Abstract
Summary
We assessed vitamin D status and its correlates in the population-based Canadian Multicentre Osteoporosis Study (CaMos). Results showed that serum 25-hydroxyvitamin D levels <75 nmol/L were common. Given Canada’s high latitude, attention should be given to strategies for enhancing vitamin D status in the population.
Introduction
Inadequate vitamin D has been implicated as a risk factor for several clinical disorders. We assessed, in a Canadian cohort, vitamin D status and its correlates, based on serum 25-hydroxyvitamin D [25(OH)D], the best functional indicator of vitamin D status.
Methods
We studied 577 men and 1,335 women 35+ years from seven cities across Canada in the randomly selected, population-based Canadian Multicentre Osteoporosis Study (CaMos). Participants completed a comprehensive questionnaire. Serum 25(OH)D was measured by immunoassay. Multivariate linear regression modeling assessed the association between 25(OH)D and determinants of vitamin D status.
Results
Participants (2.3%) were deficient in 25(OH)D (<27.5 nmol/L); a further 18.1% exhibited 25(OH)D insufficiency (27.5–50 nmol/L). Levels <75 nmol/L were evident in 57.5% of men and 60.7% of women and rose to 73.5% in spring (men) and 77.5% in winter (women); 25 (OH)D <50 nmol/L was ≤10% year round for those supplementing with ≥400 IU vitamin D/day but was 43.9% among those not supplementing in winter and spring. The strongest predictors of reduced 25(OH)D for both men and women were winter and spring season, BMI ≥30, non-white ethnicity, and lower vitamin D supplementation and its modification by fall and winter.
Conclusions
In this national Canadian cohort, vitamin D levels <75 nmol/L were common, particularly among non-white and obese individuals, and in winter and spring. Vitamin D intake through diet and supplementation and maintenance of normal weight are key modifiable factors for enhancing vitamin D status and thus potentially influencing susceptibility to common chronic diseases.
Keywords: Hydroxyvitamin D levels, Sunlight exposure, Vitamin D insufficiency, Vitamin D supplements
Introduction
Vitamin D is synthesized via ultraviolet irradiation of the skin or from food or supplements. It is then converted in the liver to 25-hydroxyvitamin D [25(OH)D], which is the most abundant circulating form of the vitamin [1]. Serum 25(OH)D has been recognized by the 1997 Institute of Medicine committee as the best functional indicator of vitamin D status [2], reflecting the sum of cutaneous synthesis and oral intake.
There is a critical requirement of vitamin D for bone and mineral homeostasis, and in particular in preventing rickets and osteomalacia [3]. Inadequate vitamin D has been implicated in increasing the risk of osteoporosis [4], cardiovascular disease [5], diabetes [6], cancer [7–9], and autoimmune diseases such as multiple sclerosis [10].
There is concern regarding the potential for vitamin D insufficiency in the Canadian population. Endogenous synthesis of vitamin D is minimal from October to March due to Canada’s high latitude and may be further limited in those with increased skin pigmentation. A number of Canadian studies have found a relatively high prevalence of 25(OH)D levels <75 nmol/L [11, 12], particularly in non-whites [13–15] and the elderly [16]. Seasonal variation was evident, with low 25(OH)D (<50 nmol/L) approximately 60% to 120% higher during winter and spring seasons [11, 15, 16]. The Canadian Health Measures Survey (CHMS) launched by Statistics Canada in partnership with Health Canada and the Public Health Agency of Canada, concurred, in a recent report, in finding lower levels of 25(OH)D between November and March and in those with darker skin pigmentation [12].
To date, there has been no Canadian national study investigating a broad range of purported biological (age, sex, ethnicity, body mass index (BMI)), behavioral (vitamin D from diet, supplements and medications, activity, sunscreen use), and environmental factors (season, sun exposure) to determine which may be independently associated with vitamin D status. The purpose of this study was to assess vitamin D status based on the distribution and seasonal variation of 25(OH)D in a national cohort of Canadian adults ≥35 years and examine the multivariable relationship between vitamin D status and factors which might be associated with it in this population.
Methods
Data source and subjects
Data for this study come from the Canadian Multicentre Osteoporosis Study (CaMos), an ongoing longitudinal, population-based cohort study in nine Canadian city-based centers. It includes 9,423 non-institutionalized, randomly selected men and women aged 25 years and older on entry into the study in 1995–1997; 42.5% of eligible household participants completed the full survey and 27.5% refused. A further 30% completed a refusal questionnaire which included demographic, fracture, and osteoporosis information. The full responders were found to be similar, with respect to risk factors for osteoporosis, to those additional individuals who only partially participated. Non-response bias was, therefore, negligible with respect to bone health, which is affected by 25 (OH)D concentration, and was of concern only slightly in very elderly women and men (over 80 years of age) [17]. For a more detailed description of CaMos, see Kreiger et al. [18].
The analyses for this cross-sectional study are of data and sera gathered from participants at seven centers (Vancouver, Calgary, Saskatoon, Toronto, Kingston, Quebec City, and Halifax) between 2005 and 2007 (at 10-year follow-up) are restricted to adults aged 35+ years. A small proportion of participants (5.8%, n=110) were assessed after 2007. Of those eligible to participate at year 10 follow-up, the full survey completion rate for these seven centers was 83.1%. When excluding known deaths after baseline (1995–1997), 66.2% of the participants from the previous seven centers were still in the study after 10 years. The cohort included the 577 male and 1,335 female full participants who gave blood samples, were measured for height and weight, and completed a comprehensive questionnaire which included data on demographics, diet and supplement use, sun exposure, and physical activity. Partial participants who did not give blood were excluded.
All participating centers received ethics approval to participate in the blood and urine collection project. Signed informed consent was obtained from all participants who agreed to give samples.
Blood collection and serum 25(OH) vitamin D
All sera were analyzed at the same laboratory, using the identical technique, between March and September 2009. Fasting blood samples from participants had been aliquoted and stored at −80°C. Serum total 25(OH)D was measured using the Liaison (Diasorin Incorporated) assay, which employs chemiluminescent immunoassay technology. The detection limit was 10 nmol/L; linearity <375 nmol/L; inter-and intra-assay variability were 2.9–5.5% and 6.3–12.9%, respectively. In addition to quality controls supplied by Diasorin, external quality controls of low, intermediate, and high levels of 25 (OH)D (Biorad Laboratories, Irvine, Ca) were always used as the samples were run on the instrument. Multiple repeat determinations using the three different standards were performed over the relatively short period that the samples were assayed, and assay drift was found to be negligible. The laboratory participates in the international Vitamin D External Quality Assessment Scheme which aids in harmonizing 25(OH)D assays among different laboratories [19]. We also performed a small assessment using the standard reference material in human serum (SRM 972) from the National Institute of Standards and Technology (NIST), NIST SRM-972 level 1, that is suitable for analysis by immunoassay [20, 21]. Overall, therefore, it is unlikely that the assay drifts noted by the Center for Disease Control and the National Center for Health Statistics in their technical advisory on the Diasorin assays [22] affect the comparability of the values in this study.
