Abstract
The stiffness of myocardial tissue changes significantly at birth and during neonatal development, concurrent with significant changes in contractile and electrical maturation of cardiomyocytes. Previous studies by our group have shown that cardiomyocytes generate maximum contractile force when cultured on a substrate with a stiffness approximating native cardiac tissue. However, effects of substrate stiffness on the electrophysiology and ion currents in cardiomyocytes have not been fully characterized. In this study, neonatal rat ventricular myocytes were cultured on the surface of flat polyacrylamide hydrogels with elastic moduli ranging from 1 to 25 kPa. Using whole-cell patch clamping, action potentials and L-type calcium currents were recorded. Cardiomyocytes cultured on hydrogels with a 9 kPa elastic modulus, similar to that of native myocardium, had the longest action potential duration. Additionally, the voltage at maximum calcium flux significantly decreased in cardiomyocytes on hydrogels with an elastic modulus higher than 9 kPa, and the mean inactivation voltage decreased with increasing stiffness. Interestingly, the expression of the L-type calcium channel subunit α gene and channel localization did not change with stiffness. Substrate stiffness significantly affects action potential length and calcium flux in cultured neonatal rat cardiomyocytes in a manner that may be unrelated to calcium channel expression. These results may explain functional differences in cardiomyocytes resulting from changes in the elastic modulus of the extracellular matrix, as observed during embryonic development, in ischemic regions of the heart after myocardial infarction, and during dilated cardiomyopathy.
Introduction
The elastic properties of cardiac tissue will change significantly during neonatal development [1] and in areas of myocardial ischemia [2]. These changes in environmental mechanical properties may impact physiological functions of cardiomyocytes, such as cell contractile strength or conduction of ion currents across the plasma membrane [3].
Previous studies from our laboratory showed that the contractile force of neonatal rat ventricular myocytes (NRVM) varied with the stiffness of the underlying substrate, resulting in a maximal contractile force in cells cultured on gels with an elastic modulus of 10 kPa ([4]). On substrates both softer and stiffer than native cardiac tissue, the force decreased along with the peak of the calcium transient. Additionally, cardiomyocyte excitability decreased with increasing elastic modulus, as measured by the percentage of cells that beat when electrically stimulated.
Subsequent studies have found similar relationships between substrate stress, cardiomyocyte calcium levels, and force generation, as reviewed in [5]. Using cardiomyocytes from quail chick embryos, one study found that the spontaneous beating frequency, as well as the percentage of beating cells, increased as the stiffness of the underlying substrate decreased ([6]). Another study, focused on neonatal rat ventricular myocytes on substrates with microposts, found that calcium transients and sarcomere organization both increased as the effective elastic modulus increased from very soft (3kPa) to physiologic (15kPa) levels. In hints as to the mechanism of substrate stiffness effects, investigators have found signaling through the RhoA GTP-ase and Rho kinase (ROCK) pathways, integrin and vinculin-mediated signal transduction, and transduction involving titin or other sarcomeric structural proteins[7, 8]. As evidence of substrate stiffness affecting electrophysiology at the ion channel level, studies have found that applying strain to maturing cardiomyocytes causes deviations in potassium channel expression, resulting in shorter action potentials ([9, 10]). Similar results have also been shown in the behavior of non-cardiac muscle cells, such as immature myotubes [11]. However, studies have not yet found a relationship between the stiffness of the cardiac extracellular matrix and the action potential duration and calcium flux, both of which can influence arrhythmias in a fibrotic heart. A fuller understanding of these areas can indicate novel targets and treatments for mitigating effects of fibrosis-induced stiffening and can motivate the development of novel therapies focused on maintenance of myocardial stiffness.
We hypothesized that changes in substrate stiffness will alter the action potential dynamics of cardiomyocytes and that the differences in action potential dynamics will result from alterations in calcium flux, related to activity of the L-type calcium channel. In order to test this hypothesis, we cultured NRVM on polyacrylamide gels of varying stiffness for 1 week, and used patch clamp techniques to record action potential voltage and calcium flux. We then measured the expression of the L-type calcium channel subunit α gene using qRT-PCR and imaged channel localization using immunocytochemistry. We observed a significant effect of substrate stiffness on cardiomyocyte calcium currents, leading to changes in action potential duration, with longer action potentials on substrates with near native stiffness.
Materials and Methods
Polyacrylamide gel
In order to ease comparison with previous studies characterizing the effects of elastic modulus on the force generation and calcium storage in cardiomyocytes [4], we have designed this experiment to evaluate cardiomyocyte behavior on gels spanning the physiologic range from approximately 1 to 25 kPa elastic modulus and over one week of cardiomyocyte maturation. Polyacrylamide (PAAm) hydrogels were synthesized as in a previous study ([4]). Briefly, 80 µL of a solution of acrylamide (EMD Millipore, Billerica, MA) monomer concentration from 3% to 7%, with a 20:1 ratio of acrylamide to N,N’-methylenebisacrylamide (Amresco Inc., Solon, OH), 0.1% ammonium persulfate (BioRad Laboratories Inc., Hercules, CA) and 0.5% N,N,N′,N′-tetramethylethylenediamine (TEMED, BioRad) was allowed to polymerize for 45 minutes between a 25 mm diameter, 1 mm thick glass slide treated with 3-aminopropyltrimethoxysilane (Sigma-Aldrich, St. Louis, MO) and 0.5% glutaraldehyde (Sigma) and another 25 mm glass slide coated with Sigmacote (Sigma). The flat gels were functionalized with 0.5 mg/mL N-sulfosuccinimidyl-6-[4’-azido-2’-nitrophenylamino] hexanoate (sulfo-SANPAH, Pierce Biotechnology, Rockford, IL) in 50 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid (HEPES) buffer (Sigma) under 365 nm UV light for 5 minutes. The gels were coated with 0.5 mg/mL calf skin collagen (MP Biomedicals, LLC, Solon, OH) in 0.2% acetic acid for 2 hours at room temperature before plating with NRVM.
