Abstract
Klebsiella oxytoca is the second most frequently identified species of Klebsiella isolated from hospitalized patients. Klebsiella spp. is difficult to identify using conventional methods and is often misclassified in clinical microbiology laboratories. K. oxytoca is responsible for an increasing number of multi-resistant infections in hospitals because of insufficient detection and identification. In this study, we propose a new simple method called pehX-LM PCR/XbaI, which simultaneously indicates K. oxytoca species and genotype by the fingerprint pattern. The pehX-LM PCR/XbaI is a combination of the following two methods: species-specific amplification of pehX gene and non-specific amplification of short restriction fragments by the LM PCR method. The specificity and the discrimination power of the pehX-LM PCR/XbaI method were determined by typing 209 K. oxytoca strains (included 9 reference strains), 28 K. pneumoniae, and other 25 strains belonging to the Enterobacteriaceae. The typing results were confirmed by the PCR melting profile method. Unlike the known fingerprinting methods, the pehX-LM PCR/XbaI leads to a clear pattern (approx. 3–5 bands) with a sufficient, relatively high discriminatory power. As a result, the time and cost of a single analysis are lower. The method can be used both in clinical and environmental research.
Electronic supplementary material
The online version of this article (doi:10.1007/s00253-016-7881-1) contains supplementary material, which is available to authorized users.
Keywords: Klebsiella spp., Klebsiella oxytoca, Enterobacteriaceae, pehX gene, PCR, LM PCR typing methods
Introduction
According to the Orskov (1984) taxonomic classification, the Klebsiella genus includes Klebsiella pneumoniae subsp. pneumoniae, K. pneumoniae subsp. ozaenae, K. pneumoniae subsp. rhinoscleromatis, Klebsiella oxytoca, Klebsiella terrigena, Klebsiella planticola, and Klebsiella ornithinolytica. Klebsiella spp. belong to clinical and environmental species/subspecies with a varying degree of virulence. K. pneumoniae and K. oxytoca are the most commonly isolated Klebsiella species in hospital infections (Asensio et al. 2000). These opportunistic human pathogens are involved in serious infections such as pneumonia, sepsis, neonatal septicemia, wound infections and urinary tract infections (Biehle et al. 2015; Brisse and Verhoef 2001). K. pneumoniae is the most common cause of hospital respiratory infections in premature neonates in Intensive Care Units and the second most common cause of urinary tract infections and bacteraemia (Giamarellou et al. 2013; Girometti et al. 2014; Han et al. 2015). Whereas, K. oxytoca can also be cultured from skin, mucous membranes, oropharynx, intestines and other tissues in healthy humans and animals. It is thought to be an opportunistic pathogen responsible for nosocomial infections in hospitalized patients and a causative organism for antibiotic-associated hemorrhagic colitis (AAHC) in humans. K. oxytoca may also be especially pathogenic due to the secretion of cytotoxin (Darby et al. 2014). Klebsiella spp. are difficult to identify by conventional methods and often misclassified in clinical microbiology laboratories because they share a similar biochemical profile with several other species (Martínez et al. 2004; Monnet et al. 1991; Monnet and Freney 1994; Podschun and Ullmann 1998; Rosenblueth et al. 2004; Westbrook et al. 2000).
K. oxytoca can be detected using a combination of the API 20E test (bioMérieux, France) and other molecular tests for a complete identification. Several studies used the sequencing analysis of 16S rRNA (Lopes et al. 2007), 16S-23S rDNA intergenic spacer region (García-Martínez et al. 1999) and housekeeping genes including the rpoB gene (RNA polymerase β-subunit), nifH (nitrogenase reductase), gyrA (DNA gyrase A subunit), phoE (phosphoporine E), mdh (malate dehydrogenase), infB (translation initiation factor 2), pehX (polygalacturonase) (Kovtunovych et al. 2003; Rosenblueth et al. 2004) to identify bacterial species and subspecies. K. oxytoca is responsible for an increasing number of multi-resistant infections in hospitals; therefore, epidemiology studies are essential to the understanding of the transmission dynamics in infections caused by these bacteria. It is of utmost importance to determine the degree of relatedness with the use of the genotyping methods. The clonality and phylogenetic diversity of these strains has been established by 16S rRNA gene, rpoB, gyrA, gapDH, and blaOXY sequencing (Fevre et al. 2005) and by molecular fingerprinting methods such as Pulsed Field Gel Electrophoresis (PFGE) (Izdebski et al. 2015), Multi Locus Sequences Typing (MLST) (Izdebski et al. 2015), Internal Transcribed Spacer Polymerase Chain Reaction (ITS PCR) (Stojowska et al. 2009a, b), Amplified Fragment Length Polymorphism (AFLP) (Jonas et al. 2004), Randomly Amplified Polymorphic DNA (RAPD) analyses (Brisse and Verhoef 2001) and PCR Melting Profile (PCR MP) (Stojowska et al. 2009a, b). Although PFGE is considered to be the gold standard for the detection of clonality in outbreaks, PCR-based methods are cheaper and provide faster results. The AFLP technique can be used to determine the inter- and intraspecies relatedness; however, as the use of it requires a high level of technical competence it is not recommended for routine bacteria identification. The ITS PCR method has a low level of discriminatory power and was chosen to study the genetic similarities and relationships between K. oxytoca strains isolated over long periods of time and across large geographical ranges (Stojowska et al. 2009b). The RAPD analysis is faster and technically less demanding but for the results to be reproducible the PCR conditions need to be carefully standardized (the choice of a primer, DNA and primer concentrations, polymerase type or a thermal cycler profile are critical to producing informative patterns) (Brise and Verhoef 2001). Another method, the PCR MP is sensitive to temperature fluctuations and for this reason, to generate reliable and repeatable data the thermal cycler needs to be calibrated regularly (Krawczyk et al. 2006; Stojowska et al. 2009a). If patterns are too complex, fast typing is difficult to achieve, especially in hospital settings. In addition, fingerprint patterns do not provide species- or genus-specific information regarding the tested strains. The purified DNA from correctly identified species of bacteria needs to be used. Closely related species of the same genus may make the correct diagnosis difficult.
