Abstract
Since liver function is changed by chronic liver diseases, chronic liver disease can lead to different hemorheological alterations during the course of the progression. This study aims to compare alterations in whole blood viscosity in patients with chronic liver disease, focusing on the gender effect. Chronic liver diseases were classified into three categories by patient’s history, serologic markers, and radiologic findings: nonalcoholic fatty liver disease (NAFLD) (n = 63), chronic viral hepatitis B and C (n = 50), and liver cirrhosis (LC) (n = 35). Whole blood viscosity was measured by automated scanning capillary tube viscometer, while liver stiffness was measured by transient elastography using FibroScan®. Both systolic and diastolic whole blood viscosities were significantly lower in patients with LC than NAFLD and chronic viral hepatitis (P < 0.001) in male patients, but not in female patients. In correlation analysis, there were inverse relationships between both systolic and diastolic whole blood viscosity and liver stiffness (systolic: r = −0.25, diastolic: r = −0.22). Whole blood viscosity was significantly lower in male patients with LC than NAFLD or chronic viral hepatitis. Our data suggest that whole blood viscosity test can become a useful tool for classifying chronic liver disease and determining the prognosis for different types of chronic liver diseases.
Keywords: Hemorheology, Whole Blood Viscosity, Chronic Liver Disease
Graphical Abstract
INTRODUCTION
The liver is an organ that plays an important role in hemorheology (1,2). It is widely known that the liver synthesizes plasma proteins, lipids, and coagulation factors, and controls the cellular composition of the blood, all of which are important determinants of hemorheology (1,3). In chronic liver diseases (CLDs), liver function is adversely affected, leading to hemorheological alterations (1,4). Since the blood vessels in the liver including sinusoid are small, the altered hemorheology disturbs the microcirculation in the liver. Hence, it is not clear whether the altered hemorheology is the cause or the result of the damaged liver.
Blood viscosity is a physical property of blood, representing the thickness or stickiness of blood. It is defined as the ratio of shear stress to shear rate (5). The shear rate is determined by the ratio of flow velocity to lumen diameter. Blood viscosity is the inherent resistance of blood against the flow within blood vessels, and is affected by factors such as hematocrit (Hct), deformability and aggregability of red blood cell (RBC), plasma proteins (i.e., fibrinogen, globulins, albumin), lipids, and plasma viscosity (5,6,7,8). Traditionally, a rotational cone-and-plate viscometer was used to measure blood viscosity, but this has not been widely used in clinical practice because of the risk of blood contamination and the need to calibrate the spring sensor. Recently, a new scanning capillary tube viscometer with disposable U-tubes is introduced, which measures blood viscosity over a wide range of shear rates (i.e., 1–1,000 1/s) (9,10,11,12).
A number of studies have reported the hemorheology in various liver diseases. In alcoholic liver cirrhosis (LC) patients, both whole blood viscosity (WBV) and plasma viscosity as well as Hct and fibrinogen concentration were significantly lower compared to the controls (1,2,13). On the other hand, in patients with nonalcoholic fatty liver disease (NAFLD), WBV was significantly higher than those in the control group (1,14,15,16,17). In addition, the WBV and RBC aggregation index were significantly higher in hepatitis B patients than those in the control group (13).
Although the previous studies clearly reported that WBV in the NAFLD and hepatitis B was significantly higher than those in the control, whereas WBV in the LC was significantly lower than those in the control (1,2,13,14,15,16,17), these studies did not investigate the effect of gender. Since the effect of gender on the WBV is one of the most important variables, it is not clear whether or not the conclusions from the previous studies are valid for both genders. Thus, we aimed to examine WBV in patents with CLDs including NAFLD, chronic viral hepatitis (CVH) B and C, and LC using an automated scanning capillary tube viscometer to identify any gender effect of WBV on the types of liver diseases.