NRVM isolation and culture
All vertebrate animal protocols were approved by the Institutional Animal Care and Use Committees of both Rice University and Baylor College of Medicine. NRVM were isolated from the hearts of 1–3 day old Sprague-Dawley rats (Harlan Sprague Dawley Inc., Indianapolis, IN), using an isolation kit (Cellutron, Highland Park, NJ) [12]. Fibroblasts were separated from myocytes by placing the cells suspended in D3 media from the isolation kit in a tissue culture Petri dish, incubating at 37°C and 5% CO2 for 2 hours, and collecting the media and suspended cells. Cells were seeded at a density of 20,000 cells/cm2 in high serum plating media consisting of 67% Dulbecco Modified Eagle Media (Thermo Fisher Scientific Inc., Waltham, MA), 17% M199 (Thermo Fisher), 10% donor horse serum (Gemini Bio-Products, West Sacramento, CA), 5% fetal bovine serum (Gemini), and penicillin/streptomycin (Gemini). Cells were incubated at 37°C and 5% CO2. High serum plating media was used for 1 day, and the media was replaced every 2 or 3 days with maintenance media (74% DMEM, 19% M199, 5% donor horse serum, 1% fetal bovine serum, 1% Pen/Strep). Experimental results presented are from 3 separate preparations.
Mechanical Testing
The elastic moduli of the polyacrylamide hydrogels were measured using previously published methods [13]. Cylindrical gels were formed by pouring 1 mL of gel solution into 12-well suspension culture wells coated with Sigmacote. Gels were hydrated in Phosphate Buffered Saline (PBS) before mechanical testing. The thickness of the hydrated cylindrical gels ranged between 2.9 mm and 3.2 mm. A 3.175 mm radius steel bearing ball weighing 1.04 g was used to compress the gel cylinders, and compression distance was measured with a DMI 6000B fluorescent microscope (Leica Microsystems Inc., Buffalo Grove, IL). The Young’s moduli of the hydrogels were calculated by using the method in Dimitriadis, 2002, as a function of ball weight, radius, indentation height, gel height, and Poisson’s ratio of polyacrylamide gels which was assumed to be 0.485 [14].
Patch Clamping
Action potentials and L-type calcium currents (ICa,L) were recorded as per previously published techniques [15]. Whole-cell patch clamp pipettes were pulled from thin-walled borosilicate glass (Harvard Apparatus, March-Hugstetten, Germany) and had resistances between 1 and 3 MΩ when filled with pipette solution. Briefly, whole-cell patch clamp was performed using an Axopatch 700A patch-clamp amplifier and pClamp9.0 software (Molecular Device, CA, USA) with a Bessel low-pass filter (cut-off frequency: 10 kHz) and with a sampling frequency of 10 kHz at room temperature. Cardiac myocytes were spontaneously contracting, and no myosin inhibition, such as blebbistatin or BDM, was used during patch clamp recording. Patch clamp action potentials were induced with a minimum necessary current pulse.
For action potential recording, the perforated current-clamp patch clamp technique with amphotericin B (240 mg/L, Sigma, MO) was used. The pipette solution for action potential recording contained: (mmol/L): 120 K-Asparatate, 10 Na2ATP, 2 MgCl2, 10 EGTA and 10 HEPES (pH 7.35 adjusted with KOH). The bath solution was normal Tyrode’s solution containing (mmol/L):126 NaCl, 5.4 KCl, 0.8 MgCl2, 10 glucose, and 10 HEPES, (pH 7.4 adjusted with NaOH). A holding current between −0.1 to −0.5pA was used to adjust the resting membrane potential to −80mV, as detailed in previous studies [16, 17]. Reported action potential duration (APD) values are APD50, calculated using pClamp9.0 software as the time between the action potential upstroke and repolarization to 50% of the peak membrane voltage.
In order to measure potassium currents at the depolarized voltage of 20 mV, the same pipette and bath solutions were used as for measuring action potentials with the addition of nifedipine (2 nmol/L) to block the calcium currents and atropine (200 nmol/L) to block acetylcholine-dependent potassium current. A pre-pulse from holding potential −80 mV to −40 mV for 20 msec was used to inactive sodium current, followed by a step pulse protocol to 20 mV.
For ICa,L recording, the conventional ruptured patch-clamp method was used. The pipette solution contained (mmol/L): 85 K-Asparatate, 20 TEACl, 2 MgCl2, 10 EGTA, 10 HEPES, 5 Mg-ATP, and 5 Na2-GTP (pH 7.2 adjusted with KOH). The extracellular buffer contained (mmol/L): 135 NaCl, 1 MgCl2, 1.8 CaCl2, 5.4 KCl, 10 HEPES, and 10 glucose (pH 7.35 with NaOH). Whole-cell ICa, L trace was induced by a step pulse protocol (between −40 and 80 mV for 500 ms from a holding potential of −80 mV after 40 ms prepulse at −40 mV). For voltage-dependence of peak conductance of ICa,L, conductance G (V) was calculated by the equation: G (V) = I / (Vm − Erev), where I is the peak current, Erev is the measured reversal potential, and Vm is the membrane potential. The normalized peak conductance was plotted against membrane potential. Steady-state inactivation of ICa,L was estimated by a prepulse protocol (between −70 mV and −35 V for 500 ms with interval of 5 mV) followed by a testing potential of 5 mV for 500 ms. The normalized peak currents tested at −40 mV were plotted as a function of pre-pulse potentials. Steady state activation and inactivation were fitted with the Boltzmann equation: y = [1 + exp ((Vh − Vm)/k)]−1, where y represents variables; Vh, midpoint; k, slope factor; Vm, membrane potential [15].
