Abstract
Background
Major depressive disorder is associated with disturbed circadian rhythms. To investigate the causal relationship between mood disorders and circadian clock disruption, previous studies in animal models have employed light/dark manipulations, global mutations of clock genes, or brain area lesions. However, light can impact mood by noncircadian mechanisms; clock genes have pleiotropic, clock-independent functions; and brain lesions not only disrupt cellular circadian rhythms but also destroy cells and eliminate important neuronal connections, including light reception pathways. Thus, a definitive causal role for functioning circadian clocks in mood regulation has not been established.
Methods
We stereotactically injected viral vectors encoding short hairpin RNA to knock down expression of the essential clock gene Bmal1 into the brain's master circadian pacemaker, the suprachiasmatic nucleus (SCN).
Results
In these SCN-specific Bmal1-knockdown (SCN-Bmal1-KD) mice, circadian rhythms were greatly attenuated in the SCN, while the mice were maintained in a standard light/dark cycle, SCN neurons remained intact, and neuronal connections were undisturbed, including photic inputs. In the learned helplessness paradigm, the SCN-Bmal1-KD mice were slower to escape, even before exposure to inescapable stress. They also spent more time immobile in the tail suspension test and less time in the lighted section of a light/dark box. The SCN-Bmal1-KD mice also showed greater weight gain, an abnormal circadian pattern of corticosterone, and an attenuated increase of corticosterone in response to stress.
Conclusions
Disrupting SCN circadian rhythms is sufficient to cause helplessness, behavioral despair, and anxiety-like behavior in mice, establishing SCN-Bmal1-KD mice as a new animal model of depression.
Keywords: Circadian clocks, Corticosterone, Depression, Learned helplessness, Mouse model, Suprachiasmatic nucleus
Our environment is characterized by recurring daily changes caused by the rotation of the earth. To optimally adjust their behavior, metabolism, and physiology to such predictable environmental changes, most living organisms evolved internal timekeeping systems—so-called circadian clocks. Cells of most tissues express clock genes in an approximately 24-hour rhythm. The hypothalamic suprachiasmatic nucleus (SCN) is the center of this clock network (1). The SCN regulates the period and the phase of all other clocks in the brain and the rest of the body and synchronizes them with the external light/dark cycle. However, neuronal pathways conveying retinal photic input also reach other brain regions as well as peripheral tissues, such as the adrenal, and recent evidence suggests that these pathways may allow non-SCN clocks to remain synchronized even when the SCN clock is nonfunctional (2–6).
Major depressive disorder (MDD) and bipolar disorder are associated with disturbed circadian rhythms, indicated by disruptions of daily processes such as sleep, appetite, and hormonal release (7–11) and weaker rhythms of clock gene expression in brains of patients with MDD (12). However, whether disruptions of circadian clocks are causes or consequences of mood disorders remains elusive (13). On one hand, exposing rats to chronic mild stress to induce depression-like behavior reduces amplitude of locomotor activity, body temperature, and corticosterone rhythms (14,15). In mice, chronic mild stress reduces circadian rhythm amplitude in the SCN and basolateral amygdala but increases amplitude in the nucleus accumbens (16,17). On the other hand, other studies suggest that circadian rhythm abnormalities may cause mood disorders. In humans, environmental disruption of circadian rhythms, such as in shift work, increases the risk of depression (18). Mice with genetically disturbed circadian clocks often show depression-like or manic-like behavior. However, owing to pleiotropic, noncircadian functions of clock genes, mood phenotypes of such mice cannot be attributed unequivocally to circadian disruption. Moreover, clock gene mutations producing similar circadian phenotypes do not always produce similar mood-related phenotypes (13). Manipulating lighting conditions also leads to changes of both circadian and mood-related behavior (19). However, light/dark manipulations can have a substantial impact on mood regulation independent of circadian clocks (e.g., by causing cell death or neurotransmitter switching of neurons in mood-regulating brain areas) or increased inflammatory factors (19–21). Lesions of the SCN lead to reduced behavioral despair (22). However, SCN lesions also destroy neurons and disrupt neuronal connections, including photic input pathways to other brain regions that have functions other than circadian rhythm regulation (23). In fact, SCN lesions can actually reverse the behavioral effects of SCN rhythm disruption in memory tasks, perhaps because an anatomically intact but dysrhythmic SCN produces an actively disruptive output signal (24).
In this study, we present a mouse model with Bmal1 short hairpin RNA (shRNA) induced suppression of circadian rhythms restricted to the SCN. These mice developed normally; were kept under normal lighting conditions; and had intact light input pathways, intact SCN neurons and efferent connections, and intact molecular clocks outside the SCN; therefore, we can exclude many of the sorts of noncircadian effects caused by global knockouts of clock genes, light/dark manipulations, or SCN lesions. Our data reveal that selectively disturbing rhythms in the SCN master circadian pacemaker causes helplessness, behavioral despair, and anxiety-like behavior as well as increased sensitivity to stress.
Methods and Materials
Animals
All experiments were done in male mPer2Luciferase (PER2∷LUC) mice 8–14 weeks old. In PER2∷LUC knockin mice, the wild-type circadian clock gene Per2 has been replaced by homologous recombination with a construct incorporating the firefly luciferase Luc gene in tandem with wild-type Per2, such that a bioluminescent PER2∷LUC fusion protein is expressed under control of all Per2 regulatory elements (25). In our mice, the reporter construct also incorporated an SV40 polyadenylation site to enhance expression levels (26), and mice were back-crossed to produce a congenic C57BL/6J background. Unless otherwise stated, mice were maintained in 12:12 light/dark cycles (12 hours light, 12 hours dark). Beginning 6 days before stereotactic adeno-associated virus (AAV) injections, mice were singly housed with continuous access to water and food. We attempted to minimize the number of animals used and animal pain and distress. Mouse studies were conducted in accordance with regulations of the Institutional Animal Care and Use Committee at the University of California, San Diego.
