Abstract
Objective
To evaluate for potential causes of delayed loss of residual hearing that variably occurs with hybrid cochlear implants.
Study design
Histopathological evaluation of 29 human temporal bone (HTB) with cochlear implant.
Setting
The Neurotology and House HTB Laboratory of UCLA (House-UCLA).
Subjects and methods
HTB from cochlear implant patients from the House-UCLA HTB Laboratory (n = 28) and one courtesy of Massachusetts Eye and Ear Infirmary (MEEI). Histopathological analysis to identify the location of cochleostomy, fibrosis and bone formation in the scala vestibuli and tympani, and endolymphatic hydrops. Spiral ganglion neuron counts were obtained. Statistical analysis compared presence of cochleostomy and location with the histopathological findings.
Results
Seventeen of 29 bones with fibrosis in the scala vestibule (SV) and tympani had evidence of a cochleostomy involving the SV containing the ductus reunions, all of which had hydrops. Ten of eleven bones had no SV fibrosis, and a cochleostomy limited to the scala tympani, of which all had no hydrops. One HTB had moderate SV fibrosis not involving the ductus reuniens, and was without hydrops. One HTB had a SV cochleostomy but the electrode ruptured Reissner’s membrane, and was without hydrops. Cochleostomy was significantly associated with SV fibrosis and hydrops (p<0.01), those without hydrops had no SV atrophy (p<0.01). Round window insertion was associated with no fibrosis and no hydrops.
Conclusion
We hypothesize that cochleostomies involving scala vestibuli incite fibrosis, compromising the ductus reuniens, causing hydrops which may cause the delayed loss of residual low frequency hearing in cochlear implant.
Introduction
It is now possible to perform cochlear implantation surgery with successful retention of residual low frequency hearing using the hybrid cochlear implant (CI). The FDA has approved the hybrid CI for patients with severe to profound high frequency sensorineural hearing loss, yet normal to moderately impaired low frequency hearing. However, in up to a third of hybrid CI patients, there is the loss of residual hearing often months after stable or fluctuating hearing. There have been many hypotheses to explain the cause of this delayed hearing loss but explanation of the cause of the hearing loss was not forthcoming (1). Human temporal bone studies of patients implanted with the conventional CI electrode arrays demonstrate fibrosis and ossification of the perilymphatic spaces, and also variably endolymphatic hydrops although there was no history suggestive of Meniere’s disease or cochlear hydrops prior to implantation (2). Therefore, we undertook a histopathological analysis of 29 HTB with a history of cochlear implantation to determine the frequency of endolymphatic hydrops and its correlation with fibrosis of the scala vestibuli in the area of the ductus reuniens. Evidence for the ductus reuniens, in a 3-dimensional reconstruction, was demonstrated to transmits the endolymph from the scala media to the saccule and on to the endolymphatic duct where it is absorbed. (3). A low frequency hearing loss is characteristic of endolymphatic hydrops may be due to a distortion of the organ of Corti caused by the increased endolymphatic pressure upon the tectorial membrane decreasing the angle between the longer hair cells of the cochlear apex and basilar membrane, making the hair cells less able to amplify the incoming signal (Kalinec, personal communication).
Methods
The House-UCLA HTB database was used to identify bones from patients that had undergone cochlear implants with electrodes of varying lengths and insertion techniques. Twenty-seven were identified that were satisfactory for the study. The bones had been removed in the morgue and placed in ten percent neutral buffered formalin for three weeks. They were then decalcified in EDTA until shown by X-ray to be free of calcium. Embedding was done in increasingly concentrated celloidin to allow complete penetration. To minimize extraction movement, the electrode was removed just before the specimen was placed in hardening chloroform. The celloidin block was cut into 20-micron sections of which every tenth was mounted and stained with hematoxylin and eosin (H&E); the remaining sections were stored for specialized procedures such as immunohistochemistry. The Massachusetts Eye and Ear Infirmary of Harvard medical school provided images and the clinical record of one additional HTB. The microscopic sections were examined for evidence of hydrops manifested by distention of Reissner’s membrane into the scala vestibuli excluding the apical segment (4). Special attention was given to the basal segment of the cochlea and the round window area for the location of a cochleostomy and the fibrosis of one of more of the three scala.
