Abstract
Goal
To validate an intracochlear piezoelectric sensor for its ability to detect intracochlear pressure and function as a microphone for a fully implantable cochlear implant.
Methods
A PVDF piezoelectric pressure sensor was inserted into a human fresh cadaveric round window at varying depths. An external sound pressure stimulus was applied to the external auditory canal (EAC). EAC pressure, stapes velocity, and piezoelectric sensor voltage output were recorded.
Results
The PVDF sensor was able to detect the intracochlear sound pressure response to an acoustic input to the EAC. The frequency response of the pressure measured with the intracochlear sensor was similar to that of the pressure at the EAC, with the expected phase delay of the middle ear transmission. The magnitude of the response increased and smoothened with respect to frequency as the sensor was inserted more deeply into the scala tympani. Artifact measurements, made with the sensor in air near the round window, showed flat frequency response in both magnitude and phase, which were distinct from those measured when the sensor was inserted in the round window.
Conclusion
This study describes a novel method of measuring intracochlear pressure for an otologic microphone composed of a piezoelectric polymer, and demonstrates feasibility. Our next goal is to improve device sensitivity and bandwidth. Our long-term objective is to imbed the piezoelectric sensor within a conventional cochlear implant electrode, to enable a device to both measure intracochlear sound pressure and deliver electrical stimulus to the cochlea, for a fully implantable cochlear implant.
Introduction
Cochlear implants have become the standard of care for profound sensorineural hearing loss. The outcomes of cochlear implants have been greatly improved with advances in both speech processing and electrode array technology. However, cochlear implantation still requires the user to wear external components. This results in both functional and cosmetic drawbacks for the wearer. (7, 21, 24)
With these drawbacks in mind, several groups have focused on designing and building a fully implantable cochlear implant (9, 23, 24). Before this can be achieved, there are technical hurdles that must be overcome, including power constraints, miniaturization of the speech processor, reliable connectors, and finally an implantable microphone. In regards to an implantable microphone, while there are several types used in active middle ear devices, there are drawbacks and limitations to each. Subcutaneous microphones, implanted near the temple (Carina of Otologics, LLC, Boulder, CO) or ear canal (Totally Integrated Cochlear Amplifier [TICA] of Implex American hearing Systems, now owned by Cochlear Corporation, Sydney Australia) result in damping of the sound stimulus by the adjacent soft tissue and unwanted noise from tissue motion (due to chewing, speaking, etc.). The Esteem (Envoy Medical Corporation, USA) senses ossicular motion at the incus body with a needle-tipped sensor. Sensors at the epitympanum for sensing incus body or malleus head vibrations are limited by several factors including instability of the connection between the sensor tip and the bone due to the rounded surface of ossicles, the complex modes of osscular motion at mid-to-high frequencies, and the need to sever the ossicular chain (1, 2, 3, 4, 8, 12, 13).
The intracochlear pressures in scala vestibuli and tympani in response to sound stimulation in gerbil ears and human temporal bones have been reported in our previous studies (5, 14–17, 22). This time-varying pressure is the output of the mechanical system of the middle ear and is the driving force for intracochlear motion, which leads to mechano-electrical transduction by cochlear hair cells. We have investigated whether this intracochlear sound pressure could be measured and used as the input to the processor of a cochlear implant. In addition to cosmetic and activity-based advantages, this approach leverages the gain, and directional cues provided from the external ear. Members of our group recently demonstrated early stage feasibility of the concept with a piezoelectric sensor positioned just within the round window opening in gerbil (25). Here we take the study further by inserting the sensor into the scala tympani of human temporal bone, and measuring its response to sound stimuli applied to the external auditory canal (EAC).
Methods
Piezoelectric Sensor Preparation
The piezoelectric sensor was constructed of a Polyvinylidene fluoride (PVDF) polymer film strip embedded in a cylindrical PDMS (silicone) housing fabricated by injection molding. Fig. 1 shows a schematic of the sensor, shaped and sized similarly to a cochlear implant. The sensor had an outer diameter of 600μm and was ~ 12mm in length.
Figure 1.