Virus Production
To make complementary DNA encoding our shRNAs and suitable to produce AAV vectors, we first used polymerase chain reaction to amplify a sequence encoding the U6 promoter, the scrambled 19-nucleotide shRNA sequence, and the cytomegalovirus–green fluorescent protein (CMV-GFP) cassette from pLL3.7 vector (pLL3.7 Scr shRNA was kindly provided by Dr. Ulrike Heberlein, University of California, San Francisco [Addgene plasmid no. 59299]) and subcloned it into the cDNA pAAV-DIO-ChR2(H134R)-eYFP (kindly provided by Dr. Karl Deisseroth, Stanford University), using MluI and XhoI sites (the latter destroyed during cloning) to produce the vector pAAV-U6-SCRshRNA-CMV-GFP-WPRE. Sequencing confirmed gene sequence integrity. Two 19-nucleotide shRNA sequences targeting Bmal1 messenger RNA (GenBank: 11865) were kindly provided by Dr. Andrew C. Liu, University of Memphis: Bmal1-shRNA1 = 5′-GTCGATGGTTCAGTTTCAT and Bmal1-shRNA2 = 5′-GCATCGATATGATAGATAA. As controls, we used two scrambled shRNAs (SCR-shRNA = 5′-GCGCTTAGCTGTAGGATTC from the vector pLL3.7 and 19-nucleotide SCR2-shRNA = 5′-GCAACAAGATGAAGAGCAC) showing no significant alignment with any mouse messenger RNA. Complementary oligonucleotides encoding shRNAs were synthesized (Invitrogen, Grand Island, NY), annealed, and cloned in pAAV-U6-SCRshRNA-CMV-GFP-WPRE by ligation after HpaI and XhoI vector digestion.
Recombinant AAV-U6-SCR1shRNA-CMV-GFP, AAV-U6-SCR2shRNA-CMV-GFP, AAV-U6-Bmal1AshRNA-CMV-GFP, and AAV-U6-Bmal1BshRNA-CMV-GFP were transfected into the 293A cell line (Invitrogen) using the polyethylenimine transfection method along with the Ad helper vector pXX680 and a plasmid encoding capsid serotype 8. The 293A cells were collected 72 hours after transfection and lysed by three freeze/thaw cycles. Recombinant AAVs from the cleared supernatant were purified on an iodixanol gradient and subsequently concentrated in an Amicon Ultra-4 Centrifugal Filter 50K device (Millipore, Billerica, MA).
Virus Injection
Mice were anesthetized with ketamine/dexmedetomidine in 0.9% saline (ketamine, 70 mg/kg body weight; dexmedetomidine, 0.3 mg/kg body weight) before bilateral stereotactic injection of AAV particles in the SCN (anteroposterior, 0.46 mm posterior to bregma; mediolateral, 0.2 mm lateral to midline; dorsoventral, 5.5 mm below dura mater). About 0.5–0.8 μL of virus was injected into each position over ∼30 minutes using a Picospritzer (Parker Hannifin, Hollis, NH). All mice received a mixture of two shRNAs: either both Bmal1 or both scrambled shRNAs. To allow diffusion of the virus, the injection pipette remained immobile for 1 minute before moving more ventral and for 3 minutes before removing it. After surgery, mice were injected with 0.05 mg atipamezole for reversal of dexmedetomidine. Efficiency of Bmal1-knockdown (Bmal1-KD) was determined as described in Supplemental Methods and Materials.
Behavioral Tests
To measure locomotor activity rhythms, SCN-Bmal1-KD mice and control mice were singly housed in running wheel-equipped cages, and locomotor activity was monitored under different lighting conditions. During the first 6 days, mice were maintained in 12:12 light/dark cycles (12 hours light, 12 hours dark) with light levels at 200 lux. Subsequently, mice were kept in constant darkness (dark/dark) for 13 days. Sucrose preference, open field test, tail suspension test, and learned helplessness were performed as described previously (27). Detailed descriptions of these and other behavioral tests are provided in Supplemental Methods and Materials. Sucrose preference tests were performed twice per week with 1% sucrose water starting 6 days before AAV injections and continuing until mice were sacrificed at the end of all experiments. All other behavioral tests started 3 weeks after AAV injections to allow time for full expression of Bmal1 shRNA. Testing began with the least stressful tests, followed by the more stressful tests, and finally the most stressful procedure, induction of learned helplessness.
Brain Slice Culture and PER2∷LUC Measurements
After all behavioral tests were complete, mice were anesthetized with isoflurane and killed by cervical dislocation, and organotypic SCN explants were prepared as described previously (28). PER2∷LUC expression patterns were measured using a LumiCycle luminometer (Actimetrics, Wilmette, IL) as described previously (28).
Blood Sampling and Corticosterone Measurements
Details about the preparation of blood samples are provided in Supplemental Methods and Materials. Corticosterone concentrations were measured using a commercially available radio-immunoassay kit (MP Biomedicals, Santa Ana, CA). Plasma samples were diluted 1:200.
24-Hour Profile
Mice were transferred to a separate room 1 day before blood sampling began. Lights were turned off at the regular time of the 12:12 light/dark cycle and then remained off until all blood samples were collected. Blood was sampled every 4 hours beginning at 7:00 am under dim red light. During sampling, mice were not restrained and were allowed to explore the bench area freely.
Restraint Stress
To measure stress-related corticosterone release, mice were transferred to mouse restrainers (Plas-Labs Inc., Lansing, MI) for 30 minutes. Blood was collected immediately before restraint stress (0 minutes) and after 10 minutes, 30 minutes, 60 minutes, and 90 minutes of restraint. All blood samples were collected between 6 and 9 hours after lights on.