Polymerase Chain Reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to evaluate the expression of the Cacna1c gene, encoding the L-Type calcium channel α-1C subunit. RNA samples were collected from cells 7 or 8 days after the isolation. RNA collection and purification was performed using the RNeasy mini kit (Qiagen, Valencia, CA) with a volume of 350 µL buffer RLT. RNA purification steps were followed as provided by Qiagen. CDNA was synthesized from the RNA using the High Capacity cDNA RT kit (Life Technologies, Grand Island, NY) with volumes of 10 µL RT Master Mix and 10 µL purified RNA in water. CDNA was synthesized in a thermocycler (BioRad) for 10 minutes at 25°C, 120 minutes at 37°C, and 5 minutes at 85°C. Quantitative RT PCR was performed using TaqMan Gene Expression Assay and Master Mix reagents (Life Technologies) and a ViiA7 quantitative PCR machine (Life Technologies). The TaqMan Gene Expression Assay targeted the Cacna1c gene in rats using Gene Expression Assay Rn00709287_m1. The reaction ran for 2 minutes at 50°C, 10 minutes at 95°C, and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Expression was normalized to GAPDH using TaqMan Gene Expression Assay Rn01775763_g1. Data was analyzed using the comparative CT method with software from Applied Biosystems, with all samples normalized to GAPDH.
Statistical Analysis
Data are expressed as mean ± standard deviation. The sample numbers for each experiment are stated in their respective figures. Analyses of variance followed by a post-hoc Student’s t-test with a Dunn-Bonferroni correction for multiple comparisons were performed for all comparisons. A value of p < 0.05 was considered significant in all tests.
Results
Polyacrylamide Gel Elastic Modulus
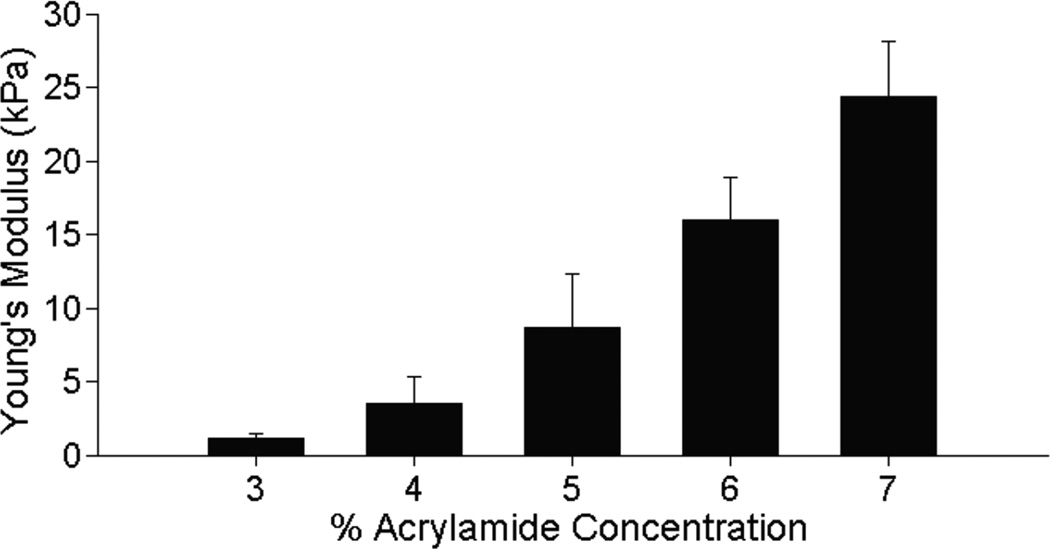
Polyacrylamide hydrogels were manufactured using varying acrylamide concentrations, and elastic moduli were calculated for acrylamide concentrations: 3% acrylamide (1.12 ± 0.358 kPa), 4% acrylamide (3.49 ± 1.89 kPa), 5% acrylamide (8.77 ± 3.58 kPa), 6% acrylamide (16.1 ± 2.91 kPa), and 7% acrylamide (24.6 ± 3.71 kPa) (Fig. 1). For simplicity, in the rest of the manuscript these gels will be referec to as 1kPa, 3kPa, 9kPa, 16kPa, and 25kPa.
Figure 1.
Elastic modulus of polyacrylamide hydrogels measured by imaging indentation depth of a steel ball bearing, and assuming Hertzian contact, shows that these hydrogels span the range of 1 to 25 kPa. n = 3 for all measurements.