Results and conclusions
Seventeen of the twenty-nine bones had endolymphatic hydrops, fibrosis of the three scala, cochlear endolymphatic hydrops, and a cochleostomy that included the scala vestibuli. (Figs.1 and 2) (Table 1). There are a total of twelve temporal bones without hydrops. Ten of eleven bones without hydrops did not exhibit scala vestibuli fibrosis (Table 2), out of which two bones had cochleostomy limited to the scala tympani (Fig. 3). Of the twelve without hydrops, eight had the round window electrode insertion and all temporal bones with round window insertion did not have hydrops nor fibrosis (Fig. 4). One of the temporal bones without hydrops had scala vestibuli fibrosis that did not obstruct the ductus reuniens and therefore had no hydrops. There was an additional bone with scala vestibuli cochleostomy but the electrode had ruptured the Reissner’s membrane and no endolymphatic hydrops was detected. Electrode insertion through the round window membrane did not produce any perilymph fibrosis or hydrops. (Fig. 4). The case from MEEI is illustrative of the problem. The bone was from a 70-year-old donor with a bilateral high frequency progressive loss presumed to be genetically predetermined and exacerbated by factory noise. The left ear had been implanted with an Iowa/ Nucleus Hybrid S8 electrode, 10 mm, through a cochleostomy at age 63. Four weeks after implantation he had preserved low frequency hearing but at 18 weeks was found to have a profound loss. (Fig. 5)
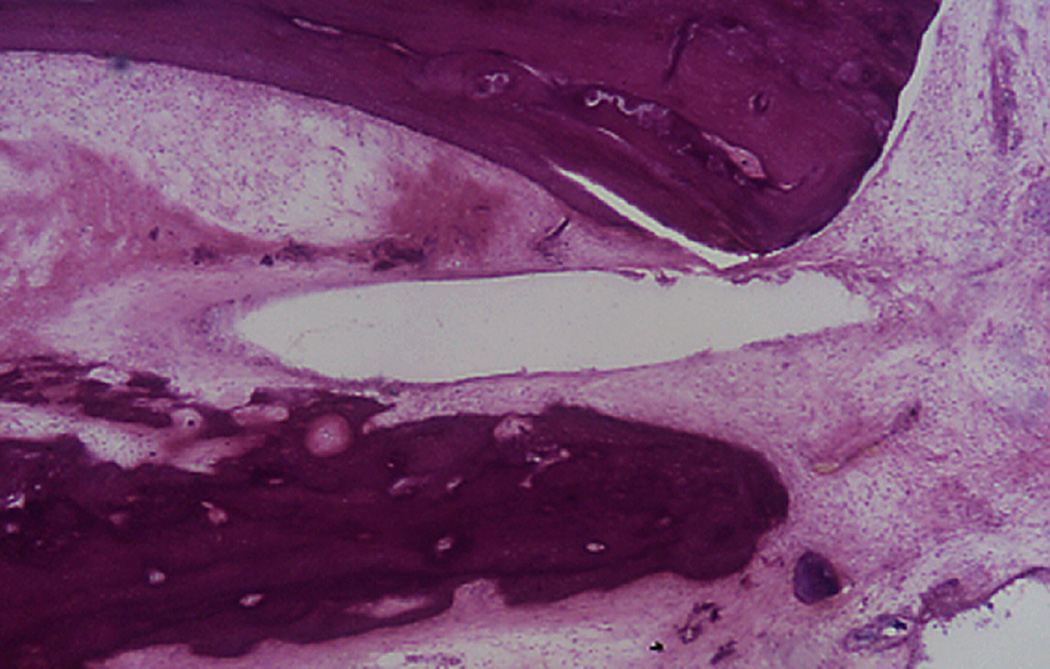
Figure 1.
Cochleostomy into the scala vestibuli. The electrode path is surrounded by fibrosis and new bone. There is hydrops in the upper segments of the cochlea (Hematoxylin and Eosin (H&E) × 100)
Figure 2.