MATERIALS AND METHODS
Study patients and collection of clinical data
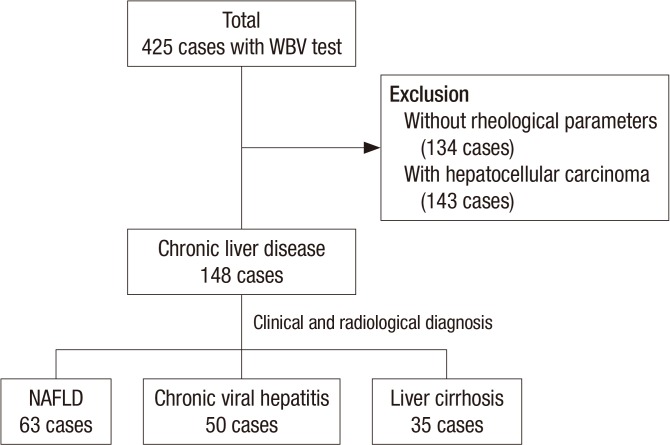
A total of 425 patients who underwent assessment for WBV at the clinic of liver disease, Seoul St. Mary’s Hospital, Catholic University of Korea between July 2015 and May 2016 were included by retrospective manner (Fig. 1). Patients without the clinical data of rheological parameters, including unknown Hct, mean corpuscular volume, lipid profile, and prothrombin time were excluded (n = 134). Patients with hepatocellular carcinoma were also excluded (n = 143).
Fig. 1.
Classification of patients with chronic liver disease who underwent whole blood viscosity test according to clinical and radiological diagnosis.
WBV = whole blood viscosity, NAFLD = nonalcoholic fatty liver disease.
The baseline clinical history and laboratory data of the 148 patients from medical records review were obtained. The baseline characteristics included age, gender, and body mass index (BMI), as well as history of alcohol, smoking, diabetes, hypertension, and cardiovascular disease. Diagnosis of CLD was based on clinical and radiologic findings measured by ultrasonography or CT and classified into three categories; NAFLD was defined that there is evidence of hepatic steatosis by radiologic tests and there are no causes for secondary hepatic fat accumulation such as significant alcohol consumption or viral hepatitis (n = 63) (18), CVH was defined as irregular and coarse hepatic parenchymal echogenicity with hepatitis B surface antigen or antibody to hepatitis C virus positive (n = 50), and LC was defined as nodular and very coarse hepatic parenchymal echogenicity (n = 35). Liver stiffness was measured by transient elastography using FibroScan®, and expressed in kilopascals (kPa). All included patients underwent blood sampling in the stable, sitting position, and the blood was stored in sterile ethyl-enediaminetetraacetic acid (EDTA)-treated plastic tubes. Hct; hemoglobin (Hb); mean corpuscular volume (MCV); international normalized ration (INR); Platelet (PLT) count; total/high/low density cholesterol (TC/HDL/LDL); triglyceride (TG); and liver function tests, including serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin were determined through blood tests.
Measuring whole blood viscosity
In addition to the baseline blood tests, we measured WBV by using the automated scanning capillary tube viscometer (Hemovister, Ubiosis, Seongnam, Korea) at a low shear rate (5s−1) and a high shear rate (300s−1), respectively (9). WBV at a low shear rate reflects hemodynamical status with very low velocity as in microcirculation, in which RBCs can aggregate and thus increase the flow resistance at blood vessels. WBV at a high shear rate reflects hemodynamical status with high velocity as in a large artery. Therefore, WBV at a low shear rate indicate blood viscosity at the diastolic status, and WBV at a high shear rate indicate blood viscosity at the systolic status. The reference intervals for WBV were 3.36–5.16 cP at 300 s−1 and 8.25–14.28 cP at 5s−1 as previously described (9). Detail theory and operating principle of the automated scanning capillary tube viscometer are given elsewhere (10).
Statistical analyses
All categorical factors were analyzed using the χ2 test, and the Fisher’s exact test. Continuous variables were analyzed using the Mann-Whitney U-test, or the analysis of variance (ANOVA) test. The linear regression analysis was used to identify correlation between the value of transient elastography and WBV. Statistical significance was defined as a P value < 0.05. SPSS version 19 software (SPSS Science, Chicago, II, USA) was used for all analyses.
Ethics statement
This study was approved by the institutional review board of Seoul St. Mary’s Hospital (KC16RISI0532), and informed consent was not required due to the retrospective nature of this study.
RESULTS
Baseline characteristics according to the liver parenchymal status and gender
The baseline characteristics of patients with CLDs are summarized in Table 1. Among the 148 included patients, 63 patients had NAFLD, 50 patients had CVH, and 35 patients had LC. The 148 CLD patients were composed of 73 males and 75 females. Patients with LC tended to be older in age. Patients with NAFLD had higher BMI than patients without NAFLD. Patients with LC had lower levels of albumin, Hct and PLT, and higher INR than patients without LC.