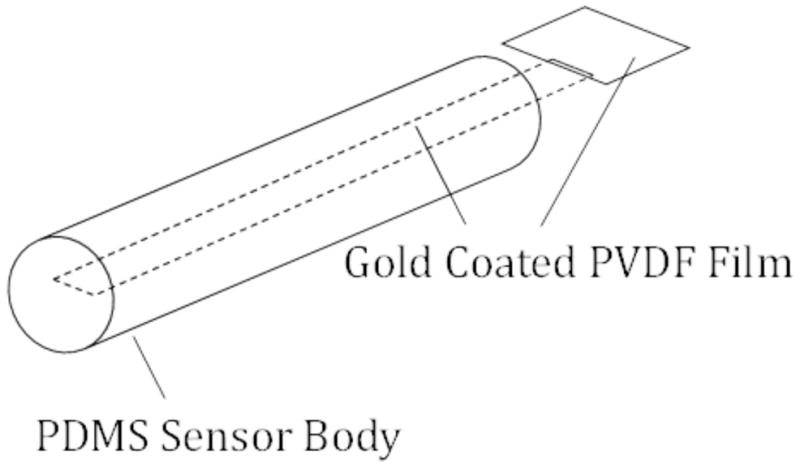
Schematic of the PVDF sensor
Temporal Bone Preparation
The Institutional Review Board of the Massachusetts Eye and Ear approved our temporal bone experiments. Temporal bones were obtained from fresh cadavers, soft tissue was removed and they were immediately frozen. Specimens were stored and defrosted in 0.9% saline. The bony external canal was shortened to approximately 1cm. An extended facial recess approach was drilled to allow access to the round window and stapes. Bony overhang was removed from the round window niche to provide wide access to the round window membrane.
Testing of PVDF Sensor within Scala Tympani
The PVDF sensor was inserted into the scala tympani via a round window insertion, similar to that used in cochlear implantation (Fig. 2). During opening of the cochlea, saline submerged the round window to ensure that no air was introduced into the inner ear. After opening of the round window, a micropositioner was used to place the PVDF sensor into the scala tympani until a full insertion of 12mm was reached. The PVDF sensor was then withdrawn at 2mm increments.
Figure 2.
Sensor entering scala tympani on the right via the round window, and reflector on stapes footplate on the left.
Tones of 10 ms duration between 100 Hz – 10 kHz (63 frequencies) and 80–110 dB SPL were presented to the sealed ear canal with a speaker (Beyer DT48). For each insertion depth of the PVDF sensor, the response to sound stimulation of the PVDF sensor, ear canal pressure, and stapes velocity were measured simultaneously. A charge amplifier (B&K 2525) with a gain of 60 dB was used to condition the output signal of the PVDF sensor. Ear canal pressure was recorded 1 to 3 mm from the umbo with a calibrated probe-tube microphone (ER-7C, Etymotic). No averaging was performed.
To determine whether insertion of the PVDF sensor changed the cochlear input impedance and middle ear sound transmission, stapes motion was measured with a laser Doppler vibrometer system (Polytec CLV 700) aimed at a ~ 0.2 mm2 reflector (consisting of 50 μm diameter reflective polystyrene microbeads on plastic tape) applied to the footplate or posterior crus of the stapes. The angle between the laser beam and the piston-motion direction of the footplate was ~ 30° to 45°. Prior to opening the round window membrane, the stapes velocity response to sound stimulus in the ear canal was recorded. Repeat measurements were made after introduction of the PVDF sensor into the scala tympani through the round window.
As a control and to check for possible electromagnetic coupling between speaker and sensor recording, the output of the PVDF sensor was also measured while the sensor was held in the air of the middle ear space or submerged in fluid and resting on the round window membrane.
A block diagram of our entire experimental setup can be seen in Figure 3.
Figure 3.
Block diagram of experimental setup showing LabView generated tones presented to the EAC, EAC pressure measured by microphone placed closed to TM, Laser Dopplear Vibrometer measurement of stapes motion, and measurement of voltage output of PVDF sensor.
