Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is a tumorigenic delta retrovirus and the causative infectious agent of a non-Hodgkin’s peripheral T-cell malignancy called adult T-cell leukemia/lymphoma (ATL). ATL develops in approximately 5% of infected individuals after a significant clinical latency period of several decades. Clinical classifications of ATL include smoldering, chronic, lymphoma, and acute subtypes, with varying median survival ranges of a few months to several years. Depending on the ATL subtype and disease symptoms, treatment options include ‘watchful waiting’, chemotherapy, antiviral therapy, allogeneic hematopoietic stem cell transplantation (alloHSCT), and targeted therapies. Herein we review the characteristics and development of ATL, as well as current and future treatment options and perspectives.
Introduction
HTLV-1 infection is found in most parts of the world with clusters of endemic infection in southwestern Japan, the Caribbean basin, equatorial Africa, parts of South America, the Middle East, and parts of Melanesia [1]. The number of HTLV-1 carriers has been estimated at 10-20 million people worldwide [2, 3]. In Japan, seroprevalence rates in HTLV-1 endemic areas can range from roughly 5% to 30-40% [4-6].
Viral transmission
HTLV-1 infection is heavily dependent upon cell-to-cell transmission [7-10] and new infections require the transfer of virus-infected cells by several routes: mother-to-child, sexual contact, and through infected blood products. Mother-to-child transmission via breastfeeding is a common route of transmission in HTLV-1-endemic areas. Studies show approximately 10-25% of breast-fed infants born to HTLV-1-seropositive mothers seroconvert [3], with seroconversion rates increasing with duration of breastfeeding and high proviral loads in breast milk [11-13]. There is small risk (<3%) of HTLV-1 transmission even without breastfeeding; vertical transmission can occur transplacentally or during delivery [14]. Importantly, because disease symptoms typically take upwards of four or five decades to present, ATL occurs almost exclusively in individuals who acquire infection during infancy. Since 2010, serological screening for HTLV-1 antibodies has been available for all pregnant women in Japan, especially in endemic areas of the country [15]. However, routine screening in other developed countries has not been commonplace given the low rate of HTLV-1 seroprevalence. While no international guidelines exist on breastfeeding and HTLV-1 prevention, research suggests HTLV-1-positive mothers who avoid breastfeeding have reduced vertical transmission [16]. Unfortunately, this may not always be feasible due to socioeconomic circumstances in resource-poor, developing countries. In these cases, short-term breastfeeding for 3 to a maximum of 6 months is recommended [17]. HTLV-1 can also be transmitted during sexual contact via HTLV-1-infected cells in bodily fluids [18-22]. While male-to-female sexual transmission is most efficient, there is a low rate of sexual transmission from female-to-male [22, 23]. Finally, another important route of HTLV-1 transmission is through exposure to infected blood products by blood transfusion and sharing of blood-contaminated needles. Prior to routine blood testing, contaminated blood transfusions have been associated with ~20% seroconversion rate in the US [24] and 44-63% seroconversion rate in HTLV-1 endemic regions [25, 26]. Routine testing of donor blood products for HTLV-1 has been commonplace in the US since 1988. Occupational exposure to HTLV-1 has not been well documented to date. In one study, no seroconversions were reported over a 10-year period in 53 individuals exposed to HTLV-1-infected blood in a Central Australian hospital [27]. However, one case was reported involving a healthcare worker in Japan who seroconverted after exposure to blood from an ATL patient [28].
HTLV-1 genome
As a complex retrovirus, HTLV-1 has a genomic structure similar to, but distinct, from simple retroviruses. In addition to the standard structural and enzymatic genes of all retroviruses, gag, pol, and env, HTLV-1 encodes additional regulatory and accessory genes derived from multiple spliced transcripts. Upon infection, proviral double-stranded DNA is generated from the genomic viral RNA, which is then integrated into the host genome at random chromosomal integration sites [29]. At least two viral gene products, tax and hbz, are individually linked to oncogenic transformation and play a role in the pathogenesis of ATL [30, 31]. Tax is the major driver of viral transcription and transformation [32, 33]. Tax also functions at transcriptional and post-transcriptional steps during cellular transformation by upregulating several interleukins and their receptors, cytokines, adhesion molecules, growth promoting factors, and apoptosis inhibiting factors [34]. However, Tax expression is frequently lost in a significant majority of ATL cases [35]. Conversely, ATL tumors always express hbz [30, 36]. Hbz RNA and protein have been shown to support the proliferation of ATL tumor cells in vivo and in vitro [37, 38]. Our current understanding of HTLV-1-mediated tumorigenesis suggests that Tax is responsible for initiating transformation, while HBZ provides maintenance or cell survival signals. There is no evidence to indicate that HTLV-1 causes insertional mutagenesis disrupting tumor suppressor genes or activating proto-oncogenes. Nonetheless, the low incidence and long latency period prior to ATL onset suggests a combination of cellular genetic and epigenetic changes that are required for disease development, in addition to HTLV-1 viral gene effects. Several molecular studies have found a wide spectrum of disrupted/mutated cellular genes and pathways commonly affected in ATL cells [34, 35].