Table 1. Baseline characteristics according to diagnosis.
| Diagnosis | NAFLD (n = 63) | Chronic viral hepatitis (n = 50) | Liver cirrhosis (n = 35) | P |
|---|---|---|---|---|
| Gender (male) | 34 (54.0) | 20 (40.0) | 19 (54.3) | 0.269 |
| Age, yr | 46.7 ± 16.6 | 52.5 ± 15.5 | 60.7 ± 9.4 | 0.000 |
| Etiology | 0.000 | |||
| Alcohol | 0 (0.0) | 0 (0.0) | 9 (25.7) | |
| HBV | 0 (0.0) | 20 (40.0) | 9 (25.7) | |
| HCV | 0 (0.0) | 30 (60.0) | 13 (37.1) | |
| NAFLD | 63 (100.0) | 0 (0.0) | 0 (0.0) | |
| Others | 0 (0.0) | 0 (0.0) | 4 (11.4) | |
| BMI, kg/m2 | 26.5 ± 4.2 | 23.1 ± 3.5 | 23.8 ± 3.2 | 0.000 |
| HTN | 13 (20.6) | 10 (20.0) | 5 (14.3) | 0.723 |
| DM | 8 (12.7) | 5 (10.0) | 7 (20.0) | 0.402 |
| CVD | 3 (4.8) | 2 (4.0) | 2 (5.7) | 0.935 |
| TE, kPa | 5.6 ± 2.0 | 7.2 ± 3.7 | 27.3 ± 18.2 | 0.000 |
| TB, mg/dL | 0.9 ± 0.8 | 0.8 ± 0.3 | 2.0 ± 3.6 | 0.017 |
| Alb, g/dL | 4.4 ± 0.3 | 4.1 ± 0.3 | 3.4 ± 0.6 | 0.000 |
| INR | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.3 | 0.000 |
| Hb, g/dL | 14.5 ± 1.4 | 14.0 ± 1.5 | 12.5 ± 1.7 | 0.000 |
| Hct, % | 42.6 ± 3.8 | 41.2 ± 4.4 | 37.1 ± 4.8 | 0.000 |
| MCV, fL | 89.6 ± 5.4 | 92.6 ± 7.8 | 94.6 ± 11.2 | 0.002 |
| PLT, ×103/mm3 | 238.8 ± 54.5 | 197.4 ± 68.8 | 112.3 ± 52.5 | 0.000 |
| TG, mg/dL | 162.2 ± 171.7 | 123.8 ± 92.5 | 95.4 ± 48.2 | 0.011 |
| TC, mg/dL | 200.8 ± 36.7 | 180.7 ± 32.0 | 156.2 ± 40.8 | 0.000 |
| AST, U/L | 70.7 ± 205.0 | 58.7 ± 97.3 | 70.4 ± 69.0 | 0.928 |
| ALT, U/L | 84.8 ± 88.3 | 100.5 ± 246.2 | 43.2 ± 32.1 | 0.292 |
| GGT, U/L | 111.1 ± 260.2 | 66.5 ± 82.7 | 143.1 ± 273.5 | 0.671 |
| Cr, mg/dL | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.867 |
| ESR, mm/hr | 8.3 ± 4.1 | 15.9 ± 20.2 | 41.0 ± 34.1 | 0.011 |
| CRP, mg/dL | 0.3 ± 0.3 | 0.4 ± 2.1 | 0.7 ± 1.6 | 0.466 |
All values are expressed as mean (± standard deviation) or number (%).
HBV = hepatitis B virus, HCV = hepatitis C virus, NAFLD = non-alcoholic fatty liver disease, BMI = body mass index, HTN = hypertension, DM = diabetes mellitus, CVD = cardiovascular disease, TE = transient elastography, kPa = kilopascals, TB = total bilirubin, Alb = albumin, INR = international normalized ratio, Hb = hemoglobin, Hct = hematocrit, MCV = mean corpuscular volume, PLT = platelet, TG = triglyceride, TC = total cholesterol, AST = aspartate transaminase, ALT = alanine transaminase, GGT = gamma-glutamyl transpeptidase, Cr = creatinine, ESR = erythrocyte sedimentation rate, CRP = C-reactive protein.