Results
Intracochlear sound pressure measured in Scala Tympani
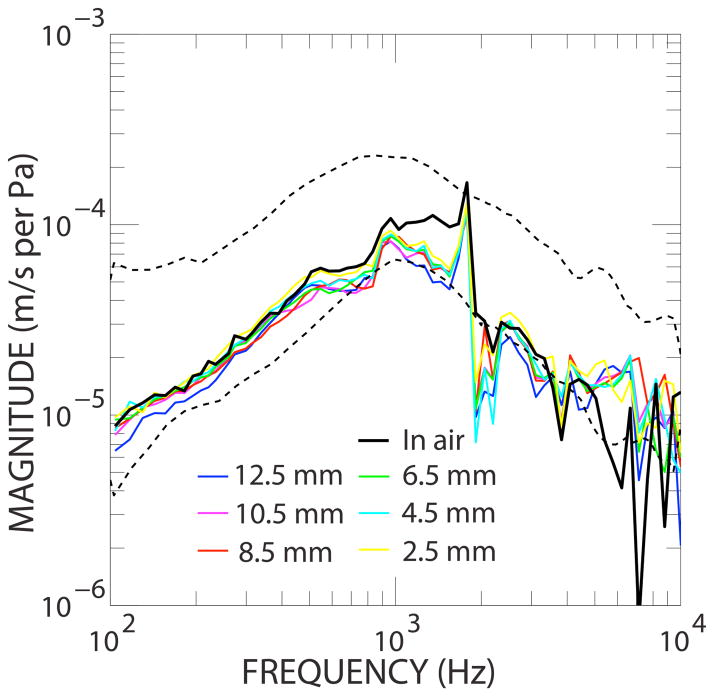
Figure 4 shows the output frequency response of the PVDF sensor while it was fully inserted in the scala tympani (blue thick-dashed line). For comparison, sound pressure in the ear canal is also plotted (black thick solid line). As shown in the left panel of Fig. 4, magnitude of the sensor resembled ear canal pressure over the range of 100 Hz to 4 kHz. At higher frequencies the sensor’s output level dropped into the vicinity of the “artifact” measure (blue thin dashed line). This artifact was measured with the sensor positioned in free air near the cochlea during sound stimulation.
Figure 4.
(Left) Simultaneous responses of the PVDF sensor in scala tympani (full insertion) and sound pressure measured in the ear canal. The artifact of the sensor was measured with the sensor in free air. (Right) Phase measurements showing phase delay between ear canal pressure and sensor output with full insertion.
The phase response of the sensor recording and the ear canal pressure (referenced to drive voltage of the speaker) are plotted on a linear frequency scale in the right panel of Fig. 4. The steeper slope of the intracochlear pressure versus frequency indicates there was a delay of approximately 0.1 ms between the sound pressure in the ear canal and the pressure in the scala tympani measured with the sensor.
The artifact measured had both magnitude and phase that were flat over all the tested frequencies. This measure is likely dominated by electric pick-up, though possibly sensing some air-conducted sound from the speaker attached to a sealed ear canal. The “noise” level (red thin dotted line) was measured with electrical stimulus disconnected to the speaker. The phase of the noise level (not plotted) had a randomly varying frequency response (as would be expected of noise).
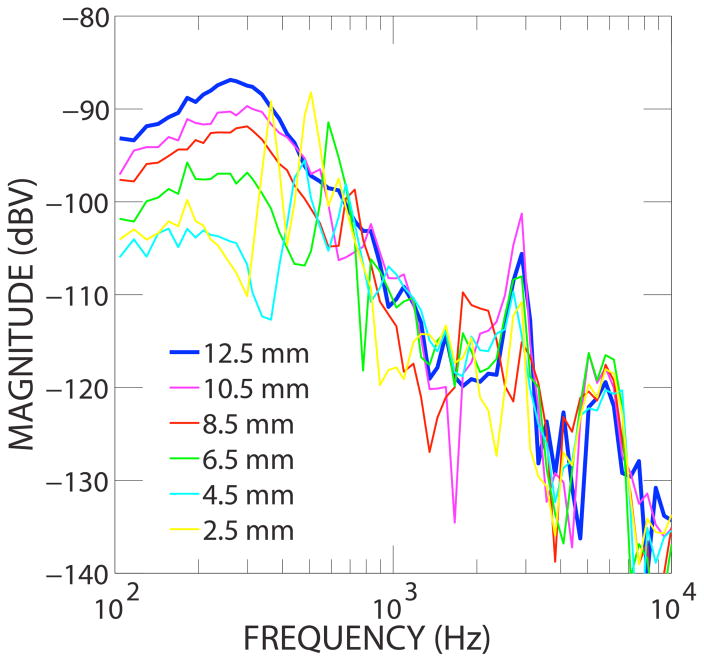
Responses of the sensor measured at different depths of insertion in the scala tympani are shown in Fig. 5. The ear canal pressure during the measurements was kept at the same level as that shown in Fig. 4. Generally, the sensor output increased and smoothened with respect to frequency as the sensor was inserted more deeply into the scala tympani. At depths greater than 6.5 mm, the output generally paralleled EAC pressure. However, at depths shallower than 6.5 mm, large sharp fluctuations in response with respect to frequency occurred.
