Abstract
Purpose
Rhinitis is a nasal inflammatory disease in children and adolescents. However, little is known about the phenotypes and characteristics of allergic rhinitis (AR) in Korean children and adolescents. The objective of this study was to analyze the symptoms and comorbidities of rhinitis, to compare AR to non-allergic rhinitis (NAR), and to reveal the phenotypes and features of AR in a Korean pediatric population.
Methods
Patients under 18 years of age with rhinitis symptoms were recruited from January 2013 to January 2015 by pediatric allergists. We analyzed symptoms, phenotypes, comorbidities, and allergen sensitization in this cross-sectional, multicenter study.
Results
Medical records were collected from 11 hospitals. The AR group has 641 (68.3%) patients, with 63.2% of boys and 7.5 (±3.4) years of mean age. The NAR group has 136 (14.5%) patients, with 55.1% of boys and 5.5 (±2.9) years of mean age. Moderate-severe persistent AR affected 41.2% of AR patients. Nasal obstruction was more common in NAR patients (P<0.050), whereas AR patients sneezed more (P<0.050) and more commonly had conjunctivitis, asthma, and otitis media (P<0.050). Sinusitis was the most common comorbidity in both groups. Allergen sensitization was caused by house dust mites (HDMs) (90.2%), pollen (38.7%), and animal dander (24.8%) in AR patients. Pollen and animal dander sensitization significantly increased age-dependently (P<0.050), but 91.9% of AR patients were already sensitized to HDMs before 5 years old.
Conclusions
Our study revealed that AR was more prevalent than NAR and that 41.2% of AR presented with moderate-severe disease in Korean pediatric populations. Sinusitis was the most common comorbidity, and sleep disturbance was associated with the severity of rhinitis. The majority of AR patients were sensitized to HDMs in preschool ages. Further studies, including nationwide and longitudinal data, will help understand the relationship between these diseases.
Keywords: Rhinitis, allergic rhinitis, children, comorbidity, phenotype
INTRODUCTION
Rhinitis is a chronic inflammatory disease of the upper respiratory airways characterized by the cardinal symptoms of nasal congestion, rhinorrhea, sneezing, and nasal itching.1,2,3 The prevalence of rhinitis in Korean elementary school students increased from 15.5% in 1995 to 28.5% in 2006.4,5 According to data gathered via a questionnaire from the International Study of Asthma and Allergies in Childhood (ISSAC), in 2010 the prevalences of rhinitis were 28.5%, 38.0%, 38.5%, and 35.9% in children 0 to 3 years of age, 4 to 6, 7 to 9, and 10 to 13, respectively.6 Allergic rhinitis (AR) is classified as a type of rhinitis and defined as rhinitis symptoms plus positive allergen sensitization.2 The prevalence of AR is combined with measures of overall rhinitis' prevalence, and it is difficult to differentiate AR from other types of rhinitis in most data sets.7
Rhinitis has a harmful influence on the physical, social, and psychological health of children and adolescents.3,8,9 Comorbidities of rhinitis are common and also have a significant influence on quality of life and health.3 These comorbidities, which affect both children and adolescents, include asthma, conjunctivitis, otitis media, rhinosinusitis, and pollen-food syndrome, and can be used as markers of possible rhinitis.3,7 Sleep disturbance is a comorbidity that often results in impaired school performance and has negative effects on the quality of life of adolescents.3,10 Moreover, some of the principal allergic diseases in children often coexist with rhinitis.6,11
Therefore, the impacts and costs associated with comorbidities of rhinitis should be considered in the management of rhinitis in the pediatric population. However, there are few studies on the comorbidities of rhinitis in children. In addition, little is known about the phenotypes, characteristics, and epidemiology of rhinitis, including AR, in Korean children and adolescents.
We sought to analyze the comorbidities and phenotypes of rhinitis in Korean children and adolescents and to investigate sensitized allergens and their variety according to age through a cross-sectional, multicenter study design based on a physician-targeted questionnaire.
MATERIALS AND METHODS
Study design and population
This is an epidemiological, cross-sectional, multicenter study intended to evaluate the comorbidities and phenotypes of rhinitis in children and adolescents. Data were collected retrospectively from patients aged less than 18 years who were diagnosed with rhinitis between January 2013 and January 2015 by pediatric allergy specialists from 11 hospitals in South Korea. We analyzed symptoms, classification according to the Allergic Rhinitis and its Impact on Asthma (ARIA) guideline, comorbidities, and allergen sensitization. This study was approved by the Institutional Review Board of all participating hospitals.