Action Potential Dynamics
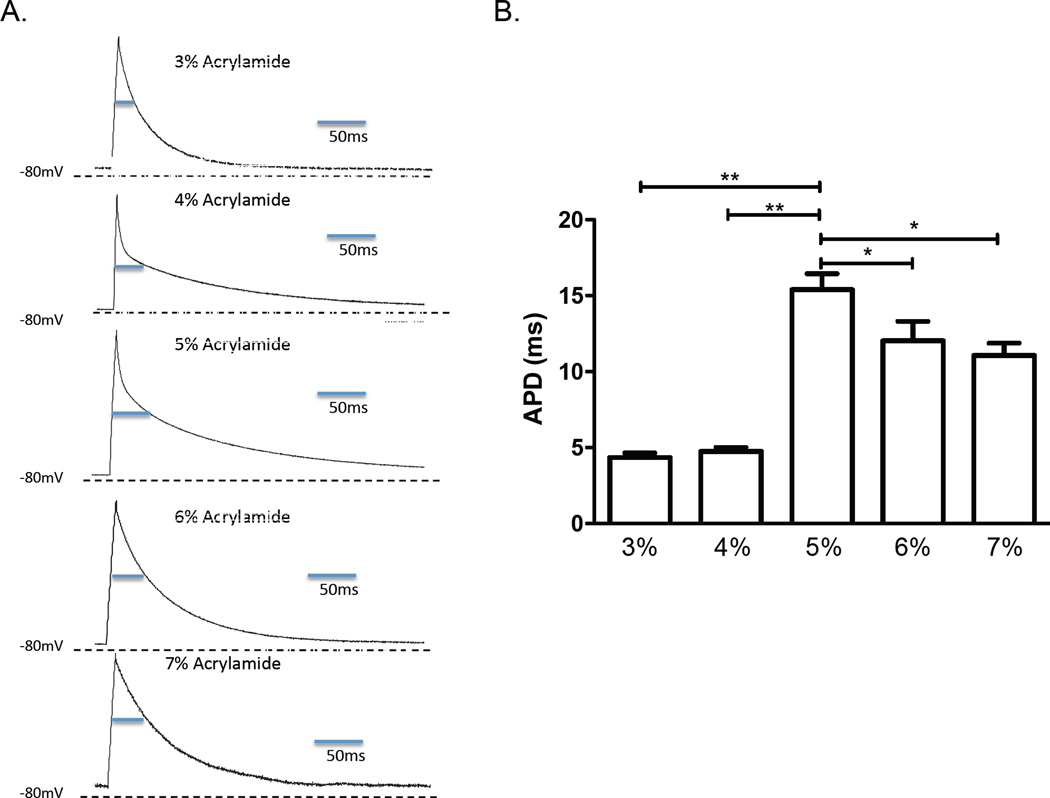
Current clamp measurements of action potentials revealed differences in the action potential duration (APD) in NRVM cultured on the hydrogels for 7 days (Fig. 2A). The longest APD in NRVM occurred on gels with 5% acrylamide (elastic modulus around 9 kPa) with APD50 of 15 ± 1.25 ms. Shorter APDs were found in cells on softer gels with 3% and 4% acrylamide with APD50 of 4 ± 0.03 ms (p < 0.01 vs. 5%) and 4.6 ± 0.25 ms (p < 0.01 vs. 5%). The NRVM on stiffer gels with 6% and 7% acrylamide showed mildly shortened APD50 of 12 ± 0.70 ms (p < 0.05 vs. 5%) and 11 ± 0.06 ms (p < 0.05 vs. 5%).(Fig. 2B).
Figure 2.
Patch clamp-measured action potentials. A. Representative action potential traces of individual neonatal rat ventricular myocytes cultured for 7 days on hydrogels of varying elastic modulus. B. Composite data of action potential durations (APDs) at the 50th percentile (lines indicate significant differences with p* < 0.05 and p** < 0.01 compared to the APD on the 5% acrylamide gel; n = 5 for all reported values).
Potassium Currents
Voltage-clamp measurements revealed no significant difference in potassium current (IK) at 20 mV testing voltage for any of the acrylamide concentrations after 7 days in culture (Fig. 3).
Figure 3.
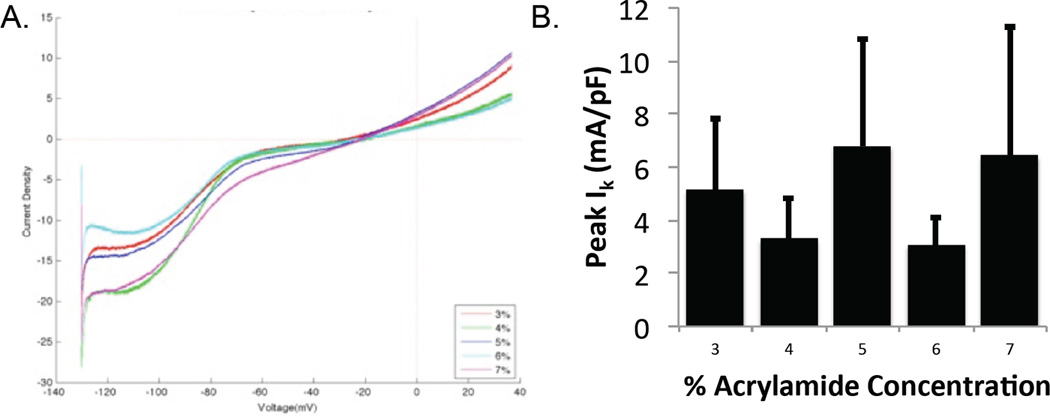
Patch voltage clamp-measured potassium currents. A. Representative potassium current traces of individual NRVM cultured for 7 days on hydrogels of varying elastic modulus; B. Peak potassium current (Ik) at holding potential of 20 mV on each hydrogel (no significant difference).
