Abstract
In this study, the seroprevalences of measles, mumps, and rubella antibodies in infants were determined to assess the immunization strategy and control measures for these infectious diseases. Serum samples from infants < 1 year of age and their mothers were collected to measure the concentrations of specific IgG antibodies to measles, mumps, and rubella by enzyme-linked immunosorbent assay. For selected infant serum samples, measles-specific neutralizing antibody levels were determined by using the plaque reduction neutralization test. The sera from 295 of infants and 80 of their mothers were analyzed. No infants had past measles, mumps, or rubella infections. Almost all infants < 2 months of age were positive for measles and rubella IgG antibodies. However, seroprevalence of measles and rubella antibodies decreased with age, and measles IgG and rubella IgG were barely detectable after 4 months of age. The seroprevalence of mumps antibodies was lower than that of measles and rubella antibodies in infants ≤ 4 months old, and mumps IgG was barely detectable after 2 months of age. The seropositivity of measles-specific neutralizing antibody was 63.6% in infants aged 2 months and undetectable in infants ≥ 6 months old. Because the seropositivity rates of measles, mumps, and rubella antibodies were low after the first few months of age in Korean infants, active immunization with vaccines is strongly recommended for infants aged 6–11 months when measles is epidemic. Timely administration of the first dose of measles-mumps-rubella vaccine at 12 months of age should be encouraged in non-epidemic situations.
Keywords: Measles, Mumps, Rubella, Seroprevalence, Infant, Korea
Graphical Abstract
INTRODUCTION
Even though primary protection against various infectious diseases is provided mainly by maternal antibodies at birth, these antibodies could hamper humoral immune responses of infants to vaccination. The presence of maternal antibodies should be considered when determining the appropriate age of immunization (1,2). In many countries, including Korea and the United States, the first dose of MMR vaccine is recommended after 12 months of age (3,4). Some countries recommend that infants receive their first measles-containing vaccine at 9 months of age (5). Currently in Korea, the first dose of MMR is administered to children aged 12–15 months and may be recommended for infants aged 6–11 months when there is a community-wide outbreak involving infants with ongoing risk for exposure or before departure for international travel to an area with endemic and epidemic levels of disease (4,6). Even in this situation, these infants should be revaccinated with two doses of MMR vaccine, the first at ≥ 12 months of age and the second dose at least 4 weeks later.
After the introduction of measles vaccination in 1965, vaccine coverage has been highly maintained, and the incidence of measles markedly decreased in Korea. Since measles elimination was achieved in 2006, there have been only small outbreaks that could boost immunity to measles in the community (6,7). Most Korean women of childbearing age are thought to have achieved their immunity against measles by immunization rather than by natural infection in recent years, and thus, they have lower titers of measles antibody than before (8). However, there have been few studies about the seroprevalence of measles antibodies in Korean infants who are less than 1 year of age, and seroprevalence data for mumps and rubella, the other components of MMR vaccine, are also scarce.
The purpose of this study was to determine the seroprevalence of measles, mumps, and rubella antibodies in infants < 1 year of age and their mothers. Additionally, we estimated the duration of maternal antibodies against measles, mumps, and rubella in infants.
MATERIALS AND METHODS
Subjects
We collected serum samples from infants < 1 year of age from September 2009 to December 2010. Age groups were stratified by 1-month intervals from 0 month to 11 months. We collected blood samples from the 295 infants when they underwent blood tests for health evaluation. We simultaneously obtained sera from 80 mothers of the infant participants, who also volunteered, to compare the presence of antibodies against measles, mumps, and rubella between the infants and their mothers. Collected samples were stored frozen at −70°C until testing. Individuals with known immune deficiencies and infants born premature (gestational age < 37 weeks at birth) were excluded.