Study outcomes
NAR and AR
If the patients had more than one of the symptoms of rhinitis, such as sneezing, rhinorrhea, nasal obstruction, and nasal itching, they were diagnosed with rhinitis.2 AR was diagnosed by doctors if the patient tested positive for sensitization to inhaled allergens.2 Rhinitis without sensitization to any of the allergens tested was defined as NAR.2 Using the ARIA guideline, AR was classified into intermittent/persistent and mild/moderate-severe types. Duration of AR was classified as intermittent (sympsymptoms <4 days a week or for <4 consecutive weeks) or persistent (symptoms lasting more than 4 days a week and for more than 4 consecutive weeks).2 Severity of AR was classified as mild or moderate/severe depending on sleep disturbance; impairment of daily activities, leisure, sport, school, and/or work; and troublesome symptoms.2
Definition of comorbidities
Rhinitis in children and adolescents can frequently present with associated comorbidities, such as conjunctivitis, asthma, sinusitis, sleep disturbance, atopic dermatitis, otitis media, and oral allergy syndrome. Conjunctivitis was defined by symptoms (i.e., red, itchy, watery eyes and eye rubbing) and signs.3 Asthma was defined by symptoms (cough, wheeze, and exercise-induced bronchospasm) and spirometry results for those >6 years of age.3 Atopic dermatitis was diagnosed by doctors according to the criteria proposed by Hanifin and Rajka13 and the Korean diagnostic criteria.12 Otitis media was diagnosed after ear examination by physicians.14 Rhinosinusitis was defined as prolonged symptoms of nasal obstruction, purulent rhinorrhea or postnasal drainage, and complaints, such as headache, facial pain, or cough.3 Sleep problems comprised a history of disturbed sleep, snoring, apnea, tiredness, and irritability.3 Oral allergy syndrome (OAS, pollen–food syndrome) was defined as oral pruritus or swelling that occurred due to cross-reactivity between aeroallergens, such as birch pollen, and fruits and vegetables, such as apples.15
Allergic sensitization
Allergic sensitization was defined as a positive skin prick test (SPT) and/or positive specific IgE test for house dust mites (HDMs), animal dander (mostly cat and dog), and pollens by a multiple antigen simultaneous test (MAST) or an Immuno-CAP. In SPT, common environmental allergens as well as positive and negative controls (histamine and saline, respectively) are introduced into the skin by a needle, and any immediate reaction (wheal and erythema) were read after 15 to 20 minutes.2,16 Result of the SPT is considered positive when the wheal size of allergen (A) is larger than that of histamine (H) (A/H ratio ≥1), the grade higher than 3+, and ≥3 mm in average of the longest and the shortest diameters.16,17,18 A specific IgE level of 0.35 kUA/L or greater on the Immuno-CAP system19 or more than class 3 by MAST was defined as a positive result.20
Statistical analysis
We investigated the relationship among symptoms, comorbidities, allergen sensitization, and severity and/or duration of NAR and AR in children and adolescents. All data are presented as number (n) and frequencies (%) for categorical variables, and mean and standard deviation (SD) for quantitative variables. Non-test patients were excluded when we compared the NAR and AR groups.
Pearson's χ2 test was used to evaluate 2×2 cross-table analyses. For 2×N cross-table analyses, the linear to linear χ2 test was used. All statistical analyses were performed using IBM SPSS ver. 22.0 (IBM Co., Armonk, NY, USA).
RESULTS
Demographic characteristics of subjects
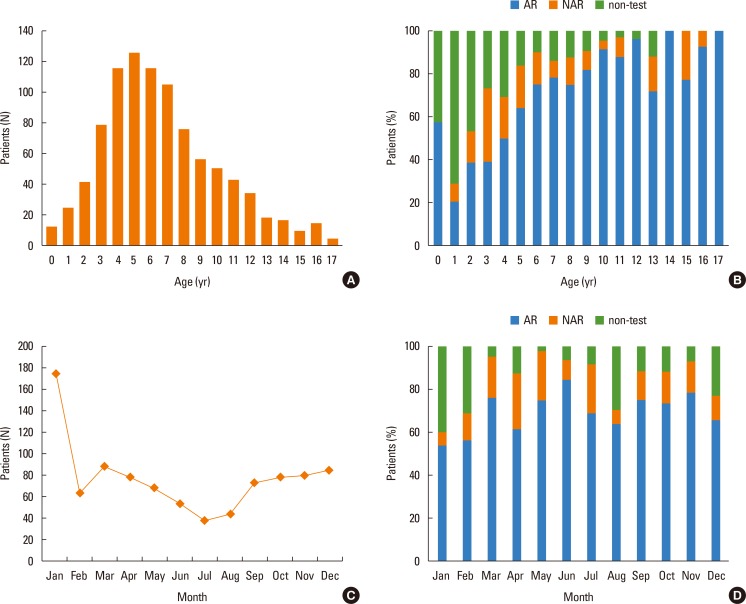
Eleven hospitals participated and 939 patients were recruited. Seven hospitals located in Seoul enrolled 645 patients, 3 hospitals located in Gyeonggi-do enrolled 203 patients, and 1 hospital located in Deagu enrolled 91 patients. All participating hospitals were located in an urban area. The patients consisted of 14.5% with NAR, and 68.3% with AR. The majority of the patients were boys (61.4% overall, 55.1% NAR and 63.2% AR). The mean age at presentation was higher in the AR than in the NAR group (7.5±3.4 vs 5.5±2.9 P<0.001; see Table 1). The peak age was 5 years old (126 patients, 13.4%) (Fig. 1A). A history of allergic disease in the family was reported for 681 patients (72.9%) and significantly more patients with a familial history had NAR (P=0.014) (Table 1). Twelve patients (1.3%) presented in infancy, and 514 (54.7%) patients were less than 6 years old (Fig. 1A). There were more AR patients even among those less than 1 year old (Fig. 1B). Most patients presented during the winter (December 9.2%, January 18.7% vs July 4.2%, August 4.8%) (Fig. 1C), and the distribution of AR and NAR did not vary by season (Fig. 1D).
