Abstract
Objective
To characterize the risk and predictors of growth during observation of vestibular schwannomas (VS).
Study Design
Retrospective case series.
Setting
Single academic, tertiary care center.
Patients
564 consecutive VS patients who underwent at least two MRI studies prior to intervention.
Intervention(s)
Serial MRI studies
Main outcome measure(s)
Tumor growth, defined as a ≥2 mm increase in the maximum tumor diameter between consecutive MRI studies, or between the first and last study.
Results
A total of 1,296 patients (1995–2015) with VS were identified. Of those, 564 patients (median age 59.2 years; 53.5% female) were initially observed and underwent multiple MRI studies (median follow-up 22.9 months, interquartile range [IQR] 11.7 – 42.7). The median maximum tumor diameter at presentation was 1.00 cm (IQR 0.6 – 1.51 cm). In all, 40.8% of tumors demonstrated growth and 32.1% underwent intervention (21.5% microsurgery, 10.5% radiation) during the surveillance period. Multivariable Cox regression analysis showed that for each tumor, the risk of growth or intervention was significantly increased for larger initial VS diameters (HR=2.22; 95% CI: 1.90 – 2.61) and when disequilibrium was a presenting symptom (HR=1.70; 95% CI: 1.30 – 2.23). Patient age, gender, aspirin use and presenting symptoms of asymmetric hearing loss, tinnitus, and vertigo, were not associated with tumor growth.
Conclusions
To date, this is the largest series of observed VS reported in the literature. Risk of VS growth is significantly increased among patients who present with larger tumors and who have concomitant disequilibrium.
IRB
151481
Define Professional Practice Gap & Educational Need
No cohort with this sample size has assessed vestibular schwannoma growth rates in conjunction with this number of variables.
Learning Objective
To characterize vestibular schwannoma growth rates and predictors of growth.
Keywords: acoustic neuroma, natural history, observation, skull base, vertigo, vestibular schwannoma
Introduction
A vestibular schwannoma (VS) is a benign neoplasm of Schwann cells originating from one of the vestibular nerves. They can originate anywhere laterally from the glial-schwannian junction, up until their nerve terminations, with earlier reports suggesting Scarpa’s ganglion, located laterally in the internal auditory canal (IAC), or more medially at the glial-schwannian cell junction, also known as the Obersteiner-Redlich zone.(1,2) Temporal bone studies report VS incidence rates of around 1%; however, epidemiological studies report lower incidences, ranging between 1–20 VS per million inhabitants per year. The recent increase in VS incidence has been linked to greater MRI use and its improved availability, image precision and fidelity.(3–7)
Historically, VS were surgically resected shortly following diagnosis. However, studies have suggested that a significant percentage of VS either do not grow, or grow very slowly. Combined with quality of life studies that have shown that intervention should be reserved for patients who demonstrate significant growth or “intractable symptoms amenable to intervention,” more clinicians and patients are collectively electing to initially observe VS.(8,9) This is supported by two independent Surveillance, Epidemiology and End Results (SEER) database studies, which have documented a trend toward increased rates of VS observation, rather than microsurgery or radiation therapy.(10,11)
With this paradigm shift towards observation, several studies have investigated VS growth rates in an attempt to help improve patient counseling. However, significant heterogeneity exists in reported growth rates, ranging from 15–85%.(12–20) Reasons for this variability include inconsistent definitions of growth and measurement techniques. Two systematic reviews synthesized this disparate evidence and reported 43% and 46% composite growth rates.(3,21) Prior to this study, the largest series to date of 552 VS, reported growth rates of 17.0% and 28.9% for intrameatal and extrameatal tumors, respectively.(18)
Uncertainty regarding VS growth rates underscores the need for more robust data to help inform patient-centered decision-making and manage expectations in a patient population that is increasingly choosing surveillance as opposed to initial intervention. The present study leverages the senior author’s 20-year experience managing VS with observation to quantify the risk of, and predictors for, VS growth.
Methods
After obtaining Institutional Review Board approval (#151481), all patients diagnosed with a VS between 1995 and 2015 were identified in the electronic medical record (EMR). Patients were included if they had at least two MRI studies available for review. Excluded, were patients with previously treated VS, neurofibromatosis type 2 (NF2) diagnoses, MRI studies demonstrating lesions other than a VS, or less than 2 MRI studies available for review prior to undergoing treatment (surgery or radiation) or being lost to follow-up.