Characteristics and development of ATL
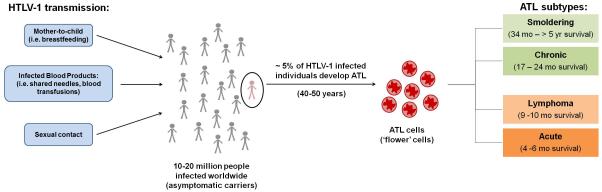
HTLV-1 is the causative infectious agent responsible for the development of an aggressive non-Hodgkin’s peripheral T-cell malignancy, ATL [6, 39, 40]. Although most HTLV-1-infected individuals remain asymptomatic, approximately 5% of infected individuals will develop ATL after a long clinical latency period of four to five decades [41]. Diverse clinical features that include lymphadenopathy, skin lesions, increased abnormal lymphocytes, frequent blood and bone marrow involvement, hypercalcemia, and lytic bone lesions characterize ATL. Although ATL most commonly is a CD4+ T-cell leukemia/lymphoma, occasional examples of CD8+ lymphoma have been described [42].
As a heterogeneous disease, ATL has been divided into four clinical subtypes based on analysis of 854 ATL patients in the 1980s known as Shimoyama classification: acute, lymphoma, smoldering and chronic [43]. Briefly, the smoldering and chronic subtypes, also known as indolent ATL, are characterized by rash and minimal blood involvement. The acute and lymphoma subtypes, also known as aggressive ATL, are characterized by large tumor burden, lymph node and blood involvement, and hypercalcemia. Classification of ATL subtype greatly influences the treatment regimen and prognosis of patients. Patients with aggressive ATL have a dismal prognosis with survival rates of 4 to 6 months for acute subtype and 9 to 10 months for lymphoma subtype. Those with indolent ATL have a more encouraging prognosis with survival rates of 17 to 24 months for the chronic subtype and 34 months to >5 years for the smoldering subtype. Aggressive ATL generally carries a poorer prognosis because of multidrug resistance, a larger tumor burden (compared to indolent forms), multiorgan failure, hypercalcemia, and/or frequent opportunistic infections due to intrinsic T-cell immunodeficiency [43, 44]. Otherwise healthy carriers of HTLV-1 with abnormal peripheral blood lymphocytes typical of ATL cells are classified as having a borderline state of disease described as pre-ATL. Patients with pre-ATL can revert spontaneously to an asymptomatic state or progress to ATL malignancy [45].
Other HTLV-1-associated diseases
In addition to ATL, HTLV-1 infection is associated with several other diseases and conditions including HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), HTLV-1-associated uveitis (HU), and various dermatological conditions. HAM/TSP develops in approximately 3-5% of HTLV-1-infected individuals, commonly women, with an average age of disease onset of 40 years [46]. It is characterized by lower limb spasticity, bowel and bladder disturbances, and slow but steady progression, without remission, over several years [47]. As a neurological condition, HAM/TSP is precipitated by lesions in the central nervous system associated with infiltrates of CD8+ T-cells [48]. HU is most commonly found in middle-aged men and women in areas of Japan with endemic HTLV-1 infection. Characterized by ‘floaters’ and foggy vision in the eye, this condition is classified as an intermediate uveitis and can be the only symptom of HTLV-1 infection or associated with HAM/TSP [49]. Infective dermatitis associated with HTLV-1 (IDH) is a pediatric form of severe, recurrent eczema commonly associated with endemic HTLV-1 infection [50]. Importantly, IDH has been considered an indicator of future ATL or HAM/TSP development [51]. Research has suggested that many of these HTLV-1-associated diseases are related to high viral load and/or overstimulation of dendritic cells, resulting in chronic production of high levels of type 1 interferons and interferon-stimulated gene expression [52-54]. While these clinical disorders are associated with HTLV-1 infection, this review will focus on ATL, the only known malignancy caused by the retrovirus HTLV-1.
Diagnosis of ATL
HTLV-1 infection is confirmed through laboratory blood tests that detect antibodies to HTLV-1 infection, such as ELISA and western blotting. Additional diagnostic tests include PCR to confirm the presence of proviral sequences and quantify proviral load. Individuals with HTLV-1 infection in the absence of any known symptoms are known as asymptomatic carriers. There is currently no established method to predict or monitor the risk of ATL development in asymptomatic HTLV-1 carriers. However, a recent prospective study from the Joint Study on Predisposing Factors of ATL Development found that patients with a higher proviral load (>4 copies/100 peripheral blood mononuclear cells) were in a high-risk category for developing ATL [55]. This study also concluded that advanced age, family history of ATL, and having the first opportunity to learn of HTLV-1 infection during treatment of other diseases were independent risk factors for ATL development. This study further confirms the observation that ATL occurs almost exclusively in individuals who acquired HTLV-1 as a result of breastfeeding, since four to five decades are generally required before disease development.
