Abstract
Diabetic retinopathy (DR) is a multifactorial disease with complex pathophysiology. It is the main cause of blindness among the people in productive age. The purpose of this literature review is to highlight recent achievements in the genetics of diabetic retinopathy with particular focus on candidate gene studies. We summarized most of the available published data about candidate genes for diabetic retinopathy with the goal to identify main genetic aspects. We conclude that genetic studies reported contradictory findings and no genetic variants meet criteria of a diagnostic marker, or significantly elucidate the root of DR development. Based on these findings it is important to continue with the research in the field of DR genetics, mainly due to the fact that currently new possibilities and approaches associated with utilization of next-generation sequencing are available.
Keywords: Diabetic retinopathy, DNA variants, Sequencing, Diabetes mellitus, Genetic studies
Introduction
Diabetes mellitus (DM) is one of the most significant health problems worldwide. It is a metabolic disorder in which elevated blood sugar levels are present as a result of the inability to produce a sufficient amount of insulin (type 1) or because of cellular insulin resistance (type 2). Both types of diabetes are associated with hyperglycaemia, oxidative stress, inflammation and macrovascular (coronary artery disease, atherosclerosis, hypertension and stroke) and microvascular complications such as retinopathy, nephropathy and neuropathy [1].
The number of patients with diabetes mellitus is rapidly increasing every year. Global mortality resulting from diabetes in adults was estimated to be 1.5 million deaths in 2012 (World Health Organization). It is estimated that there will be 418 million patients with impaired glucose tolerance and 380 million patients with T2DM by 2025 [2].
Diabetic retinopathy (DR) is a leading cause of visual impairment in patients at productive age. These alarming numbers highlight the necessity of optimization of diagnostic methods that will allow early identification of diabetic patients with significantly elevated risk of DR development that will help start optimal prevention and intervention. DR has an overall prevalence of 22–37 % in individuals with known diabetes. It leads to damage of the retina microvasculature as a result of prolonged exposure to metabolic changes induced by diabetes. If left untreated, it may lead to blindness on account of continuous blood leakage due to the loss of retinal pericytes and fenestration [1]. DR is classified into two categories based on severity, namely less-severe nonproliferative diabetic retinopathy (NPDR) and severe proliferative diabetic retinopathy (PDR). The key changes of the retina in NPDR, as a result of hypoxia and venous bleading, are microaneurysms, vascular leakage, hard exudates, intraretinal microvascular abnormalities and cotton wool spots (Fig. 1). Retinal neovascularization induced by ischemia is the main characteristic of PDR [3].
Fig. 1.

Symptoms and pathological processies typical for diabetic retinopathy leading to vision lost. EBM endothelial basal membrane, BRB blood retinal barrier, EC endothelial cell
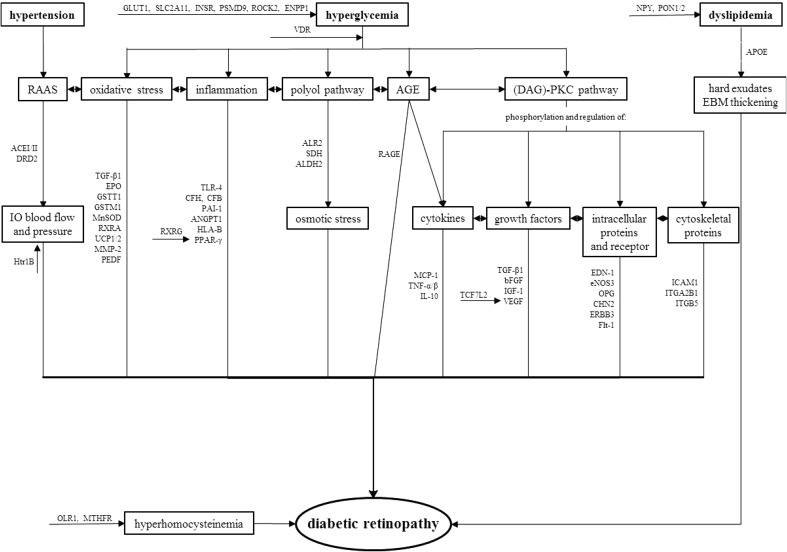