Vitamin D status was categorized by three commonly used cutoffs and definitions of serum 25(OH)D: deficient <27.5 nmol/L [2] and insufficient 27.5–49.9 nmol/L [23–25]. Suboptimal included all measured levels <75.0 nmol/L, and optimal, ≥75 nmol/L [25–27].
Seasonality
Winter was defined as January through March, spring as April through June, summer as July through September, and fall as October through December.
Skin pigmentation
Race/color (ethnicity) was dichotomized to “white” and “other” as proxies for lighter and darker skin pigmentation. The “other” category included black (African, Haitian, Jamaican), American aboriginal, Asian (e.g., Chinese, Vietnamese, Japanese), Latin American, Arab, or Middle Eastern (e.g., Armenian, Turkish, Egyptian).
Vitamin D intake from the diet, supplements, and medications
Dietary vitamin D intake was estimated from reported usual intake of vitamin D-fortified fluid milk, soya beverage, and yogurt. This was collected using an interviewer-administered abbreviated semiquantitative food frequency questionnaire (FFQ). Milk (primarily) and milk products are the main sources of vitamin D in the Canadian diet, contributing 49% of dietary vitamin D [28]. The content in vitamin D was calculated using Canada’s Food and Drugs regulations on fortification standards and vitamin D quantity from food labels. Vitamin D was categorized as no vitamin D from diet, from 1–200 or >200 IU/day. Participants were asked to bring medication and supplements used regularly. The vitamin D content of medication and supplements was determined using Health Canada’s drug product database [29]. Supplement and medication intake was summed and categorized as none, from 1–400 or >400 IU/day. Details related to food and supplement data collection were previously reported [30].
Sun-related variables
Sun exposure was estimated using the question, “In the past 12 months, did you ever expose a considerable part of your body to direct sunlight?” The possible answers to the sun exposure question were never, seldom, regularly, and often. A considerable part of the body was defined as an exposure for 30 min or more in a swimsuit or equivalent without sunscreen. The sunscreen question was “In the past 12 months, have you used sunscreen or face cream with SPF to protect your skin against sunlight?” Possible responses were no, sometimes, usually, and always. We confined the interval to 12 months to facilitate recall of the participants regarding sun exposure and sunscreen use.
Statistical analyses
25(OH)D levels found to be “<10 nmol/L” were fixed to 10 nmol/L in the database. By design, CaMos oversampled older participants and also oversampled women. Using the 2006 Canadian census data, and keeping the same total sample size (n=1,912), weights were generated and applied to all our full participants in order to represent the Canadian age and sex distribution. Therefore, all results reported as frequencies or averages (and standard errors) were weighted to the Canadian population using the 2006 Canadian census data, with the exception of sample characteristics where both weighted and non-weighted results are reported on the same participants. Logistic regression was used to determine characteristics that were associated with full participants versus partial participants. Differences were noted to be statistically important if 95% confidence intervals excluded 1. Multivariable linear regression modeling was used to assess the association between 25(OH)D levels and possible determinants of vitamin D status. The variables included vitamin D intake from selected dietary sources, supplements and medication, sun exposure, sun-screen use, sociodemographic characteristics (age group, “white” or “other” ethnicity, education, family income), BMI (weight (kg)/height (m2)), season of blood draw, center, participation in a regular program or activity, walking, smoking, alcohol intake, and energy expended per day. Prior to the multivariable regression modeling, correlations and univariate associations between the variables and 25(OH)D were examined to refine the list of predictors identified above, removing any for which there was no evidence to support their inclusion. Interactions between body mass index and season and between vitamin D supplementation and season were investigated. All analyses were performed using SAS 9.1 (Cary, NC, USA).
Results
Description of the study participants
In total, 5,569 individuals participated in the 2005–2007 CaMos survey, of which 4,283 were part of the seven centers collecting blood. Of these, 1,912 (44.6%) were full participants who gave blood samples. When excluding known deaths after baseline, 29.6% of the original CaMos sample donated blood at year 10. Respondent characteristics are summarized in Table 1.
Table 1.