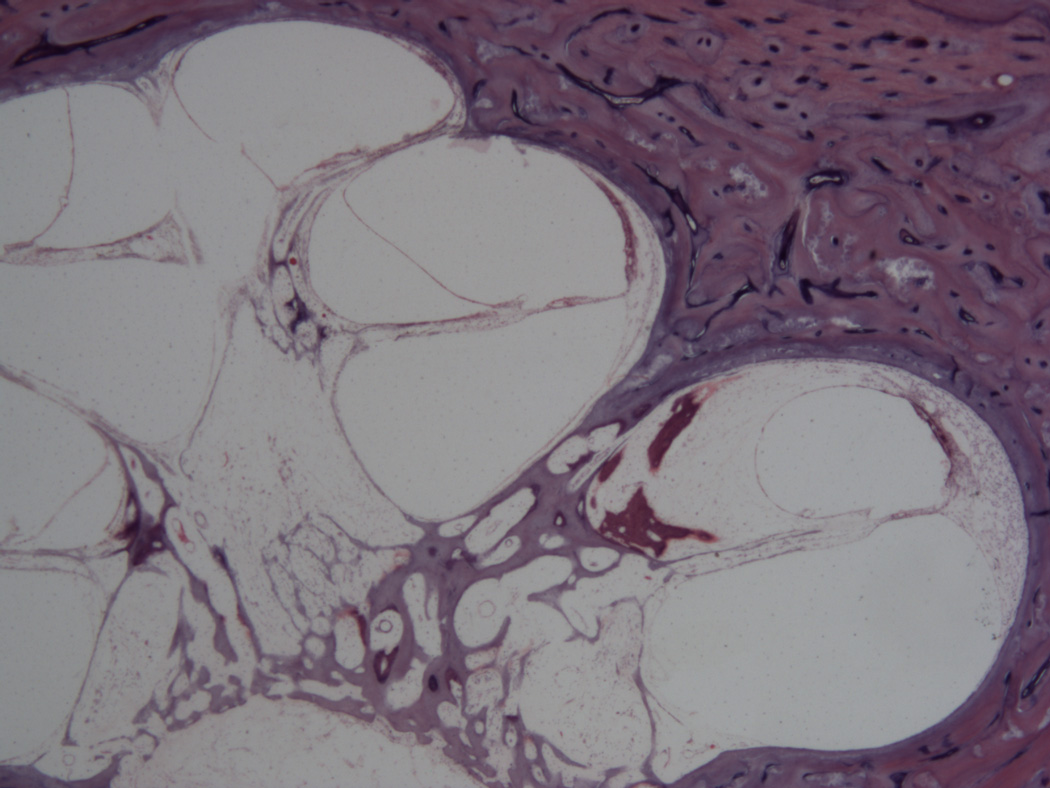
Cochleostomy into the scala vestibuli. (Fig. 2). There is fibrosis in the scala vestibuli and shows endolymphatic hydrops in all of the turns of the cochlea. (Hematoxylin and Eosin (H&E) × 100)
Table 1.
Descriptive data on hTb cases with evidence of cochlear hydrops present on histological examination.
| Case# | Age | Sex M=1 F=2 |
Ear R=1 L=2 |
Elect- Length |
Sp Gang Af ear |
Sp Gang non-Af ear |
Ostomy 1=yes 2=no |
SV Fib 1=yes 2=no |
Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 421 | 54 | 1 | 2 | 18 | 5,013 | 10,889 | 1 | 1 | Meningitis |
| 510 | 58 | 2 | 2 | 6 | 16,415 | 16,416 | 1 | 1 | Streptomycin toxicity |
| 569 | 75 | 1 | 2 | 20 | 6,194 | 7.011 | 1 | 1 | Cochlear otosclerosis |
| 586 | 67 | 1 | 1 | 6 | 14,022 | 10,678 | 1 | 1 | Cochlear otosclerosis |
| 687 | 74 | 1 | 2 | 21 | 7,767 | 25,722 | 1 | 1 | Cochlear otosclerosis |
| 727 | 71 | 2 | 1 | 18 | 19,741 | 24,588 | 1 | 1 | Sensorineural, cause undetermined |
| 735 | 87 | 2 | 1 | 20 | 14,004 | 19,845 | 1 | 1 | Cochlear otosclerosis |
| 758 | 92 | 2 | 1 | 6 | 14,283 | 11,760 | 1 | 1 | Cochlear otosclerosis |
| 761 | 87 | 2 | 1 | 20 | 18,486 | artifact | 1 | 1 | Sensorineural, cause undetermined |
| 761 | 87 | 2 | 2 | 7 | artifact | 14,265 | 1 | 1 | Sensorineural, cause undetermined |
| 775 | 76 | 2 | 1 | 20 | 2,446 | artifact | 1 | 1 | Sensorineural, cause undetermined |
| 798 | 90 | 1 | 2 | 18 | 10,104 | 6,480 | 1 | 1 | Cochlear otosclerosis |
| 808 | 64 | 2 | 1 | 20 | artifact | artifact | 1 | 1 | Sensorineural, cause undetermined |
| 808 | 64 | 2 | 2 | 18 | artifact | artifact | 1 | 1 | Sensorineural, cause undetermined |
| 822 | 81 | 2 | 1 | 25 | 11,520 | artifact | 1 | 1 | Typhoid fever |
| 834 | 89 | 1 | 2 | 20 | 935 | Missing | 1 | 1 | Cochlear otosclerosis |
| MEEI | 70 | 1 | 2 | 10 | 13,722 | 15,138 | 1 | 1 | Genetic and noise |
hTB = human temporal bone; M = male; F = female; Sp Gang Af Ear = spiral ganglion neuron count in the ipsilateral implanted ear for which data is entered on this line (some were bilaterally implanted); Sp Gang Non-af ear = spiral ganglion neuron count in the contralateral ear for which data is entered on this line; Ostomy = evidence of a cochleostomy insertion was found via temporal bone histological examination, as defined in the methods; SV Fib = stria vascularis fibrosis; missing indicates that the contralateral hTB was either not received or the modiolus was destroyed so that count could not be determined (e.g., transotic tumor removal, etc.); artifact: the modiolus was morphologically disrupted during fixation to the extent that an accurate spiral ganglion neuron count could not be determined.
