Abstract
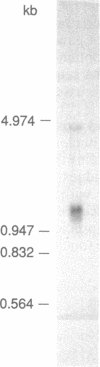
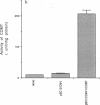
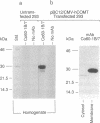
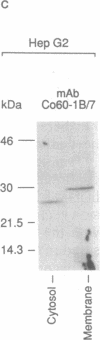
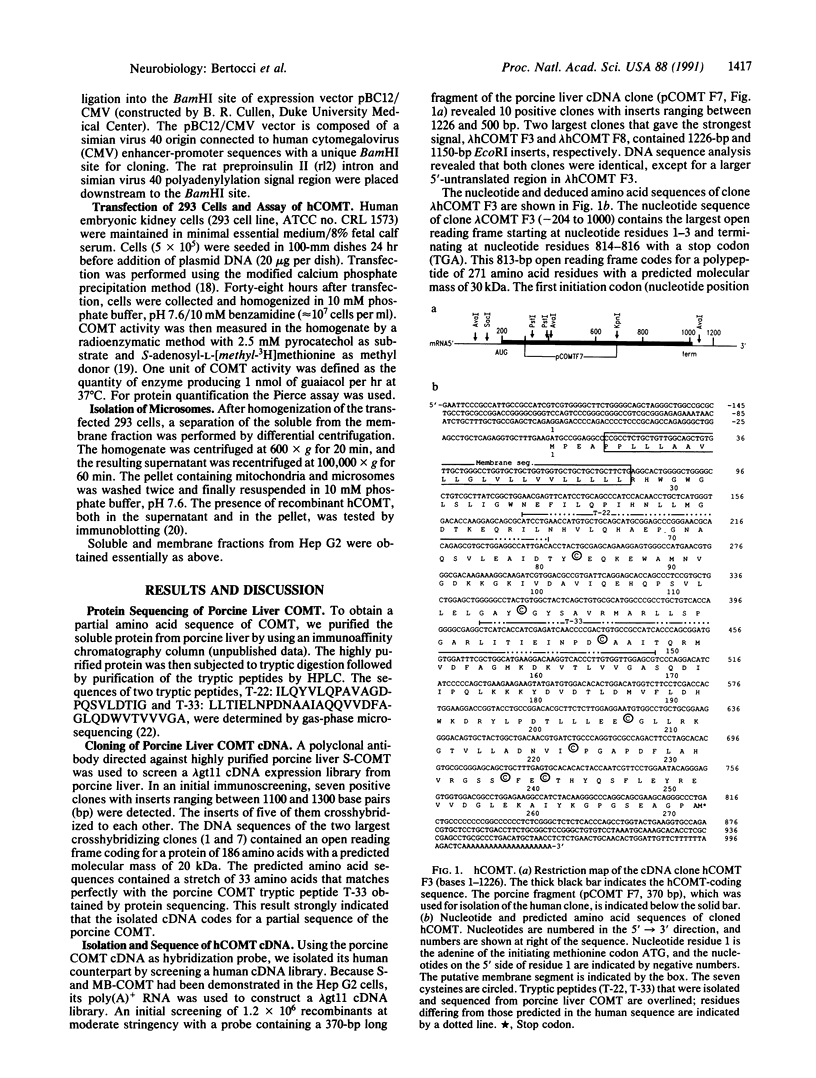
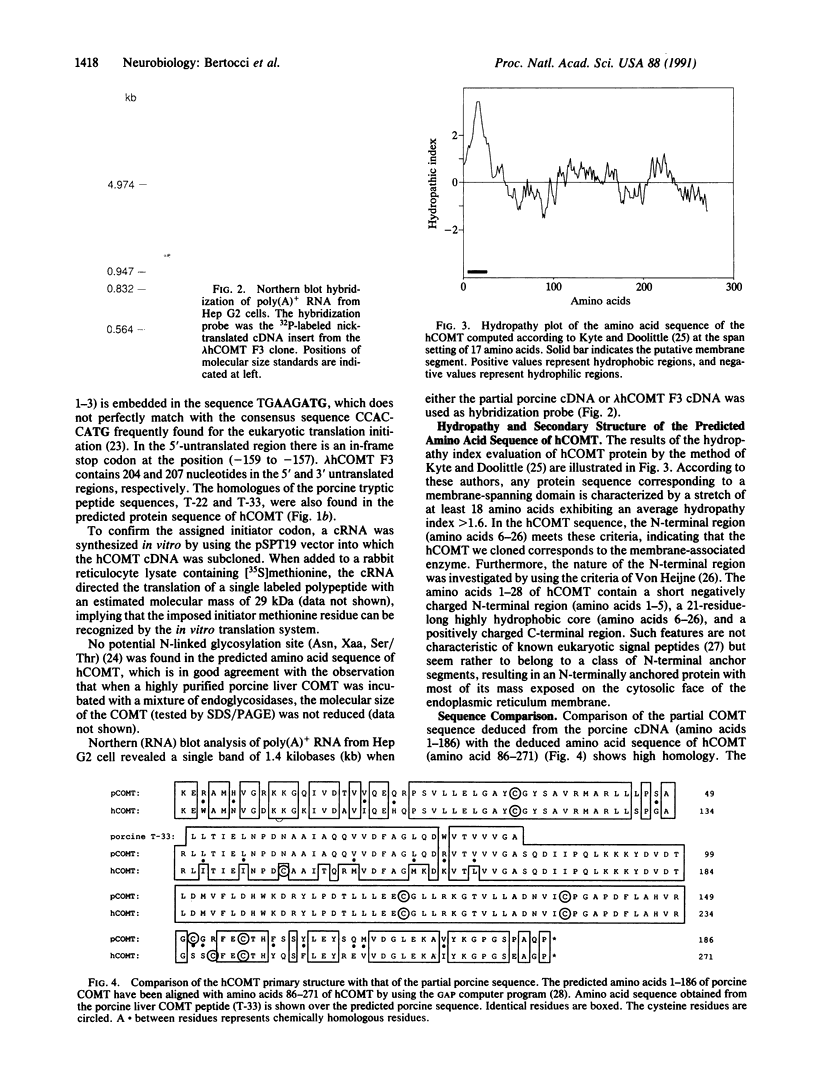
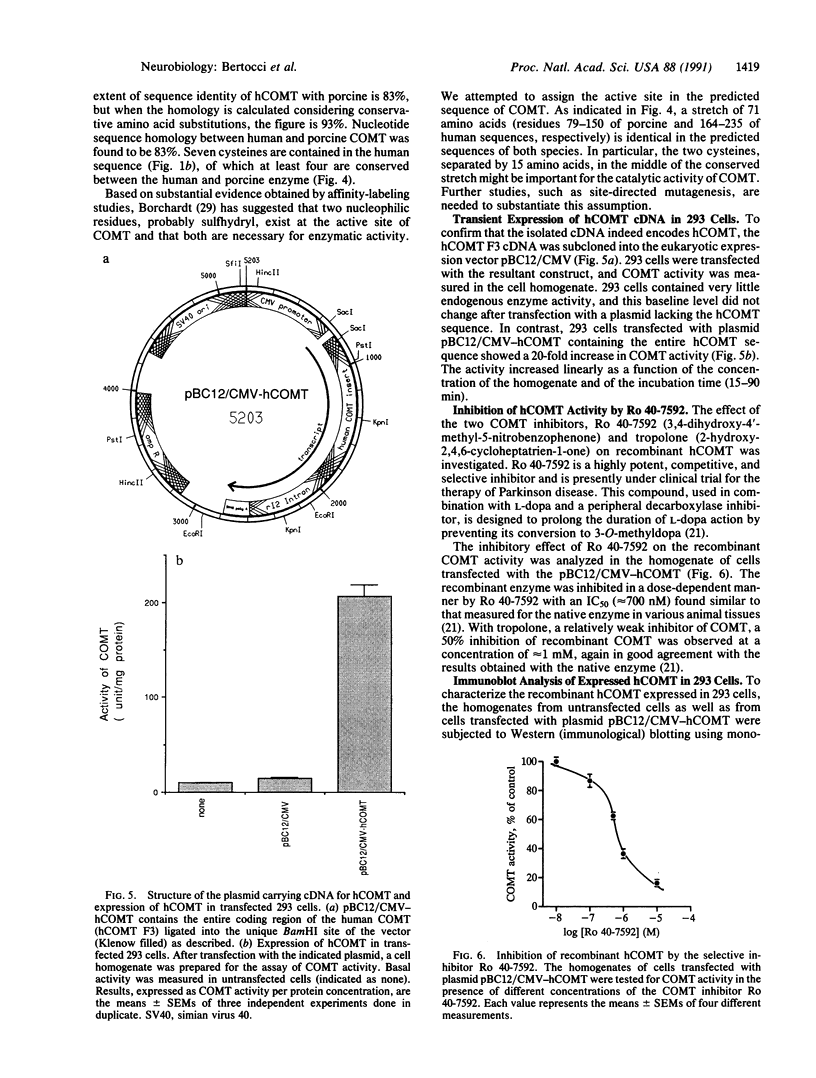
A cDNA clone for human catechol-O-methyltransferase (hCOMT; S-adenosyl-L-methionine:catechol O-methyltransferase; EC 2.1.1.6) was isolated from a human hepatoma cell line (Hep G2) cDNA library by hybridization screening with a porcine cDNA probe. The cDNA clone was sequenced and found to have an insert of 1226 nucleotides. The deduced primary structure of hCOMT is composed of 271 amino acid residues with the predicted molecular mass of 30 kDa. At its N terminus it has a hydrophobic segment of 21 amino acid residues that may be responsible for insertion of hCOMT into the endoplasmic reticulum membrane. The primary structure of hCOMT exhibits high homology to the porcine partial cDNA sequence (93%). The deduced amino acid sequence contains two tryptic peptide sequences (T-22, T-33) found in porcine liver catechol-O-methyltransferase (COMT). The coding region of hCOMT cDNA was placed under the control of the cytomegalovirus promoter to transfect human kidney 293 cells. The endogenous COMT activity, which was approximately 9.98 units per mg of protein in the untransfected cells, increased to 206 units per mg of protein upon transfection with a plasmid containing the COMT cDNA. The COMT activity of recombinant protein was inhibited competitively (IC50 = 700 nM) by the selective COMT inhibitor Ro 40-7592. An anti-COMT monoclonal antibody recognized, on immunoblots, a major polypeptide with apparent molecular mass of 29 kDa, in reasonable agreement with the