Abstract
Insects have developed highly sophisticated and sensitive olfactory systems to find animal or plant hosts for feeding. Some insects vector pathogens that cause diseases in hundreds of millions of people and destroy billions of dollars of food products every year. There is great interest, therefore, in understanding how the insect olfactory system can be manipulated to reduce their contact with hosts. Here, we review recent advances in our understanding of insect olfactory detection mechanisms, which may serve as a foundation for designing insect control programs based on manipulation of their behaviors by using odorants. Because every insect species has a unique set of olfactory receptors and olfactory-mediated behaviors, we focus primarily on general principles of odor detection that potentially apply to most insects. While these mechanisms have emerged from studies on model systems for study of insect olfaction, such as Drosophila melanogaster, they provide a foundation for discovery of odorants to repel insects or reduce host-seeking behavior.
INTRODUCTION
Volatile chemicals have been used to repel insects since ancient times, when certain plant or animal extracts were found to ward off biting insects, and were used for personal protection. Thus, people burned plant extracts to create smoke (Charlwood 2003) or anointed themselves with vinegar or oil. The primary path to discovery of effective insect repellents was trial-and-error. This type of empirical testing approach continued into the 20th century, and was expanded with the development of chemical synthesis techniques, which enabled the production of potential repellents on a large scale. Many commonly used repellents, including DEET and IR3535, were the result of these concerted efforts to synthesize and screen large numbers of compounds as potential repellents (Mccabe et al. 1954).
The 1950s brought the advent of analytical tools that could greatly expedite insect olfaction research. The modern gas chromatograph, developed in 1952, provided a means to rapidly separate complex mixtures of volatiles into individual compounds (James and Martin 1952). This meant that biologically active molecules could be isolated from their sources and tested individually. Electrophysiological developments such as the electroantennogram (EAG) led to another great leap forward, allowing neural activity in insects to be recorded in real time while the insect was exposed to individual compounds (Schneider and Kaissling 1957). The power of this method was increased still further by the coupling of gas-chromatography with electroantennogram detection (GC-EAD), providing a method of separating a complex odor blend into its individual components while simultaneously testing the antennal responses to each component. This technique greatly accelerated the identification of biologically active compounds in complex mixtures such as plant or insect extracts. The development of molecular modeling in the latter half of the 20th century allowed for more efficient compound discovery, leading to the identification of the repellent Icaridin in the 1980s. Despite these major advances in analytical methods, physiological recording methods, and molecular modeling, discovery and development of new insect repellents for applications in human health and agriculture has not yet followed. Effective and affordable repellents are largely unavailable in tropical countries that bear the brunt of vector-borne diseases, and use of repellents in agriculture is almost non-existent worldwide. However, the advent of modern genomics and molecular biology tools has led to a much better understanding of the insect olfactory system, and consequently, a deeper knowledge of the mechanisms by which repellents affect insect behavior. More recently, chemical informatics can even screen and predict active compounds, so that bioassays can be focused on the subsets of compounds that are most likely to be active (Boyle et al. 2016a).
OVERVIEW OF THE INSECT OLFACTORY SYSTEM
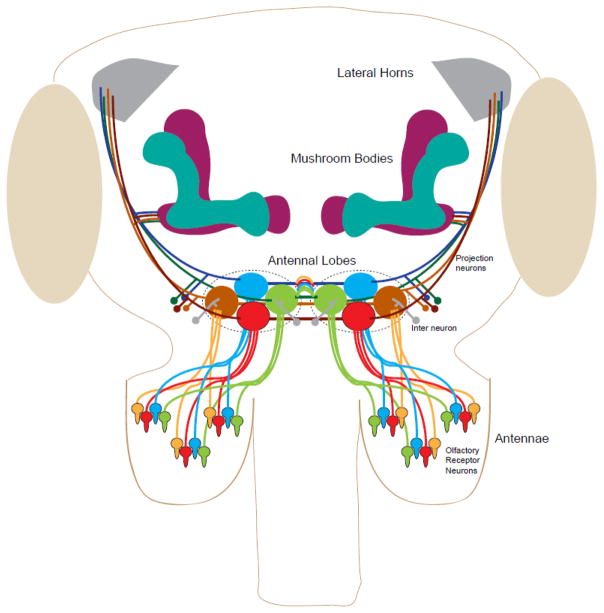
Volatile chemicals that repel insects typically act on the olfactory system. The principal olfactory organs in insects are the antennae and maxillary palps. These organs are covered with hair-like projections called sensilla that house a small number of olfactory receptor neurons (ORNs), the exact number varying by sensillar type, as well as various support cells (Shanbhag et al. 1995, 1999). The sensillar sheath is perforated with pores that allow compounds to make contact with the inner lymph bathing the neurons. Each ORN expresses a small number of olfactory receptor proteins, which pass ion current through the membrane upon binding odorant molecules (Benton et al. 2009; Sato et al. 2008). The olfactory receptor proteins are encoded by 3 different families of genes: the Odorant Receptor (Or), the Ionotropic Receptor (Ir), and a few members of the Gustatory Receptor (Gr) family (Benton et al. 2009; Clyne et al. 1999, 2000; Vosshall et al. 1999). The ORNs send projections to the antennal lobes (AL) where they synapse with the dendrites of second order projection neurons (PNs) (Fig. 1). ORNs expressing the same receptor(s) will synapse in the same neurite conglomeration, called a glomerulus (Vosshall et al. 2000). From the antennal lobe, the information is carried to the mushroom bodies and lateral horns for higher processing (Jefferis et al. 2001). Both excitatory and inhibitory interneurons provide input to the olfactory circuit (Ng et al. 2002; Wilson and Mainen 2006; Wilson et al. 2004). In Drosophila melanogaster, after detection in the antenna or maxillary palps, and processing in the antennal lobes, information about most innate olfactory behaviors are thought to be routed through the lateral horn (Strutz et al. 2014). The behavioral outcome of odor-induced neural activation, therefore, depends on which ORNs are activated and to what extent. This also can, in principle, be manipulated by using odorants that affect the activity of relevant olfactory receptors and ORNs.
Figure 1. An overview of the stereotypic connections in the insect olfactory system.
Olfactory processing begins with transduction in the olfactory receptor neurons, which are grouped in sensilla on the antennae and maxillary palps (not shown). All ORNs expressing the same olfactory receptors send an axon which fasciculates with other axons of the same ORN type. Each fasciculation terminates in a specific glomerulus of the antennal lobe, where it synapses with the dendrites of specific second order projection neurons. Excitatory and inhibitory interneurons also innervate the antennal lobes. The projection neurons in turn connect to the mushroom bodies, which are involved in olfactory learning and memory, and the lateral horns, which primarily route information instructing innate behaviors.
Although thousands of odorants potentially can interact with an insect’s olfactory system, there are only four general mechanisms that have been shown so far to reduce an insect’s contact with hosts: 1) the activation of olfactory receptors dedicated to aversion, 2) activation of pheromone receptors that cause aversion, 3) the inhibition of odorant receptors dedicated to attraction, and 4) prolonged activation of odorant receptors participating in attraction. In this review, we discuss findings that support these four mechanisms and suggest ways to exploit these pathways in developing methods for reduction of host-seeking behavior using odorants. We also discuss how the internal physiological state of the insect, such as hunger, thirst, mating, and egg-laying, can influence neural activity, and be targeted to alter host-seeking behavior.