Figure 5.
Sensor output in scala tympani with different insertion length.
Effect of the sensor on stapes velocity
Figure 6 shows the stapes velocity normalized to the ear canal pressure. The area between the two dashed lines indicates, with a 95% confidence interval, the American Society for Testing and Materials (ASTM) international normal human ear stapes velocity (19). The black line shows the stapes velocity without the sensor in the cochlea, and stapes velocity at each sensor insertion depth is also shown with colored lines.
Figure 6.
Ratio of stapes velocity to ear canal pressure at varying sensor insertion depths in the scala tympani. Dashed lines indicate 95% confidence interval of normal human ear stapes velocity.
Discussion
Here we report the feasibility of a PVDF-based piezoelectric sensor to accurately detect intracochlear sound pressure. Our results indicate that the PVDF sensor housed in PDMS silicone, shaped like a cochlear implant electrode, can detect cochlear pressure variations caused by acoustic input into the EAC. Figure 3 shows that the PVDF sensor’s frequency response followed the EAC pressure response. It is important to note that although the sensor had a frequency response similar to the stimulus sound, it does not ensure that the output recorded was from intracochlear changes. Recordings could have been the result of transmission through routes other than the middle and inner ear (e.g. electromagnetic pickup between the speaker and sensor recording). We were able to determine if the output of the sensor was from intracochlear pressure variations via middle ear transmission by measuring the response of the output both when the sensor was inserted into the scala tympani and in air nearby the round window. In air, the magnitude frequency response was flat and significantly lower than the recording inside the cochlea. Figure 3 shows a phase delay of about 0.1 ms between ear canal pressure and intracochlear pressure, which is similar to previously published reports of the time delay of the middle ear ossicular chain (15). Furthermore, the flat frequency phase response when the sensor was in air indicates that the contrasting phase delay recorded when the sensor was inserted into the scala tympani was indeed due to intracochlear pressure, and that the sensor was not simply detecting mechanical vibrations of the temporal bone, air transmission or electromagnetic coupling.
Our results also indicate that insertion of the PVDF sensor does not significantly impact the function of the middle ear. As this sensor depends on a functioning middle ear system, it is important that the device itself does not reduce the middle ear transmission. Our results show that after insertion of the sensor into the scala tympani stapes velocity remained in the normal range for human middle ears. There was a narrow frequency band near 2kHz where there was a slight decrease in stapes velocity below normal values. Given that it is not unusual to have “normal” bones with deviations over small frequency bands, and that the remainder of frequencies fell well within normal ranges we did not view these findings as significant. (19)
Finally, with full insertion the sensor output was well above the artifact level (which may include sensor response of air-conducted sound) for frequencies from 100 Hz to 4 kHz as can be seen in Figure 3. While this would not allow for the full range of human hearing, it would allow detection of human speech, which is typically between 300 Hz and 3400 Hz. The sensor did have high signal-to-noise for noise measured without sound input. Future improvements will be directed at increasing the signal-to-noise and bandwidth of the sensor.
For this prototype, the intracochlear sensor frequency response was smooth and similar to the EAC acoustic input response when the sensor was inserted fully. However, shallower cochlear insertion (with more of the sensor exposed to air) resulted in lower sensitivity and less smooth frequency response. This was possibly due to the design of the sensor where the electrical attachment site, external to the cochlea, was compliant and sensitive to change in motion with complex modes. Also, with less of the device in the cochlea with shallow insertion, less of the device is stimulated, reducing the response. Our future design will stiffen and reduce the sensitivity near the electrical connections to the sensor. We also have plans to vary the regions of piezoelectric sensitivity along the sensor for optimal response with varying insertion depths.
