Abstract
Objectives. Apo E genes involved in lipoprotein synthesis and metabolism are considered one of the candidates to CHD. However, the results remain conflicting. Methods. We performed this meta-analysis based on 30 published studies including 11,804 CHD patients and 17,713 controls. Results. Compared with the wild genotype E3/3, the variant genotypes ApoEE3/4 and E4/4 were associated with 22% and 45% increased risk of CHD, respectively (E3/4 versus E3/3: OR = 1.22, 95% CI = 1.15–1.29; E4/4 versus E3/3: OR = 1.45, 95% CI = 1.23–1.71). Besides, compared with ε3 allele, carriers with the ε4 allele had a 46% increased risk of CHD (OR = 1.46, 95% CI = 1.28–1.66), while the ε2 had no significantly decreased risk of CHD. In the subgroup analysis by ethnicity, ε4 had a 25% increased risk of CHD in Caucasians (OR = 1.25, 95% CI = 1.11–1.41), and the effects were more evident in Mongolians (OR = 2.29, 95% CI = 1.89–2.77). The ε2 allele had a decreased risk of CHD in Caucasians (OR = 0.84, 95% CI = 0.74–0.96), but not in Mongolians. Conclusions. The analysis suggested that ApoEε4 mutation was associated with the increased risk of CHD, while ApoEε2 allele had a decreased risk of CHD just in Caucasians.
1. Introduction
Coronary heart disease (CHD) as a multifactorial disease caused by genetic and environmental factors became one of the leading causes of mortality and morbidity worldwide, especially in the developed countries [1]. Previous studies provided evidence that risk factors for CHD, including diabetes mellitus, smoking, and arterial hypertension, would contribute to rapid process of clinical events such as myocardial infarction (MI), ischemic heart failure, and death [2, 3]. Moreover, apart from the above risk factors, population-based studies have reported that genetic susceptibility accounts for around 50% of the risk for CHD, which suggested that the host genetic background plays an important role in the onset and development of CHD as well [4, 5]. The determination of blood lipid and lipoprotein levels is one of the coronary risk factors. Apolipoprotein genes involved in lipoprotein synthesis and metabolism play imperative roles in studying the susceptibility to CHD and cerebrovascular disease [6–11].
In 1992, Dallongeville et al. tested the consistent relationship between plasma TG levels and Apo E phenotype among 45 different populations in a meta-analysis [12]. Another meta-analysis including 14 studies showed that subjects with ε4 allele were associated with 26% increased risk of CHD compared with ε3 allele [13]. Since the publication of these two meta-analyses, numerous studies have appeared in recent years [14–17]. However, differences in study design, end point validation, choice of subjects, and limited statistical power have led to different results of Apo E genotypes on CHD risk in the general population. Therefore, the present meta-analysis is designed to derive a more plausible estimation.
2. Methods
2.1. Studies Selection
We search the electronic databases PubMed, Web of Science, Embase, Wanfang, China National Knowledge Internet (CNKI), and VIP for relevant English language articles from Jan 1, 2000, to Mar 1, 2016, using the following index terms: Apo E and polymorphism(s) single nucleotide polymorphism (SNP), variant, mutation and coronary artery disease, coronary heart disease, CAD, and CHD. Hand searches were also performed.
2.2. Inclusion Criteria
The inclusion criteria were as follows: (1) Apo E gene polymorphism in CAD or CHD; (2) case-control studies; (3) studies with full-text articles; (4) sufficient data for calculating an odds ratio (OR) with 95% confidence interval (CI); (5) not republished data.
2.3. Data Extraction
All the data were carefully extracted from all eligible publications independently by two authors. The following information was collected: the first author's name, date of publication, country, study design, major CHD end point, selection of the controls, genotyping methods, allele frequencies of ε2, ε3, and ε4, and genotype distribution in case-patients and controls. Definition of CHD end points includes MI, angina pectoris, percutaneous transluminal coronary angioplasty, coronary artery bypass graft surgery, and severe stenosis on coronary angiography. In most studies that included case-patients with coronary artery disease, diagnosis was based on angiographically documented luminal stenosis (≥50%) in at least 1 of the 3 major coronary arteries.
2.4. Quality Score Assessment
To determine the methodological quality of each study, we used the Newcastle-Ottawa scale (NOS). The NOS ranges between zero (worst) up to nine stars (best). Two authors of this article independently assessed the qualities of included studies. Disagreement was resolved by discussion.
2.5. Meta-Analysis
The risks (odds ratios, ORs) of CHD associated with the Apo E polymorphisms were estimated for each study with the software Stata12.0. The risk of the variant genotypes E2/2, E2/3, E2/4, E4/3, and E4/4 was estimated compared with the genotype E3/3 homozygotes. In addition to comparisons for total subjects, studies were categorized into different subgroup analyses according to the ethnicity. We estimated the between-study heterogeneity across the eligible comparisons using the Cochrane Q-test and the heterogeneity was considered significant for P < 0.1. Fixed effect or random effect was used to calculate pooled effect estimates. Random effects incorporate an estimate of the between-study variance and tend to provide wider confidence intervals, when the results of the constituent studies differ among themselves. In the absence of between-study heterogeneity, the two methods provide identical results.
Publication bias was evaluated by funnel plot and Begg's and Egger's tests. The Hardy-Weinberg equilibrium (HWE) was tested by a goodness-of-fit χ 2 test to compare the observed genotypes frequencies with the expected ones among control subjects. For sensitivity analyses, we examined whether the excluding studies with substantial deviation from HWE among controls affected our pooled estimates of ORs.
Finally, we use the following formula to estimate the fail-safe number:
| (1) |
K is the number of included studies and Z is the Z value of the independent study. The result is obtained from the software SAS 9.2.
3. Result
3.1. Studies Characteristics
714 studies were searched, among which 30 studies were included in the final meta-analysis [18–47]. The study selection process is detailed in Figure 1.
Figure 1.
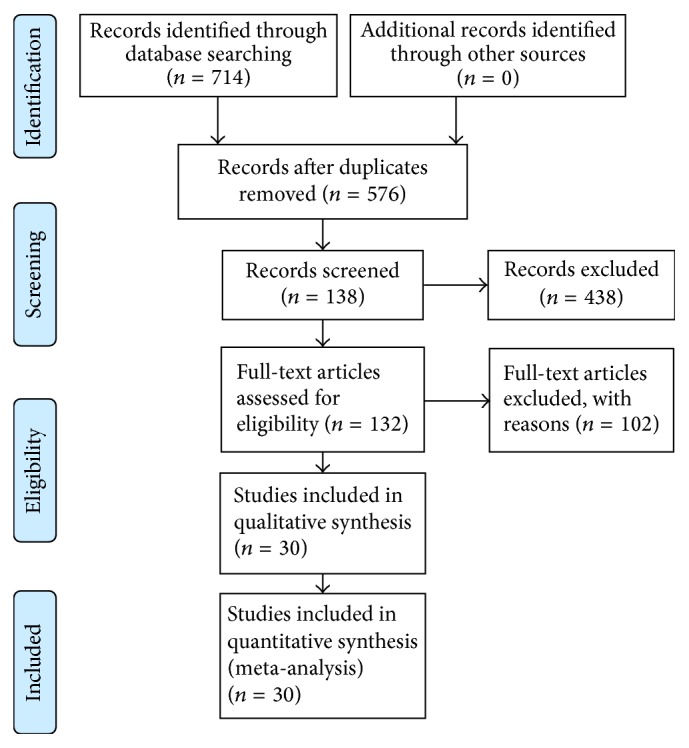
The flow diagram of study selection.
Given in Table 1 were the lists of number of cases, controls, HWE, and the NOS score of these 30 case-control studies. All studies indicated that the distribution of genotypes in the controls was consistent with HWE, except for 7 studies [29, 35, 37, 39, 40, 45, 46] (P < 0.05). According to the quality criteria, there were 21 studies with high quality (NOS score > 6).