Table 1. Characteristics of the subjects (N=939).
| Characteristics | Total | AR | NAR | P value |
|---|---|---|---|---|
| Numbers | 939 | 641 (68.3) | 136 (14.5) | |
| Boys, n (%) | 577 (61.4) | 405 (63.2) | 75 (55.1) | 0.080 |
| Age mean (SD), (yr) | 6.7 (3.4) | 7.5 (3.4) | 5.5 (2.9) | <0.001* |
| Familial history of allergic disease, n (%) | 681 (72.9) | 436 (68.0) | 107 (79.3) | 0.014* |
| ARIA classification, n (%) | ||||
| Mild | ||||
| Intermittent | 77 (12.1) | |||
| Persistent | 178 (27.9) | |||
| Moderate-severe | ||||
| Intermittent | 121 (18.9) | |||
| Persistent | 263 (41.2) | |||
| Rhinitis symptoms, n (%) | ||||
| Rhinorrhea | 725 (77.2) | 480 (74.9) | 99 (72.8) | 0.612 |
| Sneezing | 583 (62.1) | 407 (63.5) | 71 (52.2) | 0.014* |
| Nasal obstruction | 837 (89.1) | 556 (86.7) | 127 (93.4) | 0.031* |
| Nasal itching | 591 (62.9) | 385 (60.3) | 89 (65.4) | 0.268 |
| Rhinitis comorbidities, n (%) | ||||
| Conjunctivitis | 266 (31.6) | 219 (37.5) | 11 (11.2) | <0.001* |
| Asthma | 223 (24.1) | 189 (29.9) | 14 (10.3) | <0.001* |
| Atopic dermatitis | 238 (29.7) | 172 (29.1) | 46 (35.1) | 0.171 |
| Otitis media | 98 (11.5) | 60 (10.1) | 4 (3.8) | 0.041* |
| Sinusitis | 449 (48.4) | 272 (42.8) | 52 (38.2) | 0.324 |
| Sleep disturbance | 357 (38.6) | 211 (33.4) | 37 (27.2) | 0.162 |
| Oral allergy syndrome | 29 (4.8) | |||
| Atopic sensitization, n (%) | ||||
| House dust mites | 570 (73.1) | 570 (89.1) | ||
| Animal danders | 155 (20.3) | 155 (24.8) | ||
| Pollens | 230 (30.3) | 230 (37.0) |
Number (%) or Mean (SD); The number in the non-test group is 162 patients (17.3%); Eight patients of oral allergy syndrome did not have a specific allergen test result.
ARIA, allergic rhinitis and its impact on asthma; AR, allergic rhinitis; NAR, non-allergic rhinitis.
*P value <0.05.
Fig. 1. Frequency distribution of rhinitis in Korean children and adolescents. (A) The mean age of rhinitis patients is 6.7 (±3.4) at the time of presentation. The peak age is 5. The number of children who visit hospitals between ages 4 to 8 is higher than for other ages. (B) The number of patients with AR is higher than NAR at all ages. AR represents more than 70% of all cases ≥6 years. AR is also higher among those ≤1 year old. (C) Rhinitis patients visit mainly in the winter, and the peak month is January with fewer in the summer (June-August). (D) The percentage of patients with AR is higher than 50% regardless of season.
Clinical characteristics of rhinitis
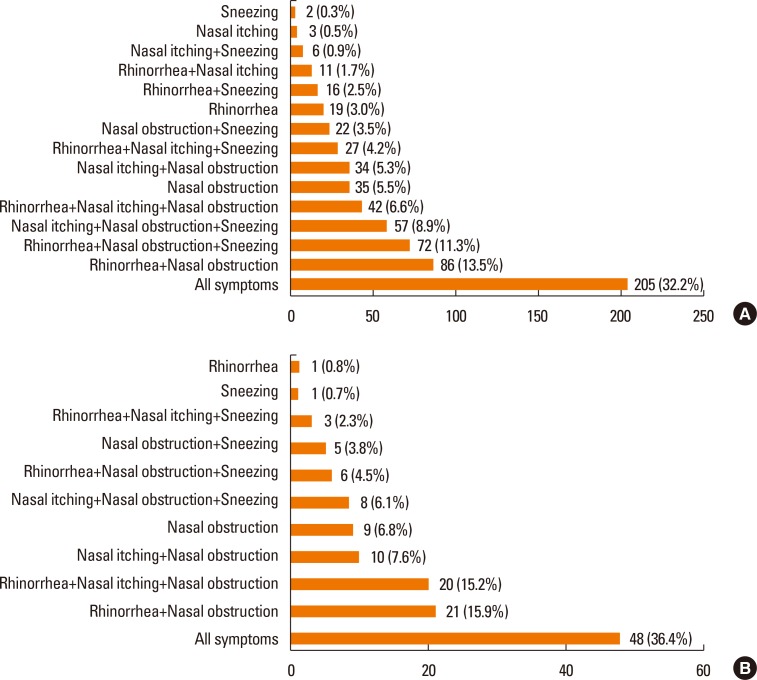
Nasal obstruction was the most common symptom in both NAR and AR, but significantly more patients with NAR (93.4%) than AR (86.7%) were affected (P=0.031) (Table 1). The number of patients who complained of sneezing was significantly larger in the AR (63.5%) than in the NAR group (52.2%) (P= 0.014) (Table 1). The frequency of rhinorrhea and nasal itching was not different between the NAR and AR groups. Multiple symptoms were present in most rhinitis patients. Two hundred and five (32.2%) AR patients and 48 (36.4%) NAR patients complained of all symptoms, including rhinorrhea, sneezing, nasal obstruction, and nasal itching (Fig. 2A and B).
Fig. 2. Frequency of rhinitis symptoms. (A) Presence of all symptoms (rhinorrhea, sneezing, nasal obstruction, and nasal itching) is the most common pattern in children with AR (32.2%). Sneezing only is the least common AR patients' symptom (0.3%). (B) Presence of all symptoms (rhinorrhea, sneezing, nasal obstruction, and nasal itching) is also the most common pattern in children with NAR (36.4%). 4 AR and 4 NAR patients were excluded due to absence of symptoms' description.