Technical details regarding the development of the sensor, its fabrication, material properties and electronics will be communicated in an engineering journal article in preparation.
While the results of this early stage experiment are encouraging, there are several challenges ahead. For the PVDF sensor to function as a microphone for cochlear implantation, it will have to be fabricated within the lumen of an existing cochlear implant electrode, which will be another step in our group’s work. Finally, while our initial study indicates that there is minimal change in the middle ear system with initial insertion, previous studies of hybrid cochlear implants raise the concern that inflammatory responses over time might affect intracochlear pressure changes and this will need to be investigated in the future (6, 11, 18, 20).
Despite these concerns, our initial outcomes clearly indicate that this piezoelectric sensor prototype can accurately detect pressure changes in the cochlea in response to acoustical input and can do so over an acceptable frequency range for speech perception.
Conclusions
This study shows the feasibility of a PVDF piezoelectric sensor housed in silicone, similar in shape to a cochlear implant electrode, and inserted into the scala tympani as a novel method for measuring intracochlear pressure in response to sound pressure presented to the external auditory canal. This device could be used in the future to function as a microphone and provide input to a speech processor for a fully implantable cochlear implant. Future studies will focus on increasing sensitivity, increasing bandwidth, decreasing noise, and developing a sensor imbedded into the lumen of existing cochlear implant electrodes.
Acknowledgments
We are grateful for the contributions of John Roswoski, Diane Jones, Jean Phillips, Mike Ravicz, Melissa McKinnon, and the staff of the Otolaryngology Department and Eaton-Peabody Laboratory at MEE as well as Abhijit Kulkarni.
Footnotes
This paper is associated with a poster presentation, “Investigation of Piezoelectric Sensors for a Middle Ear Microphone”, to be presented at the American Neurotological Society Meeting as The Combined Otolaryngology Sections Meeting in Chicago May of 2016
References
- 1.Beleites T, Neudert M, Bornitz M, Zahnert T. Sound Transfer of Active Middle Ear Implants. Otolaryngol Clin North Am. 2014;47(6):859–891. doi: 10.1016/j.otc.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Carlson ML, Pelosi S, Haynes DS. Historical Development of Active Middle Ear Implants. Otolaryngol Clin North Am. 2014;47:893–914. doi: 10.1016/j.otc.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Chen Da, Backous DD, Arriaga MA, et al. Phase 1 clinical trial results of the Envoy System: a totally implantable middle ear device for sensorineural hearing loss. Otolaryngol Head Neck Surg. 2004;131(6):904–916. doi: 10.1016/j.otohns.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 4.Decraemer WF, Khanna SM. Measurement, visualization and quantitative analysis of complete three-dimensional kinematical data sets of human and cat middle ear. In: Gyo K, Wada H, Hato N, Koike T, editors. Proceedings of the 3rd Symposium on Middle Ear Mechanics in Research and Otology. World Scientific; New Jersey, USA: 2004. pp. 3–10. [Google Scholar]
- 5.de La Rochefoucauld O, Decraemer WF, Khanna SM, Olson ES. Simultaneous measurements of ossicular velocity and intracochlear pressure leading to the cochlear input impedance in gerbil. JARO - J Assoc Res Otolaryngol. 2008;9(2):161–177. doi: 10.1007/s10162-008-0115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly N, Bibas A, Jiang D, et al. Effect of cochlear implant electrode insertion on middle-ear function as measured by intra-operative laser Doppler vibrometry. J Laryngol Otol. 2009;123(7):723–729. doi: 10.1017/S0022215109004290. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins Ha, Uhler K. Speech perception comparisons using an implanted and an external microphone in existing cochlear implant users. Otol Neurotol. 2012;33(1):13–19. doi: 10.1097/MAO.0b013e31823c9335. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins Ha, Uhler K. Otologics Active Middle Ear Implants. Otolaryngol Clin North Am. 2014;47(6):967–978. doi: 10.1016/j.otc.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Lawand NS, Ngamkham W, Nazarian G, et al. An improved system approach towards future cochlear implants. Annu Int Conf IEEE Eng Med Biol Soc. 2013;2013:5163–5166. doi: 10.1109/EMBC.2013.6610711. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre PP, Martin C, Dubreuil C, et al. A pilot study of the safety and performance of the Otologics fully implantable hearing device: transducing sounds via the round window membrane to the inner ear. Audiol Neurootol. 2009;14(3):172–180. doi: 10.1159/000171479. [DOI] [PubMed] [Google Scholar]