Data on immunization status and previous measles, mumps, and rubella infections were obtained by questionnaires. We obtained immunization data for the study subjects through individual immunization records or hospital records
Assays
Specific IgG antibody levels for measles, mumps, and rubella were studied using commercially available enzyme-linked immunosorbent assay (ELISAs) kits (Enzygnost®; Dade Behring, Schwalbach, Germany), according to the manufacturer’s instructions. Differences in optical density (∆A) were corrected by an internal control factor and used qualitatively analyze antibody presence. Values of ∆A < 0.100, 0.100 < ∆A < 0.200, and ∆A > 0.200 corresponded to negative, equivocal, and positive classification, respectively. Samples with equivocal results were retested in duplicate. If similar results were obtained, the samples were classified as equivocal. Otherwise, they were classified as positive or negative. Quantitative titers were obtained from optical density values using the equation: log10 titer = α * ΔAβ, where α and β were specific constants for the kit’s lot. The cut-off values for positivity were 150 mIU/mL, a titer of 230, and 4 IU/mL for measles, mumps, and rubella, respectively. ELISAs were performed at the Center for Vaccine Evaluation and Study within Ewha Medical Research Institute at Ewha Womans University School of Medicine.
In addition, measles-specific neutralizing antibody concentrations were studied using the plaque reduction neutralization test (PRNT) for selected sera because of the limited volume for analysis. PRNTs were performed at the National Research Institute of Health as previously described (9). The protective measles neutralizing antibody level was ≥ 120 mIU/mL.
Statistical analysis
We analyzed the data using SPSS statistical software (version 18.0; SPSS Inc., Chicago, IL, USA). The percentages of positive, negative, and equivocal sera were calculated. The geometric means of antibody concentrations and titers were computed as arithmetic means of logarithmically transformed antibody titers for positive or equivocal results. Seronegative samples were considered to have a half value of the threshold for detection.
Ethics statement
This study was approved by the institutional review board of Ewha Womans University Mokdong Hospital (IRB No. ECT 204-24). Written informed consent was submitted by the parents or legal guardians of subjected infants before bleeding. .
RESULTS
Seroprevalence of measles, mumps, and rubella in infants
