Abstract
Functional magnetic resonance imaging (fMRI) research is routinely criticized for being statistically underpowered due to characteristically small sample sizes and much larger sample sizes are being increasingly recommended. Additionally, various sources of artifact inherent in fMRI data can have detrimental impact on effect size estimates and statistical power. Here we show how specific removal of non-BOLD artifacts can improve effect size estimation and statistical power in task-fMRI contexts, with particular application to the social-cognitive domain of mentalizing/theory of mind. Non-BOLD variability identification and removal is achieved in a biophysical and statistically principled manner by combining multi-echo fMRI acquisition and independent components analysis (ME-ICA). Without smoothing, group-level effect size estimates on two different mentalizing tasks were enhanced by ME-ICA at a median rate of 24% in regions canonically associated with mentalizing, while much more substantial boosts (40–149%) were observed in non-canonical cerebellar areas. Effect size boosting occurs via reduction of non-BOLD noise at the subject-level and consequent reductions in between-subject variance at the group-level. Smoothing can attenuate ME-ICA-related effect size improvements in certain circumstances. Power simulations demonstrate that ME-ICA-related effect size enhancements enable much higher-powered studies at traditional sample sizes. Cerebellar effects observed after applying ME-ICA may be unobservable with conventional imaging at traditional sample sizes. Thus, ME-ICA allows for principled design-agnostic non-BOLD artifact removal that can substantially improve effect size estimates and statistical power in task-fMRI contexts. ME-ICA could mitigate some issues regarding statistical power in fMRI studies and enable novel discovery of aspects of brain organization that are currently under-appreciated and not well understood.
Keywords: Multi-echo EPI, Statistical power, Denoising, Task-fMRI, Mentalizing, Cerebellum
Highlights
-
•
ME-ICA enhances effect size estimates in block-design task-based fMRI studies
-
•
Effect sizes in canonical mentalizing regions are boosted at median rate of 24%
-
•
Cerebellar effect size estimates are boosted by 40–149%
-
•
Enhanced effect size enables highly-powered studies at traditional sample sizes
-
•
Enables potential for novel discoveries hidden in small underpowered studies
Introduction
A common criticism of neuroscience research in general (Button et al., 2013) and functional MRI (fMRI) in particular (Yarkoni, 2009), is that studies are characteristically statistically underpowered. Low statistical power by definition means that a study will have less of a chance for detecting true effects, but also means that observed statistically significant effects are less likely to be true and will be more susceptible to the biasing impact of questionable research practices (Button et al., 2013, Ioannidis, 2005). This problem is important given the emergent ‘crisis of confidence’ across many domains of science (e.g., psychology, neuroscience), stemming from low frequency of replication and the pervasive nature of questionable research practices (Button et al., 2013, Ioannidis, 2005, Simmons et al., 2011).
Low statistical power can be attributed to small sample sizes, small effect sizes, or a combination of both. The general recommended solution is primarily to increase sample size (though other secondary recommendations also include increased within-subject scan time). These recommendations are pragmatic mainly because these variables are within the control of the researcher during study design. While these recommendations are important to consider (Desmond and Glover, 2002, Friston, 2012, Lindquist et al., 2013, Mumford and Nichols, 2008, Yarkoni, 2009), other considerations such as dealing with substantial sources of non-BOLD noise inherent in fMRI data also need to be evaluated before the field assumes increasing sample size or scan time to be the primary or only means of increasing statistical power. These considerations are especially poignant when mandates for large-N studies and increased within-subject scan time are practically limiting due to often cited reasons such as prohibitively high imaging costs for all but the most well-funded research groups or in situations where the focus is on studying sensitive, rare, and/or less prevalent patient populations and where increasing scan time is impractical (e.g., children, patients with neuropsychiatric conditions).
On the issue of non-BOLD noise variability, it is well known that fMRI data are of variable quality. Poor and variable quality data can significantly hamper ability to achieve accurate and reproducible representations of brain organization. The poor sensitivity of fMRI often arises from high levels of subject motion (often task correlated), cardiopulmonary physiology, or other types of imaging artifact (Murphy et al., 2013). These artifacts are problematic because they are often inadequately separable from the functional blood oxygenation level dependent (BOLD) signal when using conventional fMRI methods. Given an advance in fMRI methodology that allows enhanced detection and removal of these artifacts, the situation regarding statistical power and sample size may change markedly. Such advances could create viable experimental alternatives or supplements to the recommendation for increasing sample size/scan time to boost statistical power, and concurrently make for an fMRI approach that can more reliably enable discovery of subtle but potentially key aspects of typical and atypical brain function.
In this study, we address problems related to statistical power through specific targeting of the problems related to non-BOLD artifact variability. We have applied an approach that integrates fMRI data acquisition using multi-echo EPI with the decomposition method of independent components analysis (ICA), towards more principled removal of non-BOLD signals from fMRI data without arbitrary temporal or spatial smoothing (e.g. bandpass filtering). The integration of these techniques is implemented in a pipeline called multi-echo independent components analysis or ME-ICA (Kundu et al., 2012). ME-ICA utilizes multi-echo fMRI to acquire both fMRI signal time series and their NMR signal decay, towards distinguishing functional BOLD from non-BOLD signal components based on relaxometry of their respective and differentiable signatures in the decay domain. BOLD and non-BOLD signals are markedly differentiable in data analysis of the echo time (TE) domain and independent of gross similarity in the spatial and temporal domains. BOLD-related signals specifically show linear dependence of percent signal change on TE, whereas non-BOLD signal amplitudes demonstrate TE-independence. This suggests ME-ICA could be a principled bottom-up approach towards identifying and retaining BOLD-related variability while systematically removing non-BOLD variation. ME-ICA has been successfully applied to fMRI resting state connectivity acquisition and analysis and has been shown to enable improvements regarding increased temporal signal-to-noise ratio (tSNR) and enhanced ability to remove motion and other artifactual sources of variability. ME-ICA has also been used to improve seed-based connectivity analysis, enhance specificity, and holds translational potential for use at high-field strength and within animal models (Kundu et al., 2013, Kundu et al., 2014, Kundu et al., 2015). ME-ICA can also be applied alongside multi-band acquisition (Olafsson et al., 2015) and has recently been applied to identify ultra-slow temporally-extended task-related responses (Evans et al., 2015). However, one very necessary yet unexamined niche within the space of uses for fMRI is within the highly utilized context of traditional task-based fMRI studies and the potential impact that ME-ICA innovations could have on effect size estimation and consequently statistical power.
Here we conduct the first assessment of how ME-ICA performs with regard to effect size estimation and statistical power in task-related activation mapping settings with block-designs. ME-ICA can be flexibly applied to both task- and resting state fMRI contexts. This unified approach is advantageous since conventional resting state and task data processing and denoising use disjoint pipelines that often may require different assumptions, analytical premises and skillsets. However, it is important and currently not well understood if and to what extent generalized non-BOLD removal as targeted by ME-ICA enhances the elucidation of task effects, compared to current task activation analysis with inline denoising based on linear artifact models and arbitrary filtering done in a study specific manner. In this study we specifically examined how ME-ICA performs against a conventional task-based imaging analysis pipeline involving baseline regression of motion parameters (i.e. TSOC+MotReg) and another prominent yet more recent task-based denoising procedure, GLMdenoise (Kay et al., 2013). We utilized two separate tasks (i.e. the ‘SelfOther’ and ‘Stories’ tasks) assessing neural systems supporting the social-cognitive domain or mentalizing and theory of mind and highlight the effects of ME-ICA in terms of effect size estimation and statistical power. We also evaluate the impact of the method on two sets of brain regions; ‘canonical’ regions typically highlighted as important in the neural systems for mentalizing (Frith and Frith, 2003, Lombardo et al., 2010b, Saxe and Powell, 2006, Schaafsma et al., 2015, Schurz et al., 2014, Spunt and Adolphs, 2014, van Overwalle, 2009) and ‘non-canonical’ regions in the cerebellum (van Overwalle et al., 2014).
Materials and methods
Participants
This study was approved by the Essex 1 National Research Ethics committee. Parents gave informed consent for their child to participate and each child also gave assent to participate. Participants were 69 adolescents (34 males, 35 females, mean age = 15.45 years, sd age = 0.99 years, range = 13.22–17.18 years) sampled from a larger cohort of individuals whose mothers underwent amniocentesis during pregnancy for clinical reasons (i.e. screening for chromosomal abnormalities). The main focus for sampling from this cohort was to study the fetal programming effects of steroid hormones on adolescent brain and behavioral development. At amniocentesis, none of the individuals screened positive for any chromosomal abnormalities and were thus considered typically developing. Upon recruitment for this particular study, we additionally checked for any self- or parent-reported neuropsychiatric conditions. One individual had a diagnosis on the autism spectrum. The remaining participants did not have any other kind of neurological or psychiatric diagnosis. Analyses were done on the full sample of 69 individuals, as analyses leaving out the one patient with an autism diagnosis did not change any of the results.