In this study, we propose a new one-step assay for the simultaneous species identification/confirmation and strain genotyping with the use of a PCR reaction.
Materials and methods
Bacterial strains
The K. oxytoca we used for the purposes of the study were provided to us by the State Institute of Hygiene (Poland). We studied nine reference K. oxytoca K serological type from Ørskov’s collection (K26, K29, K32, K41, K44, K65, K66, K68 and K72) (Ørskov and Ørskov 1984) and more than 200 K. oxytoca strains isolated from patients hospitalized in various clinical facilities across Poland between 1954 and 2007 (14 of them were isolated in 1999 from patients of Neonatal Intensive Care Unit of a single hospital in Bydgoszcz and were marked as closed related strains from nosocomial infection). All the strains were previously classified as Klebsiella oxytoca by the Department of Bacteriology at the National Institute of Public Health (Poland) and tested for genetic diversity in 2009 (Stojowska et al. 2009a, b). We used K. pneumoniae subsp. ozaenae (ATCC 25926), K. pneumoniae subsp. pneumoniae (ATCC 700603™) and K. pneumoniae subsp. rhinoscleromatis (ATCC® 13884™) as reference strains, and 25 clinical K. pneumoniae which are not Klebsiella oxytoca. Furthermore, we tested 25 clinical strains of other Enterobacteriaceae species (10 strains of Escherichia coli, 10 strains of Serratia marcescens and 5 strains of Proteus vulgaris) from the Molecular Biotechnology and Microbiology Department of the Gdańsk University of Technology. All the clinical isolates were identified biochemically using the Mini API ID 32E systems (bioMèrieux, France). K. pneumoniae clinical strains were put to the indole test (negative result), the growth test at 10 °C (negative result), and were tested for the production of gas from lactose at 44.5 °C (positive reaction).
DNA isolation
DNA isolations (from a single colony on an agar plate) were carried out with the Genomic DNA Kit (Bioline, A Meridian Life Science Company). The DNA concentrations were measured using NanoDrop ND-100 (Thermo Fisher Scientific, Wilmington, USA) and were at a level of 10–15 ng per microlitre.
Species-specific sequence amplification (pehX-PCR)
We have amplified a 344 bp fragment of the pehX gene by PCR according to the Kovtunovych et al. (2003). We used pehX-PCR on 25-μl samples using 10 pmol of each pehX-specific primer (PEH-A: 5′-GGACTACGCCGTCTATCGTCAAG-3′ and PEH-D: 5′ TAGCCTTTATCAAGCGGATACTGG 3′), 2.5 μl of a deoxynucleoside triphosphate mixture (a 2 mM concentration of each), 1 U of Taq polymerase (1 U/μl, Fermentas UAB, Vilnius, Lithuania), 2.5 μl of 10× PCR buffer Taq with (NH4)2SO4 (Fermentas UAB, Vilnius, Lithuania), 750 mM Tris-HCl pH 8.8 at 25 °C, 200 mM (NH4)2SO4, 0.1 % (v/v) Tween 20), 2 μl of 20 mM MgCl2, and 1 ng of the genomic DNA. The PCR conditions were as follows: 5 min of initial denaturation at 94 °C followed by 25 cycles of 30 s at 94 °C, 30 s of annealing at 60 °C with extension step at 72 °C for 30 s. After the final cycle, the samples were incubated for 5 min at 72 °C. The PCR products were confirmed for all K. oxytoca strains using agarose gel electrophoresis. The pehX-PCR products were sequenced for several reactions to ensure that the correct DNA target was amplified (Genomed, Poland). Following this, the sequences were compared with pehX gene sequences present in the GenBank database (access number AY065648). For the purposes of the assay, we designed a new reverse primer—pehX8: 5′-CACCGTAAAGGCATACTCCGTATC-3′ which was different from PEH-D that used by Kovtunovych et al., (2003) whilst the forward primer was the same as PEH-A and we called it pehX1. The reaction profile of pehX-PCR was performed as described above. The PCR product had a length of 193 bp. The specificity of PCR assay was examined for 209 strains of K. oxytoca and 28 K. pneumoniae.
Adapter preparation
The double-stranded adapter (5′ adXbaI) was formed by mixing 20 pmol of each adapter oligonucleotide (aXbaLIG 5′-CTCACTCTCACCAACGTCGAC-3′; and aXbaHELP 5′-CTAGGTCGACGTTGG-3′) with water to bring the sample volume to 50 μl. The mixture was then heated up to 70 °C for 2 min and, following this, slowly cooled down to room temperature, with the result that the adapter was formed at a final concentration of 1 pmol/μl.