Data Analysis
Statistical analyses were conducted using GraphPad Prism (GraphPad Software, La Jolla, CA). Corticosterone release profiles were fit to Fourier curves with two harmonics using CircWave (developed by Roelof Hut, University of Groningen, Netherlands). Bioluminescence data were analyzed using LumiCycle Analysis software (Actimetrics, Wilmette, IL). Amplitude was determined over 5 days by fitting a sine wave [LM fit (Sin)] to 24-hour running average baseline-subtracted data. To normalize amplitude to the size of cultured tissues, the sine wave amplitude was divided by the baseline bioluminescence brightness of each individual explant. The first day of measurement was excluded from analyses. Wheel-running activity was analyzed using ClockLab software (Actimetrics). The first 3 days of the dark/dark condition were excluded from analysis. Data that were significant outliers (p < .05) in the Grubb test and >3 SDs from the mean of the remaining values were excluded. Details about statistical tests used for individual experiments can be found in the figure legends.
Results
SCN Bmal1-KD In Vivo Suppresses PER2∷LUC Rhythmicity In Vitro
To test the effect of disrupted SCN clocks on mood-related behaviors in mice, we generated AAV vectors encoding shRNAs designed to target and downregulate Bmal1 expression (Figure 1A). The Bmal1-shRNA reduces BMAL1 protein levels by ∼60% (Figure 1B, C). After all behavioral experiments were completed, we sacrificed the animals and prepared organotypic SCN explants. We used these explants to confirm the correct injection site of the AAV particles and to determine the efficiency of the Bmal1-KD for each individual animal. The injection site was identified by the GFP reporter that was encoded by all our AAV vectors (Figure 1D and Supplemental Figure S1A, B). Animals that showed no GFP expression in the SCN were excluded from analyses (Supplemental Figure S1C). Reduced amplitude of PER2∷LUC rhythms in SCN explants provided a quantitative estimate of the efficiency of Bmal1 knockdown. In mice injected with Bmal1 shRNA, amplitude of SCN PER2∷LUC rhythms was decreased by ∼80%, and period was lengthened compared with control mice injected with scrambled sequence shRNA (Figure 1E, F). Of 10 mice with successfully targeted Bmal1 shRNA, SCN rhythms were of drastically reduced amplitude in nine and completely absent in one.
Figure 1.
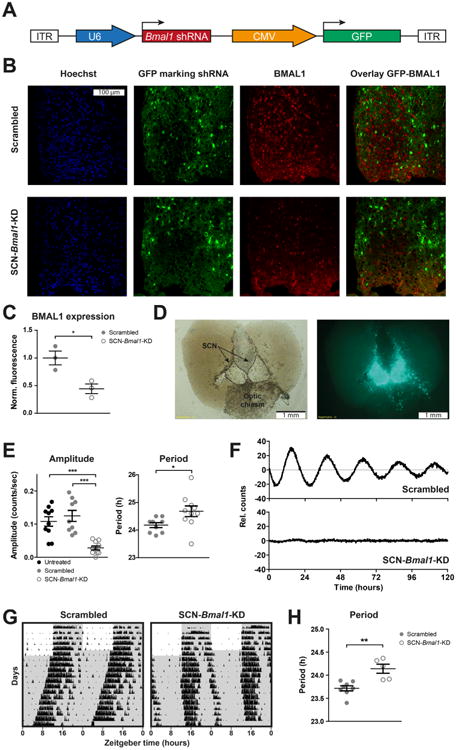
Knockdown of Bmal1 expression abolishes circadian rhythms in suprachiasmatic nucleus (SCN). (A) Adeno-associated virus (AAV) expression constructs encoding green fluorescent protein (GFP) and short hairpin RNAs (shRNAs) targeting Bmal1. (B) SCN of mice were injected with AAVs carrying Bmal1-knockdown (Bmal1-KD) or scrambled shRNA sequences as well as a GFP reporter. Fluorescence images of representative fields show cell nuclei marked by Hoechst staining (blue), transduced cells marked by GFP (green), and BMAL1 protein levels marked by immunolabeling (red) from SCN-Bmal1-KD and control mice. The overlay shows that most Bmal1-shRNA transfected cells show reduced BMAL1 levels. (C) Immunofluorescence labeling reveals an ∼60% reduction of BMAL1 protein levels in the SCN of mice injected with Bmal1-shRNA relative to control mice. Data are shown as mean ± SEM; *p ≤ .05, t4 = 3.657 (Student t test); n = 3. (D) After AAV injections and subsequent behavioral experiments, coronal organotypic SCN explants of all mice were prepared (left) to confirm the correct location of injections based on GFP expression patterns (right) and to determine the efficiency of Bmal1 knockdown based on amplitude of mPer2Luciferase (PER2∷LUC) rhythms. (E) On average, knockdown of Bmal1 expression reduces PER2∷LUC rhythm amplitude by ∼80% and significantly lengthens the PER2∷LUC circadian rhythm period. SCN explants from mice that received scrambled sequence control injections show PER2∷LUC rhythms similar in amplitude to PER2∷LUC rhythms from untreated mice. Amplitude data are shown as mean ± SEM; F22,6 = 15.98 (one-way analysis of variance with Bonferroni posttest; ***p ≤ .001); n = 9–10. Period data are shown as mean ± SEM; t17 = 2.279 (Student t test); n = 9–10. (F) Representative PER2∷LUC rhythms of SCN explants injected with AAV particles encoding scrambled shRNA sequences (top) and shRNA targeting Bmal1 RNA (bottom). (G) Representative actograms showing wheel-running activity during entrainment in a 12:12 light/dark cycle and subsequent constant darkness. Gray shading represents times of darkness. (H) SCN-Bmal1-KD mice display longer circadian free-running periods of locomotor activity in constant darkness. Data are shown as mean ± SEM; **p ≤ .01, t11 = 4.104 (Student t test); scrambled, n = 8; SCN-Bmal1-KD, n = 5. CMV, cytomegalovirus; ITR, inverted terminal repeat.