Typical criteria examined for the diagnosis of ATL include a variety of criteria discussed below. Often, the diagnosis of ATL is made following the detection of ATL cells in the peripheral blood [43, 56]. Patients with aggressive ATL typically display >5% abnormal T-cells, called ATL cells or ‘flower’ cells. Flower cells have multilobular nuclei with homogeneous and condensed chromatin, small or absent nucleoli, and an agranular and basophilic cytoplasm. ATL diagnosis can also be made from lesion biopsy. This is helpful with asymptomatic patients who develop lesions following a watchful waiting approach (to be discussed). HTLV-1 proviral integration should be assessed from either blood or lesion biopsy. Monoclonal proviral integration is found in all ATL cases [56, 57]. Integrated defective proviruses can also be an indicator of clinical subtype and prognosis [58]. Serum LDH level measurement and immunophenotypic analysis of flower cells also are typically performed in suspected ATL cases [56]. Other clinical ATL diagnostic criteria that help classify disease subtype and direct therapeutic approaches include bone marrow examination, radiologic imaging and endoscopy to determine GI involvement, cytogenetics to observe karyotypic abnormalities, and analysis of host tumor suppressor genes, such as p53, which may predict AZT/IFN-α response. [59-66].
Treatment of ATL
Treatment strategies for ATL greatly depend upon the clinical subtype of ATL and associated symptoms/diagnostic criteria present. Current treatment options are described below and include ‘watchful waiting’, chemotherapy, antiviral therapy, allogeneic hematopoietic stem cell transplantation (alloHSCT), and targeted therapies.
Watchful waiting
Patients with indolent forms of ATL, displaying few if any symptoms, are often referred for a watchful waiting approach [59, 67]. Those with indolent ATL and presenting symptoms are also advised to utilize watchful waiting and to consider treatment with AZT/IFN-α. A recent study analyzed 90 patients with indolent ATL who were mostly prescribed watchful waiting and/or chemotherapy [68]. The results found a poorer prognosis than initially expected with median survival time of 4.1 years, suggesting indolent ATL should be closely monitored in the clinical setting with practices other than watchful waiting.
Chemotherapy
Several chemotherapy regimens have been utilized to treat patients with ATL with variable degrees of success. A 2007 phase III clinical study suggested that treatment with high doses of VCAP-AMP-VECP (vincristine, cyclophosphamide, doxorubicin, and prednisone; doxorubicin, ranimustine, and prednisone; vindesine, etoposide, carboplatin, and prednisone) was a more effective regimen than biweekly CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) treatment in newly diagnosed patients with aggressive ATL subtypes [69]. The rate of complete response was 40% compared to 25%, while the overall survival rate at 3 years was 24% compared to 13% for VCAP-AMP-VECP and CHOP, respectively. Unfortunately, the median survival time was low compared to other hematological malignancies at roughly 13 months. Other problems with this combination chemotherapy regimen are its complexity and the lack of availability of several of these drugs in many countries. An alternative combination regimen with similar efficacy is DA-EPOCH (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) [70, 71].
Antiviral therapy
Since the mid-1990s, the combination of zidovudine (AZT) and interferon-alpha (IFN-α) has been tested in several clinical studies with varying degrees of success. Two reports described high major response rates of 58% (11/19 patients) [72] and 100% (5/5 patients) [73] in patients with the acute form of ATL when treated with a combination of AZT and IFN-α. However, other investigators reported lower response rates using this approach, possibly due to the amount and type of prior treatment the ATL patients had received [74]. Nevertheless, encouraging results from a worldwide meta-analysis of 254 ATL patients treated with AZT/IFN-α therapy have been reported [75]. Patients treated with first-line AZT/IFN-α therapy had a 46% 5-year overall survival rate compared to a 20% 5-year overall survival rate for first-line chemotherapy. Patients with lymphoma subtype ATL did not significantly benefit from first-line AZT/IFN-α therapy compared to first-line chemotherapy. Conversely, patients with acute ATL had an 82% 5-year survival rate, while indolent forms of ATL exhibited a 100% 5-year survival rate when treated with first-line AZT/IFN-α therapy. Together, these reports support the continued use and development of AZT/IFN-α therapies for acute and indolent subtypes of ATL.
alloHSCT
The use of alloHSCT has emerged as a promising, yet understudied, treatment option for aggressive ATL. Initial studies reported high treatment-related mortality; however, the estimated 3-year overall survival rate was 45% [76]. In a recent retrospective study, Japanese investigators found a 3-year overall survival rate of 33% for a cohort of 386 ATL patients who received alloHSCT from different graft sources [77]. This study revealed several factors that significantly lowered survival rates: age of patient/recipient (>50), sex, status other than complete remission, and use of unrelated cord blood compared to HLA-matched related grafts. Another retrospective study recently reported a 3-year overall survival rate of 39.9% for patients treated with alloHSCT [78]. Unfortunately, in this study, the use of alloHSCT had limited success in chemorefractory ATL patients. Overall, alloHSCT remains a hopeful treatment option for young patients with aggressive subtypes of ATL.