LM PCR/XbaI template preparation
Twenty-five nanograms of genomic DNA was digested using 5 U of the restriction enzyme XbaI (10 U/μl, Fermentas UAB, Vilnius, Lithuania) in 20 μl reaction volumes containing a 1× Y/Tango restriction buffer (3.3 mM Tris-CH3COOH (pH 7.9 at 37 °C), 1 mM (CH3COO)2Mg, 6.6 mM CH3COOK, 0.1 mg/ml BSA; Fermentas UAB, Vilnius, Lithuania) for 30 min at 37 °C. To achieve ligation, we used a master mix containing 1 U of T4 DNA ligase (2 U/μl; Fermentas UAB, Vilnius, Lithuania), 1× ligation buffer (40 mM Tris-HCl pH 7.8, 10 mM MgCl2, 10 mM DTT, 0.5 mM ATP; Fermentas UAB, Vilnius, Lithuania), and 1 pmol adapter (adXbaI) in each of 5 μl volumes. 5 μl of the ligation mixture was added to 20 μl of digested DNAs and the tubes were incubated for 30 min at 18–22 °C, and then to inactivate the T4 DNA ligase for 10 min at 70 °C.
pehX-LM PCR/XbaI amplification
PCRs were performed in 25-μl volumes using 10 pmol of the pehX-specific primers (pehX1 and pehX8), 5 pmol of adapter-specific primer (p-adXbaI; 5′-CTCACTCTCACCAACGTCGACCTAGA-3′), 2.5 μl of a deoxynucleoside triphosphate mixture (2 mM concentration of each), 1 U of Taq polymerase (1 U/μl, Fermentas UAB, Vilnius, Lithuania), 2.5 μl of 10× PCR buffer Taq with (NH4)2SO4 (Fermentas UAB, Vilnius, Lithuania), 2 μl of 20 mM MgCl2, and 1 μl of adapter-ligated DNA fragments. The PCR was performed as follows: 5 min at 94 °C to release an unligated helper oligonucleotide, 5 min at 72 °C to fill in the single-stranded ends of the adapter and create amplicons, 25 cycles of denaturation at 94 °C for 30 s, 30 s of annealing at 60 °C and an extension step at 72 °C for 180 s. After the last cycle, the samples were incubated at 72 °C for 10 min.
Test to verify the specificity of the amplification in the pehX-LM PCR/XbaI method
Two extra PCR samples were prepared for each of the K. oxytoca reference strains and for 50 randomly selected clinical strains: one sample contained only a pair of the pehX-specific primers (pehX1 and pehX8) and the other contained only an adapter-specific primer (p-adXbaI). The PCR conditions for pehX-LM PCR/XbaI amplification were as described above. To check that there is no specific pehX-PCR product in the pehX-LM PCR/XbaI analysis, we tested 53 strains of other species of Enterobacteriaceae family (including 25 clinical and 3 reference Klebsiella pneumoniae strains (ATCC 25926; ATCC 700603™; ATCC® 13884™) 10 clinical strains of E. coli, 10 of S. marcescens, 5 of P. vulgaris) using the same protocol as for K. oxytoca.
PCR MP genotyping
All K. oxytoca were genotyped by PCR MP according to the procedure described by Stojowska et al. (2009a).
Detection and fingerprint pattern analysis
We analysed 10 μl out of 25 μl of a PCR mixture by electrophoresis (2 % agarose gels consisting of 0.5 μg/ml of ethidium bromide, 100 V, for 1 h in 1× TAE buffer). We studied and archived gel images using Versa Doc 1000 Imaging System (Bio-Rad Laboratories, Hercules, USA). The patterns we obtained from the electropherograms were converted and analysed using the Quantity One software package, version 4.3.1 (Bio-Rad, San Francisco, CA, USA). Band positions in each gel were normalised using the DNA ladder (the molecular DNA size marker: 100–1000 bp, BLIRT SA, DNA Gdańsk, POLAND). Band matching and isolate similarity were accomplished using the Dice band-based coefficient of similarity. Tolerance and optimisations settings were 2.0 % for both pehX-LM PCR/XbaI and PCR MP. A dendrogram was constructed using the Unweighted Pair Group Method with Arithmetic Mean (UPGMA).
Reproducibility of the pehX-LM PCR/XbaI method
To check the reproducibility of the pehX-LM PCR/XbaI method, ten randomly selected strains were genotyped by this method in three independent experiments performed with DNA isolated from different cultures of one strain. The percentage similarity among fingerprint patterns for each gel was recorded and the average across the gels was calculated. Moreover, two different thermal cyclers (a Biometra Tgradient cycler and an Eppendorf MasterCycler EP gradient) and two different Taq polymerases (Fermentas UAB, Vilnius, Lithuania and BLIRT SA, DNA Gdańsk, POLAND) were used by two independent persons.
Method sensitivity-detection limit
A single colony of bacteria is sufficient for DNA isolation. 10–25 ng of genomic DNA is required in a single reaction to obtain DNA patterns.