Table 2.
Descriptive data on hTB cases with no hydrops evident throughout the cochlear duct.
| Case# | Age | Sex M=1 F=2 |
Ear R=1 L=2 |
Elect- length |
Sp Gang Af Ear |
Sp Gang Non-af ear |
Ostomy 1=yes 2=no |
SV Fib 1=yes 2=no |
Diagnosis |
|---|---|---|---|---|---|---|---|---|---|
| 441 | 69 | 1 | 2 | 18 | 16,620 | Missing | 2 | 2 | Ototoxicity drug unknown |
| 554 | 59 | 2 | 2 | 13 | 27,972 | NA | 2 | 2 | Sensorineural, cause undetermined |
| 558 | 61 | 2 | 1 | 16 | 20,177 | Implant | 2 | 2 | Sensorineural, cause undetermined |
| 558 | 61 | 2 | 2 | 20 | 24,398 | Implant | 2 | 2 | Sensorineural, cause undetermined |
| 658 | 80 | 1 | 2 | 6 | 6,435 | 3,135 | 2 | 2 | Sensorineural, hereditary |
| 709 | 70 | 2 | 2 | 20 | 4,095 | Missing | 1 | 1 | Sensorineural, cause undetermined |
| 736 | 74 | 1 | 2 | 6 | 9,453 | 10,521 | 2 | 2 | Cochlear otosclerosis |
| 796 | 90 | 2 | 1 | 16 | 2,525 | 1998 | 1 | 2 | Sensorineural, cause undetermined |
| 814 | 60 | 1 | 2 | 22 | 11,694 | Missing | 2 | 2 | SN vicodin |
| 820 | 72 | 1 | 2 | 18 | 9,621 | 13,374 | 2 | 2 | Hereditary progressive |
| 846 | 87 | 2 | 2 | 16 | 3,230 | Ossified | 1 | 2 | Hereditary progressive, ostomy in scala tympani |
| 775 | 76 | 2 | 2 | 22 | 2,244 | implant | 1 | 1? | Hereditary. Scala vest. ostomy. |
| Electrode ruptured Reissner’s memb. |
hTB = human temporal bone; M = male; F = female; Sp Gang Af Ear = spiral ganglion neuron count in the ipsilateral implanted ear for which data is entered on this line (some were bilaterally implanted); Sp Gang Non-af ear = spiral ganglion neuron count in the contralateral ear for which data is entered on this line; Ostomy = evidence of a cochleostomy insertion was found via temporal bone histological examination, as defined in the methods; SV Fib = stria vascularis fibrosis; missing indicates that the contralateral hTB was either not received or the modiolus was destroyed so that count could not be determined (e.g., transotic tumor removal, etc.)
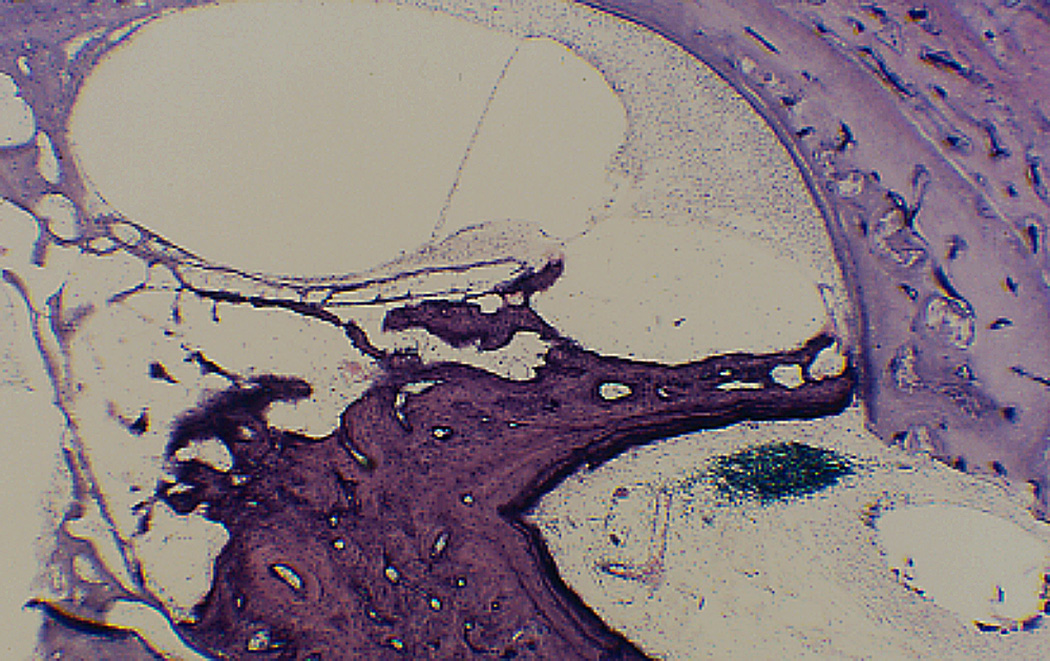
Figure 3.