Targeted therapies
Several promising targeted therapies against ATL have been part of recent, ongoing, and proposed clinical trials. These therapies include the proteasome inhibitor, Bortezomib; HDAC inhibitors such as vorinostat, romidepsin, and panobinostat; the JAK1/2 inhibitor Ruxolitinib; arsenic trioxide and IFN-α; monoclonal antibodies directed against ATL/T-cell surface molecules (anti-PD-1, anti-CD25, anti-CD2, anti-CD52, and CCR4); novel antifolates; the purine nucleotide phosphorylase inhibitor Forodesine; antiangiogenic therapy; and the immunomodulatory drug Lenalidomide [41]. Initially seen as an attractive candidate for an anti-HTLV-1 vaccine, the HTLV-1 envelope glycoproteins mediate infection and elicit both a humoral and cellular immune response. Unfortunately, early studies using viral envelope-recombinant vaccinia virus or adenoviruses and viral envelope chimeric peptides only provided partial protection to animals challenged with HTLV-1-infected cells [79, 80]. Recently, several studies have suggested the potential therapeutic use of Tax or HBZ peptides to provide protective immunity against HTLV-1 infection and/or ATL development [81, 82].
‘Humanized’ mouse model
The development of a ‘humanized’ mouse model to support HTLV-1 infection and subsequent ATL disease development has been a recent and significant advance within the field. Previously, rabbits and transgenic mice were used to productively study viral persistence/immune responses and importance/function of viral genes, respectively [83]. However, none of these animal models has offered the ability to study HTLV-1 viral infection and leukemia/lymphoma development. So-called ‘humanized’ mice are immune-deficient mice injected with human CD34+ or CD133+ umbilical cord stem cells (HUSC) that subsequently develop a reconstituted human immune system. While the lymphocytes that develop are phenotypically normal to human lymphocytes, they lack the ability to develop a functional adaptive immune response. These ‘humanized’ mice consistently develop leukemia or lymphoma (>95% incidence) after injection with lethally irradiated HTLV-1-producing cells and subsequent infection [84]. The mice possess a T cell population skewed towards oligoclonality, typical of what is observed during ATL progression, and increased viral load during infection. Also available are immune-deficient mice injected with HUSC and transplanted human fetal liver and thymus [85]. This type of ‘humanized’ mouse does develop a functional adaptive immune response. While the advantages of these types of ‘humanized’ mice are obvious, the adaptive immune response might also be a disadvantage by hindering efficient viral replication and leukemogenesis. In summary, in spite of their limitations, these ‘humanized’ mouse models are ideal systems to develop vaccines and HTLV-1-targeted therapies.
Future perspectives
ATL is a rare non-Hodgkin’s peripheral T-cell malignancy caused by the human retrovirus HTLV-1 (Fig. 1). Although only approximately 5% of HTLV-1-infected individuals develop ATL after several decades of infection, the clinical prognosis is poor for fast-growing, aggressive subtypes of ATL. Recent advances in treatment options for this devastating disease have improved overall patient response/survival. However, the heterogeneous nature of this disease, coupled with diverse prognostic factors, makes it difficult to classify and assess the best available treatment for each individual patient. As researchers continue to expand their knowledge of HTLV-1 cellular transformation and the molecular events leading to ATL development, our repertoire of treatment options will undoubtedly increase. A need exists to identify novel targets and develop related therapies that prevent or slow the progression of ATL. Undoubtedly, the use of animal models for studying HTLV-1 infection and disease progression will be beneficial for initial in vivo characterization of these novel targeted therapies.
Figure 1.
Transmission and development of ATL.
Highlights.
ATL is a peripheral T-cell malignancy induced by the human retrovirus HTLV-1.
Clinical classifications of ATL include acute, lymphoma, smoldering, and chronic.
Current therapy recommendations improve patient survival and depend on ATL subtype.
Novel and innovative approaches are needed to advance ATL treatment options.