Descriptive statistics of full participants
| Variable | Categories | Unweighted (%) | Weighteda (%) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Males (n=577) | Females (n=1,335) | Males (n=914) | Females (n=998) | ||
| Age group | 35–50 | 10.6 | 6.6 | 43.9 | 42.3 |
| 51–70 | 49 | 51 | 41.9 | 39.4 | |
| >70 | 40.4 | 42.4 | 14.2 | 18.3 | |
| Ethnicity (versus other) | White | 93.1 | 96.4 | 92.9 | 96.1 |
| Body mass index | <25 | 29.1 | 38 | 28.4 | 47.3 |
| 25–30 | 49.2 | 36.6 | 52.3 | 27.7 | |
| ≥30 | 21.7 | 25.4 | 19.3 | 25 | |
| Season | Winter | 20.3 | 19.2 | 14.3 | 15 |
| Spring | 28.6 | 31.4 | 28 | 24.5 | |
| Summer | 22.2 | 22.9 | 24.7 | 26 | |
| Fall | 28.9 | 26.5 | 33 | 34.5 | |
| Sun exposure | Never | 35.4 | 45.2 | 27.7 | 38.9 |
| Seldom/Regularly | 57.7 | 50.4 | 67.8 | 56.5 | |
| Often | 6.9 | 4.4 | 4.5 | 4.6 | |
| Sunscreen use | No | 49.2 | 31.6 | 35.9 | 22.4 |
| Sometimes | 22.9 | 22.1 | 27 | 24.1 | |
| Usually/Always | 27.9 | 46.3 | 37.1 | 53.5 | |
| Vitamin D intake from supplements | None | 57.8 | 32 | 61.3 | 42.5 |
| 0–400 IU | 26.9 | 30.3 | 27.6 | 31.7 | |
| >400 IU | 15.3 | 37.7 | 11.1 | 25.8 | |
| Vitamin D intake from milk, yogurt, or soy beverage | None | 9.2 | 11 | 8.3 | 11 |
| 0–200 IU | 74.7 | 71.2 | 69 | 70.8 | |
| >200 IU | 16.1 | 17.8 | 22.7 | 18.2 | |
| Participation in regular activity/program | Yes | 53.4 | 54.8 | 56.2 | 51.7 |
| Center | Vancouver | 4 | 6.6 | 2.6 | 5 |
| Calgary | 20.5 | 19.2 | 26.5 | 16.8 | |
| Saskatoon | 20 | 20.7 | 17.8 | 18.4 | |
| Toronto | 16.3 | 12.4 | 16.2 | 15.7 | |
| Kingston | 11.5 | 13.3 | 9.1 | 11.2 | |
| Quebec city | 23.2 | 24.1 | 25 | 30.8 | |
| Halifax | 4.5 | 3.7 | 2.8 | 2.1 | |
| Education | Less than high school | 23.1 | 31 | 16.5 | 19.2 |
| High school graduation | 12.8 | 14.4 | 12.6 | 14.8 | |
| Trade/professional certification | 20.3 | 21.8 | 23.5 | 24.9 | |
| University without degree | 7.8 | 8.3 | 6.3 | 9.2 | |
| University with certificate/degree | 36 | 24.5 | 41.1 | 31.9 | |
Both unweighted and weighted columns refer to the same sample. By design, CaMos oversampled women and elderly. Therefore, the weighted sample sizes (n) in women and men are different than the unweighted sample sizes
Differences between full participants and partial participants (those not giving blood) were examined. There were no statistically significant differences between the two groups with regard to “white” or “other” ethnicity, use of sunscreen, vitamin D intake from supplements and selected vitamin D fortified foods, and participation in regular activity. Those willing to participate fully were more likely to be female, aged 51 to 70 years (versus 35 to 50 years), have a BMI of 25 to <30 (versus BMI <25), to have less than a high school education (versus high school or trades), report often getting sun exposure (versus never), be married/partnered versus single, separated, or divorced. Some aspects were potentially biased in favor of higher values, e.g., often getting sun exposure while others were in favor of lower values, e.g., more full participants over age 50 years. For others, the effect is variable or unknown: e.g., sex, education.
Normative data
Serum 25(OH)D distributions (Table 2) and means for males and females aged 35+ years were similar. Younger males, 35–50 years, however, exhibited higher median levels than those 51–70 and 70+ years (77.1 versus 66.7 and 68.5 nmol/L, respectively). Females exhibited similar median levels over all age groups. Among all participants, serum 25(OH)D values ranged from 10 to 212.1 nmol/L.
Table 2.
Normative data for 25(OH)D (nmol/L)—weighted by sex and (10-year) age group to the Canadian census
| Sex | Age group (years) | Non-weighted N | Weighted N | Mean (SE) nmol/L | Percentiles (nmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Min (0) | 5 | 10 | 25 | 50 | 75 | 90 | 95 | Max (100) | |||||
| Males | 35–50 | 61 | 401 | 73.37 (3.11) | 12.07 | 30.1 | 39.53 | 56.08 | 77.09 | 91.14 | 104.38 | 112.45 | 122.35 |
| 51–70 | 283 | 384 | 66.26 (1.36) | 15.67 | 28.46 | 33.88 | 50.68 | 66.72 | 79.78 | 96.17 | 107.34 | 136.05 | |
| >70 | 233 | 130 | 71.74 (1.80) | 20.98 | 33.92 | 40.98 | 54.03 | 68.5 | 86.9 | 97.86 | 108.1 | 209.17 | |
| 35+ | 577 | 915 | 70.16 (1.02) | 12.07 | 30.1 | 36.35 | 53.52 | 71.99 | 85.65 | 100.3 | 107.34 | 209.17 | |
| Females | 35–50 | 88 | 422 | 71.01 (2.47) | 11.24 | 43.6 | 44.31 | 51.48 | 69.06 | 85.03 | 102.25 | 109.53 | 135.97 |
| 51–70 | 681 | 393 | 69.74 (0.89) | 10 | 33.21 | 40.14 | 53.48 | 69.56 | 84.25 | 95.62 | 104.66 | 206.23 | |
| >70 | 566 | 182 | 71.25 (1.08) | 11.41 | 34.14 | 41.61 | 55.48 | 69.79 | 82.6 | 99.81 | 118.24 | 212.05 | |
| 35+ | 1,335 | 997 | 70.55 (0.65) | 10 | 35.7 | 43.89 | 53.16 | 69.06 | 84.25 | 100.09 | 109.53 | 212.05 | |
| All | 1,912 | 1,912 | 70.36 (0.55) | 10 | 32.88 | 40.88 | 53.18 | 70.06 | 85.22 | 100.09 | 109.31 | 212.05 | |
Vitamin D status
Vitamin D status is given in Table 3. Suboptimal levels of serum 25(OH)D (<75 nmol/L) were evident in 57.5% of males and 60.7% of females. This varied for males of different age groups with the lowest prevalence of values <75 nmol/L being 50.0% for those 35–50 years; the highest prevalence was 65.5% for 51–70-year-olds. For females, this prevalence was more stable over age, ranging from 59.1% to 62.5%.
Table 3.