Cochleostomy into scala tympani. There is extensive fibrosis and new bone in the scala tympani but none in the scala vestibuli; and no hydrops. (H&E × 100)
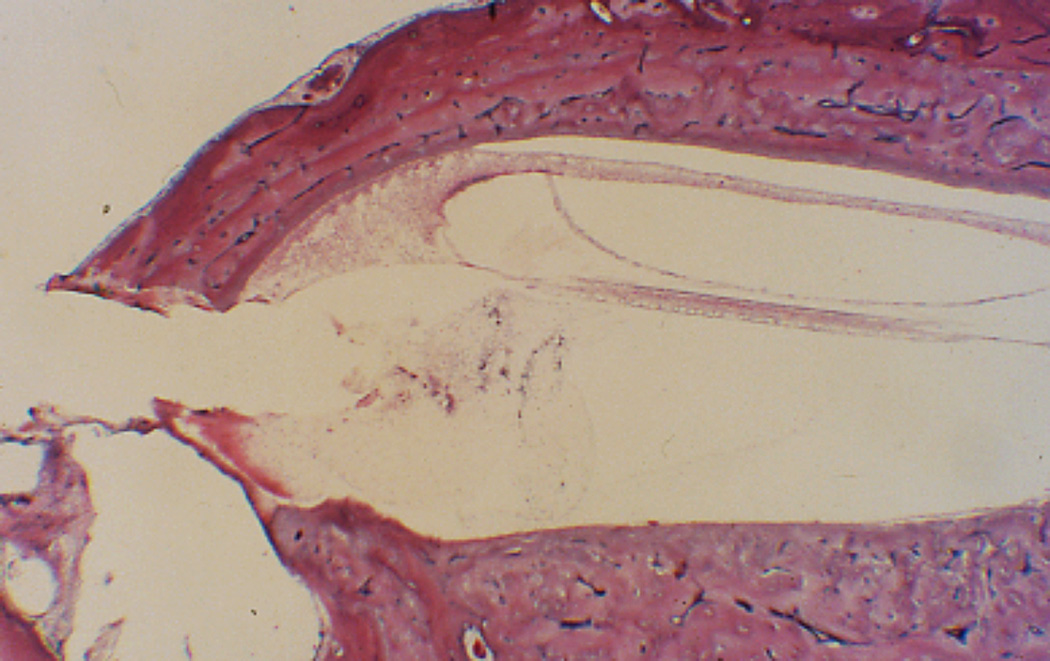
Figure 4.
Round window insertion after removal of the operculum. There is some debris in the scala tympani but no fibrosis. (H&E × 100)
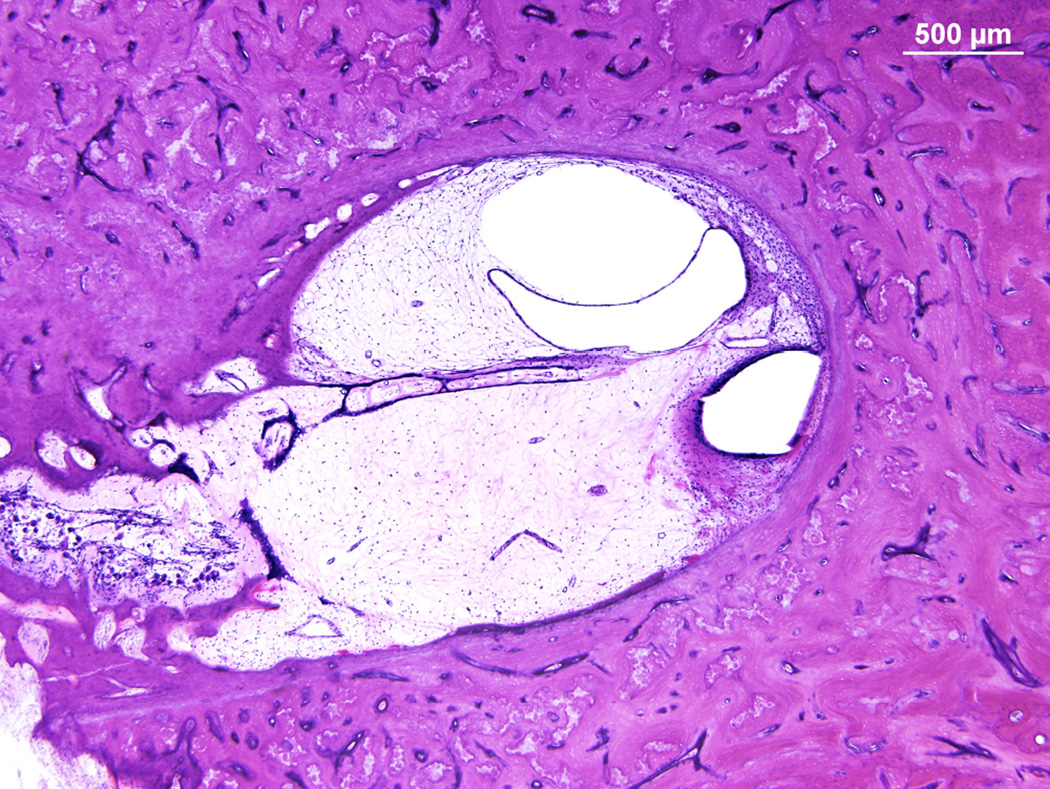
Figure 5.