Table 1.
Distribution of the CHD cases and controls selection in the studies of APOE gene polymorphisms and CHD risk.
| First author (year) |
Ethnic | Case distribution | Percentage (%) | Control distribution | Percentage (%) | HW (Pvalue) | NOS score |
|---|---|---|---|---|---|---|---|
| (1) Wang (2004) | Mongoloid (China) | 146 cases of coronary heart disease confirmed by coronary angiography: 90 male cases and 56 females, mean ages 64 ± 11 years | 30.0 | 340 control people had noncardiovascular disease and removed the body lipid metabolism disorder, cancer, and cerebrovascular disease population; 184 men and 156 women, mean age 63 ± 12 years | 70.0 | 0.410 | 6 |
| (2) Bai (2001) | Mongoloid (China) | All 65 patients with coronary heart disease patients were men, aged 47.16 ± 8.13 years on average | 58.0 | 47 controls were noncardiovascular disease; mean age 46 ± 6 years | 42.0 | 0.939 | 7 |
| (3) Wang (2004) | Mongoloid (China) | 110 cases were male and 76 females, aged 41 to 88 years, mean ages 65.0 ± 10.6 years | 34.7 | 350 controls (185 males, 165 females), aged 40 to 87 years, mean 63.56 ± 8.32 years, were in the same hospital during the same period by the past medical history, physical examination, and ECG and other methods of noncardiac diagnosis of vascular disease cases | 65.3 | 0.432 | 7 |
| (4) Peng (2001) | Mongoloid (China) | 213 cases of coronary heart disease and 52 cases of early-onset CHD and 161 cases of late CHD; early-onset group included 30 males and 22 females, mean age 47.4 ± 3.9 years; the late CHD group included 97 male cases and 64 female cases, mean age 69.8 ± 7.9 years | 54.2 | 180 healthy persons acted as controls: 94 males and 86 females, mean age 53.1 ± 5.7 years | 45.8 | 0.436 | 8 |
| (5) Yang (2001) | Mongoloid (China) | 204 cases of myocardial infarction and angina pectoris (coronary angiography showed stenosis greater than or equal to 70%) patients, 55 cases of early-onset group, the average age of 45.14 ± 8.18 years, 136 cases of late-onset group, the average age of 70.1 ± 6.4 years | 60 | 136 control patients from the local companies and confirmed noncoronary heart disease; the average age of 52.15 ± 12.18 years | 40 | 0.738 | 7 |
| (6) Ouyang (2005) | Mongoloid (China) | A total of 200 cases of coronary heart disease; 105 cases were male and 95 cases were female; average age (63.6 ± 5.8) years | 66.7 | 100 healthy people as control group: male 55 and female 45; average age 62.1 ± 7.8 years; no coronary heart disease symptoms; ECG and echocardiography tests were normal | 33.3 | 0.399 | 7 |
| (7) Heide (2009) | Caucasian (Germany) | 121 cases of coronary heart disease: 100 cases of men, average age 33.7 ± 5.8 years; 21 females, mean age 39.1 ± 5.4 years | 32.6 | 250 blood donors as controls, mean age 31.8 ± 6.7 years | 67.4 | 0.529 | 8 |
| (8) Kolovou (2009) | Caucasian (Greek) | 359 cases were male patients with coronary heart disease; coronary angiography showed occlusion greater than or equal to 50% | 59 | 248 health people were recruited as control, with no cardiovascular disease and no family history of cardiovascular disease | 41 | 0.579 | 7 |
| (9) Bahri (2008) | Caucasian (Tunisian) | 80 cases (diagnosed after coronary angiography and ECG): 64 males and 16 females, mean age 57.42 ± 8.37 years; 56 were smoking, 51 had diabetes, 35 had hypertension, and 22 had high blood lipid | 44.4 | 100 controls without coronary heart disease and other cardiovascular diseases, and cases from the same region in which 24 men and 76 women were randomly selected | 55.6 | 0.815 | 6 |
| (10) Akanji (2007) | Caucasian (Kuwaiti) | 50 cases had evidence of acute myocardial infarction, the average age of 54 years | 43.5 | 65 controls were randomly selected blood donors; the average age was 39 years without CHD family history; physical examination and blood examination ruled out other systemic diseases | 56.5 | 0.441 | 7 |
| (11) Aydogan (2009) | Caucasian (Turkey) | 41 cases: 19 women and 22 men have undergone coronary angiography; patients with atherosclerosis risk factors such as diabetes, high blood pressure, and smoking | 55.4 | 33 controls: 12 males and 11 females, no family history of coronary heart disease and coronary heart disease symptoms | 44.6 | 0.754 | 7 |
| (12) Ranjith (2004) | Caucasian (India) | 195 cases were patients with acute myocardial infarction | 39.4 | 300 controls from the same community, without cardiovascular disease and no associated risk factors | 60.6 | <0.001 | 7 |
| (13) Peng (2003) | Mongoloid (China) | 150 cases were diagnosed after coronary angiography or myocardial infarction | 48.9 | 157 control patients from the hospital's medical examination, age and sex matched with cases | 51.1 | 0.424 | 8 |
| (14) Singh (2008) | Caucasian (India) | 193 cases are diagnosed after coronary angiography; the average age of 54.94 ± 11.43 years | 56.3 | 150 patients: 105 males and 45 females, mean age 53.42 ± 12.47 years | 43.7 | 0.327 | 6 |
| (15) Keavney (2003) | Caucasian | 4484 cases of patients, mean age 50.5 ± 7.3 years, of which 1706 are smokers | 43.8 | 5757 cases were of an average age of 45.7 ± 9.7 years, including 1151 smokers | 56.2 | 0.463 | 8 |
| (16) Freitas (2002) | Caucasian | 640 cases were diagnosed by coronary angiography and (or) a history of myocardial infarction, mean age 44 ± 4 years, male 561, female 79, and 300 smokers |
50.6 | 624 healthy people were randomly selected from the same community; no history of cardiovascular disease, mean age 40 ± 6 years, 322 men, 302 women, 183 smokers | 49.4 | 0.767 | 8 |
| (17) Kawakami (2001) | Mongoloid (Japan) | 225 cases, of which 76 were vasospasm angina patients and 149 were ischemic heart disease, male 171, female 54 | 51.4 | 213 controls, average age 58.4 years, 152 male, 61 female; they were randomly selected from the hospital when they had an annual physical examination; no history of cardiovascular disease | 48.6 | 0.510 | 7 |
| (18) Attila (2001) | Caucasian (Turkey) | 107 patients have undergone coronary angiography: 73 men and 34 women; the average age of 55.1 ± 10.2 years, 53 patients with hypertension, 22 with diabetes, 52 smokers | 53.8 | 92 controls, after coronary angiography for the exclusion of noncoronary heart disease, of whom 51 were men and 41 women, average age 51.6 ± 9.5 years, 33 patients with hypertension, 6 with diabetes mellitus, 29 smokers | 46.2 | <0.001 | 6 |