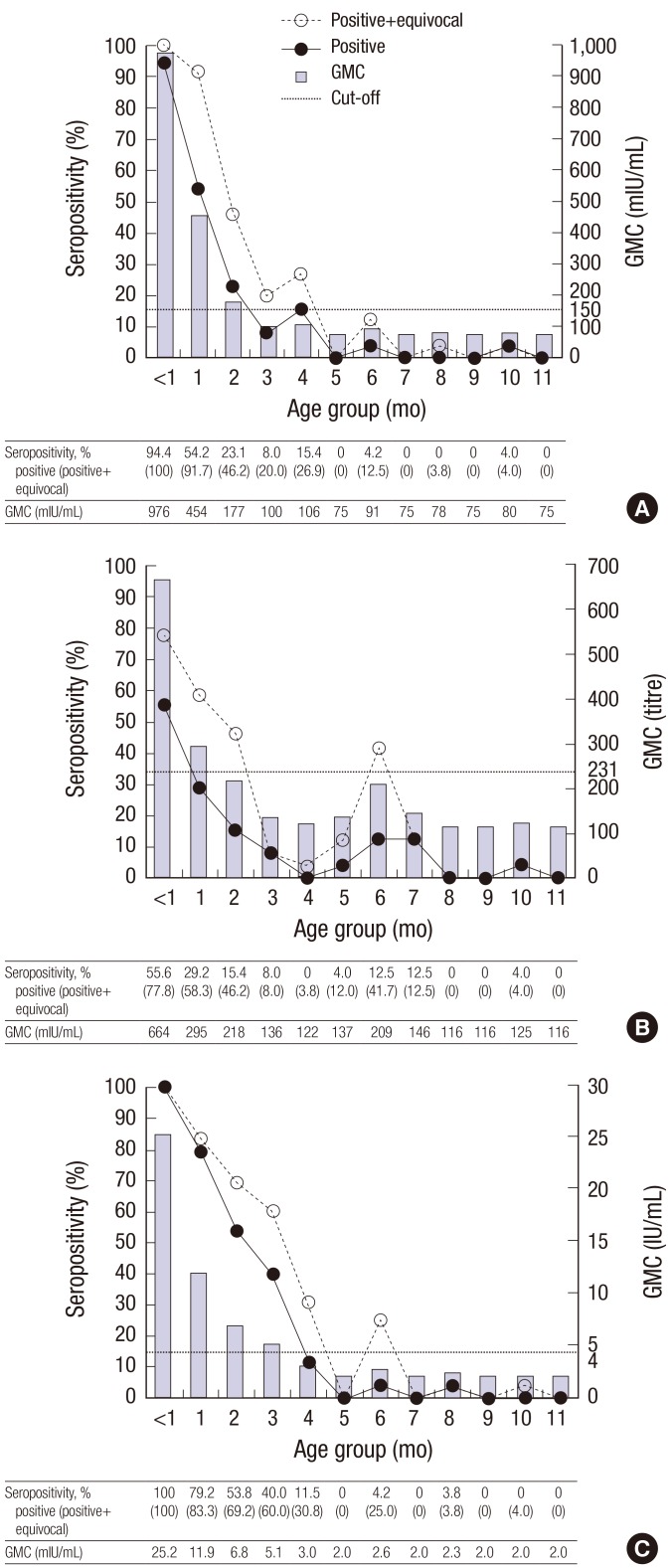
Seroprevalences and geometric mean concentrations (GMCs) of measles, mumps, and rubella IgG according to age group by month in 295 infants are shown in Fig. 1. For all subjects, seroprevalences for measles, rubella, and mumps were 14.9%, 22.4%, and 10.5%, respectively. For measles, the seroprevalence was 94.4% for infants < 1 month old, and seroprevalence dropped thereafter, from 54.2% of infants aged 1 month to 15.4% of infants aged 4 months. Almost no infants aged ≥ 5 months tested positive for the measles antibody (Fig. 1A). For rubella, all infants < 1 month old had rubella antibodies, which decreased with age, from 53.8% of infants aged 2 months to 11.5% of infants aged 4 months. Almost no infants aged ≥ 5 months tested positive for the rubella antibody (Fig. 1C). The seroprevalence of mumps was lower than those of measles and rubella in almost age groups. For mumps, seroprevalence was only 55.6% in infants < 1 month old and 29.2% in infants aged 1 month. Even though the seroprevalence of mumps in infants aged 6–7 months was slightly higher than those of measles and rubella, no infants aged 4 months tested positive for the mumps antibody (Fig. 1B). In terms of antibody concentrations, the GMCs of anti-measles and anti-rubella antibodies were above the thresholds in infants aged ≤ 2 months and ≤ 3 months, respectively. In subjects older than these, the GMCs of the anti-measles and anti-rubella antibodies were below the thresholds. The GMC of the anti-mumps antibody dropped more rapidly. It was above the threshold only for infants ≤ 1 month old. For all groups of infants older than 1 month old, the GMCs of the anti-mumps antibody were lower than the threshold.
Fig. 1.
Seroprevalence (left axis) and geometric mean concentrations (right axis) of IgG antibodies by infant age. (A) Measles. (B) Mumps. (C) Rubella.
Measles neutralizing antibody concentrations in infants
The sera of 33 subjects were tested for neutralizing antibodies against measles by PRNT. The seropositivity was 63.6% in subjects aged 2 months and decreased to 27.3% in subjects aged 4 months and 16.7% in those aged 6 months. Of note, no of subjects aged > 6 months exhibited any anti-measles activity (Table 1). The geometric mean titer (GMT) of measles neutralizing antibody was 225.1 mIU/mL for subjects aged 2 months, and this dropped abruptly to 82.4 mIU/mL in subjects aged 4 months and 25.9 mIU/mL in subjects aged 6 months, which were lower than protective antibody levels.
