for OC (n = 284) compared to the increase for heroin (n = 995) annually.
Cassidy et al. reported that overall prescription opioid abuse did not change after OC reformulation, but increased buprenorphine products used to treat opioid addiction/dependence accounted for most of the increased opioid abuse after OC reformulation37 during which buprenorphine prescriptions for opioid dependence increased substantially.
One potential benefit of OADPs may be deterrence of progression from oral to nonoral abuse among patients using an OADP, since prescription opioid abuse typically begins with oral use and progresses in some people to nonoral abuse.3 Nonoral abuse is associated with more serious health outcomes, such as HIV, hepatitis C, and other infections. In addition, abusers report being “grabbed” by addiction which starting to snort or inject opioids.5 This warrants further research.25, 26
The reason for decreasing nonpatient accidental exposures for OC after its reformulation among 12–30‐month‐old children is not clear. One possibility is that toddlers who put tablets in their mouths that are harder to crush or dissolve and remain intact longer may result in fewer calls to poison centers for emergency assistance than toddlers whose parents found dissolved or partially ingested tablets in their mouths. There were decreases in accidental exposures by chewing in the RADARS System poison center study.
The studies have limitations. Several studies relied on respondents' self‐report of specific drugs abused. This could lead to misclassification of OC as other opioids and other opioids as OxyContin. Given that OC is ∼3% of all Schedule II opioid prescriptions and 10% of oxycodone prescriptions and that OC is a brand (OxyContin) well‐recognized by abusers, it is more likely that other opioids would be misclassified as OC than OC would be misclassified as other opioids. If reported abuse of OC was due to abuse of other opioids, e.g., oxycodone IR single entity 30 mg tablets, this would underestimate the effect of the abuse‐deterrent formulation. This limitation of self‐reporting is complemented in the study program by those studies that use more accurate measures of medicines abused (e.g., prescription record databases, reports by healthcare providers or law enforcement officers), all of which yielded similar results.
Another potential limitation is decreased OC prescribing and, consequently, decreased availability for abuse. However, OC prescriptions decreased 13% in the 3 years after reformulation, according to IMS National Prescription Audit data analyzed for this study program, relative to larger decreases in OC abuse outcomes. In addition, prescription‐adjusted rates showed similar, although slightly smaller, decreases in OC abuse than population‐adjusted rates.
Another limitation is potential confounding if prescribers became more cautious in prescribing OC, especially to high‐risk patients, or used prescription monitoring programs more simultaneous to reformulation of OC. However, data suggest this is unlikely. Abuse of OC increased for several years preceding reformulation, yet decreased precipitously shortly after reformulation, while abuse of other oxycodone tablets increased 20%24 and of ER oxymorphone increased 236%.15 Decreased supply from less prescribing should lead to increased street price and thefts, yet OC's street price decreased, as did thefts, robberies, and black market sales of OC, as OC abuse decreased after OC reformulation.19 A study of Internet chat for drug abuse identified that posts about OC abuse increased in negativity after OC reformulation and became more positive for other opioids.44
Another potential limitation is the selection of comparator opioids. Comparators are used as a measure of prescription opioid abuse changes from general abuse interventions, e.g., prescription monitoring programs, so the effect of OC's abuse‐deterrent properties could be differentiated from general opioid interventions. To reflect changes in abuse of the overall prescription opioid therapeutic class, we used all Schedule II opioids as a comparator. To reflect changes in abuse of oxycodone, the active ingredient in OC, IR oxycodone was used which had a similar pattern of opioid abuse as OC/ER oxycodone.14, 42 Space limited inclusion of other comparators, such as ER morphine or ER oxymorphone, but these were used in individual studies. Abuse and addiction diagnoses decreased 27% for OC, increased 17% for ER morphine, and 16% for ER oxymorphone in commercially insured patients (Figure 4 b). In drug treatment centers, ER oxymorphone abuse increased 236%, ER morphine abuse increased 1%, and OC abuse decreased 33% within 2 years after OC reformulation.15 In poison centers, ER morphine abuse decreased by 22% and prescriptions increased by 28% by 2013, so prescription‐adjusted ER morphine abuse decreased 33%.
