Abstract
The Rhesus rotavirus (RRV) induced murine model of biliary atresia (BA) is a useful tool in studying the pathogenesis of this neonatal biliary obstructive disease. In this model, the mitogen associated protein kinase pathway is involved in RRV infection of biliary epithelial cells (cholangiocytes). We hypothesized that extracellular signal-related kinase (ERK) phosphorylation is integral to calcium influx, allowing for viral replication within the cholangiocyte. Utilizing ERK and calcium inhibitors in immortalized cholangiocytes and BALB/c pups, we determined that ERK inhibition resulted in reduced viral yield and subsequent decreased symptomatology in mice. In vitro, the RRV VP6 protein induced ERK phosphorylation, leading to cellular calcium influx. Pre-treatment with an ERK inhibitor or Verapamil resulted in lower viral yields. We conclude that the pathogenesis of RRV-induced murine BA is dependent on the VP6 protein causing ERK phosphorylation and triggering calcium influx allowing replication in cholangiocytes.
Keywords: MAPK pathway, ERK, calcium, rhesus rotavirus, RRV, VP6, biliary atresia, cholangiocyte
Introduction
Biliary atresia (BA) is a progressive, obstructive cholangiopathy of the newborn that affects between 1 in 5000 to 18000 infants (Balistreri et al., 1996; Sokol et al., 2007) . This inflammatory process results in cholestasis, hepatic fibrosis, and ultimately death. Although early surgical intervention with Kasai portoenterostomy may restore biliary drainage, infants often progress to cirrhosis and end-stage liver disease (Bessho and Bezerra, 2011). Consequently, BA is the most common cause of pediatric liver transplantation, accounting for 50% of all liver transplants in the pediatric population (Salzedas-Netto et al., 2014).
While the etiology of BA remains unclear, one proposed mechanism is a perinatal viral infection triggering a host mediated immunologic response resulting in the end phenotype of clinical biliary atresia (Landing, 1974). Evidence in support of this proposed mechanism includes the detection of multiple viruses such as rotavirus group c (Riepenhoff-Talty et al., 1996), reovirus type 3 (Glaser et al., 1984), cytomegalovirus (Domiati-Saad et al., 2000) human papillomavirus (Drut et al., 1998) and Epstein-Barr virus (Fjaer et al., 2005) in livers of infants with biliary atresia. Additional supportive evidence has been obtained using the murine model of biliary atresia where infection of BALB/c pups with rhesus rotavirus (RRV) results in portal tract inflammation and extrahepatic biliary obstruction, paralleling human biliary atresia (Riepenhoff-Talty et al., 1993). In the murine model of BA, RRV has been shown to target the biliary epithelial cell (cholangiocyte) for infection, initiating the disease process (Allen et al., 2007). To elucidate the pathway RRV uses to infect the cholangiocyte, we have employed an in vitro model, utilizing immortalized cholangiocytes derived from BALB/c mice (Coots et al., 2012).
Rhesus rotavirus is an icosahedral, non-enveloped, double-stranded RNA virus with a triple-layered protein capsid. This virus consists of eleven gene segments encoding for twelve proteins. The triple-layered protein capsid is composed of viral proteins including VP6, VP4 and VP7 (Li et al., 2009). RRV infection is dependent upon a complete viral infectious cycle, including attachment to a host cell surface, viral entry into the cell, viral replication and the release of newly generated viral progeny. The entry process of RRV is a multi-step process involving sialic acid labeled glycolipids and cell surface proteins that act as receptors for the virus (Lopez and Arias, 2004, 2006). These receptors include the integrins α2β1, α4β1, αVβ3, αXβ2 (Graham et al., 2003; Guerrero et al., 2000; Hewish et al., 2000). In the cholangiocyte, we have shown that the α2β1 integrin is essential for RRV attachment and infection (Jafri et al., 2008). Once RRV enters the cell, it uncoats in a calcium-dependent manner (Zhou et al., 2009). Replication then ensues utilizing both viral and host cell machinery, creating viral progeny. The new viral particles are released from the cell in either cytolysis or by exocytosis, depending on the cell type.
The mitogen activated protein kinase (MAPK) pathway is a series of signaling networks involved in multiple cellular processes, including growth, differentiation and apoptosis (Sumbayev and Yasinska, 2006). Included in the family of MAPKs, are extracellular signal-regulated kinase (ERK 1/2), p38 and c-JUN NH2-terminal kinase (JNK (1/2). Through these proteins, the MAPK pathway helps govern cellular response to stress (Tadlock et al., 2003). These pathways are activated by various stimuli, including those which may be involved in the pathogenesis of the murine model of BA. Activation of this pathway by RRV dsRNA (Tadlock et al., 2003), viral capsid proteins (LaMonica et al., 2001), and integrin mediated processes (Sanders and Basson, 2004) has resulted in phosphorylation of downstream targets (Clarke et al., 2005; Sumbayev and Yasinska, 2006). Previous studies have shown that RRV replication in intestinal epithelial cells to be dependent on JNK 1/2 and p38 protein kinases (Holloway and Coulson, 2006). We previously demonstrated that activation of the MAPK pathway played an important role governing RRV infection of the cholangiocyte (Jafri et al., 2007). Following cholangiocyte infection in vivo and in vitro by RRV, we found an increase in phosphorylation of all three kinases (ERK 1/2, p38 and JNK 1/2). In vitro, we demonstrated this to be a dose-dependent process. Inhibition of ERK 1/2 and p38 kinases led to diminished viral replication without affecting viral binding or entry into the cell (Jafri et al., 2007).
The purpose of this study was to further determine the mechanistic basis by which ERK phosphorylation governs the murine model of BA. Using complimentary in vivo and in vitro studies, we found that the ERK pathway facilitates RRV replication in the cholangiocyte (Jafri et al., 2007) via a calcium influx effect, primarily driven by the rotavirus VP6 protein.
Methods
Cells and virus
A murine cholangiocyte cell line obtained from the laboratory of Dr. James Boyer (Yale Liver Care Center, Hartford, CT), was derived from primary cholangiocytes harvested from BALB/c mice and immortalized with SV-40 large T-cell antigen. These cells express GGT and cytokeratin-7, consistent with their biliary epithelium origin (data not shown). Cholangiocyte cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM) (Cellgro, Harndon, VA), supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Invitrogen Corporation, Carlsbad, Calif), 1% penicillin (10,000 U/mL), streptomycin (10,000μg / mL) (Invitrogen), 1% L-glutamine (Invitrogen) and 0.5% amphotericin B (250μg/mL) (Cellgro). MA104 cells (Bio Whittaker, Walkersville, MD), Hela cells (ATCC, Manassas, VA), CMT-93 cells (ATCC), IEC-6 cells, IEC-18 cells and Caco-2 cells, (generous gifts from Dr. Mitch Cohen; Cincinnati Children's Hospital, Cincinnati, OH) were also cultured as described above.
RRV stock was originally obtained from the laboratory of Dr. Harry Greenberg (Stanford University, Palo Alto, CA). This stock was maintained by passage in MA104 cells. RRV grown in MA104 cells was pelleted by ultracentrifugation and the supernatant collected after centrifugation was used as virus-depleted stock.
Experimental Model of Biliary Atresia
Breeding pairs of BALB/c mice (Envigo, Indianapolis, IN) were kept in micro-isolator cages in a virus free environment with free access to sterilized chow and water. Dams were separated approximately one week before estimated delivery. Intraperitoneal (I.P.) inoculation of pups with RRV at a dose of 1.5 × 106 FFU/gram) was performed on day of life (DOL) one. A second cohort of pups was inoculated with ERK Activation Inhibitor Peptide I or JNK Inhibitor I (CALBIOCHEM, San Diego, CA) on DOL 0, DOL 2 and DOL 4. RRV was injected at a dose of 1.5 × 106 on DOL 1. Mice were monitored every other day for weight and obstructive symptomatology, including bilirubinuria, acholic stools and jaundice. They were considered “symptomatic” if they presented two or more obstructive symptoms. Pups used for symptom analysis were monitored for 21 days. Extrahepatic bile ducts of DOL 7 mice were harvested using micro-dissection and homogenized in 0.5ml of Earle's balanced salt solution (EBSS) supplemented with Ca+2. Samples were stored at −80°C until used to determine viral titer by Fluorescent Focus forming assay (FFA).