ACTIVATION OF OLFACTORY RECEPTORS DEDICATED TO AVERSION
Identification of dedicated receptors and ORNs for aversion in a target insect species can serve as an excellent way to identify powerful species-specific insect repellents. The idea of such labeled line codes – that is, the existence of separate neural circuits dedicated to the processing of specific and fixed sensations – has been a staple of theory in sensory neuroscience since Johannes Peter Muller first proposed its less-refined predecessor in the mid-nineteenth century. Evidence from the model species Drosophila melanogaster suggests that in insects a labeled line code exists for some aversive odors (Stensmyr et al. 2012; Suh et al. 2004). These odors can originate from a variety of sources such as plants, microbes, or, in the case of pheromones or carbon dioxide, other flies.
CO2
One of the first olfactory circuits identified as causing innate aversion is that which detects the gas CO2 in Drosophila melanogaster (Suh et al. 2004). This pathway is unusual among antennal lobe inputs in that its primary sensory neurons express members of the Gustatory Receptor (Gr) family Gr63a and Gr21a, instead of members of the more typical Odorant Receptor (Or) family (Jones et al. 2007; Suh et al. 2004). Genetically silencing the Gr21a/Gr63a+ neurons abolished aversion to CO2. Artificial activation by optogenetics of the CO2 circuit using channelrhodopsin (ChR2) is also sufficient to cause aversion (Suh et al. 2007), further corroborating its role in innate aversion behavior in Drosophila. When channelrhodopsin (ChR2) was expressed in Gr21a-expressing neurons, flies avoided the illuminated arm of a T-maze. Since the gaseous CO2 is difficult to formulate for delivery, other odorants that also activate this aversive labeled line could serve as effective repellents. Odorants that activate the CO2 receptor (Gr21a+Gr63a) such as pyridine, cyclopentanone, and (E)-2-methylbut-2-enal were identified using electrophysiological recording techniques (Lu et al. 2007; Tauxe et al. 2013). As expected, when pyridine was tested in a T-maze with Drosophila melanogaster, it showed a strong repellent effect similar to CO2 (Pham and Ray 2015). Comparative analysis across 5 related Drosophila species showed that the CO2 receptor is conserved as a sensitive detector of CO2 and pyridine; however, its role in aversive behavior was species-specific. Two of the 5 species tested with CO2 and with pyridine, Drosophila suzukii and Drosophila virilis, showed little avoidance in a T-maze assay (Pham and Ray 2015). In fact, more distantly related dipterans like mosquitoes are attracted to CO2 plumes, suggesting that the aversion response is not well conserved among Diptera (Cardé and Gibson 2010; Ray 2015).
The T-maze assays used in the laboratory test walking flies in a narrow tube, and when flying Drosophila were tested for longer-term behaviors by using a 2-choice trap assay, D. melanogaster did not show significant avoidance of the pyridine-treated trap, suggesting that the aversion is dependent on the context of the assay design and duration of the assay. Thus, CO2-receptor-activating odorants such as pyridine are unlikely to act as broad-spectrum repellents for Drosophila species, particularly for the agriculturally important pest D. suzukii. Free-flying Drosophila have not been tested for instantaneous responses to CO2, but tethered D. melanogaster that can flap their wings and turn directionally did not turn away from a puff of 100% CO2 (Wasserman et al. 2013).
Because species-specificity of the labeled aversive line was observed, one of the questions that emerges is why D. melanogaster would be averse to low concentrations of CO2 in the first place, particularly since CO2 is ever-present at low levels in the atmosphere (Gillies 1980; Pham and Ray 2015; Turner and Ray 2009), and is emitted by food sources such as fermenting fruit on which flies feed and oviposit. While stressed flies emit a small amount of CO2 as part of their stress response (Suh et al. 2004), it is released at much higher levels by ripening fruit (Faucher et al. 2006). One possibility is that this ability equips flies to avoid less ripe fruit, which emit higher levels of CO2, in favor of fruits further along in the decaying process (Pham and Ray 2015; Turner and Ray 2009).
Organic Acids
Calcium imaging of neuronal activity in the antennal lobe of Drosophila revealed that the DC4 glomeruli, innervated by coeloconic neurons expressing the ionotropic receptor IR64a, showed increased activity in response to carbonic acid and other short-chain organic acids (Ai et al. 2010). The IR64a protein was determined to be necessary, but not sufficient, for acid detection, but activity of the IR64a-expressing neurons was required to achieve a strong aversion to acids.
Geosmin
A third labeled-line aversive pathway was discovered in D. melanogaster that comprises ORNs expressing the Or56a receptor, which is activated by the microbe-produced bicyclic compound geosmin (Stensmyr et al. 2012). In addition to being narrowly tuned so as to be dedicated to geosmin detection, these neurons were shown to be sensitive to low, ecologically relevant concentrations and to confer aversion. For example, addition of geosmin to balsamic vinegar caused approximately a one-third reduction in the attraction index (Stensmyr et al. 2012).
Multiple Receptors
As qualified as these circuits are to be considered labeled lines, a more prominent form of odor detection is combinatorial coding, where one odor can elicit varying responses from multiple receptors and glomeruli (de Bruyne et al. 1999, 2001; Galizia et al. 1999; Hallem and Carlson 2006; Semmelhack and Wang 2009; Wang et al. 2003). This means that a single odor may activate both attractive and repellent pathways, and it is the integration of such activity that determines the odor quality and subsequent behavior. In general, adult flies are averse to high concentrations of odorants, even if those odorants are attractive at lower concentrations (Semmelhack and Wang 2009). One way this can occur is via recruitment of additional neural populations with increasing odor concentration. Drosophila melanogaster is attracted to apple cider vinegar due to activation of the DM1 and VA2 glomeruli, innervated by ORNs expressing Or42b and Or92a, respectively. However, these flies avoid higher concentrations of vinegar by recruitment of additional activated neurons, including those innervating the DM5 glomerulus, which express Or85a. This study indicated that DM5 is an innately aversive glomerulus, and its activation can override the attractive input of other glomeruli like DM1 and VA2 (Semmelhack and Wang 2009). In order to test whether this basic discovery can be utilized to identify novel repellents, odorants that were identified as activators of this neuron were used in behavioral tests. Indeed, an odorant that activates the Or85a receptor, such as ethyl 3-hydroxybutyrate (Hallem and Carlson 2006) causes aversion in D. melanogaster, although the behavior is not conserved across other Drosophila species such as D. suzukii, D. yakuba, D. pseudoobscura, and D. virilis (Pham and Ray 2015).