The aetiology of this complex disease remains unclear and poorly understood. It is associated with both environmental and genetic factors. The possibility of developing and progression of DR is closely related to the duration of DM [1]. Almost all patients with T1DM and >60 % of patients with T2DM are anticipated to have some type of retinopathy within the first 10 years of diabetes being diagnosed. The Diabetes Control and Complications Trial (DCCT) and United Kingdom Prospective Diabetes Study (UKPDS) clinical trials have also confirmed significant association between chronic hyperglycaemia and development and progression of DR, however, the fundamentals of how hyperglycaemia causes microvascular changes in the retina has not been fully elucidated [4]. Involvement of several biochemical pathways which can elucidate the role of hyperglycaemia in DR pathophysiology has been proposed, including activation of diacyl-glycerol (DAG)-PKC pathway, accelerated formation of advanced glycation endproducts (AGE), increased polyol pathway flux, increased expression of growth factors (VEGF, IGF-1), haemodynamic changes, renin-angiotensine-aldosterone system (RAAS), leukostasis, subclinical inflammation, and oxidative stress that leads to increased expression of several proinflammatory genes (NF-κB, TGF-β, NOX4, Nrf2, etc.). Other risk contributors for DR development are dyslipidemia [5] and possibly blood pressure, but studies are contradictory about these risk modifiers [4, 6]. Pathways contributing to DR pathologies alongside with relevant genes are summarized in Fig. 2. In summary, increased vascular permeability, haemostatic abnormalities, endothelial dysfunction, increased tissue ischemia, angiogenesis and neovascularization is typical for overall DR pathophysiology [1].
Fig. 2.
Putative roles of genes identified by candidate genes studies in pathophysiological processies during diabetic retinopathy. RAAS renin–angiotensin–aldosterone system, AGE advanced glycation end-product, IO intraoccular, EBM endothelial basal membrane, BRB blood retinal barrier
Genetic aspects of the diabetic retinopathy
The above mentioned risk factors are not solely responsible for susceptibility to DR. Clinical studies have revealed considerable variations in the retinopathy onset and severity that cannot be fully explained by known risk factors such as the duration of diabetes, the level of glycemic control, or concomitant vascular disease [7]. For instance, some people might have DR even when they have good glycemic control and duration of DM is short. In contrast, other patients have poor glycemic control and prolonged duration of DM and yet may not develop DR. The probability of DR development also depends on the ethnicity; the Hispanics, the individuals of African descent and the Asians are more susceptible to DR [8, 9]. A study from 2008 reported retinopathy symptoms, including retinal microaneurysms, in nondiabetic patients with an optimal glucose level (glycosylated haemoglobin levels <5.0 %) [10] and these microvascular changes were indistinguishable from lesions in diabetic DR. Therefore there is evidence that additional risk factors and genetic predispositions have a part in the development and progression of DR and that these factors are independent of DM.
This is a solid confirmation of genetic contribution to the development and progression of DR. Over the past several years, progress has been made in identifying some of the susceptibility loci associated with DR through twin studies, family studies, candidate gene studies, linkage studies and small-scale GWAS (genome-wide association study). Twin and family studies have demonstrated that risk of DR emergence is three times higher for patients with a family history of DR than in patients without it for both T1DM and T2DM. Concordance is dramatically higher among monozygotic twins when compared to dizygotic twins [11]. One of the first twin studies has reported DR concordance of 68 % in T1DM and 95 % in T2DM [12]. Heritability score increases with the severity of DR, and has been estimated to be 18 and 52 % for DR and PDR, respectively [13].