Task design
Participants were scanned using two block-design fMRI paradigms. The first paradigm, which we call the ‘SelfOther’ task, is a 2 × 2 within-subjects factorial design which contains two contrasts that tapped either self-referential cognition and mentalizing and was similar in nature to previously published studies (Lombardo et al., 2010a, Lombardo et al., 2010b, Lombardo et al., 2011). Briefly, participants are asked to make reflective judgments about either themselves or the British Queen that varied as either a mentalistic (e.g., “How likely are [you/the Queen] to think that it is important to keep a journal?”) or physical judgment (e.g., “How likely are [you/the Queen] to have bony elbows?”). Participants make judgments on a 1–4 scale, where 1 indicated ‘not at all likely’ and 4 indicated ‘very likely’. All stimuli are taken from Jason Mitchell's lab and have been used in prior studies on mentalizing and self-referential cognition (Jenkins et al., 2008, Mitchell et al., 2006). The SelfOther task is presented in 2 scanning runs (8:42 duration per run; 261 volumes per run). Within each scanning run there are 4 blocks per condition, and within each block there are 4 trials of 4 s duration each. Task blocks are separated from each other by a 16 s fixation block. The first 5 volumes of each run are discarded to allow for equilibration effects.
The second paradigm, which we call the ‘Stories’ task, is also a block-design and contains two contrasts tapping mentalizing and language domains. The paradigm is identical to a study by Gweon and colleagues (Gweon et al., 2012), utilizing the same stimuli and presentation scripts provided directly by Gweon and colleagues. Briefly, participants listen to a series of stories whereby the stories differ in content. The content of the stories vary in terms of mentalistic, social, or physical content. The social stories contain descriptions of people and characters but make no statements that referenced mental states. Physical stories are segments of stories that describe the physical setting but do not include people. Mental stories are segments that include references to people as main characters and make references to mental states that those characters hold. The paradigm also includes blocks for two other kinds of language control conditions that are not examined in this manuscript (i.e. stories read in a foreign language (e.g., Russian, Hebrew, and Korean) and blocks of music played by different instruments (e.g., guitar, piano, saxophone, and violin). After participants heard each story segment they were given a choice of whether a specific auditory segment logically came next. This was introduced to verify that participants were paying close attention to the stories and the details inside each story segment. The Stories task is presented in 2 scanning runs (6:36 duration per run; 192 volumes per run) and within each scanning run there are 2 blocks per condition. The first 6 volumes were discarded to allow for equilibration effects.
Resting state data was also collected on each participant with a 10 min long ‘eyes-open’ run (i.e. 300 volumes), where participants were asked to stare at a central fixation cross and to not fall asleep. The multi-echo EPI sequence was identical to those used in the task paradigms.
fMRI data acquisition
All MRI scanning took place on a 3T Siemens Tim Trio MRI scanner at the Wolfson Brain Imaging Centre in Cambridge, UK. Functional imaging data during task and rest was acquired with a multi-echo EPI sequence with online reconstruction (repetition time (TR), 2000 ms; field of view (FOV), 240 mm; 28 oblique slices, descending alternating slice acquisition, slice thickness 3.8 mm; TE = 13, 31, and 48 ms, GRAPPA acceleration factor 2, BW = 2368 Hz/pixel, flip angle, 90°, voxel size 3.8 mm isotropic). Anatomical images were acquired using a T1-weighted magnetization prepared rapid gradient echo (MPRAGE) sequence for warping purposes (TR, 2300 ms; TI, 900 ms; TE, 2.98 ms; flip angle, 9°, matrix 256 × 256 × 256, field-of-view 25.6 cm).
fMRI preprocessing
Data were processed by ME-ICA using the tool meica.py as distributed in the AFNI neuroimaging suite (v2.5 beta10), which implemented both basic fMRI image preprocessing and decomposition-based denoising. For the processing of each subject, first the anatomical image was skull-stripped and then warped nonlinearly to the MNI anatomical template using AFNI 3dQWarp. The warp field was saved for later application to functional data. For each functional dataset, the first TE dataset was used to compute parameters of motion correction and anatomical-functional coregistration, and the first volume after equilibration was used as the base EPI image. Matrices for de-obliquing and six-parameter rigid body motion correction were computed. Then, 12-parameter affine anatomical-functional coregistration was computed using the local Pearson correlation (LPC) cost function, using the gray matter segment of the EPI base image computed with AFNI 3dSeg as the LPC weight mask. Matrices for de-obliquing, motion correction, and anatomical-functional coregistration were combined with the standard space non-linear warp field to create a single warp for functional data. The dataset of each TE was then slice-time corrected and spatially aligned through application of the alignment matrix, and the total nonlinear warp was applied to the dataset of each TE. No time series filtering was applied in the preprocessing phase. Data were analyzed both with no spatial smoothing and with a 6 mm full-width-half-maximum (FWHM) spatial filter. The effective smoothness from second-level group analyses are reported for each task and each analysis in Supplementary Table 2.
Multi-echo fMRI data enables analysis where time series of different TEs and thus different BOLD contrasts can be combined to synthesize time series of optimal contrast specific to each voxel, a process called “optimal combination”. For a given voxel with a particular T2* value, the signal acquisition of optimal BOLD contrast is at TE = T2*. While conventional fMRI involves data acquisition at a single TE (selected to reflect the average tissue T2*), some voxels have T2* > TE and others have T2* < TE, meaning BOLD contrast-to-noise ratio is not homogeneous throughout the brain. The greatest macro-inhomogeneities are near areas of high magnetic susceptibility such as orbitofrontal cortex or temporal bone, where conventional fMRI suffers substantial signal “drop-out.” However, with multi-echo fMRI, each voxel's T2* can be estimated specifically, and a given voxel's time series of different TEs can be averaged with weights to produce a computed signal time series with BOLD contrast approximating an acquisition of TE = T2*, done for every voxel. This procedure first involves computing an empirical map of magnetic susceptibility, where for each voxel, the TE-specific signal time series means are fit to an exponential decay model, with the rate constant parameter being the T2* estimate. The voxel-specific T2* values are then used to calculate weights for averaging time series across different TEs, with each voxel- and TE-specific weight being calculated as (Posse et al., 1999):
This procedure implemented a matched-filter that produced a contrast-optimized or “high dynamic range” time series dataset where the functional contrast-to-noise at each voxel was maximized and thermal noise is attenuated. The “optimally combined” time series dataset (abbreviated TSOC) was used in all further analysis steps (i.e. ME-ICA, TSOC+MotReg, GLMdenoise). Note that the TSOC dataset is input into all denoising procedures (i.e. after basic preprocessing) to ensure a fair comparison across techniques. While ME-ICA denoises TSOC data by further exploitation of multi-echo data through TE-dependence/independence analysis, other pipelines instead attempt to remove noise primarily through the inclusion of noise regressors in the first-level GLM (i.e. motion regressors or global noise regressors).
ME-ICA denoising
Time series denoising with ME-ICA was based on ICA decomposition of optimally combined multi-echo data and component classification informed by signal models reflecting the BOLD-like or artifact-like signal decay processes. This has been detailed in our prior work (Evans et al., 2015, Kundu et al., 2013) and is summarized in Supplementary Table 1. The decomposition path utilized in ME-ICA is designed to elucidate components specifically with the BOLD TE-dependence pattern, but is implemented similarly to other ICA treatments: dimensionality is first reduced using a PCA step, then spatial ICA is applied to dimensionally reduced data to find sparse or statistically independent sources (e.g. in MELODIC PICA, dimensionality reduction is achieved with probabilistic PCA, followed by FastICA). Dimensionality reduction is important for reducing the complexity of the ICA problem (to an optimal number of components depending on the data), and concomitantly reducing the proportion of Gaussian-distributed noise (explained by many low-eigenvalue components) that would otherwise cause the ICA solution to fail in convergence. However, ME-ICA differs from other ICA treatments in the dimensionality reduction step. Conventional automatic dimensionality estimation cannot analyze individual principal components for their mechanisms of signal origin, and instead utilizes assumptions on the statistical distribution of noise. In contrast, ME-ICA implements ME-PCA, a model-based approach to distinguishing principal components representing MR contrast versus thermal noise as, respectively, those components with high Kappa, Rho, or variance relative to corresponding spectrum elbows vs. those with none of these properties. While high-variance principal components are preserved for subsequent ICA decomposition by both conventional and ME-PCA, the latter retains low-variance components with MR contrast. In this way, ME-PCA achieves higher dimensional ICA decompositions, indicating potentially greater sensitivity in elucidating BOLD components in ICA decomposition, while utilizing a more direct detection of Gaussian distributed thermal noise, indicating potentially greater specificity and ICA stability for higher dimensional (i.e. more comprehensive) ICA solutions. Following dimensionality reduction based on ME-PCA, ME-ICA applies spatial FastICA using the tanh contrast function to identify a spatial basis of statistically independent component maps, alongside a complementary matrix of time courses (the mixing matrix).