Table 1. Seropositivity rates and GMTs of measles neutralizing antibodies according to infant age by PRNT.
| Age, mon | Seropositivity rate (%) (No. of positive tests/tested samples) | GMT, mIU/mL |
|---|---|---|
| 2 | 63.6 (7/11) | 225.1 |
| 4 | 27.3 (3/11) | 82.4 |
| 6 | 16.7 (1/6) | 25.9 |
| 7–11 | 0 (0/5) | 32.1 |
| Total | 33.3 (11/33) | 80.9 |
GMT = geometric mean titer, PRNT = plaque reduction neutralization test.
Seropositive rates for measles, mumps, and rubella antibodies among the infants of seropositive mothers
To investigate the decrease in maternal antibodies, we analyzed the seropositivity and antibody concentrations for measles, mumps, and rubella antibodies in infants born from seropositive mothers. Because none of the infants had a history of MMR vaccination, natural infection, or contact with an infected person, we assumed that their specific antibodies were passed from their mothers. The mean age of the mothers was 31.8 years (median, 31 years; range, 20–41 years). Seropositive rates for measles, mumps, and rubella antibodies for the mothers were 96.3% (77/80), 87.5% (70/80), and 98.8% (79/80), respectively. Among 80 mothers, 52, 43, and 55 subjects experienced either immunization or past infections of measles, mumps, and rubella, respectively. Seropositivities of the vaccinated mothers and the naturally immunized mothers were 100% (43/43) and 88.9% (8/9) for measles antibodies, 81.1% (30/37) and 83.3% (5/6) for mumps antibodies, and 98.0% (49/50) and 100% (5/5) for rubella antibodies, respectively.
All infants aged < 1 month who had seropositive mothers had measurable antibodies against measles, mumps, and rubella, and the seropositivities of the infants declined as they aged. Measles and rubella antibodies were undetectable in infants > 4 months old. Mumps antibody levels decreased more rapidly, and they were barely detectable among infants > 2 months old. The levels of maternal antibodies in infants diminished shortly after birth. The infant-to-mother measles antibody titer ratio was 0.65 in infants < 1 month old and further decreased to 0.12 and 0.03 in infants that were 2 months old and 4 months old, respectively. The mumps antibody titer ratio was 0.74 in infants < 1 month old and dropped to 0.13 in 2-month-old infants. The rubella antibody titer ratio was 0.65 in infants < 1 month old and fell to 0.19 and 0.07 in 2-month-old and 4-month-old infants (Table 2).
Table 2. Age-stratified seropositivity rates and antibody titer ratios (infant/mother) among the seropositive mothers and their infants for measles, mumps, and rubella-specific IgG.
| Age group, mon | Measles IgG | Mumps IgG | Rubella IgG | |||
|---|---|---|---|---|---|---|
| Rate (%) (Seropositive infants [No.]/seropositive mothers [No.]) |
Antibody titer ratio (infant/mother) | Rate (%) (Seropositive infants [No.]/seropositive mothers [No.]) |
Antibody titer ratio (infant/mother) | Rate (%) (Seropositive infants [No.]/seropositive mothers [No.]) |
Antibody titer ratio (infant/mother) | |
| 0 | 100 (3/3) | 0.65 | 100 (3/3) | 0.74 | 100 (3/3) | 0.65 |
| 1 | 100 (5/5) | 0.29 | 50.0 (2/4) | 0.24 | 100 (4/4) | 0.36 |
| 2 | 63.6 (7/11) | 0.12 | 33.3 (3/9) | 0.13 | 72.7 (8/11) | 0.19 |
| 3 | 14.3 (1/7) | 0.09 | 0 (0/5) | - | 71.4 (5/7) | 0.13 |
| 4 | 60.0 (3/5) | 0.03 | 0 (0/5) | - | 16.7 (1/6) | 0.07 |
| 5 | 0 (0/8) | - | 0 (0/8) | - | 0 (0/8) | - |
| 6 | 0 (0/4) | - | 25.0 (1/4) | 0.10 | 0 (0/4) | - |
| 7 | 0 (0/4) | - | 0 (0/4) | - | 0 (0/5) | - |
| 8 | 20.0 (1/5) | 0.07 | 0 (0/6) | - | 16.7 (1/6) | 0.11 |
| 9 | 0 (0/14) | - | 0 (0/12) | - | 0 (0/14) | - |
| 10 | 0 (0/3) | - | 0 (0/3) | - | 0 (0/3) | - |
| ≥ 11 | 0 (0/8) | - | 0 (0/8) | - | 0 (0/8) | - |
| Total | 26.0 (20/77) | 0.21 | 12.7 (9/71) | 0.30 | 27.8 (22/79) | 0.25 |
DISCUSSION