SCN-Bmal1-KD mice did not show GFP expression outside of the SCN, suggesting that non-SCN brain areas had intact molecular clocks (Supplemental Figure S2). To confirm that molecular clocks outside the SCN were not deficient, we cultured periaqueductal gray explants from SCN-Bmal1-KD mice and control mice. The periaqueductal gray was shown previously to express stable circadian rhythms in culture (28). We found that periaqueductal gray explants from SCN-Bmal1-KD mice and control mice were equally rhythmic (66.7% vs. 70% of slices rhythmic, n = 12 and n = 10, respectively), indicating that molecular clocks in brain areas outside of the SCN were still functional despite SCN-specific Bmal1-KD.
To test effects of SCN-specific Bmal1-KD on behavioral rhythms, mice were transferred to the dark/dark condition to monitor the endogenous SCN-driven (“free-running”) locomotor activity rhythm with running wheels. Similar to SCN explants from these mice, SCN-Bmal1-KD mice on running wheels exhibited a significantly longer circadian period compared with control mice but did not show any other circadian locomotor rhythm abnormalities (Figure 1G, H and Supplemental Figure S3).
To assess for possible tissue damage caused by the injection procedure or toxicity of shRNAs, injected and uninjected SCN explants were compared and showed no differences in either histologic appearance by bright field microscopy or amplitude of PER2∷LUC rhythms. To assess for possible toxicity specific to Bmal1 shRNA, SCN explants injected with Bmal1 shRNA versus scrambled sequence shRNA were compared and showed no differences in either cell morphology or 24-hour mean level of PER2∷LUC bio-luminescence, which is an adenosine triphosphate–requiring enzymatic reaction requiring cell viability. Thus, injection of shRNAs did not adversely affect anatomic structure of the SCN or viability of SCN neurons. These results confirm that AAV-directed downregulation of Bmal1 is sufficient to impair circadian rhythms in SCN and constitutes a good model to test the impact of impaired SCN circadian rhythms on mood-related behavioral changes in mice.
Disrupting SCN Rhythms Causes Helplessness, Behavioral Despair, Anxiety-like Behavior, and Weight Gain
Mice underwent behavioral tests 3 weeks after the shRNA injections to determine the effects of disrupting SCN circadian rhythms on mood. Beginning 6 days before shRNA injection, we also performed a sucrose preference test twice a week to test for gradual changes in hedonic responses to sucrose (Figure 2A). In the learned helplessness paradigm, reduced SCN rhythm amplitude induced by Bmal1-KD led to significant increases in escape latencies and number of escape failures (Figure 2A). Mice with disrupted SCN rhythms also showed a significantly higher immobility time in the tail suspension test compared with control animals (Figure 2B). In the light/dark box test, SCN-Bmal1-KD mice spent significantly less time in the light compartment (Figure 2F), indicating increased anxiety-like behavior, but were not impaired in their spatial preference or total activity in the open field test (Figure 2G).
Figure 2.
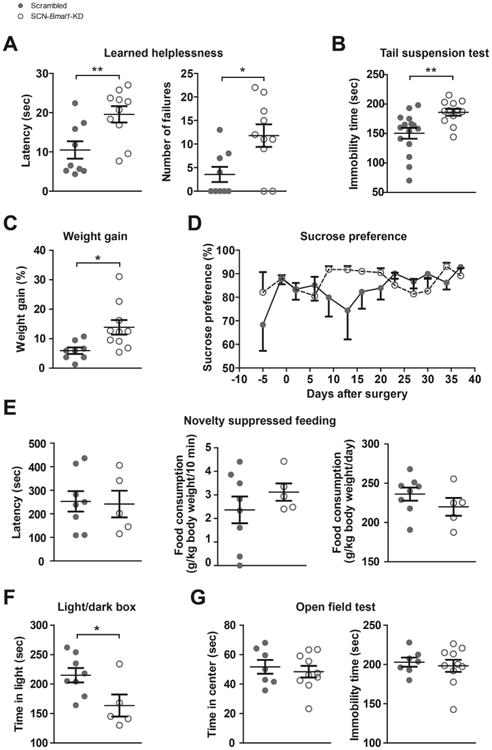
Disruption of circadian rhythms in the suprachiasmatic nucleus (SCN) leads to increased helplessness, despair, weight gain, and anxiety-related behavior. (A) In the learned helplessness paradigm, Bmal1-knockdown (Bmal1-KD) in SCN increases escape latency times (left) and number of escape failures (right). Data are shown as mean 6 SEM; *p ≤ .05, **p ≤ .01; latency time, t17 = 2.997; escape failures, t17 = 2.801 (Student t test); n = 9–10. (B) In the tail suspension test, Bmal1-KD in SCN increases immobility time. Data are shown as mean ± SEM; **p ≤ .01; t25 = 3.067 (Student t test); n = 12–15. (C) During the 5 weeks after adeno-associated virus injection, SCN-Bmal1-KD mice gain significantly more weight than control mice. Data are shown as mean ± SEM; *p ≤ .05; t16 = 2.693 (Student t test); n = 8–10. (D) In the sucrose preference test, Bmal1-KD in SCN has no significant impact on preference for sweet water. Data are shown as mean 6 SEM; interaction, F10,120 = 1.189, p = .352; time, F1,120 = 1.355, p = .2093; genotype, F10,120 = .8537, p = .3737; post hoc test, not significant (two-way repeated measures analysis of variance with Bonferroni posttest); n = 9–10. (E) Suppression of SCN rhythms does not change the aversion to eating in a novel environment (expressed as latency to begin eating) or total food intake (expressed per 10 minutes during the test or per day). Data are shown as mean ± SEM; not significant; latency time, t11 = 0.1600; food intake in novel environment, t11 = 0.9643; daily food intake, t11 = 1.173 (Student t test); n = 5–8. (F) SCN-Bmal1-KD mice spend significantly less time in the light compartment of a light/dark box, which is conventionally interpreted as an increase in anxiety-related behavior. Data are shown as mean ± SEM; *p ≤ .05; t11 = 2.430 (Student t test); n = 5–8. (G) In the open field test, Bmal1-KD in SCN does not alter spatial preference or total activity. Data are shown as mean ± SEM; not significant; time in center, t15 = 0.5319; immobility time, t15 = 0.4397 (Student t test); n = 7–10.