The mixing matrix was fit to the time series of each separate TE, producing coefficient maps for each component at each TE. The signal scaling of each component across TEs was then used to compute Kappa (κ) and Rho (ρ), which were pseudo-F statistics indicating component-level TE-dependence and TE-independence, respectively. While it is understood that ICA separates data into statistically independent components, for multi-echo fMRI these metrics were evaluated to determine the segregation of signals into components of specifically BOLD-related or non-BOLD related contrasts (1 and 2, in Supplementary Table 1), indicating a higher order decomposition than the one achieved by ME-PCA which produced mixed-contrast components (row5 in Supplementary Table 1) versus thermal noise. In addition to BOLD and non-BOLD groups, a usually small group of mixed BOLD/non-BOLD components related to draining vein physiology (row 4 in Supplementary Table 1) are elucidated and rejected as not neuronally related. Finally, for time series denoising, the full mixing matrix (including all component time courses) is fit to the optimally combined (i.e. the source data for the ME-PCA/ICA decomposition pipeline) with multiple linear least squares regression, and the time series fit corresponding to rejected components sub-model is subtracted from the optimally combined time series. The number of components selected for all subjects on all runs of both tasks can be found in Supplementary Table 2.
Task-fMRI data analysis
All first and second level statistical modeling was performed in SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), using the general linear model (GLM). First level analyses modeled the hemodynamic response function (HRF) with the canonical HRF, and used a high-pass filter of 1/128 Hz. In contrast to ME-ICA, we also ran denoising with two other prominent approaches; GLMdenoise (Kay et al., 2013) and via conventional task-based fMRI analysis that included motion regressors in the first-level GLM model (TSOC+MotReg). It is important to re-iterate that each pipeline (ME-ICA, GLMdenoise, and TSOC+MotReg) utilized TSOC data. For GLMdenoise, global noise regressors are identified with cross validation across runs and used as regressors of no interest in first-level individual subject GLMs. For TSOC+MotReg we mimicked conventional task-based fMRI analysis by using motion parameters as regressors of no interest in first-level individual subject GLMs. When running first-level GLMs on ME-ICA denoised data, we did not include motion parameters as regressors of no interest because such artifact is already removed in principled manner at the prior denoising step. All first-level individual subject GLMs modeled the specific contrast of Mentalizing > Physical, and these contrast images were input into second-level random effects GLM analyses (i.e. one sample t-test). Any whole-brain second-level group analyses we report are thresholded at a voxel-wise FDR q < 0.05 (Genovese et al., 2002).
Resting state fMRI connectivity analysis
Resting state connectivity on ME-ICA processed data was estimated using the multi-echo independent components regression (ME-ICR) technique developed by Kundu and colleagues (Kundu et al., 2013). This analysis technique effectively controls for false positives in connectivity estimation by using the number of independent components estimated by ME-ICA as the effective degrees of freedom in single-subject connectivity estimation. Once ME-ICA has the estimated number of components, these component maps are concatenated, and connectivity is estimated by computing the correlation of ICA coefficients between the seed and other brain voxels. The seed regions we have chosen are the peak voxels from the NeuroSynth ‘mentalizing’ map in right and left hemisphere cerebellum (RH MNI x = 29, y = − 82, z = − 39; LH MNI x = − 25, y = − 78, z = − 39). Connectivity GLM analyses were implemented within SPM and the second-level group connectivity maps are thresholded with a voxel-wise FDR threshold of q < 0.05.
To assess the similarity between whole-brain resting state connectivity and Mentalizing > Physical task-activation maps, we used robust regression (Wager et al., 2005) to compute the correlation between the whole-brain connectivity and activation maps. Robust regression allows for protection against the effects of outliers that are particularly pronounced in the connectivity maps, since voxels that contain or are proximally close to the seed voxel exhibit very large connectivity values.
In contrast to connectivity estimated via ME-ICA data with ME-ICR, we also ran conventional functional connectivity analyses on the TSOC data. Here we followed standard analysis procedures such as bandpass filtering and motion regression. These steps are achieved using AFNI 3dBandpass to bandpass filter the data between 0.01 and 0.1 Hz, after orthogonalizing data with respect to a baseline (motion parameters, etc.) matrix (-ort argument) to additionally remove motion-related variability all in one step. No other steps were taken to denoise the data (e.g., global signal regression, white matter regression, etc). The bandpass filtered and motion-regressed data were then inserted into GLMs in SPM8. Note here that bandpass filtering was only applied in this analysis of conventional resting state connectivity analysis and was not done in ME-ICA and ME-ICR connectivity analyses.
To compare the difference between activation-connectivity correlations for ME-ICR vs TSOC+MotReg, we use the paired.r function within the psych R library (http://cran.r-project.org/web/packages/psych/) to obtain z-statistics to describe the difference between correlations. However, no hypothesis tests (i.e. p-values) are computed for these analyses as they are not needed since the comparisons are on correlations estimated from the entire population of interest (i.e. all voxels within whole-brain volume).
Effect size estimation and power simulations
All effect size and power estimates were computed with the fmripower MATLAB toolbox (http://fmripower.org) (Mumford and Nichols, 2008). Effect size is operationalized here as a standardized measure of distance from 0 expressed in standard deviation units (i.e. mean/sd) and is analogous to Cohen's d. Here the mean refers to the contrast image produced by the second-level random effects analysis (i.e. the con*.img from an SPM analysis). The standard deviation is taken by computing the square root of the variance image produced by the second-level random effects analysis (i.e. the ResMS.img from an SPM analysis). We have made one change to the code within fmripower in how it computes effect size. This change allows us to compute effect size at each voxel and then to average the effect size across ROI voxels. This is different from the current implementation in fmripower which will first compute the average mean and standard deviation values across ROI voxels and then computes effect size based on these average mean and standard deviation values. Within fmripower the Type I error was set to 0.05 and we computed power across a sample size range from n = 5 to n = 100. All effect size and power estimates were estimated from independently defined meta-analytic ROIs identified by NeuroSynth (http://neurosynth.org) (Yarkoni et al., 2011) for the feature ‘mentalizing’. This feature contained 98 studies and 4526 activations. The NeuroSynth ‘mentalizing’ mask was first resampled to the same voxel sizes as the current fMRI datasets. Because regions surviving the NeuroSynth analysis at FDR q < 0.01 were large and contained multiple peaks (e.g., medial prefrontal cortex comprised both dorsal and ventral subregions), we constrained ROIs further by finding peak voxels within each region, and constructing a 8 mm sphere around each peak. This resulted in 11 separate ROIs. Eight of the 11 have been reported and heavily emphasized in the literature (dorsomedial prefrontal cortex (dMPFC): x = − 2, y = 60, z = 22; ventromedial prefrontal cortex (vMPFC): x = − 2, y = 48, z = − 20; right temporo-parietal junction (RTPJ): x = 59, y = − 55, z = 27; left temporo-parietal junction (LTPJ): x = − 48, y = − 55, z = 26; posterior cingulate cortex/precuneus (PCC): x = 2, y = − 52, z = 42; right anterior temporal lobe (rATL): x = 48, y = − 6, z = − 20; left anterior temporal lobe (lATL): x = − 52, y = 6, z = − 35; left temporal pole (lTP): x = − 40, y = 21, z = − 24). The remaining 3 regions are located in the cerebellum (right hemisphere cerebellar region Crus II (rCereb): x = 29, y = 82, z = − 39; medial cerebellar region IX (mCereb): x = 2, y = − 52, z = − 47; left hemisphere cerebellar region Crus II (lCereb): x = − 25, y = − 78, z = − 39) and have been relatively overlooked in the literature, with some exceptions that also rely on meta-analytic inference (van Overwalle et al., 2014).
To get an indication of how large an effect size boost due to ME-ICA was, we computed a measure of effect size percentage increase operationalized as (ESME-ICA − ESTSOC+MotReg or GLMdenoise / abs(ESTSOC+MotReg or GLMdenoise) ∗ 100. Bootstrapping (1000 resamples) was then used to re-run SPM second-level group analysis and fmripower computations in order to construct 95% confidence intervals around effect size and effect size percentage increase (i.e. ‘effect size boost’) estimates. The calculation of confidence intervals for the effect size boost metric allowed us to determine which brain regions showed robust ME-ICA related effect size boosts compared to either GLMdenoise or TSOC + MotReg pipelines. Any region that showed a lower bound 95% confidence interval estimate above 0 was considered a region whereby ME-ICA robustly improves effect size estimation over and above GLMdenoise or TSOC+MotReg pipelines.
To further describe the effects of ME-ICA over and above GLMdenoise TSOC+MotReg pipelines, we have computed the minimum sample size to achieve 80% power and the sample size and cost reduction due to using ME-ICA to achieve a study with 80% power, assuming a per subject scanning cost of $500. In cost savings computations, any regions that did not achieve requisite power before n = 100 were excluded from such calculations.