Results
Outline of the pehX-LM PCR/XbaI method
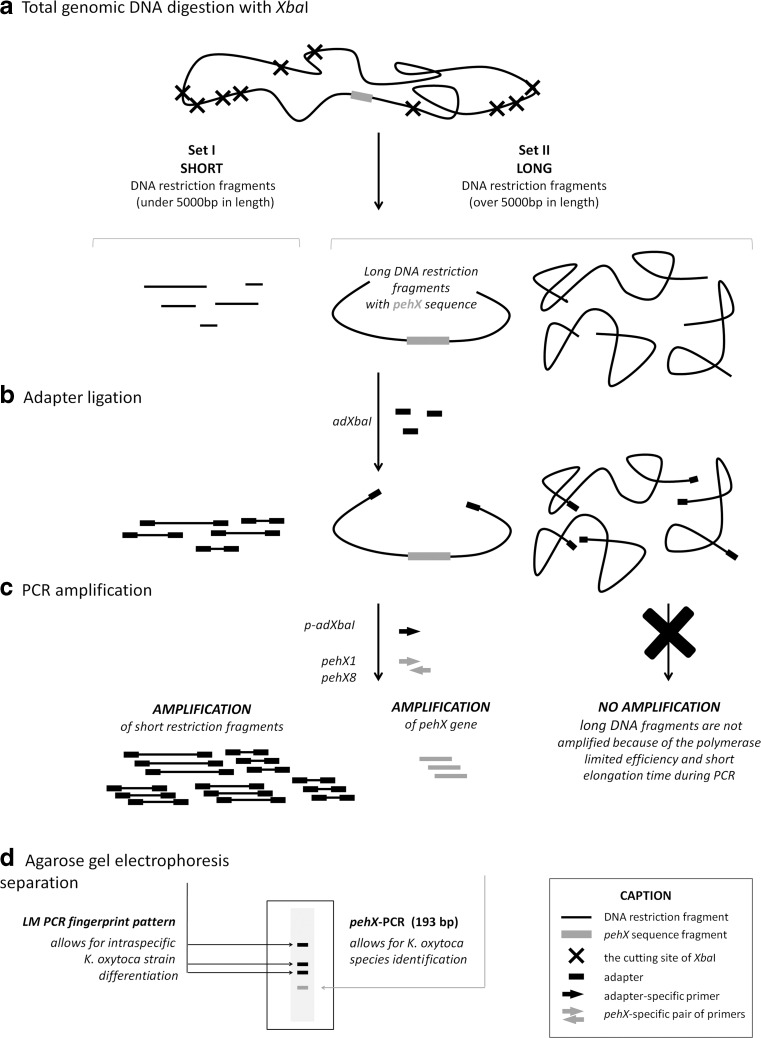
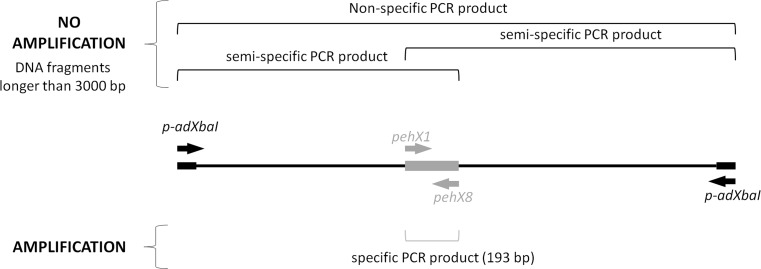
To perform the species-specific fingerprinting of K. oxytoca strains, we applied the LM PCR technique involving the ligation and selective amplification of DNA restriction fragments in combination with the specific gene amplification of pehX-genes. The method used in our experiments is shown in Fig. 1. A genomic total DNA is digested completely with the restriction enzyme (XbaI) (Fig. 1a). We chose XbaI because it cuts the genomic DNA of Enterobacteriaceae bacteria at no more than 40 restriction sites and generates up to 10 fragments from 300 to 3000 bp in length (Fig. 1a, set I, SHORT fragments). The remaining fragments are usually much longer than 5000 bp (Fig. 1a, set II, LONG fragments) and one of them contains the pehX gene. The pehX gene is located at a distance of over 3000 bp from each end of the restriction fragment which avoids a semi-specific amplification (Fig. 2). All the restriction fragments contain 5′ cohesive ends and are ligated to the synthetic adapter (adXbaI) in the next step (Fig. 1b). At first, the unligated oligonucleotides (aXbaHELP) are released and single-stranded ends are filled with DNA polymerase. Following this, DNA fragments are amplified by PCR using the adapter-specific primer (p-adXbaI) capable of hybridising with the 5′ ends of DNA fragments. Only SHORT DNA fragments are amplified (Fig. 1, set I) and after separation by agarose gel electrophoresis they form strain-specific band patterns (Fig.1d). LONG DNA fragments are not amplified because the DNA polymerase has a limited efficiency and a short elongation time (Fig. 1, set II). Addition of pehX-specific primers during the PCR amplification process (Fig. 1c) creates an extra PCR product with a length of 193-bp, an indication that K. oxytoca species are present (Fig. 1d). Three primers should be used in one PCR to receive a full K. oxytoca strain fingerprint profile: the adapter-specific primer (p-adXbaI) to amplify strain-specific restriction fragments and a pair of pehX-specific primers (pehX1 and pehX8) to amplify species-specific DNA fragments.
Fig. 1.
Outline of pehX-LM PCR/XbaI method. I, II sets of restriction fragments. Caption section (bottom right corner) shows the description of all symbols used in the pehX-LM PCR method scheme.
Fig. 2.
