Abstract
Summary
Changes in bone mineral density and bone strength following treatment with zoledronic acid (ZOL) were measured by quantitative computed analysis (QCT) or dual-energy X-ray absorptiometry (DXA). ZOL treatment increased spine and hip BMD vs placebo, assessed by QCT and DXA. Changes in trabecular bone resulted in increased bone strength.
Introduction
To investigate bone mineral density (BMD) changes in trabecular and cortical bone, estimated by quantitative computed analysis (QCT) or dual-energy X-ray absorptiometry (DXA), and whether zoledronic acid 5 mg (ZOL) affects bone strength.
Methods
In 233 women from a randomized, controlled trial of once-yearly ZOL, lumbar spine, total hip, femoral neck, and trochanter were assessed by DXA and QCT (baseline, Month 36). Mean percentage changes from baseline and between-treatment differences (ZOL vs placebo, t-test) were evaluated.
Results
Mean between-treatment differences for lumbar spine BMD were significant by DXA (7.0%, p<0.01) and QCT (5.7%, p<0.0001). Between-treatment differences were significant for trabecular spine (p=0.0017) [non-parametric test], trabecular trochanter (10.7%, p<0.0001), total hip (10.8%, p<0.0001), and compressive strength indices at femoral neck (8.6%, p=0.0001), and trochanter (14.1%, p<0.0001).
Conclusions
Once-yearly ZOL increased hip and spine BMD vs placebo, assessed by QCT vs DXA. Changes in trabecular bone resulted in increased indices of compressive strength.
Keywords: Bisphosphonates, Bone densitometry, Bone QCT, Clinical trials, Osteoporosis
Introduction
Once-yearly treatment with zoledronic acid is known to be effective in decreasing the risk of fracture in women with postmenopausal osteoporosis [1]. In a 3-year study, zoledronic acid reduced the risk of vertebral fracture by 70% and the risk of hip fracture by 41% compared with placebo persistently over 3 years [1]. In addition, zoledronic acid was associated with a significant improvement in bone mineral density (BMD) as measured by dual-energy X-ray absorptiometry (DXA).
Measurement of BMD by DXA has a number of limitations. Such measurements integrate the cortical and trabecular compartments of bone. Evaluations of spine BMD in the anteroposterior projection include the posterior elements of the vertebrae, which do not contribute to the strength of the vertebral body, and aortic calcification (if present). Also, DXA measurements are influenced by degenerative changes to the vertebrae, such as osteophytes and endplate sclerosis, and these contributions do not add to the strength of the bone. In addition, a number of studies have suggested that changes in BMD as measured by DXA with bisphosphonate treatment only partially explain the decreases in fracture risk seen with these agents. Measurement of the spine by quantitative computed tomography (QCT) however, allows study of the trabecular compartment of the vertebral body, avoiding the endplates and osteophytes, and does not include the posterior elements or aortic calcification. Such measurements may therefore give a clearer picture of the effects of a drug on the strength of the vertebrae. Similarly, QCT measurements of the proximal femur allow the study of cortical and trabecular compartments and moreover, the sites measured are relevant to fracture: femoral neck measurements reflect the region at which femoral neck fractures occur, while trochanteric measurements reflect the region at which intertrochanteric fractures occur. Importantly, QCT measurements of the proximal femur allow calculation of more biomechanically relevant indices relating to bone strength than DXA, as they are based on volumetric rather than projectional data. For example, Cheng et al. (2007) reported a case–control study of Chinese women with hip fracture and calculated indices of bending/torsion strength (BSI) and compression strength (CSI) [2]. They found that CSI, but not BSI, was decreased in patients with hip fracture, and also found that some cortical bone indices, such as the ratio of cortical to total bone volume, were associated with the risk of hip fracture independent of volumetric BMD.
The study was conducted in a subgroup of the postmenopausal women studied in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly–Pivotal Fracture Trail (HORIZON-PFT) [1]. We hypothesized that treatment with zoledronic acid 5 mg would (1) result in increases in lumbar spine and proximal femur BMD, although the changes may differ when measured by QCT as compared with DXA; (2) result in increases in trabecular and cortical bone, although the magnitude of the changes may differ; and (3) result in changes in bone strength indices, and these may help explain the reduction in hip fracture risk observed in the HORIZON-PFT study.
Methods
Patients
In the HORIZON-PFT, a total of 3,889 patients were randomly assigned to receive a single 15-min infusion of zoledronic acid (5 mg; manufactured by Novartis Pharma, Basel, Switzerland) at baseline, 12 and 24 months, and 3,876 were assigned to receive a matching placebo [1]. Patients were subsequently followed for 36 months. Overall, 233 women (mean age, 74 years; range, 65–87 years) were recruited from six clinical sites for this substudy of QCT.
Dual X-ray absorptiometry
Measurement of the hip (total hip, femoral neck, and trochanter) by DXA was performed at baseline and at months 6, 12, 24, and 36 using a Hologic (Waltham, MA) or GE Lunar (Madison, WI) or Norland (Trumbull, CT) axial bone densitometer. Measurements of BMD at the lumbar spine were obtained for a subgroup of patients at the same timepoints.