Results
ME-ICA denoising on the raw time series
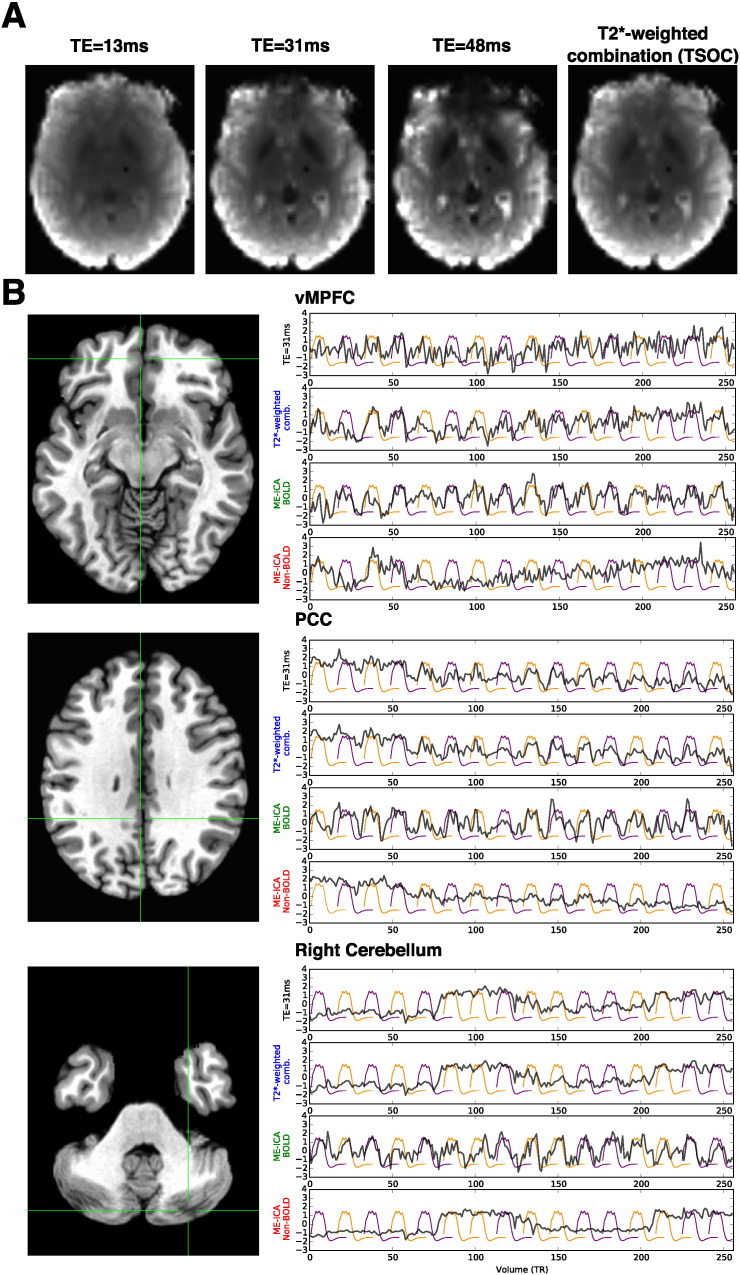
Before addressing quantitative comparisons of effect size and power due to ME-ICA, it is helpful to convey properties of the images and time series acquired with ME acquisition, as well as the effect of ME-ICA denoising directly on the time series. ME sequences capture the decay of EPI images and (time series) with increasing TE, shown in Fig. 1A. For example, ME data show the signal evolution of susceptibility artifact (i.e. signal dropout) in areas such as ventromedial prefrontal cortex (vMPFC) — it is made clear from Fig. 1A that signal dropout occurs at longer TEs, as affected regions have short T2* due to proximity to air-tissue boundaries. Additionally, gray/white signal contrast increases over longer TE due to T2* differences between these tissue types. The T2*-weighted optimal combination (TSOC) implements a matched-filter of TE images yielding a new image time series with optimized contrast (TE ~ T2*) and compensation of susceptibility artifact by weighting towards the early TE in areas with short T2*. In Fig. 1B we present time series data from ventromedial prefrontal cortex (vMPFC), posterior cingulate cortex/precuneus (PCC), and right cerebellum in order to demonstrate the effect of optimal combination on the time series, and then the effect of removing non-BOLD noise using ME-ICA relative to modeled task blocks. It is particularly apparent that ME-ICA, without prior information on task structure, recovers task-based block fluctuations while much of the middle echo, TSOC, and non-BOLD isolated signals carrying complex artifacts including drifts, step changes, and spikes.
Fig. 1.
Multi-echo signal characterization. Panel A shows the signal decay captured in multi-echo EPI images, for a single representative volume. With longer TE, gray/white contrast increases. Susceptibility artifact (e.g. dropout) also increases, as regions near in proximity to air-tissue boundaries have shorter T2*. The T2*-weighted optimal combination (TSOC) implements a matched-filter of TE images yielding a new image with optimized gray/white contrast (TE ~ T2*) and mitigation of susceptibility artifact. Panel B shows comparisons of time series data across three regions of interest; ventromedial prefrontal cortex (vMPFC), posterior cingulate cortex/precuneus (PCC), and right cerebellum. Each comparison shows the time series before model-based filtering of the middle TE image (black), TSOC image (blue), BOLD signals isolated on the basis of TE-dependence (green), and non-BOLD signals removed from the data (red). Purple and orange lines represent modeled mentalizing and physical blocks respectively.
ME-ICA boosts effect size estimation
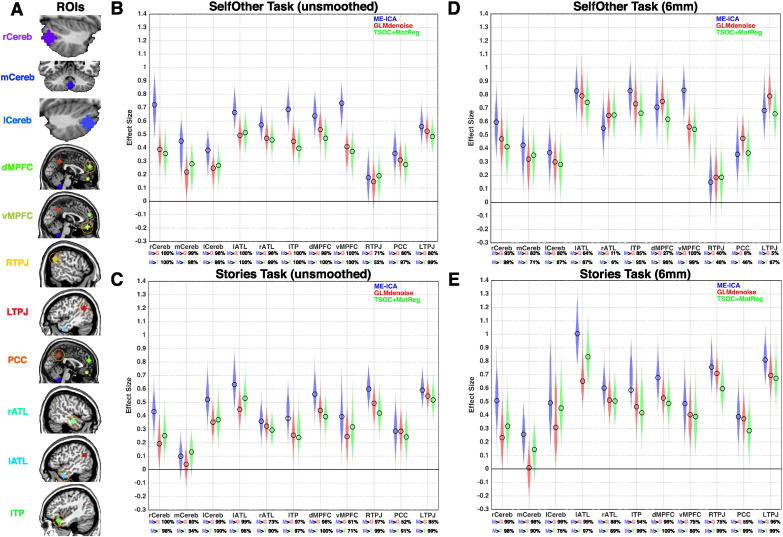
In evaluating ME-ICA-related effects on group-level inference, we examined the influence of non-BOLD denoising on effect size estimation. Effect size is operationalized here as a standardized measure of distance from 0 expressed in standard deviation units (i.e. mean/sd) and is analogous to Cohen's d. As illustrated in Fig. 2, with no explicitly applied full-width-half-max (FWHM) Gaussian image smoothing, ME-ICA outperforms conventional analysis methods for task-based fMRI analysis (TSOC+MotReg) and a prominent task-based denoising method (GLMdenoise) (Kay et al., 2013) (Fig. 2B–C). This enhanced performance is evident across both mentalizing tasks and in most regions assessed. Quantifying the magnitude of effect size boosting as the difference in effect size estimates, we find that the median ME-ICA induced boost for canonical mentalizing regions is approximately 24%. Boosts were much larger (nearly always > 50%) in areas such as vMPFC and left temporal pole (lTP) that characteristically suffer from signal dropout. Amongst cerebellar areas, right and left cerebellar Crus I/II areas showed evidence of larger effect size boosts ranging from 48 to 149% increases when compared to GLMdenoise and 40–101% increases when compared to TSOC+MotReg. Although this pattern emerges in our actual sample, under more conservative criteria of examining how consistent this effect is with bootstrap resampling, within the SelfOther task with no smoothing, 8/12 regions showed a robust ME-ICA-related boost over and above TSOC+MotReg and GLMdenoise on 97.5% or more of the bootstrap resamples. Within the Stories task, 3/12 regions show this boost for ME-ICA > GLMdenoise, while 5/12 regions show the boost for ME-ICA > TSOC+MotReg. Thus, while there are indications that ME-ICA can result in effect size boosts for several regions, there are also some instances where this was not the case from the bootstrapping analysis. Because the bootstrapping analysis applies a hard threshold at 97.5%, we also report the exact percentage of the bootstrap resamples showing a ME-ICA-related boost in Fig. 2 underneath the x-axis, in order to descriptively show how consistent such boosts may be as a continuous measure.
Fig. 2.
ME-ICA effect size boosting. This figure shows effect size estimates from all regions of interest (panel A). Panels B and C show effect sizes in unsmoothed data, while panels D and E are from 6 mm FWHM smoothed data effect sizes are expressed in standard deviation units and are analogous to Cohen's d. Colored clouds in each plot represent density of estimates obtained from 1000 bootstrap resamples, while unfilled black circles represent estimates within the true dataset. Below each region label on the x-axis are descriptive statistics indicating the percentage of bootstrap resamples where ME-ICA performed better than the alternatives (blue M, ME-ICA; red G, GLMdenoise; green T, TSOC+MotReg).
Under conditions where smoothing is done (i.e. 6 mm FWHM) we see that many of the ME-ICA-related effect size boosts have been matched relative to what was observed with no smoothing in the SelfOther task (e.g., 1/12 regions ME-ICA > GLMdenoise, 2/12 regions ME-ICA > TSOC + MotReg). However, within the Stories task, the number of regions passing the 97.5% criterion was larger than in the unsmoothed data (4/12 regions ME-ICA > GLMdenoise, 6/12 regions ME-ICA > TSOC + MotReg). In other words, the effect of smoothing was one whereby it attenuated the ME-ICA-related effect size boosts in the SelfOther tasks, but preserved such boosts when present in the Stories task. See Supplementary Table 3, Supplementary Table 4 for full characterization of effect size estimates and effect size increases.