Specific amplification of pehX gene from XbaI restriction fragment. p-adXbaI—adapter-specific primer, pehX1 and pehX8—pehX-specific primers. The pehX gene is located amongst long restriction fragments, more than 3000 bp away from each end
Verification of the pehX-LM PCR/XbaI method
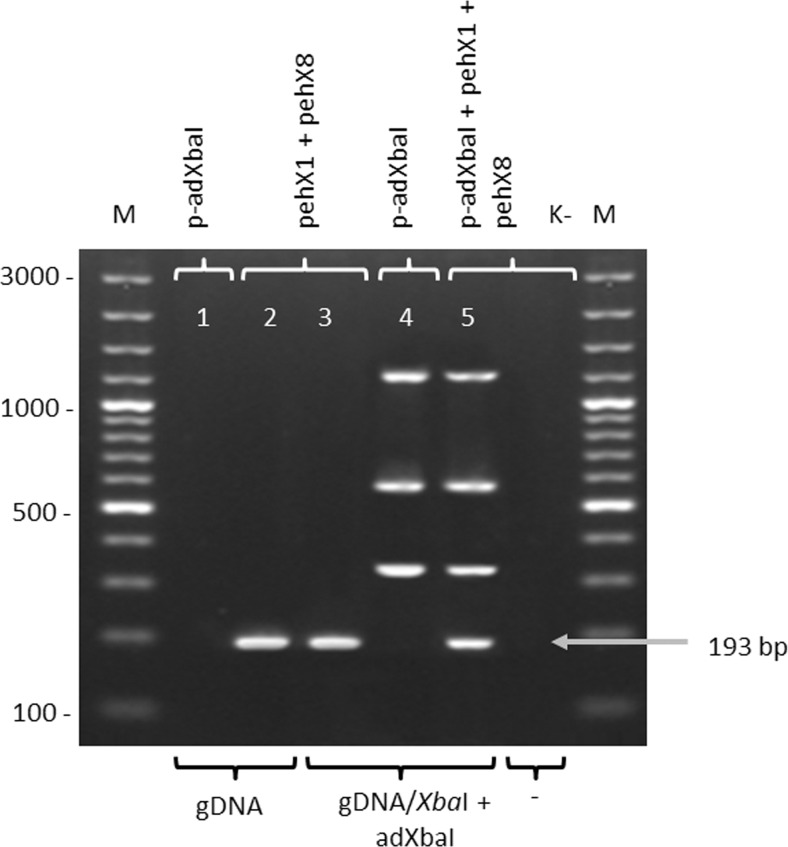
The pehX-LM PCR/XbaI method was verified. The results are shown in Fig. 3. The pehX-species-specific fragments can be amplified by PCR using the pehX1 and pehX8 primers either from undigested genomic DNA (Fig. 3, lane 2) or the DNA previously digested and ligated to form the adapter (Fig. 3, lane 3), whilst the strain-specific fingerprint profile (LM PCR) can be obtained only after the complete digestion of the genomic DNA, the ligation of the adapter to the fragments and PCR amplification with the use of the p-adXbaI primer (Fig. 3, lanes 1 and 4). The complete fingerprint profile of the K. oxytoca strain consists both of a pehX-specific product with a length of 193 bp and three well separated strain-specific LM PCR products with a length of at least 300 bp (Fig. 3, lane 5). There are no extra non-specific or semi-specific PCR products with three primers in one PCR.
Fig. 3.
Verification of the pehX-LM PCR/XbaI model system for the K. oxytoca reference strain. Electropherogram of PCR products which were obtained using a combination of pehX-specific or adapter-specific primers with digested or undigested DNA. gDNA—non digested, genomic DNA of K. oxytoca K26, gDNA/XbaI + adXbaI—digested and ligated to the adapter DNA of K. oxytoca K26; p-adXbaI—adapter-specific primer, pehX1 and pehX8—pehX-specific primers; M—DNA ladder (100–3000 bp), K-negative control (without DNA)
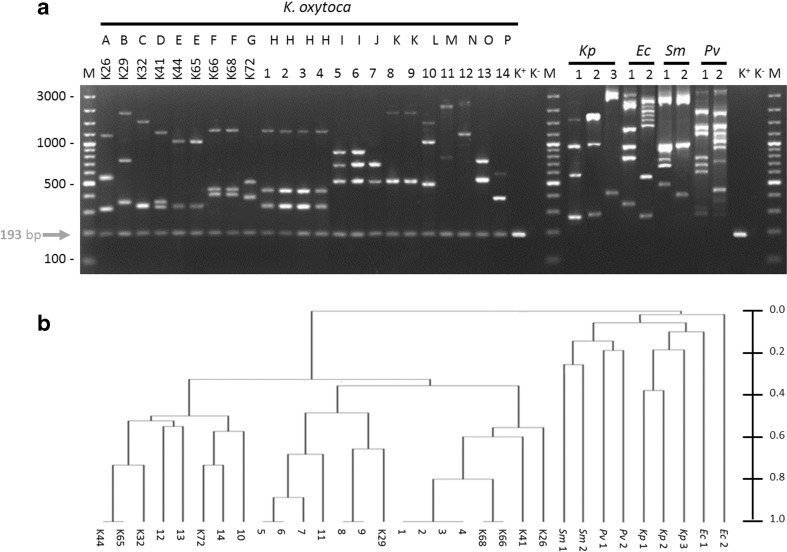
To demonstrate the usefulness and discrimination power of the pehX-LM PCR/XbaI method, we examined 200 clinical and 9 references K. oxytoca, 28 K. pneumoniae and 25 strains of other species of Enterobacteriaceae. The typing results for selected strains are shown in Fig. 4a.
Fig. 4.
a Representative typing results for Enterobacteriaceae by pehX-LM PCR/XbaI method. b Dendrogram of pehX-LM PCR/XbaI constructed under Dice band-based coefficient of similarity and the Unweighted Pair Group Method with Arithmetic Mean (UPGMA). K26-K72 reference strains of K. oxytoca from Ørskov collection, 1-14 - clonally related and unrelated K. oxytoca strains, Kp - Klebsiella pneumoniae, Ec - Escherichia coli, Sm - Serratia marcescens, Pv - Proteus vulgaris, A-P genotypes (different fingerprint profiles), K+ positive control of pehX-specific product (193-bp DNA fragment amplified from genomic DNA of K. oxytoca K26). K- negative control of PCR (without DNA), M - DNA ladder (100–3000 bp)
For all K. oxytoca strains the electrophoretic profile usually contained 2, 3 or 4 strain-specific (genotype-specific) LM PCR products with a length of at least 300 bp, and one well-separated pehX-specific product with a length of 193 bp. The electrophoretic patterns relating to K. pneumoniae and the other bacteria from Enterobacteriaceae, contained up to 10 well visible and well-separated genotype-specific LM PCR products. There was no pehX-specific band in the profiles. The presence of a pehX-specific product in all K. oxytoca strains and its absence in other Enterobacteriaceae strains in the electrophoretic profile was confirmed by comparing the results of three experiments with reference strains as shown in Fig. 3, lines 3, 4 and 5. All K. oxytoca strains tested positive on PCR with the primer pair pehX1/pehX8, whereas the negative results were obtained for all K. pneumoniae (28 strains) and S. marcescens (10 strains), E. coli (15 strains), P. vulgaris (10 strains). Furthermore, there were no semi-specific PCR products generated by pehX1/p-adXbaI or/and pehX8/p-adXbaI primers in both K. oxytoca strains and non-K. oxytoca strains.