Bone from MEEI illustrating fibrosis and hydrops in the scala vestibuli. The electrode path is in the scala tympani but ivolved the spiral ligament causing fibrosis in the scala vestibuli. (H&E × 20)
Discussion
Recent advancement in the design of cochlear implant electrodes and improved surgical techniques allow for the use of cochlear implantation aimed to preserve residual hearing in the lower frequencies. With preservation of residual hearing with cochlear implantation, these patients can wear hybrid cochlear implant processors with distinctive advantages. The advantage of being able to combine both the acoustic and electric hearing is evident in situations requiring better frequency resolution, including better hearing in background noise and in quiet, better spatial localization and improved music appreciation (5–9).
Although the goal is to perform cochlear implantation surgery and preserve hearing in the low frequencies, some patients unfortunately experience the loss of residual hearing, often occurring months following an initially successful surgery. Although the cause for this delayed hearing loss remains unknown, possible hypothesized etiologies include triggering an inflammatory cascade (10), an immunologic response (11,12), excitotoxicity due to electric stimulation (13), delayed hair cell degeneration and primary afferent neurons and synapses (14) and post-implantation conductive hearing loss (15). In order to understand the cause of delayed hearing loss following cochlear implantation, the current study was undertaken to analyze the archival human temporal bone specimens from patients who had undergone cochlear implantation surgery.
In seventeen out of twenty nine patients who had cochlear implantation, we found histologic evidence of secondary fibrosis and endosteal reaction with resultant endolymphatic hydrops when the cochleostomy involves the scala vestibuli. Ten out of eleven patients had no evidence of endolymphatic hydrops when the cochleostomy did not involve the scala vestibuli. One patient had the fibrosis of the scala vestibuli without obstructing the ductus reuniens and was without histologic evidence of endolymphatic hydrops. It is unlikely that there is a confounding factor since no patient in our study carried the diagnosis of endolymphatic hydrops at the time of surgery. There was no significant difference in length of insertion between those with and without hydrops. Therefore, our data strongly suggest that cochleostomy and disturbance of endosteum is more likely to lead to scala vestibuli fibrosis and endolymphatic hydrops. There is histopathological evidence for the fibroosseous reaction starting from the cochleostomy site. Since the endolymphatic hydrops causes low frequency sensorineural hearing loss, it is possible that secondary endolymphatic hydrops caused by the obstruction of ductus reuniens is the cause of loss of initially preserved hearing in the low frequencies following cochlear implantation.
The cause for the scala vestibuli fibrosis is the secondary endosteal reaction seen at the location of cochleostomy and it is remarkable that there is no evidence of endosteal reaction in the temporal bones when the cochlear implant electrode was inserted through the round window. Therefore, direct round window insertion of the cochlear implant electrode should be preferred to avoid the endosteal reaction caused by the cochleostomy to prevent the secondary endolymphatic hydrops to maximize the chance of preserving residual hearing. If cochleostomy needs to be performed due to technical reason, it is critical to perform the cochleostomy in the scala tympani to prevent the fibrosis of the scala vestibuli obstructing the ductus reuniens with resultant endolymphatic hydrops.