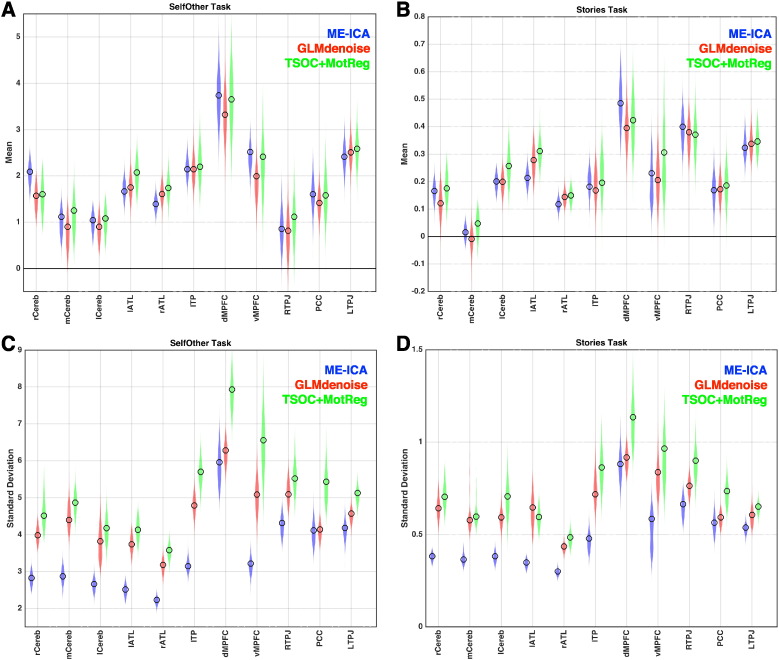
Because our operational definition of effect size is a standardized measure that incorporates both mean and variability measurements, we went further in decomposing how these boosts in effect size estimation in unsmoothed data manifested in terms of changes to either the mean and/or variability measurements. It is clear from Fig. 3 that ME-ICA induces these boosts primarily by reducing estimates of variability at the second-level group analysis. Given that at a within-subject level ME-ICA removes non-BOLD noise from the time series, it is clear that one consequence of this for group-level modeling is clear reduction of between-subject variance which works to enhance standardized effect size estimates.
Fig. 3.
ME-ICA reduction in variance in group-level analyses. This figure shows mean and standard deviation estimates from 2nd level group modeling that contribute to the standardized effect size calculations. Panels A and B show mean estimates for all regions in both tasks. Panels C and D show standard deviation estimates. Colored clouds in each plot represent density of estimates obtained from 1000 bootstrap resamples, while unfilled circles represent estimates within the true dataset.
Impact of ME-ICA on statistical power
Given a ME-ICA-related improvement in standardized effect size estimation, it follows that statistical power will also be increased, as such estimates are critical in such computations. However, for assessing the practical impact that ME-ICA may have, it is necessary to assess the impact such effect size boosting has on statistical power and sample size. Here we describe power simulations on the unsmoothed data that mainly inform what we could expect in future work given effect size estimates similar to what we have observed in the current study under ME-ICA versus other analysis pipelines.
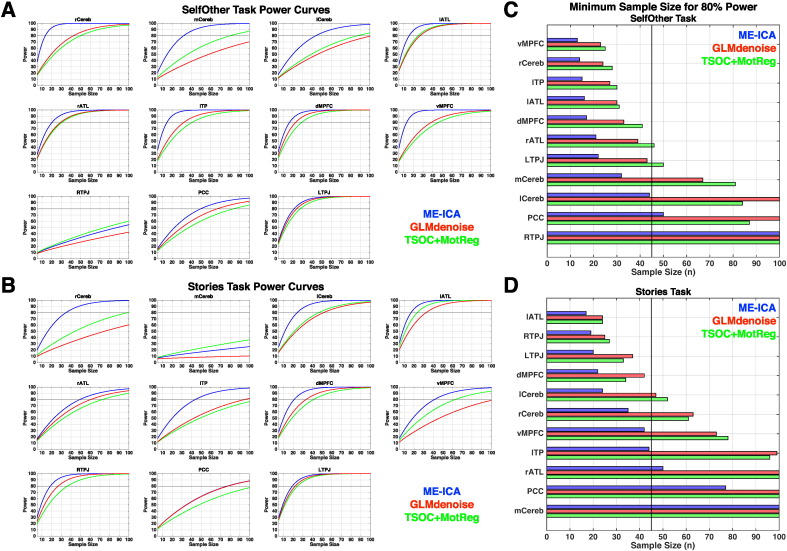
Power curves for each analysis pipeline across a range of sample sizes from n = 5 to n = 100 are illustrated in Fig. 4A–B. Minimum sample size necessary for achieving 80% power at an alpha of 0.05 is shown in Fig. 4C. Across all canonical regions and both tasks, the median minimum sample size to achieve 80% power at an alpha of 0.05 with ME-ICA is n = 22. Minimum sample sizes across nearly all regions were well within reach of current standards for sample size (e.g., n < 45). In contrast, for both GLMdenoise and TSOC+MotReg the median minimum sample size for canonical regions is n = 38.
Fig. 4.
Power simulations. This figure shows power curves constructed for each processing pipeline across a range of sample sizes from 5 to 100 (panels A–B). The minimum sample size necessary for achieving 80% power is shown in panel C for the Stories task (left) and SelfOther task (right). The dotted line indicates sample size of n = 45.
For cerebellar regions, the power benefits due to ME-ICA were more pronounced. Aside from medial cerebellar region XI (mCereb) in the Stories task which did not result in a sizeable effect (e.g., effect size < 0.14), the minimum sample size needed for the bilateral cerebellar Crus I/II areas (rCereb, lCereb) was within the range of typical functional neuroimaging study sample size when using ME-ICA (e.g., n < 45). In contrast, when using GLMdenoise and TSOC+MotReg, sample sizes of n > 40 were required and in many instances 80% power was not attained by n = 100.
For further illustration of practical impact, these boosts in statistical power and reduction in sample size necessary for achieving 80% power can be quantified into monetary savings. Assuming a scan rate of $500 per individual, if one was only interested in canonical regions, using ME-ICA would amount to median savings of $8750 compared to GLMdenoise and $5750 compared to TSOC+MotReg. If one was interested in cerebellar regions, using ME-ICA would amount to a median savings of $21,250 compared to GLMdenoise and $19,750 compared to TSOC+MotReg.
Visual examination of the power curves in Fig. 4A–B highlights a point of diminishing returns when power is > 95%, as the improvements in power for adding more subjects diminishes substantially. We term this effect ‘saturation’. When using ME-ICA, many regions quickly reach these saturation levels at sample sizes that are practically attainable (e.g., n < 45). In contrast, other pipelines like GLMdenoise and TSOC+MotReg typically require considerably larger sizes to hit these saturation levels.
Functional connectivity evidence for cerebellar involvement in neural systems supporting mentalizing
The improvements in effect size estimation particularly for cerebellar regions are important as it potentially signals the ability of ME-ICA to uncover novel effects that may have been undetected in previous research and which are likely due to natively high fMRI artifact in such areas. To further test the importance of cerebellar contributions to mentalizing, we have examined resting state functional connectivity data and the relationship that cerebellar connectivity patterns may have with task-evoked mentalizing systems. Prior work suggests that specific cerebellar regions may be integral to the default mode network (Buckner et al., 2011). The default mode network incorporates many of the regions that are highly characteristic in task-evoked systems supporting mentalizing (Andrews-Hanna et al., 2014). Meta-analytically defined cerebellar regions associated with mentalizing show some overlap with these cerebellar default mode areas (van Overwalle et al., 2015). Therefore, if cerebellar regions are integral in neural circuits associated with mentalizing, we hypothesized that such regions would be involved in the default mode network. Taking this hypothesis one step further, we also hypothesized that if these cerebellar nodes are truly important within the neural systems that support mentalizing, we should expect that cerebellar resting state functional connectivity patterns highlighted with multi-echo EPI methods would better recapitulate (compared to other analysis pipelines) observed whole-brain activational topology during mentalizing tasks.
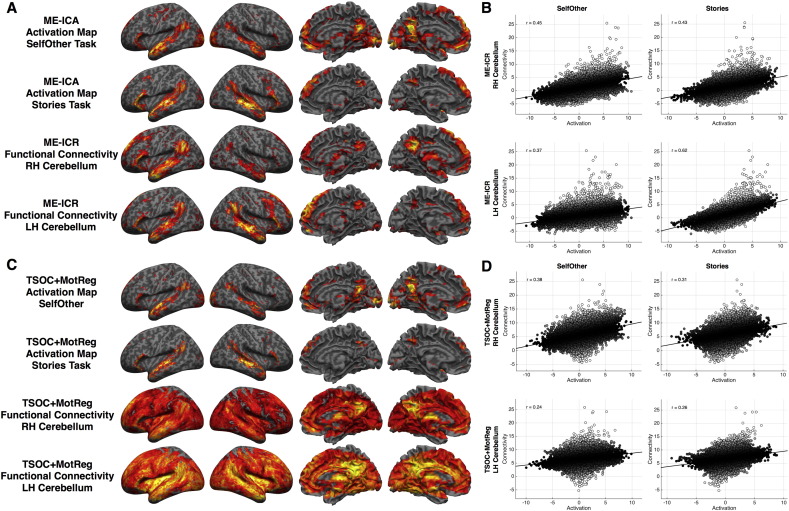
Confirming these hypotheses we find that bilateral cerebellar seeds involved in mentalizing show highly robust resting state functional connectivity patterns that resemble the default mode network within the same participants scanned on our task paradigms. Visually, the similarity between the ME-ICR connectivity maps and our Mentalizing > Physical activation maps are striking (Fig. 5A). Quantitatively we assessed this similarity through voxel-wise correlations (estimated with robust regression) across the whole-brain, and we confirm that the resting state functional connectivity maps are strikingly similar in patterning to what we observe for task-evoked mentalizing activation patterns (all r > 0.37) (Fig. 5B). Relative to the activation-connectivity similarity observed in TSOC+MotReg data, the activation-connectivity similarity obtained with ME-ICA and ME-ICR is much larger (i.e. z > 8.85) (Fig. 5B–D).