| (19) Kharrazi (2006) | Caucasian (Iran) | 115 cases of 34 men and 81 women, mean age 54.4 ± 8.9 years | 46 | 135 controls: 50 females and 85 males, mean age 55 ± 12.3 years | 54 | 0.834 | 7 |
| (20) Gamboa (2008) | Caucasian (Mexico) | 156 patients, 124 males, 32 females, mean age 56.2 ± 9.8 years | 43.8 | 200 controls: 150 males and 50 females, mean age 55.7 ± 4.16 years | 56.2 | <0.001 | 8 |
| (21) Al-Yahyaee (2007) | Caucasian | 67 cases who were diagnosed as rheumatoid arthritis patients with coronary heart disease | 27.5 | 177 controls were cases of noncoronary heart disease patients with rheumatoid arthritis | 72.5 | 0.903 | 6 |
| (22) Al-Bustan (2009) | Caucasian (Kuwaiti) | 143 patients: 91 were males and 52 females; mean age was 60.88 ± 12.1 years | 54.0 | 122 controls: 65 males and 57 females, mean age 57.18 ± 13.0 years | 46.0 | <0.001 | 6 |
| (23) Vaisi-Raygani (2010) | Caucasian (Iran) | 162 patients were diagnosed by angiographic documented CAD and without diabetes 89 males and 73 females, mean age 56.3 ± 8.5 years | 47.5 | 179 unrelated control subjects: 88 males and 91 females, mean age 55.7 ± 12.9 years | 52.5 | 0.035 | 7 |
| (24) Corella (2011) | Caucasian (Spain) | 534 cases were participants who developed an incident CHD event during follow-up |
32.2 | 1123 controls were healthy matched subjects | 67.8 | 0.169 | 8 |
| (25) Fallah (2011) | Caucasian (Iran) | 190 patients: 140 males and 50 females; age range: 49–70 years | 48.7 | 200 controls: 100 males and 100 females, age range: 36–62 years | 51.3 | 0.059 | 6 |
| (26) Gustavsson (2012) | Caucasian (Sweden) | 1735 CHD cases included the INTERGENE study with upper age limit of 75 years and the SHEEP study with age of 45–70 years | 27.2 | 4654 controls: the INTERGENE study: 3614 people aged 25–74 years; the SHEEP study: 1561 control subjects free from earlier MI diagnosis and matched for age and sex | 72.8 | 0.765 | 7 |
| (27) Heidari (2013) | Caucasian (Iran) | 66 patients with significant lesions, male gender 35, mean age ± SD 52.5 ± 7.9 | 52.4 | 60 controls with normal coronary artery, male gender 32, mean age ± SD 51.2 ± 7.1 | 47.6 | <0.001 | 6 |
| (28) Marrzoq (2011) | Caucasian (Czech) | 69 subjects (24 female and 45 male) with coronary artery disease | 50.4 | 68 controls: 35 female and 33 male | 49.6 | 0.708 | 6 |
| (29) Afroze (2015) | Caucasian (India) | 200 CAD patients (130 female and 70 male) were recruited from a cohort of patients undergoing clinically indicated coronary angiography | 30.8 | 450 control subjects (260 female and 190 male) | 69.2 | <0.001 | 8 |
| (30) Sapkota (2015) | Caucasian (USA) | 723 CAD patients were diagnosed by nitrate medication, electrocardiographic evidence of angina pain, coronary angiographic evidence of severe (greater than 50%) stenosis, or echocardiographic evidence of myocardial infarction | 37.4 | 1212 controls were healthy subjects without T2D or CAD | 62.6 | 0.08 | 7 |
Tables 2 and 3 showed the frequency distributions of the Apo E alleles and genotypes in the cases and controls.
Table 2.
Distribution of Apo E alleles in the case and control groups included in the meta-analysis.
| First author (year) | Case/control (N/N) | Distribution of apolipoprotein E alleles | |||||
|---|---|---|---|---|---|---|---|
| ε2 | ε3 | ε4 | |||||
| Case | Control | Case | Control | Case | Control | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| (1) Wang (2004) | 292/680 | 21 (7.2) | 48 (7.1) | 232 (79.4) | 583 (85.7) | 39 (13.4) | 49 (7.2) |
| (2) Bai (2001) | 100/94 | 4 (4.0) | 5 (5.3) | 90 (90.0) | 86 (91.5) | 6 (6.0) | 3 (3.2) |
| (3) Wang (2004) | 372/700 | 27 (7.3) | 50 (7.1) | 291 (78.2) | 601 (85.9) | 54 (14.5) | 49 (7.0) |
| (4) Peng (2001) | 426/360 | 34 (8.0) | 30 (8.3) | 328 (77.0) | 303 (84.2) | 64 (15.0) | 27 (7.5) |
| (5) Yang (2001) | 408/272 | 20 (4.9) | 15 (5.5) | 348 (85.3) | 239 (87.9) | 40 (9.8) | 18 (6.6) |
| (6) Ouyang (2005) | 400/200 | 31 (7.7) | 18 (9.0) | 291 (72.8) | 167 (81.5) | 78 (19.5) | 19 (9.5) |
| (7) Heide (2009) | 242/500 | 11 (4.5) | 36 (8.3) | 202 (83.5) | 406 (81.2) | 29 (12.0) | 58 (11.5) |
| (8) Kolovou (2009) | 718/496 | 37 (5.2) | 39 (7.9) | 612 (85.2) | 414 (83.4) | 69 (9.6) | 43 (8.7) |
| (9) Bahri (2008) | 160/200 | 6 (3.7) | 9 (4.5) | 140 (87.6) | 174 (87.0) | 14 (8.7) | 17 (8.5) |
| (10) Akanji (2007) | 100/130 | 6 (6.0) | 9 (6.9) | 87 (87.0) | 108 (83.1) | 7 (7.0) | 13 (10.0) |
| (11) Aydogan (2009) | 82/46 | 4 (4.9) | 4 (17.4) | 69 (84.1) | 37 (80.4) | 9 (11.0) | 5 (11.6) |
| (12) Ranjith (2004) | 390/600 | 10 (2.6) | 27 (4.5) | 330 (84.6) | 517 (86.2) | 50 (12.8) | 56 (9.3) |
| (13) Peng (2003) | 300/314 | 24 (8.0) | 16 (5.0) | 237 (79.0) | 275 (87.6) | 39 (13.0) | 23 (7.4) |
| (14) Singh (2008) | 386/300 | 21 (5.4) | 23 (7.7) | 307 (79.5) | 257 (85.6) | 58 (15.1) | 20 (6.7) |
| (15) Keavney (2003) | 8968/11514 | 609 (6.8) | 911 (7.9) | 6778 (75.6) | 8830 (76.7) | 1581 (17.6) | 1773 (15.4) |
| (16) Freitas (2002) | 1280/1248 | 61 (4.8) | 90 (7.2) | 1002 (78.2) | 958 (76.8) | 217 (17.0) | 200 (16.0) |
| (17) Kawakami (2001) | 450/426 | 13 (2.9) | 15 (3.5) | 390 (86.7) | 349 (81.9) | 47 (10.4) | 62 (14.6) |
| (18) Attila (2001) | 214/184 | 26 (12.1) | 15 (8.1) | 166 (77.6) | 163 (88.6) | 22 (10.3) | 6 (3.3) |
| (19) Kharrazi (2006) | 230/270 | 18 (7.8) | 11 (4.1) | 169 (73.5) | 250 (92.6) | 43 (18.7) | 9 (3.3) |
| (20) Gamboa (2008) | 312/400 | 5 (1.6) | 9 (2.2) | 283 (90.7) | 364 (91.0) | 24 (7.7) | 27 (6.8) |
| (21) Al-Yahyaee (2007) | 134/354 | 1 (0.7) | 6 (1.7) | 127 (94.8) | 335 (94.6) | 6 (4.5) | 13 (3.7) |
| (22) Al-Bustan (2009) | 286/244 | 21 (7.4) | 23 (9.4) | 246 (86.0) | 207 (84.9) | 19 (6.6) | 14 (5.7) |
| (23) Vaisi-Raygani (2010) | 324/357 | 31 (9.6) | 40 (11.3) | 257 (79.3) | 289 (80.8) | 36 (11.1) | 28 (7.9) |
| (24) Corella (2011) | 1035/2246 | 42 (4.1) | 123 (5.5) | 891 (86.1) | 1928 (85.8) | 102 (9.9) | 195 (8.7) |
| (25) Fallah (2011) | 380/400 | 73 (19.2) | 55 (13.8) | 141 (37.1) | 218 (54.5) | 166 (43.7) | 127 (31.8) |
| (26) Gustavsson (2012) | 3470/9308 | 199 (5.7) | 738 (7.9) | 2672 (77.0) | 7065 (75.9) | 599 (17.3) | 1505 (16.2) |
| (27) Heidari (2013) | 132/122 | 16 (12.1) | 14 (11.5) | 110 (83.3) | 108 (88.5) | 6 (4.5) | 0 (0) |
| (28) Marrzoq (2011) | 138/136 | 5 (3.6) | 7 (5.2) | 114 (82.6) | 119 (87.5) | 19 (13.8) | 10 (7.4) |
| (29) Afroze (2015) | 400/900 | 26 (6.5) | 66 (7.3) | 291 (72.8) | 732 (81.3) | 83 (20.8) | 102 (11.3) |
| (30) Sapkota (2015) | 1446/2432 | 52 (3.6) | 95 (3.9) | 1285 (88.9) | 2174 (89.4) | 109 (7.5) | 163 (6.7) |
Table 3.