The optimal age for MMR vaccination for infants is an important public health issue because infections in young infants lead to severe complications (10). Delaying vaccination may increase the risk of complication in infants while early vaccination may reduce humoral immune responses by neutralizing maternal antibodies. Maternal antibodies transferred via the placenta during late pregnancy provide primary protection against infectious diseases at birth and then disappear during the first months of life while the infants’ own immune system develops (11). Infants can be susceptible to disease between the loss of passively acquired protection and the achievement of protection by vaccination. To maintain protection against measles, mumps, and rubella in infants, it is important for each country to optimize the timing of vaccination to its own seroepidemiological situations for those infections.
In the present study, most infants aged ≥ 3 months were seronegative for measles antibodies by ELISA. Seropositivity data by PRNT was similar even though a small number of sera could be analyzed using this method due to the limited volume of each sample. The proportions of seropositive infants in our data were slightly lower those in data from other countries. Although detection may be affected by the difference in assay methods, the coverage, the time of vaccine introduction, and the extent of circulating wild-type viruses can contribute to seropositive rate. In Korea, routine two-dose measles-containing vaccination was introduced in 1991 (monovalent measles vaccine at 9 months of age and MMR vaccine at 15 months of age). Two-dose MMR vaccination at 12–15 months and 4–6 years was introduced in 1997 (6). According to the results of second MMR vaccination requirement for school entry in 2006–2011, the coverage rate of first MMR vaccine in children aged ≤ 3 years old was 99.2% (7,12). Additionally, yearly reported cases of measles were ranged 2–194 from 2002 to 2010 in Korea, in the population of 49 million (13).
According to the French study performed in 2004, maternal measles antibodies decrease dramatically by 6 months of age, and 90% of infants are not protected against measles after 6 months of age (14). Measles-containing vaccine coverage in France was 87% for one dose at 24 months of age in 2004, and there were 5,185 and 4,448 yearly reported cases of measles in 2002 and 2004 in France, respectively, in a population of 64 million (15). This suggests that France has a lower vaccination rate for MMR and a higher incidence of measles than Korea. The results of a Belgian cohort study were comparable with our data, revealing that maternal measles antibodies persisted for a median of 2.6 months (16). The vaccine coverage rate of MMR and the incidence of measles in Belgium were similar to those in Korea. The mean coverage of the first dose MMR vaccine was 92%–94%, and that of the second dose was 78%–83% from 2006–2009 in Belgium (17,18). There were less than 100 yearly reported cases of measles from 2000 to 2008 in a Belgian population of 11 million (18).
According to the results of this study, levels of maternal mumps antibodies decreased faster, compared to those of measles and rubella antibodies. The seroprevalence of mumps antibodies was lower than that of measles and rubella in almost age groups of infants, and it was less than 60% even in infants < 1 month of age. Except for a Belgian study that showed that mumps maternal antibodies persisted at a comparable level to those of measles and rubella antibodies (3.6 months vs. 2.6 months and 2.1 months, respectively), most studies have described a more rapid decline in maternal mumps antibodies compared to measles and rubella antibodies (19,20,21).
In the past, maternal antibodies persisted for more than 12 months after birth, and they lowered seroconversion rates and antibody levels obtained through vaccination (2). However, recent reports suggested that maternal antibodies actually have shorter durations. Although there have been very few Korean studies, maternal antibodies persisted for more than 6 months after birth in 2004, as seen in recent studies from other countries (16,22).