Classification of AR
According to the ARIA classification, the most frequent type of AR was moderate-severe persistent AR (41.2%), followed by mild persistent (27.9%), moderate-severe intermittent (18.9%), and mild intermittent (12.1%) (Table 1). Sneezing and itching were more common in persistent rhinitis than in intermittent rhinitis (P<0.001 and P=0.001, respectively) (Table 2). Nasal obstruction was more common in both moderate-severe cases (P=0.001) and persistent cases (P<0.005) (Table 2). Patients with persistent AR had significantly more symptoms than those with intermittent AR (P<0.001) (Table 2).
Table 2. Analysis of the relationship between nasal symptoms, comorbidities, allergen sensitization, and ARIA classification in Korean children.
| Variable | Mild, n (%) | Moderate-severe, n (%) | P value | Intermittent, n (%) | Persistent, n (%) | P value |
|---|---|---|---|---|---|---|
| Symptom | ||||||
| Rhinorrhea | 204 (80.0) | 276 (71.9) | 0.020* | 147 (74.2) | 333 (75.5) | 0.732 |
| Sneezing | 155 (60.8) | 252 (65.6) | 0.213 | 102 (51.5) | 305 (69.2) | <0.001* |
| Obstruction | 208 (81.6) | 347 (90.4) | 0.001* | 161 (81.3) | 394 (89.3) | 0.005* |
| Itching | 147 (57.9) | 237 (62.0) | 0.293 | 101 (51.0) | 283 (64.6) | 0.001* |
| No. of symptoms | 0.258 | <0.001* | ||||
| 1 | 24 (9.5) | 33 (8.6) | 25 (12.6) | 32 (7.3) | ||
| 2 | 69 (27.3) | 106 (27.7) | 71 (35.9) | 104 (23.8) | ||
| 3 | 90 (35.6) | 108 (28.3) | 64 (32.3) | 134 (30.7) | ||
| 4 | 70 (27.7) | 135 (35.3) | 38 (19.2) | 167 (38.2) | ||
| Comorbidities | ||||||
| Conjunctivitis | 84 (39.3) | 135 (36.6) | 0.522 | 65 (34.9) | 154 (38.8) | 0.372 |
| Asthma | 77 (30.6) | 112 (29.6) | 0.787 | 54 (27.4) | 135 (31.1) | 0.348 |
| Atopic dermatitis | 75 (31.0) | 96 (27.6) | 0.370 | 52 (28.1) | 119 (29.4) | 0.752 |
| Otitis media | 23 (10.6) | 37 (9.9) | 0.798 | 22 (11.7) | 38 (9.4) | 0.389 |
| Sinusitis | 114 (45.4) | 158 (41.4) | 0.313 | 84 (42.6) | 188 (43.1) | 0.910 |
| Sleep disturbance | 73 (29.1) | 138 (36.4) | 0.056 | 45 (22.8) | 166 (38.3) | <0.001* |
| Oral allergy syndrome | 11 (4.7) | 18 (4.8) | 0.957 | 8 (4.1) | 21 (5.1) | 0.579 |
| Allergen sensitization | ||||||
| House dust mites | 226 (88.6) | 342 (89.3) | 0.792 | 171 (86.4) | 397 (90.2) | 0.149 |
| Animal danders | 61 (24.7) | 93 (24.7) | 0.994 | 47 (24.4) | 107 (24.8) | 0.899 |
| Pollens | 81 (32.8) | 148 (39.8) | 0.078 | 64 (33.2) | 165 (38.7) | 0.183 |
| No. of allergen sensitizations | 0.156 | 0.098 | ||||
| 1 | 161 (63.1) | 223 (58.1) | 126 (63.6) | 258 (58.5) | ||
| 2 | 75 (29.4) | 123 (32.0) | 60 (30.3) | 138 (31.3) | ||
| 3 | 19 (7.5) | 38 (9.9) | 12 (6.1) | 45 (10.2) |
ARIA, allergic rhinitis and its impact on asthma.
*P value <0.05.
Comorbidities of rhinitis
The most frequent comorbidity for patients with AR was rhinosinusitis (42.8%), followed by conjunctivitis (37.5%), sleep disturbance (33.4%), asthma (29.9%), atopic dermatitis (29.1%), otitis media (10.1%), and oral allergy syndrome (4.8%) (Table 1). In patients with NAR, sinusitis (38.2%) was also the most frequent comorbidity, followed by sleep disturbance (27.2%), conjunctivitis (11.2%), atopic dermatitis (35.1%), asthma (10.3%), and otitis media (3.8%) (Table 1). Conjunctivitis, asthma, and otitis media were significantly more common in the AR group than in the NAR group, but there was no difference between the groups in the other comorbidities (Table 1). In patients with AR, sleep disturbances were more common in moderate-severe rhinitis than in intermittent rhinitis (P<0.001). There was no significant relationship between severity and duration of AR and any other comorbidities, such as conjunctivitis, asthma, atopic dermatitis, otitis media, and sinusitis (Table 2).