Quantitative computed tomography
All QCT acquisition used CT scanners (Pittsburgh, GE Lightspeed 16 detector; Sheffield, Siemens Somatom 16 detector scanner; Hong Kong, GE Hi Speed advantage, GE LightSpeed; Buenos Aires, Picker PQ5000, Toshiba Xpress Gx; Quebec, GE LightSpeed). Patients were positioned supine on the CT table. An Image Analysis QCT calibration phantom (Image Analysis, Columbia KY, USA) was placed under the patient between the hips. The superior aspect of the helical scan was 5 mm above the acetabulum and the inferior limit was 5 mm below the lesser trochanter. Scan parameters were 3-mm section thickness (pitch=1), 80 kVp, and 140 and 280 mAs for spine and hip, respectively, with an in-plane pixel size of 0.94–0.97 mm depending on the model of scanner. The CT images were archived to DICOM CD-ROM and forwarded to the University of California, San Francisco (UCSF) for analysis by the UCSF QCT Reading Center.
The QCT image analysis of the left hip was performed as previously described, [2, 3] using an image analysis program. The software defined the periosteal boundaries of the hip, and defined measurement regions encompassing the greater and lesser trochanters, the femoral neck, and the entire hip. Within each region, the program characterized the volumetric BMD, volume and bone mineral content of the total tissue envelope, the cortical bone, and the trabecular bone. Areal BMD was computed from each region by projecting each region into the anteroposterior plane and dividing the projected region area into the total bone mineral content for that region. The program searched along the femoral neck axis between the lateral aspect of the femoral head and the lateral edge of the proximal femur and computed the cross-sectional area within the periosteal boundary as a function of position along the neck axis. The minimum of this function (MNCS) occurred at the femoral neck, and the maximum (MXCS) is the plane between the lesser and greater trochanters. Femoral neck BSI was computed from the elastic modulus-weighted effective polar moment of inertia of the MNCS cross-section divided by the calculated bone width. Femoral neck and trochanteric compressive strength indices (NCSI and BCSI) were then computed from the square of volumetric BMD of each region multiplied by the cross-sectional area values for those regions (MNCS and MXCS, respectively).
Statistical analysis
Analysis of the data from the QCT subgroup was carried out at UCSF by the Department of Epidemiology and Biostatistics. Group means (unadjusted) and 95% confidence intervals (CI) were calculated for the percentage changes from baseline in variables measured by DXA and QCT. These values were then used to assess the significance of changes within each treatment group. The statistical significance of between-treatment differences in baseline risk factors was evaluated using a two-sample t test. When data were not normally distributed, non-parametric Wilcoxon rank-sum tests were performed. Interactions between treatments and baseline values were assessed at the p<0.05 level.
We limited this analysis to the cohort of 233 participants who had an evaluable spine or hip QCT measurement at baseline. The numbers included for DXA are the subset among these 233 with DXA available for each measurement site or time. Where specific measurements for either DXA or QCT were not available, the denominators are indicted in the tables.
Given the sample size and the standard deviations of percentage change seen in this study, with a power of 0.9 (calculated post hoc), changes of approximately 3% would have been detected for the DXA measurements. For QCT, there was sufficient power to see differences of approximately 4% for trabecular spine BMD or for integral hip BMD, 6% for hip cortical volume and about 5% for compressive strength indices.
Results
At baseline, 232 and 230 patients had evaluable QCT scans at the hip and spine, respectively. Of these, 177 patients had evaluable QCT scans for both hip and spine at year 3, while an additional two patients had evaluable data at the hip only, and one had evaluable data at the spine only. Most baseline characteristics were similar between the groups (Table 1), and similar to those of the overall HORIZON-PFT population [1]. The subgroup with QCT had slightly greater femoral neck BMD at baseline (0.56 vs. 0.53 g/cm2, respectively) and a slightly lower proportion of prevalent vertebral fractures (56% vs. 63%) compared with the overall population [1]. At baseline, most QCT parameters were comparable between the two treatment groups (Table 2). Safety was assessed for the HORIZON-PFT population as previously reported [1]. Zoledronic acid (5 mg) was generally safe and well tolerated.
Table 1.
Baseline characteristics
| Zoledronic acid (n=122) | Placebo (n=111) | |
|---|---|---|
| Age at randomization, years | 74.2±5.6 | 74.3±6.4 |
| Age at time of menopause, years | 48.2±5.8 | 46.8±5.7 |
| Race | ||
| Caucasian | 90 (73.8) | 89 (80.2) |
| Asian | 32 (26.2) | 22 (19.8) |
| Body mass index, kg/m2 | 25.0±4.3 | 24.8±3.9 |
| Prior bisphosphonate usage | ||
| Yes | 108 (88.5) | 91 (82.0) |
| No | 14 (11.5) | 19 (17.1) |
| Missing | 0 | 1 (0.9) |
| Baseline vertebral fracture | ||
| Yes | 68 (55.7) | 63 (56.8) |
| No | 54 (44.3) | 48 (43.2) |
| Patients with three infusions | 92 (75.4) | 83 (74.8) |
| Corrected BMD, g/cm3 | ||
| Lumbar spine | 0.807±0.119 (n=67) | 0.802±0.137 (n=68) |
| Femoral neck | 0.565±0.081 (n=97) | 0.560±0.083 (n=86) |
| Total hip | 0.681±0.101 (n=97) | 0.672±0.097 (n=86) |
Data are expressed as n (%) or mean+SD
Table 2.