L-Type Calcium Currents
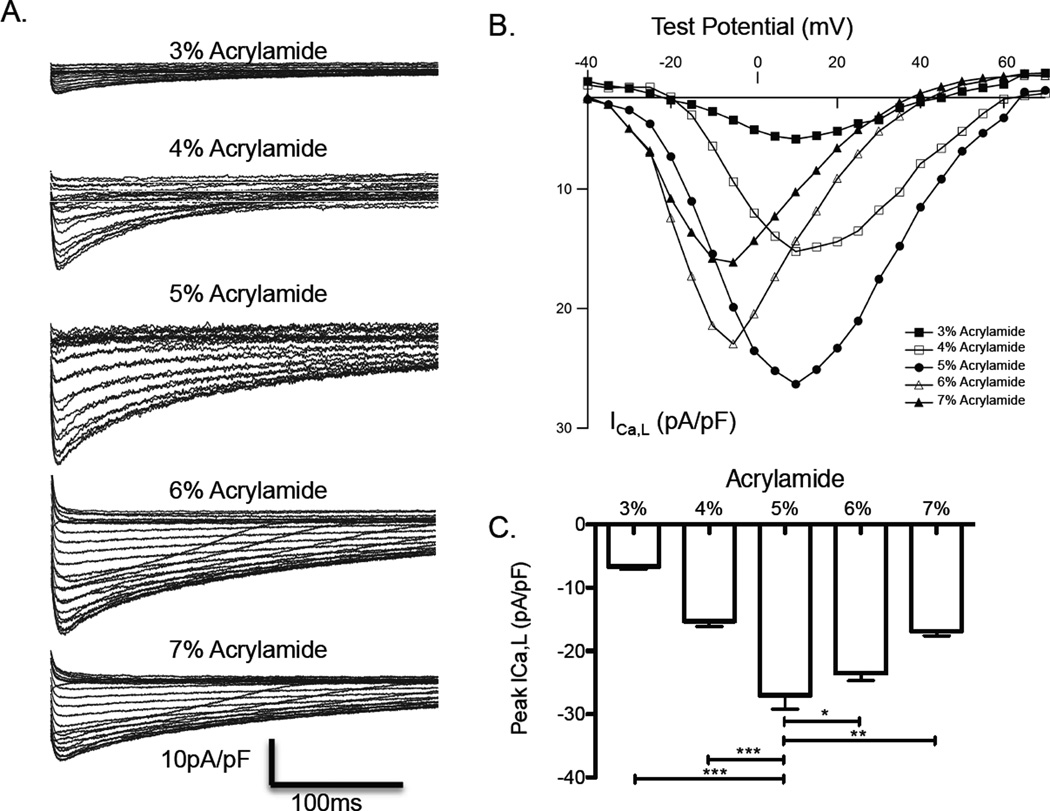
Voltage-clamp measurements revealed that NRVM cultured for 7 days on hydrogels with 5% acrylamide (10 kPa) had the greatest inward L-type calcium current (ICa,L) with a peak current density of −27.01 ± 2.15 pA/pF. The smallest ICa,L was found in NRVM on 3% acrylamide with peak current density of −6.66 ± 0.43 pA/pF. NRVM on 4%, 6% and 7% had moderately lower current density of ICa,L, −15.28 ± 0.84 pA/pF (p < 0.05 vs. 5%), −23.53 ± 1.18 pA/pF (p < 0.05 vs. 5%), and −16.84 ± 1.02 pA/pF (p < 0.05 vs. 5%) (Fig. 4 A–C), respectively.
Figure 4.
Patch voltage clamp-measured L-type calcium currents. A. Representative calcium current traces of individual NRVM cultured for 7 days on hydrogels of varying elastic modulus; B. Composite plot of voltage-mediated transmembrane L-type calcium currents (ICa,L) at holding potentials of −20 to 70mV. C. Peak calcium current on each hydrogel (p* < 0.05, p** < 0.01, p*** < 0.001). n = 4 for all reported values.
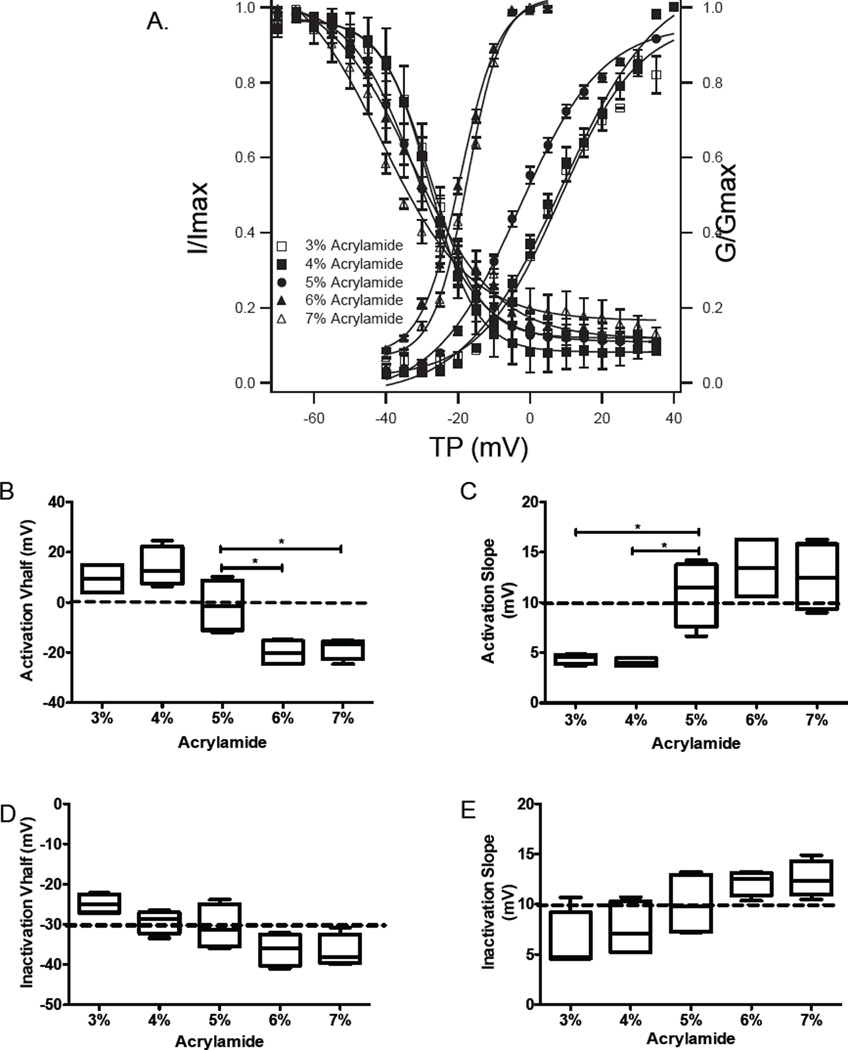
More importantly, as shown in Fig. 5A, the steady state voltage dependence of the activation curve of ICa,L was shifted negatively and became significantly steeper in NRVM on the stiffer gels, while the curve shifted positively and became flatter in NRVM on softer gels of 3% and 4%, compared to 5% polyacrylamide gels. The half activation voltage (Vh) of activation curves in NRVM on 6% and 7% were more negative (−18.25 ± 2.16 mV and −20.06 ± 2.53 mV) than its half activation voltage on the 5% gel (−1.31 ± 5.18 mV) (p<0.01). However, the Vh was positive on 3% (9.4 ± 3.19 mV) and on 4% (14.1 ± 3.95 mV) (p<0.05 vs. 5%), respectively (Fig. 5B). The slope of the activation curve in NRVM on softer gels of 4% (4.44 ± 0.26) and 3% (4.05 ± 0.21) were flatter than the slope on gels of 5% (10.9 ± 1.64), while the slopes on 6% (13.4 ± 1.64) and 7% (12.53 ± 1.71) were steeper (Fig. 5C). In addition, there was no significant difference between inactivation curves in either Vh and slope across the different concentrations of gel (Fig. 5D and E).