Distribution of Apo E genotypes in case and control groups included in the meta-analysis.
| First author (year) | Case/control (N/N) | Distribution of apolipoprotein E genotype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E2/2 | E2/3 | E2/4 | E3/3 | E3/4 | E4/4 | ||||||||
| Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | Case | Control | ||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| (1) Wang (2004) | 146/340 | 0 (0.0) | 3 (0.9) | 19 (13.0) | 41 (12.1) | 2 (1.3) | 1 (0.3) | 89 (61.0) | 249 (73.2) | 35 (24.0) | 44 (12.9) | 1 (0.7) | 2 (0.6) |
| (2) Bai (2001) | 50/47 | 0 (0.0) | 0 (0.0) | 4 (8.0) | 5 (10.6) | 0 (0.0) | 0 (0.0) | 40 (80.0) | 39 (82.99) | 6 (12.0) | 3 (6.4) | 0 (0.0) | 0 (0.0) |
| (3) Wang (2004) | 186/350 | 0 (0.0) | 3 (0.9) | 25 (13.4) | 43 (12.3) | 2 (1.1) | 1 (0.3) | 108 (58.1) | 257 (73.4) | 50 (26.9) | 44 (12.6) | 1 (0.5) | 2 (0.6) |
| (4) Peng (2001) | 213/180 | 0 (0.0) | 0 (0.0) | 29 (13.6) | 27 (15.0) | 5 (2.3) | 3 (1.7) | 123 (57.7) | 126 (70.0) | 53 (24.9) | 24 (13.3) | 3 (1.4) | 0 (0.0) |
| (5) Yang (2001) | 204/136 | 1 (0.5) | 1 (0.7) | 18 (8.8) | 12 (9.0) | 0 (0.0) | 1 (0.7) | 153 (25.0) | 106 (77.9) | 24 (11.8) | 15 (11.0) | 8 (3.9) | 1 (0.7) |
| (6) Ouyang (2005) | 200/100 | 1 (0.5) | 0 (0.0) | 28 (14.0) | 17 (17.0) | 1 (1.0) | 1 (0.5) | 98 (49.0) | 66 (66.0) | 67 (33.5) | 14 (14.0) | 5 (2.5) | 2 (2.0) |
| (7) Heide (2009) | 121/250 | 0 (0.0) | 0 (0.0) | 7 (5.8) | 31 (12.4) | 4 (3.3) | 5 (2.0) | 88 (72.7) | 163 (65.2) | 19 (15.7) | 49 (19.6) | 3 (2.5) | 2 (0.8) |
| (8) Kolovou (2009) | 359/248 | 2 (0.6) | 0 (0.0) | 26 (7.2) | 36 (14.5) | 7 (1.9) | 3 (1.2) | 268 (74.7) | 171 (69.0) | 50 (13.9) | 36 (14.5) | 6 (1.7) | 2 (0.8) |
| (9) Bahri (2008) | 80/100 | 0 (0.0) | 0 (0.0) | 6 (7.5) | 8 (8.0) | 0 (0.0) | 1 (1.0) | 61 (76.3) | 78 (78.0) | 13 (16.3) | 13 (13.0) | 0 (0.0) | 0 (0.0) |
| (10) Akanji (2007) | 50/65 | 0 (0.0) | 0 (0.0) | 6 (12.0) | 9 (13.8) | 0 (0.0) | 0 (0.0) | 37 (74.0) | 43 (66.2) | 7 (14.0) | 13 (20.0) | 0 (0.0) | 0 (0.0) |
| (11) Aydogan (2009) | 41/23 | 0 (0.0) | 0 (0.0) | 4 (9.8) | 3 (13.0) | 0 (0.0) | 1 (4.3) | 28 (68.3) | 15 (65.2) | 9 (22.0) | 4 (17.4) | 0 (0.0) | 0 (0.0) |
| (12) Ranjith (2004) | 195/300 | 0 (0.0) | 3 (1.0) | 7 (4.0) | 18 (6.0) | 3 (1.0) | 3 (1.0) | 139 (71.0) | 228 (76.0) | 45 (83.0) | 43 (14.0) | 1 (1.0) | 5 (2.0) |
| (13) Peng (2003) | 150/157 | 1 (0.7) | 1 (0.6) | 21 (14.0) | 13 (8.3) | 1 (0.7) | 1 (0.6) | 93 (62.0) | 122 (77.7) | 30 (20.0) | 18 (11.5) | 4 (2.7) | 2 (1.7) |
| (14) Singh (2008) | 193/150 | 1 (0.5) | 1 (0.7) | 15 (7.8) | 19 (12.7) | 4 (2.1) | 2 (1.3) | 123 (63.7) | 112 (74.7) | 46 (23.8) | 14 (9.7) | 4 (2.1) | 2 (1.3) |
| (15) Keavney (2003) | 4484/5757 | 34 (0.8) | 44 (0.8) | 440 (9.8) | 686 (11.9) | 101 (2.3) | 137 (2.4) | 2566 (57.2) | 3384 (58.8) | 1206 (26.9) | 1376 (23.9) | 137 (13.0) | 130 (2.2) |
| (16) Freitas (2002) | 640/624 | 5 (0.8) | 4 (0.6) | 37 (5.8) | 67 (10.7) | 14 (2.2) | 15 (2.4) | 396 (62.0) | 372 (60.0) | 173 (27.0) | 147 (24.0) | 15 (2.3) | 19 (3.0) |
| (17) Kawakami (2001) | 225/213 | 3 (1.2) | 0 (0.0) | 6 (2.7) | 13 (6.1) | 1 (0.4) | 2 (0.9) | 177 (78.7) | 140 (65.7) | 30 (1.3) | 56 (26.3) | 8 (3.6) | 2 (0.9) |
| (18) Attila (2001) | 107/92 | 9 (8.4) | 4 (4.3) | 7 (6.5) | 7 (7.6) | 0 (0.0) | 0 (0.0) | 71 (66.4) | 75 (81.6) | 18 (16.8) | 5 (5.4) | 2 (1.9) | 1 (1.1) |
| (19) Kharrazi (2006) | 115/135 | 0 (0.0) | 0 (0.0) | 18 (15.7) | 11 (8.1) | 0 (0.0) | 0 (0.0) | 54 (47.0) | 115 (85.2) | 43 (37.4) | 9 (6.7) | 0 (0.0) | 0 (0.0) |
| (20) Gamboa (2008) | 156/200 | 0 (0.0) | 4 (2.0) | 5 (3.2) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 127 (81.4) | 168 (84.0) | 24 (15.4) | 27 (13.5) | 0 (0.0) | 0 (0.0) |