Infection of Cells
RRV infection of cell lines was performed in 24 well culture plates containing cells seeded at 4 × 105/well and incubated at 37°C for three days. Confluent plates were washed twice with EBSS and infected with RRV at varying multiplicities of infection (MOIs). An MOI of one indicates that the amount of infectious virus is equal to the number of cells in the culture condition. Incubation was performed at 37°C on a plate rocker for one hour. The cultures were washed with EBSS and incubated with serum-free DMEM + 4μg trypsin / mL at 37°C for varying amounts of times (1 to 24 hours).
Measurement of viral titers using a focus forming assay (FFA)
Cholangiocytes were analyzed for amount of live virus at 24 hour time points according to previously established methods (Walther et al., 2013). Virus quantity was expressed as focus forming units (ffu)/ml, with each ffu representing one infectious virus.
Detection of MAPK proteins by Western blot analysis
Immortalized cholangiocytes were lysed and protein extracted in 1X Loading Buffer (50mM Tris-HCl pH 6.8, 2% SDS, 10% Glycerol, 0.05% Bromo-Blue, 0.4M 2-mercaptoethanol). Equal amounts of cell lysate per sample were loaded on a 4-20% tris-glycine gel (Invitrogen Corporation, Carlsbad, CA). Gels were transferred overnight to PVDF membranes (GE Healthcare, Pittsburg, PA) and blocked with 5% milk solution. Membranes were stained with a 1:1000 dilution of rabbit anti-mouse p-ERK 1/2 antibody and total p38 antibody as loading control (Cell Signaling Technology Inc. Danvers, MA). Densitometry was utilized to determine fold change between groups.
Assays for ERK phosphorylation in cholangiocytes under various experimental conditions: Cholangiocyte treatment with inactivated RRV
ERK phosphorylation was determined in cholangiocytes at one and eight hours post treatment with RRV that had been purified by cesium chloride centrifugation and inactivated by Psoralen with long-wave UV light (McNeal et al., 1992).
Cholangiocyte treatment with Poly I:C
ERK phosphorylation was determined in cholangiocytes at one hour after transfection using Lipofectamine (Invitrogen) and Poly I:C (double-stranded RNA analog Invitrogen) in a dose dependent manner (0 to 50ug).
Cholangiocyte treatment with double-layer particles
ERK phosphorylation was determined in cholangiocytes at one hour after treatment with different doses (0 to 10ug) of RRV double-layer particles (purified by cesium chloride centrifugation). These double-layer particles were validated by Western Blot analysis (data not shown).
Cholangiocyte treatment with VLP 2/6
ERK phosphorylation was determined in cholangiocytes at one hour after treatment with a dose response (0 to 25ug) of virus like particles (VLP) 2/6, containing VP2 and VP6 (generously gifted by Dr. Linda Saif, The Ohio State University, Columbus, Ohio).
Cholangiocyte treatment with VP6 protein
The intermediate layer of rotavirus particles consists of the VP6 protein which is highly conserved across all group A rotavirus strains (Knipe, 2013). ERK phosphorylation was determined in cholangiocytes at one hour after treatment with purified VP6 protein (generous gift from the laboratory of Dr. Richard Ward, Cincinnati Children's Medical Center, Cincinnati, Ohio). This protein was synthesized as a chimera of EDIM (Epizootic Diarrhea of Infant Mice) VP6 with maltose binding protein (VP6::MBP) (Choi et al., 1999). Since the VP6 protein was expressed as a fusion with maltose-binding protein, cholangiocytes were also treated with maltose-binding protein (Prospec Protein Specialists, East Brunswick, NJ) alone as negative control.
Cholangiocyte treatment with calcium-free media
Cholangiocytes were incubated for two hours with calcium-free media. They were then washed with the same media, prior to treatment with RRV for one hour. The presence of ERK phosphorylation was determined through Western Blot analysis as described above.
Treatment with Calcium channel and MAPK inhibitors
Cholangiocytes were pre-treated for one hour with ERK inhibitor: U0126 (ERK 1/2 inhibitor, Cell Signaling Technology), Verapamil (Ca++ inhibitor, Sigma Aldrich) or Diltiazem HCl (Ca++ inhibitor, Tocris bioscience, Minneapolis, MN) at concentrations of 20μmol/L and 1μg/ml, respectively. All the inhibitors were dissolved in dimethyl-sulfoxide (DMSO). After one hour of pre-incubation, cells were exposed to RRV at an MOI of 160, followed by overlay with media containing inhibitor for 24 hours (the approximate length of RRV replication cycle). Between each step, cells were washed with 1mL/well of serum-free media to remove any unbound viral particles. After 24 hours of culture the cells then underwent two freeze/thaw cycles and the viral content was determined using FFA. In a second set of experiments, inhibitors were used only during pre-inoculation or only in the overlay media.
Detection of intracellular calcium using Fluo-4 dye
Cholangiocytes were cultured on 8-well Ibidi micro-slide (ibiTreat plates, Ibidi, Klopferspitz, Germany). They were then pre-treated for one hour with 2.5μg of fluo-4, AM (Invitrogen), ERK inhibitor, Verapamil, Diltiazem HCl or Calcium-free media. After one hour of pre-treatment, cells underwent three washes with 200μL serum free or calcium-free media. They were then imaged every minute for three minutes using 10X objective on a Nikon Eclipse Ti; NIS elements software to obtain background fluorescence level. Cholangiocytes were then treated with MOI of 160 RRV, 25μg of VP6 protein, triple-layer particles, virus-depleted stock or 10mM Ionomycin (Fisher Scientific, Pittsburgh, PA). Wells were then imaged every minute for 30 minutes to observe calcium influx through fluorescence. Fluorescence values were reported as ΔF/F0, (ΔF= Ft – F0) where Ft is observed fluorescence at time t and F0 is fluorescence at t=0. Experiments were carried out at room temperature (21 – 23°C).
Immunohistochemistry for detection of L-type Calcium Channels in cholangiocytes
Cholangiocytes were isolated and fixed to slides using cytospin and 4% paraformaldehyde. Following two washes in 1X PBS the slides were blocked for 1 hour with 5% donkey serum + 3% bovine serum albumin in 1X PBS. Slides were then incubated overnight at 4°C with 1:100 diluted primary antibodies of rabbit anti- CaV1. 1, CaV1.2, CaV1.3, (Bioss Antibodies Woburn, MA), or CaV1.4 (Biorbyt Ltd., San Francisco, CA). Following washing, the cells were incubated with donkey anti-rabbit DyLight 488-conjugated antibody (1:500; Jackson Immunoresearch Laboratories, West Grove, PA) for 1 h at room temperature. After a final wash, the nuclei were counterstained with DAPI (Vector Laboratories US) and observed under a Nikon Eclipse TI Microscope.
Statistics
Analysis of non-continuous variables was performed using z-test. Results of continuous variables were expressed as mean +/− standard error and analyzed using ANOVA with post hoc testing where appropriate. Comparisons were made using ANOVA on ranks with Dunn's method for post hoc testing. A p < 0.05 was considered significant. Error bars on graphs represent standard error. Each experiment was repeated three times for statistical power.