Baseline QCT measurements
| Bone measurement | Zoledronic acid | Placebo |
|---|---|---|
| Spine | (n=120) | (n=110) |
| Spine BMD | ||
| Single slice integral, g/cm3 | 0.145±0.027 | 0.142±0.032 |
| AP view: DXA-like vertebrae, g/cm3 | 0.158±0.027 | 0.156±0.031 |
| Lateral view DXA-like vertebrae, g/cm3 | 0.121±0.026 | 0.114±0.024 |
| Total vertebrae trabecular bone, g/cm3 | 0.071±0.023 | 0.062±0.023* |
| Hip | (n=121) | (n=111) |
| Femoral neck BMD | ||
| Integral, g/cm3 | 0.207±0.030 | 0.202±0.031 |
| Trabecular, g/cm3 | 0.030±0.031 | 0.022±0.027* |
| Cortical, g/cm3 | 0.473±0.034 | 0.476±0.033 |
| Trochanter BMD | ||
| Integral, g/cm3 | 0.190±0.030 | 0.182±0.031* |
| Trabecular, g/cm3 | 0.066±0.025 | 0.060±0.025* |
| Cortical, g/cm3 | 0.463±0.030 | 0.457±0.031 |
| Total hip BMD | ||
| Integral, g/cm3 | 0.193±0.029 | 0.185±0.030* |
| Trabecular, g/cm3 | 0.063±0.024 | 0.057±0.023* |
| Cortical, g/cm3 | 0.457±0.028 | 0.453±0.029 |
| Cortical volume measurements | (n=121) | (n=111) |
| Femoral neck cortical bone volume, cm3 | 4.96±1.17 | 4.98±1.34 |
| Trochanter cortical bone volume, cm3 | 18.10±4.74 | 18.20±5.23 |
| Total hip cortical bone volume, cm3 | 24.36±6.00 (n=120) | 24.53±6.81 (n=109) |
| Strength indices | (n=121) | (n=111) |
| MNCS, cm | 9.65±1.33 | 9.84±1.56 |
| MXCS, cm | 27.06±3.87 | 27.69±4.05 |
| Femoral neck BSI | 202187±441190 | 215660±521461 |
| Femoral neck CSI | 0.42±0.12 (n=120) | 0.41±0.11 (n=109) |
| Trochanter CSI | 1.01±0.32 (n=120) | 0.95±0.33 (n=109) |
Data are presented as mean ± SD
BSI bending/torsion strength index, CSI compression strength index
p<0.05 vs zoledronic acid group
Change in BMD
Mean percentage changes in BMD from baseline in the zoledronic acid and placebo groups are shown in Table 3. The mean difference between treatment groups for lumbar spine BMD by DXA was 7.0% and this was similar to the percentage change in QCT-derived AP view DXA-like, for which the mean difference was 5.7%. The mean between-treatment differences for femoral neck, trochanter, and total hip BMD by DXA were 5.1%, 7.5%, and 5.1%, respectively. These were similar to the measurements obtained at the same sites by QCT of 4.0%, 6.5%, and 6.0% (Table 3, Fig. 1).
Table 3.