Acknowledgements
We thank Kate Hayes-Ozello for editorial comments on the manuscript. This work was supported by a grant from the National Institutes of Health (CA100730) to PLG.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matsuoka M, Jeang KT. Human T-cell leukemia virus type I at age 25: a progress report. Cancer Res. 2005;65(11):4467–70. doi: 10.1158/0008-5472.CAN-05-0559. [DOI] [PubMed] [Google Scholar]
- 2.DeThe G, Bomford R. An HTLV-I vaccine: why, how, for whom? AIDS Res Hum Retroviruses. 1993;9:381–386. doi: 10.1089/aid.1993.9.381. [DOI] [PubMed] [Google Scholar]
- 3.Gessain A, Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Frontiers in Microbiology. 2012;3:388. doi: 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kohakura M, et al. Seroepidemiology of the human retrovirus (HTLV/ATLV) in Okinawa where adult T-cell leukemia is highly endemic. Japanese journal of cancer research : Gann. 1986;77(1):21–3. [PubMed] [Google Scholar]
- 5.Tajima K, et al. Epidemiological features of HTLV-I carriers and incidence of ATL in an ATL-endemic island: a report of the community-based co-operative study in Tsushima, Japan. International journal of cancer. 1987;40(6):741–6. doi: 10.1002/ijc.2910400605. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida M, Miyoshi I, Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci USA. 1982;79:2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igakura T, et al. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299(5613):1713–6. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- 8.Barnard AL, et al. Engagement of specific T cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood. 2005 doi: 10.1182/blood-2004-07-2850. [DOI] [PubMed] [Google Scholar]
- 9.Derse D, et al. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. Journal of virology. 2001;75(18):8461–8. doi: 10.1128/JVI.75.18.8461-8468.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazurov D, et al. Quantitative comparison of HTLV-1 and HIV-1 cell-to-cell infection with new replication dependent vectors. PLoS pathogens. 2010;6(2):e1000788. doi: 10.1371/journal.ppat.1000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi K, et al. Inhibitory effect of maternal antibody on mother-to-child transmission of human T-lymphotropic virus type I. The Mother-to-Child Transmission Study Group. Int J Cancer. 1991;49(5):673–7. doi: 10.1002/ijc.2910490508. [DOI] [PubMed] [Google Scholar]
- 12.Hino S, et al. Intervention of maternal transmission of HTLV-1 in Nagasaki, Japan. Leukemia. 1994;8(Suppl 1):S68–70. [PubMed] [Google Scholar]
- 13.Ureta-Vidal A, et al. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: implication of high antiviral antibody titer and high proviral load in carrier mothers. International journal of cancer. 1999;82(6):832–6. doi: 10.1002/(sici)1097-0215(19990909)82:6<832::aid-ijc11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14*.Carneiro-Proietti AB, et al. Mother-to-Child Transmission of Human T-Cell Lymphotropic Viruses-1/2: What We Know, and What Are the Gaps in Understanding and Preventing This Route of Infection. Journal of the Pediatric Infectious Diseases Society. 2014;3(Suppl 1):S24–9. doi: 10.1093/jpids/piu070. The authors review what is curently known concerning HTLV-1 maternal tranmission and discuss methods for prevention. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki S, et al. Prevalence of human T-cell leukemia virus type 1 carrier in Japanese pregnant women in 2013. Journal of clinical medicine research. 2015;7(6):499–500. doi: 10.14740/jocmr2097w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashiwagi K, et al. A decrease in mother-to-child transmission of human T lymphotropic virus type I (HTLV-I) in Okinawa, Japan. The American journal of tropical medicine and hygiene. 2004;70(2):158–63. [PubMed] [Google Scholar]
- 17.Mylonas I, et al. HTLV infection and its implication in gynaecology and obstetrics. Archives of gynecology and obstetrics. 2010;282(5):493–501. doi: 10.1007/s00404-010-1559-1. [DOI] [PubMed] [Google Scholar]
- 18.Nakano S, et al. Search for possible routes of vertical and horizontal transmission of adult T-cell leukemia virus. Gann. 1984;75:1044–1045. [PubMed] [Google Scholar]
- 19.Tajima K, et al. Epidemiological analysis of the distribution of antibody to adult T-cell leukemia-virus-associated antigen: Possible horizontal transmission of adult T-cell leukemia virus. Gann. 1982;73:893–901. [PubMed] [Google Scholar]
- 20.Murphy EL, et al. Sexual transmission of human T-lymphotropic virus type I (HTLV-I) Ann Intern Med. 1989;111:555–560. doi: 10.7326/0003-4819-111-7-555. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan JE, et al. Male-to-female transmission of human T-cell lymphotropic virus types I and II: association with viral load. The Retrovirus Epidemiology Donor Study Group. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(2):193–201. doi: 10.1097/00042560-199606010-00014. [DOI] [PubMed] [Google Scholar]
- 22.Roucoux DF, et al. A prospective study of sexual transmission of human T lymphotropic virus (HTLV)-I and HTLV-II. The Journal of infectious diseases. 2005;191(9):1490–7. doi: 10.1086/429410. [DOI] [PubMed] [Google Scholar]