Using Optogenetics to Identify Aversive Neurons
A recent paper used optogenetics and systematically expressed a UV-light-sensitive Channelrhodopsin-2 protein in specific ORN types, and tested behavior activated by light (Bell 2016). Surprisingly, activation of the Or56a+, Or85a+ and the Ir64a+ ORNs led to attraction instead of aversion as would have been expected from previous studies. The Gr63a+ neurons showed aversion as before. Interestingly this study also reported that airflow within the behavior arena was required for a behavioral response (Bell 2016). These results suggest that the design of laboratory assays can impact the behavioral outcome, and that several lines of experimentation are necessary for identifying aversive ORN lines.
Citronellal
Another widely known insect repellent is the monoterpenoid citronellal. The olfactory receptor activated by citronellal is not known; however, electrophysiological studies show both the ab11a and ab12a neurons are activated by citronellal (Kwon et al. 2010), and citronellal apparently does not activate other neurons in the antenna. In further screening of mutant flies, activation of ab11a was shown to be independent of Orco, whereas activation of ab12a was dependent on Orco. TrpA1, a cation channel that activates at temperatures over ~26 degrees C, was implicated in mediating the aversive response in one of the neurons in the mutant analyses, which showed a defect in the temporal kinetics of the response.
Orco Ligand that Activates Broadly
Because each insect species will have its own repellent olfactory receptor pathways that are challenging to identify, an interesting alternative approach to identify repellents would be to find chemicals that show widespread activation of olfactory receptors in general. That is, these chemicals would probably non-specifically activate at least some repellent pathways as well. One group of such molecules, VUAA1 and its analogs, were identified as activators of the co-receptor Orco by using large-scale small molecule screening from a synthetic chemistry library (Jones et al. 2011). However, these molecules are nonvolatile, and behavioral testing to evaluate reduction in attraction to hosts has been difficult. Identification of volatile variants of these compounds in the future have great potential for testing whether host seeking is reduced, and also whether the widespread activation acts as a confusing cue to an insect.
As expected, aversion to odorants is blocked when synaptic transmission is blocked in all ORNs by expressing shibirets1 (Gao et al. 2015). However, selectively rescuing only a small subset of these silenced ORNs – as few as one – was sufficient to restore aversive behavior. Furthermore, the authors discovered that aversion was unaffected by silencing GH146+ excitatory projection neurons (ePNs), which are thought to form 2/3 of the total ePN population. This indicates that, like the primary ORNs, relatively few antennal lobe output neurons are required to convey aversion information to higher brain centers. These experiments indicate that aversive channels are possibly widespread.
DEET
The aversive mechanism that has received a great deal of interest recently is for the repellent with the most widespread commercial use, N,N-Diethyl-meta-toluamide (DEET). It is a weak repellent, likely due to its low volatility, showing almost no repellency in Drosophila melanogaster when tested in a T-maze and requiring special close-range assays. Even in mosquitoes, DEET repels only at close range and is used in formulations at high concentrations (5%–100%). It has very limited use in tropical countries where there is a great need for effective repellents, and it is not used at all in crop protection. Nevertheless, the main advantages of DEET include long-lasting repellency for 2 or more hours, perhaps due to the low volatility and high concentrations used, its non-specific action across many insect species including Diptera (Pham and Ray 2015). These two attributes make it one of the most commonly used insect repellents in the developed world. However DEET has also been shown to dissolve nylons and plastics, blocks human acetylcholinesterase, activates human M3 muscarinic GPCR causing anti-angiogenesis, inhibits mammalian TRPM8, and inhibits mammalian CNGA2 channels, the health consequences of none of which are well understood (Abd-Ella 2015; Corbel 2009; Ditzen 2008; Legeay 2016; Swale 2015). The notion that identification of the mechanisms of action of DEET could help in the development of improved broad-spectrum repellents has resulted in much recent research in this area (Ray 2015). However, no mechanism uncovered so far fully explains its properties and conservation of those repellent properties across numerous species.
There is some evidence that DEET acts in part through Or family members in an Orco-dependent manner (DeGennaro et al. 2013; Ditzen et al. 2008). There also are data from Culex quinquefasciatus that show it activates specific ORNs directly (Syed and Leal 2008; Xia et al. 2008). Other lines of evidence suggest that DEET may also inhibit responses to attractive odors (Bohbot and Dickens 2012). The C. quinquifasciatus receptor CquiOr136 was shown to impart response to DEET and other repellents when expressed in Xenopus oocytes (Xu et al. 2014). The RNAi knockdown of this one receptor led to mosquitoes with significantly reduced repellency to DEET, suggesting that a sole receptor type may be responsible for repellency in this species. The CquiOr136 receptor does not have orthologs in other mosquito or insect species that also avoid DEET, raising the possibility that different insects have evolved different receptor pathways to detect this molecule and convey aversion (Xu et al. 2014). In another study, authors co-expressed the Aedes aegypti Ors AaOR2 or AaOR8 together with the co-receptor AaOR7 (now called Orco) in Xenopus oocytes and exposed them to four different insect repellents, including DEET (Bohbot and Dickens 2010). They found that when presented alone, DEET activated AaOR2 but not AaOR8. However, when presented as a mixture, DEET inhibited the activity elicited by 1-octen-3-ol, a ligand known to activate AaOR8. This effect also was observed when a DEET/indole mixture was presented to AaOR2. Two other repellents, IR3535 and picaridine, inhibited the activity of indole and 1-octen-3-ol on their cognate Ors but induced no activity themselves. Given these confusing results, clearly more research is needed to clarify the mechanism of DEET detection across species.
Genetic approaches using CRISPR-Cas9 also have been useful in addressing mechanistic questions about DEET repellency. For example, Orco mutant Aedes aegypti no longer avoid a human arm covered with DEET placed outside a cage full of mosquitoes (DeGennaro et al. 2013). Interestingly, when a 37°C heat source is used as an attractant instead of an arm, Orco mutants continue to avoid DEET, suggesting that repellent mechanisms other than Or receptors also are active in aversion to DEET (Guda et al. 2015). An alternative receptor pathway such as the ionotropic pathway could be involved. Hopefully, further experimentation to identify the non-Or receptor pathways as well as Or pathways that detect DEET will help to solve some of these conundrums.
The variety of different effects seen with DEET suggests that DEET could be a broadly tuned ligand that has binding sites on multiple olfactory receptors, albeit with lower affinity than those of cognate odors. One variation of this model was proposed as an unusual “odorant scramble” hypothesis which posited that DEET is not itself detected by the insect olfactory receptors but, when presented alongside weak ligands, causes differences in levels of activation and inhibition of multiple Or/ORNs, leading to a “scrambling” of the odor responses and the resulting disorientation (Pellegrino et al. 2011). This hypothesis was developed based on electrophysiology analyses in large basiconic sensilla of the adult D. melanogaster antennae when exposed to a mixture of DEET and various odorants. While 1-octen-3-ol normally suppresses activity of the ab2A neuron, a DEET + 1-octen-3-ol mixture activated the ab2A neuron. These effects were observed with other odorants that were weak ligands as well. Further experimentation revealed that only the inhibition of the Or59b receptor by odorants was impaired; the activation by compounds that stimulate the neuron was not affected. This hypothesis has not yet been tested with behavioral bioassays and it is still possible that DEET’s repellency is caused by its activation of one or a few critical, aversive pathways.