Variables Collected
Extracted from each VS patient’s EMR was age at initial presentation (years), gender (M/F), presenting symptoms (asymmetric hearing loss (Y/N), tinnitus (Y/N), disequilibrium (Y/N), vertigo (Y/N), and aspirin use at presentation (Y/N)). “Vertigo” was documented if the patient described a “spinning sensation,” while “disequilibrium” collectively represented patients who reported dizziness, unsteadiness, imbalance, or disequilibrium.
Primary Outcome
MRI studies, with particular emphasis on the gadolinium-enhanced T1-weighted sequence, were reviewed to confirm the VS diagnosis. The primary outcome was tumor growth, defined as 2 mm or greater increase in the largest axial tumor diameter between any two MRI studies, or between the first and last MRI studies.
Management Algorithm
Recognizing that management of VS is tailored to an individual patient’s unique clinical presentation and personal preferences regarding treatment, our general institutional algorithm for managing VS is shown in Figure 1. In brief, patients who present with VS are presented the option to pursue surgery, radiation, or observation. If observation is chosen initially, another MRI study is obtained approximately 12 months following the initial MRI study. However, those patients with VS larger than 2 cm in their maximum diameter and/or with brainstem compression are offered either microsurgery or radiosurgery, taking into account a multitude of other patient-specific factors (e.g., age, medical comorbidities, patient preference). Intervention is also typically recommended if serial imaging demonstrates VS growth, with reconsideration of the same factors mentioned above.
Figure 1.

Generalized treatment algorithm for all presenting vestibular schwannomas
Statistical Analysis
A total of 564 patients had complete data and were included in the analyses. Patients were classified as having experienced tumor growth if the size of the tumor increased by at least 2 mm, resulting in two comparison groups (tumor growth vs. no tumor growth). We then calculated descriptive statistics for each group and tested the significance of differences between the groups using the Wilcoxon rank sum test for continuous variables and the Chi-square test for categorical variables. Results were summarized as medians and interquartile ranges (IQR) for continuous variables and as percentages for categorical variables.
Next, we defined follow-up as the time between a patient’s first and last MRI study for those without intervention or growth, or the time between the first MRI and the MRI just prior to growth or intervention. Censoring occurred at the time point when a patient had tumor growth or intervention, whichever occurred first.
To test whether tumor size at baseline determined tumor growth, we categorized tumor diameter at diagnosis in 0.5 cm increments (≤0.50 cm, 0.51–1.00 cm, 1.01–1.50 cm, 1.51–2.00 cm, and ≥2.00 cm) and performed Kaplan-Meier (KM) analyses to estimate the probability of tumor growth within each category. We used the log-rank test to determine the significance of the differences in the tumor growth curves.
To identify variables that may be independently associated with tumor growth, we fitted Cox regression models to calculate hazard ratios (HR) and associated 95% confidence intervals (CI) for tumor growth. Age and sex were included in all models, while presenting symptoms at baseline (e.g., asymmetric hearing loss, tinnitus, disequilibrium, vertigo, and aspirin use) were evaluated as potential cofounders/risk factors for tumor growth. In secondary analyses, we treated tumor size at diagnosis as a continuous variable and estimated HR (95% CI) for tumor growth associated with 1 cm increases in tumor size. P-values ≤0.05 were considered statistically significant. All analyses were performed with STATA 12MP (StataCorp; College Station, TX) or SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
A total of 1,296 patients were diagnosed with VS (1995 – 2015). Of the 564 that met inclusion criteria, 53.5% were female and most were between 50 to 66 years of age (median 59.2 years, IQR 50.5 – 66.9). Median follow-up was 22.9 months (IQR 11.7 – 42.7 months), over which time most patients had between 2 – 4 MRI scans (median 3, IQR 2 – 4). At diagnosis, the median maximum tumor diameter was 1.00 cm (IQR 0.6 – 1.51 cm). Growth (≥2 mm) was observed in 40.8% (n=230) during follow-up. Approximately 1/3 of patients diagnosed with VS ultimately underwent intervention (32.1%, n=182), with a median time to treatment of 16.2 months (IQR 9.6 – 28.4). Descriptive statistics for the whole study sample are shown in Table 1.
Table 1.