predicted molecular mass. The recombinant hCOMT was shown by immunoblot analysis to be mainly associated with the membrane fraction. RNA blot analysis revealed one COMT mRNA transcript of 1.4 kilobases in Hep G2 poly(A)+ RNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assicot M., Bohuon C. Presence of two distinct catechol -O- methyltransferase activities in red blood cells. Biochimie. 1971;53(8):871–874. doi: 10.1016/s0300-9084(71)80150-5. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Cohn C. K. Methyltransferase enzymes in red blood cells. J Pharmacol Exp Ther. 1971 Mar;176(3):650–654. [PubMed] [Google Scholar]
- Axelrod J. Methylation reactions in the formation and metabolism of catecholamines and other biogenic amines. Pharmacol Rev. 1966 Mar;18(1):95–113. [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Guldberg H. C., Marsden C. A. Catechol-O-methyl transferase: pharmacological aspects and physiological role. Pharmacol Rev. 1975 Jun;27(2):135–206. [PubMed] [Google Scholar]
- Gustavson K. H., Wetterberg L., Bäckström M., Ross S. B. Catechol-O-methyltransferase activity in erythrocytes in Down's syndrome. Clin Genet. 1973;4(3):279–280. doi: 10.1111/j.1399-0004.1973.tb01156.x. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Inscoe J. K., Daly J., Axelrod J. Factors affecting the enzymatic formation of O-methylated dihydroxy derivatives. Biochem Pharmacol. 1965 Aug;14(8):1257–1263. doi: 10.1016/0006-2952(65)90303-5. [DOI] [PubMed] [Google Scholar]
- Jeffery D. R., Roth J. A. Characterization of membrane-bound and soluble catechol-O-methyltransferase from human frontal cortex. J Neurochem. 1984 Mar;42(3):826–832. doi: 10.1111/j.1471-4159.1984.tb02755.x. [DOI] [PubMed] [Google Scholar]