Computational Approaches to Repellent Discovery
Given that DEET’s repellent effect could work through several receptor pathways, it is extremely challenging to devise a screening method to identify improved DEET. To circumvent this hurdle, a computational approach has been tested and proven to be effective (Boyle et al. 2016a; Katritzky et al. 2008, 2010; Oliferenko et al. 2013). These approaches generally rely on identifying physicochemical descriptors that can be predictive of repellent behavioral activity from previously identified compounds like DEET and picaridin, among others. Some form of learning such as neural networks or machine learning is then used so that the computer can learn to discriminate between repellents and non-repellents. In one instance, this chemical informatics method was utilized to screen hundreds of thousands of chemicals, and several hundred compounds were predicted to be novel repellents. What made this approach particularly useful was the ability to screen a set of natural compounds to identify repellents with excellent safety properties. Behavioral testing revealed that many of the predicted compounds are indeed strong repellents and have potential in protecting against mosquitoes (Boyle et al. 2016a). Since many of these chemicals are non-toxic and already used in food, they also were tested as potential repellents for use in agriculture. It was found that indeed they might offer protection against agricultural pests like the spotted wing Drosophila, Drosophila suzukii (Pham and Ray 2015).
Taken together, these discoveries show that members of all three olfactory receptor gene families, Ors, Irs, and Grs, can act as labeled line aversive receptors. These studies also highlight that discovery of labeled aversive receptors and neurons require a significant number of genetic experiments that are challenging with vector insects. However, the recently developed CRISPR-Cas9 mutagenesis method potentially enables these types of experiments to be carried out in a variety of different insect species. The conclusions of these studies also reveal that very few inputs, as little as a single class of ORNs, can be sufficient to cause aversion even if the ligands that activate them also activate other ORN classes. These Or pathways and ORNs will likely be different across species, and their identification could lead to development of species-specific repellents.
Larvae
A special case to consider for repellent research is that of insect larvae. Many herbivorous insects damage their host plants in the larval stage, so it is important to understand olfactory repellent pathways in this life stage as well. The D. melanogaster larval olfactory system is simpler than that of the adult, and thus has provided a window into understanding hardwired olfactory processing (Fishilevich et al. 2005; Kreher et al. 2005, 2008; Ramaekers et al. 2005). Efforts to discover the valence encoded by individual larval ORNs were encumbered by the fact that most odorants activate multiple ORNs (Kreher et al. 2008). A number of odorants that activate Ors in a combinatorial manner have been identified in larvae using large-scale deorphanization efforts (Kreher et al. 2005, 2008; Mathew et al. 2013). Knowledge of how odorants activate nearly the entire larval repertoire of odorant receptors allowed for modeling of behavior. Based on these foundational studies, it was shown that the vast majority of odorants and Ors mediate attraction, with only a few imparting weak to moderate repellency. Modeling the experimentally observed behavior to the electrophysiologically determined activities of each receptor suggested that Or74a and Or82a may contribute to aversion, as geranyl acetate, an aversive compound, only activates Or82a at the concentration tested, and 1-nonanol, another aversive compound, acts most strongly on Or74a (Kreher et al. 2008). In a complimentary approach, transgenic larvae were created that selectively expressed either the light-sensitive channel channelrhodopsin-2 or adenylyl cyclase Pac-alpha in specific ORN types via the control of Or-specific drivers. Their behavior was evaluated upon activation of these selected neurons using blue light (Bellmann et al. 2010). This study highlighted two important points: First, most larval ORNs likely carry a positive valence, because ten of the twelve lines had reduced aversion to light. Second, the ORN types expressing Or33b and Or45a are much weaker conveyers of attractive valence, being either indifferent or possibly conveying repulsive behavior (Bellmann et al. 2010). To determine if the Or33b and Or45a expressing ORNs were indeed mediators of repulsion, larvae were placed in an arena with blue light in two quadrants and the Or45a ligand octyl acetate in the other two quadrants. Wild-type larvae avoided the octyl acetate quadrants in favor of the illuminated quadrants, indicating that octyl acetate is even more repulsive than light. However, transgenic lines expressing channelrhodopsin-2 in both Or33b and Or45a avoided the light quadrants, indicating that the Or33b and Or45a neurons indeed convey a negative valence. Interestingly, when ChR2 or Pac-alpha was expressed in all ORNs simultaneously, larvae became attracted to blue light (Bellmann et al. 2010). This indicates that activity of Or33b and Or45a can be overridden by attractive input of the other ORNs. These studies suggest that identifying repellents for insect larvae may pose a challenging problem, and if other insect larvae behave like those of D. melanogaster, pursuing dedicated aversive olfactory receptors may not be the most promising route to repellent discovery.
ACTIVATION OF PHEROMONE RECEPTORS DEDICATED TO AVERSION
An interesting target to exploit in the future for behavior modification could be pheromone receptors typically involved in mate location. Both heterospecific and intrasexual courtship in insects often are prevented through chemically established barriers (Billeter et al. 2009; Coyne et al. 1994; Savarit et al. 1999). For example, males of other Drosophila species will not court D. melanogaster females because 7,11-heptacosadiene, a cuticular hydrocarbon excreted by the female’s oenocytes, is aversive. If the oenocytes are ablated, these males will court D. melanogaster females. Additionally, these males will not court females of their own species if the females are treated with 7,11-heptacosadiene (Billeter et al. 2009).
cVA
One of the best-studied volatile pheromones in Drosophila is the long-chain lipid cis-vaccenyl acetate (cVA), which is produced in the ejaculatory bulb of the male and transferred to females during copulation (Brieger and Butterworth 1970; Butterworth 1969). This compound is detected by two receptor proteins, Or67d and Or65a (Ha and Smith 2006; van Naters and Carlson 2007), and elicits different responses depending on the context, dosage, and sex of the detecting fly. Males will not court other males upon which cVA has accumulated, but Or67d mutants will do so at a rate three times higher (Kurtovic et al. 2007). cVA also acts as an aphrodisiac in female flies (Kurtovic et al. 2007). How can cVA bind to the same receptors in the same neurons of both males and females, and yet lead to entirely opposite behaviors? The Or67d-expressing neurons are the first components of a sexually dimorphic circuit that is characterized by the fruitless transcription factor (Datta et al. 2008; Ruta et al. 2010). The Or67d-expressing ORNs project to the DA1 glomerulus and synapse with projection neurons (PNs). These PNs project to the lateral horn where they overlap with dendrites of aSP-f neurons in males, but not females, and aSP-g neurons in females, but not males (Cachero et al. 2010). Functional characterization of these dimorphic neuronal populations found that aSP-f neurons responded to cVA in males, but not in females, whereas aSP-g neurons responded to cVA in females, but not in males (Kohl et al. 2013). These neurons did not respond to cVA in Or67d-mutants, indicating that Or65a does not contribute to their input. The fruitless protein product, FruM, was found to specify the circuit dimorphism, feminizing the circuit in males such that aSP-f neurons no longer overlapped with DA1 PNs when FruM was knocked out, and masculinizing the circuit in females such that aSP-f neurons overlapped with DA1 PNs when FruM was expressed. This emerging understanding of pheromone detection and processing could in principle lead to development of sex-specific repellents for insects using pheromones or compounds that mimic their activities.