Whole blood viscosity in patients with chronic liver disease
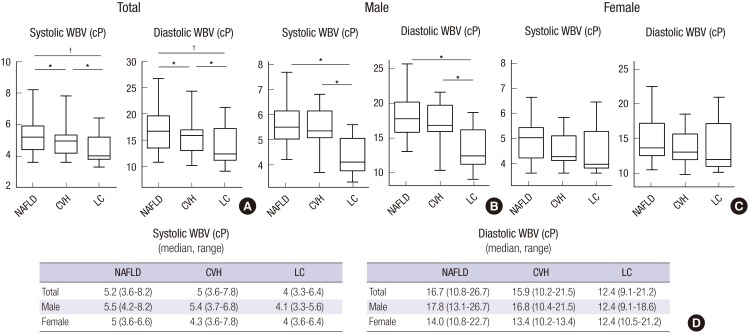
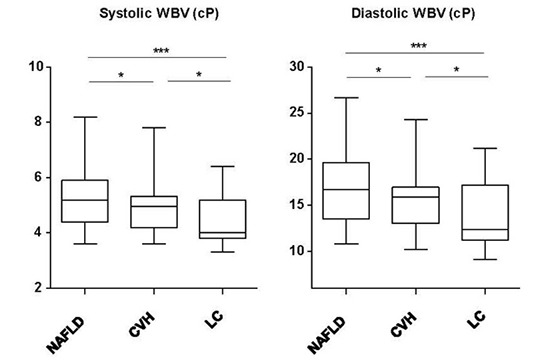
Next, we analyzed systolic and diastolic WBV according to the diagnosis and gender (Fig. 2). Systolic and diastolic WBV was significantly lower in total and male patients with LC than NAFLD and CVH. However, there were no statistically significant differences in systolic and diastolic WBV between liver diseases in female patients. Of note, systolic and diastolic WBV in patients with CLD enrolled in this study tended to be higher than those in healthy Koreans (systolic: median, 5.1 cP vs. 4.03 cP, diastolic: median, 15.8 cP vs. 10.63 cP) (9). These findings suggest that patients with CLD have higher blood viscosity than healthy controls, indicating the disturbance of microcirculation in liver; however, WBV decreases with progression of liver fibrosis. This change in blood viscosity was found to be affected by gender, which have distinct clinical and rheological characteristics. For example, in female patients, there was no significant difference in both systolic and diastolic WBVs among NAFLD, CVH, and LC. The significant difference in WBV between NAFLD/CVH and LC reported by all the previous studies (1,2,9,14,16,17,18,19) was only found in male patients, not in female patients in the present study.
Fig. 2.
Total (A), male (B) and female (C) systolic and diastolic whole blood viscosity according to diagnosis. The boxplots show systolic and diastolic whole blood viscosity are significantly lower in patients with liver cirrhosis than other patients. Median (range) values of total, male and female whole blood viscosity according to diagnosis; systolic and diastolic whole blood viscosity (D).
cP = centipoises, NAFLD = nonalcoholic fatty liver disease, CVH = chronic viral hepatitis, LC = liver cirrhosis, WBV = whole blood viscosity.
*P < 0.05, †P < 0.001.
Correlation between transient elastography and whole blood viscosity
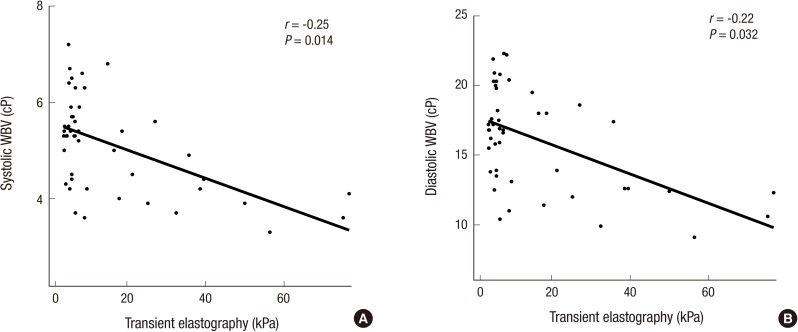
The level of liver stiffness measured by transient elastography using FibroScan® became higher in patients with CLD along with the progression of the liver fibrosis (median, NAFLD: 5.6 kPa, CVH: 7.2 kPa, LC: 27.3 kPa). Using correlation analysis, we found that there was statistically significant, inverse correlations between both systolic and diastolic WBV and liver stiffness (Fig. 3).
Fig. 3.
