Abstract
Hereditary angioedema is a disease of congenital deficiency or functional defect in the C1 esterase inhibitor (C1-INH) consequent to mutation in the SERPING1 gene, which encodes C1-INH. This disease manifests as recurrent, non-pitting, non-pruritic subcutaneous, or submucosal edema as well as an erythematous rash in some cases. These symptoms result from the uncontrolled localized production of bradykinin. The most commonly affected sites are the extremities, face, gastrointestinal tract, and respiratory system. When the respiratory system is affected by hereditary angioedema, swelling of the airway can restrict breathing and lead to life-threatening obstruction. Herein, we report a case of a 24-year-old woman with type 2 hereditary angioedema who presented with recurrent episodic abdominal pain and swelling of the extremities. She had no family history of angioedema. Although her C4 level was markedly decreased (3.40 mg/dL; normal range: 10-40 mg/dL), she presented with a very high C1-INH level (81.0 mg/dL; normal range: 21.0-39.0 mg/dL) and abnormally low C1-INH activity (less than 25%; normal range: 70%-130%). The SERPING1 gene mutation was confirmed in this patient. She was treated with prophylactic tranexamic acid, as needed, and subsequently reported fewer and less severe episodes. To our knowledge, this is the first reported case of type 2 hereditary angioedema in Korea that was consequent to SERPING1 mutation and involved a significantly elevated level of C1-INH as well as a low level of C1-INH activity.
Keywords: Hereditary angioedema, Type 2, SERPING1 gene mutation
INTRODUCTION
Hereditary angioedema (HAE) is a rare autosomal dominant disorder characterized by recurrent attacks of non-pitting submucosal and/or subcutaneous edema that usually involves the skin, gastrointestinal tract, and upper airways.1 An attack can persist for some time or improve spontaneously.2 In some cases, however, laryngeal edema may lead to asphyxiation and death if it is not properly managed.3
HAE is a disease of congenital deficiency or functional defect of the C1 esterase inhibitor (C1-INH) expressing gene SERPING1.1 It is classified into 3 phenotypes: HAE type I which features low C1-INH level and activity; HAE type II which features normal or high C1-INH level, but low activity; and type III which is a newly described, estrogen-dependent, inherited form that features normal protein and activity levels.4 Types I and II are consequent to mutations in SERPING1, whereas missense mutations in the gene encoding human coagulation factor XII have been reported in Type III cases.5
Although some cases of adult and pediatric HAE with low C1-INH levels have been reported in Korea,6,7,8 a case involving both a high C1-INH level and decreased C1-INH function has not yet been described. Herein, we describe the case of a 24-year-old woman with SERPING1-mutated HAE that was characterized by a high level, but diminished activity of serum C1-INH.
CASE REPORT
A 24-year-old woman was referred to our Allergy Asthma Center with a history suggestive of HAE. Approximately 2 years earlier, she had experienced migrating soft tissue swelling in both hands, thighs, and feet twice monthly. She had also visited a private clinic for recurrent abdominal pain without altered bowel habits. Although medications were prescribed (ranitidine, rebamipide, and loperamide), most of her episodic symptoms disappeared without intervention. One year after symptom onset, she began working as a kindergarten teacher and experienced considerable stress; her symptoms subsequently began to emerge at approximately 1-week intervals. Shortly before visiting our clinic, she developed abdominal pain, edema of both hands, and dyspnea due to upper airway swelling. Her symptoms did not improve with antihistamine medication, but they subsequently spontaneously resolved. She had experienced these episodes only in the last 2 years, and she had had no prior attacks. Furthermore, she had no other medical history, and neither parent had a history of angioedema.