We made a comparison of the number of spiral ganglia neurons from the implanted ear and the control unimplanted ear showing no statistically significant differences. This finding demonstrates that delayed hearing loss in the low frequencies is not caused by the degeneration of spiral ganglia neurons. Recently, a single case of human temporal bone study was published of a patient who had received the Iowa/Nucleus8 hybrid implant electrode (16). This patient experienced progression of loss of residual hearing and at18 weeks following surgery he had profound sensorineural hearing loss in the operated ear. He died 7 years later due to unrelated cause. The cochlear implant electrode was inserted through a small 0.5 mm cochleostomy created and it was placed into scala tympani. There was evidence of fibroosseous tissue filling the scala tympani but loose fibrous tissue was also found in the scala vestibuli at the basal turn of the cochlea. There was also evidence of endolymphatic hydrops in the implanted ear. The quantification of the spiral ganglia neurons from the implanted ear did not differ when compared to the unoperated ear. Thus, it is very likely that the cause of delayed hearing loss 18 weeks after the surgery was also caused by the endolymphatic hydrops secondary to the obstruction of the ductus reuniens.
Adunka et al. (17) found that patients who underwent round window cochlear implant electrode insertion demonstrated comparable residual hearing preservation compared with the traditional cochleostomy insertion group among twenty patients who underwent prospective clinical trial using Med-El cochlear implants. However, they reported negative impact on the speech perception ability when the cochlear implant electrode was inserted through the scala vestibuli. However, others have advocated round window cochlear implant electrode insertion since it causes less trauma to the cochlea. (12,17). Li el al. (18) demonstrated that the degree of new tissue formation in cochlea correlated with the degree of trauma to the ear. Every attempt should be made to minimize the trauma and associated secondary changes in the cochlea especially when attempting to preserve the residual hearing.
It has been shown that immediate sealing of cochlear implant electrode insertion site is critical to minimize the degree of fibroosseous tissue within the cochlea. In addition, the use of autologous tissue at the electrode insertion site is always preferred since materials such as bone cement have been shown to decrease the speech perception performance due to an extensive new bone formation, sometimes even resulting in expulsion of the electrode (19).
Recent animal experiment using guinea pigs reported that bony cochleostomy did not cause progressing hearing loss but round window membrane incision and use of a muscle seal resulted in progressing hearing loss at 2000Hz (20). Rowe et al., (20) postulated that the cause of progressing post cochlear implant hearing loss maybe due to fibrosis in the round window niche associated with the muscle tissue seal. We were not able to identify the type of tissue or substance that was used to seal the cochlear implant insertion site as it was not clearly mentioned in the operative report. Therefore, it appears that application of “soft surgery” technique such with minimal trauma of the tissues around the round window membrane and endosteum is important to avoid the alteration of ductus reuniens function resulting in endolymphatic hydrops. As laser is used to minimize trauma in stapedectomy, there may be a role to apply similar technique to create a well-controlled incision at the round window membrane when hearing preservation is attempted in cochlear implantation.
A second mechanism that may play a role in the development of endolymphatic hydrops is the trauma associated with the cochleostomy inciting increased permeability of the blood-labyrinthine barrier, known to be demonstrated on magnetic resonance imaging of Meniere’s diseased ears. These studies demonstrate the association of endolymphatic hydrops with an increased gadolinium uptake in the perilymph, with a significantly higher degree of gadolinium uptake in Meniere’s compared with sudden hearing loss (21). In both cases, the degree of trauma to the tissues and the endosteum is critical to avoid the formation of endolymphatic hydrops, and the resultant low frequency hearing loss.
Conclusion
The ideal insertion is through the round window membrane after drilling off the operculum if necessary, being careful not to enter the scala vestibuli. If anatomical constraints prevent round window insertion, then a cochleostomy should be made in the scala tympani using a ‘soft surgery’ technique to avoid damaging the endosteum. Damage to the scala vestibuli endosteum produces proliferation of the spiral ligament cells leading to impaired function of the ductus reuniens, limiting the egress of endolymph and resulting in an insidious loss of low frequency hearing characteristic of-endolymphatic hydrops.
Acknowledgments
We thank Dr. Karen Berliner, PhD, for her expertise on data organization and statistical analysis of the data.