Other Pheromones
Interestingly, both D. melanogaster larvae and adults can smell and avoid semiochemicals emitted by another insect species, the parasitic wasp Leptopilina, that can parasitize up to 80% of fly larvae in nature (Ebrahim et al. 2015). Both larvae and adults use the Or49a receptor to detect iridomyrmecin, whereas adult flies use Or85f to detect the wasp odors actinidine and nepetalactol. Identification of aversive receptors for semiochemicals might also have enormous value in reducing contact of insect larvae with hosts.
INHIBITION OF ODORANT RECEPTORS DEDICATED TO ATTRACTION
Or
Many insects rely on their olfactory system to find hosts, thus volatile chemicals that inhibit receptors that detect host odorants may offer a potential strategy to reduce host-seeking behavior. In an electrophysiological study with D. melanogaster, 11% of tested odorants inhibited the spontaneous activity of Odorant Receptors (Or), acting as inverse agonists (Hallem and Carlson 2006). However, when such odorants were tested in mixtures with an activating odorant, they are unable to overcome the activity to a sufficient extent to be considered candidates for preventing attraction (Su et al. 2011).
Orco
Artificial antagonists for the co-receptor Orco however, have been identified through high-throughput screening of compounds (Jones et al. 2012; Pask et al. 2013). These compounds, VUANT1 and amiloride derivatives can inhibit responses to odorants when applied to cells expressing odorant receptors. In principle, these chemicals should inhibit Orco-dependent receptors efficiently across most insect species because insects have a relatively well-conserved Orco. Presently these chemicals are not volatile enough for use in behavioral studies with hosts, but one would expect to observe a decrease in attraction of insects to odor sources that are detected primarily using Or-family receptors.
Gr
One of the few receptors that can be inhibited efficiently by volatile odorants is the heteromeric CO2-receptor (Gr1,2,3). The effects of inhibitors on behavior have been tested in mosquitoes where CO2 is one of the few known strong attraction cues to humans (Turner et al. 2011; Turner and Ray 2009). For example, an inhibitory odorant like ethyl pyruvate significantly reduced the entry of mosquitoes into a CO2-baited trap tested overnight in a greenhouse. The same Gr1,2,3 receptor also detects other odorants from skin, and ethyl pyruvate inhibited this response and also reduced the number of mosquitoes attracted to a human arm as odor source (Tauxe et al. 2013). However, in order to block attraction to a host completely one needs to find inhibitors for most of the attractive neuronal pathways that an insect uses, making this approach somewhat practically difficult compared to repellents. One possibility would be to identify volatile compounds that have effects like VUANT1 for all three classes of odor receptors (Ors, Irs, and Grs) to mask the host-odor detection completely.
PROLONGED ACTIVATION OF ODORANT RECEPTORS PARTICIPATING IN ATTRACTION
A few odorants can cause prolonged tonic responses in olfactory neurons expressing Gr receptors for CO2 (Tauxe et al. 2013; Turner et al. 2011) or Or receptors (Boyle et al. 2016b; Martelli et al. 2013). Thus, the neurons are unable to respond strongly to subsequent exposure to odorant activators (Boyle et al. 2016b; Tauxe et al. 2013; Turner et al. 2011). Elevated activity in the olfactory receptor neuron also is likely to cause a depression in synaptic transmission to the projection neurons (PNs) (Bhandawat et al. 2007). Both these effects would substantially undermine the ability of the neurons to accurately convey detection of subsequent activators, and for the insect to behave appropriately. It was shown that pre-exposure to a prolonged activator blend for the CO2 receptor (Gr1,2,3) can significantly reduce the navigation of A. gambiae, C. quinquefasciatus, and A. aegypti mosquitoes towards a CO2 source in a wind-tunnel. In semi-field greenhouse trials, this blend also significantly reduced the entry of mosquitoes into a fake hut with a CO2-trap placed inside (Turner et al. 2011). In experiments with D. melanogaster larvae, pre-exposure to a prolonged activator odorant for the Or42b receptor significantly reduced the navigation of the larvae to ethyl acetate or apple cider vinegar (Boyle et al. 2016b).
For blocking odorant receptor detection pathways, prolonged activators are more likely to produce behavior modifications than inhibitory odorants, which so far are unable to override activators. There also appears to be structural similarities between prolonged activators of a given receptor because a chemical informatics method can learn to identify new prolonged activators from structures of known prolonged activators (Boyle et al. 2016b). Moreover, identifying prolonged activators may be more straightforward in non-model insects that are important disease vectors.
EFFECTS OF INTERNAL STATE ON AVERSION
Mating
In some cases, an odorant can elicit different behaviors depending on the internal physiological state of the insect. For example, preference of D. melanogaster for food rich in amino acids varies in accordance with their nutritional state (Toshima and Tanimura 2012). Additionally, virgin female Drosophila prefer sugars to yeast extract, but this preference is reversed once they have mated (Ribeiro and Dickson 2010; Vargas et al. 2010). This preference reversal appears to be mediated by the seminal fluid protein sex peptide (SP), because mutant flies lacking functional SP did not show the preference switch upon mating (Ribeiro and Dickson 2010). However, exactly how the peripheral or central processing mechanisms are altered upon mating is unknown. Similarly, female mosquitos show host-seeking behavior only in the window between mating and consumption of a blood meal, after which they are primarily interested in finding an oviposition site (Takken et al. 2001). As the primary means by which insects detect food sources, the olfactory system is a potent mediator of state-dependent valence changes to odor signals (Beshel and Zhong 2013; Bracker et al. 2013; Root et al. 2011).
Starvation
It has been demonstrated that starved fruit flies exhibit a reduced avoidance of CO2 when it is presented along with vinegar as a mimic of food odor (Bracker et al. 2013). Interestingly, this reduction in aversion was not generalizable to another aversive odor, 3-octanol, suggesting the CO2 detection was processed differently. Electrophysiological recordings revealed that the CO2-detecting ORNs did not function differently upon starvation, meaning that the processing difference may occur downstream of the primary sensory neurons. Indeed, blocking the output of the a′/B′ neurons of the mushroom body abolished CO2 aversion in starved, but not fed, flies. This indicates that the a′/B′ neurons of the mushroom body are involved in context-dependent odor processing in addition to olfactory learning. A combination of genetic and imaging techniques led to the discovery that a bilateral ventral projection neuron was required for the state-dependent CO2 avoidance: blocking its output abolished CO2 aversion in starved, but not fed, flies. However, in another study it was argued that this bilateral PN, which the authors referred to as PNv-1, was dependent on CO2 concentration, as it responded to lower concentrations than other PNs of the V-glomerulus (Lin et al. 2013). While not directly contradictory, this finding warrants a more careful investigation into the purposes of these differential-processing pathways in the brains of insects that could influence behaviors. A follow-up study found that dopamine and specific projection neuron circuits were involved in context-dependent behavior differences (Bracker et al. 2013; Siju et al. 2014). Blocking dopaminergic signaling caused starved flies to be as repelled by CO2 as fed flies, while artificially activating dopaminergic signaling caused fed flies to have a similarly reduced aversion as starved flies. Calcium imaging observations in the mushroom body were consistent with the behavior, as dopamine application reduced responses of a′/B′ neuron to CO2.