Figure 5.
A. Steady state activation and inactivation of L-type calcium channels represented by the transmambrane calcium current normalized to maximum current (I/Imax) and the membrane conductance normalized to maximal conductance (G/Gmax). B–E. Half-voltage and slope for activation and inactivation curves.
L-Type Calcium Channel Expression
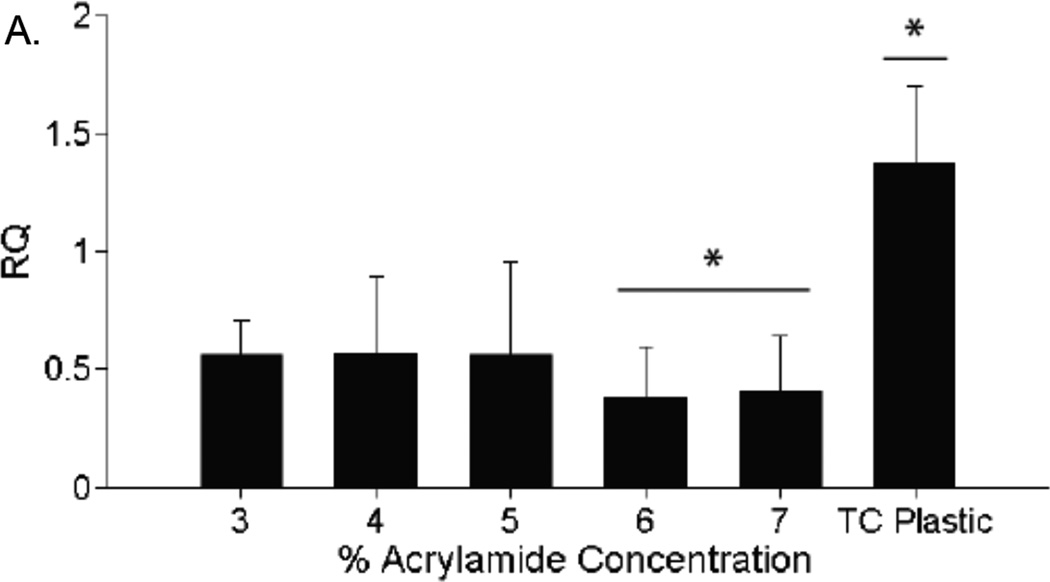
The expression of the Cacna1c gene, encoding for the L-type calcium channel, was measured using qRT-PCR. The expression of Cacna1c did not differ significantly between gel concentrations (Fig. 6).
Figure 6.
Expression of L-type calcium channels. PCR results after amplifying RNA segments transcribed from the Cacna1c gene, which codes for the L-type calcium channel. Gene expression is not significantly different between any acrylamide gel groups. Gene expression in cells on polystyrene tissue culture plastic was significantly higher than in cells on acrylamide gels (p* < 0.05). n = 3 for all expression values.
Discussion
In this study, we found that NRVM cultured on soft substrates with a Young’s modulus around 9 kPa have longer action potentials than those cultured on softer and stiffer matrices, indicating a more mature phenotype. These differences in action potential durations result, at least in part, from higher peak calcium currents on 9 kPa hydrogels. The calcium current alterations are not related to changes in expression of the L-type calcium channel subunit alpha gene, but correspond to changes in the calcium current voltage response and kinetics.
This study investigated NRVM response on PAAm hydrogels ranging in elastic modulus from 1.1 to 24.6 kPa, with formulations similar to those in the seminal 1997 manuscript by Pelham & Wang demonstrating cell response to substrate stiffness on a 2-dimensional hydrogel [18]. Note that this study used a constant ratio of acrylamide to bisacrylamide crosslinker, varying both to achieve desired elastic properties, as opposed to the higher constant acrylamide concentration (10%) and lower, varying bisacrylamide concentrations used by Pelham & Wang, though elastic modulus was still in the same order of magnitude range. Other studies have demonstrated that a constant crosslinker ratio will preserve a large range of elastic properties of PAAm hydrogels [19]. Previous studies have also investigated the micro-scale structure and roughness of PAAm hydrogels using scanning electron microscopy and have found that structures are significantly altered in the bulk [20], though, when manufactured between coverslips, as in this study, and covalently coated with sulfo-SANPAH and collagen, the surface of the hydrogels had equivalent smoothness and collagen coating density independent of crosslinker density and stiffness [21]. Additionally, studies using fluorescent attachment proteins have demonstrated that protein attachment density is independent of hydrogel stiffness in these PAAm systems [11, 22, 23].