Descriptive Statistics and Presenting Symptom Incidences
| N/Median | Frequency/IQR | ||
|---|---|---|---|
| Patients | 564 | ||
| Patient Age (years) | 59.2 | 50.5 – 66.9 | |
| Sex (Female) | 302 | 53.5% | |
| Follow-Up (months) | 22.9 | 11.7 – 42.7 | |
| MRI Studies/Patient | 3 | 2 – 4 | |
| Maximum Tumor Diameter | 1.00 cm | 0.6 – 1.51 cm | |
| Growing Tumors | 230 | 40.8% | |
| Intervention | 182 | 32.1% | |
| Presenting Symptoms | Asymmetric Hearing Loss | 436 | 77.3% |
| Tinnitus | 353 | 62.6% | |
| Disequilibrium | 219 | 38.8% | |
| Vertigo | 106 | 18.8% | |
| Aspirin Use | 158 | 28.0% |
Univariate Analysis
Patient and tumor characteristics were compared between those that did or did not experience tumor growth (Table 2). In this analysis, VS growth was associated with older patients (60.2 years vs. 58.2 years, p=0.02), those presenting with symptoms of asymmetric hearing loss (82.6% vs. 73.7%, p=0.01), disequilibrium (46.5% vs. 33.5%, p=0.002), and larger initial tumor diameter (p<0.001).
Table 2.
Univariate analysis.
| No Growth (<0.2 cm; n=334) |
Growth (≥0.2 cm; n=230) |
P-value | ||
|---|---|---|---|---|
| Age [years] (median, IQR) | 58.2 (49.3 – 65.1) | 60.2 (50.8 – 69.8) | 0.02* | |
| Sex (% Female) | 51.5% | 56.5% | 0.24 | |
| Presenting Symptoms | Asymmetric HL (n=436) | 73.7% | 82.6% | 0.01* |
| Tinnitus (n=353) | 62.9% | 62.2% | 0.86 | |
| Disequilibrium (n=219) | 33.5% | 46.5% | 0.002* | |
| Vertigo (n=106) | 20.1% | 17.0% | 0.35 | |
| Aspirin Use | 27.0% | 30.0% | 0.5 | |
| Presenting Tumor Diameter | ≤0.5 cm | 24.9% | 10.4% | <0.001*† |
| 0.51 – 1.0 cm | 33.5% | 30.9% | ||
| 1.01 – 1.50 cm | 18.0% | 28.7% | ||
| 1.51 – 2.0 cm | 13.2% | 18.3% | ||
| >2.0 cm | 10.5% | 11.7% | ||
| Intervention | 15.9% | 56.1% | <0.001* |
P-values < 0.05 are considered statistically significant.
Data analysis was conducted with ANOVA. IQR: interquartile range; HL: hearing loss
Kaplan-Meier Analyses
KM analyses were performed to illustrate the risk of growth or intervention in this cohort over the follow-up period. Two analyses were completed. In the first, the risk of growth among all included VS was considered (Figure 2). By 22 months from baseline, 50% of the tumors had experienced growth or had undergone intervention. We observed a significant (Log Rank p<0.0001) monotonic increase in risk of tumor growth or intervention with increase in tumor size at baseline (Figure 3). For example, within 9.4 months of follow-up, 50% of tumors ≥2.00 cm at baseline had grown or underwent intervention, while it took 77 months for 50% of tumors ≤0.50 cm to grow or undergo intervention. Data by year are provided in Table 3.
Figure 2.
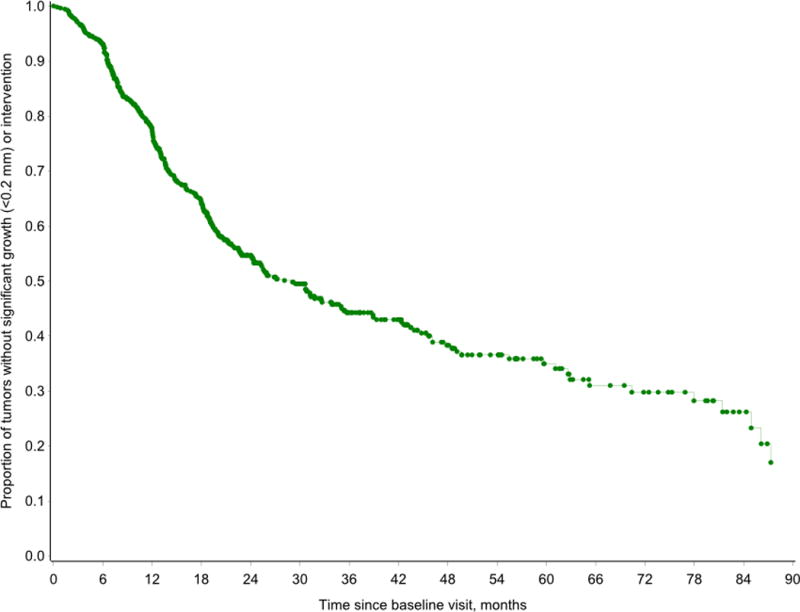
Kaplan-Meier analysis demonstrating the risk of growth or intervention among all included vestibular schwannomas. By 22 months from baseline, 50% of the tumors had experienced growth or had undergone intervention.