25(OH)D Groupings (vitamin D status)—weighted by sex and (10 yr) age group to the Canadian census
| Sex | Age group (years) | 25(OH)D (nmol/L) groupings | Total | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| <27.5 | 27.5–49.9 | 50–74.9 | ≥75 | ||||||||||||
|
|
|
|
|
|
|||||||||||
| n | % | SE of % | n | % | SE of % | n | % | SE of % | n | % | SE of % | na | % | ||
| Males | 35–50 | 16 | 3.9 | 2.7 | 46 | 11.6 | 4.2 | 138 | 34.5 | 6.1 | 201 | 50 | 6.5 | 401 | 100 |
| 51–70 | 10 | 2.5 | 0.9 | 86 | 22.3 | 4.1 | 155 | 40.4 | 4.5 | 134 | 34.9 | 4.3 | 384 | 100 | |
| >70 | 3 | 2.6 | 1.1 | 24 | 18.7 | 2.7 | 48 | 36.5 | 3.2 | 55 | 42.2 | 3.3 | 130 | 100 | |
| 35+ | 28 | 3.1 | 1.2 | 156 | 17.1 | 2.6 | 341 | 37.3 | 3.3 | 389 | 42.6 | 3.4 | 915 | 100 | |
| Females | 35–50 | 3 | 0.7 | 0.7 | 90 | 21.4 | 4.6 | 171 | 40.5 | 5.7 | 158 | 37.5 | 5.6 | 422 | 100 |
| 51–70 | 8 | 2.1 | 0.8 | 70 | 17.7 | 2.1 | 155 | 39.3 | 2.6 | 161 | 41 | 2.7 | 393 | 100 | |
| >70 | 5 | 2.5 | 0.7 | 29 | 16.0 | 1.7 | 76 | 41.6 | 2.2 | 73 | 39.8 | 2.2 | 182 | 100 | |
| 35+ | 16 | 1.6 | 0.4 | 189 | 19.0 | 2.1 | 401 | 40.2 | 2.6 | 392 | 39.3 | 2.6 | 997 | 100 | |
| Total | 44 | 2.3 | 0.6 | 345 | 18.1 | 1.7 | 742 | 38.8 | 2.1 | 781 | 40.8 | 2.1 | 1912 | 100 | |
The total number of participants in each age category of vitamin D status may not sum to the total number of participants per age group due to rounding
Serum 25(OH)D in the deficient range (<27.5 nmol/L) was low in the total group at 2.3%. An additional 18.1% exhibited 25(OH)D in the insufficient range (27.5–50 nmol/L).
Variability of 25(OH)D by month of year and season
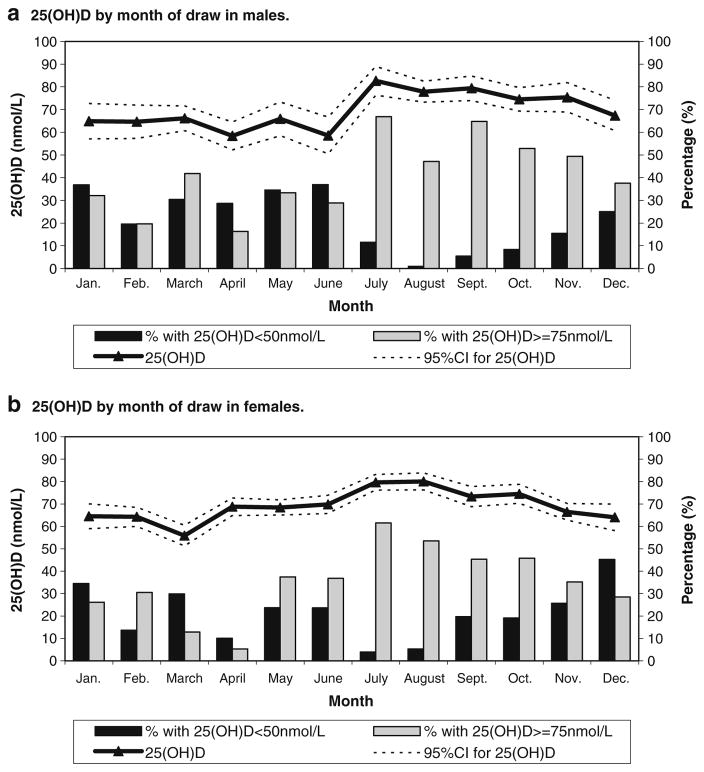
25(OH)D varied over the course of the year (Fig. 1a, b). The percent with 25(OH)D levels ≥75 nmol/L sharply increased in July, reaching 66.9% for males and 61.5% for females. In the month of April, the percentage with 25(OH)D levels ≥75 nmol/L decreased to 16.4% for males and 5.3% for females. Deficient or insufficient level (<50 nmol/L) prevalence was lowest from July to October for males (1.0–11.5%) and in July (3.9%) and August (5.3%) for females. Peak prevalence of insufficiency/deficiency occurred in January (36.9%) for males and December (45.3%) for females.
Fig. 1.
a 25(OH)D by month of draw in males. b 25(OH)D by month of draw in females
When months were grouped into “seasons,” the prevalence of 25(OH)D levels ≥75 nmol/L peaked for both males and females at 59.0% and 54.3%, respectively, in summer and were lowest in spring for males (26.5%) and in winter for females (22.5%). The prevalence of levels <50 nmol/L was greatest in the winter and spring for males (30.9–33.7%) and in fall, winter, and spring for females (25.5%, 26.1%, and 22.7%, respectively). As expected, lowest levels were observed in the summer for both males and females (5.7% and 8.7%, respectively).
Factors associated with lower 25(OH)D levels
Elevated BMI and low/no vitamin D supplementation were among the strongest risk factors for lower 25(OH)D. Among females with BMI <25, 25–29.9 (overweight), and ≥30 (obese), 25(OH)D <50 was 15.7%, 21.0%, and 29.1%, respectively. For males, estimates were 13.4%, 23.9%, and 20.2%, respectively; 38.7% of males and 57.5% of females supplemented their diet with vitamin D to some degree. Milk, soy beverages, and yogurt accounted on average for 48.1% of the vitamin D intake (from milk, soy beverages, yogurt, supplements, and drugs). In winter and spring, of those taking no supplementary vitamin D, at least 40% had insufficient 25(OH)D concentrations (<50 nmol/L; Table 4), the overall percentage in winter and spring being 43.9. Although the prevalence of 25(OH)D <50 nmol/L was low in summer, it was higher for those taking no vitamin D supplement. The prevalence of 25(OH)D <50 nmol/L was at or below 10% year-round among those who took a vitamin D supplement at or above 400 IU/day.
Table 4.
Seasonal variation in 25(OH)D <50 nmol/L vitamin D supplementation
| Vitamin D Supplementation (mean daily) | 25(OH)D <50nmol/L (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Winter | Spring | Summer | Fall | |||||||||
|
|
|
|
|
|||||||||
| n | % | SE of % | n | % | SE of % | n | % | SE of % | n | % | SE of % | |
| 0 | 66 | 48.2 | 7.0 | 83 | 40.9 | 6.6 | 26 | 9.2 | 2.4 | 80 | 22.2 | 4.3 |
| ≤400 IU | 10 | 12.6 | 3.5 | 48 | 25.5 | 6.6 | 6 | 4.6 | 1.7 | 45 | 26.8 | 7.2 |
| >400 IU | 3 | 4.8 | 1.9 | 11 | 10.0 | 4.3 | 4 | 5.0 | 2.0 | 7 | 5.9 | 4.1 |
In sex-specific multivariate linear regression analyses, several independent factors predicted 25(OH)D levels (Table 5). Degree of vitamin D supplementation, together with its modification by season, were important contributors to 25(OH)D level. The greatest effects of this interaction were evident in fall and winter. Ethnic background as “other,” winter and spring season, and BMI ≥30 (obese) were strongly associated with lower levels for both males and females. For females, determinants also included the following: BMI of 25–30 (overweight); not often exposing a considerable part of the body to sunlight; lower intake of vitamin D from supplements and fortified milk, soy beverages, or yogurt; and lack of regular activity. For males, the interaction between vitamin D supplementation and spring season was associated with higher 25(OH)D. Age and sunscreen use were not identified as independently associated factors.