| (21) Al-Yahyaee (2007) | 67/177 | 0 (0.0) | 0 (0.0) | 1 (1.5) | 6 (3.4) | 0 (0.0) | 0 (0.0) | 60 (89.6) | 158 (89.3) | 6 (9.0) | 13 (7.3) | 0 (0.0) | 0 (0.0) |
| (22) Al-Bustan (2009) | 143/122 | 8 (5.6) | 9 (7.3) | 3 (2.1) | 2 (1.6) | 2 (1.4) | 3 (2.5) | 114 (79.7) | 98 (80.3) | 15 (10.5) | 9 (7.4) | 1 (0.7) | 1 (0.8) |
| (23) Vaisi-Raygani (2010) | 162/179 | 0 (0) | 6 (3.4) | 31 (19.1) | 26 (14.5) | 0 (0) | 2 (1.1) | 99 (61.1) | 119 (66.5) | 28 (17.3) | 24 (13.4) | 4 (2.5) | 2 (1.1) |
| (24) Corella (2011) | 534/1123 | 1 (0.1) | 1 (0) | 33 (6.2) | 105 (9.3) | 7 (1.3) | 16 (1.4) | 400 (74.9) | 828 (73.7) | 91 (17.0) | 167 (14.8) | 2 (0.4) | 6 (0.5) |
| (25) Fallah (2011) | 190/200 | 13 (6.8) | 7 (3.5) | 10 (5.3) | 22 (11.0) | 37 (19.5) | 19 (9.5) | 35 (18.4) | 59 (29.5) | 61 (32.1) | 78 (39.0) | 34 (17.9) | 15 (7.5) |
| (26) Gustavsson (2012) | 1735/4654 | 11 (0.6) | 32 (0.7) | 133 (7.8) | 547 (11.8) | 44 (2.5) | 127 (2.7) | 1044 (60.2) | 2689 (57.8) | 451 (26.0) | 1140 (24.5) | 52 (3.0) | 119 (2.6) |
| (27) Heidari (2013) | 66/61 | 8 (12.1) | 7 (11.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 52 (78.8) | 54 (88.5) | 6 (9.1) | 0 (0) | 0 (0) | 0 (0) |
| (28) Marrzoq (2011) | 69/68 | 0 (0) | 0 (0) | 5 (7.2) | 7 (10.3) | 0 (0) | 0 (0) | 45 (65.2) | 51 (75.0) | 19 (27.5) | 10 (14.7) | 0 (0) | 0 (0) |
| (29) Afroze (2015) | 200/450 | 2 (1) | 9 (2) | 18 (9) | 36 | 4 (2) | 12 (2.7) | 110 (55) | 315 (7) | 53 (26.5) | 66 (14.7) | 13 (6.5) | 12 (2.7) |
| (30) Sapkota (2015) | 723/1212 | 1 (0.1) | 1 (0) | 46 (6.4) | 83 (6.8) | 4 (0.5) | 10 (0.8) | 572 (79.1) | 977 (80.6) | 95 (13.1) | 137 (11.3) | 5 (0.7) | 4 (0.3) |
3.2. Meta-Analysis Results
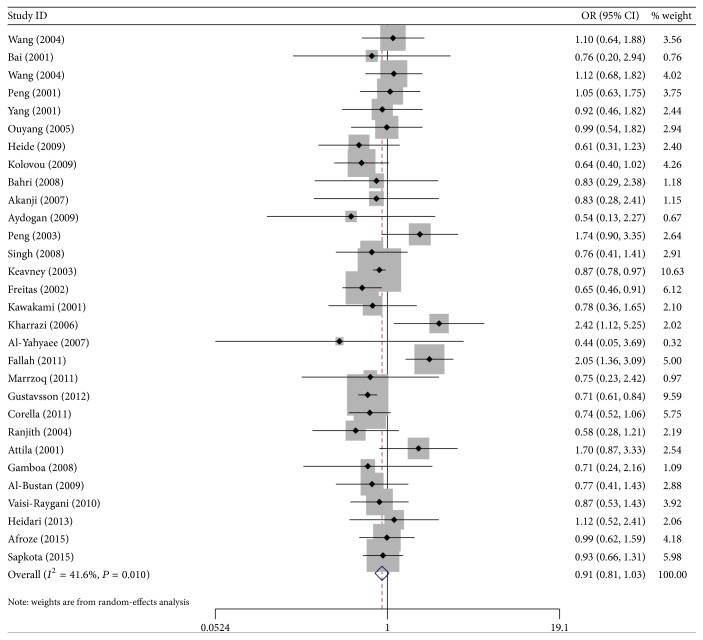
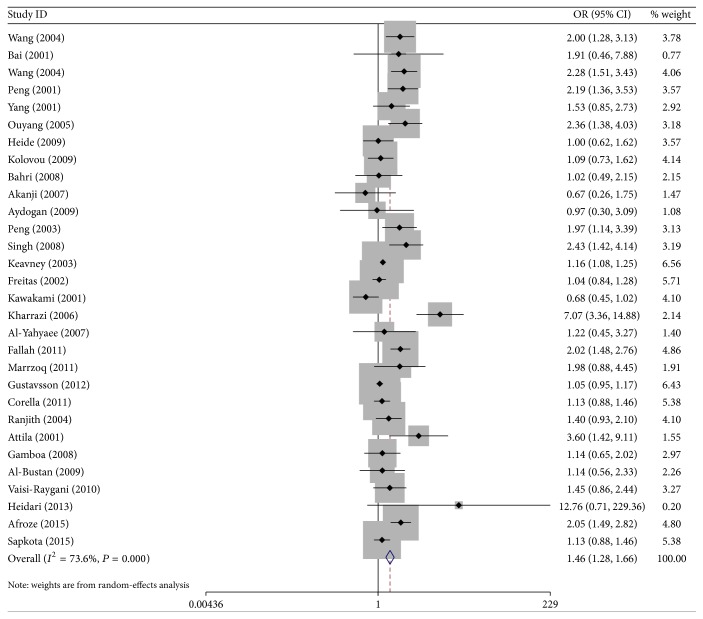
Figures 2 and 3 showed the ORs on CHD associated with ApoEε2 alleles and ApoEε4 alleles compared with the ε3 alleles in individual studies.
Figure 2.
ORs of CHD associated with Apo E for the allele ε2 compared with the ε3.
Figure 3.
ORs of CHD associated with Apo E for the allele ε4 compared with the ε3.
Overall, in the dominant model, individuals carrying the ApoEE2/2, E2/3, and E2/4 genotypes did not have significant risk of CHD compared with individuals with the E3/3 genotype (E2/2 versus E3/3: OR = 1.03, 95% CI = 0.79–1.34; E2/3 versus E3/3: OR = 0.89, 95% CI = 0.76–1.06; E2/4 versus E3/3: OR = 1.04, 95% CI = 0.87–1.23), However, compared with E3/3 genotype, the variant genotypes ApoEE4/4 and E3/4 were associated with increased risk of CHD in 30 studies in the genetic models (E3/4 versus E3/3: OR = 1.48, 95% CI = 1.26–1.75; E4/4 versus E3/3: OR = 1.45, 95% CI = 1.23–1.71).
In the subgroups, the results showed evidence of significant association between Apo E gene polymorphism and CHD risk in the genetic model of E3/4 and E4/4 compared with the genotype E3/3 in eight Mongolian studies (E3/4 versus E3/3: OR = 1.73, 95% CI = 1.02–2.93; E4/4 versus E3/3: OR = 2.78, 95% CI = 1.35–5.72), while the variant genotypes ApoEE2/2, E2/3, and E2/4 were not associated with the increased risk of CHD (E2/2 versus E3/3: OR = 1.08, 95% CI = 0.39–3.00; E2/3 versus E3/3: OR = 1.16, 95% CI = 0.91–1.49; E2/4 versus E3/3: OR = 1.38, 95% CI = 0.62–3.11). In 22 Caucasians studies, significant associations were found in three genetic models (E2/3 versus E3/3: OR = 0.81, 95% CI = 0.67–0.98; E3/4 versus E3/3: OR = 1.38, 95% CI = 1.17–1.62; E4/4 versus E3/3: OR = 1.51, 95% CI = 1.12–2.04); carriers with ApoEE2/2 and E2/4 were not associated with the significant risk of CHD (E2/2 versus E3/3: OR = 1.03, 95% CI = 0.78–1.35; E2/4 versus E3/3: OR = 1.02, 95% CI = 0.86–1.22).