Figure 3.
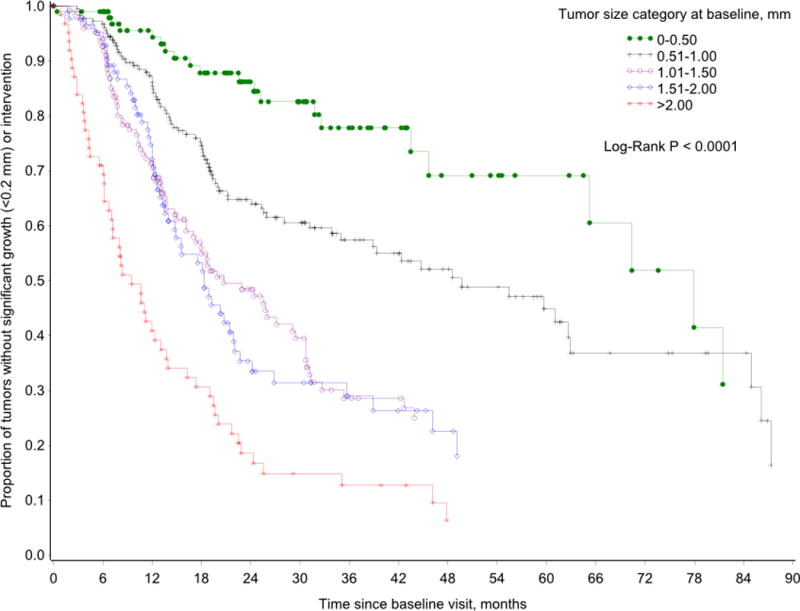
Kaplan-Meier analysis demonstrating the risk of growth or intervention for vestibular schwannomas categorized based on their presenting tumor diameter. For tumors ≥2.00 cm at baseline, 50% had grown or underwent intervention within 9.4 months of follow-up. Similarly, it took 77 months for 50% of presenting tumors ≤0.50 cm to grow or undergo intervention.
Table 3.
Observed rates of tumor growth or intervention stratified by presenting tumor diameter. All tumors in the first row are listed based on their maximum tumor diameter at presentation. The percentages are the observed rates of growth for each stratified tumor diameter at presentation. As one moves down the table, tumors are removed when they either demonstrated growth, underwent intervention, or no further MRI study was obtained. For example, looking at the bottom row, while 19 patients had demonstrated no tumor growth with at least 9 years of follow-up, one patient’s tumor still grew for the first time in the 10th year of surveillance.
| Presenting Tumor Diameter | |||||
|---|---|---|---|---|---|
| Follow-up year (tumors) | ≤0.5 cm (growth or intervention/tumors) | 0.51 – 1.0 cm | 1.01 – 1.5 cm | 1.51 – 2.0 cm | >2.0 cm |
| 1 (564) | 3.7% (4/107) |
9.8% (18/183) |
23.8% (30/126) |
19.8% (17/86) |
27.4% (17/62) |
| 2 (419) | 8.0% (7/88) |
19.9% (31/156) |
20.8% (20/96) |
38.2% (21/55) |
25.0% (6/24) |
| 3 (268) | 6.7% (4/60) |
7.2% (8/111) |
23.6% (13/55) |
6.7% (2/30) |
25.0% (3/12) |
| 4 (179) | 4.7% (2/43) |
5.3% (4/76) |
5.9% (2/34) |
5.6% (1/18) |
12.5% (1/8) |
| 5 (114) | 0.0% (0/26) |
8.0% (4/50) |
0.0% (0/22) |
8.3% (1/12) |
0.0% (0/4) |
| 6 (74) | 10.0% (2/20) |
9.1% (3/33) |
0.0% (0/12) |
0.0% (0/6) |
0.0% (0/3) |
| 7 (53) | 11.8% (2/17) |
0.0% (0/21) |
0.0% (0/8) |
0.0% (0/5) |
0.0% (0/2) |
| 8 (32) | 0.0% (0/12) |
23.1% (3/13) |
20.0% (1/5) |
0.0% (0/1) |
0.0% (0/1) |
| 9 (31) | 5.6% (1/18) |
0.0% (0/9) |
0.0% (0/3) |
0.0% (0/1) |
|
| 10 (19) | 11.1% (1/9) |
0.0% (0/7) |
0.0% (0/3) |
||
Multivariable Analyses
In Cox regression analyses adjusted for age, gender, disequilibrium, and presenting tumor diameter, only presenting tumor diameter and disequilibrium at presentation were associated with increased risk of tumor growth or intervention (Table 4). Specifically, compared to tumors ≤0.50 cm in diameter, the risk of growth or intervention was 2.05-fold higher (95% CI: 1.26 – 3.32) for tumors measuring 0.51 – 1.00 cm and 7.61-fold higher (95% CI: 4.55 – 12.72) for those tumors ≥2.00 cm. Presenting tumor diameter was also analyzed as a continuous variable, showing a 2.22-fold increased risk of growth or intervention for every centimeter increase in size at presentation (HR=2.22; 95% CI: 1.90 – 2.61) (p<0.0001). Patients who presented with disequilibrium had 68% increased risk (95% CI: 32 – 214%) of tumor growth or intervention when compared to those without it at initial presentation. Though disequilibrium and tumor size at baseline were independently associated with tumor growth or intervention, there was no evidence for interaction between the two variables (p>0.05).