Candidate genes studies
Our knowledge of pathophysiology of DR allows us to propose possible candidate genes, which could play a role in the development and progression of DR. Candidate gene studies compare the frequency of a particular genetic variant in subjects with or without DR. This approach has revealed several genes with a possible key role in DR. These genes are part of different physiological and pathophysiological processes in organism, often associated with inflammation, such as RAAS (renin–angiotensine–aldosterone system), glucose induced pathways, remodeling of extracellular matrix (ECM), vascular endothelial dysfunction, and angiogenesis. It has been proposed that a great number of factors and genes with modest effect, as a part of different biochemical pathways, invoke pathological processes leading to DR.
Polyol pathway and its role in DR
Polyol pathway represents the main metabolic link between hyperglycaemia and damages caused by DM. Aldose reductase (ALR2) is the essentialenzyme in the pathway. ALR2 converts glucose to sorbitol in an NADPH-dependent reaction. During the hyperglycaemia sorbitol accumulates in cells and induces osmotic stress and cellular damage. The above mentioned process leads to the destruction of retinal cells, microaneurysms, thickening of the basement membrane and loss of pericytes in animal models, which are also typical symptoms of DR in humans [14]. Three DR-associated ALR2 polymorphisms have been identified in different populations (see Table 1). The first polymorphism located at the 5′ end of the gene, the Z-2 allele of the (CA)n microsatellite located at the 5′ end of the gene increases risk of DR. In contrast, Z + 2 and Z alleles show protective effect against DR [15]. Another polymorphism, rs759853, has shown association with DR where T allele confers protection against DR in T1DM [15, 16]. However, the use of AKR (aldo–keto reductase) inhibitors did not confirm expected results in clinical trials and they could not prevent progression of the disease [17]. But these clinical trials disregarded the genetic variants in the AKR2 gene which could have had negative impact on the function of AKR inhibitors. Another enzyme in the polyol pathway, sorbitol dehydrogenase (SDH) converts sorbitol into fructose in NAD+-dependent reaction. Amano et al. found that SDH overexpression potentiated glucose toxicity to cultured retinal pericytes, thus leading to acceleration of pericyte loss, a typical trait of DR [18]. Polymorphisms rs2055858 and rs3759890 were identified in Polish, Japanese and Caucasian-Brazilian population and it is possible that they could affect the promoter activity of the SDH gene and have role in onset of DR [18, 19].
Table 1.
Summary of genes identified by candidate genes studies with possible role in pathophysiology of DR
| Gene symbol | Gene name | Function/cellular role | Polymorphism | Ch. | Type of DM | Population | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| AKR1B1/ALR2 | Aldose reductase gene | Polyol pathway—conversion of glucose to sorbitol | rs35839483 [(CA)n dinucleotide repeats] | 7 | 1 and 2 | Chinese, Japanese, Indians, Chileans, Brazilians | z-2 microsatellite confers risk in all DR, z2 microsatellite against all DR | [20–27] |
| rs759853 (c. C-106T) | 7 | 2 | Euro-Brazilian, Mainland Chinese, Han Chinese, Japanese | T allele protective against DR but according to some studies it is weak association | [25, 26, 28–30] | |||
| rs9640883 | 7 | 2 | Australian | Association with onset of diabetes | [25] | |||
| SDH | Sorbitol dehydrogenase | Polyol pathway—conversion of sorbitol to fructose | rs2055858 (c. C-1214G) | 15 | 2 | Poland | Weak associations; polymorphism possibly affect promoter activity | [18, 19] |
| rs3759890 (c. G-888C) | 15 | 2 | Japan, Poland, Caucasian-Brazilians | Inconsistent finding, polymorphism possibly affect promoter activity | [18, 31] | |||