NRVM cultured for 7 days on PAAm hydrogels with an elastic modulus near 9 kPa, near that of native myocardium, had significantly longer action potential durations than cardiomyocytes on softer (< 5kPa) or stiffer (> 15 kPa) gels. Cardiomyocytes on 9 kPa hydrogels also had higher maximum calcium currents, which we quantified using peak current density. This result is consistent with the findings of our previous study, which found that NRVM on hydrogels around 10 kPa stiffness have larger intracellular free calcium transients compared to NRVM on softer and stiffer gels (1). Additionally, cardiomyocytes on gels stiffer than 9 kPa both activated and inactivated L-type calcium channels at significantly lower voltages than cells on softer gels. Because the L-type calcium current is primarily responsible for action potential elongation in cardiomyocytes, a lower inactivation voltage would be expected to cause earlier inactivation of calcium current and result in the observed shorter action potentials.
Interestingly, the changes in calcium current are not reflected by changes in expression of the L-type calcium channel subunit α. All cells cultured upon acrylamide gels had lower expression levels of the Cacna1c gene than NRVM cultured upon tissue culture plastic, and the expression did not change with respect to gel stiffness.
An effect of extracellular matrix stiffness on the amplitude or force of cardiac contraction would be expected from classical Frank-Starling (length-force) and Hill (velocity-force) effects. Various studies have identified higher contraction amplitude with lower total cardiac force generation on soft substrates [4, 24]. The importance of calcium in the cardiomyocyte response to substrate stiffness, with lower calcium transients in environments softer than native myocardium, has been previously shown by studies from our laboratory and others [4, 8], though a specific link to membrane calcium flux had not previously been quantified. This is the first study to identify a link between the behavior of membrane calcium current, calcium channel activation voltage, and action potential duration mediated by substrate stiffness in cultured cardiomyocytes.
A previous study from our laboratory (6) found a general trend of increased cell area with increasing stiffness, and also that cells were more elongated when cultured on gels with a Young’s modulus of 10 kPa than on both stiffer and softer gels. This could indicate more mature myocytes on the substrates near 10 kPa, which may result in the electrophysiological observations. Cell size and cell elongation have been shown to have significant effects on action potential propagation in multi-cell networks, which could indicate significant tissue-based electrophysiological effects of these observed phenomena, as reviewed in [7]
These findings likely have significance both in development and in myocardial ischemia and other fibrotic conditions in the heart. Previous studies from our group have identified a significant stiffening of cardiac tissue in a mouse model at birth [1], and other studies have quantified this continued stiffening during prenatal development, coinciding with an increase in cardiac force [3, 8]. Further stiffening, to levels that have been shown here to decrease calcium current density, occurs in areas of myocardial infarction [1, 2], and this electrophysiological effect could lead to decreased function in infarcted areas.
The mechanism leading to the change in calcium channel activity and activation is still unknown. However, studies have previously found that membrane strain during cardiomyocyte contraction leads to changes in current density, activation, and inactivation of calcium channels (17,18). In addition, other studies have identified that cardiomyocyte strain modulates the expression levels of potassium channel proteins (4). The sensing mechanism and signal transduction involved in the cardiomyocyte response to substrate stiffness likely involves various cytoskeletal and associated proteins that can link the cytoskeletal structure to external forces and resistance. Studies have shown effects mediated by extracellular matrix proteins that can be deformed by mechanical stress, transmembrane protein receptors, large flexible intracellular membrane-associated proteins such as talin and filamin, cytoskeletal filaments, and motor proteins, as reviewed in [25]. Specifically, studies have found significant remodeling of focal adhesions on soft substrates that are likely involved in cell signaling [26] and investigations into the trans-sarcomeric structural protein titin show its flexibility and interactions in the signal transduction of sarcomeric forces [27]. However, the effect of cardiomyocyte strain on calcium channel expression has not yet been identified. Further studies are needed to determine whether changes in the localization of calcium channels or effects of membrane or cytoskeletal strain on calcium channels are responsible for the changes in action potential duration and calcium flux observed in this study. Additionally, this study found that calcium current inactivation occurred at a lower voltage in NRVM cultured on the stiffest substrate compared to those on the softest substrate. Based on the hypothesis that lower calcium inactivation voltage leads to shorter action potentials, one would expect shorter action potentials on the stiffest gels. However, we observed APD on the stiffest gels to be around twice as long as on the softest gels, suggesting greater complexity to the control of action potential duration.
An additional mechanism leading to the electrophysical changes observed could involve a stiffening or softening of the internal NRVM cytoskeleton. Studies have found that an increase in substrate stiffness will lead to increased actin polymerization and crosslinking, resulting in an increase in cell stiffness, in 2D cultured fibroblasts [28] and 2D cultured endothelial cells [29]. Though investigators have published methods of measuring the complex spatial and dynamic elasticity of cardiomyocytes [30], and have shown changes in cardiomyocyte stiffness when attached to various extracellular matrix molecules [31], we can find no study that has examined changes in cardiomyocyte stiffness with changes in substrate stiffness. We suggest this as a future follow-up investigation to this current study.
One recognized limitation of this study is the inability to directly measure the elastic modulus of thin, coated gels used in cell culture with the ball indentation method used here. Previous studies have also used bulk measurement of larger uncoated hydrogels to estimate elastic modulus [4, 18, 23], and results are comparable with other studies. Additionally, The exact elastic modulus leading to the observed differences in action potential dynamics and calcium flus is likely a function of cell attachment and, when extrapolated to in vivo tissues, is likely different for that 3-dimensional and anisotropic environment. A complete future study that can directly relate effects observed in this study to physiologic function should examine exact local and anisotropic modulus of native and engineered cellular environments.
In summary, we observed a significant effect of substrate stiffness on cardiomyocyte calcium currents, leading to changes in action potential duration, with longer action potentials on substrates with near native stiffness. This could relate to in vivo differences both during development and in infarcted areas of the myocardium and further studies should investigate contribution of this electrophysiological effect of substrate stiffness on cardiac regulation and arrhythmogenicity
Acknowledgments
Funding for this research was provided by the NIH/NHLBI (1R21HL110330-01 to JGJ) and by Texas Children’s Hospital.