Another example of starvation increasing tolerance for aversive odors is the role of tachykinin (DTK) in suppressing the activity of the DM5 glomerulus in D. melanogaster (Ko et al. 2015). Knocking down DTK reduced attraction to medium concentrations of apple cider vinegar in starved flies, but not fed flies. Two-photon microscopy revealed that the aversive DM5 glomerulus, which is required for aversion to high concentrations of vinegar (Semmelhack and Wang 2009), became reduced in sensitivity and required higher concentrations of vinegar to become active in starved flies than in fed flies. This modulation co-occurred with the effect of starvation-induced increases of short neuropeptide F (sNPF) signaling in the innately attractive DM1 glomerulus (Ko et al. 2015; Root et al. 2011). This suggests a dual mechanism for starvation-dependent reduction in aversion.
If the physiological state of an insect can modulate attractiveness of odors, can it cause a complete valence reversal? Evidence from the haematophagous assassin bug Rhodnius prolixus indicates that such valence reversals do occur. It was found that during a window of a few days immediately after feeding, the insects would show no behavioral response to CO2, an odorant to which they were attracted when seeking hosts (Bodin et al. 2009). This period was followed by another period also lasting a few days in which the insects would actively avoid CO2. This effect was observed in both larvae and adult females, and is not attributable to differences in amount of ambulation between the different periods post-feeding. Although the exact mechanisms of this valence reversal are not known, it seems that components in the blood of the host are necessary, as a saline meal could not recapitulate the effect (Bodin et al. 2009).
SUMMARY AND FUTURE DIRECTIONS
In conclusion, the olfactory system continues to provide a fascinating template to understand behavior and its modulation using odorants. As complex as it may be to unravel, we have discussed a few basic mechanisms of odor detection that can be targeted for odor-mediated behavior disruption (Fig. 2). While all these approaches have promise, the ability to use chemical informatics to rapidly screen large numbers of compounds may also prove very effective in identifying and developing novel repellents. Prolonged activators for olfactory neurons may also have considerable promise in disrupting behaviors by masking detection of attractive cues from the host. However, the most effective repellents will likely be discovered by identification of the dedicated aversive receptors in insect species. A better understanding of basic odor detection and processing mechanisms along with advances in computational techniques will further accelerate efforts to identify odorants that can repel or mask attraction effectively in low doses and in an affordable and environmentally safe manner.
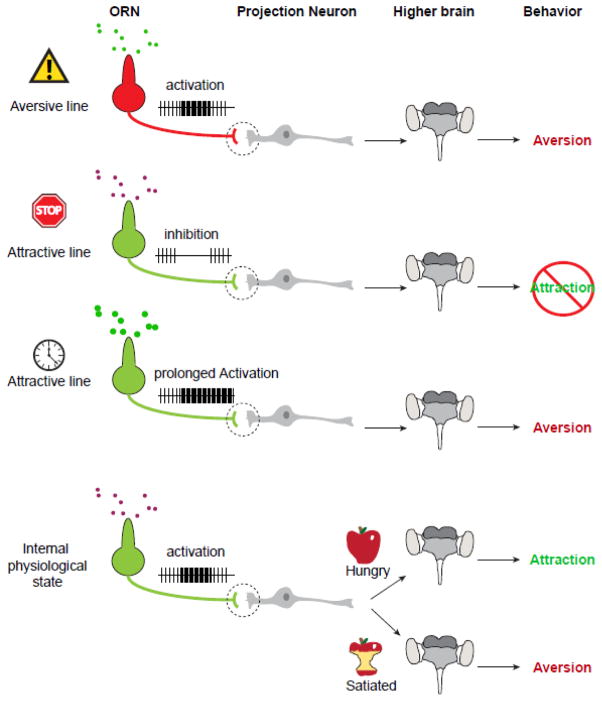
Figure 2. Olfactory mechanisms that can reduce attraction of insects to hosts.
Activation of labeled-line aversive circuits will lead to innate aversive behavior. Some odorants can inhibit ORNs mediating attractive behavior and therefore “mask” the target odor source. Other compounds can act as prolonged activators of ORNs, meaning that they increase the baseline neural firing rate for extended periods of time. This elevated activity reduces the ability of the neuron to detect attractive odorant stimuli. The internal state of an insect can affect how it responds to attractive host-odors. Starved insects will often process the same olfactory signal differently than satiated insects, leading to differences in behavior. The mechanisms underlying this processing can potentially be used to discover ways to reduce host-seeking behavior in insect vectors.
Acknowledgments
We thank Christine Pham for helpful suggestions. We acknowledge funding support from the National Institutes of Health (R01AI087785, R01DC014092). A. Ray is founder of Sensorygen Inc.