Another consideration is how to account for opioid availability in the community, as recommended by FDA researchers.38 We did not require that changes for OC be significantly different from changes for all opioid analgesic groups using prescription‐adjusted rates. One issue with dividing abuse cases by prescriptions when calculating changes is it assumes that the change in abuse is proportional to the change in prescriptions, which is not consistent with observed data.39 Prescription changes for a specific opioid due to health plan formulary decisions may not result in proportional increases in availability in the distribution channel of abusers, and might not result in proportional increases in abuse, especially for less desirable opioids for abuse. Appropriate methods to account for adjusting for prescription changes over time are needed.40
In conclusion, after the introduction of reformulated OC with abuse‐deterrent properties, there were decreases in associated abuse, overdose diagnoses, and diversion that occurred consistently across 10 studies that used different measures of abuse and its consequences. Decreases in observations of abuse began within a few months after the introduction of reformulated OC and persisted over the 3‐year assessment period. There were reductions in both nonoral and oral abuse of OC, although greater decreases were observed for nonoral abuse, consistent with the physicochemical abuse‐deterrent properties. The decreases for OC were both larger and occurred earlier than that for comparator opioids without abuse‐deterrent properties, suggesting that the decreases for OC were not due to general opioid interventions such as prescription monitoring programs or environmental trends such as less opioid prescribing. Individual studies had limitations, but limitations in one study were generally offset by other studies without that limitation. Abuse of OC persisted, albeit at lower rates. These results are applicable to the particular abuse‐deterrent technology assessed and may not be generalizable to other formulations intended to be abuse deterrent. Abuse‐deterrent opioids may potentially mitigate abuse and overdose risks as part of a multifaceted approach, among patients with pain who benefit from opioid analgesics.41
METHODS
Details of the program design are previously described in several study‐specific publications.19, 24, 25, 42, 43, 44, 45 Abuse was defined as the intentional use of drugs for nontherapeutic purposes of achieving positive psychological or physical effects.46 Misuse was defined as the intentional inappropriate use of drugs for therapeutic purposes outside of directions in labels, prescriptions, or directions by healthcare practitioners.46 Diversion, which describes any intentional act that results in transferring a prescription medication from lawful to unlawful distribution or possession, was used as a measure of demand for abuse.
Design overview
A quasi‐experimental design was used to assess changes from 1 year before (3Q2009–2Q2010, baseline) to 3 years after introduction of reformulated OC (1Q2011–4Q2013, intervention period). A 6‐month transition period (3Q2010–4Q2010) was excluded from calculation to allow for circulating original OC to be depleted. To determine whether changes resulted from reformulation of OC rather than temporal trends affecting all opioid analgesics, changes for OC were compared to changes for comparator opioids. Two analyses were used: 1) quantification of the change from 1 year before to 3 years after (“means” analysis) and 2) a descriptive trend to compare the timing of abuse decreases for OC vs. comparator opioids. Abuse cases of original OC persisted into the postreformulation period. Three studies did not include abuse cases of original OC that persisted after reformulation because of difficulties in differentiating between original and reformulated OC (NAVIPPRO and two poison center studies), while the remaining studies included both original and reformulated (any) OC abuse cases after reformulation.
Settings and populations
A total of 10 studies were conducted that addressed five outcomes required by the FDA Guidance.7 The study program covered a broad range of different study populations (Table 1). The data sources for these studies consisted of 10 databases:
National Poison Data System (NPDS), which collects reports from all US poison centers;
RADARS System Poison Center (PC) program, which covers 87% of the US population with quality‐control review of structured interview notes from each poison center encounter;
National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO) Addiction Severity Index‐Multimedia Version (ASI‐MV) system covering over 1,000 substance abuse treatment centers in 36 states;
RADARS System Outpatient Treatment Program (OTP) Drug Treatment Study covering over 70 public methadone maintenance clinics;
RADARS System Study of Key Informants' Patients (SKIP) Program, which collects opioid abuse and misuse among individuals at private substance abuse treatment centers;
A study of individuals in rural Kentucky who abused OC conducted by the University of Kentucky;
MarketScan, an administrative healthcare database of ∼100 million commercially insured individuals;
Fatal adverse events reported to the manufacturer and subsequently to FDA;
RADARS System Drug Diversion Program, a national network of law enforcement officials reporting drug diversion events in their jurisdictions;
IMS Health prescription database,47 a national prescription database used to assess doctor/pharmacy shopping and national trends in prescribing volume.