Table 5.
Multivariate regression for independent determinants of 25(OH)D (nmol/L)
| Correlates | Parameter estimates (95% CI) | |
|---|---|---|
|
| ||
| Females | Males | |
| Age group (years) | ||
| 71+ | −0.78 (−5.66; 4.10) | 1.31 (−5.39; 8.01) |
| 51–70 | 0.96 (−3.77; 5.68) | −1.79 (−8.26; 4.69) |
| 35–50 | Reference | Reference |
| Ethnic background | ||
| Other | −9.08 (−15.14; −3.02) | −15.22 (−22.69; −7.75) |
| White | Reference | Reference |
| Body Mass Index (kg/m0) | ||
| 30+ | −11.12 (−14.04; −8.21) | −8.17 (−13.49; −2.85) |
| 25–29.9 | −5.83 (−8.45; −3.21) | −3.78 (−8.20; 0.64) |
| <25 | Reference | Reference |
| Season | ||
| Fall | −3.48 (−7.18; 0.22) | −9.62 (−15.19; −4.06) |
| Winter | −13.51 (−17.44; −9.58) | −17.31 (−23.87; −10.75) |
| Spring | −6.12 (−9.64; −2.61) | −13.22 (−18.85; −7.60) |
| Summer | Reference | Reference |
| Vitamin D supplementation (per 400 IU) | 3.71 (2.82; 4.60) | 0.47 (−1.74; 2.67) |
| Vitamin D supplementation (per 400 IU) by seasona | ||
| Vitamin D by fall | 1.84 (0.4; 3.29) | 5.27 (2.18; 8.35) |
| Vitamin D by winter | 2.37 (1.02; 3.72) | 10.81 (5.37; 16.24) |
| Vitamin D by spring | 0.39 (−0.85; 1.62) | 3.66 (1.07; 6.25) |
| Vitamin D by summer | Reference | Reference |
| Vitamin D—dietaryb (per 200 IU) | 3.24 (1.27; 5.22) | 2.21 (−0.74; 5.17) |
| Sun exposure | ||
| Never | −8.58 (−14.20; −2.95) | −3.61 (−11.27; 4.04) |
| Seldom/Regularly | −8.05 (−13.62; −2.48) | 2.23 (−5.26; 9.72) |
| Often | Reference | Reference |
| Sunscreen use | ||
| Usually/always | −1.33 (−4.05; 1.39) | 0.987 (−3.72; 5.45) |
| Sometimes | 0.92 (−2.24; 4.09) | 1.07 (−3.78; 5.91) |
| None | Reference | Reference |
| Regular activity participation | ||
| No | −2.94 (−5.26; −0.63) | −3.55 (−7.34; 0.23) |
| Yes | Reference | Reference |
Interaction between vitamin D supplementation and season
Vitamin D from fortified milk, soy beverages, and yogurt
Discussion
If associations between vitamin D levels and several chronic diseases are determined to be causal, then achieving and maintaining optimal vitamin D levels would represent a major correctable goal for ameliorating a number of common and debilitating diseases. In this study, levels of serum 25(OH)D <75 nmol/L [22] were evident in 59% of Canadians. Overall estimates of 25(OH)D levels, however, may be misleading, as they are derived by averaging blood samples drawn over the high and lower risk seasons. Thus, in our study, in winter and spring, nearly one third of males and one quarter of females had insufficient or deficient concentrations of 25(OH)D (<50 nmol/L), and in spring, approximately three quarters of males and females displayed 25(OH)D levels <75 nmol/L. These results are consistent with the seasonal variability noted in other Canadian studies [11, 12, 15, 31] and in other countries [32]. Consequently, it is likely that a large proportion of Canadians would have suboptimal vitamin D status at some point during the year.
The prevalence of vitamin D insufficiency and suboptimal level is dependent on the definition used (usually <50 or <75 nmol/L, respectively), and there are currently no standard international reference levels for insufficient and optimal status. The Institute of Medicine is currently reviewing vitamin D requirements and associated 25(OH) D cutoff points for health outcomes. Part of the uncertainty over defining levels of insufficiency may reside with standardization and operator variability in 25(OH)D assays [33], because a variety of methods to measure 25(OH)D are available and different extraction procedures are used. Irrespective of the assay used, as serum 25(OH)D levels falls, levels of parathyroid hormone (PTH) in the serum increase. Consequently, a standard definition of an optimal 25(OH)D level has been the concentration above which serum PTH levels cannot be suppressed further. Estimates of optimal 25(OH)D concentrations reached using the PTH suppression criterion vary widely. Overall, however, the minimum level considered by many to be optimal is 75 nmol/L [1, 26, 27], and 59% of subjects in our study fell below this threshold.