The typeability, reproducibility and discriminatory power of pehX-LM PCR/XbaI technique were evaluated. The typeability of the pehX-LM PCR/XbaI method was at 100 % (for each K. oxytoca strain electrophoretic pattern with species-specific and genotype-specific bands was obtained), whilst reproducibility was estimated at 96,8 %. We have proved that genotyping results do not depend on the thermostable DNA polymerase in PCR and the thermal cycler used (Fig. S1). The same results were obtained by two independent researchers giving the same genetic groups.
The new pehX-LM PCR/XbaI method was shown to have a high discriminatory power. Most of 209 K. oxytoca strains showed a single-specific pattern. We distinguished 187 different genotypes at a cut-off similarity level of 95 %, which conforms to the PCR MP results (cut-off values 90 %) (Stojowska et al. 2009b). The genotyping results for selected K. oxytoca by the pehX-LM PCR/XbaI method and the PCR MP method are shown in Fig. 4a and Fig. S2, respectively. Typing by pehX-LM PCR/XbaI lead to the same electrophoretic patterns for clonally related strains but clonally unrelated strains generated different electrophoretic profiles.
All the patterns for K. oxytoca were different from those observed for K. pneumoniae and other Enterobacteriaceae (Fig. 4a). Measured with the use of the Dice band-based coefficients the UPGMA method, K. oxytoca strains had a similarity of over 30 %, whilst similarity between K. oxytoca and other Enterobacteriaceae strains did not exceed 5 % (Fig. 4b).
Discussion
Currently, the complete identification of Klebsiella spp. and the reliable strain typing analysis can only be obtained with the use of separate molecular tests. A number of different molecular techniques have been developed for genetic typing including ribotyping (ITS PCR) (Stojowska et al. 2009b), pulsed field gel electrophoresis (PFGE, using XbaI restriction enzyme) (Fujita et al. 2015; Izdebski et al. 2015; Krawczyk et al. 2005), multilocus sequence typing (MLST, based on ropB, gapA, mdh, pgi, phoE and tonB genes) (Fujita et al. 2015; Izdebski et al. 2015), DNA fingerprinting based on PCR such as random amplified polymorphic DNA (RAPD) (Brisse and Verhoef 2001; Krawczyk et al. 2005; Vogel et al. 1999), AFLP (using restriction enzymes EcoRI + MseI) (Donnarumma et al. 2012; Jonas et al. 2004; van der Zee et al. 2003), Amplification of DNA fragments Surrounding Rare Restriction Sites (ADSRRS) (Krawczyk et.al. 2005), and PCR MP (using restriction enzyme HindIII) (Stojowska et al. 2009a, b). None of these methods will not provide information about the species or genus of the tested strains. For this reason, they require the use of purified DNA from the correctly identified species of bacteria. Closely related species which belong to the same genus may hinder the diagnosis.
In this study, we propose a new simple method called pehX-LM PCR/XbaI, which uses fingerprint patterns to show whether the tested strain belongs to the K. oxytoca, and identify its genotype. pehX-LM PCR/XbaI is a combination of the following two methods: the species-specific amplification of the pehX gene and the non-specific amplification of short restriction fragments (LM PCR). The only way to make this combination work was to select suitable restriction enzyme XbaI, which (i) rarely cuts genomic DNA of Enterobacteriaceae, (ii) generates a small number of relatively short restriction fragments (up to 3000–5000 bp) and may be amplified using the standard DNA polymerases and (iii) is able to detect a strain-specific restriction fragment length polymorphism using gel electrophoresis. Lack of amplification of long, non-specific DNA restriction fragments and both semi-specific fragments generated with the adapter-specific and pehX-specific primers can be attributed to the limited efficiency of the DNA polymerase and short elongation times during the PCR cycles.
As with any other LM PCR method, the pehX-LM PCR/XbaI requires the use of a purified genomic DNA from one species/strain of bacteria (typically, DNA isolated from one colony of bacteria). If a sample contains a mix of bacteria, the fingerprint patterns will be distorted. If this happens, the number of bands in the profile will increase (more than four bands) and the bands will not be separated well after gel electrophoresis. When there is no K. oxytoca DNA in a sample (a mix or a single strain), there will be no amplification of a specific fragment of the pehX gene (193 bp). The length of PCR products in the fingerprint pattern will not be less than 300 bp. The pehX-LM PCR/XbaI method may help identify K. oxytoca in a sample and determine the purity of the sample (a single strain or a mix of bacteria), which is impossible using other LM PCR methods.
The new pehX-LM PCR/XbaI method has a high discriminatory power, comparable to other LM PCR methods. Fingerprint patterns consist of up to five bands and they are easy to read and interpret. Simultaneous species identification allows avoiding mistaken identities or contamination which could lead to the misinterpretation of fingerprint patterns. Analyses (without DNA isolation) take no more than 4 h. This is of extreme importance, especially in hospital settings where time may play a crucial role in diagnosis and treatment. The pehX-LM PCR/XbaI method does not require a high degree of technical competency. To be reproducible, analyses do not require careful standardization of the PCR conditions and are not sensitive to small temperature fluctuations during PCR.
The rarely cutting restriction enzymes, e.g., XbaI (as in pehX-LM PCR/XbaI method) give a small number of the short restriction fragments and the number and the size (length) of them depend on the genetic events (deletion, insertion, mutation). Our studies have indicated that this is sufficient for differentiation of K. oxytoca strains.