Bibliography
- 1.Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. J Biomech. 2010;43:93–98. doi: 10.1016/j.jbiomech.2009.09.014. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006;290:H2196–H2203. doi: 10.1152/ajpheart.01017.2005. 2006. [DOI] [PubMed] [Google Scholar]
- 3.Young JL, Engler AJ. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacot JG, McCulloch AD, Omens JH. Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J. 2008;95:3479–3487. doi: 10.1529/biophysj.107.124545. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tallawi M, Rai R, Boccaccini AR, Aifantis KE. Effect of substrate mechanics on cardiomyocyte maturation and growth. Tissue Eng Part B Rev. 2015;21:157–165. doi: 10.1089/ten.teb.2014.0383. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spach MS, Heidlage JF, Barr RC, Dolber PC. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm. 2004;1:500–515. doi: 10.1016/j.hrthm.2004.06.010. 2004. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez AG, Han SJ, Regnier M, Sniadecki NJ. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys J. 2011;101:2455–24 64. doi: 10.1016/j.bpj.2011.09.057. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saygili E, Rana OR, Saygili E, Reuter H, Frank K, Schwinger RH, Muller-Ehmsen J, Zobel C. Losartan prevents stretch-induced electrical remodeling in cultured atrial neonatal myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H2898–H2905. doi: 10.1152/ajpheart.00546.2006. 2007. [DOI] [PubMed] [Google Scholar]
- 10.Rana OR, Zobel C, Saygili E, Brixius K, Gramley F, Schimpf T, Mischke K, Frechen D, Knackstedt C, Schwinger RH, Schauerte P, Saygili E. A simple device to apply equibiaxial strain to cells cultured on flexible membranes. Am J Physiol Heart Circ Physiol. 2008;294:H532–H540. doi: 10.1152/ajpheart.00649.2007. 2008. [DOI] [PubMed] [Google Scholar]
- 11.Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pok S, Benavides OM, Hallal P, Jacot JG. Use of Myocardial Matrix in a Chitosan-Based Full-Thickness Heart Patch. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2013.0620. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitriadis EK, Horkay F, Maresca J, Kachar B, Chadwick RS. Determination of elastic moduli of thin layers of soft material using the atomic force microscope. Biophys J. 2002;82:2798–2810. doi: 10.1016/S0006-3495(02)75620-8. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudou T, Ohayon J, Picart C, Tracqui P. An extended relationship for the characterization of Young's modulus and Poisson's ratio of tunable polyacrylamide gels. Biorheology. 2006;43:721–728. 2006. [PubMed] [Google Scholar]
- 15.Xi Y, Wu G, Ai T, Cheng N, Kalisnik JM, Sun J, Abbasi S, Yang D, Fan C, Yuan X, Wang S, Elayda M, Gregoric ID, Kantharia BK, Lin SF, Cheng J. Ionic mechanisms underlying the effects of vasoactive intestinal polypeptide on canine atrial myocardium. Circ Arrhythm Electrophysiol. 2013;6:976–983. doi: 10.1161/CIRCEP.113.000518. 2013. [DOI] [PubMed] [Google Scholar]
- 16.Osorio N, Delmas P. Patch clamp recording from enteric neurons in situ. Nat. Protocols. 2011;6:15–27. doi: 10.1038/nprot.2010.172. 2011. [DOI] [PubMed] [Google Scholar]
- 17.Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. J Neurosci. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calvet D, Wong JY, Giasson S. Rheological monitoring of polyacrylamide gelation: Importance of cross-link density and temperature. Macromolecules. 2004;37:7762–7771. 2004. [Google Scholar]
- 20.Peyton SR, Kim PD, Ghajar CM, Seliktar D, Putnam AJ. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials. 2008;29:2597–2607. doi: 10.1016/j.biomaterials.2008.02.005. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–1160. doi: 10.1002/bit.22647. 2010. [DOI] [PubMed] [Google Scholar]
- 22.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. 2006. [DOI] [PubMed] [Google Scholar]
- 23.Leach JB, Brown XQ, Jacot JG, Dimilla PA, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26–34. doi: 10.1088/1741-2560/4/2/003. 2007. [DOI] [PubMed] [Google Scholar]
- 24.Shapira-Schweitzer K, Seliktar D. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater. 2007;3:33–41. doi: 10.1016/j.actbio.2006.09.003. 2007. [DOI] [PubMed] [Google Scholar]
- 25.Janmey PA, Winer JP, Murray ME, Wen Q. The hard life of soft cells. Cell Motil Cytoskeleton. 2009;66:597–605. doi: 10.1002/cm.20382. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153:881–888. doi: 10.1083/jcb.153.4.881. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008;77:637–648. doi: 10.1016/j.cardiores.2007.03.029. 2008. [DOI] [PubMed] [Google Scholar]
- 28.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byfield FJ, Reen RK, Shentu TP, Levitan I, Gooch KJ. Endothelial actin and cell stiffness is modulated by substrate stiffness in 2D and 3D. J Biomech. 2009;42:1114–1119. doi: 10.1016/j.jbiomech.2009.02.012. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Azeloglu EU, Costa KD. Cross-bridge cycling gives rise to spatiotemporal heterogeneity of dynamic subcellular mechanics in cardiac myocytes probed with atomic force microscopy. Am J Physiol Heart Circ Physiol. 2010;298:H853–H860. doi: 10.1152/ajpheart.00427.2009. 2010. [DOI] [PubMed] [Google Scholar]
- 31.Deitch S, Gao BZ, Dean D. Effect of matrix on cardiomyocyte viscoelastic properties in 2D culture. Mol Cell Biomech. 2012;9:227–249. 2012. [PMC free article] [PubMed] [Google Scholar]