Table 4.
Multivariable analyses assessing hazard ratios of tumor growth or undergoing intervention.
| Hazard Ratio | 95% CI | ||
|---|---|---|---|
| Tumor Diameter | 0.51 – 1.0 cm | 2.045* | 1.259 – 3.322 |
| 1.01 – 1.50 cm | 3.770* | 2.302 – 6.172 | |
| 1.51 – 2.0 cm | 3.966* | 2.368 – 6.643 | |
| >2.0 cm | 7.606* | 4.549 – 12.720 | |
| Age | 0.997 | 0.988 – 1.006 | |
| Sex | 1.206 | 0.946 – 1.538 | |
| Disequilibrium | 1.683* | 1.321 – 2.144 | |
| Asymmetric Hearing Loss | 1.167 | 0.845 – 1.611 |
Considered statistically significant
When censoring those patients who underwent intervention, both presenting tumor diameter and disequilbirum were still associated with increased risk of growth. When compared to tumors ≤0.50 cm in diameter, the risk of growth was 1.79-fold higher (95% CI: 1.11 – 2.90) for tumors measuring 0.51 – 1.00 cm, 3.20-fold higher (95% CI: 1.95 – 5.25) for tumors 1.01 cm – 1.50 cm, 2.94-fold higher (95% CI: 1.73 – 4.99) for tumors 1.51 cm – 2.00 cm, and 3.67-fold higher (95% CI: 2.06 – 6.51) for those tumors ≥2.00 cm. Patients who presented with disequilibrium had a 70.2% increased risk (95% CI: 30.0 – 123%) of tumor growth when compared to those without it at initial presentation.
Discussion
Current VS management trends primarily involve surveillance for most tumors, recognizing that a substantial percentage do not grow and will not need extirpation.(10,11) Growth is often used as a metric for which surgeons begin to counsel patients regarding the possible need for intervention. But the natural history of this rare tumor has been difficult to characterize due to its low incidence (15:1,000,000). As such, identification of factors that predict growth would assist in clinical decision-making and patient counseling.
The present large case series, which represents the largest to date, harnessed the senior author’s 20-year experience at our high-volume VS center to provide important prognostic information on the risk and predictors of tumor growth. We observed that disequilibrium and tumor size at presentation are the strongest variables significantly associated with tumor growth. While the two variables were independently associated with VS growth, there was no evidence for their interaction. In Cox regression analyses, an apparent dose-response relationship was observed with tumor size; for every centimeter greater the tumor was at baseline, the risk of growth (≥2 mm) was increased by over 2-fold. Analyzing categorized baseline tumor sizes and using tumors ≤0.5 cm as the reference tumor, those tumors sized 0.51 – 1.00 cm, 1.01 – 1.50 cm, 1.51 – 2.00 cm, and ≥2 cm, had 1.79, 3.20, 2.94 and 3.67 times higher risk of growth, respectively.