Antibody levels in cord blood are known to be higher than maternal antibody levels (23). In this study, the infant-to-mother antibody titer ratios for infants aged < 1 month were 0.65 for measles antibodies, 0.74 for mumps antibodies, and 0.65 for rubella antibodies. These ratios showed that maternal antibody levels decreased soon after the birth and dropped quickly as infants aged. The persistence of maternal antibodies in infants is affected by several factors. First, as vaccine coverage of a population increases and more women of childbearing age acquire their immunity from vaccines rather than from natural infection, more infants are born with vaccine-induced maternal antibodies. Although vaccine-induced immunity appears to be long-term and probably lifelong, vaccine-induced antibodies are often seen at lower levels and disappear more quickly than antibodies acquired after natural infection (16,24). Therefore, infants born more recently would be protected for a shorter period than those born in the pre-vaccine era. Boosting by wild-type viruses occurs less often as vaccine coverage increases and wild virus circulation declines. This can lead to lower antibody levels in childbearing-aged women and lower persistence of antibodies in their infants. In fact, certain infectious agents, such as pertussis, are circulating again in adolescents and young adults in the United States and some European countries, as populations lose their vaccine-induced immunity over time (25,26). In addition, the childbearing age of women is increasing in Korea as well as in the Western population, meaning the interval between childhood vaccination and childbirth is also increasing. For this reason, it is possible that duration and level of transmitted maternal protection decrease.
Early loss of maternal antibodies leads to an increasing gap of susceptibility in the time between the loss of maternal protection and the administration of a first MMR vaccine. Some authors suggest that early administration (< 12 months) of the MMR vaccine is needed (14). Because mumps and acquired rubella cases in infants under 12 months of age are very rare and without major complications, early vaccination with the MMR vaccine to combat mumps and rubella does not seem necessary to prevent those infections in infants aged < 12 months (13,27,28). As far as measles is concerned, early MMR vaccination can be considered to prevent measles in infants less than 12 months of age. However, recommendation for the age of vaccination must balance between the earliest age at which high rates of seroconversion can be obtained and the age group with the greatest risk of morbidity and mortality (29). Previous studies have found that infants 6 months of age had lower seroconversion rates and GMTs than older infants and toddlers. This phenomenon was evident even among 6-month-old infants who lacked maternal measles antibodies, suggesting an age-related difference in humoral immune responses unrelated to passively transferred maternal antibodies (30,31). Korean immunization guidelines recommend the administration of a monovalent measles vaccine or the MMR vaccine in infants from 6 to 11 months of age only during a measles outbreak. In these cases, the infants must be immunized with the MMR vaccine again after 12 months of age (6).
There are a few limitations in our study. First, because until a few years ago, data concerning immunization status was not registered via a national program in Korea, most vaccination data for the mothers relied only on memory. Second, we could not analyze the seropositivity rates and antibody levels between mothers with vaccine-induced immunity and those with naturally acquired immunity for MMR because the populations naturally immunized or vaccinated mothers were too small for each infant age group. However, this study is the first attempt to analyze the seroprevalence of MMR antibodies in Korean infants along with their mothers’ seroprevalences. These data would assist in the efforts to make an informed decision on the vaccination strategies and to create disease control measures when outbreaks occur.
In conclusion, seroprevalences of measles, mumps, and rubella vaccines were low after the first few months of age in Korean infants. Active immunization with vaccines is strongly recommended for infants aged 6–11 months when measles is epidemic. Timely administration of the first dose of the measles-mumps-rubella vaccine at 12 months of age should be encouraged in non-epidemic situations.
ACKNOWLEDGMENT
We thank the study participants who provided the samples and Soo Young Lim, who assisted with laboratory work.
Footnotes
Funding: This study was funded by the Ministry of Food and Drug Safety (09122MFDS424) to Kyung-Hyo Kim. For the remaining authors, none was declared.