Change in DXA and QCT measures over 36 months in response to zoledronic acid (5 mg) or placebo
| Variable | Zoledronic acid (95% CI) | Placebo (95% CI) | Between-treatment difference (95% CI) | p value |
|---|---|---|---|---|
| Spine | (n=67) | (n=68) | ||
| Lumbar spine BMD (DXA) | 8.81 (7.31, 10.31) | 1.83 (0.34, 3.32) | 6.98 (4.86, 9.10) | <0.0001 |
| Spine BMD (QCT) | (n=92) | (n=88) | ||
| AP view DXA-like | 5.98 (4.51, 7.45) | 0.31 (−1.20, 1.81) | 5.67 (3.56, 7.77) | <0.0001. |
| Lateral view DXA-like | 9.80 (7.40, 12.20) | 1.44 (−1.01, 3.90) | 8.36 (4.93, 11.78) | <0.0001 |
| Total bone trabeculara | 0.006381 (0.003515, 0.009247) | 0.000627 (−0.002304, 0.003558) | 0.005754 (0.001654, 0.009853) | 0.0062. |
| Hip | (n=97) | (n=86) | ||
| Femoral neck BMD (DXA) | 4.03 (2.85, 5.22) | −1.06 (−2.32, 0.20) | 5.10 (3.36, 6.83) | <0.0001 |
| Trochanter BMD (DXA) | 5.84 (4.67, 7.02) | −1.62 (−2.86, −0.37) | 7.46 (5.74, 9.17) | <0.0001 |
| Total hip BMD (DXA) | 3.53 (2.62, 4.44) | −1.54 (−2.50, −0.57) | 5.07 (3.74, 6.39) | <0.0001 |
| Femoral neck BMD (QCT) | (n=93) | (n=86) | ||
| Integral | 0.92 (−0.39, 2.22) | −3.10 (−4.46, −1.74) | 4.02 (2.13, 5.90) | <0.0001. |
| Trabeculara | −0.00576 (−0.00946, −0.00206) | −0.00559 (−0.00944, −0.00174) | −0.000168 (−0.0055, 0.00517) | 0.951 |
| Cortical | −0.86 (−1.94, 0.21) | −1.81 (−2.92, −0.69) | 0.94 (−0.61, 2.50) | 0.2323 |
| Trochanter BMD (QCT) | (n=93) | (n=86) | ||
| Integral | 3.27 (2.00, 4.54) | −3.26 (−4.58, −1.94) | 6.53 (4.70, 8.36) | <0.0001. |
| Trabecular | 2.98 (−0.08, 6.04) | −7.76 (−10.94, −4.57) | 10.74 (6.32, 15.20) | <0.0001. |
| Cortical | −0.54 (−1.26, 0.18) | −1.46 (−2.21, −0.71) | 0.92 (−1.22, 1.96) | 0.0834. |
| Total hip BMD (QCT) | (n=93) | (n=86) | ||
| Integral | 2.86 (1.65, 4.07) | −3.15 (−4.41, −1.90) | 6.02 (4.27, 7.76) | <0.0001 |
| Trabecular | 2.04 (−1.30, 5.38) | −8.73 (−12.20, −5.26) | 10.77 (5.95, 15.59) | <0.0001. |
| Cortical | −0.43 (−1.14, 0.27) | −1.43 (−2.16, −0.70) | 1.00 (−0.02, 2.02) | 0.0541 |
| Cortical volume measurements(QCT) | (n=93) | (n=86) | ||
| Femoral neck cortical bone volume | 3.31 (0.62, 6.00) | 1.11 (−1.69, 3.91) | 2.20 (−1.68, 6.09) | 0.2640. |
| Trochanter cortical bone volume | 8.83 (5.70, 11.95) | 0.13 (−3.12, 3.37) | 8.70 (4.20, 13.20) | 0.0002 |
| Total hip cortical bone volume | 7.20 (4.53, 9.86) | −0.02 (−2.79, 2.79) | 7.22 (3.38, 11.06) | 0.0003 |
| Strength indices(QCT) | (n=93) | (n=86) | ||
| MNCS | 2.44 (0.68, 4.19) | 2.75 (0.92, 4.57) | −3.10 (−2.84, 2.22) | 0.8097 |
| MXCS | 2.65 (1.53, 3.78) | 1.83 (0.66, 3.00) | 0.82 (−0.80, 2.44) | 0.3188 |
| Femoral neck BSI | −2.25 (−3.42, −1.08) | −2.21 (−3.43, −1.00) | −0.03 (−1.72, 1.65) | 0.9674 |
| Femoral neck CSI | 4.91 (2.06, 7.76) | −3.70 (−6.67, −0.74) | 8.61 (4.50, 12.72) | 0.0001 |
| Trochanter CSI | 9.83 (7.12, 12.55) | −4.25 (−7.08, −1.43) | 14.08 (10.16, 18.00) | <0.0001 |
Data are presented as mean (95% CI) percentage change, with p values showing the differences between the two groups
Since baseline values are very low, data are shown as absolute rather than relative changes
n number of patients with baseline and follow up DXA or QCT data for a specific measurement and timepoint
BSI bending/torsion strength index
CSI compression strength index
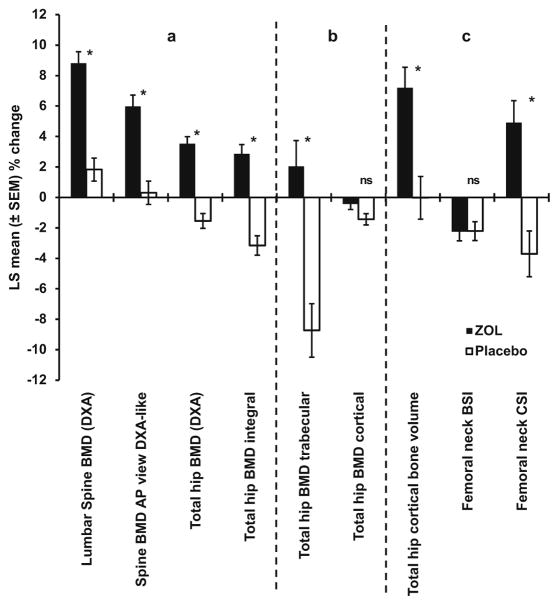
Fig. 1.