References
- Abd-Ella A, Stankiewicz M, Mikulska K, Nowak W, Pennetier C, Goulu M, Fruchart-Gaillard C, Licznar P, Apaire-Marchais V, List O, Corbel V. The repellent DEET potentiates carbamate effects via insect muscarinic receptor interactions: An alternative strategy to control insect vector-borne diseases. PLoS ONE. 2015;10:e0126406. doi: 10.1371/journal.pone.0126406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JS, Wilson R. Behavior reveals selective summation and max pooling among olfactory processing channels. Neuron. 2016;91:425–438. doi: 10.1016/j.neuron.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmann D, Richardt A, Freyberger R, Nuwal N, Schwarzel M, Fiala A, Stortkuhl KF. Optogenetically induced olfactory stimulation in Drosophila larvae reveals the neuronal basis of odor-aversion behavior. Front Behav Neurosci. 2010;4:27. doi: 10.3389/fnbeh.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshel J, Zhong Y. Graded encoding of food odor value in the Drosophila brain. J Neurosci. 2013;33:15693–15704. doi: 10.1523/JNEUROSCI.2605-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter JC, Atallah J, Krupp JJ, Millar JG, Levine JD. Specialized cells tag sexual and species identity in Drosophila melanogaster. Nature. 2009;461:987–991. doi: 10.1038/nature08495. [DOI] [PubMed] [Google Scholar]
- Bodin A, Vinauger C, Lazzari CR. Behavioural and physiological state dependency of host seeking in the blood-sucking insect Rhodnius prolixus. J Exp Biol. 2009;212:2386–2393. doi: 10.1242/jeb.030668. [DOI] [PubMed] [Google Scholar]
- Bohbot JD, Dickens JC. Insect repellents: Modulators of mosquito odorant receptor activity. PLoS One. 2010;5:e12138. doi: 10.1371/journal.pone.0012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohbot JD, Dickens JC. Odorant receptor modulation: Ternary paradigm for mode of action of insect repellents. Neuropharmacology. 2012;62:2086–2095. doi: 10.1016/j.neuropharm.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Boyle SM, Guda T, Pham CK, Tharadra SK, Dahanukar A, Ray A. Natural DEET substitutes that are strong olfactory repellents of mosquitoes and flies. 2016a doi: 10.1101/060178. bioRxiv. [DOI] [Google Scholar]
- Boyle SM, McInally S, Tharadra S, Ray A. Short-term memory trace mediated by termination kinetics of olfactory receptor. Sci Rep. 2016b;6:19863. doi: 10.1038/srep19863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracker LB, et al. Essential role of the mushroom body in context-dependent CO2 avoidance in Drosophila. Curr Biol. 2013;23:1228–1234. doi: 10.1016/j.cub.2013.05.029. [DOI] [PubMed] [Google Scholar]
- Brieger G, Butterworth FM. Drosophila melanogaster: Identity of male lipid in reproductive system. Science. 1970;167:1262. doi: 10.1126/science.167.3922.1262. [DOI] [PubMed] [Google Scholar]
- Butterworth FM. Lipids of Drosophila: A newly detected lipid in the male. Science. 1969;163:1356–1357. doi: 10.1126/science.163.3873.1356. [DOI] [PubMed] [Google Scholar]
- Cachero S, Ostrovsky AD, Yu JY, Dickson BJ, Jefferis GS. Sexual dimorphism in the fly brain. Curr Biol. 2010;20:1589–1601. doi: 10.1016/j.cub.2010.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardé RT, Gibson G. Long-distance orientation of mosquitoes to host odours and other host-related cues. In: Takken W, Knols BGJ, editors. Ecology of vector-borne diseases. Vol. 2. Wageningen Academic Publishers; Wageningen: 2010. pp. 115–141. [Google Scholar]
- Charlwood D. Did Herodotus describe the first airborne use of mosquito repellents? Trends Parasitol. 2003;19:555–556. doi: 10.1016/j.pt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Clyne P, Warr C, Carlson J. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR. A novel family of divergent seven-transmembrane proteins: Candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- Corbel V, Stankiewicz M, Pennetier C, Fournier D, Stojan J, Girard E, Dimitrov M, Molgó J, Hougard JM, Lapied B. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent DEET. BMC Biol. 2009;7:47. doi: 10.1186/1741-7007-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Crittenden AP, Mah K. Genetics of a pheromonal difference contributing to reproductive isolation in Drosophila. Science. 1994;265:1461–1464. doi: 10.1126/science.8073292. [DOI] [PubMed] [Google Scholar]
- Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: The Drosophila maxillary palp. J Neurosci. 1999;19:4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. Orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 2013;498:487–491. doi: 10.1038/nature12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838–1842. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209:2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- Fishilevich E, Domingos AI, Asahina K, Naef F, Vosshall LB, Louis M. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Galizia CG, Sachse S, Rappert A, Menzel R. The glomerular code for odor representation is species specific in the honeybee Apis mellifera. Nature Neurosci. 1999;2:473–478. doi: 10.1038/8144. [DOI] [PubMed] [Google Scholar]
- Gao XJ, Clandinin TR, Luo L. Extremely sparse olfactory inputs are sufficient to mediate innate aversion in Drosophila. PLoS One. 2015;10:e0125986. doi: 10.1371/journal.pone.0125986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies MT. The role of carbon dioxide in host-finding by mosquitoes: A review. Bull Entomol Res. 1980;70:525–532. [Google Scholar]
- Guda T, Kain P, Sharma KR, Pham CK, Ray A. Repellent compound with larger protective zone than DEET identified through activity-screening of Ir40a neurons, does not require Or function. 2015 doi: 10.1101/017145. bioRxiv. [DOI] [Google Scholar]
- Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- James AT, Martin AJ. Gas-liquid partition chromatography: The separation and micro-estimation of ammonia and the methylamines. Biochem J. 1952;52:238–242. doi: 10.1042/bj0520238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis GS, Marin EC, Stocker RF, Luo L. Target neuron prespecification in the olfactory map of Drosophila. Nature. 2001;414:204–208. doi: 10.1038/35102574. [DOI] [PubMed] [Google Scholar]
- Jones PL, Pask GM, Rinker DC, Zwiebel LJ. Functional agonism of insect odorant receptor ion channels. Proc Natl Acad Sci USA. 2011;108:8821–8825. doi: 10.1073/pnas.1102425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PL, Pask GM, Romaine IM, Taylor RW, Reid PR, Waterson AG, Sulikowski GA, Zwiebel LJ. Allosteric antagonism of insect odorant receptor ion channels. PLoS One. 2012;7:e30304. doi: 10.1371/journal.pone.0030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- Katritzky AR, Wang Z, Slavov S, Dobchev DA, Hall CD, Tsikolia M, Bernier UR, Elejalde NM, Clark GG, Linthicum KJ. Novel carboxamides as potential mosquito repellents. J Med Entomol. 2010;47:924–938. doi: 10.1603/me09284. [DOI] [PubMed] [Google Scholar]
- Katritzky AR, Wang Z, Slavov S, Tsikolia M, Dobchev D, Akhmedov NG, Hall CD, Bernier UR, Clark GG, Linthicum KJ. Synthesis and bioassay of improved mosquito repellents predicted from chemical structure. Proc Natl Acad Sci USA. 2008;105:7359–7364. doi: 10.1073/pnas.0800571105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko KI, Root CM, Lindsay SA, Zaninovich OA, Shepherd AK, Wasserman SA, Kim SM, Wang JW. Starvation promotes concerted modulation of appetitive olfactory behavior via parallel neuromodulatory circuits. Elife. 2015;4:e08298. doi: 10.7554/eLife.08298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl J, Ostrovsky AD, Frechter S, Jefferis GS. A bidirectional circuit switch reroutes pheromone signals in male and female brains. Cell. 2013;155:1610–1623. doi: 10.1016/j.cell.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreher SA, Kwon JY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- Kwon Y, Kim SH, Ronderos DS, Lee Y, Akitake B, Woodward OM, Guggino WB, Smith DP, Montell C. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr Biol. 2010;20:1672–1678. doi: 10.1016/j.cub.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legeay S, Clere N, Hilairet G, Do QT, Bernard P, Quignard JF, Apaire-Marchais V, Lapied B, Faure S. The insect repellent N,N-diethyl-m-toluamide (DEET) induces angiogenesis via allosteric modulation of the M3 muscarinic receptor in endothelial cells. Sci Rep. 2016;6:28546. doi: 10.1038/srep28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Chu LA, Fu TF, Dickson BJ, Chiang AS. Parallel neural pathways mediate CO2 avoidance responses in Drosophila. Science. 2013;340:1338–1341. doi: 10.1126/science.1236693. [DOI] [PubMed] [Google Scholar]