Table 1.
Study program to assess impact of reformulated OxyContin
| Study | Abuse | Misuse | Addiction | Overdose | Death | Diversion | Study Population |
|---|---|---|---|---|---|---|---|
| National Poison Data System | ✓ | ✓ | General US population | ||||
| RADARS System Poison Centers | ✓ | ✓ | General US population | ||||
| NAVIPPRO ASI‐MV | ✓ | Abusers at treatment centers | |||||
| RADARS Drug Treatment: OTP and SKIP | ✓ | Abusers at treatment centers | |||||
| Abuser Cohort in Kentucky | ✓ | Cohort of oxycodone abusers | |||||
| Retrospective Cohort in MarketScan Claims Database | ✓ | ✓ | ✓ | Patients dispensed opioids for pain | |||
| Fatalities reported to pharmacovigilance system | ✓ | ✓ | General US population | ||||
| RADARS: Drug Diversion/Street Diversion | ✓ | Law enforcement agents' reports | |||||
| Doctor/pharmacy‐Shopping Patients | ✓ | Patients dispensed opioids | |||||
| Drug Utilization Study | Contextual | National prescription database | |||||
The FDA considered some studies as primary studies, due to their national scope, clear route‐specific abuse measures, and statistical power; however, the studies considered primary evolved over time. In addition, changes in reported OxyContin abuse were assessed using data from the National Survey of Drug Use and Health.48
Outcomes
Outcomes used to measure abuse, misuse, overdose, death, and diversion included: 1) calls to poison centers for opioid abuse problems by any route, 2) abuse by route obtained via self‐report, 3) reported diversion events (arrests and drug seizures), 4) doctor/pharmacy shopping (overlapping prescriptions by ≥2 prescribers and ≥3 pharmacies) using a validated definition,48 5) ICD‐9 diagnosis codes for opioid overdose (965.0x) and opioid use disorder consisting of opioid abuse (305.5x) or dependence/addiction (304.0x and 304.7x), 6) poison center exposures for opioid misuse (therapeutic errors, misuse, and accidental ingestion by nonpatients), and 7) spontaneous reports of fatalities and overdose fatalities associated with OC. These are collectively referred to as abuse‐related outcomes in this article. Medical examiner and national vital statistics databases were not used because they do not differentiate between ER and IR oxycodone and do not reliably contain product‐specific information.
Statistical analysis
All analyses were performed using SAS v. 9.2 (SAS Institute, Cary, NC) and MS Excel 2010. Rate, ratios, means, and percent changes with 95% confidence intervals and P‐values were calculated using Poisson regression (link = log) and the following model:
The dependent variable was abuse cases, covariates included were time (0/1 for pre‐ and postreformulation), opioid groups (0/1 for OC/comparator), and opioid group by time, and the offset used the log of census population, prescription numbers or person‐years of exposure. The percent change and confidence intervals were calculated by subtracting rate ratios from 1 and multiplying by 100. P‐values were not used to formally test hypotheses but more descriptively to assess whether observed changes were likely due to chance.
CONFLICT OF INTEREST
Paul Coplan, Aditi Kadakia, Venkatesh Harikrishnan, and J. David Haddox are full‐time employees of Purdue Pharma L.P. Howard Chilcoat was an employee of Purdue Pharma L.P. and is currently employed by Indivior. The studies were supported by Purdue Pharma L.P.
AUTHOR CONTRIBUTIONS
P.M.C., H.D.C., S.B., and V.H. wrote the article; P.M.C., H.D.C., S.B., E.M.S., J.D.H., and R.C.D. designed the research; P.M.C., H.D.C., S.B., A.K., V.H., and R.C.D. performed the research; P.M.C., H.D.C., S.B., E.M.S., A.K., V.H., and R.C.D. analyzed the data; P.M.C. and A.K. contributed new reagents/analytical tools.