The percentage of tumors that demonstrated growth in the present series differs from rates observed in a previous large case series (n=552).(18) In particular, the previous study reported 17.0% and 28.9% growth rates in intrameatal and extrameatal VS, respectively. The current study found that over 40% of tumors grew over the follow-up period. It is possible that these differences relate to methodological and definitional differences since the median age in the study by Stangerup et al. (59 years) is identical to our case series. In addition, they defined intrameatal tumor growth if an intrameatal tumor showed extrameatal extension. Though extrameatal growth was defined as a size increase ≥2 mm, similar to that used herein, this variable growth definition risks underestimating VS growth percentage by ignoring tumors limited to the IAC that may enlarge, but fail to develop extrameatal extension.
Importantly, there were consistent findings between Stangerup et al. and the present study.(18) Specifically, they reported extrameatal tumors demonstrated significantly more growth than those tumors limited to the IAC.(18) The implication, similar to our analyses, is that larger tumors had greater propensity to grow than smaller tumors. This finding has also been corroborated by other studies. Agrawal et al. found that tumor size at diagnosis is predictive of growth, suggesting that larger tumors at diagnosis are reflective of past growth and thus indicative of a biological inclination to grow.(22) In another study, while not necessarily predicting growth, Malhotra et al. reviewed 202 conservatively managed VS patients, reporting that for every 1-mm increase of the initial tumor size, the chance the patient would require intervention increased 14%.(23)
To graphically demonstrate the risk of VS growth as a function of time, we used KM analyses. Using a similar approach with a smaller sample size, Solares et al. reviewed 110 VS patients and found that 70.6% tumors did not grow at 5-years.(24) Similarly, in another study, Jethanamest et al. applied KM analyses to 94 patients to assess the risk of intervention, reporting that 69.1% of patients continued tumor observation at 5 years.(25) In our study, 50% of tumors had experienced growth or had undergone intervention by 22 months since the initial MRI. However, it is important to note that KM analyses are not adjusted for covariates. Thus in our study, growth differences by baseline tumor size observed in KM analyses were tested in the Cox regression model. This showed that the association was robust independent of age, sex and other covariates.
We also noted, following adjusted analysis, that patients who presented with disequilibrium (i.e., dizziness, imbalance, or unsteadiness not associated with a “spinning sensation”) had 70.2% increased risk of tumor growth compared to those without this symptom at presentation. This is in agreement with a number of other studies.(23,25,26) Beenstock et al. performed a post hoc multivariable analysis and reported that symptoms of vertigo and unsteadiness were significantly related to tumor growth.(26) In addition, Jethanamest et al. found that disequilibrium at presentation was also significantly associated with tumor growth, with an adjusted odds ratio of 2.96.(25) And while Malhotra et al. did not define disequilibrium, they also showed that patients presenting with disequilibrium had 3.42 greater odds of tumor growth.(23) However, while we calculated an adjusted hazard ratio of tumor growth of 1.70 in the present study in those patients presenting with disequilibrium, it is important to note that odds ratios are not directly comparable to the hazard ratios due to the underlying assumptions surrounding logistic regression models. Odds ratios do not account for variable follow-up times and instead presume homogeneous follow-up among included subjects. Thus, we employed Cox regression analysis to more accurately represent covariate associations with tumor growth.
To the best of our knowledge, this is the largest study of observed VS to date. Not only do our results reinforce previously documented relationships between the maximum tumor diameter at presentation and disequilibrium with tumor growth, but lack of association between patient age(9,19,22,27), gender(9,19,27), hearing loss(9,27) and tinnitus(25) corroborate results from previously smaller studies. Nonetheless, our findings contradict a few previously reported statistically significant associations with VS growth; these include tinnitus(9,22) and aspirin use(28). While there may truly be no association between tumor growth and tinnitus or aspirin use, sampling error, growth definitions, and recall bias, among other issues, could account for the discrepancy between our results and previous findings. In addition, our large sample size with long-term follow-up enabled us to identify patients whose tumors first demonstrated growth after upwards of 8, 9, and 10 years following the initial MRI study, which has not been previously reported. While we tend to obtain MRI studies on an annual basis in first 5 years of observation, we begin to lengthen the interval between studies beyond 5 years if tumors have remained stable in size. Nonetheless, our results demonstrate that tumors may grow at a significant later date following presentation, which can help guide long-term surveillance MRI studies. This highlights the need for long-term surveillance of patients with VS and provides useful information regarding the need for continued MRI surveillance, even if prior serial imaging failed to demonstrate significant tumor growth.