However, we cannot exclude that the clonally unrelated strains will obtain the same electrophoretic pattern and will be classified as the same genotype. It is a disadvantage of all typing methods that use the restriction enzymes for whole genome digestion. It is recommended to use another method for confirmation (e.g., identification of nosocomial infection) for doubtful results.
We cannot ensure that for non-K. oxytoca strains belonging to another Enterobacteriaceae; there will be an unspecific 190–200 bp band in the fingerprint pattern, and that it may hinder the proper identification. In this case K. oxytoca-species identification requires confirmation. It is recommended to repeat the PCR without pehX-specific primers (as it was shown on Fig. 3, lane 4). At the time the species-specific fragment for K. oxytoca should not be observed.
A small number of strain-specific PCR products make fingerprint patterns well readable.
It enables to generate a dendrogram graph to show genetic diversity between tested strains but it could be insufficient to measure the level of genetic similarity, to study bacterial population dynamic or transmission and sources of bacterial infections (more advanced clinical study). This method allows to distinguish clonally related strains from clonally unrelated strains. It may be used as a screening method for the initial identification of nosocomial infection in hospitals.
The pehX-LM PCR/XbaI method was designed for K. oxytoca typing. Like with other LM PCR methods, it is not universal and cannot be applied for other species. By changing the species-specific primers (specific DNA target) and/or restriction enzymes in the pehX-LM PCR/XbaI method, it is possible to design similar assays for other species or genera of bacteria.
To sum up:
The pehX-LM PCR/XbaI method was designed to simultaneously type K. oxytoca strains and confirm the species.
The method combines the merits of species-specific PCR primers, carefully selecting sequences which are present only in K. oxytoca, enabling a quick confirmation of the species with the use of the LM PCR method for the genotyping of strains.
Unlike in most fingerprinting methods, we obtain a clear pattern (approx. 3–5 bands) with a sufficient, relatively high discriminatory power.
A small amount of DNA is used as a template for PCR. This reduces the duration and cost of a single analysis.
The method after validation process could be used in clinical and environmental research.
Electronic supplementary material
(PDF 354 kb)
Acknowledgments
Authors’ contributions
KS-S carried out many of the experiments as a PhD student, participated in field work and drafted the manuscript; BK was the supervisor and chief designer of the project, participated in the conception, design, supervision of the experiments, in the drafting and revision of the manuscript. All authors read and approved the final manuscript before submission.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Karolina Stojowska-Swędrzyńska, Email: karolinastojowska@gmail.com.
Beata Krawczyk, Phone: +48 58347 23 83, Email: beakrawc@pg.gda.pl.
References
- Asensio A, Oliver A, González-Diego P, Baquero F, Pérez-Díaz JC, Ros P, Cobo J, Palacios M, Lasheras D, Cantón R. Outbreak of a multiresistant Klebsiella pneumoniae strain in an intensive care unit: antibiotic use as risk factor for colonization and infection. Clin Infect Dis. 2000;30:55–60. doi: 10.1086/313590. [DOI] [PubMed] [Google Scholar]
- Biehle LR, Cottreau JM, Thompson DJ, Filipek RL, O’Donnell JN, Lasco TM, Mahoney MV, Hirsch EB. Outcomes and risk factors for mortality among patients treated with carbapenems for Klebsiella spp bacteremia. PLoS One. 2015;10:e0143845. doi: 10.1371/journal.pone.0143845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisse S, Verhoef J. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int J Syst Evol Microbiol. 2001;51:915–924. doi: 10.1099/00207713-51-3-915. [DOI] [PubMed] [Google Scholar]
- Darby A, Lertpiriyapong K, Sarkar U, Seneviratne U, Park DS, Gamazon ER, Batchelder C, Cheung C, Buckley EM, Taylor NS, Shen Z, Tannenbaum SR, Wishnok JS, Fox JG. Cytotoxic and pathogenic properties of Klebsiella oxytoca isolated from laboratory animals. PLoS One. 2014;9:e100542. doi: 10.1371/journal.pone.0100542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnarumma F, Indorato C, Mastromei G, Goti E, Nicoletti P, Pecile P, Fanci R, Bosi A, Casalone E. Molecular analysis of population structure and antibiotic resistance of Klebsiella isolates from a three-year surveillance program in Florence hospitals, Italy. Eur J Clin Microbiol Infect Dis. 2012;31:371–378. doi: 10.1007/s10096-011-1319-6. [DOI] [PubMed] [Google Scholar]