DISCLOSURE: In Tae Kim is an employee of the Seegene Medical Foundation in Seoul, Korea, but his relationship to this company or its products had no influence on this work. Other authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study conception and design: Kim KH. Data acquisition: Lee H, Kim KH. Sample preparation: Kim HW, Kim IT. Neutralizing assays: Kim SS, Kang HJ Analysis and interpretation of data: Cho HK, Kim KH. Writing: Cho HK. Critical revision: Lee H, Kim KH. Approval of final manuscript: all authors.
References
- 1.Glezen WP. Effect of maternal antibodies on the infant immune response. Vaccine. 2003;21:3389–3392. doi: 10.1016/s0264-410x(03)00339-6. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht P, Ennis FA, Saltzman EJ, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91:715–718. doi: 10.1016/s0022-3476(77)81021-4. [DOI] [PubMed] [Google Scholar]
- 3.Cherry JD. Measles virus. In: Cherry JD, Harrison GJ, Kaplan SL, Hotez PJ, Steinbach WJ, editors. Feigin & Cherry's Textbook of Pediatric Infectious Diseases. 7th ed. Philadelphia, PA: Elsevier/Saunders; 2014. pp. 2723–2795. [Google Scholar]
- 4.American Academy of Pediatrics. Measles. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2015. pp. 535–547. [Google Scholar]
- 5.World Health Organization, authors. Immunization, vaccines and biological [Internet] [accessed on 19 June 2016]. Available at http://www.who.int/immunization/monitoring_surveillance/data/en/
- 6.The Korean Pediatric Society, authors; The Korean Pediatric Society, editors. Immunization Guideline. 8th ed. Seoul: The Korean Pediatric Society; 2015. Measles, mumps, rubella vaccine; pp. 141–162. [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Elimination of measles--South Korea, 2001-2006. MMWR Morb Mortal Wkly Rep. 2007;56:304–307. [PubMed] [Google Scholar]
- 8.Gans H, DeHovitz R, Forghani B, Beeler J, Maldonado Y, Arvin AM. Measles and mumps vaccination as a model to investigate the developing immune system: passive and active immunity during the first year of life. Vaccine. 2003;21:3398–3405. doi: 10.1016/s0264-410x(03)00341-4. [DOI] [PubMed] [Google Scholar]
- 9.Cohen BJ, Audet S, Andrews N, Beeler J. WHO working group on measles plaque reduction neutralization test. Plaque reduction neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine. 2007;26:59–66. doi: 10.1016/j.vaccine.2007.10.046. [DOI] [PubMed] [Google Scholar]
- 10.Filia A, Brenna A, Panà A, Cavallaro GM, Massari M. Ciofi degli Atti ML. Health burden and economic impact of measles-related hospitalizations in Italy in 2002-2003. BMC Public Health. 2007;7:169. doi: 10.1186/1471-2458-7-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine. 2007;25:6296–6304. doi: 10.1016/j.vaccine.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Hwang I. Results of 2nd MMR entry requirement, 2006-2011. Public Health Weekly Report. 2011;4:749–751. [Google Scholar]
- 13.Korea Centers for Disease Control and Prevention, authors. Disease web statistics system [Internet] [accessed on 19 June 2016]. Available at http://stat.cdc.go.kr.
- 14.Gagneur A, Pinquier D, Aubert M, Balu L, Brissaud O, De Pontual L, Gras Le Guen C, Hau-Rainsard I, Mory O, Picherot G, et al. Kinetics of decline of maternal measles virus-neutralizing antibodies in sera of infants in France in 2006. Clin Vaccine Immunol. 2008;15:1845–1850. doi: 10.1128/CVI.00229-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization, authors. WHO vaccine-preventable diseases: monitoring system. 2016 global summary. Coverage time series for France [Internet] [accessed on 19 June 2016]. Available at http://apps.who.int/immunization_monitoring/globalsummary/coverages?c=FRA.