Besides, carriers with ApoEε2 allele had no significantly decreased risk of CHD compared with individuals with the ε3 allele in the random-effect model (OR = 0.91; 95% CI = 0.81–1.03). Stratified analysis on the descent also showed no evidence on the ε2 allele variant and CHD risk in Mongolians (OR = 1.18, 95% CI = 0.94–1.46), but there had a decrease risk in Caucasians (OR = 0.84, 95% Cl = 0.74–0.96). In addition, compared with the ApoEε3 allele, carriers with the ε4 allele had a 46% increased risk of CHD in the random-effect model (OR = 1.46, 95% CI = 1.28–1.66), and the effects were more evident in the Mongolians (the random-effects model OR = 2.29, 95% CI = 1.89–2.77) and mild in the Caucasians (the fixed-effects model OR = 1.25, 95% CI = 1.11–1.41).
3.3. Sensitivity Analysis
We conducted a sensitivity analysis on the Apo E polymorphisms and risk of CHD excluding studies deviating from HWE among controls. The pooled ORs estimates were similar with that of excluded studies, so the results were not shown.
3.4. Bias Diagnostics
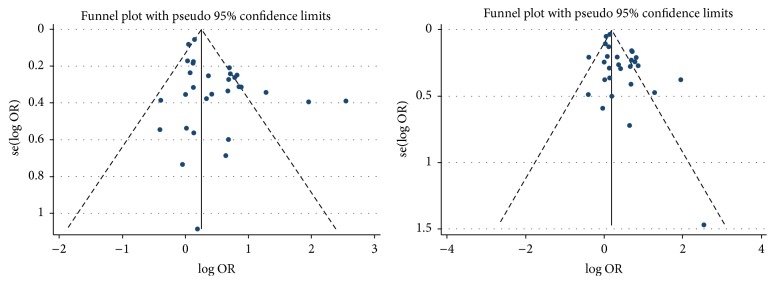
For the ApoEε2 versus ε3 allele, the shape of the funnel plot seemed symmetrical (Figure 4), and then Egger's test showed no evidence of publication bias (P = 0.211), and the fail-safe number also showed no publication bias in this meta-analysis (Nfs(0.05) = 381.51, Nfs(0.01) = 173.87).
Figure 4.
The funnel plot of the Apo E allele ε2 and ε4 compared with the ε3.
For the ApoEε4 compared with ε3 allele, the shape of the funnel plots seemed asymmetrical (Figure 4), and Egger's test revealed there was a significant publication bias (P = 0.003). By using the trim and fill method, we showed that OR and 95% CI did not change. Besides, the fail-safe number also suggested that the result of the meta-analysis was stability (Nfs(0.05) = 1218.31, Nfs(0.01) = 588.44).
4. Discussion
Apolipoprotein E (Apo E) is one of the most major apolipoproteins in the central nervous system, with functions of neurons repair. Apo E genetic variants showed significant associations with the risks of nervous system degenerative diseases, including Alzheimer's disease, vascular dementia, and cerebrovascular disease [48, 49]. Apo E gene as a receptor-binding ligand mediating the clearance of chylomicron and remnants of very-low-density lipoprotein cholesterol from plasma also plays a major role in the metabolisms of cholesterol and triglyceride. Functional variants of genes encoding lipoproteins are responsible in part for between-individual variation in the plasma levels of lipoproteins.
Apo E with 3 major isoforms, E2, E3, and E4, which are coded by the corresponding alleles, ε2, ε3, and ε4, has 6 genotypes, E2/2, E2/3, E2/4, E3/3, E3/4, and E4/4. Compared with ε3 carriers, carriers of the ε2 allele, which has defective receptor-binding ability, have lower circulating cholesterol levels and higher triglyceride levels, whereas carriers of the ε4 allele appear to have higher plasma levels of total and low-density lipoprotein cholesterol [50–52]. In serum or plasma cholesterol of healthy Caucasians, Apo E polymorphisms accounted for approximately 2%–11% of the total [53]. Recent evidences also indicated that Apo E may play additional roles in the development of CHD through macrophage cholesterol efflux, platelet aggregation, and allele-specific antioxidant and immune activities [53–55].
A lot of epidemiologic studies have investigated the relation between Apo E genotypes and CHD risk in the general population. Apo E polymorphisms are believed to confer susceptibility to CHD risk. In 1992, a meta-analysis of 27 studies reported that the subjects carrying the ε2 and ε4 alleles had, respectively, lower and higher plasma cholesterol values than subjects carrying the E3/3 genotype, which suggested that the ApoEε4 allele may, in individuals with the ApoEE4/3 phenotype, be a risk factor of cardiovascular disease [12]. The last meta-analysis of 14 published case-control studies in 2015 showed that carriers with POEε2 allele were associated with the decreased risk for CHD (OR = 0.82, 95% CI: 0.75–0.90) compared with those carrying ε3 allele, while those with ε4 allele had a significant increased risk for CHD (OR = 1.34, 95% CI: 1.15–1.57) [15].
In this 30 studies' meta-analysis including 11,804 CHD patients and 17,713 controls, we identified a significant increased risk for CHD among carriers of the ApoEE3/4 and E4/4 genotypes compared with carriers of the E3/3 genotype, but no significant evidence was found between the variant genotypes of ApoEE2/3, E2/4, and E2/2 and CHD risk. In the stratified analyses by descent, for the ε4 allele genetic variant, the effect was more evident in the Mongolians group and mild in Caucasians group, which showed that the ApoEε4 allele genetic variant modulated the increased risk of CHD with the differences of genetic background. Moreover, there was a decreased risk in Caucasians between the ε2 allele variant and CHD risk but no evidence in Mongolians; further studies should be conducted to verify it.
Our study has several limitations. First, as with all meta-analyses, although we did Egger's test and calculated the fail-safe number to evaluate the publication bias, it might have occurred because our analyses were all based on published studies. For the ApoEε4 compared with ε3 allele, the Egger test showed existence of publication bias, but from the results of the trim and fill method and the fail-safe number, the publication bias and the possibility of false positive were relatively small. Second, the control group of some studies was not in conformity with Hardy-Weinberg equilibrium. But, in sensitivity analysis, the pooled estimates were similar after we excluded studies deviating from Hardy-Weinberg equilibrium among controls; therefore these studies were included in the final analysis. Gene-environment interactions may have contributed to the CHD. Apo E gene is a candidate gene and a common one to study gene-environment interactions. However, because of lack of the original data of the meta-analysis, further evaluation of potential gene-gene and gene-environment interactions was limited.
In conclusion, it was showed in this meta-analysis that ApoEε4 allele polymorphism may contribute to the individual susceptibility of CHD. Further rigorous design, large sample of case-control, or prospective study are required to continue in-depth evaluation on gene-gene and gene-environment interactions on Apo E polymorphisms and CHD risk.
Acknowledgments
This study was supported by the “Five-Twelfth” National Science and Technology Support Program (no. 2012BAI41B08 and no. 2013BAI12B01) and the National Natural Science Foundation of China (81673259).
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Min Xu and Jun Zhao equally contributed to the article.