Results
The in vivo effect of JNK and ERK inhibition in the murine model of BA
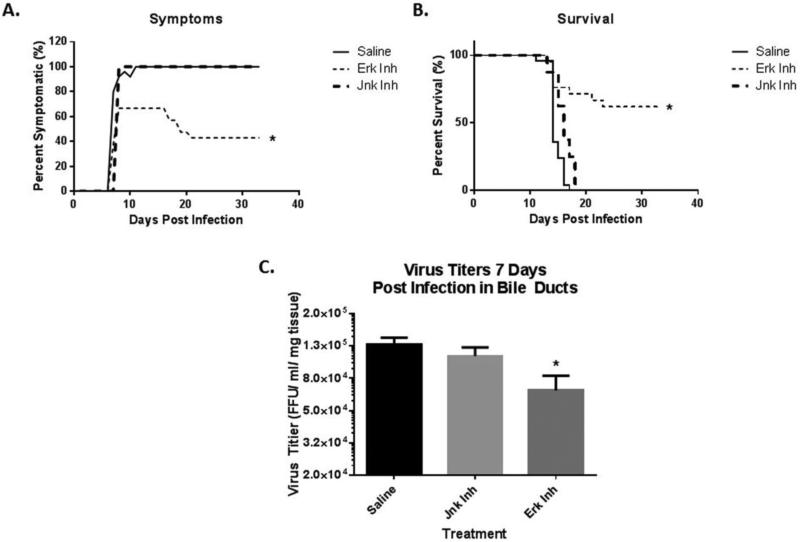
BALB/c mice inoculated with saline (n=25) or JNK inhibitor (n=8) on DOL 0 followed by RRV on DOL 1 developed 100% symptoms of biliary obstruction by DOL 7. Inhibition of the ERK pathway using ERK Activation Inhibitor Peptide I resulted in only 66.7% of mice manifesting symptoms of biliary obstruction (n=21). (Fig 1A). The mortality rate at 21 days was also significantly reduced with ERK inhibition, decreasing it from 100% in RRV infected mice pre-treated with saline or JNK inhibitor to 43% in pups pre-treated with ERK inhibitor. (Fig. 1B).
Fig. 1. Analysis of symptoms and viral titers after treatment with ERK 1/2 inhibitors.
(A) Mice were inoculated with saline, ERK 1/2 or JNK 1/2 inhibitors on DOL 0. Following this, they were injected with RRV (1.5×106 FFU/pup) on DOL 1 to initiate the murine model of biliary atresia. Inhibitor and saline were again injected on DOL 2 and DOL 4. Symptoms were observed for 35 days post-injection and revealed reduced symptoms in ERK 1/2 treated mice (66.7%) as compared to saline (100%) or JNK inhibitor (100%) pre-treatment. (B) Survival was noted for mice injected with saline, JNK inhibitor and ERK 1/2 inhibitor followed by RRV. This showed that mice pre-treated with ERK 1/2 inhibitor had an improved survival (57%) when compared to saline or JNK inhibitor (0%). (C) Virus titers were obtained from mice injected with RRV and ERK 1/2 inhibitor at 7 days post injection. This revealed significantly lower titers in those injected with ERK 1/2 inhibitor (6.68 × 104) than those who received RRV with saline control (1.28 × 105) or JNK inhibitor (1.09 × 105). (* = p<0.05)
Levels of infectious rotavirus in extra-hepatic bile ducts of RRV-injected mice treated with MAPK inhibitors
Previous studies have shown that the peak titer of RRV was detected within the extra-hepatic bile duct at DOL 7 after injection (Jafri et al., 2007). Mice pre-treated with saline and JNK inhibitor followed by injection with RRV resulted in viral titers of 1.28 ± 0.13 × 105 and 1.09 ± 0.14 × 105 respectively, in the extrahepatic bile duct. However, viral titers in the bile ducts from mice treated with ERK 1/2 inhibitor (6.68 ±1.58 × 104) were noted to be significantly lower than both no inhibitor and JNK inhibition (p<0.05) (Fig. 1C). These in vivo studies suggest that the ERK pathway affects the murine model of BA. To better understand the mechanistic basis of the ERK pathway, we studied its role in RRV infection of the cholangiocyte in vitro.
ERK phosphorylation in various cell lines
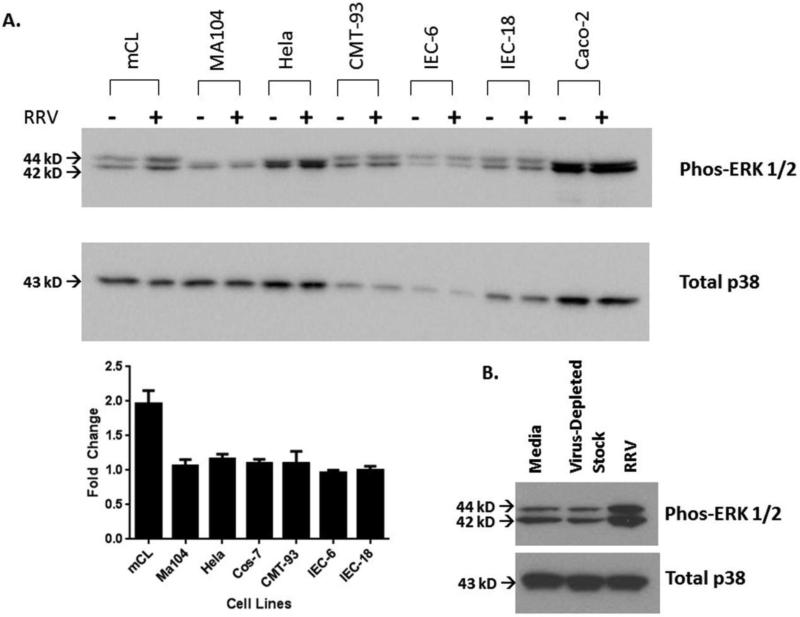
Using the previous knowledge that RRV infection in cholangiocytes results in ERK phosphorylation, we determined if the activation occurred in other cell types susceptible to RRV infection, including MA104 (Simian kidney), Hela (human cervical carcinoma), CMT-93 (murine rectal), IEC-6 (murine intestinal), IEC-18 (murine intestinal) and Caco-2 (human intestinal) cells (Fig. 2A). ERK phosphorylation after RRV infection was found to be specific to cholangiocytes as shown by a 2.0±0.2-fold increase in cholangiocytes with no changes in the other cell types. This ERK phosphorylation was also witnessed in primary cholangiocytes harvested from 2 day old pups (data not shown), demonstrating it was not a result of the immortalization process.
Fig. 2. ERK phosphorylation in various cell lines.
(A) Mouse cholangiocytes, MA104, Hela, CMT-93, IEC-6, IEC-18 and Caco-2 cells were infected with RRV at an MOI 160. Densitometry was used to determine fold change between cell lines. At one-hour post infection, western blot analysis revealed ERK phosphorylation occurring only in cholangiocytes, as can be seen by a 2.0±0.2 fold change from serum free control. Total p38 acts as the standard loading control. (B) Cholangiocytes treated with virus-depleted media for one hour demonstrated no increase in ERK 1/2 phosphorylation over media alone.
ERK phosphorylation is not due to viral lysate bioactive compounds
In order to prove that ERK phosphorylation was not the result of bioactive compounds present in the RRV viral lysate, cholangiocytes were treated with virus-depleted stock. This did not result in ERK phosphorylation, thus concluding that ERK phosphorylation is not the result of bioactive compounds found in the virus lysate (Fig. 2B).
ERK phosphorylation is not due to virus replication
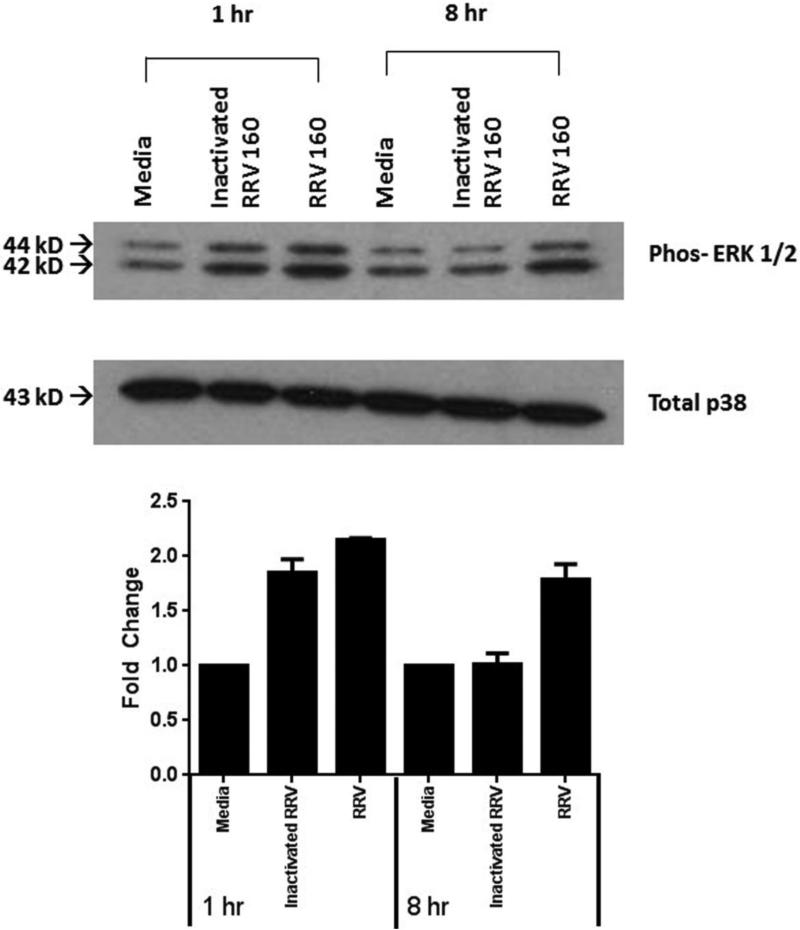
The role of viral replication on ERK phosphorylation was assessed using inactivated virus. Virus treated with Psoralen and long wave UV light is inactivated and can bind and enter a cell but cannot replicate. Inactivated virus was used to determine if viral replication caused ERK phosphorylation. One hour after virus infection, ERK phosphorylation was observed in both RRV (2.1±0.1- fold change from media control) and inactivated virus (1.8±0.1 fold change from media control). At eight hours post-infection however, ERK phosphorylation was observed only in the presence of replication-competent virus (1.8±0.1-fold change from media). This temporal pattern suggests that replication was not essential for early ERK phosphorylation, but it must occur for the late ERK phosphorylation (Fig. 3). Serum free media was utilized as control. These time points were chosen based on our previously study illustrating that ERK phosphorylation occurred in the cholangiocyte at 1 hour post infection, decreased by 4 hours, and increases again by 8 hours (Jafri et al., 2007).