ACKNOWLEDGMENTS
We thank Craig Landau, MD, Angela DeVeaugh‐Geiss, PhD, Hrishikesh Kale, MS, Nelson Sessler, PharmD, Melinda Philbrook, Louis Alexander, PhD, Gary Stiles, MD, PhD, Todd Baumgartner, MD, MS, and Gail Cawkwell, MD, PhD, from Purdue Pharma L.P. for their work on the study program and review or preparation of the article, and Jennifer Havens, PhD, and Carl Leukefeld, PhD, at the University of Kentucky for work on the Kentucky study.
References
- 1. Jones, C.M. , Mack, K.A. & Paulozzi, L.J. Pharmaceutical overdose deaths, United States, 2010. JAMA. 309, 657–659 (2013). [DOI] [PubMed] [Google Scholar]
- 2. American Academy of Pain Medicine . Use of Opioids for the Treatment of Chronic Pain. Chicago: American Academy of Pain Medicine; 2013. [Google Scholar]
- 3. Hays, L. , Kirsh, K.L. & Passik, S.D. Seeking drug treatment for OxyContin abuse: a chart review of consecutive admissions to a substance abuse treatment facility in Kentucky. J. Natl. Compr. Canc. Netw. 1, 423–428 (2003). [DOI] [PubMed] [Google Scholar]
- 4. Katz, N. et al Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am. J. Drug Alcohol Abuse 37, 205–217 (2011). [DOI] [PubMed] [Google Scholar]
- 5. Wawrzyniak, K.M. et al Root cause analysis of prescription opioid overdoses. J. Opioid. Manag. 11, 127–137 (2015). [DOI] [PubMed] [Google Scholar]
- 6. Mastropietro, D.J. & Omidian, H. Abuse‐deterrent formulations: part 2: commercial products and proprietary technologies. Expert. Opin. Pharmacother. 16, 305–323 (2015). [DOI] [PubMed] [Google Scholar]
- 7. FDA . Abuse‐Deterrent Opioids — Evaluation and Labeling Guidance for Industry. <http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm334743.pdf> (2015). Accessed 20 August 2015.
- 8. Epidemic: Responding to Americas Prescription Drug Abuse Crisis. Executive Office of the President of the United States, 2011.
- 9. Health Canada: Draft Guidance Document — tamper‐resistant formulations of opioid drug product submissions. <http://www.hc‐sc.gc.ca/dhp‐mps/consultation/drug‐medic/draft_guid_opioid_ebauche_ld‐eng.php>. Accessed 17 August 2015.
- 10. Alexander, L. , Mannion, R.O. , Weingarten, B. , Fanelli, R.J. & Stiles, G.L. Development and impact of prescription opioid abuse deterrent formulation technologies. Drug Alcohol. Depend. 138, 1–6 (2014). [DOI] [PubMed] [Google Scholar]
- 11. Abramowicz, M. , Zuccotti, G. & Pflomm, J.M. Abuse‐deterrent opioid formulations. JAMA. 314, 1744–1745 (2015). [DOI] [PubMed] [Google Scholar]
- 12. de Wit, H. , Bodker, B. & Ambre, J. Rate of increase of plasma drug level influences subjective responses in humans. Psychopharmacology 107, 352–358 (1992). [DOI] [PubMed] [Google Scholar]
- 13. OxyContin [package insert]. Stamford, CT: Purdue Pharma L.P. <http://app.purduepharma.com/xmlpublishing/pi.aspx?id=o> (2015). Accessed 26 February 2015.