- Karege F., Bovier P., Gaillard J. M., Tissot R. The decrease of erythrocyte catechol-O-methyltransferase activity in depressed patients and its diagnostic significance. Acta Psychiatr Scand. 1987 Sep;76(3):303–308. doi: 10.1111/j.1600-0447.1987.tb02899.x. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. Glycoproteins. Annu Rev Biochem. 1972;41:673–702. doi: 10.1146/annurev.bi.41.070172.003325. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needleman S. B., Wunsch C. D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970 Mar;48(3):443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Rivett A. J., Eddy B. J., Roth J. A. Contribution of sulfate conjugation, deamination, and O-methylation to metabolism of dopamine and norepinephrine in human brain. J Neurochem. 1982 Oct;39(4):1009–1016. doi: 10.1111/j.1471-4159.1982.tb11490.x. [DOI] [PubMed] [Google Scholar]
- Roth J. A. Presence of membrane-bound catechol-O-methyltransferase in human brain. Biochem Pharmacol. 1980 Nov 15;29(22):3119–3122. doi: 10.1016/0006-2952(80)90458-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher G., Da Prada M. Rapid and sensitive single-step radiochemical assay for catechol-O-methyltransferase. J Neurochem. 1982 Jan;38(1):191–195. doi: 10.1111/j.1471-4159.1982.tb10871.x. [DOI] [PubMed] [Google Scholar]
- Zürcher G., Keller H. H., Kettler R., Borgulya J., Bonetti E. P., Eigenmann R., Da Prada M. Ro 40-7592, a novel, very potent, and orally active inhibitor of catechol-O-methyltransferase: a pharmacological study in rats. Adv Neurol. 1990;53:497–503. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Towards a comparative anatomy of N-terminal topogenic protein sequences. J Mol Biol. 1986 May 5;189(1):239–242. doi: 10.1016/0022-2836(86)90394-3. [DOI] [PubMed] [Google Scholar]