Fig. 3. The role of viral replication on ERK phosphorylation.
Inactivated virus was used to determine if viral replication is necessary for ERK phosphorylation. One hour after RRV infection, ERK phosphorylation occurred with both infectious virus (2.1±0.1-fold change from control) and inactivated virus (1.8±0.1 fold change from control). At eight hours post infection however, only infectious virus was capable of phosphorylating ERK (1.8±0.1-fold change from control). This reveals that replication is not essential for early phosphorylation of ERK but must occur for later ERK phosphorylation. Total p38 was used as a loading control on all western blots.
ERK phosphorylation was not due to activation of innate immune response to double-stranded RNA
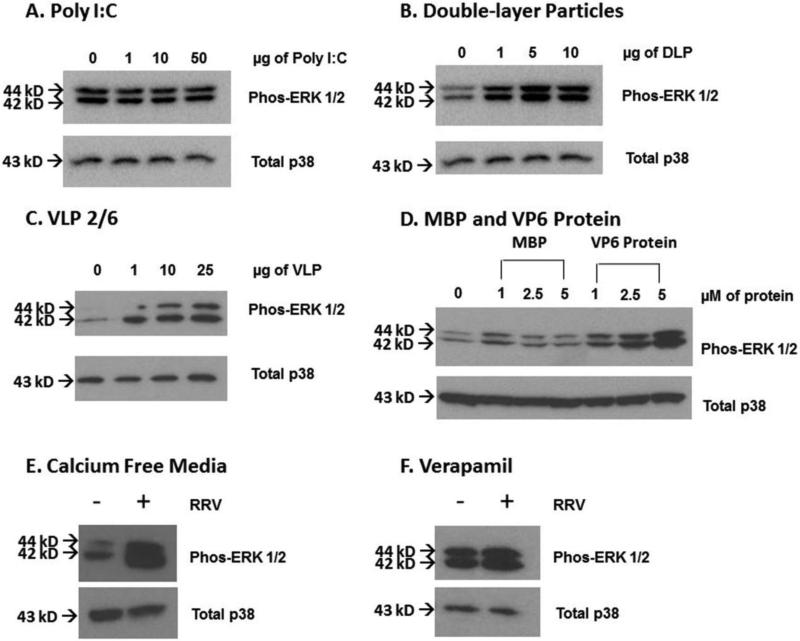
Poly I:C was transfected into cholangiocytes in a dose-dependent fashion to determine if ERK phosphorylation was the result of an immune response to double-stranded RNA (0-50μg) (Fig. 4A). Treatment with Poly I:C did not cause ERK phosphorylation, independent of dosage employed, at one hour (greatest densitometry change 1.0±0.1 fold at 50μg), thus demonstrating that ERK phosphorylation is not a consequence of host response to double-stranded RNA.
Fig. 4. Role of innate immune response and RRV capsid proteins on ERK phosphorylation.
(A) Cholangiocytes were transfected with Poly I:C, a double-stranded RNA analog, in a dose-dependent fashion (0-50 μg) to evaluate if innate immune activation plays a role in ERK phosphorylation. Poly I:C did not phosphorylate ERK at any dosage. (B) Double-layer particles at varying dosages were used to determine the role of VP4 and VP7 in ERK phosphorylation. These particles were able to cause ERK phosphorylation despite absence of VP4 and VP7. (C) Virus like particles consisting of rotavirus proteins VP2 and VP6 (VLP 2/6) were able to induce a dose dependent ERK phosphorylation. (D) VP6 protein was assessed in a similar manner. With increasing dosages (1--5μM) of VP6, a greater ERK phosphorylation was noted compared to Maltose-binding protein (MBP) control. (E) Cholangiocytes treated with calcium free media for two hours and infected with RRV in calcium free media still demonstrated ERK phosphorylation. (F) Verapamil (1μg/ml) was used to block calcium channels on cholangiocytes. Following this, the cells were treated with RRV at an MOI of 160. ERK phosphorylation was noted, despite the presence of this L-type calcium channel blocker. Total p38 was used as a loading control for all studies.
The role of RRV capsid proteins in ERK phosphorylation
Double-layer RRV particles (lacking the VP4 and VP7 cell surface proteins) were utilized at varying dosages to determine if VP4 or VP7 are responsible for ERK phosphorylation. The double layered particles were able to cause ERK phosphorylation at one hour, indicating that ERK phosphorylation was not dependent on VP4 or VP7 surface proteins (Fig. 4B). Densitometry demonstrated the greatest fold change at 10μg, resulting in a 2.2±0.3-fold change.
ERK phosphorylation with Viral-like Proteins (VLP) 2 and 6
After determining that the two outer binding proteins (VP4 and VP7) did not cause ERK phosphorylation, we sought to determine the role of the remaining capsid proteins (VP2 and VP6) in this pathway. VLP 2/6 particles were able to induce ERK phosphorylation in cholangiocytes in a dose dependent manner 1 hour after treatment (Fig. 4C). This suggested that either the VP2 or VP6 protein was responsible for ERK phosphorylation.
ERK phosphorylation is due to VP6 protein
In order to determine the specific RRV protein responsible for ERK phosphorylation, cholangiocytes were treated with purified VP6 protein. This protein was able to cause ERK phosphorylation in a dose dependent fashion, with the greatest phosphorylation observed at 5μM resulting in a 5.8±0.6-fold change over media control (Fig. 4D). Cholangiocytes treated with maltose-binding protein served as a negative control.
The role of calcium in ERK phosphorylation
The involvement of calcium in rotavirus infection has been previously established. Rotavirus infection triggers an increase in intracellular calcium, which is required for rotavirus replication and morphogenesis (Hyser et al., 2010). Our investigations have implicated VP6 in ERK phosphorylation. In order to determine if VP6-mediated ERK phosphorylation governs calcium influx or if ERK phosphorylation is the result of calcium influx, calcium-free media was utilized. In the presence of calcium-free media, ERK phosphorylation was still observed after cholangiocyte treatment with RRV (Fig. 4E). Additionally, Verapamil was used to block calcium channels on the cholangiocyte cell surface. ERK phosphorylation occurred despite the presence of Verapamil (Fig. 4F). The results of these two experiments indicate that ERK phosphorylation was not due to cellular calcium influx.