Both the current study and the report recently released by Statistics Canada from the 2007–2009 CHMS [12] included participants from the five regions of Canada—Atlantic, Quebec, Ontario, Prairies, and British Columbia. Both national surveys included sites with larger and smaller population densities. CaMos included seven sites and CHMS included 15. Both studies did not include residents of Indian reserves, those in remote northern regions, and those in institutions. The studies differed with respect to age groups. The age focus of CHMS was 6 to 79 years whereas that of this CaMos study was adults aged 35 and over. In the CaMos study, there was particular strength (i.e., unweighted numbers of participants that exceeded that of corresponding CHMS groups) for 51–70-year-old females, males that were 70 years and older, and females that were 70 years and older. Females were overrepresented in the CaMos sampling to allow for sufficient sample size to estimate factors related to osteoporosis. The CaMos age groups for the present study were chosen to correspond with those used for the Dietary Reference Intakes (DRI). Because of this, other studies which examine adequacy of vitamin D intake (which vary by DRI age group) can be easily compared with the results of CaMos. In both studies, participants within their respective age groups were weighted to represent the Canadian population. For those participants age 40 to 79, both the CaMos and CHMS studies showed similar mean 25(OH)D levels, and similar percentages of participants age 40 to 79 had 25(OH)D levels ≥75 nmol/L. Median values were below 75 nmol/L in most age–sex groups in our study as well as in adults in the CHMS [12]. The exceptions to this were males age 35–50 years in our study (median 77.1 nmol/L). Similar percentages (below 3%) of 25(OH)D levels <27.5 nmol/L were also noted in women aged 40 to 59 and men aged 60 to 79. In CaMos, greater numbers of women in the 60–79-year group allowed for an estimate of women with deficient (<27.5 nmol/L) levels (below 3%) whereas this estimate was not possible in CHMS due to their extreme sampling variability or small sample size. Additionally, fewer men aged 40–59 in the CaMos study had levels <27.5 nmol/L than in the CHMS study (below 3% versus approximately 6%, respectively). Twenty percent in our study had values below 50 nmol/L, whereas this percentage was not determined in the CHMS study.
In our study, among males aged 35–50 years, median 25 (OH)D was approximately 10 nmol/L higher than for those aged 50+ years. This is consistent with findings of decreased cutaneous production of vitamin D3 with age [34, 35]. Median 25(OH)D was not higher for females aged 35–50 years. This may be related to dietary intake and/or supplement use or to other external issues, and further studies are required to explore this. In other studies, lower [36, 37], similar [13], and higher [12] 25(OH)D levels have been noted in women compared to men.
Both CaMos and CHMS studied the seasonal influences on 25(OH)D levels and vitamin D consumption from dietary sources. CHMS considered two seasons of blood collection (November to March and April to October) whereas CaMos extended the CHMS findings by studying seasonal variation of 25(OH)D in more detail, i.e., by month and by four seasons. CHMS estimated frequency of consumption of milk/enriched milk substitutes using a non-quantitative FFQ, whereas CaMos extended these findings by using a semiquantitative FFQ to estimate dietary intake of vitamin D from milk/enriched milk substitutes, as well as enriched yogurt. Furthermore, in contrast to CHMS, CaMos also addressed the use of vitamin D supplements, body mass index, sun exposure, sunscreen use, and physical activity. The CaMos results, therefore, are comparable to the CHMS results with respect to mean 25OHD levels and percentages of participants with 25(OH)D levels ≥75 nmol/L but extend the CHMS findings by having increased power to assess low 25(OH)D levels in older women and men, by investigating more predictors of 25(OH)D and by using a multivariable analysis (rather than a bivariate analysis) to determine independent effects of the factors.
“Other” ethnicity, a proxy for darker skin pigmentation in this study, was a strong and independent risk factor for low 25(OH)D in the CaMos study. This finding concurs with other Canadian studies [12, 13, 15, 38–40] and with international [32] findings. The population of Canadians with darker skin pigmentation now stands at one in five Canadians, and the need for people of darker skin to take more vitamin D is increasingly recognized [41].
Overweight and obesity have been rising among adults [42, 43] and children [44, 45] in Canada, particularly over the past 35 years. Adiposity is a risk factor for low 25(OH) D [11], perhaps due to sequestration of fat-soluble vitamin D in adipose tissue [46]. Excess adiposity was an independent predictor of low 25(OH)D in this study. Obesity alone was predictive for males and females in our study, but being overweight was predictive for females only. This is consistent with studies among youth in Canada [47] and New Zealand [48] in which an inverse association between serum 25(OH)D and higher BMI was observed in females but not males. This may be related to the greater amount of body fat per unit weight among females compared to males [49] which can serve as a depot for the fat soluble vitamin D. In our study, regular physical activity was a surrogate to estimate time spent outdoors, and lack of regular activity was associated with reduced 25 (OH)D for females. However, physical activity may have benefits on 25(OH)D independent of exposure to the outdoors.
In our study, the effect of vitamin D supplementation was highly modified by season. Fall and winter supplementation, in particular, emerged as independent predictors of 25(OH)D concentrations. Low intake of vitamin D supplements (<400 IU/day) had the least impact in summer; however, 400 IU or more was needed to keep 25(OH)D above 50 nmol/L year round in 90% of participants. Few foods naturally contain vitamin D, and our study would confirm that Canadians appear to be dependent on fortified dietary sources or supplements to maintain vitamin D status.
Strengths of this study are that it offers a recent, large sample of adults, and data on an array of biological, behavioral, and environmental correlates. It uses a randomly selected sample of Canadians, from predominantly urban sites across Canada. A limitation of the study is that non-whites are underrepresented, likely due to their lower proportion in the population at entry into the study in 1995–1997. Furthermore, self-reported ethnicity was used as a proxy to surmise variations in skin pigmentation. Nevertheless, “other” (non-white) ethnicity was identified as an independent risk factor in the multivariate analysis because of the strong effect of this characteristic. Another limitation is that seasonal effects were studied using cross-sectional data instead of multiple measurements in the same person. Questions on sun exposure, although limited, were included to provide a relative estimate of sun exposure rank. Household non-response bias related to bone health was slight, although some may still have been evident [17]. In this study’s sample, the response rate of the full participants (who gave blood samples) was modest, but there was no overt bias toward low or high values when full and partial participants were compared. The results for this study are based on cross-sectional data; therefore, it is not possible to infer causal links for some determinants of low vitamin D.
In summary, while frank deficiency was low in the population, combined deficient and insufficient levels (<50 nmol/L) affected one in five Canadians adults 35 years of age or older. Specific risk groups include those with excess adiposity and darker skin pigmentation. Seasonal variation had strong effects on 25(OH)D levels: vitamin D levels <75 nmol/L approached and surpassed 75% for several months in winter (for females) and spring (for males). Vitamin D supplementation was a strong determinant of 25(OH)D level, and ≥400 IU/day was highly protective against levels below 50 nmol/L. Achievement of adequate vitamin D intake through diet and supplementation, particularly during the winter and spring months, and maintenance of normal weight appear to be key modifiable factors to consider when developing strategies for enhancing vitamin D status in the population.
Acknowledgments
The authors would like to thank all the participants in the Canadian Multicentre Osteoporosis Study.