- 16.Leuridan E, Hens N, Hutse V, Ieven M, Aerts M, Van Damme P. Early waning of maternal measles antibodies in era of measles elimination: longitudinal study. BMJ. 2010;340:c1626. doi: 10.1136/bmj.c1626. [DOI] [PubMed] [Google Scholar]
- 17.Andrews N, Tischer A, Siedler A, Pebody RG, Barbara C, Cotter S, Duks A, Gacheva N, Bohumir K, Johansen K, et al. Towards elimination: measles susceptibility in Australia and 17 European countries. Bull World Health Organ. 2008;86:197–204. doi: 10.2471/BLT.07.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization, authors. WHO vaccine-preventable diseases: monitoring system. 2016 global summary. Coverage time series for Belgium [Internet] [accessed on 19 June 2016]. Available at http://apps.who.int/immunization_monitoring/globalsummary/coverages?c=BEL.
- 19.Nicoara C, Zäch K, Trachsel D, Germann D, Matter L. Decay of passively acquired maternal antibodies against measles, mumps, and rubella viruses. Clin Diagn Lab Immunol. 1999;6:868–871. doi: 10.1128/cdli.6.6.868-871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desgrandchamps D, Schaad UB, Glaus J, Tusch G, Heininger U. Seroprevalence of IgG antibodies against measles, mumps and rubella in Swiss children during the first 16 months of life. Schweiz Med Wochenschr. 2000;130:1479–1486. [PubMed] [Google Scholar]
- 21.Leuridan E, Hens N, Hutse V, Aerts M, Van Damme P. Kinetics of maternal antibodies against rubella and varicella in infants. Vaccine. 2011;29:2222–2226. doi: 10.1016/j.vaccine.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Oh SH, Kim HW, Lim Y, Lee H. Measles antibodies measured by plaque reduction neutralization test in infants during the first year of life. Korean J Pediatr. 2004;47:827–832. [Google Scholar]
- 23.Kohler PF, Farr RS. Elevation of cord over maternal IgG immunoglobulin: evidence for an active placental IgG transport. Nature. 1966;210:1070–1071. doi: 10.1038/2101070a0. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (US), authors Measles. In: Hamborsky J, Kroger A, Wolfe C, editors. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Washington, D.C.: Public Health Foundation; 2015. pp. 209–230. [Google Scholar]
- 25.Rohani P, Drake JM. The decline and resurgence of pertussis in the US. Epidemics. 2011;3:183–188. doi: 10.1016/j.epidem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Hellenbrand W, Beier D, Jensen E, Littmann M, Meyer C, Oppermann H, Wirsing von König CH, Reiter S. The epidemiology of pertussis in Germany: past and present. BMC Infect Dis. 2009;9:22. doi: 10.1186/1471-2334-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang HR, Kim SY, Cha HH, An YM, Park IA, Kang HJ, Eun BW. An outbreak of mumps in a high school, Seoul, 2013. Pediatr Infect Vaccine. 2015;22:1–6. [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Update: mumps outbreak - New York and New Jersey, June 2009-January 2010. MMWR Morb Mortal Wkly Rep. 2010;59:125–129. [PubMed] [Google Scholar]
- 29.Orenstein WA, Markowitz L, Preblud SR, Hinman AR, Tomasi A, Bart KJ. Appropriate age for measles vaccination in the United States. Dev Biol Stand. 1986;65:13–21. [PubMed] [Google Scholar]
- 30.Kumar ML, Johnson CE, Chui LW, Whitwell JK, Staehle B, Nalin D. Immune response to measles vaccine in 6-month-old infants of measles seronegative mothers. Vaccine. 1998;16:2047–2051. doi: 10.1016/s0264-410x(98)00083-8. [DOI] [PubMed] [Google Scholar]
- 31.Gans H, Yasukawa L, Rinki M, DeHovitz R, Forghani B, Beeler J, Audet S, Maldonado Y, Arvin AM. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis. 2001;184:817–826. doi: 10.1086/323346. [DOI] [PubMed] [Google Scholar]