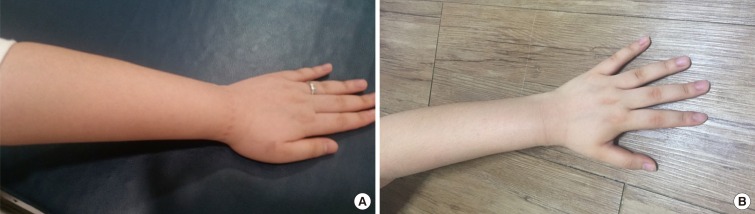
Her initial physical examination revealed the following. Her vital signs were non-significant. Both hands and forearms swelled, but did not show overlying erythema, urticaria, pitting, redness, or tenderness (Figure). The swelling persisted >24 hours and subsided without specific treatment. Laboratory findings included leukocyte, eosinophil, and platelet counts of 6,300/mm3, 120/µL, and 381,000/mm3, respectively; a hemoglobin level of 13.5 g/dL; and serum total IgE level of 12.7 kUA/L. Serum cryoglobulin, rheumatoid factor, or anti-CCP was not detected. An antinuclear-antibody test was positive (1:80, speckled). However, further autoantibody evaluation yielded negative anti-Smith Ab, anti-SSA/Ro, anti-SSB/La, anti-cardiolipin IgG and IgM, anti-β2 glycoprotein IgG and IgM, and anti-dsDNA IgG. Her C4 (3.40 mg/dL, normal: 10.0-40.0 mg/dL) and CH50 levels (4.9 U/mL; normal: 36.2-69.6 U/mL) were low, and her C3 (143 mg/dL; normal: 77.0-195.0 mg/dL) and circulating immune complex (<1.5 µg/mL; normal: 0.0-3.0 µg/mL) levels were within normal ranges. However, her C1-INH level was high, at 81.90 mg/dL (normal: 21.0-39.0 mg/dL). A C1-INH functional assay and genetic testing for the presence of mutation in SERPING1 revealed a decreased functional activity level of C1-INH (less than 25%, normal: 70%-130%) and a missense mutation in exon 8 of SERPING1. This heterozygous 1396 C→T mutation caused an Arg→Cys substitution in 466th amino acid of C1-INH.
Figure. The left hand of the patient. Swelling of the patient's forearm was evident on the day following an attack of angioedema (A). Her normal condition after treatment is shown for comparison (B).
The patient took a leave of absence from work after developing laryngeal edema. She was averse to danazol treatment because of its side effects, such as increased sebum production, rapid weight gain, virilization, and hyposexuality. Hence, we prescribed tranexamic acid for prophylaxis. Eight weeks later, she continued to experience HAE-related symptoms, though both the frequency and severity of attacks had lessened.
DISCUSSION
The C1-INH deficiency that is characteristic of HAE results from a mutation in SERPING1 on the eleventh chromosome (11q11–q13.1).9 C1-INH is a protease inhibitor belonging to the serpin superfamily.10 The main role of C1-INH is inhibition of the complement system, thus avoiding spontaneous activation. 11 Subcutaneous and submucosal non-pitting edema without pruritus is characteristic of HAE. These self-limiting lesions are thought to be triggered by environmental factors and pathophysiologic mechanisms.9 They resolve spontaneously within several days and recur.11 Edema results from a transient increase in capillary endothelial permeability, consequent to excessive bradykinin release.11,12
The estimated general prevalence of HAE is approximately 1 case per 30,000-50,000 persons.13 However, the prevalence of HAE in Korea remains unknown. According to a study of physicians who were members of The Korean Academy of Asthma, Allergy and Clinical Immunology, only 13 physicians in Korea reported confirmed HAE cases among their patients, suggesting underdiagnosis.14 Furthermore, to date, only cases of type I HAE have been reported in Korea.14
Mutations that affect the reactive-center arginyl residue on exon 8 (Arg444Cys) are well known in patients with HAE.15 Although most HAE patients have a classic family history, approximately 25% (including our patient) have none, suggesting the presence of de novo mutations of SERPING1.1 However, the detection of an autosomal dominant mutation in our patient indicated that genetic counseling was needed for her family, to assess risks and explore potential morbidities.
Two types of treatment are available for HAE: acute symptomatic (i.e., for laryngeal edema) and prophylactic treatments.4 Acute treatment consists of the administration of C1-INH concentrates, kallikrein inhibitors, or fresh frozen plasma to Type I and II HAE patients.16 However, patients with Type III HAE do not benefit from C1-INH infusion.16 Although plasma-derived C1-INH was previously only available from the Korea Orphan Drug Center, it has been supplied by pharmaceutical companies since 2013. Patients with frequent episodes and those at high risk of developing laryngeal edema require long-term prophylaxis with danazol or antifibrinolytic agents.17 We prescribed a prophylactic drug for our patient, to treat the gastrointestinal and respiratory symptoms associated with monthly recurrences.