- 14. Butler, S.F. , Black, R.A. , Cassidy, T.A. , Dailey, T.M. & Budman, S.H. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm. Reduct. J. 8, 29–45 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butler, S.F. et al Abuse rates and routes of administration of reformulated extended‐release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J. Pain 14, 351–358 (2013). [DOI] [PubMed] [Google Scholar]
- 16. Cone, E.J. , Giordano, J. & Weingarten, B. An iterative model for in vitro laboratory assessment of tamper deterrent formulations. Drug Alcohol Depend. 131, 100–105 (2013). [DOI] [PubMed] [Google Scholar]
- 17. Harris, S.C. et al Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse‐deterrent controlled‐release tablets in recreational opioid users. J. Clin. Pharmacol. 54, 468–477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration . Joint Meeting of the Anesthetic and Life Support Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee. October 21‐22 2010. Summary Minutes. <http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/AnestheticAndLifeSupportDrugsAdvisoryCommittee/UCM236242.pdf>. Accessed 10 June 2014.
- 19. Severtson, S.G. et al Reduced abuse, therapeutic errors, and diversion following reformulation of extended‐release oxycodone in 2010. J. Pain 14, 1122–1130 (2013). [DOI] [PubMed] [Google Scholar]
- 20. LaRochelle, M.R. , Zhang, F. , Ross‐Degnan, D. & Wharam, J.F. Rates of opioid dispensing and overdose after introduction of abuse‐deterrent extended‐release oxycodone and withdrawal of propoxyphene. JAMA Intern. Med. 175, 978–987 (2015). [DOI] [PubMed] [Google Scholar]
- 21. Cassidy, T. , DasMahapatra, P. , Black, R. , Wieman, M.S. & Butler, S.F. Changes in prevalence of prescription opioid abuse after introduction of an abuse‐deterrent opioid formulation. Pain Med. 15, 440–451 (2014). [DOI] [PubMed] [Google Scholar]
- 22. Compton, W.M. , Jones, C.M. & Baldwin, G.T. Relationship between nonmedical prescription‐opioid use and heroin use. N. Engl. J. Med. 374, 154–163 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cicero, T.J. , Ellis, M.S. & Surratt, H.L. Effect of abuse‐deterrent formulation of OxyContin. N. Engl. J. Med. 367, 187–189 (2012). [DOI] [PubMed] [Google Scholar]
- 24. Coplan, P.M. , Kale, H. , Sandstrom, L. , Landau, C. & Chilcoat, H.D. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended‐release oxycodone with abuse‐deterrent characteristics. Pharmacoepidemiol. Drug Saf. 22, 1274–1282 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dart, R.C. et al Trends in opioid analgesic abuse and mortality in the United States. N. Engl. J. Med. 372, 241–248 (2015). [DOI] [PubMed] [Google Scholar]
- 26. Cicero, T.J. & Ellis, M.S. Abuse‐deterrent formulations and the prescription opioid abuse epidemic in the United States: lessons learned From OxyContin. JAMA Psychiatry 72, 424–430 (2015). [DOI] [PubMed] [Google Scholar]
- 27. Substance Abuse and Mental Health Services Administration . Results from the 2014 National Survey on Drug Use and Health. Detailed Tables. http://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs2014/NSDUH-DetTabs2014.pdf. [Google Scholar]
- 28. Degenhardt, L. et al Evaluating the potential impact of a reformulated version of oxycodone upon tampering, non‐adherence and diversion of opioids: the National Opioid Medications Abuse Deterrence (NOMAD) Study Protocol. Addiction 110, 226–237 (2015). [DOI] [PubMed] [Google Scholar]
- 29. Delcher, C. , Wagenaar, A.C. , Goldberger, B.A. , Cook, R.L. & Maldonado‐Molina, M.M. Abrupt decline in oxycodone‐caused mortality after implementation of Florida's prescription drug monitoring program. Drug Alcohol Depend. 150, 63–68 (2015). [DOI] [PubMed] [Google Scholar]
- 30. Daubresse, M. et al Impact of a drug utilization review program on high‐risk use of prescription controlled substances. Pharmacoepidemiol. Drug Saf. 23, 419–427 (2014). [DOI] [PubMed] [Google Scholar]
- 31. Fulton‐Kehoe, D. et al Opioid poisonings in Washington State Medicaid: trends, dosing, and guidelines. Med. Care. 53, 679–685 (2015). [DOI] [PubMed] [Google Scholar]
- 32. FDA News Release . FDA introduces new safety measures for extended‐release and long‐acting opioid medications. <http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm310870.htm>. (2012). Accessed 26 February 2015.