Correlation between transient elastography and whole blood viscosity; systolic (A) and diastolic (B) whole blood viscosity. The linear regression analyses show that there are weak inverse correlation between liver stiffness and whole blood viscosity, both systolic and diastolic.
WBV = whole blood viscosity, cP = centipoises, kPa = kilopascals.
DISCUSSION
In this study, we found that the WBV was highest in NAFLD patients, and it was relatively low in LC patients particularly in male patients. WBV also observed to decrease in LC patients with more severe fibrosis. Among the three groups, patients with NAFLD had the highest blood viscosity, and this is consistent with the results of previous studies (16,17,19,20,21). It is commonly known through many previous studies that increased blood viscosity is related to increased incidence and mortality of cardio-cerebrovascular disease (22,23,24,25,26,27,28,29).
In this study, among the three different CLD, the LC group had the lowest values for WBV. In addition, WBV negatively correlated with transient elastography using FibroScan®, and decreased as fibrosis progressed. As White blood cell, RBC, Hct, PLT and albumin levels decrease in these patients, WBV is also expected to decrease (30). Liu et al. (31) reported that Hct levels and PLT count decreased in patients with advanced cirrhosis, and cirrhosis patients with esophageal variceal hemorrhage accompanied by shock had lower WBV values than patients who had hemorrhage without shock. Therefore, WBV values can be used to classify the severity of cirrhosis (32), and also to make a prognosis for esophageal hemorrhage.
In this study, men tended to have higher WBV values than women for all types of CLD, and this is consistent with results of previous studies (9,33,34,35). In a study by Jung et al. (9) involving 297 healthy Korean subjects, men showed higher WBV at all shear rates than women, and showed a significant difference in Hct levels. The big question is why both systolic and diastolic WBVs in LC are not significantly different from those in NAFLD/CVH in female patients. Conversely, one can ask why the WBVs in LC are significantly lower with the progression of liver disease than those in NAFLD/CVH in male patients. One can speculate that in male NAFLD/CVH patients, WBV is significantly elevated (Fig. 2B), leading to the disturbance of microcirculation such that the normal function of liver to generate plasma proteins and lipids is compromised. Subsequently, with the progression of disease to LC, the WBV significantly decreases. On the other hand, in female NAFLD/CVH patients, WBV is not significantly elevated (Fig. 2C), such that the normal function of liver is not compromised compared to male patients. Subsequently, with the progression of disease to LC, the WBV does not significantly decrease.
The limitations of this study are that the patient sample size was small, and it is retrospective study. In addition, since we did not measure the levels of plasma proteins, it was difficult to evaluate the impacts of these proteins on blood viscosity. Moreover, it was impossible to exclude bias since we did not include healthy controls in our study.
In conclusion, WBV values varied across the CLD patients depending on gender, the different types and etiologies of the disease. We suggest that a WBV test can become a useful tool for classifying CLD and determining the prognosis for different types of CLD particularly in male patients.
Footnotes
Funding: This work was supported by grants of the National Research Foundation of Korea grant funded by the Korean government (NRF-2015R1A2A1A15052783) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Research conception and design: Jang B, Han JW, Yoon SK. Data acquisition: Jang B, Han JW. Data analysis and interpretation: Jang B, Han JW, Sung PS. Statistical analysis: Jang B, Han JW, Sung PS. Drafting of the manuscript: Jang B, Han JW, Sung PS. Critical revision of the manuscript: Jang JW, Bae SH, Choi JY, Cho YI, Yoon SK. Receiving grant: Yoon SK. Approval of final manuscript: all authors.