In conclusion, we reported a case involving a 24-year-old woman with angioedema that was typical of HAE, a significantly high C1-INH level, and low C1-INH activity. To our knowledge, this is the first case of type 2 HAE in Korea.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Agostoni A, Aygören-Pürsün E, Binkley KE, Blanch A, Bork K, Bouillet L, et al. Hereditary and acquired angioedema: problems and progress: proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. J Allergy Clin Immunol. 2004;114:S51–S131. doi: 10.1016/j.jaci.2004.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008;359:1027–1036. doi: 10.1056/NEJMcp0803977. [DOI] [PubMed] [Google Scholar]
- 3.Shibuya M, Takahashi N, Yabe M, Iwamoto K, Hide M. Hereditary angioedema as the cause of death from asphyxia: postmortem computed tomography study. Allergol Int. 2014;63:493–494. doi: 10.2332/allergolint.13-LE-0655. [DOI] [PubMed] [Google Scholar]
- 4.Bowen T, Cicardi M, Farkas H, Bork K, Kreuz W, Zingale L, et al. Canadian 2003 international consensus algorithm for the diagnosis, therapy, and management of hereditary angioedema. J Allergy Clin Immunol. 2004;114:629–637. doi: 10.1016/j.jaci.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 5.Cichon S, Martin L, Hennies HC, Müller F, Van Driessche K, Karpushova A, et al. Increased activity of coagulation factor XII (Hageman factor) causes hereditary angioedema type III. Am J Hum Genet. 2006;79:1098–1104. doi: 10.1086/509899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YS, Chung JH, Cho KH, Youn JI. A case of hereditary angioedema. Korean J Dermatol. 1994;32:115–118. [Google Scholar]
- 7.Kim SH, Lee BJ, Chang YS, Kim YK, Cho SH, Min KU, et al. A case of hereditary angioedema associated with idiopathic hypoparathyroidism. Korean J Intern Med. 2001;16:281–283. doi: 10.3904/kjim.2001.16.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin M, Ahn K. A case of hereditary angioedema in a 7-year-old Korean girl. Allergy Asthma Immunol Res. 2013;5:59–61. doi: 10.4168/aair.2013.5.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gower RG, Busse PJ, Aygören-Pürsün E, Barakat AJ, Caballero T, Davis-Lorton M, et al. Hereditary angioedema caused by c1-esterase inhibitor deficiency: a literature-based analysis and clinical commentary on prophylaxis treatment strategies. World Allergy Organ J. 2011;4:S9–S21. doi: 10.1097/1939-4551-4-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis AE., 3rd Biological effects of C1 inhibitor. Drug News Perspect. 2004;17:439–446. doi: 10.1358/dnp.2004.17.7.863703. [DOI] [PubMed] [Google Scholar]
- 11.Bas M, Adams V, Suvorava T, Niehues T, Hoffmann TK, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy. 2007;62:842–856. doi: 10.1111/j.1398-9995.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- 12.Fine LM, Bernstein JA. Guideline of chronic urticaria beyond. Allergy Asthma Immunol Res. 2016;8:396–403. doi: 10.4168/aair.2016.8.5.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicardi M, Bork K, Caballero T, Craig T, Li HH, Longhurst H, et al. Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy. 2012;67:147–157. doi: 10.1111/j.1398-9995.2011.02751.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee SY, Kang HR, Jung JW, Jang GC, Lee SY, Ahn Y, et al. Clinical experience in managing patients with hereditary angioedema in Korea: questionnaire survey and a literature review. Allergy Asthma Respir Dis. 2014;2:277–284. [Google Scholar]
- 15.Rijavec M, Korošec P, Šilar M, Zidarn M, Miljković J, Košnik M. Hereditary angioedema nationwide study in Slovenia reveals four novel mutations in SERPING1 gene. PLoS One. 2013;8:e56712. doi: 10.1371/journal.pone.0056712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waytes AT, Rosen FS, Frank MM. Treatment of hereditary angioedema with a vapor-heated C1 inhibitor concentrate. N Engl J Med. 1996;334:1630–1634. doi: 10.1056/NEJM199606203342503. [DOI] [PubMed] [Google Scholar]
- 17.Zuraw BL. Hereditary angiodema: a current state-of-the-art review, IV: short- and long-term treatment of hereditary angioedema: out with the old and in with the new. Ann Allergy Asthma Immunol. 2008;100:S13–S18. doi: 10.1016/s1081-1206(10)60581-9. [DOI] [PubMed] [Google Scholar]