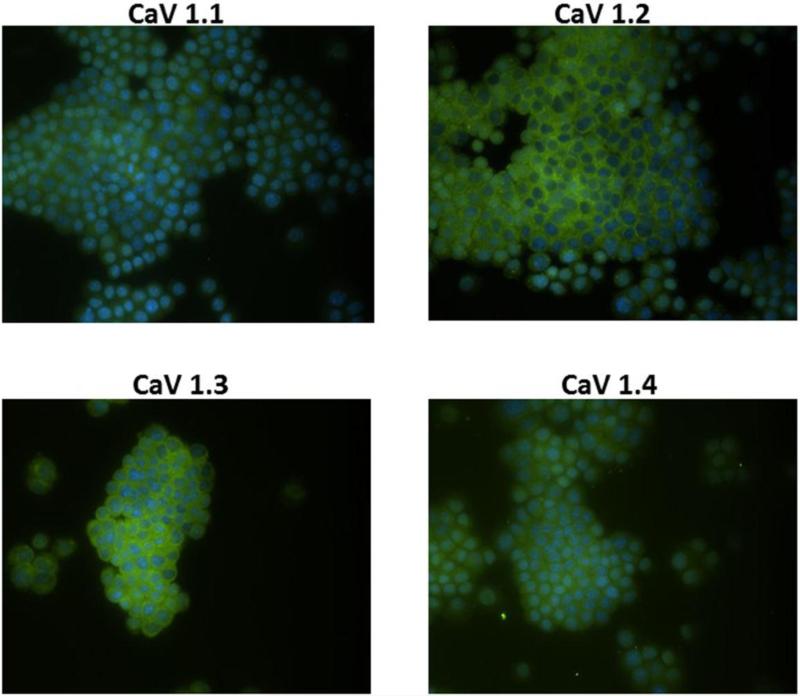
Detection of L-type Calcium Channels in cholangiocytes
To validate the use of Verapamil, a known L-type calcium channel inhibitor, immunohistochemistry was performed on the cholangiocytes to demonstrate the presence of L-type calcium channels. Cholangiocytes were stained positive for the four L-type voltage activated calcium channels (CaV1.1, CaV1.2, CaV1.3 and CaV1.4) (Fig. 5).
Fig. 5. Immunohistochemistry for L-type Calcium Channels.
Cholangiocytes were stained for different L-type calcium channels shown in green and the nucleus counterstained with DAPI shown in blue. Magnification 40x.
Calcium influx assays
Cholangiocytes were pre-loaded with Fluo-4 dye, which fluoresces in the presence of calcium, to determine if ERK regulates calcium influx in the RRV infection of cholangiocytes. As expected, cholangiocytes infected with RRV demonstrated an increase in intracellular calcium from control, as reflected by increased fluorescent activity (Fig. 6A, B, F). Treatment with ERK inhibitor (Fig. 6C, D, F), both in the presence and absence of RRV, had a decreased in fluorescent activity and thus, decreased calcium, suggesting that RRV-induced ERK phosphorylation results in calcium influx into the cell. To determine if VP6 causes calcium influx, cholangiocytes were treated with purified VP6 protein. We found that intracellular calcium influx in VP6 protein-treated cholangiocytes was similar to RRV-treated cells (Fig. 6B, E, F).
Fig. 6. Calcium influx in treated cholangiocytes.
Cells were pre-treated for one hour with 2.5μg of fluo-4 along with no inhibitor or ERK 1/2 inhibitor, followed by infection with RRV at an MOI of 160 or 25μg of VP6 protein and imaged every minute for 30 minutes to observe calcium influx through fluorescence. (A, B) Cholangiocytes treated with RRV displayed increased calcium influx, as detected by fluorescence when compared to cells treated with media alone. (C, D) Treatment of cholangiocytes with ERK 1/2 inhibitor both in the presence and absence of virus showed a reduction in calcium influx as compared to RRV alone (B). (E) Cholangiocyte treatment with VP6 protein had similar results to RRV treatment (B). (F) Change in immunofluorescence intensity over time (ΔF/F0, (ΔF= Ft – F0) is demonstrated, where Ft is observed fluorescence at time t and F0 is fluorescence at t=0) (* = p<0.05)
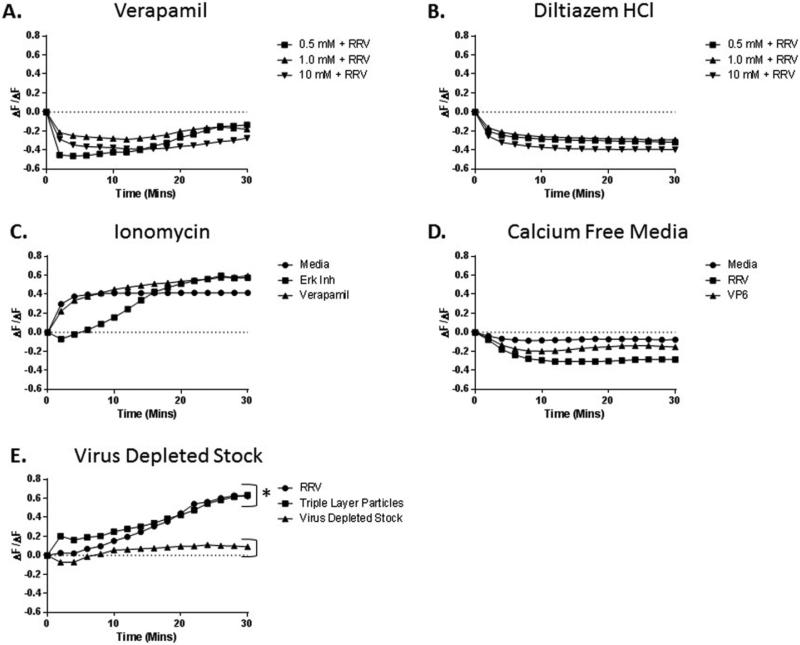
Calcium influx into cholangiocytes following calcium channel blocking
In order to determine if calcium channels are involved in ERK-driven calcium influx, Verapamil was utilized. Pre-treatment of cholangiocytes with this L-type calcium channel blocker resulted in reduced calcium influx into the cell in a dose-dependent manner (Fig 7A). Similar results were observed with use of Diltiazem HCl, a second L-type calcium channel blocker (Fig 7B).
Fig. 7. Role of calcium inhibition on calcium influx.
(A) Fluo-4 loaded cholangiocytes were treated with increasing doses of Verapamil followed by infection with RRV. This resulted in a dose dependent reduction of calcium influx into the cells. (B) Similar results were observed when cholangiocytes were treated with Diltiazem HCl, a second calcium channel inhibitor. (C) In order to determine fluo-4 loading in the presence of ERK 1/2 inhibitor and Verapamil, cells were treated with 10mM Ionomycin to open calcium channels. No reduction in the ability to fluoresce in the presence of calcium was witnessed with Verapamil or ERK inhibitor. (D) Cholangiocytes were pre-treated with calcium-free media followed by infection with RRV in calcium-free media to determine if the fluo-4 was detecting intracellular calcium release or extracellular influx. No fluorescence was observed following infection with RRV or exposure to VP6 protein in calcium-free media. (E) Cholangiocytes treated with virus-depleted stock displayed no calcium influx, while those treated with the triple-layer particles of RRV reacted similarly to the RRV virus lysate. (* = p<0.05)
Effective Fluo-4 dye loading
To prove that ERK or calcium inhibition with Verapamil did not result in decreased Fluo-4 dye loading of the cells, cholangiocytes were treated with ERK inhibitor and Verapamil, followed by treatment with Ionomycin. Ionomycin is an ionophore which transports calcium across biological membranes (Beeler et al., 1979). This treatment resulted in calcium influx, similar to that seen in media treated cells (Fig. 7C).
ERK phosphorylation leads to extracellular calcium influx
In order to demonstrate the source of calcium after ERK phosphorylation, cholangiocytes were pre-treated with calcium-free media, followed by treatment with RRV or purified VP6 protein. These cells demonstrated no calcium influx, thus suggesting that calcium does not originate from an intracellular source and is required in the external cellular environment (Fig 7D).
Calcium influx was not the result of bioactive components from virus lysate
In order to verify that the calcium influx observed in cholangiocytes following exposure to RRV was not the result of bioactive components within the virus lysate, cells where treated with cesium chloride-purified triple-layer particles and virus-depleted stock. Cholangiocytes treated with triple-layer particles displayed similar amounts of calcium influx when compared to those exposed to RRV lysate, while those treated with virus-depleted stock did not display calcium influx (Fig 7E).