Because mood disorders are often associated with metabolic disorders, we also tested weight gain and found that over the course of 5 weeks, SCN-Bmal1-KD mice gained significantly more weight than control mice (Figure 2C). Knockdown of Bmal1 in the SCN had no significant impact on preference for sweet sucrose water at any time point (sucrose preference test) (Figure 2D) or on the latency to approach and eat food in a novel environment (novelty-suppressed feeding test) (Figure 2E). In this test, total food uptake was similar in SCN-Bmal1-KD mice and control mice.
Inescapable Stress Is Not Required for Helpless Behavior in SCN-Bmal1-KD Mice
Because some mood-related behavior changes in SCN-Bmal1-KD mice were evident before inescapable stress (IS), we investigated the importance of stress in more detail by testing for helplessness even in the absence of IS in a new group of SCN-Bmal1-KD mice and control mice. In these experiments, we tested active avoidance by performing only the testing phase of the learned helplessness paradigm, in which animals always had the opportunity to escape. Even without any previous exposure to IS, SCN-Bmal1-KD mice displayed decreased active avoidance, with significantly longer escape latencies and more failures compared with control mice (Figure 3). Thus, helplessness appears to develop spontaneously in SCN-Bmal1-KD mice. When testing sensitivity to gradually increasing electric shock intensity, SCN-Bmal1-KD mice and control mice displayed no differences, demonstrating that the differences in active avoidance were not due to altered pain sensitivity (Supplemental Figure S4).
Figure 3.
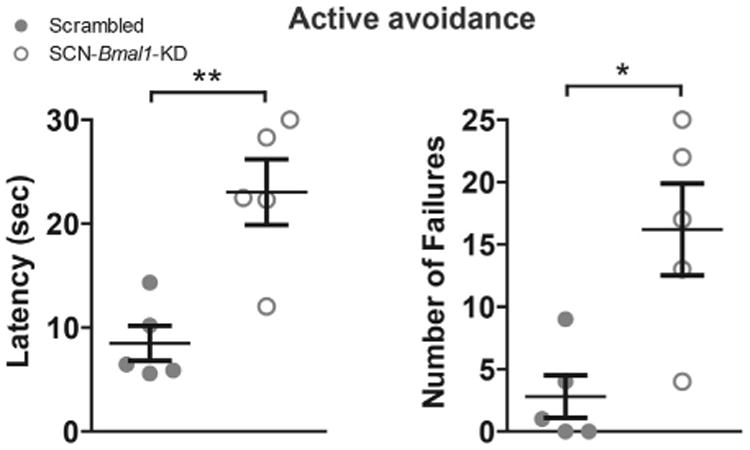
Disruption of suprachiasmatic nucleus (SCN) circadian clock function leads to a state of helplessness as manifested by decreased active avoidance of foot shock. Even without previous exposure to inescapable stress, Bmal1-knockdown (Bmal1-KD) in SCN leads to higher escape latency times (left) and increased numbers of escape failures during testing in the learned helplessness shuttle boxes. Data are shown as mean ± SEM; *p ≤ .05, **p ≤ .01; latency time, t8 = 4.069; escape failures, t8 = 3.301 (Student t test); n = 5.
Stress Response System Is Altered in SCN-Bmal1-KD Mice
The circadian clock and the stress response system are closely related, and abnormal glucocorticoid levels are associated with the development of mood disorders (29). For this reason, we investigated diurnal plasma corticosterone rhythms of SCN-Bmal1-KD mice and control mice under constant environmental conditions as well as corticosterone release in response to restraint stress. Control animals showed a single clear corticosterone peak at the beginning of their subjective night when they normally become active (25 hours after “lights off”). In contrast, SCN-Bmal1-KD mice showed a second, even more pronounced, increase of corticosterone toward the end of their subjective night (33 hours after “lights off”). A two-way analysis of variance revealed a significant difference between the two groups over the time course studied (p = .01) (Figure 4A). When we stressed the mice by transferring them into a restrainer, SCN-Bmal1-KD mice and control mice showed a similar increase of corticosterone after the first 10 minutes of stress. However, after 30 minutes of stress, SCN-Bmal1-KD mice displayed significantly lower corticosterone levels than control mice. Analyses of these data by two-way analysis of variance indicated a statistically significant difference between SCN-Bmal1-KD mice and control mice (p = .0242) (Figure 4B).
Figure 4.
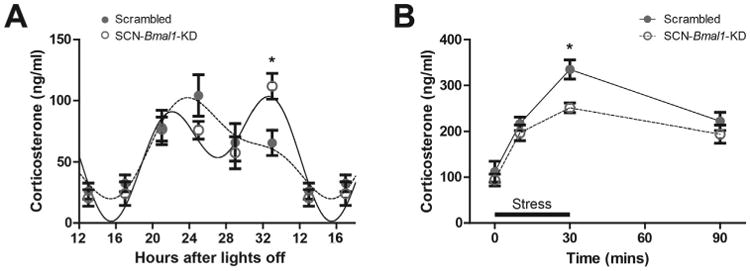
Suprachiasmatic nucleus–specific Bmal1-knockdown (SCN-Bmal1-KD) mice have altered stress hormone function. (A) Circadian patterns of corticosterone release in SCN-Bmal1-KD and control mice kept in constant darkness. In addition to the normal increase of corticosterone at the beginning of the subjective night (25 hours after “lights off”), SCN-Bmal1-KD mice have a second corticosterone peak at the end of subjective night (33 hours after “lights off”). Data are shown as mean ± SEM and Fourier-curve fits with two harmonics; interaction, F5,70 = 3.279, p = .0102; stress, F1,70 = 18.45, p ≤ .0001; genotype, F5,70 = 0.01066, p = .9192; post hoc test, *p ≤ .05 (two-way repeated measures analysis of variance with Bonferroni posttest); n = 8. (B) Corticosterone release of SCN-Bmal1-KD and control mice in response to acute restraint stress for 30 minutes. SCN-Bmal1-KD mice show an attenuated corticosterone increase. Data are shown as mean ± SEM; interaction, F3,39 = 1.23, p = .3101; stress, F1,39 = 38.45, p ≤ .0001; genotype, F3,39 = 6.500, p = .0242; post hoc test, *p ≤ .05 (two-way repeated measures analysis of variance with Bonferroni posttest); n = 7–8.