Fig. 5.
Resting state functional connectivity from cerebellar seed regions and pattern similarity with mentalizing > physical activation maps. This figure shows resting state connectivity from right and left cerebellar seed voxels (i.e. peak voxels from the NeuroSynth ‘mentalizing’ map) and their similarity to mentalizing > physical activation maps. Panel A shows activation and resting state functional connectivity maps when using ME-ICA and multi-echo independent components regression (ME-ICR). All data are visualized at thresholded of voxelwise FDR q < 0.05. Panel B shows scatterplots and robust regression correlations between whole-brain activation and connectivity patterns when using ME-ICA and ME-ICR. Robust regression was used to calculate the correlation in a way that is insensitive to the outliers in the connectivity map which are voxels that are proximally close to the seed region. Panel C shows activation and cerebellar functional connectivity maps for data when using conventional analysis approaches on TSOC data. Activation maps are thresholded at FDR q < 0.05. Connectivity maps are thresholded at the same t-statistic threshold for defining FDR q < 0.05 in ME-ICR analyses (which were already much higher than the FDR q < 0.05 cutoff estimated from TSOC data), and were shown in this manner to show connectivity at the exact same t-threshold cutoff. Panel D shows activation and connectivity similarity estimated with robust regression in TSOC data.
Discussion
Task-based fMRI studies are characteristically of small sample size and thus likely underpowered for all but the most robust effects. Furthermore, typical task-based fMRI studies do not apply advanced methods to mitigate substantial non-BOLD noise that is generally known to be inherent in such data. Combining small underpowered studies with little to no consideration of pervasive non-BOLD noise that is present in the data even after typical pre-processing and statistical modeling creates a situation where most task-based studies are potentially missing key effects and makes for impractical conditions for most researchers where massive sample sizes are required to overcome this limitation. In this study we showed that ME-ICA enhances standardized effect size estimation and statistical power in task-based block-design studies for many key hypothesized regions as well as several other potentially overlooked regions. We should note here that although effect size increases were prominent for some regions, there were some instances where increases were less substantial and did not meet our specific criterion for significance of showing increases in > 97.5% of the bootstrap resamples. This shows that although ME-ICA has the ability to make for sizeable improvements in effect size estimation, it is clear that there may be some regions and circumstances where ME-ICA results can be statistically indistinguishable to other methods.
ME-ICA-related improvements to effect size and statistical power tend to be pronounced even without any spatial smoothing. However, when data is smoothed (e.g., 6 mm FWHM), we observe task-specific differences in smoothing-related effects. For instance, under 6 mm smoothing the SelfOther task had fewer ROIs that showed ME-ICA-related effect size boosts, compared to when the data was unsmoothed. In contrast, ME-ICA effect size improvements were largely retained with or without smoothing in the Stories task. Given this task-specific difference, a general statement regarding the effects of smoothing on ME-ICA-related improvements in effect size estimation cannot be made. Because ME-ICA generally provided boosts to many regions without smoothing, it may be important to assess how ME-ICA could enhance applications of task-based fMRI analysis where smoothing is typically less frequently employed. Analysis approaches such as multi-voxel pattern analysis (MVPA) (Norman et al., 2006) or representational similarity analysis (RSA) (Kriegeskorte et al., 2008) treat smoothing as an important analytic consideration (Kamitani and Sawahata, 2010, Kriegeskorte et al., 2010, Op de Beeck, 2010) and may benefit from analytic improvements that enhance sensitivity without smoothing. It will be important in future work to explore whether ME-ICA could enhance such analysis approaches.
There are several practical points of impact that these results underscore. First, addressing the problem of statistical power in neuroscience, particularly fMRI studies (Button et al., 2013, Yarkoni, 2009), is a complicated matter as most recommendations for this problem rely on increasing the amount of data collected both at the within- and between-subject levels. A practical barrier for most research labs is that increasing the scale of data collection (e.g. massive sample size studies) is typically cost prohibitive. Our work here takes a different perspective on the problem of low statistical power in fMRI studies by attenuating non-BOLD noise, which directly has impact on the sensitivity of fMRI, and thus statistical power. In practical terms, we show that ME-ICA allows for increases in effect size estimation and consequently statistical power whereby in many cases (i.e. canonical and cerebellar regions investigated here) requisite levels of statistical power become attainable in a range of traditional sample sizes. Therefore, employing a multi-echo imaging approach alongside collecting much larger sample sizes could be strategically advantageous for enhancing future work.
It is particularly important to underscore here that we are not suggesting that ME-ICA is a panacea to the small sample size problem and that as a result, researchers could continue the tradition of small sample size studies. Rather, we advocate that there are always compelling reasons to collect more data and that if funds permit, researchers should make every effort to ensure that their studies are sufficiently powered for a given effect size. Such emphasis will ensure that canonical large effects are robust and replicable. However, boosts in the sensitivity of fMRI at the subject-level can open up a range of previously practically unattainable possibilities for new discoveries. Such new discoveries could take the form of enhanced sensitivity for detecting smaller and more subtle effects in brain regions that are currently not well understood or which are methodologically hampered by being continually veiled underneath blankets of non-BOLD noise. New discoveries could also be enabled with parsing apart further variability such as subgroups that may have important translational implications (Lombardo et al., 2015), parsing apart heterogeneity mapped onto individual differences (Laumann et al., 2015), and/or more fine grained hypotheses/methods that result in much smaller effects than could be detected in the typical and more basic activation mapping paradigm. All of these situations could be substantially improved with a methodological approach that improves statistical power, but at the same time does not inhibit researchers from collecting larger samples than what is typically characteristic.
As an empirical demonstration of ME-ICA's ability to enhance new discoveries for human brain functional organization, we have uncovered robust evidence that there are discrete cerebellar regions that should hold more prominence in discussions about the neural systems supporting mentalizing/theory of mind and the ‘social brain’. The cerebellum is already a neglected and poorly understood brain area, particularly in the context of its potential role in higher-level cognition (Buckner, 2013, Schmahmann, 1997, Stoodley and Schmahmann, 2009, van Overwalle et al., 2014, Wang et al., 2014). Prior indications that these cerebellar regions may be plausible candidates for neural systems supporting mentalizing come from meta-analytic evidence (van Overwalle et al., 2014). However, while meta-analytic evidence alone might suggest plausibility for these regions, it was still unclear as to the exact reasons for why these cerebellar regions have not been the topic of more extensive focus.
In this study, one of the novel findings that may help explain why these cerebellar regions are missed, is that they are typically veiled in substantial amounts of non-BOLD noise that obscure such effects given traditional acquisition approaches and analysis pipelines. Effect sizes for these regions under more traditional analysis approaches (e.g., TSOC+MotReg) are typically small and the sample size necessary for detecting those effects with high power are much greater than what is typical for fMRI research. However, after acquiring multi-echo data and applying ME-ICA, we found effects could be boosted by > 40%. As we show in this study, ME-ICA primarily boosts effect size estimation via noise reduction at the within-subject level and consequently has impact for reduction of variance at the group level. Assuming similar effect sizes in future work, power simulations suggest that discovery of these novel cerebellar effects will remain nonetheless hidden at characteristically small sample sizes and without the multi-echo denoising innovations we report here. Therefore, it is clear that these regions are typically highly saturated in non-BOLD noise and this problem helps to obscure these effects from traditional research practices of using small sample sizes and conventional fMRI acquisition and denoising procedures that do not fully identify and remove such non-BOLD noise variability.
The ME-ICA application we present here should help researchers to gain a more stable foothold on cerebellar effects in the context of mentalizing and enable better circumstances for parsing apart how their role can further our understanding of such complex social cognitive processes. A promising avenue for future work on this topic would be to further understand the computational role the cerebellum plays in simulative processes that may be important in mentalizing (Ito, 2008, Mitchell et al., 2006). Translationally, the link between cerebellum and mentalizing is also particularly intriguing, given the longstanding, yet independent, literatures in autism regarding the cerebellum (Courchesne et al., 1988) and mentalizing (Baron-Cohen et al., 1985). Wang and colleagues have recently argued that atypical developmental processes within the cerebellum may be particularly important for understanding autism (Wang et al., 2014). Autism is well known for hallmark deficits in the domain of social-communication (Lai et al., 2014) and impairments in the development of mentalizing/theory of mind and self-referential cognition in autism (Lombardo and Baron-Cohen, 2010, Lombardo and Baron-Cohen, 2011) as well as atypical functioning of neural mechanisms that bolster such processes (Lombardo et al., 2010a, Lombardo et al., 2011) are thought to be important as explanations behind social-communication deficits in autism. Thus, the intersection of developmental abnormalities in cerebellar development and their relationship to the development of mentalizing in autism will be an interesting new avenue of research enabled by these kinds of novel discoveries.