Change in DXA and QCT measures over 36 months in response to zoledronic acid (5 mg) or placebo at the spine and hip. a Change in bmd at the spine and hip as measured by DXA and QCT. b Change in trabecular and cortical bone at the hip measured by QCT. c Change in bone strength indicies measured by QCT. *p<0.001. ns non-significant
Changes in cortical and trabecular compartments
Mean percentage changes from baseline in cortical and trabecular components are summarized in Table 3. The cortical bone component for the spine is too thin and cannot be separated accurately from the trabecular component and so this result is not shown.
Mean between-treatment percentage differences for integral spine, but not the trabecular component, were statistically significant; however, the 3.3% mean between-treatment difference for the trabecular component was significant when analyzed by non-parametric test (p= 0.0017). Similarly the absolute between-treatment change, 0.00575 g/cm3, was significant (p=0.0062). Integral BMD is defined as the mean BMD within the periosteal contour of the specific region. It includes both the cortical and trabecular bone envelopes.
The mean percentage change from baseline in femoral neck trabecular BMD shown in Table 3 differs from the absolute change from baseline, where no significant difference was observed between the zoledronic acid and placebo groups. There was a significant mean between-treatment difference for femoral neck integral BMD (4.0%; p<0.0001), but not cortical BMD. Significant mean between-treatment differences were observed for trochanter and total hip integral (6.5% and 6.0%, respectively) and trabecular BMD (10.7% and 10.8%, respectively) (all p< 0.0001), but not for the corresponding cortical components (Fig. 1). The changes in integral bone at the femoral neck, trochanter, and total hip all showed decreases in the placebo group of approximately 3%. The changes in trabecular bone at the trochanter and total hip showed decreases in the placebo group of about 8% (Table 3, Fig. 1).
Changes in cortical volume and indices of strength
There was a significant mean between-treatment increase in cortical bone volume of the trochanter of 8.7% and of the total hip of 7.2% (all p<0.0005). There was no significant change in cortical bone volume of the femoral neck, although there was a significant mean between-treatment increase in femoral neck ratio of cortical to total volume of 4.8%. There was no mean between-treatment increase in MNCS or BSI of the femoral neck. However, CSI showed a mean between-treatment increase of 8.6% at the femoral neck and 14.1% at the trochanter (all p<0.0001; Table 3, Fig. 1).
Discussion
Overall, the results of this study show that treatment with zoledronic acid (5 mg) leads to significant increases in BMD at the spine, femoral neck, trochanter, and hip, compared with placebo as measured by both DXA and QCT. It is widely acknowledged that QCT has several advantages over DXA, as it allows the separate analysis of trabecular and cortical bone compartments, cortical bone volume and indices of bending/torsional and compressive strength. Such measurements can give a more detailed picture of the effects of a drug on bone strength.
In general, the present study found that the increases in BMD observed in response to treatment with zoledronic acid corresponded to changes in both trabecular and cortical compartments, with trabecular bone density showing large percentage increases. Although cortical bone density did not differ significantly between groups, cortical bone volume increased in the treatment group, contributing to the difference in integral BMD measures by QCT and DXA. Bone turnover is higher in trabecular than cortical bone, and the effects of antiresorptive therapy, such as zoledronic acid, would therefore be expected to be greater in trabecular bone. In this study, the femoral neck trabecular and cortical measures by QCT were observed to be the same in both treatment groups as were the strength indices MNCS and femoral neck BSI. However, the femoral neck CSI was observed to be higher in response to treatment with zoledronic acid than with placebo. This can be explained by the fact that trabecular BMD is quite variable due to a small region size and in this study had very low baseline BMD, meaning that the absolute changes of a few mg/cm3 translate into huge percentage changes and large variations. The cortical BMD in the neck showed a trend towards an intergroup difference (slight increase to the same in the zoledronic acid treatment group, with a decrease in the placebo-treatment group) but this was not significant. Femoral neck CSI is based on neck integral BMD which showed a significant change. Since femoral neck CSI is neck integral BMD2 multiplied by MNCS, there was a large change in femoral neck CSI even though MNCS did not change.
Only a few other studies have examined the effect of bisphosphonates on changes in bone as assessed by QCT. In the PaTH (Parathyroid Hormone and Alendronate) study, for example, the effect of parathyroid hormone 1 to 84 (PTH1–84) compared with alendronate or the combination of both agents over 1 year on the spine and hip was evaluated by QCT [4]. The PaTH study also used the same analysis program as used in this study so the results can be broadly compared [2, 3]. In an extension to the PaTH study, 1 year of alendronate therapy was compared with 1 year of placebo, after 1 year of parathyroid hormone treatment [5]. In agreement with our findings, alendronate treatment resulted in significant increases in trabecular BMD, but no significant increases in cortical BMD compared with placebo. One group in the study received 2 years of alendronate therapy, and the pattern of BMD response was broadly similar to our findings in that trabecular, but not cortical BMD was increased (although the increases in trabecular bone from baseline did not reach significance). The PaTH study also reported significant increases in cortical bone volume in response to alendronate treatment, and similarly we found that zoledronic acid resulted in significant increases in cortical bone volume at the trochanter and hip.