Discussion
In this study, we investigated whether disrupting rhythms in the central SCN circadian clock alters mood-related behavior. Mechanistic connections between disturbed circadian clocks and mood regulation have been evaluated before in rodent models (7,8,11). However, in previous studies, it was unclear whether circadian or noncircadian changes led to abnormal mood-related behavior (13). Manipulations of lighting conditions, light input pathways, or expression of genes involved in circadian rhythms always involve consequences that are independent of the actual diurnal oscillations produced by intrinsic circadian clocks. In contrast, our study provides an animal model with normally developed, anatomically intact brain and light input pathways and intact molecular clock gene machinery in all non-SCN brain regions and peripheral tissues, under normal 12:12 light/dark lighting conditions. To create such a model, we performed an shRNA-induced knockdown of Bmal1 expression restricted to the SCN. The efficiency of the knockdown was determined individually for each animal by the amplitude of PER2∷LUC expression rhythms of SCN explants prepared after completion of all behavioral experiments.
The Bmal1-KD SCN explants generally still showed significant circadian rhythms but with ∼80% lower amplitude and longer period compared with control SCN explants. The SCN-Bmal1-KD mice still showed circadian activity rhythms in the dark/dark condition but with longer free-running periods. Many patients with MDD display decreased circadian rhythm amplitude (30) and abnormally delayed melatonin (31,32) and behavioral rhythms (33). It is well known that a longer free-running period is typically associated with delayed phase of entrainment, suggesting that patients with MDD may also have abnormally long circadian periods. However, the locomotor activity rhythm is just one output of the central clock in the SCN. In view of strongly attenuated SCN rhythm amplitude, it is possible that other SCN-regulated rhythms are more strongly affected than locomotor activity rhythms. Furthermore, a long circadian SCN period in SCN-Bmal1-KD mice may promote internal desynchrony among brain areas and other tissues whose clocks are genetically intact. Circadian misalignment among brain regions could contribute to the development of mood-related abnormalities (9,28). Thus, in our mouse model, circadian rhythms in the SCN were greatly weakened and the period was lengthened, but rhythms were not completely eliminated, which is a realistic model of the kind of disruption most likely to occur in humans.
Disrupting circadian rhythms of the central circadian pacemaker in the SCN causes several robust mood-related behavioral changes. In particular, compared with control mice injected with a scrambled sequence shRNA, SCN-Bmal1-KD mice exhibit increases in behavioral despair, anxiety-like behavior, and helplessness. Even before exposure to the IS of the learned helplessness induction procedure, SCN-Bmal1-KD mice already show significantly increased immobility times in the tail suspension test, conventionally interpreted as a sign of increased behavioral despair (although this interpretation is controversial) (23,34). In addition, disrupting central circadian rhythms leads to increased anxiety-like behavior in the light/dark box test, manifesting as avoidance of a potentially dangerous environment. Furthermore, SCN-Bmal1-KD mice are more helpless than control mice, showing increased latencies or failures to escape foot shock. Running wheel activity measurements and the open field test revealed that the SCN-specific Bmal1-KD procedure does not alter levels of general locomotor activity. Just as for behavioral despair and anxiety-like behavior, helplessness in SCN-Bmal1-KD mice is already present at baseline, even before they are subjected to IS training. Although exposure to IS usually is seen as being essential for “learned helplessness,” “congenitally helpless” rats have been characterized that display increased active avoidance latencies and other signs of depression-like behavior without induction by IS (35). Accordingly, SCN-Bmal1-KD mice represent a new genetic animal model of helplessness, despair, and anxiety.
Not all mood-related behaviors are altered in SCN-Bmal1-KD mice. Hedonic behavior in the sucrose preference test, spatial preference in the open field test, and aversion to eating in a novel environment all are similar in SCN-Bmal1-KD mice and control mice. This indicates that specific aspects of mood-related behavior are influenced differently by the suppression of SCN circadian rhythms.
Because the SCN is not known to be directly involved in mood regulation, it is likely that disruption of the central clock affects mood by causing disturbed circadian rhythms in downstream systems that regulate mood more directly (9). One such system is the hypothalamic-pituitary-adrenal axis, which is tightly associated with both the circadian clock and mood regulation (29). In this study, we show that mice with disrupted SCN rhythms have abnormally high corticosterone levels toward the end of their subjective night, manifesting as a second peak of corticosterone release in addition to the expected increase at the beginning of the subjective night. Another study investigated corticosterone levels in a different SCN clock–deficient mouse model achieved by neuron-specific knockout of Bmal1 using Cre recombinase expressed under a Syt10 promoter (3). In that study, performed in a light/dark cycle, corticosterone rhythms were not different in SCN clock–deficient mice and control mice, suggesting that the light/dark cycle may compensate for the loss of SCN rhythms by directly modulating corticosterone release (3–5). Because our experiment was carried out in constant darkness, without potential “masking” influences of light, we were able to detect more directly the impact of SCN rhythms on rhythmic release of corticosterone from the adrenals. We found that SCN-Bmal1-KD mice display a reduced corticosterone response to stress, suggesting an essential role of the SCN clock in this response. This blunted corticosterone response to stress could explain the helpless behavior of SCN-Bmal1-KD mice, as attenuated levels of corticosterone were previously associated with escape deficits in learned helplessness (36-38). Thus, low-amplitude SCN rhythms may cause changes in mood-related behavior at least in part by altering corticosterone levels at baseline and in response to stress.