References
- 1.Omoti CE, Omuemu CE, Olu-Eddo AN. The rheological profile of chronic liver disease patients in Nigeria. Clin Hemorheol Microcirc. 2009;42:279–284. doi: 10.3233/CH-2009-1198. [DOI] [PubMed] [Google Scholar]
- 2.Tamer S, Cefle K, Gokkusu C, Ademoglu E, Ozturk S, Vatansever S, Palanduz S, Guler K. Comparison of rheological parameters in patients with post hepatitic and alcoholic cirrhosis. Clin Hemorheol Microcirc. 2007;36:247–252. [PubMed] [Google Scholar]
- 3.Anwar MA, Rampling MW. Abnormal hemorheological properties in patients with compensated and decompensated hepatic cirrhosis. Clin Hemorheol Microcirc. 2003;29:95–101. [PubMed] [Google Scholar]
- 4.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med. 2011;365:147–156. doi: 10.1056/NEJMra1011170. [DOI] [PubMed] [Google Scholar]
- 5.Baskurt OK, Meiselman HJ. Blood rheology and hemodynamics. Semin Thromb Hemost. 2003;29:435–450. doi: 10.1055/s-2003-44551. [DOI] [PubMed] [Google Scholar]
- 6.Chen G, Zhao L, Liu Y, Liao F, Han D, Zhou H. Regulation of blood viscosity in disease prevention and treatment. Chin Sci Bull. 2012;57:1946–1952. [Google Scholar]
- 7.Reinhart WH. Molecular biology and self-regulatory mechanisms of blood viscosity: a review. Biorheology. 2001;38:203–212. [PubMed] [Google Scholar]
- 8.Coppola L, Caserta F, De Lucia D, Guastafierro S, Grassia A, Coppola A, Marfella R, Varricchio M. Blood viscosity and aging. Arch Gerontol Geriatr. 2000;31:35–42. doi: 10.1016/s0167-4943(00)00063-7. [DOI] [PubMed] [Google Scholar]
- 9.Jung JM, Lee DH, Kim KT, Choi MS, Cho YG, Lee HS, Choi SI, Lee SR, Kim DS. Reference intervals for whole blood viscosity using the analytical performance-evaluated scanning capillary tube viscometer. Clin Biochem. 2014;47:489–493. doi: 10.1016/j.clinbiochem.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Cho YI, Hogenauer WN, Kensey KR. A method of isolating surface tension and yield stress effects in a U-shaped scanning capillary-tube viscometer using a Casson model. J Nonnewton Fluid Mech. 2002;103:205–219. [Google Scholar]
- 11.Kim H, Cho YI, Lee DH, Park CM, Moon HW, Hur M, Kim JQ, Yun YM. Analytical performance evaluation of the scanning capillary tube viscometer for measurement of whole blood viscosity. Clin Biochem. 2013;46:139–142. doi: 10.1016/j.clinbiochem.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Shin S, Ku Y, Park MS, Suh JS. Measurement of blood viscosity using a pressure-scanning capillary viscometer. Clin Hemorheol Microcirc. 2004;30:467–470. [PubMed] [Google Scholar]
- 13.Wang K, Wang DS, Fan XP, Li Y. Hemorheologic changes in patients with liver diseases. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2003;17:280–282. [PubMed] [Google Scholar]
- 14.Tamer S, Cefle K, Palanduz S, Vatansever S. Rheological properties of blood in patients with chronic liver disease. Clin Hemorheol Microcirc. 2002;26:9–14. [PubMed] [Google Scholar]
- 15.Yang Y, Wang K, Han LY, Li XH, Wang HM. Hemorheologic changes in patients with chronic hepatitis B. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2005;19:61–63. [PubMed] [Google Scholar]
- 16.Yu XY, Li Y, Liu T, Wang RT. Association of whole blood viscosity with non-alcoholic fatty liver disease. Clin Hemorheol Microcirc. 2015;62:335–343. doi: 10.3233/CH-151974. [DOI] [PubMed] [Google Scholar]
- 17.Zhao HY, Li J, Xu M, Wang TG, Sun WW, Chen Y, Bi YF, Wang WQ, Ning G. Elevated whole blood viscosity is associated with insulin resistance and non-alcoholic fatty liver. Clin Endocrinol (Oxf) 2015;83:806–811. doi: 10.1111/cen.12776. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 19.Yu KJ, Zhang MJ, Li Y, Wang RT. Increased whole blood viscosity associated with arterial stiffness in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:540–544. doi: 10.1111/jgh.12368. [DOI] [PubMed] [Google Scholar]
- 20.Dong H, Lu F, Wang N, Zou X, Rao J. Type 2 diabetic patients with non-alcoholic fatty liver disease exhibit significant haemorheological abnormalities. Front Med. 2011;5:288–293. doi: 10.1007/s11684-011-0127-9. [DOI] [PubMed] [Google Scholar]