An important caveat for this study is that our findings are based on block-design activation paradigms, utilizing relatively long-duration changes in susceptibility weighting. This differs from event-related paradigms, whereby activations may be associated with a significant inflow component that is S0-weighted. Future studies will involve assessing the suitability of ME-ICA for the analysis of event-related studies as well as other more novel task-designs. With regard to novel task-designs such as temporally extended tasks, we have previously shown that ME-ICA also has the ability to separate ultra-slow BOLD effects from slow non-BOLD effects (Evans et al., 2015), and this opens up a range of possibilities for new paradigms that may be particularly well-suited for temporally-extended and continuous tasks, such as more naturalistic paradigms for social cognition (Schilbach et al., 2013, Zaki et al., 2009).
One limitation of the current study is the lack of comparison between multi-echo and traditional single-echo fMRI as well as emerging single-echo multi-band data. While our prior publications suggest that optimally combined multi-echo data is at least a fair proxy to single-echo data, there is some chance that the present comparison is conservative regarding the benefits of ME-ICA. We have previously shown that optimally combined time series data (TSOC) can readily double signal-to-noise ratio relative to unaccelerated single-echo fMRI (Kundu et al., 2013), via homogenizing functional contrast across the brain while attenuating thermal noise (combination is a weighted average implementing a matched- filter). Thus, in our view the most conservative comparison to make against ME-ICA should also be multi-echo data that benefits from enhanced tSNR over and above single-echo data. Recent work by Kirilina and colleagues provides direct comparisons of single- and multi-echo data in a task-fMRI context. These authors found that both kinds of acquisitions produced very similar group-level results in a task-fMRI context (Kirilina et al., 2016). This suggests that there is little benefit in group-level analyses for multi-echo acquisition (without ME-ICA) over and above traditional single-echo acquisitions. Applied to our work, these observations would suggest that analyses on TSOC data (i.e. TSOC+MotReg, GLMdenoise) are a useful approximation of what would be expected if we also compared ME-ICA directly to single-echo data. However, given all these important caveats, it will be important for future work on this topic to directly make this comparison of ME-ICA to single-echo data in task-fMRI contexts to confirm this prediction. Our prediction would be that given there is a boost in tSNR simply by acquiring multi-echo data and utilizing our T2* optimal combination method (Kundu et al., 2013) that ME-ICA would similarly outperform single-echo data.
It also should be noted that acquiring multi-echo data has some notable trade-offs. Mainly, acquiring multiple TE images requires time that could otherwise be spent in acquiring higher resolution images or increasing temporal resolution by acquiring more volumes. Single-echo or even dual-echo (one early, one standard TE) approaches will have greater spatial or temporal resolution than a multi-echo acquisition given the same level of in-plane acceleration. If a single-echo approach does not utilize in-plane acceleration (such as in the Human Connectome Project) whereas a multi-echo approach does, then the multi-echo approach will be better matched in terms of spatial and temporal resolution, but may still have a disadvantage in native resolution. Increases in noise associated with higher in-plane acceleration and requisite bandwidth increases will also increase the per-echo noise in a multi-echo experiment, although T2* weighted combination of echoes can compensate and lead to equal or better temporal signal-to-noise ratio as previously described. The advantage of a multi-echo approach then becomes chiefly the ability to denoise fMRI data in a more flexible and generable manner than needing to determine particular spatial or temporal filters specific to an experiment type such as in rs-fMRI, specific task designs, anatomy, or requiring classifier training etc. At the level of group analysis, if inter-individual differences constitute the dominant noise source in an analysis, which may be the case for more compliant subject groups expressing low levels or artifact or for very large studies, a single-echo approach could ultimately lead to results with high anatomical specificity from high resolution, limited mainly by inter-individual factors such as effectiveness of standard space normalization. In addition, while ME-ICA aims to use T2* relaxometry and TE-dependence models to automate much of the fMRI denoising process, relaxometric analysis does fundamentally increase the level of complexity of the analysis. An alternative advancement may be increasing the number of time points in fMRI acquisitions by using multi-band acceleration. One metric for characterizing aggregate statistical power of an fMRI scan pertaining particularly to multi-band acceleration, Power ~ tSNR × sqrt(N) (Smith et al., 2013), indicates that acquiring more volumes in the same acquisition time can compensate for per-volume increase in noise due to multi-band acceleration. Such an approach may be the preferred route for increasing the sensitivity and capability of an imaging experiment given adequate sample size, effect size, and appropriate considerations of experiment design. Ongoing work also combines multi-echo acquisition with multi-band acceleration, towards leveraging some of the statistical benefits of acquiring more volumes per imaging time in the context of multi-echo denoising (Olafsson et al., 2015).
In this work we show that the multi-echo approach could offer substantial improvements that can largely affect how the field conducts fMRI research. We have developed a fully integrated toolset based on open-source software implementing all the techniques described in this paper, distributed with the AFNI tools, as meica.py. We hope that the community will experiment with multi-echo methodology in task and rest conditions, as well as in pharmacological contexts, further innovate in this space, towards enhancing the flexibility and reliability of fMRI research and bolstering discovery science on human brain function in health and disease.
The following are the supplementary data related to this article.
Classes of signal sources decomposed by ME-ICA. Signal sources elucidated through combination of multivariate decomposition (PCA, ICA in order) and T2* decay analysis of multi-echo fMRI data as implemented in ME-ICA. κ is pseudo-F statistic component-level TE-dependent scaling suggesting network BOLD origin. ρ is pseudo-F statistic component-level TE-independent scaling suggesting artifact.
Number of BOLD-related components identified by ME-ICA for all subjects across both tasks. Effective smoothness of 2nd-level group analyses for each task, each analysis, and under 0 mm or 6 mm.
Effect size, power, and effect size boosting statistics for data with no smoothing. This table provides effect sizes and 95% CIs for all analysis pipelines and all regions when data is not smoothed. It also provides estimates of sample size needed to achieve 80% power. This table also provides information about effect size boosting statistics and 95% confidence intervals, impact on sample size estimates to achieve 80% power and impact on monetary savings.
Effect size, power, and effect size boosting statistics for data with 6 mm FWHM smoothing. This table provides effect sizes and 95% CIs for all analysis pipelines and all regions when data is smoothed at 6 mm FWHM. This table also provides information about effect size boosting statistics and 95% confidence intervals for all regions in both tasks.
Acknowledgments
This work was supported by a Wellcome Trust (091774/Z/10/Z) project grant to SB-C and ETB. MVL was supported by the Wellcome Trust and fellowships from Jesus College, Cambridge and the British Academy. PK was supported by the National Institutes of Health–Cambridge Scholars Program. ETB is employed half-time by the University of Cambridge and half-time by GlaxoSmithKline (GSK).
Contributor Information
Michael V. Lombardo, Email: mvlombardo@gmail.com.
Prantik Kundu, Email: prantik.kundu@mssm.edu.