Funding CaMos was funded by the Canadian Institutes of Health Research (CIHR), Merck Frosst Canada Ltd., Eli Lilly Canada Inc., Novartis Pharmaceuticals Inc., The Alliance for Better Bone Health: Sanofi-Aventis and Procter and Gamble Pharmaceuticals Canada Inc., Amgen, The Dairy Farmers of Canada, The Arthritis Society.
Footnotes
CaMos Research Group David Goltzman (co-principal investigator, McGill University, Montreal), Nancy Kreiger (co-principal investigator, University of Toronto, Toronto), Alan Tenenhouse (principal investigator emeritus, Toronto), CaMos Coordinating Centre, McGill University, Montreal, Quebec: Suzette Poliquin (national coordinator), Suzanne Godmaire (research assistant), Claudie Berger (statistician), Wei Zhou (statistician).
Memorial University, St. John’s Newfoundland: Carol Joyce (director), Christopher Kovacs (co-director), Emma Sheppard (coordinator).
Dalhousie University, Halifax, Nova Scotia: Susan Kirkland, Stephanie Kaiser (co-directors), Barbara Stanfield (coordinator).
Laval University, Quebec City, Quebec: Jacques P. Brown (director), Louis Bessette (co-director), Marc Gendreau (coordinator).
Queen’s University, Kingston, Ontario: Tassos Anastassiades (director), Tanveer Towheed (co-director), Barbara Matthews (coordinator).
University of Toronto, Toronto, Ontario: Bob Josse (director), Sophie Jamal (co-director), Tim Murray (past director), Barbara Gardner-Bray (coordinator)
McMaster University, Hamilton, Ontario: Jonathan D. Adachi (director), Alexandra Papaioannou (co-director), Laura Pickard (coordinator).
University of Saskatchewan, Saskatoon, Saskatchewan: Wojciech P. Olszynski (director), K. Shawn Davison (co-director), Jola Thingvold (coordinator).
University of Calgary, Calgary, Alberta: David A. Hanley (director), Jane Allan (coordinator).
University of British Columbia, Vancouver, British Columbia: Jerilynn C. Prior (director), Millan Patel (co-director), Brian Lentle (radiologist), Yvette Vigna (coordinator).
Conflicts of interest D. Goltzman has received honoraria from and served on the advisory boards of Amgen, Eli Lilly, Proctor & Gamble, Merck Frosst, Novartis and Servier. D.A. Hanley has received honoraria from Amgen, Proctor & Gamble, sanofi-aventis, Merck Frosst, and Servier. L Greene-Finestone, C Berger, M de Groh, N Hidiroglou, K Sarafin, S Poliquin, J Krieger, and JB Richards have no conflicts of interest.
Contributor Information
L. S. Greene-Finestone, Public Health Agency of Canada, Ottawa, Ontario, Canada
C. Berger, CaMos Coordinating Centre, McGill University, Montreal, Quebec, Canada
M. de Groh, Public Health Agency of Canada, Ottawa, Ontario, Canada
D. A. Hanley, University of Calgary, Calgary, Alberta, Canada
N. Hidiroglou, Health Canada, Ottawa, Ontario, Canada
K. Sarafin, Health Canada, Ottawa, Ontario, Canada
S. Poliquin, CaMos Coordinating Centre, McGill University, Montreal, Quebec, Canada
J. Krieger, Health Canada, Ottawa, Ontario, Canada
J. B. Richards, Department of Medicine, Jewish General Hospital, McGill University, Montreal, Quebec, Canada
D. Goltzman, CaMos Coordinating Centre, McGill University, Montreal, Quebec, Canada. Department of Medicine, McGill University, Montreal, Quebec, Canada. CaMos, Royal Victoria Hospital, 687 Pine Ave West, Room E1-59, Montréal, Québec, Canada H3A 1A1
CaMos Research Group, CaMos, Royal Victoria Hospital, 687 Pine Ave West, Room E1-59, Montréal, Québec, Canada H3A 1A1.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Standing committee on the Scientific Evaluation of Dietary Intakes FaNBIoM. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D and fluoride. National Academy Press; Washington, DC: 1997. pp. 250–287. [Google Scholar]
- 3.Balsan S, Garabedian M. Rickets, osteomalacia, and osteopetrosis. Curr Opin Rheumatol. 1991;3:496–502. doi: 10.1097/00002281-199106000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, et al. Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res. 2009;24:935–942. doi: 10.1359/JBMR.081242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krause R, Buhring M, Hopfenmuller W, et al. Ultraviolet B and blood pressure. Lancet. 1998;352:709–710. doi: 10.1016/S0140-6736(05)60827-6. [DOI] [PubMed] [Google Scholar]
- 6.Mathieu C, Gysemans C, Giulietti A, et al. Vitamin D and diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 7.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32:210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Garland CF, Mohr SB, Gorham ED, et al. Role of ultraviolet B irradiance and vitamin D in prevention of ovarian cancer. Am J Prev Med. 2006;31:512–514. doi: 10.1016/j.amepre.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF. Calcium plus vitamin D and the risk of colorectal cancer. N Engl J Med. 2006;354:2287–2288. doi: 10.1056/NEJMc060753. [DOI] [PubMed] [Google Scholar]
- 10.Munger KL, Levin LI, Hollis BW, et al. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 11.Rucker D, Allan JA, Fick GH, et al. Vitamin D insufficiency in a population of healthy western Canadians. CMAJ. 2002;166:1517–1524. [PMC free article] [PubMed] [Google Scholar]
- 12.Langlois K, Greene-Finestone L, Little J, et al. Vitamin D status of Canadians as measured in the 2007 to 2009 Canadian Health Measures Survey. Statistics Canada; Ottawa: 2010. [PubMed] [Google Scholar]
- 13.Gozdzik A, Barta JL, Wu H, et al. Low wintertime vitamin D levels in a sample of healthy young adults of diverse ancestry living in the Toronto area: associations with vitamin D intake and skin pigmentation. BMC Public Health. 2008;8:336. doi: 10.1186/1471-2458-8-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiler HA, Leslie WD, Bernstein CN. Parathyroid hormone is predictive of low bone mass in Canadian Aboriginal and White women. Bone. 2008;42:498–504. doi: 10.1016/j.bone.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Vieth R, Cole DE, Hawker GA, et al. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr. 2001;55:1091–1097. doi: 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- 16.Liu BA, Gordon M, Labranche JM, et al. Seasonal prevalence of vitamin D deficiency in institutionalized older adults. J Am Geriatr Soc. 1997;45:598–603. doi: 10.1111/j.1532-5415.1997.tb03094.x. [DOI] [PubMed] [Google Scholar]