In an 18-month, randomized, parallel, double-blind study that compared the effects of once-daily doses of teriparatide and alendronate on BMD and biomarkers of bone turnover, trabecular lumbar spine BMD and trabecular and cortical femoral neck BMD were measured by QCT [6]. Alendronate treatment resulted in significant increases in trabecular lumbar spine BMD and cortical femoral neck BMD, but not trabecular femoral neck BMD, after 18 months [6]. This is in contrast to our study, which found a significant increase in absolute lumbar spine trabecular BMD, but no significant increase in cortical or trabecular BMD at the femoral neck. In addition, we observed the greatest increases in BMD in the trabecular rather than cortical compartments of the total hip and trochanter.
In the MORE (Multiple Outcomes of Raloxifene Evaluation) study; a 3-year, randomized, double-blind, placebo-controlled trial of raloxifene in postmenopausal women, QCT of the spine was evaluated in a subgroup of patients [7]. In agreement with our study, the results indicated an effect of raloxifene treatment on both integral bone and vertebral trabecular BMD. However, in contrast to our findings, the MORE trial showed decreases in BMD in the placebo groups at both integral and trabecular spine.
The present study found that for similar sites, QCT gave similar results to DXA. The QCT-based measurements showed no bone loss at the spine (except in absolute values for antero-posterior view DXA-like). Similarly, DXA measurement of lumbar spine BMD showed an increase in the placebo group, possibly due to artifacts. Interestingly, the lack of bone loss at the spine is often attributed to the development of degenerative changes. However, use of QCT measurements should mean that the effects of such changes on BMD measurement are avoided as QCT measurements avoid endplates and osteophytes, and do not include the posterior elements or aortic calcification.
A promising finding in the present analysis is the response to zoledronic acid observed in parameters that are believed to be related to bone strength. Femoral neck cortical to total volume ratio and femoral neck and trochanter CSI have all been linked to lower hip fracture risk in a case control study [2]. From basic mechanical engineering principles, the compressive strength of an object is mean elastic modulus (Ea) multiplied by cross-sectional area (CSA). In this study, we modeled Ea as BMD2 where BMD is the integral BMD of a region and where NCSI equals BMD2 multiplied by MNCS and TCSI equals BMD2 multiplied by MXCS. TCSI has been validated against femoral strength in vitro by Lotz et al. [8] and for vertebrae by Buckley et al. [9]. Although prospective studies are needed, our results suggest a significant treatment effect with zoledronic acid on all three of these key indices, and the increase in volumetric BMD is consistent with expectation of an increase in the compressive strength. In addition, a 7% increase in total hip cortical volume with zoledronic acid was observed, with cortical volume increasing more in the trochanter than the femoral neck. This apparent increase in cortical bone volume could be due to three changes: (1) trabecular bone in the endocortical region is increasing in density and so is misclassified by the image processing program as cortical, (2) there is a decrease in cortical porosity and hence cortical bone that is porous at the first visit is counted as trabecular but at the second visit it is less porous and so counted as cortical, and (3) there is a decrease in subendosteal resorption causing a relative increase in cortical thickness.
In conclusion, we found that treatment with zoledronic acid 5 mg (1) resulted in increases in lumbar spine and proximal femur BMD, and the changes did not differ when measured by QCT as compared with DXA, (2) resulted in increases in trabecular but not cortical bone, and 3) resulted in increases in compressive bone strength index, and this may help explain the reduction in hip fracture risk observed in the HORIZON-PFT study.