Allergen sensitization in AR
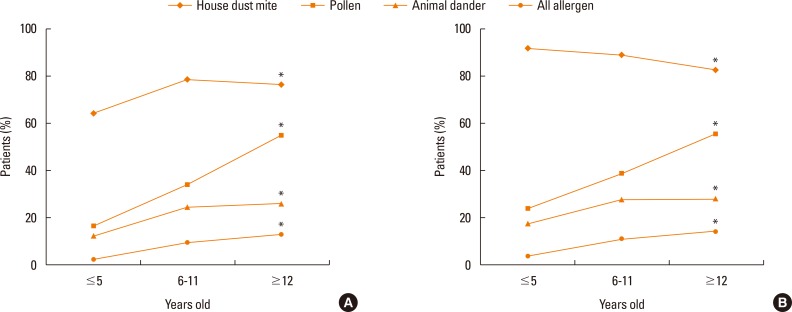
The proportions of children and adolescents sensitized to HDMs, pollen, animal dander, and allergens increased significantly from less than 5 years old to more than 12 years old (Fig. 3, 4A and B). However, it was noteworthy that the proportion of patients sensitized to HDMs peaked in 6 to 11 year olds. Furthermore, in the AR group, the proportion sensitive to HDMs decreased in the older children (P=0.027) (Fig. 4B).
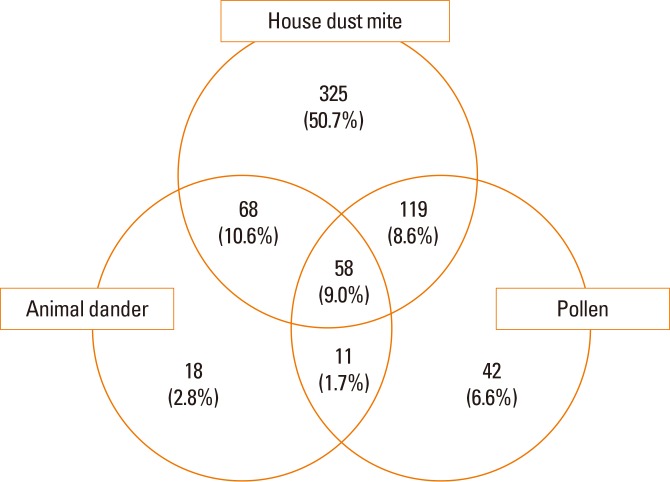
Fig. 3. Frequency of allergen sensitization. Many allergic rhinitis patients have multiple allergen sensitizations. Some patients are sensitive to all kinds of allergens, which are house dust mites, animal dander, and pollen (9.0%). Sensitization to house dust mites only is the most common pattern (50.7%). The least is the co-occurrence of sensitization to animal dander and pollen (1.7%).
Fig. 4. Change of the prevalence of allergen sensitization in AR patients. (A) This graph contains all cases, including NAR and non-test groups. Types of sensitized allergens vary according to the ages of the children. For house dust mites, 64.1% of children ≤5 years old are sensitized and there is a plateau of the sensitized rate at 6-11 years old (P<0.05). The percentage sensitized to pollen rapidly increases in patients ≥12 years old (16.6% to 54.9%) (P<0.050). The number sensitive to animal dander gradually increases at 6-11 years old (P<0.05). There is an increase in the number of all-allergen sensitized patients from ≤5 years old to ≥12 years old (P<0.050). (B) This graph only includes the AR group. Types of sensitized allergens varies according to the ages of the children. For house dust mites, 91.9% of patients ≤5 years old are sensitized. The number then significantly decreases to 82.6% for patients ≥12 years old (P<0.050). The number of patients sensitized to pollen swiftly increases to 55.5% among those ≥12 years old (P<0.050). There is also an increase in numbers for sensitivity to animal dander, but the percentage reaches a plateau at 6-11 years old (P<0.05). The number of all-allergen sensitized patients increases from ≤5 years old to ≥12 years old (P<0.050).
*P<0.005.
DISCUSSION
There have been few large-scale investigations of clinical manifestations, including comorbidities and phenotypes, of rhinitis in Korean children and adolescents. Moreover, research including Korean adolescents is rare. As far as we know, this is the first multicenter study that showed a difference in comorbidities between NAR and AR in Korean children and adolescents. Patients with AR had more conjunctivitis (37.5%), asthma (29.9%), and otitis media (10.1%). Sleep disturbance was a critical comorbidity related to the persistence of AR in Korean children and adolescents. Moderate-severe persistent AR was the most common type of AR (using the ARIA classification) in our patients, and nasal obstruction was the most common symptom of AR in Korean children. We also found a significant association between age and inhalant allergens, which suggests the necessity of early diagnosis and intervention.
It is difficult to distinguish between AR and NAR through use of the ISAAC questionnaire because its diagnosis is not supported by specific IgE tests. In our study, AR and NAR were clearly defined by specific IgE tests. AR was more common, affecting 641 (68.3%) patients as compared to 135 (14.5%) patients with NAR, a trend that existed across most of the age groups. A Norwegian study reported a 72.8% prevalence of AR and 27.2% of NAR in 10 year-olds.7 A Korean study reported 76.9% AR vs 23.1% NAR.21 The prevalence reported in our study may be different those in from other studies due to not having inhalant allergen test data from all patients, particularly ≤5 years old. However, all adolescents more than 14 years old were examined with allergy tests, and 100% were positive in the 14 to 17 years old group. Considering that AR increased and NAR underwent remission with advancing years,22 our results are similar to other studies.