- 11.Li PMMC, Somdas Ma, Eddington DK, Nadol JB. Analysis of intracochlear new bone and fibrous tissue formation in human subjects with cochlear implants. Ann Otol Rhinol Laryngol. 2007;116(10):731–738. doi: 10.1016/S1041-892X(09)79445-4. [DOI] [PubMed] [Google Scholar]
- 12.Marzo SJ, Sappington JM, Shohet JA. The Envoy Esteem Implantable Hearing System. Otolaryngol Clin North Am. 2014;47(6):941–952. doi: 10.1016/j.otc.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Memari F, Asghari A, Daneshi A, Jalali A. Safety and patient selection of totally implantable hearing aid surgery: Envoy system, Esteem. Eur Arch Otorhinolaryngol. 2011;268(10):1421–1425. doi: 10.1007/s00405-011-1507-0. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima H. Evaluation of round window stimulation using the FMT by intracochlear sound pressure measurements in Human Temporal Bones. Otol Neurotol. 2010;31(3):506–511. doi: 10.1097/MAO.0b013e3181c0ea9f.Evaluation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakajima HH, Dong W, Olson ES, Merchant SN, Ravicz ME, Rosowski JJ. Differential intracochlear sound pressure measurements in normal human temporal bones. JARO - J Assoc Res Otolaryngol. 2009;10(1):23–36. doi: 10.1007/s10162-008-0150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson ES. Direct measurement of intra-cochlear pressure waves. Nature. 1999;402(6761):526–529. doi: 10.1038/990092. [DOI] [PubMed] [Google Scholar]
- 17.Olson ES. Observing middle and inner ear mechanics with novel intracochlear pressure sensors. J Acoust Soc Am. 1998;103(6):3445–3463. doi: 10.1121/1.423083. [DOI] [PubMed] [Google Scholar]
- 18.Quesnel AM, Nakajima HH, Rosowski JJ, Hansen MR, Gantz BJ, Nadol JB. Delayed loss of hearing after hearing preservation cochlear implantation: Human temporal bone pathology and implications for etiology. Hear Res. 2015:1–10. doi: 10.1016/j.heares.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosowski JJ, Manuscript A, With IM. NIH Public Access. Society. 2008;28(2):943–951. doi: 10.1097/01.mao.0000244370.47320.9a.Measurements. [DOI] [Google Scholar]
- 20.Seyyedi M, Nadol JB. Intracochlear Inflammatory Response to Cochlear Implant Electrodes in Humans. Otol Neurotol. 2014;35(9):1545–1551. doi: 10.1097/MAO.0000000000000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shinners MJ, Hilton CW, Levine SC. Implantable hearing devices. Curr Opin Otolaryngol Head Neck Surg. 2008;16(5):416–419. doi: 10.1097/MOO.0b013e32830a49f0. [DOI] [PubMed] [Google Scholar]
- 22.Stieger C, Rosowski JJ, Nakajima HH. Comparison of forward (ear-canal) and reverse (round-window) sound stimulation of the cochlea. Hear Res. 2013;301:105–114. doi: 10.1016/j.heares.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sudano A, Accoto D, Francomano MT, Salvinelli F, Guglielmelli E. Optimization of kinetic energy harvesters design for fully implantable Cochlear Implants. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:7678–7681. doi: 10.1109/IEMBS.2011.6091892. [DOI] [PubMed] [Google Scholar]
- 24.Yip M, Rui J, Nakajima HH, Stankovic KM, Chandrakasan AP. A Fully-Implantable Cochlear Implant SoC With Piezoelectric Middle-Ear Sensor and Arbitrary Waveform Neural Stimulation. Solid-State Circuits, IEEE J. 2015;50(1):214–229. doi: 10.1109/JSSC.2014.2355822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang A, Kale S, Olson ES, Kymissis I. Progress report on a piezoelectric-polymer intra-cochlear pressure sensor and a novel fluid-assisted cochlear implant insertion method. Abstracts of the 37th midwinter research meeting; Association for Research in Otolaryngology; 2014. [Google Scholar]