IRB# 14-001753 UCLA
Supported by NIDCD/NIH: U24DC011962-04
REFERENCES
- 1.Dedhia K, Worman T, Meredith MA, et al. Patterns of Long-term Hearing Loss in Hearing Preservation Cochlear Implant Surgery. Program and abstracts of the one hundredth forty-eighth annual meeting, American Olological Society Inc. 2015 Apr 24–26; doi: 10.1097/MAO.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 2.Richard C, Fayad JN, Doherty J, et al. Round window versus cochleostomy technique in cochlear implantation: histologic findings. Otol Neurotol. 2012;33(7):1181–1187. doi: 10.1097/MAO.0b013e318263d56d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Linthicum FH, Jr, Doherty J, Webster P, et al. The periductal channels of the endolymphatic duct, hydrodynamic implications. Otolaryngol Head Neck Surg. 2014;150(3):441–447. doi: 10.1177/0194599813516420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makarem A, Fayad JN, Linthicum FH., Jr Endolymphatic pseudohydrops of the cochlear apex. Otolaryngol Head Neck Surg. 2010;143:269–273. doi: 10.1016/j.otohns.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gantz BJ, Hansen MR, Turner CW, et al. Hybrid 10 clinical trial. Audio Neurotol. 2009;14:32–38. doi: 10.1159/000206493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner CW, Reiss LAJ, Gantz BJ. Combined acoustic and electric hearing: preserving residual acoustic hearing. Hearing Res. 2008;242:164–171. doi: 10.1016/j.heares.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gantz BJ, Turner CW. Combining acoustic and electrical speech processing:Iowa/Nucleus hybrid implant. Acta Oto Laryngol. 2004;124:344–347. doi: 10.1080/00016480410016423. [DOI] [PubMed] [Google Scholar]
- 8.Gifford RH, Grantham DW, Sheffield SW, et al. Localization and interaural time difference (ITD) thresholds for cochlear implant recipients with preserved acoustic hearing in the implanted ear. Hearing Res. 2014;312:28–37. doi: 10.1016/j.heares.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiefer J, Pok M, Adunka OF, et al. Combined electric and acoustic stimulation of the auditory system: results of a clinical study. Audiol Neurotol. 2005;10(3):134–144. doi: 10.1159/000084023. [DOI] [PubMed] [Google Scholar]
- 10.O’Leary SJ, Monksfield P, Kel G, et al. Relations between cochlear histopathology and hearing loss in experimental cochlear implantation. Hearing Res. 2013;298:27–35. doi: 10.1016/j.heares.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Nadol JB, Eddington DK, Burgess BJ. Foreign body or hypersensitivity granuloma of the inner ear after cochlear implantation: one possible cause of a soft failure? Eur Acad Otol Neurotol. 2008;29(8):1076–1084. doi: 10.1097/MAO.0b013e31818c33cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burghard A, Lenarz T, Kral A, et al. Insertion site and sealing technique after residual hearing and tissue formation after cochlear implantation. Hearing Res. 2014;312:21–27. doi: 10.1016/j.heares.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Kopelovich JC, Reissn LAJ, Etler CP, et al. Hearing Loss After Activation of hearing preservation cochlear implants might be related to afferent cochlear innervation injury. Otol Neurotol. 2015;36:1035–1044. doi: 10.1097/MAO.0000000000000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshraghi AA, Wang J, Adil E, et al. Blocking c-Jun-N-terminal kinase signaling can prevent hearing loss induced by both electrode insertion trauma and neomycin ototoxicity. Hearing Res. 2007;226:168–177. doi: 10.1016/j.heares.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Chole RA, Hullar TE, Potts LC. Conductive component after cochlear implantation in patients with residual hearing conservation. Am J Audiol. 2014;23:359–364. doi: 10.1044/2014_AJA-14-0018. [DOI] [PubMed] [Google Scholar]
- 16.Quesnel AM, Nakajima HH, John J, et al. Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implication for etiology. Hearing Res. 2016;333:325–334. doi: 10.1016/j.heares.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adunka OF, Dillon MT, Adunka MC, et al. Cochleostomy versus round window insertion: influence on functional outcomes in electric-acoustic stimulation of the auditory system. Otol Neurotol. 2014;35:613–618. doi: 10.1097/MAO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 18.Li PM, Somdas MA, Eddington DK, et al. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007;116:731–738. doi: 10.1177/000348940711601004. [DOI] [PubMed] [Google Scholar]
- 19.Mcelveen JT, Wolford RD, Miyamoto RT. Implications of bone pate in cochlear implant surgery. Otolaryngol Head Neck Surg. 1995;112:457–460. doi: 10.1016/S0194-59989570284-9. [DOI] [PubMed] [Google Scholar]
- 20.Rowe D, Chambers S, Hampson A, et al. Delayed low frequency hearing loss caused by cochlear implantation interventions via the round window but not cochleostomy. Hear Res. 2015;333:49–57. doi: 10.1016/j.heares.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Pakdaman MN*, Ishiyama G*, Ishiyama A, et al. Blood-labyrinth barrier permeability in Meniere’s disease and idiopathic sudden sensorineural hearing loss: findings on delayed post-contrast 3D-FLAIR MRI. Am J Neuroradiology. doi: 10.3174/ajnr.A4822. In press *authors contributed equally. [DOI] [PMC free article] [PubMed] [Google Scholar]