L-type calcium channel blockers attenuate RRV replication
RRV replication in cholangiocytes was assayed following 1-hour pre-treatment with Verapamil or Diltiazem HCl. Through this investigation, it was determined that use of L-type calcium channel blockers results in a significant dose dependent reduction of viral replication from media control (Fig 8A). The timing of calcium channel blockade through cholangiocyte treatment with Verapamil was assessed prior to infection with RRV, after infection or both. Pre-treatment with Verapamil resulted in a 30.2% reduction of viral titer from control. Pre-treatment with inhibitor followed by additional Verapamil in the overlay resulted in 33.4% reduction compared to control. Cholangiocytes receiving Verapamil in overlay media only however, had no reduction in viral titer (Fig. 8B). This showed that calcium channel blockade acts similarly to ERK inhibition. Our previous studies have shown that although pre-treatment alone with ERK inhibitor resulted in less viral replication, treatment with ERK inhibitor after RRV infection had little effect (Jafri et al., 2007). The combination of pre-treatment with both ERK inhibitor and Verapamil resulted in no further reduction of viral titer (35.06% decrease; p=0.331), implying signaling of calcium and ERK may be in the same pathway (Fig. 8C).
Fig. 8. Role of calcium blockade on RRV replication.
(A) Dose dependent treatment of cholangiocytes with L-type calcium channel blockers Verapamil or Diltiazem HCl resulted in significant decrease in virus titers. (B) The timing of calcium channel blockade was evaluated through treatment of cholangiocytes prior to infection with RRV, after infection or both. Pre-treatment and the combination of pre- and post-treatment with 1.0uM Verapamil resulted in significant reductions in viral titers. Post-treatment with the calcium channel blocker however, did not result in a reduction of live virus. (C) The combination of pre-treatment with both ERK 1/2 inhibitor and Verapamil resulted in no further reduction of viral titer. * = p<0.05 versus control.
Discussion
The pathogenesis of biliary atresia remains largely unknown. It has been hypothesized that a viral insult, triggering an un-checked, autoimmune inflammatory response governs the disease process (Bezerra, 2005; Mack, 2007). The murine model of BA has provided important supportive evidence in favor of this hypothesis where RRV in newborn BALB/c mice, has tropism for the cholangiocyte triggering a host immune response causing biliary obstruction (Czech-Schmidt et al., 2001).
This study using in vivo and in vitro mechanisms investigates the role of ERK, a member of the MAPK family, in rotavirus infection of the biliary epithelial cell. MAPK signaling is a multifunctional pathway that is pivotal in the innate immune response and viral infection. Jafri et al reported that MAPK signal activation occurs through ERK phosphorylation in RRV infection (Jafri et al., 2007). We further investigated this cascade as it pertains to the murine model of biliary atresia. Through utilization of MAPK inhibitors, we were able to determine that ERK plays an integral role in mortality after RRV infection in vivo. Inhibition of this kinase decreased the amount of extrahepatic bile duct viral content, suggesting a mechanistic basis for its role in vivo. To study how it governs viral replication in the cholangiocyte, we expanded on previous studies using the in vitro model of RRV cholangiocyte infection where we had shown a temporally-dependent inhibition of ERK phosphorylation resulted in decreased viral titer. (Jafri et al., 2007).
Interestingly, RRV-induced ERK phosphorylation was unique to cholangiocytes. Holloway and Coulson have previously shown that RRV infection of MA104, Caco-2 and HT-29 cells resulted in activation of MAPK pathway proteins p38 and JNK (Holloway and Coulson, 2006). Our study confirmed their results of an absence of ERK phosphorylation in other cells in which RRV has tropism, finding it to occur only in cholangiocytes.
Initial RRV-cell attachment occurs through the VP4 protein, attachment to cell surface receptors through proteoglycans (Lopez and Arias, 2006). The virus then interacts with α2β1, αVβ3, and αXβ2 and heat shock protein-70 (Arias et al., 2015). During entry into the cholangiocyte cytoplasm, the virus is liberated from its outer capsid (composed of VP4 and VP7), producing active double-layer particles. Through our investigations, we determined that the replication process was not responsible for early ERK phosphorylation.
The structural composition of RRV has allowed us to study which portion of the virus was responsible for driving the ERK phosphorylation pathway. RRV is comprised of three protein layers (Estes et al., 1983). VP6 is a highly-conserved 780-molecule protein forming the intermediate layer of the viral particle (Ward and McNeal, 2010). Our study established that VP6 initiates the phosphorylation of ERK through the MAPK pathway in the cholangiocyte. This drives subsequent calcium influx.
Calcium is a well-established signaling molecule involved in an array of different cellular processes (Berridge et al., 2003). Previous investigations have demonstrated that voltage-gated calcium channel activation and subsequent cellular calcium influx resulted in ERK phosphorylation (Chen et al., 2016; Li et al., 2005; Selway et al., 2012). However, this was not the case in our system. Through the use of calcium-channel blockers, we illustrate that calcium influx in cholangiocytes was not a prerequisite of ERK phosphorylation. This finding was confirmed using calcium-free media treated cholangiocytes still expressing ERK phosphorylation in presence of RRV.
Calcium is involved in viral structure formation, entry, gene expression, protein processing and release (Zhou et al., 2009). Calcium influx is required for establishing a favorable viral replication environment in multiple viruses, including HIV-1 (Kinoshita et al., 1997), HTLV-1(Ding et al., 2002), HCV(Bergqvist et al., 2003), HHV-8 (Lee et al., 2005) and rotavirus (Perez et al., 1999; Ruiz et al., 2000). For many of these viral pathways, calcium release from the sarcoplasmic reticulum allows for mitochondrial calcium absorption, creating energy for viral replication through ATP-dependent mechanisms (Zhou et al., 2009). Rotavirus infection, however, involves external calcium entry through plasma membrane calcium channels (Michelangeli et al., 1991).
NSP4 is an endoplasmic reticulum transmembrane glycoprotein which is recognized as driving calcium influx in MA104 and HEK 293T cells and acts as a viroporin. The mechanistic aspects of NSP4-induced calcium influx have been somewhat defined. This protein is released upon completion of a rotavirus replication cycle and amplifies the viral infection. It then activates a transient calcium influx through store-operated calcium channels (Dong et al., 1997; Hyser et al., 2010; Hyser et al., 2013). Although early ERK phosphorylation-dependent calcium influx was identified in our study, this was achieved in the absence of NSP4, as one replication cycle could not be completed in one hour. Double-layer particles (those lacking VP4, VP7) along with VLPs 2/6 and purified VP6 protein were able to achieve ERK phosphorylation at one hour. This established that NSP4 is not the sole driving force for calcium influx in the cholangiocyte, though may play a role in later ERK phosphorylation and calcium influx.
Although calcium is not necessary for cell binding (Keljo and Smith, 1988), its influx is integral for RRV uncoating. Ludert et al observed that calcium driven viral uncoating does not occur when calcium concentration is equilibrated across the plasma membrane (Ludert et al., 1987). Furthermore, Shahrabadi et al showed that calcium reduction leads to impaired viral replication and titer (Shahrabadi et al., 1987). Our results are consistent with these observations, showing that impairment of calcium influx through the blockade of L-type calcium channels with Verapamil results in decreased viral titer. Through use of fluo-4 loaded cells, we were also able to illustrate the calcium influx that occurs with RRV infection of the cholangiocyte.
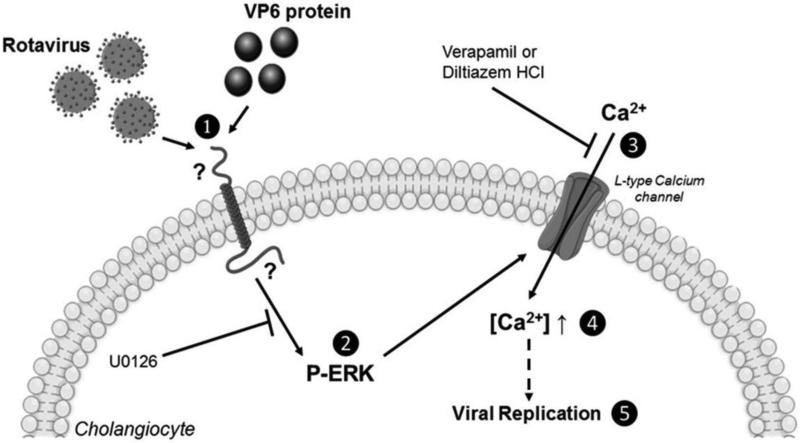
This data expounds on a mechanistic basis for RRV-induced cholangiocyte injury. The MAPK pathway, specifically ERK phosphorylation and calcium influx appears to be essential to RRV infection (Fig 9). Although we determined that the VP6 protein drives ERK phosphorylation, the protein binding calcium and those downstream of ERK require further investigation. Additionally, studies are undergoing to determine a correlation with our in vivo model of biliary atresia.