Despite the large size of this study and the number of variables we analyzed, several limitations must be addressed. Retrospectively reviewing patient medical records limits the accuracy of patient self-report and weakens the associations one can make with presenting symptoms. In addition, to account for different imaging parameters between MRI studies, it is generally accepted that VS growth is defined as a ≥2 mm increase in the greatest tumor diameter. Thus, extremely slow growing tumors, less than 2 mm between scans, would be excluded from analyses. Though we tried to accommodate slower growing tumors by comparing the first and last MRI study, tumors growing even slower would probably not require intervention. Lastly, though our median follow-up length was 22.9 months, our mean follow-up was 31.6 months, the latter of which is the more commonly reported descriptor of central tendency. While our mean follow-up is consistent with prior studies, the median is more appropriate because mean values can be strongly influenced by outliers in follow-up.
Conclusion
In the largest study of observed VS to date, we found that less than half (40.8%) of tumors demonstrated significant growth at a median follow-up of 23 months. Larger maximum tumor diameter at diagnosis was predictive of tumor growth; specifically, for every centimeter increase in tumor diameter at presentation, the likelihood of growth increased more than two-fold. Furthermore, we found that disequilibrium at presentation independently increased the likelihood of growth by 70.2% when compared to those patients who do not report disequilibrium at the time of presentation. These findings provide clinically useful information when counseling patients regarding management strategies for VS.
Acknowledgments
The authors would like to thank Amy Woosley, Loretta Russell, Forrest Tallent, and David Whelan with their help identifying and pulling records from storage.
Financial Material & Support: Internal departmental funding was utilized without commercial sponsorship or support. Salary support for DOF was provided by National Institute of Health – National Institute for Deafness and Communication Disorders K23DC013559 and L30DC012687.
Footnotes
Conflict(s) of Interest to Declare: DSH is a consultant for Advanced Bionics Corp., Cochlear Corp., MED-EL GmbH, Stryker, Synthes, Grace Medical and Oticon. GBW is a consultant for Advanced Bionics Corp., Cochlear Corp., and MED-EL GmbH, and Oticon. AR is a consultant for Advanced Bionics Corp., Cochlear Corp., and MED-EL GmbH, Grace Medical, and Olympus.
Institutional Review Board Approval: Data Integrated Study Console of Vanderbilt’s Research Enterprise (DISCOVR-E) IRB – 151481
References
- 1.Xenellis JE, Linthicum FH., Jr On the myth of the glial/schwann junction (Obersteiner-Redlich zone): origin of vestibular nerve schwannomas. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2003;24:1. doi: 10.1097/00129492-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Roosli C, Linthicum FH, Jr, Cureoglu S, et al. What is the site of origin of cochleovestibular schwannomas? Audiology & neuro-otology. 2012;17:121–5. doi: 10.1159/000331394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshimoto Y. Systematic review of the natural history of vestibular schwannoma. Journal of neurosurgery. 2005;103:59–63. doi: 10.3171/jns.2005.103.1.0059. [DOI] [PubMed] [Google Scholar]
- 4.Tos M, Charabi S, Thomsen J. Incidence of vestibular schwannomas. The Laryngoscope. 1999;109:736–40. doi: 10.1097/00005537-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Tos M, Stangerup SE, Caye-Thomasen P, et al. What is the real incidence of vestibular schwannoma? Arch Otolaryngol Head Neck Surg. 2004;130:216–20. doi: 10.1001/archotol.130.2.216. [DOI] [PubMed] [Google Scholar]
- 6.Moffat DA, Kasbekar A, Axon PR. Growth characteristics of vestibular schwannomas. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2012;33:1053–8. doi: 10.1097/MAO.0b013e3182595454. [DOI] [PubMed] [Google Scholar]
- 7.Kurland LT. The frequency of intracranial and intraspinal neoplasms in the resident population of Rochester, Minnesota. Journal of neurosurgery. 1958;15:627–41. doi: 10.3171/jns.1958.15.6.0627. [DOI] [PubMed] [Google Scholar]
- 8.Carlson ML, Tveiten OV, Driscoll CL, et al. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. Journal of neurosurgery. 2015;122:833–42. doi: 10.3171/2014.11.JNS14594. [DOI] [PubMed] [Google Scholar]