Until now, there has been little research into the comorbidities of AR in children in Korea. A recent investigation of comorbidities of AR from the Korea National Health and Nutrition Extermination Survey (KNHANES) reported the prevalence, risk factors, and comorbidities of rhinitis in an adult population.23 They reported asthma, chronic rhinosinusitis, and olfactory dysfunction were more prevalent in the AR group, and asthma was correlated to severity and atopic dermatitis. According to a study of a birth cohort of 2,024 children from Sweden, AR and NAR were associated with asthma, eczema, and food hypersensitivity, and 25% of 8-year-olds with AR also had oral allergy syndrome.22 A cross-sectional population survey in Korea found the percentage of patients with AR and asthma, and those with AR and atopic dermatitis were 4.7% and 8.7%, respectively, among 31,201 children between 0 and 13 years of age.6 Atopic dermatitis, asthma, and oral allergy syndrome were found in 29.7%, 24.1%, and 4.6%, respectively, of our patients. These high rates of comorbidities are probably due to the study's hospital-based design. A position paper from the European Academy and Allergy Clinical Immunology Taskforce on Rhinitis in Children recognized comorbidities of rhinitis, including conjunctivitis, asthma, impaired hearing, rhinosinusitis, sleep problems, and pollen-food syndrome.3
In our study, most rhinitis patients had one or more comorbidities, and patients with AR had significantly more comorbidities than patients with NAR. One study reported 11.8% of children with rhinitis had conjunctivitis, asthma, or atopic dermatitis.7 Although there was no significant association between conjunctivitis and age or severity of AR in our study, conjunctivitis was the most common comorbidity, affecting more than half of the children with AR in several European studies.7,24
Bronchial eosinophilic inflammation could be associated with an increase in nasal eosinophilic chemotactic factors and bronchial hyper-responsiveness (BHR), which are significantly related to AR.7,25 Our findings support the concept that AR and asthma are one airway disease.26 If allergen exposure in the nasopharynx is associated with secretion of histamine and allergic mediators, the Eustachian tube could be obstructed and sequentially cause middle ear effusion.27,28 Allergic inflammation also contributes to the hypertrophy of lymphoid tissues, such as the adenoids and tonsils,27 explaining how AR could contribute to the development of otitis media and rhinosinusitis.7
The higher prevalence of moderate-severe persistent patients in our study may be the result of selection bias, as patients were recruited from secondary or tertiary hospitals. Unlike our results, intermittent AR was more prevalent in a population-based study in Spain.24
In our study, the most common AR symptom was nasal obstruction (86.7%), and the presence of nasal obstruction was significantly associated with both the severity and persistence of AR (Table 2). The importance of nasal obstruction is also noted in the Pediatric Allergies in an American survey as the main symptom in children.29 The authors collected information in on symptom burden, quality of life, productivity, disease management, and medication, and concluded that its burden on children in the United States has been significantly underestimated.29 Because nasal congestion is associated with sleep-disordered breathing and seems to be a major cause of sleep impairment in patients with AR, treatment to reduce nasal congestion has a positive influence on sleep.30
Like other chronic diseases, AR affects the quality of life in children by causing bothersome symptoms and comorbidities and by requiring medication use. There have been some trials to assess the quality of life in Korean children with AR by questionnaires.31,32 Contents of the questionnaire involved the degree of discomfort caused by main symptoms, daily activity or sleep disturbance, and psychosocial problems. A substantial proportion of subjects (38.6%) complained of sleep disturbances, which can be caused by nasal obstruction.30 Sleep disturbance was the only comorbidity that was significantly associated with the persistence of AR (P<0.001) and marginally significantly associated with severity (P=0.056) in our study. The other comorbidities were not significantly related to AR classifications according to the ARIA guideline (Table 2).
The nose is anatomically and physiologically linked to the paranasal sinuses, pharynx, middle ear, larynx, and lower airway. Therefore, presentation of AR may be associated with mouth breathing and snoring with sleep problems, and chronic cough.33 First-generation H1 antihistamines can also cause sleep problems and performance impairment.33,34,35,36 In our study, a sleep disturbance questionnaire was filled out by patients and/or their parents, but objective tests, such as polysomnography, should be performed for accurate evaluation of sleep disturbance.
We identified an age-dependent increase in sensitization to inhalant allergens, but there was no significant correlation between the severity of AR and allergen sensitization. An Italian multicenter study comprising 1,360 children with AR showed that 84.9% were sensitized to more than 3 allergens and that there was a strong association between pollen induced AR duration and severity.17 Young children, including those less than 1 year of age, already had allergic rhinitis due to sensitization to HDMs (7 children, 58.3%) and pollen (3 children, 25%). HDM sensitization occurred from less than 1 year of age and increased until 6-11 years of age. The rate of HDM sensitization was 73.1% in all rhinitis patients, and 89.1% in AR patients. Sensitization rates to pollen or animal dander increased significantly with advancing age. The prevalence and changing patterns of allergen sensitization in our study are similar to those in other Korean studies.21 Although sensitization to pollen is reported to be related to AR, we did not confirm this finding in our study.37,38
Atopic sensitization may be influenced by several environmental exposures occurring after infancy.39 The National Health and Nutrition Examination Survey (NHANES) in the USA announced that atopic sensitization to dust mites, plant-related allergens, or pets (dog or cat) affected 20.3%, 27.1%, and 15.7% of children, respectively.40 This implies that there is a big difference in the allergen sensitization rate and pattern in children between Korea and the USA, not surprising if the environment is supposed to be an important factor. On the basis of our study, both HDMs and pollen allergens should be evaluated in all Korean children even if less than 1 year old when doctors suspect the child has allergic sensitization.
Our study might be influenced by some limitations. First, 10 of the 11 hospitals are located in Seoul metropolitan area including Gyeonggi-do, and the geographic distribution of patients was biased toward Seoul. Therefore the study population is not truly representative of the Korean general population.
Secondly, there might be some heterogeneity across the methods of skin prick tests or serologic tests for specific IgE due to its multicentricity, and some patients were not evaluated by allergy tests, so that we could not confirm AR or NAR. Thirdly, a cross-sectional study design cannot clarify the role of risk factors in the natural course of a disease. However, our study investigated comorbidities of rhinitis and allergic sensitization from less than 1 year to 18 years old, and was a multicenter study. Therefore, it can be used as representative data in allergic rhinitis in children and adolescents in Korea.