- 33. Reifler, L.M. et al Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 13, 434–442 (2012). [DOI] [PubMed] [Google Scholar]
- 34. Sauber‐Schatz, E.K. , Mack, K.A. , Diekman, S.T. & Paulozzi, L.J. Associations between pain clinic density and distributions of opioid pain relievers, drug‐related deaths, hospitalizations, emergency department visits, and neonatal abstinence syndrome in Florida. Drug Alcohol Depend 133, 161–166 (2013). [DOI] [PubMed] [Google Scholar]
- 35. Strathdee, S.A. & Beyrer, C. Threading the needle — how to stop the HIV outbreak in rural Indiana. N. Engl. J. Med. 373, 397–399 (2015). [DOI] [PubMed] [Google Scholar]
- 36. Paulozzi, L.J. , Strickler, G.K. , Kreiner, P.W. & Koris, CM . Controlled substance prescribing patterns — prescription behavior surveillance system, eight states, 2013. MMWR. 64, 1–14 (2015). [DOI] [PubMed] [Google Scholar]
- 37. Cassidy, T. , DasMahapatra, P. , Black, R. , Wieman, M.S. & Butler, S.F. Changes in prevalence of prescription opioid abuse after introduction of an abuse‐deterrent opioid formulation. Pain Med. 15, 440–451 (2014). [DOI] [PubMed] [Google Scholar]
- 38. Secora, A.M. , Dormitzer, C.M. , Staffa, J.A. & Dal Pan, G.J. Measures to quantify the abuse of prescription opioids: a review of data sources and metrics. Pharmacoepidemiol. Drug Saf. 23, 1227–1237 (2014). [DOI] [PubMed] [Google Scholar]
- 39. Secora, A. , Trinidad, J. , Dormitzer, C. & Staffa, J. Metrics and methodologies to quantify the abuse of prescription opioids. Poster presented at College on Problems of Drug Dependence, San Diego, CA, 20 June 2013.
- 40. Secora, A. , Trinidad, J. , Zhang, R. , Wu, Y. & Gill, R. Drug availability adjustments in population‐based studies of prescription opioid abuse. Ann. Epidemiol. 24, 694 (2014). [DOI] [PubMed] [Google Scholar]
- 41. Califf, R.M. , Woodcock, J. & Ostroff, S. A proactive response to prescription opioid abuse. N. Engl. J. Med. 2016; e‐pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42. Havens, J.R. , Leukefeld, C.G. , DeVeaugh‐Geiss, A. , Coplan, P. & Chilcoat, H.D. Changes in opioid abuse in rural Kentucky after the reformulation of extended release oxycodone. Drug Alcohol. Depend. 139, 9–17 (2014). [DOI] [PubMed] [Google Scholar]
- 43. Sessler, N.E. et al Reductions in reported deaths following the introduction of extended‐release oxycodone (OxyContin) with an abuse‐deterrent formulation. Pharmacoepidemiol. Drug Saf. 23, 1238–1246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McNaughton, E.C. et al Monitoring of Internet forums to evaluate reactions to the introduction of reformulated OxyContin to deter abuse. J. Med. Internet Res. 16, e119 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Coplan, P. , Chilcoat, H. , Baumgartner, T. & Landau, C. Design of a post‐marketing study program to assess the effects of a reformulated extended‐release oxycodone tablet on its abuse. Pharmacoepidemiol. Drug Saf. 21(suppl. s3), 446 [Abstract #963] (2012). [Google Scholar]
- 46. Smith, S.M. et al Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 154, 2287–2296 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. IMS Health National Prescription Audit. <http://www.imshealth.com/portal/site/imshealth>. Accessed 26 February 2015.
- 48. Substance Abuse and Mental Health Services Administration . Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration. <http://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf>. (2015).
- 49. Cepeda, M.S. , Fife, D. , Chow, W. , Mastrogiovanni, G. & Henderson, S.C. Assessing opioid shopping behavior: a large cohort study from a medication dispensing database in the US. Drug Saf. 35, 325–334 (2012). [DOI] [PubMed] [Google Scholar]