- Fevre C, Jbel M, Passet V, Weill F-X, Grimont PAD, Brisse S. Six groups of the OXY β-lactamase evolved over millions of years in Klebsiella oxytoca. Antimicrob Agents Chemother. 2005;49:3453–3462. doi: 10.1128/AAC.49.8.3453-3462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Kimura K, Yokoyama S, Jin W, Wachino J, Yamada K, Suematsu H, Yamagishi Y, Mikamo H, Arakawa Y. Characterization of iperacillin/tazobactam-resistant Klebsiella oxytoca recovered from a nosocomial outbreak. PLoS One. 2015;10:e0142366. doi: 10.1371/journal.pone.0142366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Martínez J, Acinas SG, Antón AI, Rodríguez-Valera F. Use of the 16S-23S ribosomal genes spacer region in studies of prokaryotic diversity. J Microbiol Meth. 1999;36:55–64. doi: 10.1016/S0167-7012(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Giamarellou H, Galani L, Baziaka F, Karaiskos I. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother. 2013;57:2388–2390. doi: 10.1128/AAC.02399-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girometti N, Lewis RE, Giannella M, Ambretti S, Bartoletti M, Tedeschi S, Tumietto F, Cristini F, Trapani F, Gaibani P, Viale P. Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore) 2014;93:298–309. doi: 10.1097/MD.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SB, Lee SC, Lee SY, Jeong DC, Kang JH. Aminoglycoside therapy for childhood urinary tract infection due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae. BMC Infect Dis. 2015;15:414. doi: 10.1186/s12879-015-1153-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izdebski R, Fiett J, Urbanowicz P, Baraniak A, Derde LP, Bonten MJ, Carmeli Y, Goossens H, Hryniewicz W, Brun-Buisson C, Brisse S, Gniadkowski M. MOSAR WP2, WP3 and WP5 study groups; phylogenetic lineages, clones and β-lactamases in an international collection of Klebsiella oxytoca isolates non-susceptible to expanded-spectrum cephalosporins. J Antimicrob Chemother. 2015;70:3230–3237. doi: 10.1093/jac/dku359. [DOI] [PubMed] [Google Scholar]
- Jonas D, Spitzmüller B, Daschner FD, Verhoef J, Brisse S. Discrimination of Klebsiella pneumoniae and Klebsiella oxytoca phylogenetic groups and other Klebsiella species by use of amplified fragment length polymorphism. Res Microbiol. 2004;155:17–23. doi: 10.1016/j.resmic.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Kovtunovych G, Lytvynenko T, Negrutska V, Lar O, Brisse S, Kozyrovska N. Identification of Klebsiella oxytoca using a specific PCR assay targeting the polygalacturonase pehX gene. Res Microbiol. 2003;154:587–592. doi: 10.1016/S0923-2508(03)00148-7. [DOI] [PubMed] [Google Scholar]
- Krawczyk B, Samet A, Czarniak E, Szczapa J, Kur J. Extended-spectrum beta-lactamase-producing Klebsiella pneumoniae in a neonatal unit: control of an outbreak using a new ADSRRS technique. Pol J Microbiol. 2005;54:105–110. [PubMed] [Google Scholar]
- Krawczyk B, Samet A, Leibner J, Sledzinska A, Kur J. Evaluation of a PCR melting profile technique for bacterial strain differentiation. J Clin Microbiol. 2006;44(7):2327–2332. doi: 10.1128/JCM.00052-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes AC, Rodrigues JF, Clementino MB, Miranda CA, Nascimento AP, de Morais Júnior MA. Application of PCR ribotyping and tDNA-PCR for Klebsiella pneumoniae identification. Mem Inst Oswaldo Cruz. 2007;102:827–832. doi: 10.1590/S0074-02762007005000113. [DOI] [PubMed] [Google Scholar]
- Martínez J, Martínez L, Rosenblueth M, Silva J, Martínez-Romero E. How are gene sequence analyses modifying bacterial taxonomy? The case of Klebsiella. Int Microbiol. 2004;7:261–268. [PubMed] [Google Scholar]
- Monnet D, Freney J. Method for differentiating Klebsiella planticola and Klebsiella terrigena from other Klebsiella species. J Clin Microbiol. 1994;32:1121–1122. doi: 10.1128/jcm.32.4.1121-1122.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet D, Freney J, Brun Y, Boeufgras JM, Fleurette J. Difficulties in identifying Klebsiella strains of clinical origin. Zentralbl Bakteriol. 1991;274:456–464. doi: 10.1016/S0934-8840(11)80081-2. [DOI] [PubMed] [Google Scholar]
- Ørskov I. Genus v Klebsiella. In: Krieg NR, Holt JG, editors. Bergey’s manual of systematic bacteriology. Baltimore, Md: Williams & Wilkins; 1984. pp. 461–465. [Google Scholar]
- Ørskov I, Ørskov F. Serotyping of Klebsiella. In: Bergan T, editor. Methods in microbiology. New York, NY: Academic Press Inc; 1984. pp. 143–164. [Google Scholar]
- Podschun R, Ullmann U. Klebsiella spp as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblueth M, Martínez L, Silva J, Martínez-Romero E. Klebsiella variicola, a novel species with clinical and plant-associated isolates. Syst Appl Microbiol. 2004;27:27–35. doi: 10.1078/0723-2020-00261. [DOI] [PubMed] [Google Scholar]
- Stojowska K, Kałużewski S, Krawczyk B. Usefulness of PCR melting profile method for genotyping analysis of Klebsiella oxytoca isolates from patients of a single hospital unit. Pol J Microbiol. 2009;58:247–253. [PubMed] [Google Scholar]
- Stojowska K, Krawczyk B, Kałużewski S, Kur J. Retrospective analysis of the genetic diversity of Klebsiella oxytoca isolated in Poland over a 50-year period. Eur J Clin Microbiol Infect Dis. 2009;28:1263–1266. doi: 10.1007/s10096-009-0768-7. [DOI] [PubMed] [Google Scholar]
- van der Zee A, Steer N, Thijssen E, Nelson J, van’t Veen A, Buiting A. Use of multienzyme multiplex PCR amplified fragment length polymorphism typing in analysis of outbreaks of multiresistant Klebsiella pneumoniae in an intensive care unit. J Clin Microbiol. 2003;41:798–802. doi: 10.1128/JCM.41.2.798-802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel L, Jones G, Triep S, Koek A, Dijkshoorn L. RAPD typing of Klebsiella pneumoniae, Klebsiella oxytoca, Serratia marcescens and Pseudomonas aeruginosa isolates using standardized reagents. Clin Microbiol Infect. 1999;5:270–276. doi: 10.1111/j.1469-0691.1999.tb00140.x. [DOI] [PubMed] [Google Scholar]
- Westbrook GL, O’Hara CM, Roman SB, Miller JM. Incidence and identification of Klebsiella planticola in clinical isolates with emphasis on newborns. J Clin Microbiol. 2000;38:1495–1497. doi: 10.1128/jcm.38.4.1495-1497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 354 kb)