Another possibility is that knockdown of the SCN central circadian pacemaker leads to disturbed rhythms in downstream brain areas that are more directly involved in mood regulation (9,28). Such brain areas are likely to harbor less stable clocks than the SCN (39), and their circadian oscillations may be extremely disturbed in the presence of low-amplitude SCN rhythms. In a recent study, we found that certain brain regions implicated in mood regulation show disrupted rhythms in helpless mice (28). Most mice that become helpless in the learned helplessness procedure exhibit an absence of circadian rhythms in the nucleus accumbens and the periaqueductal gray. However, it remains to be determined whether disrupting circadian rhythms specifically in these areas, individually or in combination, is sufficient to cause changes in mood-related behavior.
Finally, we found that SCN-Bmal1-KD mice display increased weight gain, despite unchanged food intake. Many psychiatric disorders, including MDD, are associated with metabolic disturbances and obesity (40). Because circadian clocks control both affective and metabolic functions, we previously suggested that disturbed circadian rhythms may play an important role in mediating the comorbidity of metabolic and psychiatric disorders (40). Although our results are preliminary, they support the hypothesis that disturbed circadian rhythms lead to mood-related and metabolic abnormalities simultaneously in SCN-Bmal1-KD mice.
In conclusion, our data provide evidence that disturbed circadian clocks are sufficient for the development of depression-like behavior in different behavioral tests, helplessness at baseline as well as after stress, anxiety-like behavior, and weight gain. By using a mouse model with genetic disruption of circadian rhythms specific to the SCN but normal brain development and anatomy and a normal light/dark environment, we can exclude pleiotropic effects of clock genes in mood-regulating systems, loss of neuronal connections, and light-related factors in the development of mood-related behavioral abnormalities. Our data suggest that disturbed clocks affect the stress system and probably also circadian oscillators in mood-regulating brain areas, which may explain the increase of helplessness, behavioral despair, and anxiety-like behavior in SCN-Bmal1-KD mice (Figure 5). Future studies of non-SCN clocks in the stress system and other brain regions may provide more targeted chronotherapies for patients with mood disorders.
Figure 5.
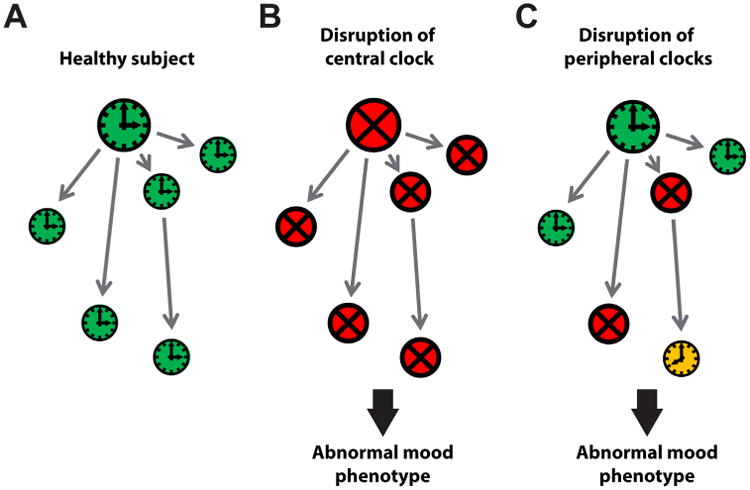
Alternative models of how disruption of the clock network could lead to abnormal mood phenotypes. (A) In healthy subjects, the clock network is intact and synchronized (green), including the suprachiasmatic nucleus master pacemaker (big clock) and various peripheral clocks, including brain clocks and clocks in other tissues such as the adrenal (small clocks). (B) If the suprachiasmatic nucleus master pacemaker is rendered non-rhythmic (red), all downstream peripheral clocks, including clocks important for mood regulation, are disturbed as well because their component cellular circadian oscillators are no longer synchronized by the suprachiasmatic nucleus. This leads to abnormal mood. (C) An abnormal mood phenotype may occur when only a subset of peripheral clocks is disturbed. This may involve loss of rhythms as a result of asynchronous component cellular oscillators (red) or loss of synchronization with other tissues, such as adrenal (yellow).
Supplementary Material
Acknowledgments
This work was supported by a Veterans Affairs Merit Award Grant No. 1I01BX001146 (to DKW) and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to DKW).
We thank Sandra Braun and Alan Turken from Dr. Richard Hauger's laboratory (Veterans Affairs San Diego Healthcare System) for their help with corticosterone measurements.
Footnotes
Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2016.03.1050.