- 9.Ferri GG, Modugno GC, Pirodda A, et al. Conservative management of vestibular schwannomas: an effective strategy. The Laryngoscope. 2008;118:951–7. doi: 10.1097/MLG.0b013e31816a8955. [DOI] [PubMed] [Google Scholar]
- 10.Carlson ML, Habermann EB, Wagie AE, et al. The Changing Landscape of Vestibular Schwannoma Management in the United States–A Shift Toward Conservatism. Otolaryngology–head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;153:440–6. doi: 10.1177/0194599815590105. [DOI] [PubMed] [Google Scholar]
- 11.Babu R, Sharma R, Bagley JH, et al. Vestibular schwannomas in the modern era: epidemiology, treatment trends, and disparities in management. Journal of neurosurgery. 2013;119:121–30. doi: 10.3171/2013.1.JNS121370. [DOI] [PubMed] [Google Scholar]
- 12.Battaglia A, Mastrodimos B, Cueva R. Comparison of growth patterns of acoustic neuromas with and without radiosurgery. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2006;27:705–12. doi: 10.1097/01.mao.0000226302.59198.87. [DOI] [PubMed] [Google Scholar]
- 13.Anand VT, Kerr AG, Byrnes DP, et al. Non-surgical management of acoustic neuromas. Clin Otolaryngol Allied Sci. 1992;17:406–10. doi: 10.1111/j.1365-2273.1992.tb01683.x. [DOI] [PubMed] [Google Scholar]
- 14.Charabi S, Tos M, Thomsen JC, et al. [Vestibular schwannoma. A new interpretation of tumor growth] Ugeskr Laeger. 2000;162:5497–500. [PubMed] [Google Scholar]
- 15.Wiet RJ, Zappia JJ, Hecht CS, et al. Conservative management of patients with small acoustic tumors. The Laryngoscope. 1995;105:795–800. doi: 10.1288/00005537-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Massick DD, Welling DB, Dodson EE, et al. Tumor growth and audiometric change in vestibular schwannomas managed conservatively. The Laryngoscope. 2000;110:1843–9. doi: 10.1097/00005537-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Strasnick B, Glasscock ME, 3rd, Haynes D, et al. The natural history of untreated acoustic neuromas. The Laryngoscope. 1994;104:1115–9. doi: 10.1288/00005537-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Stangerup SE, Caye-Thomasen P, Tos M, et al. The natural history of vestibular schwannoma. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2006;27:547–52. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- 19.Fucci MJ, Buchman CA, Brackmann DE, et al. Acoustic tumor growth: implications for treatment choices. The American journal of otology. 1999;20:495–9. [PubMed] [Google Scholar]
- 20.Fayad JN, Semaan MT, Lin J, et al. Conservative management of vestibular schwannoma: expectations based on the length of the observation period. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2014;35:1258–65. doi: 10.1097/MAO.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 21.Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. The Laryngoscope. 2005;115:450–4. doi: 10.1097/00005537-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Agrawal Y, Clark JH, Limb CJ, et al. Predictors of vestibular schwannoma growth and clinical implications. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2010;31:807–12. doi: 10.1097/MAO.0b013e3181de46ae. [DOI] [PubMed] [Google Scholar]
- 23.Malhotra PS, Sharma P, Fishman MA, et al. Clinical, radiographic, and audiometric predictors in conservative management of vestibular schwannoma. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2009;30:507–14. doi: 10.1097/MAO.0b013e31819d3465. [DOI] [PubMed] [Google Scholar]
- 24.Solares CA, Panizza B. Vestibular schwannoma: an understanding of growth should influence management decisions. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2008;29:829–34. doi: 10.1097/MAO.0b013e318180a4c4. [DOI] [PubMed] [Google Scholar]
- 25.Jethanamest D, Rivera AM, Ji H, et al. Conservative management of vestibular schwannoma: Predictors of growth and hearing. The Laryngoscope. 2015;125:2163–8. doi: 10.1002/lary.25159. [DOI] [PubMed] [Google Scholar]
- 26.Beenstock M. Predicting the stability and growth of acoustic neuromas. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2002;23:542–9. doi: 10.1097/00129492-200207000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Bakkouri WE, Kania RE, Guichard JP, et al. Conservative management of 386 cases of unilateral vestibular schwannoma: tumor growth and consequences for treatment. Journal of neurosurgery. 2009;110:662–9. doi: 10.3171/2007.5.16836. [DOI] [PubMed] [Google Scholar]
- 28.Kandathil CK, Dilwali S, Wu CC, et al. Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otology & neurotology: official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2014;35:353–7. doi: 10.1097/MAO.0000000000000189. [DOI] [PubMed] [Google Scholar]