Acknowledgments
Supported by Novartis Pharma
Dr. Boonen is senior clinical investigator of the Fund for Scientific Research, Flanders, Belgium (F.W.O.-Vlaanderen). Dr. Isra Saeed at UCSF carried out the QCT image analysis and provided substantial support in managing data for the Reading centre. Special thanks are due Zeb Horowitz (now at Savient Pharmaceuticals), John Orloff (Novartis), and the following: Steering Committee members—Dennis Black, Steven Cummings, Pierre Delmas, Richard Eastell, Ian Reid, Steven Boonen, Jane Cauley, Felicia Cosman, Péter Lakatos, Ping C. Leung, Zulema Man, Erik Fink Eriksen (Novartis), Peter Mesenbrink (Novartis); Past Steering Committee members—Edith Lau, Saloman Jasqui, Carlos Mautalen, Theresa Rosario-Jansen (Novartis), John Caminis (Novartis); Data Safety Monitoring Board—Lawrence Raisz (chairman), Peter Bauer, Juliet Compston, David DeMets, Raimund Hirschberg, Olof Johnell, Stuart Ralston, Robert Wallace; DSMB Consultants—Michael Farkough; Novartis—Mary Flood; University of California, San Francisco (UCSF) Coordinating Center—Douglas Bauer, Lisa Palermo; UCSF Radiology (QCT analysis)—Thomas Lang. We are indebted to the HORIZON-PFT Clinical Site Investigators: Argentina: Eduardo Kerzberg, Zulema Man, Carlos Mautalen, Maria Ridruejo, Guillermo Tate, Jorge Velasco; Australia: Michael Hooper, Mark Kotowicz, Peter Nash, Richard Prince, Anthony Roberts, Philip Sambrook; Austria: Harald Dobnig, Gerd Finkenstedt, Guenter Hoefle, Klaus Klaushofer, Martin Pecherstorfer, Peter Peichl; Belgium: Jean Body, Steven Boonen, Jean-Pierre Devogelaer, Piet Geusens, Jean Kaufman; Brazil: João Brenol, Jussara Kochen, Rubem Lederman, Sebastiao Radominski, Vera Szejnfeld, Cristiano Zerbini; Canada: Jonathan Adachi, Jacques Brown, Denis Choquette, David Hanley, Robert Josse, David Kendler, Richard Kremer, Frederic Morin, Wojciech Olszynski, Alexandra Papaioannou, Chiu KinYuen; China: Baoying Chen, Shouqing Lin; Colombia: Nohemi Casas, Monique Chalem, Juan Jaller, Jose Molina; Finland: Hannu Aro, Jorma Heikkinen, Heikki Kröger, Lasse Mäkinen, Juha Saltevo, Jorma Salmi, Matti Välimäki; France: Claude-Laurent Benhamou, Pierre Delmas, Patrice Fardellone, Georges Werhya; Germany: Bruno Allolio, Dieter Felsenberg, Joachim Happ, Manfred Hartard, Johannes Hensen, Peter Kaps, Joern Kekow, Ruediger Moericke, Bernd Ortloff, Peter Schneider, Siegfried Wassenberg; Hong Kong: Ping Chung Leung; Hungary: Adam Balogh, Bela Gomor, Tibor Hidvégi, Laszlo Koranyi, Péter Lakatos, Gyula Poór, Zsolt Tulassay; Israel: Rivka Dresner Pollak, Varda Eshed, A. Joseph Foldes, Sophia Ish-Shalom, Iris Vered, Mordechai Weiss; Italy: Silvano Adami, Antonella Barone, Gerolamo Bianchi, Sandro Giannini, Giovanni Carlo Isaia, Giovanni Luisetto, Salvatore Minisola, Nicola Molea, Ranuccio Nuti, Sergio Ortolani, Mario Passeri, Alessandro Rubinacci, Bruno Seriolo, Luigi Sinigaglia; Korea (Republic of): Woong-Hwan Choi, Moo-II Kang, Ghi-Su Kim, Hye-Soon Kim, Yong-Ki Kim, Sung-Kil Lim, Ho-Young Son, Hyun-Koo Yoon; Mexico: Carlos Abud, Pedro Garcia, Salomon Jasqui, Luis Ochoa, Javier Orozco, Javier Santos; New Zealand: Ian Reid; Norway: Sigbjørn Elle, Johan Halse, Arne Høiseth, Hans Olav, Høivik Ingun Røed, Arne Skag, Jacob Stakkestad, Unni Syversen; Poland: Janusz Badurski, Edward Czerwinski, Roman Lorenc, Ewa Marcinowska-Suchowierska, Andrzej Sawicki, Jerzy Supronik; Russia: Eduard Ailamazyan, Lidiya Benevolenskaya, Alexander Dreval, Leonid Dvoretsky, Raisa Dyomina, Vadim Mazurov, Galina Melnichenko, Ashot Mkrtoumyan, Alexander Orlov-Morozov, Olga Ostroumova, Eduard Pikhlak, Tatiana Shemerovskaya, Nadezhda Shostak, Irina Skripnikova, Vera Smetnik, Evgenia Tsyrlina, Galina Usova, Alsu Zalevskaya, Irina Zazerskaya, Eugeny Zotkin; Sweden: Osten Ljunggren, Johan Lofgren, Mats Palmér, Maria Saaf, Martin Stenström; Switzerland: Paul Hasler, Olivier Lamy, Kurt Lippuner, Claude Merlin, René Rizzoli, Robert Theiler, Alan Tyndall, Daniel Uebelhart; Taiwan: Jung-Fu Chen, Po-Quang Chen, Lin-show Chin, Jawl-Shan Hwang, Tzay-Shing Yang, Mayuree Jirapinyo; Thailand: Mayuree Jirapinyo, Rojanasthien Sattaya, Sutin Sriussadaporn, Soontrapa Supasin, Nimit Taechakraichana, Kittisak Wilawan; United Kingdom: Hugh Donnachie, Richard Eastell, William Fraser, Alistair McLellan, David Reid; United States: John Abruzzo, Ronald Ackerman, Robert Adler, John Aloia, Charles Birbara, Barbara Bode, Henry Bone, Donald Brandon, Jane Cauley, Felicia Cosman, Daniel Dionne, Robert Downs, Jr., James Dreyfus, Victor Elinoff. Ronald Emkey, Joseph Fanciullo, Darrell Fiske, Palmieri Genaro, M. Gollapudi, Richard Gordon, James Hennessey, Paul Howard, Karen Johnson, Conrad Johnston, Risa Kagan, Shelly Kafka, Jeffrey Kaine, Terry Klein, William Koltun, Meryl Leboff, Bruce Levine, E. Michael Lewiecki, Cora Elizabeth Lewis, Angelo Licata, Michael Lillestol, Barry Lubin, Raymond Malamet, Antoinette Mangione, Velimir Matkovic, Daksha Mehta, Paul Miller, Sam Miller, Frederik T. Murphy, Susan Nattrass, David Podlecki, Christopher Recknor, Clifford Rosen, Daniel Rowe, Robert Rude, Thomas Schnitzer, Yvonne Sherrer, Stuart Silverman, Kenna Stephenson, Barbara Troupin, Joseph Tucci, Reina Villareal, Nelson Watts, Richard Weinstein, Robert Weinstein, Michael Weitz, Richard White.