References
- 1.Zhou J., Chen X., Ye H., et al. An association study between genetic polymorphism in the interleukin-6 receptor gene and coronary heart disease. BioMed Research International. 2014;2014:6. doi: 10.1155/2014/504727.504727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halim E. F. A. E., Reda A. A., Hendi A. A. K., Zaki S. A., Essa E. S., Khalifa A. S. Apolipoprotein E gene variants as a risk factor for coronary artery disease in type 2 diabetic Egyptian patients. The Egyptian Journal of Immunology. 2012;19(1):1–10. [PubMed] [Google Scholar]
- 3.Kolovou G. D., Panagiotakos D. B., Kolovou V., et al. Common variants of apolipoprotein E and cholesteryl ester transport protein genes in male patients with coronary heart disease and variable body mass index. Angiology. 2015;66(2):169–173. doi: 10.1177/0003319713517927. [DOI] [PubMed] [Google Scholar]
- 4.Roberts R., Stewart A. F. R. Genes and coronary artery disease: where are we? Journal of the American College of Cardiology. 2012;60(18):1715–1721. doi: 10.1016/j.jacc.2011.12.062. [DOI] [PubMed] [Google Scholar]
- 5.Mi X., Eskridge K. M., George V., Wang D. Structural equation modeling of gene-environment interactions in coronary heart disease. Annals of Human Genetics. 2011;75(2):255–265. doi: 10.1111/j.1469-1809.2010.00634.x. [DOI] [PubMed] [Google Scholar]
- 6.Jeemon P., Pettigrew K., Sainsbury C., Prabhakaran D., Padmanabhan S. Implications of discoveries from genome-wide association studies in current cardiovascular practice. World Journal of Cardiology. 2011;3(7):230–247. doi: 10.4330/wjc.v3.i7.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bañares V., Wyszynski D., Schreier L., Tavella M. APOE -219G/T polymorphism related to serum lipid levels in atherosclerotic patients from Argentina. Investigacion Clinica. 2010;51(1):17–26. [PubMed] [Google Scholar]
- 8.Bazzaz J. T., Nazari M., Nazem H., et al. Apolipoprotein E gene polymorphism and total serum cholesterol level in Iranian population. Journal of Postgraduate Medicine. 2010;56(3):173–175. doi: 10.4103/0022-3859.68629. [DOI] [PubMed] [Google Scholar]
- 9.Ken-Dror G., Talmud P. J., Humphries S. E., Drenos F. APOE/C1/C4/C2 gene cluster genotypes, haplotypes and lipid levels in prospective coronary heart disease risk among UK healthy men. Molecular Medicine. 2010;16(9-10):389–399. doi: 10.2119/molmed.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takeuchi F., Isono M., Katsuya T., et al. Association of genetic variants influencing lipid levels with coronary artery disease in Japanese individuals. PLoS ONE. 2012;7(9, article e46385) doi: 10.1371/journal.pone.0046385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waltmann M. D., Basford J. E., Konaniah E. S., Weintraub N. L., Hui D. Y. Apolipoprotein E receptor-2 deficiency enhances macrophage susceptibility to lipid accumulation and cell death to augment atherosclerotic plaque progression and necrosis. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease. 2014;1842(9):1395–1405. doi: 10.1016/j.bbadis.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallongeville J., Lussier-Cacan S., Davignon J. Modulation of plasma triglyceride levels by apoE phenotype: a meta-analysis. Journal of Lipid Research. 1992;33(4):447–454. [PubMed] [Google Scholar]
- 13.Wilson P. W. F., Schaefer E. J., Larson M. G., Ordovas J. M. Apolipoprotein E alleles and risk of coronary disease: a meta-analysis. Arteriosclerosis, Thrombosis, and Vascular Biology. 1996;16(10):1250–1255. doi: 10.1161/01.atv.16.10.1250. [DOI] [PubMed] [Google Scholar]
- 14.Cheema A. N., Bhatti A., Wang X., et al. APOE gene polymorphism and risk of coronary stenosis in Pakistani population. BioMed Research International. 2015;2015:5. doi: 10.1155/2015/587465.587465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Tang H. Q., Peng W. J., Zhang B. B., Liu M. Meta-analysis for the association of apolipoprotein E epsilon2/epsilon3/epsilon4 polymorphism with coronary heart disease. Chinese Medical Journal. 2015;128:1391–1398. doi: 10.4103/0366-6999.156803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang M.-D., Gu W., Qiao S.-B., Zhu E.-J., Zhao Q.-M., Lv S.-Z. Apolipoprotein e gene polymorphism and risk for coronary heart disease in the chinese population: a meta-analysis of 61 studies including 6634 cases and 6393 controls. PLoS ONE. 2014;9(4, article e95463) doi: 10.1371/journal.pone.0095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marrzoq L. F., Sharif F. A., Abed A. A. Relationship between ApoE gene polymorphism and coronary heart disease in Gaza Strip. Journal of Cardiovascular Disease Research. 2011;2(1):29–35. doi: 10.4103/0975-3583.78584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C.-H., Zhou X., Zhou G.-D., Han D.-F., Zheng F. Interaction of ApoE and LDL-R gene polymorphisms and alcohol drinking and smoking on coronary heart disease. Zhonghua Yi Xue Za Zhi. 2004;84(7):554–558. [PubMed] [Google Scholar]
- 19.Bai X., Zhao M., Wang B. Dyslipidemia-related risk factors for myocardial infarction and polymorphism of ApoE gene among myocardial infarction patients and their siblings. Zhonghua yi xue za zhi. 2001;81(6):340–343. [PubMed] [Google Scholar]
- 20.Wang C.-H., Zhou X., Zhou G.-D., et al. Genetic association of apoE and apoCI gene polymorphisms with coronary heart disease. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(11):982–985. [PubMed] [Google Scholar]
- 21.Peng S., Peng J., Gong W. Association of apolipoprotein E gene polymorphism with early-onset coronary heart disease and its effect on plasma lipid levels. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2001;18(5):375–378. [PubMed] [Google Scholar]
- 22.Yang Z., Zhu T., Ma G., et al. Apolipoprotein E polymorphism in the early onset of coronary heart disease. Chinese Medical Journal. 2001;114(9):983–985. [PubMed] [Google Scholar]
- 23.Ouyang T., Song J.-N., Miao Y., et al. Study on relationship between polymorphism of apolipoprotein E gene and syndromes of phlegm and blood stasis in patients with coronary heart disease. Zhong Xi Yi Jie He Xue Bao. 2005;3(6):438–442. doi: 10.3736/jcim20050605. [DOI] [PubMed] [Google Scholar]
- 24.Heide S., Manfred K., Gläser C., Schulz S. Apolipoprotein E (apoE) polymorphism: a risk factor for fatal coronary sclerosis? Forensic Science International. 2009;192(1–3):62–66. doi: 10.1016/j.forsciint.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Kolovou G. D., Anagnostopoulou K. K., Kostakou P., et al. Apolipoprotein E gene polymorphism and obesity status in middle-aged men with coronary heart disease. In Vivo. 2009;23(1):33–39. [PubMed] [Google Scholar]
- 26.Bahri R., Esteban E., Moral P., Hassine M., Hamda K. B., Chaabani H. Apolipoprotein gene polymorphisms and plasma levels in healthy Tunisians and patients with coronary artery disease. Lipids in Health and Disease. 2008;7, article 46 doi: 10.1186/1476-511x-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akanji A. O., Suresh C. G., Fatania H. R., Al-Radwan R., Zubaid M. Associations of apolipoprotein E polymorphism with low-density lipoprotein size and subfraction profiles in Arab patients with coronary heart disease. Metabolism: Clinical and Experimental. 2007;56(4):484–490. doi: 10.1016/j.metabol.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Aydogan H. Y., Isbir S., Kurnaz O., Gormus U., Isbir T. Associations of lipoprotein lipase S447X and apolipoprotein E genotypes with low-density lipoprotein subfractions in Turkish patients with coronary artery disease. In Vivo. 2009;23:155–161. [PubMed] [Google Scholar]