In conclusion, this study revealed that AR was more prevalent than NAR and that moderate-severe persistent AR was the most frequent type in Korean pediatric populations. Nasal obstruction was an important symptom associated with the severity and persistence of AR. Sinusitis was the most common comorbidity, and sleep disturbance was associated with the severity of rhinitis. These findings may be helpful in developing a plan for the prevention and treatment of rhinitis and in improving the quality of life of Korean children and adolescents suffering from AR. Further nationwide and longitudinal studies will help understand the relationship between these diseases.
ACKNOWLEDGMENTS
This study was supported by a 2011-Grant from Korean Academy of Medical Sciences. The study was presented in 2015 at the KAPARD Spring Conference (Oral Presentation Number: 15S-033).
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Seo JH, Kim HY, Jung YH, Lee E, Yang SI, Yu HS, et al. Interactions between innate immunity genes and early-life risk factors in allergic rhinitis. Allergy Asthma Immunol Res. 2015;7:241–248. doi: 10.4168/aair.2015.7.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen) Allergy. 2008;63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x. [DOI] [PubMed] [Google Scholar]
- 3.Roberts G, Xatzipsalti M, Borrego LM, Custovic A, Halken S, Hellings PW, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68:1102–1116. doi: 10.1111/all.12235. [DOI] [PubMed] [Google Scholar]
- 4.Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008;51:343–350. [Google Scholar]
- 5.Jee HM, Kim KW, Kim CS, Sohn MH, Shin DC, Kim KE. Prevalence of asthma, rhinitis and eczema in Korean children using the International Study of Asthma and Allergies in Childhood (ISAAC) Questionnaires. Pediatr Allergy Respir Dis. 2009;19:165–172. [Google Scholar]
- 6.Hong S, Son DK, Lim WR, Kim SH, Kim H, Yum HY, et al. The prevalence of atopic dermatitis, asthma, and allergic rhinitis and the comorbidity of allergic diseases in children. Environ Health Toxicol. 2012;27:e2012006. doi: 10.5620/eht.2012.27.e2012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertelsen RJ, Carlsen KC, Carlsen KH. Rhinitis in children: co-morbidities and phenotypes. Pediatr Allergy Immunol. 2010;21:612–622. doi: 10.1111/j.1399-3038.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- 8.Silva CH, Silva TE, Morales NM, Fernandes KP, Pinto RM. Quality of life in children and adolescents with allergic rhinitis. Braz J Otorhinolaryngol. 2009;75:642–649. doi: 10.1016/S1808-8694(15)30511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roberts G, Hurley C, Lack G. Development of a quality-of-life assessment for the allergic child or teenager with multisystem allergic disease. J Allergy Clin Immunol. 2003;111:491–497. doi: 10.1067/mai.2003.138. [DOI] [PubMed] [Google Scholar]
- 10.Walker S, Khan-Wasti S, Fletcher M, Cullinan P, Harris J, Sheikh A. Seasonal allergic rhinitis is associated with a detrimental effect on examination performance in United Kingdom teenagers: case-control study. J Allergy Clin Immunol. 2007;120:381–387. doi: 10.1016/j.jaci.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 11.Kim HY, Kwon EB, Baek JH, Shin YH, Yum HY, Jee HM, et al. Prevalence and comorbidity of allergic diseases in preschool children. Korean J Pediatr. 2013;56:338–342. doi: 10.3345/kjp.2013.56.8.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KH. Overview of atopic dermatitis. Asia Pac Allergy. 2013;3:79–87. doi: 10.5415/apallergy.2013.3.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60(Suppl 92):44–47. [Google Scholar]
- 14.Spector SL. Overview of comorbid associations of allergic rhinitis. J Allergy Clin Immunol. 1997;99:S773–S780. doi: 10.1016/s0091-6749(97)70126-x. [DOI] [PubMed] [Google Scholar]
- 15.Webber CM, England RW. Oral allergy syndrome: a clinical, diagnostic, and therapeutic challenge. Ann Allergy Asthma Immunol. 2010;104:101–108. doi: 10.1016/j.anai.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton RG. Clinical laboratory assessment of immediate-type hypersensitivity. J Allergy Clin Immunol. 2010;125:S284–S296. doi: 10.1016/j.jaci.2009.09.055. [DOI] [PubMed] [Google Scholar]
- 17.Dondi A, Tripodi S, Panetta V, Asero R, Businco AD, Bianchi A, et al. Pollen-induced allergic rhinitis in 1360 Italian children: comorbidities and determinants of severity. Pediatr Allergy Immunol. 2013;24:742–751. doi: 10.1111/pai.12136. [DOI] [PubMed] [Google Scholar]
- 18.Kwon SE, Lim DH, Kim JH, Son BK, Park YS, Jang HJ, et al. Prevalence and allergens of allergic rhinitis in children and adolescents in Gwangju. Allergy Asthma Respir Dis. 2015;3:54–61. [Google Scholar]
- 19.Peters RL, Allen KJ, Dharmage SC, Tang ML, Koplin JJ, Ponsonby AL, et al. Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013;132:874–880. doi: 10.1016/j.jaci.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Wolthers OD, Staberg M. The usefulness of the multiple allergen simultaneous test-chemiluminescent as compared to the Phadia Immunocap IgE test panel system in children and adolescents. Recent Pat Inflamm Allergy Drug Discov. 2013;7:96–99. [PubMed] [Google Scholar]