- 17.Kmetic A, Joseph L, Berger C, et al. Multiple imputation to account for missing data in a survey: estimating the prevalence of osteoporosis. Epidemiology. 2002;13:437–444. doi: 10.1097/00001648-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Kreiger N, Tenenhouse A, Joseph L, et al. The Canadian Multicentre Osteoporosis Study (CaMos): background, rationale, methods. Can J Aging. 1999;18:376–387. [Google Scholar]
- 19.Carter GD, Carter R, Jones J, et al. How accurate are assays for 25-hydroxyvitamin D? Data from the international vitamin D external quality assessment scheme. Clin Chem. 2004;50:2195–2197. doi: 10.1373/clinchem.2004.040683. [DOI] [PubMed] [Google Scholar]
- 20.Phinney KW. Development of a standard reference material for vitamin D in serum. Am J Clin Nutr. 2008;88:511S–512S. doi: 10.1093/ajcn/88.2.511S. [DOI] [PubMed] [Google Scholar]
- 21.Wallace AM, Gibson S, de la Hunty A, et al. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75:477–488. doi: 10.1016/j.steroids.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Looker AC, Lacher DA, Pfeiffer CM, et al. Data advisory with regard to NHANES serum 25-hydroxyvitamin D data. Am J Clin Nutr. 2009;90:695. doi: 10.3945/ajcn.2009.28175. [DOI] [PubMed] [Google Scholar]
- 23.Lips P, Bouillon R, van Schoor NM, et al. Reducing fracture risk with calcium and vitamin D. Clin Endocrinol (Oxf) 2009 doi: 10.1111/j.1365-2265.2009.03701.x. in press. [DOI] [PubMed] [Google Scholar]
- 24.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 25.Pearce SH, Cheetham TD. Diagnosis and management of vitamin D deficiency. Bmj. 2010;340:b5664. doi: 10.1136/bmj.b5664. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 28.Vatanparast H, Calvo MS, Green TJ, et al. Despite mandatory fortification of staple foods, vitamin D intakes of Canadian children and adults are inadequate. J Steroid Biochem Mol Biol. 2010;121(1–2):301–303. doi: 10.1016/j.jsbmb.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 29.Canada H. Drug product database online query. Health Canada; Ottawa: 2009. [Google Scholar]
- 30.Poliquin S, Joseph L, Gray-Donald K. Calcium and vitamin D intakes in an adult Canadian population. Can J Diet Pract Res. 2009;70:21–27. doi: 10.3148/70.1.2009.21. [DOI] [PubMed] [Google Scholar]
- 31.Newhook LA, Sloka S, Grant M, et al. Vitamin D insufficiency common in newborns, children and pregnant women living in Newfoundland and Labrador, Canada. Matern Child Nutr. 2009;5:186–191. doi: 10.1111/j.1740-8709.2008.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 33.Jones G, Horst R, Carter G, et al. Contemporary diagnosis and treatment of vitamin D-related disorders. J Bone Miner Res. 2007;22(Suppl 2):V11–V15. doi: 10.1359/jbmr.07s219. [DOI] [PubMed] [Google Scholar]
- 34.Holick MF, Matsuoka LY, Wortsman J. Age, vitamin D, and solar ultraviolet. Lancet. 1989;2:1104–1105. doi: 10.1016/s0140-6736(89)91124-0. [DOI] [PubMed] [Google Scholar]
- 35.Need AG, Morris HA, Horowitz M, et al. Effects of skin thickness, age, body fat, and sunlight on serum 25-hydroxyvitamin D. Am J Clin Nutr. 1993;58:882–885. doi: 10.1093/ajcn/58.6.882. [DOI] [PubMed] [Google Scholar]
- 36.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 37.Bolland MJ, Grey AB, Ames RW, et al. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr. 2007;86:959–964. doi: 10.1093/ajcn/86.4.959. [DOI] [PubMed] [Google Scholar]
- 38.Weiler HA, Leslie WD, Krahn J, et al. Canadian Aboriginal women have a higher prevalence of vitamin D deficiency than non-Aboriginal women despite similar dietary vitamin D intakes. J Nutr. 2007;137:461–465. doi: 10.1093/jn/137.2.461. [DOI] [PubMed] [Google Scholar]
- 39.Lebrun JB, Moffatt ME, Mundy RJ, et al. Vitamin D deficiency in a Manitoba community. Can J Public Health. 1993;84:394–396. [PubMed] [Google Scholar]
- 40.Ward LM, Gaboury I, Ladhani M, et al. Vitamin D-deficiency rickets among children in Canada. CMAJ. 2007;177:161–166. doi: 10.1503/cmaj.061377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Canadian Cancer Society. Canadian Cancer Society announces Vitamin D recommendation. Canadian Cancer Society; Toronto: 2009. [Google Scholar]
- 42.Hopman WM, Leroux C, Berger C, et al. Changes in body mass index in Canadians over a five-year period: results of a prospective, population-based study. BMC Public Health. 2007;7:150. doi: 10.1186/1471-2458-7-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shields M, Tremblay MS, Laviolette M, et al. Fitness of Canadian adults: results from the 2007–2009 Canadian Health Measures Survey. Health Rep. 2010;21:21–35. [PubMed] [Google Scholar]
- 44.Tremblay MS, Willms JD. Secular trends in the body mass index of Canadian children. CMAJ. 2000;163:1429–1433. [PMC free article] [PubMed] [Google Scholar]
- 45.Tremblay MS, Shields M, Laviolette M, et al. Fitness of Canadian children and youth: results from the 2007–2009 Canadian Health Measures Survey. Health Rep. 2010;21:7–20. [PubMed] [Google Scholar]
- 46.Wortsman J, Matsuoka LY, Chen TC, et al. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 47.Mark S, Gray-Donald K, Delvin EE, et al. Low vitamin D status in a representative sample of youth from Quebec, Canada. Clin Chem. 2008;54:1283–1289. doi: 10.1373/clinchem.2008.104158. [DOI] [PubMed] [Google Scholar]
- 48.Rockell JE, Green TJ, Skeaff CM, et al. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5–14 y. J Nutr. 2005;135:2602–2608. doi: 10.1093/jn/135.11.2602. [DOI] [PubMed] [Google Scholar]
- 49.Chumlea WC, Guo SS, Kuczmarski RJ, et al. Body composition estimates from NHANES III bioelectrical impedance data. Int J Obes Relat Metab Disord. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]