- 21.Kurihara T, Deguchi S, Kato J, Furakawa M, Tsuchiya M, Akimoto M, Ishiguro H, Hashimoto H, Niimi A, Maeda A, et al. Impaired blood rheology by remnant-like lipoprotein particles: studies in patients with fatty liver disease. Clin Hemorheol Microcirc. 2001;24:217–225. [PubMed] [Google Scholar]
- 22.Pop GA, Duncker DJ, Gardien M, Vranckx P, Versluis S, Hasan D, Slager CJ. The clinical significance of whole blood viscosity in (cardio)vascular medicine. Neth Heart J. 2002;10:512–516. [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe GD, Lee AJ, Rumley A, Price JF, Fowkes FG. Blood viscosity and risk of cardiovascular events: the Edinburgh Artery Study. Br J Haematol. 1997;96:168–173. doi: 10.1046/j.1365-2141.1997.8532481.x. [DOI] [PubMed] [Google Scholar]
- 24.Junker R, Heinrich J, Ulbrich H, Schulte H, Schönfeld R, Köhler E, Assmann G. Relationship between plasma viscosity and the severity of coronary heart disease. Arterioscler Thromb Vasc Biol. 1998;18:870–875. doi: 10.1161/01.atv.18.6.870. [DOI] [PubMed] [Google Scholar]
- 25.Lee AJ, Mowbray PI, Lowe GD, Rumley A, Fowkes FG, Allan PL. Blood viscosity and elevated carotid intima-media thickness in men and women: the Edinburgh Artery Study. Circulation. 1998;97:1467–1473. doi: 10.1161/01.cir.97.15.1467. [DOI] [PubMed] [Google Scholar]
- 26.Fischer EI, Armentano RL, Pessana FM, Graf S, Romero L, Christen AI, Simon A, Levenson J. Endothelium-dependent arterial wall tone elasticity modulated by blood viscosity. Am J Physiol Heart Circ Physiol. 2002;282:H389–94. doi: 10.1152/ajpheart.00330.2001. [DOI] [PubMed] [Google Scholar]
- 27.Seki K, Sumino H, Nara M, Ishiyama N, Nishino M, Murakami M. Relationships between blood rheology and age, body mass index, blood cell count, fibrinogen, and lipids in healthy subjects. Clin Hemorheol Microcirc. 2006;34:401–410. [PubMed] [Google Scholar]
- 28.Park SK, Ryoo JH, Choi JM, Seo MW, Park CM. The risk of abdominal obesity according to the degree of non-alcoholic fatty liver disease in Korean men. J Korean Med Sci. 2016;31:410–416. doi: 10.3346/jkms.2016.31.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim MY, Jee SH, Yun JE, Baek SJ, Lee DC. Hemoglobin concentration and risk of cardiovascular disease in Korean men and women - the Korean heart study. J Korean Med Sci. 2013;28:1316–1322. doi: 10.3346/jkms.2013.28.9.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Min YW, Bae SY, Gwak GY, Paik YH, Choi MS, Lee JH, Paik SW, Yoo BC, Koh KC. A clinical predictor of varices and portal hypertensive gastropathy in patients with chronic liver disease. Clin Mol Hepatol. 2012;18:178–184. doi: 10.3350/cmh.2012.18.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu TT, Wong WJ, Hou MC, Lin HC, Chang FY, Lee SD. Hemorheology in patients with liver cirrhosis: special emphasis on its relation to severity of esophageal variceal bleeding. J Gastroenterol Hepatol. 2006;21:908–913. doi: 10.1111/j.1440-1746.2006.04266.x. [DOI] [PubMed] [Google Scholar]
- 32.Polio J, Groszmann RJ. Hemodynamic factors involved in the development and rupture of esophageal varices: a pathophysiologic approach to treatment. Semin Liver Dis. 1986;6:318–331. doi: 10.1055/s-2008-1040614. [DOI] [PubMed] [Google Scholar]
- 33.Filatova OV, Sidorenko AA, Agarkova SA. The rheological properties of blood depending on age and sex. Fiziol Cheloveka. 2015;41:110–118. [PubMed] [Google Scholar]
- 34.Galduróz JC, Antunes HK, Santos RF. Gender- and age-related variations in blood viscosity in normal volunteers: a study of the effects of extract of Allium sativum and Ginkgo biloba. Phytomedicine. 2007;14:447–451. doi: 10.1016/j.phymed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Konstantinova E, Tolstaya T, Prishchep S, Milutin A, Mironova E, Ivanova L. Plasma lipid levels, blood rheology, platelet aggregation, microcirculation state and oxygen transfer to tissues in young and middle-aged healthy people. Clin Hemorheol Microcirc. 2004;30:443–448. [PubMed] [Google Scholar]