Footnotes
Conflicts of interest Dr. Eastell serves as a consultant, has received honoraria for speaking, and has received grant funding from Novartis, Amgen, Sanofi-Aventis, Lilly, Organon, Pfizer, and Procter & Gamble Pharmaceuticals. Dr. Lang serves as a consultant for Merck and has received grant funding from Novartis. Dr. Boonen serves as a consultant, has received honoraria for speaking, and has received grant funding from Novartis. Dr. Cummings reports no conflict of interest. Dr. Cauley has received research grants from Merck, Eli Lilly, Pfizer, Novartis and Procter & Gamble, and has received honoraria from Novartis. Dr. Horowitz was an employee of Novartis until 2003. He has no other conflict of interest. Dr. Kerzberg has no conflict of interest. Dr. Bianchi has received consulting fees from Novartis. Dr. Kendler serves on advisory boards or as a consultant for Novartis, Servier, Eli Lilly, Amgen, Wyeth and Nycomed. Dr. Leung has no conflict of interest. Dr. Man has received honoraria for speaking from Sanofi-Aventis, Roche, Merck and Novartis. Dr. Mesenbrink is an employee of Novartis Pharmaceuticals Corporation who funded the study and owns stock in the company. Dr. Eriksen serves as a consultant and has received honoraria for speaking from Novartis. Dr. Black serves as a consultant for Nycomed and Zosano, and has research contracts with Novartis and Roche.
Contributor Information
R. Eastell, Academic Unit of Bone Metabolism, University of Sheffield, Sheffield, UK. Metabolic Bone Centre, Sorby Wing, Northern General Hospital, Herries Road, Sheffield, South Yorkshire S5 7AU, UK
T. Lang, Department of Radiology, University of California, San Francisco, USA
S. Boonen, Leuven University Centre for Metabolic Bone Diseases and Division of Geriatric Medicine, Katholieke Universiteit Leuven, Leuven, Belgium
S. Cummings, CPMC Research Institute, University of California, San Francisco, USA
P. D. Delmas, INSERM Research Unit 831 and University of Lyon, Lyon, France
J. A. Cauley, Department of Epidemiology, University of Pittsburgh, Pittsburgh, USA
Z. Horowitz, Savient Pharmaceuticals, New Jersey, USA
E. Kerzberg, Argentine Reference Center in Osteoporosis, Hospital Ramos Mejía, Buenos Aires, Argentina
G. Bianchi, Division of Rheumatology, La Colletta Hospital, Genoa, Italy
D. Kendler, Osteoporosis Research Centre, Vancouver, Canada
P. Leung, Faculty of Medicine, Chinese University of Hong Kong, Prince of Wales Hospital, Hong Kong, China
Z. Man, Centro TIEMPO, Universidad Favaloro, Buenos Aires, Argentina
P. Mesenbrink, Novartis Pharmaceuticals Corporation, New Jersey, USA
E. F. Eriksen, Department of Endocrinology, Aker University Hospital, Oslo, Norway
D. M. Black, Department of Epidemiology and Biostatistics, University of California, San Francisco, USA
References
- 1.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 2.Cheng X, Li J, Lu Y, Keyak J, Lang T. Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone. 2007;40:169–174. doi: 10.1016/j.bone.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 3.Lang T, Leblanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 4.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–1215. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 5.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ. One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 6.McClung MR, San MJ, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–1768. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 7.Genant HK, Lang T, Fuerst T, Pinette KV, Zhou C, Thiebaud D, Diez-Perez A. Treatment with raloxifene for 2 years increases vertebral bone mineral density as measured by volumetric quantitative computed tomography. Bone. 2004;35:1164–1168. doi: 10.1016/j.bone.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Lotz JC, Hayes WC. The use of quantitative computed tomography to estimate risk of fracture of the hip from falls. J Bone Joint Surg Am. 1990;72:689–700. [PubMed] [Google Scholar]
- 9.Buckley JM, Loo K, Motherway J. Comparison of quantitative computed tomography-based measures in predicting vertebral compressive strength. Bone. 2007;40:767–774. doi: 10.1016/j.bone.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]