Fig. 9. Diagram.
(1) The VP6 protein on RRV interacts with an unknown receptor on cholangiocytes. (2) This interactions results in the phosphorylation of ERK 1/2 MAPK which is blocked by the ERK inhibitor U0126. (3) The phosphorylation of ERK leads to opening of L-type calcium channels, resulting in an influx of calcium. (4) This influx of calcium causes the intracellular concentration of calcium to increase. (5) The increase in intracellular calcium concentration plays a role in rotavirus replication.
Highlights.
ERK inhibitors resulted in reduced rotavirus yield and subsequent decreased symptomatology of biliary atresia in mice.
Rhesus rotavirus VP6 protein induced ERK phosphorylation, leading to cellular calcium influx in cholangiocyte cells.
Pre-treatment with an ERK inhibitor or L-type calcium channel inhibitor resulted in lower viral yields in cholangiocyte cells.
Acknowledgments
Funding
Funded by National Institutes of Health (NIH) R03 DK-087974, R01 DK-091566
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen SR, Jafri M, Donnelly B, McNeal M, Witte D, Bezerra J, Ward R, Tiao GM. Effect of rotavirus strain on the murine model of biliary atresia. Journal of virology. 2007;81:1671–1679. doi: 10.1128/JVI.02094-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias CF, Silva-Ayala D, Lopez S. Rotavirus Entry: a Deep Journey into the Cell with Several Exits. Journal of virology. 2015;89:890–893. doi: 10.1128/JVI.01787-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balistreri WF, Grand R, Hoofnagle JH, Suchy FJ, Ryckman FC, Perlmutter DH, Sokol RJ. Biliary atresia: current concepts and research directions. Summary of a symposium. Hepatology. 1996;23:1682–1692. doi: 10.1002/hep.510230652. [DOI] [PubMed] [Google Scholar]
- Beeler TJ, Jona I, Martonosi A. The effect of ionomycin on calcium fluxes in sarcoplasmic reticulum vesicles and liposomes. The Journal of biological chemistry. 1979;254:6229–6231. [PubMed] [Google Scholar]
- Bergqvist A, Sundstrom S, Dimberg LY, Gylfe E, Masucci MG. The hepatitis C virus core protein modulates T cell responses by inducing spontaneous and altering T-cell receptor-triggered Ca2+ oscillations. The Journal of biological chemistry. 2003;278:18877–18883. doi: 10.1074/jbc.M300185200. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews. Molecular cell biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Bessho K, Bezerra JA. Biliary atresia: will blocking inflammation tame the disease? Annual review of medicine. 2011;62:171–185. doi: 10.1146/annurev-med-042909-093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra JA. Potential etiologies of biliary atresia. Pediatric transplantation. 2005;9:646–651. doi: 10.1111/j.1399-3046.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- Chen R, Ji G, Wang L, Ren H, Xi L. Activation of ERK1/2 and TNF-alpha production are regulated by calcium/calmodulin signaling pathway during Penicillium marneffei infection within human macrophages. Microbial pathogenesis. 2016;93:95–99. doi: 10.1016/j.micpath.2016.01.026. [DOI] [PubMed] [Google Scholar]
- Choi AH, Basu M, McNeal MM, Clements JD, Ward RL. Antibody-independent protection against rotavirus infection of mice stimulated by intranasal immunization with chimeric VP4 or VP6 protein. Journal of virology. 1999;73:7574–7581. doi: 10.1128/jvi.73.9.7574-7581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, Debiasi RL, Goody R, Hoyt CC, Richardson-Burns S, Tyler KL. Mechanisms of reovirus-induced cell death and tissue injury: role of apoptosis and virus-induced perturbation of host-cell signaling and transcription factor activation. Viral immunology. 2005;18:89–115. doi: 10.1089/vim.2005.18.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coots A, Donnelly B, Mohanty SK, McNeal M, Sestak K, Tiao G. Rotavirus infection of human cholangiocytes parallels the murine model of biliary atresia. The Journal of surgical research. 2012;177:275–281. doi: 10.1016/j.jss.2012.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czech-Schmidt G, Verhagen W, Szavay P, Leonhardt J, Petersen C. Immunological gap in the infectious animal model for biliary atresia. The Journal of surgical research. 2001;101:62–67. doi: 10.1006/jsre.2001.6234. [DOI] [PubMed] [Google Scholar]
- Ding W, Albrecht B, Kelley RE, Muthusamy N, Kim SJ, Altschuld RA, Lairmore MD. Human T-cell lymphotropic virus type 1 p12(I) expression increases cytoplasmic calcium to enhance the activation of nuclear factor of activated T cells. Journal of virology. 2002;76:10374–10382. doi: 10.1128/JVI.76.20.10374-10382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domiati-Saad R, Dawson DB, Margraf LR, Finegold MJ, Weinberg AG, Rogers BB. Cytomegalovirus and human herpesvirus 6, but not human papillomavirus, are present in neonatal giant cell hepatitis and extrahepatic biliary atresia. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2000;3:367–373. doi: 10.1007/s100240010045. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zeng CQ, Ball JM, Estes MK, Morris AP. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:3960–3965. doi: 10.1073/pnas.94.8.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drut R, Drut RM, Gomez MA, Cueto Rua E, Lojo MM. Presence of human papillomavirus in extrahepatic biliary atresia. Journal of pediatric gastroenterology and nutrition. 1998;27:530–535. doi: 10.1097/00005176-199811000-00007. [DOI] [PubMed] [Google Scholar]
- Estes MK, Palmer EL, Obijeski JF. Rotaviruses: a review. Current topics in microbiology and immunology. 1983;105:123–184. doi: 10.1007/978-3-642-69159-1_3. [DOI] [PubMed] [Google Scholar]
- Fjaer RB, Bruu AL, Nordbo SA. Extrahepatic bile duct atresia and viral involvement. Pediatric transplantation. 2005;9:68–73. doi: 10.1111/j.1399-3046.2005.00257.x. [DOI] [PubMed] [Google Scholar]
- Glaser JH, Balistreri WF, Morecki R. Role of reovirus type 3 in persistent infantile cholestasis. The Journal of pediatrics. 1984;105:912–915. doi: 10.1016/s0022-3476(84)80076-1. [DOI] [PubMed] [Google Scholar]
- Graham KL, Halasz P, Tan Y, Hewish MJ, Takada Y, Mackow ER, Robinson MK, Coulson BS. Integrin-using rotaviruses bind alpha2beta1 integrin alpha2 I domain via VP4 DGE sequence and recognize alphaXbeta2 and alphaVbeta3 by using VP7 during cell entry. Journal of virology. 2003;77:9969–9978. doi: 10.1128/JVI.77.18.9969-9978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero CA, Mendez E, Zarate S, Isa P, Lopez S, Arias CF. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14644–14649. doi: 10.1073/pnas.250299897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish MJ, Takada Y, Coulson BS. Integrins alpha2beta1 and alpha4beta1 can mediate SA11 rotavirus attachment and entry into cells. Journal of virology. 2000;74:228–236. doi: 10.1128/jvi.74.1.228-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway G, Coulson BS. Rotavirus activates JNK and p38 signaling pathways in intestinal cells, leading to AP-1-driven transcriptional responses and enhanced virus replication. Journal of virology. 2006;80:10624–10633. doi: 10.1128/JVI.00390-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyser JM, Collinson-Pautz MR, Utama B, Estes MK. Rotavirus disrupts calcium homeostasis by NSP4 viroporin activity. mBio. 2010;1 doi: 10.1128/mBio.00265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyser JM, Utama B, Crawford SE, Broughman JR, Estes MK. Activation of the endoplasmic reticulum calcium sensor STIM1 and store-operated calcium entry by rotavirus requires NSP4 viroporin activity. Journal of virology. 