- 23.Kajiyama W, et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis. 1986;154(5):851–7. doi: 10.1093/infdis/154.5.851. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan MT, et al. Transmission of human T-lymphotropic virus types I and II by blood transfusion: a retrospective study of recipients of blood components. Arch Int Med. 1991;151:2043–2048. [PubMed] [Google Scholar]
- 25.Okochi K, Sato H, Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: Seroconversion in recipients. Vox Sang. 1984;46:245–253. doi: 10.1111/j.1423-0410.1984.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 26.Manns A, et al. A prospective study of transmission by transfusion of HTLV-I and risk factors associated with seroconversion. Int J Cancer. 1992;51(6):886–91. doi: 10.1002/ijc.2910510609. [DOI] [PubMed] [Google Scholar]
- 27*.Hewagama S, et al. Human T-cell lymphotropic virus type 1 exposures following blood-borne virus incidents in central Australia, 2002-2012. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(1):85–7. doi: 10.1093/cid/ciu227. This is the first detailed, published report on occupational exposure to HTLV-1. The authors retrospectively examined hospital occupational exposure events over a 10-year period in Central Australia, finding no transmission of HTLV-1 in 53 exposed individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kataoka R, et al. Transmission of HTLV-I by blood transfusion and its prevention by passive immunization in rabbits. Blood. 1990;76(8):1657–61. [PubMed] [Google Scholar]
- 29.Derse D, et al. Human T-cell leukemia virus type 1 integration target sites in the human genome: comparison with those of other retroviruses. J Virol. 2007;81(12):6731–41. doi: 10.1128/JVI.02752-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7(4):270–80. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- 31.Matsuoka M, Green PL. The HBZ gene, a key player in HTLV-1 pathogenesis. Retrovirology. 2009;6:71. doi: 10.1186/1742-4690-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bex F, Gaynor RB. Regulation of gene expression by HTLV-I Tax protein. Methods. 1998;16(1):83–94. doi: 10.1006/meth.1998.0646. [DOI] [PubMed] [Google Scholar]
- 33.Grassmann R, Aboud M, Jeang KT. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene. 2005;24(39):5976–85. doi: 10.1038/sj.onc.1208978. [DOI] [PubMed] [Google Scholar]
- 34*.Giam CZ, Semmes OJ. HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma-A Tale of Two Proteins: Tax and HBZ. Viruses. 2016;8(6) doi: 10.3390/v8060161. The authors concisely summarize a large body of literature concerning how HTLV-1 infection progresses to ATL. They focus on the roles of two important viral proteins, Tax and HBZ, in viral replication, persistence, and oncogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35*.Kataoka K, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nature genetics. 2015;47(11):1304–15. doi: 10.1038/ng.3415. An integrated molecular study of 426 ATL cases was performed, identifying several alterations in the cellular genome which significantly overlap with the Tax interactome and are highly enriched for T-cell receptor-NF-κB signaling, T-cell trafficking and other T-cell-related pathways and immunosurveillance. These findings provide convincing evidence for new diagnostics and therapeutics in ATL patients. [DOI] [PubMed] [Google Scholar]
- 36.Satou Y, et al. HTLV-I basic leucine zipper factor gene mRNA supports proliferation of adult T cell leukemia cells. Proc Natl Acad Sci U S A. 2006;103(3):720–5. doi: 10.1073/pnas.0507631103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold J, et al. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood. 2006;107(10):3976–82. doi: 10.1182/blood-2005-11-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arnold J, et al. Human T-cell Leukemia Virus Type-1 Antisense-encoded Gene, Hbz, Promotes T Lymphocyte Proliferation. Blood. 2008;112(9):3788–97. doi: 10.1182/blood-2008-04-154286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uchiyama T, et al. Adult T-cell leukemia: Clinical and hematologic features of 16 cases. Blood. 1977;50:81–492. [PubMed] [Google Scholar]
- 40.Poiesz BJ, et al. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Ishitsuka K, Tamura K. Human T-cell leukaemia virus type I and adult T-cell leukaemia-lymphoma. The Lancet. Oncology. 2014;15(11):e517–26. doi: 10.1016/S1470-2045(14)70202-5. The authors detail an updated determination of ATL clinical subtype classification according to the 1991 Shimoyama criteria. They also outline therapeutic options for ATL treatment outside of a clinical trial setting. Finally, current clinical trials for ATL which are ongoing or under consideration are reported. [DOI] [PubMed] [Google Scholar]
- 42.Ureshino H, et al. Mogamulizumab for adult T-cell leukemia/lymphoma expressing atypical phenotype CD4-/CD8+/CCR4+ Open Journal of Hematology. 2014;5:5. [Google Scholar]
- 43.Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukemia-lymphoma. A report from the lymphoma study group (1984-1987) Br J Haematol. 1991;79:437. doi: 10.1111/j.1365-2141.1991.tb08051.x. [DOI] [PubMed] [Google Scholar]