- 29.Ranjith N., Pegoraro R. J., Rom L., Rajput M. C., Naidoo D. P. Lp(a) and apoE polymorphisms in young South African Indians with myocardial infarction. Cardiovascular Journal of South Africa. 2004;15(3):111–117. [PubMed] [Google Scholar]
- 30.Peng D.-Q., Zhao S.-P., Nie S., Li J. Gene-gene interaction of PPARγ and ApoE affects coronary heart disease risk. International Journal of Cardiology. 2003;92(2-3):257–263. doi: 10.1016/s0167-5273(03)00101-3. [DOI] [PubMed] [Google Scholar]
- 31.Singh P., Singh M., Bhatnagar D., Kaur T., Gaur S. Apolipoprotein E polymorphism and its relation to plasma lipids in coronary heart disease. Indian Journal of Medical Sciences. 2008;62(3):105–112. doi: 10.4103/0019-5359.39613. [DOI] [PubMed] [Google Scholar]
- 32.Keavney B., Parish S., Palmer A., et al. Large-scale evidence that the cardiotoxicity of smoking is not significantly modified by the apolipoprotein E ε2/ε3/ε4 genotype. The Lancet. 2003;361(9355):396–398. doi: 10.1016/s0140-6736(03)12386-0. [DOI] [PubMed] [Google Scholar]
- 33.Freitas E. M., Phan T. C. A., Herbison C. E., Christiansen F. T., Taylor R. R., Van Bockxmeer F. M. The poliovirus receptor related 2 (PRR2) and apolipoprotein E genes and coronary heart disease. Journal of Cardiovascular Risk. 2002;9(1):59–65. doi: 10.1097/00043798-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami K., Okumura K., Matsui H., et al. The apolipoprotein E genotype influences the risk for vasospastic angina. Canadian Journal of Cardiology. 2001;17(6):660–666. [PubMed] [Google Scholar]
- 35.Attila G., Acartürk E., Eskandari G., et al. Effects of apolipoprotein E genotypes and other risk factors on the development of coronary artery disease in Southern Turkey. Clinica Chimica Acta. 2001;312(1-2):191–196. doi: 10.1016/S0009-8981(01)00624-6. [DOI] [PubMed] [Google Scholar]
- 36.Kharrazi H., Vaisi Raygani A., Sabokroh A. R., Pourmotabbed T. Association between apolipoprotein E polymorphism and coronary artery disease in the Kermanshah population in Iran. Clinical Biochemistry. 2006;39(6):613–616. doi: 10.1016/j.clinbiochem.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Gamboa R., Huesca-Gómez C., Pérez-Méndez O., et al. Apolipoprotein E polymorphisms in Mexican patients with coronary artery disease. Clinical Chemistry and Laboratory Medicine. 2008;46(4):481–485. doi: 10.1515/CCLM.2008.077. [DOI] [PubMed] [Google Scholar]
- 38.Al-Yahyaee S. A. S., Ganguly S. S., Al-Kindi M. N., Al-Bahrani A. I. Apolipoprotein E polymorphism in omani dyslipidemic patients with and without coronary artery disease. Human Biology. 2007;79(1):93–102. doi: 10.1353/hub.2007.0020. [DOI] [PubMed] [Google Scholar]
- 39.Al-Bustan S. A., Alkhalaf M., Al-Rashdan I., et al. Apolipoprotein E, CI and B gene polymorphisms in a sample of patients with coronary heart disease in the Kuwaiti population. Medical Principles and Practice. 2009;18(4):294–299. doi: 10.1159/000215727. [DOI] [PubMed] [Google Scholar]
- 40.Vaisi-Raygani A., Rahimi Z., Tavilani H., Pourmotabbed T. Butyrylcholinesterase K variant and the APOE-ε4 allele work in synergy to increase the risk of coronary artery disease especially in diabetic patients. Molecular Biology Reports. 2010;37(4):2083–2091. doi: 10.1007/s11033-009-9666-4. [DOI] [PubMed] [Google Scholar]
- 41.Corella D., Portolés O., Arriola L., et al. Saturated fat intake and alcohol consumption modulate the association between the APOE polymorphism and risk of future coronary heart disease: a nested case-control study in the Spanish EPIC cohort. Journal of Nutritional Biochemistry. 2011;22(5):487–494. doi: 10.1016/j.jnutbio.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Fallah S., Seifi M., Firoozrai M., Hosainnee Ghohari L., Samadikuchaksaraei A., Samadirad B. Effect of Apolipoprotein E genotypes on incidence and development of coronary stenosis in Iranian patients with coronary artery disease. Journal of Clinical Laboratory Analysis. 2011;25(1):43–46. doi: 10.1002/jcla.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustavsson J., Mehlig K., Leander K., et al. Interaction of apolipoprotein E genotype with smoking and physical inactivity on coronary heart disease risk in men and women. Atherosclerosis. 2012;220(2):486–492. doi: 10.1016/j.atherosclerosis.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Machal J., Vasku A., Hlinomaz O., Linhartova P., Groch L., Vitovec J. Apolipoprotein E polymorphism is associated with both number of diseased vessels and extent of coronary artery disease in Czech patients with CAD. Biomedical Papers. 2012;156(2):151–158. doi: 10.5507/bp.2012.051. [DOI] [PubMed] [Google Scholar]
- 45.Heidari M. M., Foruzannia S. K., Khatami M., Hadadzadeh M., Emami Meybodi M. Apolipoprotein E gene polymorphism in Iranian coronary atherosclerosis patients candidate for coronary artery bypass graft. Iranian Journal of Basic Medical Sciences. 2013;16(7):841–844. [PMC free article] [PubMed] [Google Scholar]
- 46.Afroze D., Yousuf A., Tramboo N. A., Shah Z. A., Ahmad A. ApoE gene polymorphism and its relationship with coronary artery disease in ethnic Kashmiri population. Clinical and Experimental Medicine. 2016;16(4):551–556. doi: 10.1007/s10238-015-0389-7. [DOI] [PubMed] [Google Scholar]
- 47.Sapkota B., Subramanian A., Priamvada G., et al. Association of APOE polymorphisms with diabetes and cardiometabolic risk factors and the role of APOE genotypes in response to anti-diabetic therapy: results from the AIDHS/SDS on a South Asian population. Journal of Diabetes and Its Complications. 2015;29(8):1191–1197. doi: 10.1016/j.jdiacomp.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu M., Bian C., Zhang J., Wen F. Apolipoprotein E gene polymorphism and Alzheimer's disease in Chinese population: a meta-analysis. Scientific Reports. 2014;4, article 4383 doi: 10.1038/srep04383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y., Yu J.-T., Wang H.-F., et al. APOE genotype and neuroimaging markers of Alzheimer's disease: systematic review and meta-analysis. Journal of Neurology, Neurosurgery and Psychiatry. 2015;86(2):127–134. doi: 10.1136/jnnp-2014-307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaudhary R., Likidlilid A., Peerapatdit T., et al. Apolipoprotein E gene polymorphism: effects on plasma lipids and risk of type 2 diabetes and coronary artery disease. Cardiovascular Diabetology. 2012;11, article 36 doi: 10.1186/1475-2840-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davignon J., Gregg R. E., Sing C. F. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8(1):1–21. doi: 10.1161/01.ATV.8.1.1. [DOI] [PubMed] [Google Scholar]
- 52.Davignon J., Cohn J. S., Mabile L., Bernier L. Apolipoprotein E and atherosclerosis: insight from animal and human studies. Clinica Chimica Acta. 1999;286(1-2):115–143. doi: 10.1016/s0009-8981(99)00097-2. [DOI] [PubMed] [Google Scholar]
- 53.Moghadasian M. H., McManus B. M., Nguyen L. B., et al. Pathophysiology of apolipoprotein E deficiency in mice: relevance to apo E-related disorders in humans. The FASEB Journal. 2001;15(14):2623–2630. doi: 10.1096/fj.01-0463com. [DOI] [PubMed] [Google Scholar]
- 54.Stannard A. K., Riddell D. R., Sacre S. M., et al. Cell-derived Apolipoprotein E (ApoE) particles inhibit vascular cell adhesion molecule-1 (VCAM-1) expression in human endothelial cells. Journal of Biological Chemistry. 2001;276(49):46011–46016. doi: 10.1074/jbc.m104812200. [DOI] [PubMed] [Google Scholar]
- 55.Shewan L. G., Coats A. J. S. Ethics in the authorship and publishing of scientific articles. International Journal of Cardiology. 2010;144(1):1–2. doi: 10.1016/j.ijcard.2010.07.030. [DOI] [Google Scholar]