References
- 1.Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Husse J, Leliavski A, Tsang AH, Oster H, Eichele G. The light-dark cycle controls peripheral rhythmicity in mice with a genetically ablated suprachiasmatic nucleus clock. FASEB J. 2014;28:4950–4960. doi: 10.1096/fj.14-256594. [DOI] [PubMed] [Google Scholar]
- 4.Kiessling S, Sollars PJ, Pickard GE. Light stimulates the mouse adrenal through a retinohypothalamic pathway independent of an effect on the clock in the suprachiasmatic nucleus. PloS One. 2014;9:e92959. doi: 10.1371/journal.pone.0092959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Boyce P, Barriball E. Circadian rhythms and depression. Aust Fam Physician. 2010;39:307–310. [PubMed] [Google Scholar]
- 8.Schnell A, Albrecht U, Sandrelli F. Rhythm and mood: Relationships between the circadian clock and mood-related behavior. Behav Neurosci. 2014;128:326–343. doi: 10.1037/a0035883. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy MJ, Welsh DK. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–352. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- 10.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kronfeld-Schor N, Einat H. Circadian rhythms and depression: Human psychopathology and animal models. Neuropharmacology. 2012;62:101–114. doi: 10.1016/j.neuropharm.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 2013;110:9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Landgraf D, McCarthy MJ, Welsh DK. The role of the circadian clock in animal models of mood disorders. Behav Neurosci. 2014;128:344–359. doi: 10.1037/a0036029. [DOI] [PubMed] [Google Scholar]
- 14.Ushijima K, Morikawa T, To H, Higuchi S, Ohdo S. Chronobiological disturbances with hyperthermia and hypercortisolism induced by chronic mild stress in rats. Behav Brain Res. 2006;173:326–330. doi: 10.1016/j.bbr.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 15.Gorka Z, Moryl E, Papp M. Effect of chronic mild stress on circadian rhythms in the locomotor activity in rats. Pharmacol Biochem Behav. 1996;54:229–234. doi: 10.1016/0091-3057(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 16.Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, McClung CA. Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biol Psychiatry. 2015;78:249–258. doi: 10.1016/j.biopsych.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savalli G, Diao W, Schulz S, Todtova K, Pollak DD. Diurnal oscillation of amygdala clock gene expression and loss of synchrony in a mouse model of depression. Int J Neuropsychopharmacol. 2014 Dec 11;18(5) doi: 10.1093/ijnp/pyu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg R, Doghramji PP. Is shift work making your patient sick? Emerging theories and therapies for treating shift work disorder. Postgrad Med. 2011;123:106–115. doi: 10.3810/pgm.2011.09.2465. [DOI] [PubMed] [Google Scholar]
- 19.Monje FJ, Cabatic M, Divisch I, Kim EJ, Herkner KR, Binder BR, et al. Constant darkness induces IL-6-dependent depression-like behavior through the NF-kappaB signaling pathway. J Neurosci. 2011;31:9075–9083. doi: 10.1523/JNEUROSCI.1537-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulcis D, Jamshidi P, Leutgeb S, Spitzer NC. Neurotransmitter switching in the adult brain regulates behavior. Science. 2013;340:449–453. doi: 10.1126/science.1234152. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez MM, Aston-Jones G. Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proc Natl Acad Sci USA. 2008;105:4898–4903. doi: 10.1073/pnas.0703615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tataroglu O, Aksoy A, Yilmaz A, Canbeyli R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res. 2004;1001:118–124. doi: 10.1016/j.brainres.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 23.Cryan JF, Mombereau C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez F, Lu D, Ha P, Costacurta P, Chavez R, Heller HC, et al. Circadian rhythm. Dysrhythmia in the suprachiasmatic nucleus inhibits memory processing. Science. 2014;346:854–857. doi: 10.1126/science.1259652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, et al. PERIOD2∷LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landgraf D, Long J, Der-Avakian A, Streets M, Welsh DK. Dissociation of learned helplessness and fear conditioning in mice: a mouse model of depression. PloS One. 2015;10:e0125892. doi: 10.1371/journal.pone.0125892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landgraf D, Long JE, Welsh DK. Depression-like behaviour in mice is associated with disrupted circadian rhythms in nucleus accumbens and periaqueductal gray. Eur J Neurosci. 2015;43:1309–1320. doi: 10.1111/ejn.13085. [DOI] [PubMed] [Google Scholar]
- 29.Landgraf D, McCarthy MJ, Welsh DK. Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Curr Psychiatry Rep. 2014;16:483. doi: 10.1007/s11920-014-0483-7. [DOI] [PubMed] [Google Scholar]
- 30.Souetre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, et al. Circadian rhythms in depression and recovery: Evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28:263–278. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 31.Buckley TM, Schatzberg AF. A pilot study of the phase angle between cortisol and melatonin in major depression—a potential biomarker? J Psychiatr Res. 2010;44:69–74. doi: 10.1016/j.jpsychires.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 32.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Kitamura S, Hida A, Watanabe M, Enomoto M, Aritake-Okada S, Moriguchi Y, et al. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol Int. 2010;27:1797–1812. doi: 10.3109/07420528.2010.516705. [DOI] [PubMed] [Google Scholar]
- 34.Pollak DD, Rey CE, Monje FJ. Rodent models in depression research: Classical strategies and new directions. Ann Med. 2010;42:252–264. doi: 10.3109/07853891003769957. [DOI] [PubMed] [Google Scholar]
- 35.Henn FA, Vollmayr B. Stress models of depression: Forming genetically vulnerable strains. Neurosci Biobehav Rev. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 36.Edwards E, Harkins K, Wright G, Henn F. Effects of bilateral adrenalectomy on the induction of learned helplessness behavior. Neuropsychopharmacology. 1990;3:109–114. [PubMed] [Google Scholar]
- 37.Chover-Gonzalez AJ, Jessop DS, Tejedor-Real P, Gibert-Rahola J, Harbuz MS. Onset and severity of inflammation in rats exposed to the learned helplessness paradigm. Rheumatology (Oxford) 2000;39:764–771. doi: 10.1093/rheumatology/39.7.764. [DOI] [PubMed] [Google Scholar]
- 38.Toloknov AV, Vvedenskaia OV, Bol'shakova TD, Buniatian AF, Khitrov NK, Fisenko VP. Blood corticosterone and resistance to hypoxia during operant learning and development of learned helplessness. Biull Eksp Biol Med. 1999;128:29–31. in Russian. [PubMed] [Google Scholar]
- 39.Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barandas R, Landgraf D, McCarthy MJ, Welsh DK. Circadian clocks as modulators of metabolic comorbidity in psychiatric disorders. Curr Psychiatry Rep. 2015;17:98. doi: 10.1007/s11920-015-0637-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