- 44.Phillips AA, et al. A critical analysis of prognostic factors in North American patients with human T-cell lymphotropic virus type-1-associated adult T-cell leukemia/lymphoma: a multicenter clinicopathologic experience and new prognostic score. Cancer. 2010;116(14):3438–46. doi: 10.1002/cncr.25147. [DOI] [PubMed] [Google Scholar]
- 45.Ikeda S, et al. Detection of preleukemic state of adult T-cell leukemia (pre-ATL) in HTLV-1 carriers. Cancer detection and prevention. 1990;14(4):431–5. [PubMed] [Google Scholar]
- 46.Orland JR, et al. Prevalence and clinical features of HTLV neurologic disease in the HTLV Outcomes Study. Neurology. 2003;61(11):1588–94. doi: 10.1212/01.wnl.0000096011.92542.da. [DOI] [PubMed] [Google Scholar]
- 47.Goncalves DU, et al. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clinical microbiology reviews. 2010;23(3):577–89. doi: 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bangham CR, Osame M. Cellular immune response to HTLV-1. Oncogene. 2005;24(39):6035–46. doi: 10.1038/sj.onc.1208970. [DOI] [PubMed] [Google Scholar]
- 49.Kamoi K, Mochizuki M. HTLV-1 uveitis. Frontiers in Microbiology. 2012;3:270. doi: 10.3389/fmicb.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGill NK, et al. HTLV-1-associated infective dermatitis: updates on the pathogenesis. Experimental dermatology. 2012;21(11):815–21. doi: 10.1111/exd.12007. [DOI] [PubMed] [Google Scholar]
- 51.Primo J, et al. High HTLV-1 proviral load, a marker for HTLV-1 associated myelopathy/tropical spastic paraparesis, is also detected in patients with infective dermatitis associated with HTLV-1. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al.] 2009;42(8):761–4. doi: 10.1590/s0100-879x2009005000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagai M, et al. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J Neurovirol. 1998;4:586–593. doi: 10.3109/13550289809114225. [DOI] [PubMed] [Google Scholar]
- 53.Olindo S, et al. HTLV-1 proviral load in peripheral blood mononuclear cells quantified in 100 HAM/TSP patients: a marker of disease progression. Journal of the neurological sciences. 2005;237(1-2):53–9. doi: 10.1016/j.jns.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 54.Tattermusch S, et al. Systems biology approaches reveal a specific interferon-inducible signature in HTLV-1 associated myelopathy. PLoS pathogens. 2012;8(1):e1002480. doi: 10.1371/journal.ppat.1002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwanaga M, et al. Human T-cell leukemia virus type I (HTLV-1) proviral load and disease progression in asymptomatic HTLV-1 carriers: a nationwide prospective study in Japan. Blood. 2010;116(8):1211–9. doi: 10.1182/blood-2009-12-257410. [DOI] [PubMed] [Google Scholar]
- 56.Kikuchi M, Jaffe ES, Ralfkiaer E. In: Adult T-cell leukaemia/lymphoma, in WHO Classification of Tumours; Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Jaffe ES, Harris NL, Stein H, editors. IARC Press; Lyon, France: 2001. pp. 200–203. [Google Scholar]
- 57*.Cook LB, et al. The role of HTLV-1 clonality, proviral structure, and genomic integration site in adult T-cell leukemia/lymphoma. Blood. 2014;123(25):3925–31. doi: 10.1182/blood-2014-02-553602. Using a sensitive high-throughput method to quantify 197 ATL proviral integration sites, the authors quantified the oligoclonality in ATL and identified 91% of malignant clones contained a single provirus and that malignant clones mainly arise from low-abundance clones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsukasaki K, et al. Integration patterns of HTLV-I provirus in relation to the clinical course of ATL: frequent clonal change at crisis from indolent disease. Blood. 1997;89(3):948–56. [PubMed] [Google Scholar]
- 59.Takasaki Y, et al. Impact of visceral involvements and blood cell count abnormalities on survival in adult T-cell leukemia/lymphoma (ATLL) Leukemia research. 2007;31(6):751–7. doi: 10.1016/j.leukres.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 60.Gallamini A, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood. 2004;103(7):2474–9. doi: 10.1182/blood-2003-09-3080. [DOI] [PubMed] [Google Scholar]
- 61.Utsunomiya A, et al. Adult T-cell leukemia with leukemia cell infiltration into the gastrointestinal tract. Cancer. 1988;61(4):824–8. doi: 10.1002/1097-0142(19880215)61:4<824::aid-cncr2820610430>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 62.Tsukasaki K, et al. Comparative genomic hybridization analysis in adult T-cell leukemia/lymphoma: correlation with clinical course. Blood. 2001;97(12):3875–81. doi: 10.1182/blood.v97.12.3875. [DOI] [PubMed] [Google Scholar]
- 63.Itoyama T, et al. Cytogenetic analysis and clinical significance in adult T-cell leukemia/lymphoma: a study of 50 cases from the human T-cell leukemia virus type-1 endemic area, Nagasaki. Blood. 2001;97(11):3612–20. doi: 10.1182/blood.v97.11.3612. [DOI] [PubMed] [Google Scholar]