- Lu T, Qiu YT, Wang G, Kwon JY, Rutzler M, Kwon HW, Pitts RJ, van Loon JJ, Takken W, Carlson JR, Zwiebel LJ. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli C, Carlson JR, Emonet T. Intensity invariant dynamics and odor-specific latencies in olfactory receptor neuron response. J Neurosci. 2013;33:6285–6297. doi: 10.1523/JNEUROSCI.0426-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Martelli C, Kelley-Swift E, Brusalis C, Gershow M, Samuel AD, Emonet T, Carlson JR. Functional diversity among sensory receptors in a Drosophila olfactory circuit. Proc Natl Acad Sci USA. 2013;110:E2134–2143. doi: 10.1073/pnas.1306976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccabe ET, Barthel WF, Gertler SI, Hall SA. Insect repellents. 3. N,N-Diethylamides. J Org Chem. 1954;19:493–498. [Google Scholar]
- Ng M, Roorda RD, Lima SQ, Zemelman BV, Morcillo P, Miesenbock G. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Oliferenko PV, Oliferenko AA, Poda GI, Osolodkin DI, Pillai GG, Bernier UR, Tsikolia M, Agramonte NM, Clark GG, Linthicum KJ, Katritzky AR. Promising Aedes aegypti repellent chemotypes identified through integrated QSAR, virtual screening, synthesis, and bioassay. PLoS One. 2013;8:e64547. doi: 10.1371/journal.pone.0064547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pask GM, Bobkov YV, Corey EA, Ache BW, Zwiebel LJ. Blockade of insect odorant receptor currents by amiloride derivatives. Chem Senses. 2013;38:221–229. doi: 10.1093/chemse/bjs100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino M, Steinbach N, Stensmyr MC, Hansson BS, Vosshall LB. A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature. 2011;478:511–514. doi: 10.1038/nature10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham CK, Ray A. Conservation of olfactory avoidance in Drosophila species and identification of repellents for Drosophila suzukii. Sci Reports. 2015;5:11527. doi: 10.1038/srep11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers A, Magnenat E, Marin EC, Gendre N, Jefferis GS, Luo L, Stocker RF. Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr Biol. 2005;15:982–992. doi: 10.1016/j.cub.2005.04.032. [DOI] [PubMed] [Google Scholar]
- Ray A. Reception of odors and repellents in mosquitoes. Curr Opin Neurobiol. 2015;34:158–164. doi: 10.1016/j.conb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 2010;20:1000–1005. doi: 10.1016/j.cub.2010.03.061. [DOI] [PubMed] [Google Scholar]
- Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta V, Datta SR, Vasconcelos ML, Freeland J, Looger LL, Axel R. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Vosshall LB, Touhara K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- Savarit F, Sureau G, Cobb M, Ferveur JF. Genetic elimination of known pheromones reveals the fundamental chemical bases of mating and isolation in Drosophila. Proc Natl Acad Sci USA. 1999;96:9015–9020. doi: 10.1073/pnas.96.16.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D, Kaissling K. Der Bau der Antenne des Seidenspinners Bombyx mori. Sensillen, cuticulare Bildungen und innerer Bau. Zool Jhb Anat. 1957;76:223–250. [Google Scholar]
- Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag S, Muller B, Steinbrecht A. Atlas of olfactory organs of Drosophila melanogaster. 1. Types, external organization, innervation and distribution of olfactory sensilla. Int J Insect Morphol Embryol. 1999;28:377–397. [Google Scholar]
- Shanbhag S, Singh K, Singh R. Fine structure and primary sensory projections of sensilla located in the sacculus of the antenna of Drosophila melanogaster. Cell Tissue Research. 1995;282:237–249. doi: 10.1007/BF00319115. [DOI] [PubMed] [Google Scholar]
- Siju KP, Bracker LB, Kadow ICG. Neural mechanisms of context-dependent processing of CO2 avoidance behavior in fruit flies. Fly. 2014;8:68–74. doi: 10.4161/fly.28000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensmyr MC, Dweck HK, Farhan A, Ibba I, Strutz A, Mukunda L, Linz J, Grabe V, Steck K, Lavista-Llanos S, Wicher D. A conserved dedicated olfactory circuit for detecting harmful microbes in Drosophila. Cell. 2012;151:1345–1357. doi: 10.1016/j.cell.2012.09.046. [DOI] [PubMed] [Google Scholar]
- Strutz A, Soelter J, Baschwitz A, Farhan A, Grabe V, Rybak J, Knaden M, Schmuker M, Hansson BS, Sachse S. Decoding odor quality and intensity in the Drosophila brain. Elife. 2014;3:e04147. doi: 10.7554/eLife.04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CY, Martelli C, Emonet T, Carlson JR. Temporal coding of odor mixtures in an olfactory receptor neuron. Proc Natl Acad Sci USA. 2011;108:5075–5080. doi: 10.1073/pnas.1100369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh GS, Ben-Tabou de Leon S, Tanimoto H, Fiala A, Benzer S, Anderson DJ. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- Suh GSB, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- Swale DR, Sun B, Tong F, Bloomquist JR. Neurotoxicity and Mode of Action of N,N-Diethyl-Meta-Toluamide (DEET) PloS One. 2014;9:e103713. doi: 10.1371/journal.pone.0103713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA. 2008;105:13598–13603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W, van Loon JJ, Adam W. Inhibition of host-seeking response and olfactory responsiveness in Anopheles gambiae following blood feeding. J Insect Physiol. 2001;47:303–310. doi: 10.1016/s0022-1910(00)00107-4. [DOI] [PubMed] [Google Scholar]
- Tauxe GM, MacWilliam D, Boyle SM, Guda T, Ray A. Targeting a dual detector of skin and CO2 to modify mosquito host seeking. Cell. 2013;155:1365–1379. doi: 10.1016/j.cell.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toshima N, Tanimura T. Taste preference for amino acids is dependent on internal nutritional state in Drosophila melanogaster. J Exp Biol. 2012;215:2827–2832. doi: 10.1242/jeb.069146. [DOI] [PubMed] [Google Scholar]
- Turner SL, Li N, Guda T, Githure J, Carde RT, Ray A. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature. 2011;474:87–91. doi: 10.1038/nature10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- van Naters WVG, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas MA, Luo N, Yamaguchi A, Kapahi P. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr Biol. 2010;20:1006–1011. doi: 10.1016/j.cub.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshall L, Wong A, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell. 2003;112:271–282. doi: 10.1016/s0092-8674(03)00004-7. [DOI] [PubMed] [Google Scholar]
- Wasserman S, Salomon A, Frye MA. Drosophila tracks carbon dioxide in flight. Curr Biol. 2013;23:301–306. doi: 10.1016/j.cub.2012.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RI, Mainen ZF. Early events in olfactory processing. Ann Rev Neuroscience. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wang G, Buscariollo D, Pitts RJ, Wenger H, Zwiebel LJ. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc Natl Acad Sci USA. 2008;105:6433–6438. doi: 10.1073/pnas.0801007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Choo YM, De La Rosa A, Leal WS. Mosquito odorant receptor for DEET and methyl jasmonate. Proc Natl Acad Sci USA. 2014;111:16592–16597. doi: 10.1073/pnas.1417244111. [DOI] [PMC free article] [PubMed] [Google Scholar]