2013;87:13579–13588. doi: 10.1128/JVI.02629-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M, Donnelly B, Allen S, Bondoc A, McNeal M, Rennert PD, Weinreb PH, Ward R, Tiao G. Cholangiocyte expression of alpha2beta1-integrin confers susceptibility to rotavirus-induced experimental biliary atresia. American journal of physiology. Gastrointestinal and liver physiology. 2008;295:G16–G26. doi: 10.1152/ajpgi.00442.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafri M, Donnelly B, McNeal M, Ward R, Tiao G. MAPK signaling contributes to rotaviral-induced cholangiocyte injury and viral replication. Surgery. 2007;142:192–201. doi: 10.1016/j.surg.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Keljo DJ, Smith AK. Characterization of binding of simian rotavirus SA-11 to cultured epithelial cells. Journal of pediatric gastroenterology and nutrition. 1988;7:249–256. doi: 10.1097/00005176-198803000-00015. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Su L, Amano M, Timmerman LA, Kaneshima H, Nolan GP. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- Knipe D, Howley P. Fields Virology. 6th ed. LWW.; 2013. [Google Scholar]
- LaMonica R, Kocer SS, Nazarova J, Dowling W, Geimonen E, Shaw RD, Mackow ER. VP4 differentially regulates TRAF2 signaling, disengaging JNK activation while directing NF-kappa B to effect rotavirus-specific cellular responses. The Journal of biological chemistry. 2001;276:19889–19896. doi: 10.1074/jbc.M100499200. [DOI] [PubMed] [Google Scholar]
- Landing BH. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst--the concept of infantile obstructive cholangiopathy. Progress in pediatric surgery. 1974;6:113–139. [PubMed] [Google Scholar]
- Lee BS, Lee SH, Feng P, Chang H, Cho NH, Jung JU. Characterization of the Kaposi's sarcoma-associated herpesvirus K1 signalosome. Journal of virology. 2005;79:12173–12184. doi: 10.1128/JVI.79.19.12173-12184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DW, Liu JP, Mao YW, Xiang H, Wang J, Ma WY, Dong Z, Pike HM, Brown RE, Reed JC. Calcium-activated RAF/MEK/ERK signaling pathway mediates p53-dependent apoptosis and is abrogated by alpha B-crystallin through inhibition of RAS activation. Molecular biology of the cell. 2005;16:4437–4453. doi: 10.1091/mbc.E05-01-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Baker ML, Jiang W, Estes MK, Prasad BV. Rotavirus architecture at subnanometer resolution. Journal of virology. 2009;83:1754–1766. doi: 10.1128/JVI.01855-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez S, Arias CF. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends in microbiology. 2004;12:271–278. doi: 10.1016/j.tim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Lopez S, Arias CF. Early steps in rotavirus cell entry. Current topics in microbiology and immunology. 2006;309:39–66. doi: 10.1007/3-540-30773-7_2. [DOI] [PubMed] [Google Scholar]
- Ludert JE, Michelangeli F, Gil F, Liprandi F, Esparza J. Penetration and uncoating of rotaviruses in cultured cells. Intervirology. 1987;27:95–101. doi: 10.1159/000149726. [DOI] [PubMed] [Google Scholar]
- Mack CL. The pathogenesis of biliary atresia: evidence for a virus-induced autoimmune disease. Seminars in liver disease. 2007;27:233–242. doi: 10.1055/s-2007-985068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal MM, Sheridan JF, Ward RL. Active protection against rotavirus infection of mice following intraperitoneal immunization. Virology. 1992;191:150–157. doi: 10.1016/0042-6822(92)90176-p. [DOI] [PubMed] [Google Scholar]
- Michelangeli F, Ruiz MC, del Castillo JR, Ludert JE, Liprandi F. Effect of rotavirus infection on intracellular calcium homeostasis in cultured cells. Virology. 1991;181:520–527. doi: 10.1016/0042-6822(91)90884-e. [DOI] [PubMed] [Google Scholar]
- Perez JF, Ruiz MC, Chemello ME, Michelangeli F. Characterization of a membrane calcium pathway induced by rotavirus infection in cultured cells. Journal of virology. 1999;73:2481–2490. doi: 10.1128/jvi.73.3.2481-2490.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riepenhoff-Talty M, Gouvea V, Evans MJ, Svensson L, Hoffenberg E, Sokol RJ, Uhnoo I, Greenberg SJ, Schakel K, Zhaori G, Fitzgerald J, Chong S, el-Yousef M, Nemeth A, Brown M, Piccoli D, Hyams J, Ruffin D, Rossi T. Detection of group C rotavirus in infants with extrahepatic biliary atresia. The Journal of infectious diseases. 1996;174:8–15. doi: 10.1093/infdis/174.1.8. [DOI] [PubMed] [Google Scholar]
- Riepenhoff-Talty M, Schaekel K, Clark HF, Mueller W, Uhnoo I, Rossi T, Fisher J, Ogra PL. Group A rotaviruses produce extrahepatic biliary obstruction in orally inoculated newborn mice. Pediatric research. 1993;33:394–399. doi: 10.1203/00006450-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Ruiz MC, Cohen J, Michelangeli F. Role of Ca2+in the replication and pathogenesis of rotavirus and other viral infections. Cell calcium. 2000;28:137–149. doi: 10.1054/ceca.2000.0142. [DOI] [PubMed] [Google Scholar]
- Salzedas-Netto AA, Chinen E, de Oliveira DF, Pasquetti AF, Azevedo RA, da Silva Patricio FF, Cury EK, Gonzalez AM, Vicentine FP, Martins JL. Grade IV fibrosis interferes in biliary drainage after Kasai procedure. Transplantation proceedings. 2014;46:1781–1783. doi: 10.1016/j.transproceed.2014.05.045. [DOI] [PubMed] [Google Scholar]
- Sanders MA, Basson MD. Collagen IV regulates Caco-2 migration and ERK activation via alpha1beta1- and alpha2beta1-integrin-dependent Src kinase activation. American journal of physiology. Gastrointestinal and liver physiology. 2004;286:G547–557. doi: 10.1152/ajpgi.00262.2003. [DOI] [PubMed] [Google Scholar]
- Selway J, Rigatti R, Storey N, Lu J, Willars GB, Herbert TP. Evidence that Ca2+ within the microdomain of the L-type voltage gated Ca2+ channel activates ERK in MIN6 cells in response to glucagon-like peptide-1. PloS one. 2012;7:e33004. doi: 10.1371/journal.pone.0033004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrabadi MS, Babiuk LA, Lee PW. Further analysis of the role of calcium in rotavirus morphogenesis. Virology. 1987;158:103–111. doi: 10.1016/0042-6822(87)90242-x. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46:566–581. doi: 10.1002/hep.21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbayev VV, Yasinska IM. Role of MAP kinase-dependent apoptotic pathway in innate immune responses and viral infection. Scandinavian journal of immunology. 2006;63:391–400. doi: 10.1111/j.1365-3083.2006.001764.x. [DOI] [PubMed] [Google Scholar]
- Tadlock L, Yamagiwa Y, Marienfeld C, Patel T. Double-stranded RNA activates a p38 MAPK-dependent cell survival program in biliary epithelia. American journal of physiology. Gastrointestinal and liver physiology. 2003;284:G924–932. doi: 10.1152/ajpgi.00355.2002. [DOI] [PubMed] [Google Scholar]
- Walther AE, Mohanty SK, Donnelly B, Coots A, McNeal M, Tiao GM. Role of myeloid differentiation factor 88 in Rhesus rotavirus-induced biliary atresia. The Journal of surgical research. 2013;184:322–329. doi: 10.1016/j.jss.2013.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RL, McNeal MM. VP6: A candidate rotavirus vaccine. The Journal of infectious diseases. 2010;202(Suppl):S101–107. doi: 10.1086/653556. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Frey TK, Yang JJ. Viral calciomics: interplays between Ca2+ and virus. Cell calcium. 2009;46:1–17. doi: 10.1016/j.ceca.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]