References
- Andrews-Hanna J.R., Saxe R., Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. NeuroImage. 2014;91:324–335. doi: 10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Leslie A.M., Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21:37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Buckner R.L. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80:807–815. doi: 10.1016/j.neuron.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Krienen F.M., Castellanos A., Diaz J.C., Yeo B.T. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P., Mokrysz C., Nosek B.A., Flint J., Robinson E.S., Munafo M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Courchesne E., Yeung-Courchesne R., Press G.A., Hesselink J.R., Jernigan T.L. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N. Engl. J. Med. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Desmond J.E., Glover G.H. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J. Neurosci. Methods. 2002;118:115–128. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- Evans J.W., Kundu P., Horovitz S.G., Bandettini P.A. Separating slow BOLD from non-BOLD baseline drifts using multi-echo fMRI. NeuroImage. 2015;105:189–197. doi: 10.1016/j.neuroimage.2014.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K. Ten ironic rules for non-statistical reviewers. NeuroImage. 2012;61:1300–1310. doi: 10.1016/j.neuroimage.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003;358:459–473. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gweon H., Dodell-Feder D., Bedny M., Saxe R. Theory of mind performance in children correlates with functional specialization of a brain region for thinking about thoughts. Child Dev. 2012;83:1853–1868. doi: 10.1111/j.1467-8624.2012.01829.x. [DOI] [PubMed] [Google Scholar]
- Ioannidis J.P. Why most published research findings are false. PLoS Med. 2005;2 doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Control of mental activities by internal models in the cerebellum. Nat. Rev. Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- Jenkins A.C., Macrae C.N., Mitchell J.P. Repetition suppression of ventromedial prefrontal activity during judgments of self and others. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4507–4512. doi: 10.1073/pnas.0708785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitani Y., Sawahata Y. Spatial smoothing hurts localization but not information: pitfalls for brain mappers. NeuroImage. 2010;49:1949–1952. doi: 10.1016/j.neuroimage.2009.06.040. [DOI] [PubMed] [Google Scholar]
- Kay K.N., Rokem A., Winawer J., Dougherty R.F., Wandell B.A. GLMdenoise: a fast, automated technique for denoising task-based fMRI data. Front. Neurosci. 2013;7:247. doi: 10.3389/fnins.2013.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilina E., Lutti A., Poser B.A., Blankenburg F., Weiskopf N. The quest for the best: the impact of different EPI sequences on the sensitivity of random effect fMRI group analyses. NeuroImage. 2016;126:49–59. doi: 10.1016/j.neuroimage.2015.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Mur M., Bandettini P. Representational similarity analysis — connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2008;2:4. doi: 10.3389/neuro.06.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N., Cusack R., Bandettini P. How does an fMRI voxel sample the neuronal activity pattern: compact-kernel or complex spatiotemporal filter? NeuroImage. 2010;49:1965–1976. doi: 10.1016/j.neuroimage.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Inati S.J., Evans J.W., Luh W.M., Bandettini P.A. Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. NeuroImage. 2012;60:1759–1770. doi: 10.1016/j.neuroimage.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Brenowitz N.D., Voon V., Worbe Y., Vertes P.E., Inati S.J., Saad Z.S., Bandettini P.A., Bullmore E.T. Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proc. Natl. Acad. Sci. U. S. A. 2013;110:16187–16192. doi: 10.1073/pnas.1301725110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Santin M.D., Bandettini P.A., Bullmore E.T., Petiet A. Differentiating BOLD and non-BOLD signals in fMRI time series from anesthetized rats using multi-echo EPI at 11.7 T. NeuroImage. 2014;102(Pt 2):861–874. doi: 10.1016/j.neuroimage.2014.07.025. [DOI] [PubMed] [Google Scholar]
- Kundu P., Benson B.E., Baldwin K.L., Rosen D., Luh W.M., Bandettini P.A., Pine D.S., Ernst M. Robust resting state fMRI processing for studies on typical brain development based on multi-echo EPI acquisition. Brain Imaging Behav. 2015 doi: 10.1007/s11682-014-9346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.C., Lombardo M.V., Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- Laumann T.O., Gordon E.M., Adeyemo B., Snyder A.Z., Joo S.J., Chen M.Y., Gilmore A.W., McDermott K.B., Nelson S.M., Dosenbach N.U., Schlaggar B.L., Mumford J.A., Poldrack R.A., Petersen S.E. Functional system and areal organization of a highly sampled individual human brain. Neuron. 2015;87:657–670. doi: 10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist M.A., Caffo B., Crainiceanu C. Ironing out the statistical wrinkles in “ten ironic rules”. NeuroImage. 2013;81:499–502. doi: 10.1016/j.neuroimage.2013.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Baron-Cohen S. Unraveling the paradox of the autistic self. WIREs Cognit. Sci. 2010;1:393–403. doi: 10.1002/wcs.45. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Baron-Cohen S. The role of the self in mindblindness in autism. Conscious. Cogn. 2011;20:130–140. doi: 10.1016/j.concog.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Sadek S.A., Wheelwright S.J., Pasco G., Suckling J., Consortium M.A., Baron-Cohen S. Atypical neural self-representation in autism. Brain. 2010;133:611–624. doi: 10.1093/brain/awp306. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Wheelwright S.J., Sadek S.A., Suckling J., Consortium M.A., Baron-Cohen S. Shared neural circuits for mentalizing about the self and others. J. Cogn. Neurosci. 2010;22:1623–1635. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Chakrabarti B., Bullmore E.T., Consortium M.A., Baron-Cohen S. Specialization of right temporo-parietal junction for mentalizing and its association with social impairments in autism. NeuroImage. 2011;56:1832–1838. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- Lombardo M.V., Pierce K., Eyler L., Carter Barnes C., Ahrens-Barbeau C., Solso S., Campbell K., Courchesne E. Different functional neural substrates for good and poor language outcome in autism. Neuron. 2015 doi: 10.1016/j.neuron.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J.P., Macrae C.N., Banaji M.R. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–663. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Mumford J.A., Nichols T.E. Power calculation for group fMRI studies accounting for arbitrary design and temporal autocorrelation. NeuroImage. 2008;39:261–268. doi: 10.1016/j.neuroimage.2007.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Bandettini P.A. Resting-state fMRI confounds and cleanup. NeuroImage. 2013;80:349–359. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman K.A., Polyn S.M., Detre G.J., Haxby J.V. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn. Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Olafsson V., Kundu P., Wong E.C., Bandettini P.A., Liu T.T. Enhanced identification of BOLD-like components with multi-echo simultaneous multi-slice (MESMS) fMRI and multi-echo ICA. NeuroImage. 2015 doi: 10.1016/j.neuroimage.2015.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Beeck H.P. Against hyperacuity in brain reading: spatial smoothing does not hurt multivariate fMRI analyses? NeuroImage. 2010;49:1943–1948. doi: 10.1016/j.neuroimage.2009.02.047. [DOI] [PubMed] [Google Scholar]
- Posse S., Wiese S., Gembris D., Mathiak K., Kessler C., Grosse-Ruyken M.L., Elghahwagi B., Richards T., Dager S.R., Kiselev V.G. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magn. Reson. Med. 1999;42:87–97. doi: 10.1002/(sici)1522-2594(199907)42:1<87::aid-mrm13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Saxe R., Powell L.J. It's the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Schaafsma S.M., Pfaff D.W., Spunt R.P., Adolphs R. Deconstructing and reconstructing theory of mind. Trends Cogn. Sci. 2015;19:65–72. doi: 10.1016/j.tics.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilbach L., Timmermans B., Reddy V., Costall A., Bente G., Schlicht T., Vogeley K. Toward a second-person neuroscience. Behav. Brain Sci. 2013;36:393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. Academic Press; London: 1997. The Cerebellum and Cognition. [Google Scholar]
- Schurz M., Radua J., Aichhorn M., Richlan F., Perner J. Fractionating theory of mind: a meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014;42:9–34. doi: 10.1016/j.neubiorev.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Simmons J.P., Nelson L.D., Simonsohn U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol. Sci. 2011;22:1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Beckmann C.F., Andersson J., Auerbach E.J., Bijsterbosch J., Douaud G., Duff E., Feinberg D.A., Griffanti L., Harms M.P., Kelly M., Laumann T., Miller K.L., Moeller S., Petersen S., Power J., Salimi-Khorshidi G., Snyder A.Z., Vu A.T., Woolrich M.W., Xu J., Yacoub E., Ugurbil K., Van Essen D.C., Glasser M.F., Consortium W.U.-M.H. Resting-state fMRI in the human connectome project. NeuroImage. 2013;80:144–168. doi: 10.1016/j.neuroimage.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt R.P., Adolphs R. Validating the why/how contrast for functional MRI studies of theory of mind. NeuroImage. 2014;99:301–311. doi: 10.1016/j.neuroimage.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- van Overwalle F. Social cognition and the brain: a meta-analysis. Hum. Brain Mapp. 2009;30:829–858. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Overwalle F., Baetens K., Marien P., Vandekerckhove M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. NeuroImage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- van Overwalle F., Baetens K., Marien P., Vandekerckhove M. Cerebellar areas dedicated to social cognition? A comparison of meta-analytic and connectivity results. Soc. Neurosci. 2015:1–8. doi: 10.1080/17470919.2015.1005666. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Keller M.C., Lacey S.C., Jonides J. Increased sensitivity in neuroimaging analyses using robust regression. NeuroImage. 2005;26:99–113. doi: 10.1016/j.neuroimage.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wang S.S., Kloth A.D., Badura A. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T. Big correlations in little studies: inflated fMRI correlations reflect low statistical power — commentary on Vul et al.(2009) Perspect. Psychol. Sci. 2009;4:294–298. doi: 10.1111/j.1745-6924.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Van Essen D.C., Wager T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J., Weber J., Bolger N., Ochsner K. The neural bases of empathic accuracy. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Classes of signal sources decomposed by ME-ICA. Signal sources elucidated through combination of multivariate decomposition (PCA, ICA in order) and T2* decay analysis of multi-echo fMRI data as implemented in ME-ICA. κ is pseudo-F statistic component-level TE-dependent scaling suggesting network BOLD origin. ρ is pseudo-F statistic component-level TE-independent scaling suggesting artifact.
Number of BOLD-related components identified by ME-ICA for all subjects across both tasks. Effective smoothness of 2nd-level group analyses for each task, each analysis, and under 0 mm or 6 mm.
Effect size, power, and effect size boosting statistics for data with no smoothing. This table provides effect sizes and 95% CIs for all analysis pipelines and all regions when data is not smoothed. It also provides estimates of sample size needed to achieve 80% power. This table also provides information about effect size boosting statistics and 95% confidence intervals, impact on sample size estimates to achieve 80% power and impact on monetary savings.
Effect size, power, and effect size boosting statistics for data with 6 mm FWHM smoothing. This table provides effect sizes and 95% CIs for all analysis pipelines and all regions when data is smoothed at 6 mm FWHM. This table also provides information about effect size boosting statistics and 95% confidence intervals for all regions in both tasks.