- 64.Tawara M, et al. Impact of p53 aberration on the progression of Adult T-cell Leukemia/Lymphoma. Cancer letters. 2006;234(2):249–55. doi: 10.1016/j.canlet.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 65.Ramos JC, et al. IRF-4 and c-Rel expression in antiviral-resistant adult T-cell leukemia/lymphoma. Blood. 2007;109(7):3060–8. doi: 10.1182/blood-2006-07-036368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinpara S, et al. Interferon-alpha (IFN-alpha) suppresses HTLV-1 gene expression and cell cycling, while IFN-alpha combined with zidovudine induces p53 signaling and apoptosis in HTLV-1-infected cells. Retrovirology. 2013;10:52. doi: 10.1186/1742-4690-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsukasaki K, et al. Definition, prognostic factors, treatment, and response criteria of adult T-cell leukemia-lymphoma: a proposal from an international consensus meeting. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(3):453–9. doi: 10.1200/JCO.2008.18.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takasaki Y, et al. Long-term study of indolent adult T-cell leukemia-lymphoma. Blood. 2010;115(22):4337–43. doi: 10.1182/blood-2009-09-242347. [DOI] [PubMed] [Google Scholar]
- 69.Tsukasaki K, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(34):5458–64. doi: 10.1200/JCO.2007.11.9958. [DOI] [PubMed] [Google Scholar]
- 70*.Ratner L, et al. Dose-adjusted EPOCH chemotherapy with bortezomib and raltegravir for human T-cell leukemia virus-associated adult T-cell leukemia lymphoma. Blood cancer journal. 2016;6:e408. doi: 10.1038/bcj.2016.21. The authors report favorable results of a current phase 1/2 trial which was designed to test the tolerability and efficacy of dose-adjusted EPOCH chemotherapy with bortezomib (proteasome inhibitor) and raltegravir (antiviral) in ATL patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ratner L, et al. Human T cell leukemia virus reactivation with progression of adult T-cell leukemia-lymphoma. PLoS One. 2009;4(2):e4420. doi: 10.1371/journal.pone.0004420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gill PS, et al. Treatment of adult T-cell leukemia-lymphoma with a combination of interferon alfa and zidovudine. The New England journal of medicine. 1995;332(26):1744–8. doi: 10.1056/NEJM199506293322603. [DOI] [PubMed] [Google Scholar]
- 73.Hermine O, et al. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alfa. N Engl J Med. 1995;332(26):1749–51. doi: 10.1056/NEJM199506293322604. [DOI] [PubMed] [Google Scholar]
- 74.White JD, et al. The combination of zidovudine and interferon alpha-2B in the treatment of adult T-cell leukemia/lymphoma. Leukemia & lymphoma. 2001;40(3-4):287–94. doi: 10.3109/10428190109057927. [DOI] [PubMed] [Google Scholar]
- 75.Bazarbachi A, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(27):4177–83. doi: 10.1200/JCO.2010.28.0669. [DOI] [PubMed] [Google Scholar]
- 76.Fukushima T, et al. Allogeneic hematopoietic stem cell transplantation provides sustained long-term survival for patients with adult T-cell leukemia/lymphoma. Leukemia. 2005;19(5):829–34. doi: 10.1038/sj.leu.2403682. [DOI] [PubMed] [Google Scholar]
- 77.Hishizawa M, et al. Transplantation of allogeneic hematopoietic stem cells for adult T-cell leukemia: a nationwide retrospective study. Blood. 2010;116(8):1369–76. doi: 10.1182/blood-2009-10-247510. [DOI] [PubMed] [Google Scholar]
- 78*.Kawada H, et al. A retrospective analysis of treatment outcomes in adult T cell leukemia/lymphoma patients with aggressive disease treated with or without allogeneic stem cell transplantation: A single-center experience. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015;21(4):696–700. doi: 10.1016/j.bbmt.2014.12.020. This retrospective study compared the effects of chemotherapy with alloHSCT for treatment of ATL. The authors found the use of alloHSCT is limited in chemorefractory patients with aggressive ATL disease, meaning chemosensitive patients were more receptive to alloHSCT and overall survival. [DOI] [PubMed] [Google Scholar]
- 79.Bomford R, Kazanji M, De The G. Vaccine against human T cell leukemia-lymphoma virus type I: progress and prospects. AIDS research and human retroviruses. 1996;12(5):403–5. doi: 10.1089/aid.1996.12.403. [DOI] [PubMed] [Google Scholar]
- 80.Rodriguez SM, et al. Preventive and therapeutic strategies for bovine leukemia virus: lessons for HTLV. Viruses. 2011;3(7):1210–48. doi: 10.3390/v3071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Suehiro Y, et al. Clinical outcomes of a novel therapeutic vaccine with Tax peptide-pulsed dendritic cells for adult T cell leukaemia/lymphoma in a pilot study. British journal of haematology. 2015;169(3):356–67. doi: 10.1111/bjh.13302. [DOI] [PubMed] [Google Scholar]
- 82.Sugata K, et al. Protective effect of cytotoxic T lymphocytes targeting HTLV-1 bZIP factor. Blood. 2015;126(9):1095–105. doi: 10.1182/blood-2015-04-641118. [DOI] [PubMed] [Google Scholar]
- 83.Panfil AR, Al-Saleem JJ, Green PL. Animal Models Utilized in HTLV-1 Research. Virology : research and treatment. 2013;4:49–59. doi: 10.4137/VRT.S12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Villaudy J, et al. HTLV-1 propels thymic human T cell development in "human immune system" Rag2(−)/(−) gamma c(−)/(−) mice. PLoS pathogens. 2011;7(9):e1002231. doi: 10.1371/journal.ppat.1002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aryee KE, Shultz LD, Brehm MA. Immunodeficient mouse model for human hematopoietic stem cell engraftment and immune system development. Methods in molecular biology. 2014;1185:267–78. doi: 10.1007/978-1-4939-1133-2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]