- 21.Kim JS, Kang HS, Jang HJ, Kim JH, Lim DH, Son BK. Clinical features of allergic rhinitis in Korean children. Allergy Asthma Respir Dis. 2015;3:116–123. [Google Scholar]
- 22.Westman M, Stjärne P, Asarnoj A, Kull I, van Hage M, Wickman M, et al. Natural course and comorbidities of allergic and nonallergic rhinitis in children. J Allergy Clin Immunol. 2012;129:403–408. doi: 10.1016/j.jaci.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 23.Rhee CS, Wee JH, Ahn JC, Lee WH, Tan KL, Ahn S, et al. Prevalence, risk factors and comorbidities of allergic rhinitis in South Korea: the Fifth Korea National Health and Nutrition Examination Survey. Am J Rhinol Allergy. 2014;28:e107–e114. doi: 10.2500/ajra.2014.28.4040. [DOI] [PubMed] [Google Scholar]
- 24.Ibáñez MD, Valero AL, Montoro J, Jauregui I, Ferrer M, Dávila I, et al. Analysis of comorbidities and therapeutic approach for allergic rhinitis in a pediatric population in Spain. Pediatr Allergy Immunol. 2013;24:678–684. doi: 10.1111/pai.12126. [DOI] [PubMed] [Google Scholar]
- 25.Bonay M, Neukirch C, Grandsaigne M, Leçon-Malas V, Ravaud P, Dehoux M, et al. Changes in airway inflammation following nasal allergic challenge in patients with seasonal rhinitis. Allergy. 2006;61:111–118. doi: 10.1111/j.1398-9995.2006.00967.x. [DOI] [PubMed] [Google Scholar]
- 26.Bachert C, Vignola AM, Gevaert P, Leynaert B, Van Cauwenberge P, Bousquet J. Allergic rhinitis, rhinosinusitis, and asthma: one airway disease. Immunol Allergy Clin North Am. 2004;24:19–43. doi: 10.1016/S0889-8561(03)00104-8. [DOI] [PubMed] [Google Scholar]
- 27.Hadley JA, Derebery MJ, Marple BF. Comorbidities and allergic rhinitis: not just a runny nose. J Fam Pract. 2012;61:S11–S15. [PubMed] [Google Scholar]
- 28.Skoner DP, Doyle WJ, Fireman P. Eustachian tube obstruction (ETO) after histamine nasal provocation--a double-blind dose-response study. J Allergy Clin Immunol. 1987;79:27–31. doi: 10.1016/s0091-6749(87)80012-x. [DOI] [PubMed] [Google Scholar]
- 29.Meltzer EO, Blaiss MS, Derebery MJ, Mahr TA, Gordon BR, Sheth KK, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124:S43–S70. doi: 10.1016/j.jaci.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 30.Craig TJ, Sherkat A, Safaee S. Congestion and sleep impairment in allergic rhinitis. Curr Allergy Asthma Rep. 2010;10:113–121. doi: 10.1007/s11882-010-0091-5. [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Ahn YM, Kim HJ, Lim DH, Son BK, Kang HS, et al. Development of a questionnaire for the assessment of quality of life in Korean children with allergic rhinitis. Allergy Asthma Immunol Res. 2014;6:541–547. doi: 10.4168/aair.2014.6.6.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin JY, Yang HJ, Jeon YH, Kim KW, Kim WK, Park YM, et al. Development and validation of the questionnaire for quality-of-life specific to allergic rhinitis in Korean children (QQOL-ARK): a multicenter study. Korean J Asthma Allergy Clin Immunol. 2009;29:242–248. [Google Scholar]
- 33.Muliol J, Maurer M, Bousquet J. Sleep and allergic rhinitis. J Investig Allergol Clin Immunol. 2008;18:415–419. [PubMed] [Google Scholar]
- 34.Qidwai JC, Watson GS, Weiler JM. Sedation, cognition, and antihistamines. Curr Allergy Asthma Rep. 2002;2:216–222. doi: 10.1007/s11882-002-0022-1. [DOI] [PubMed] [Google Scholar]
- 35.Craig TJ, McCann JL, Gurevich F, Davies MJ. The correlation between allergic rhinitis and sleep disturbance. J Allergy Clin Immunol. 2004;114:S139–S145. doi: 10.1016/j.jaci.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Craig TJ, Hanks CD, Fisher LH. How do topical nasal corticosteroids improve sleep and daytime somnolence in allergic rhinitis? J Allergy Clin Immunol. 2005;116:1264–1266. doi: 10.1016/j.jaci.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 37.Bousquet J, Annesi-Maesano I, Carat F, Léger D, Rugina M, Pribil C, et al. Characteristics of intermittent and persistent allergic rhinitis: DREAMS study group. Clin Exp Allergy. 2005;35:728–732. doi: 10.1111/j.1365-2222.2005.02274.x. [DOI] [PubMed] [Google Scholar]
- 38.Warm K, Hedman L, Lindberg A, Lötvall J, Lundback B, Ronmark E. Allergic sensitization is age-dependently associated with rhinitis, but less so with asthma. J Allergy Clin Immunol. 2015;136:1559–1565.e1-2. doi: 10.1016/j.jaci.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 39.Weiland SK, von Mutius E, Hirsch T, Duhme H, Fritzsch C, Werner B, et al. Prevalence of respiratory and atopic disorders among children in the East and West of Germany five years after unification. Eur Respir J. 1999;14:862–870. doi: 10.1034/j.1399-3003.1999.14d23.x. [DOI] [PubMed] [Google Scholar]
- 40.Salo PM, Arbes SJ, Jr, Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005-2006. J Allergy Clin Immunol. 2014;134:350–359. doi: 10.1016/j.jaci.2013.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]