| ALDH2 | Mitochondrial aldehyde dehydrogenase 2 | Polyol pathway—transformation from acetaldehyde to acetic acid, prevents creation of AGE | ALDH2*2 | 12 | 2 | Japanese | Associated with protective effect against DR | [32] |
| VEGF | Vascular endothelial growth factor | Stimulation of angiogenesis and vasculogenesis | rs2010963 (c. C-634G) | 6 | 2 | Japanese, Indian, Caucasian | C allele confers risk for NPDR in T2DM | [33–41] |
| (c. C-460T) | 6 | 1 and 2 | Caucasian | Possible association with DR | [42, 43] | |||
| rs25648 | 6 | 2 | Multi-ethnic | T allele increase risk of DR but finding inconsistent | [33, 34, 37] | |||
| rs1570360 (c. A-116G) | 6 | 2 | Multi-ethnic | Inconsistent finding | [33, 34, 42] | |||
| rs3095039 | 6 | 2 | Multi-ethnic | T allele increase risk of DR but finding inconsistent | [33–36] | |||
| rs35569394 | 6 | 1 and 2 | Multi-ethnic | (− 2549) DEL increases risk but finding inconsistent | [33] | |||
| rs699947(c. A-2578C) | 6 | 2 | Multi-ethnic | A allele increases risk but finding inconsistent | [33, 36, 40, 43–46] | |||
| rs13207351 (c. A-152G) | 6 | 1 and 2 | Caucasian | Associated with PDR in some of the studies | [34, 42, 46] | |||
| rs735286 (c. C4618T) | 6 | 1 and 2 | Caucasian | Haplotype-tagged SNP associated with severity of DR | [42] | |||
| rs2146323 (c. C5092A) | 6 | 1 and 2 | Caucasian | Haplotype-tagged SNP associated with severity of DR, associated with early progression of DR | [42, 44, 47] | |||
| rs833061 (c. C-1498T) | 6 | 2 | Chinese | Inconsistent finding and weak association | [34, 37, 39, 46] | |||
| rs3025021 | 6 | 2 | Chinese | Inconclusive | [33, 46] | |||
| rs10434 | 6 | 1 and 21 and 2 | Caucasian | G allele associated with blinding DR | [33] | |||
| rs833068 | 6 | 1 and 2 | Caucasian | G allele confers risk in DR | [33] | |||
| rs833070 | 6 | 1 | Japanese | Associated with early progression of DR but weak association | [44] | |||
| rs3025039 (c. C+936T) | 6 | 2 | Caucasian | T allele increases risk | [48] | |||
| bFGF/FGF2 | Basic fibroblast growth factor/fibroblast growth factor 2 | Stimulation of angiogenesis and tissue repair | rs41456044 | 4 | 2 | Multi-ethnic | A allele increases risk but weak associations | [15] |
| rs308395 | 4 | 2 | Multi-ethnic | G allele increases risk but finding inconsistent | [15] | |||
| c. C-754G | 4 | 2 | Slovak | C allele increases level of bFGF | [49] | |||
| c. T − 553 A c. T−834A |
4 | 2 | Caucasian | AT genotype could be risk factor for PDR during T2DM | [50] | |||
| IGF-1 | Insulin-like growth factor 1 | Stimulation of cell growth and proliferation, inhibition of apoptosis | (CA)n | 4 | 2 | Southern Indian | 18-repeat of (CA) increases risk of DR | [51] |
| EPO | Erythropoietin | Control of erythropoiesis, stimulation of proliferation, migration and angiogenesis in hypoxic cells | rs1617640, rs507392, rs551238 | 7 | 1 and 2 | Multi-ethnic, European American, Australian | TTA allele associated with PDR in European American, meta-analysis hasn´t found significant association; GCC haplotype associated with DR in Australian | [15, 52, 53] |
| RAGE | Advanced glycosylation end product-specific receptor | Activation of pro-inflammatory genes | rs1800624 (c. T-374A) | 6 | 2 | Indian, Chinese, African-Brazilian, Caucasian - Scandinavian | Inconsistent finding and weak association, may be interacting with glycosylated hemoglobin | [15, 54–56] |
| rs1800625 (c. T-429C) | 6 | 2 | Caucasian, Indians, Danish | Inconsistent finding and weak association, functional studies show differences in polymorphic receptor activity | [55–60] | |||
| rs2070600 (p. G82S) | 6 | 2 | Caucasian, Indian, Chinese, Malaysian | Associated with DR, no association in Malaysian | [15, 58, 61] | |||
| ACE I | Angiotensin-I converting enzyme | Component of the renin-angiotensin system—activation of angiotensin II | rs4646994 (c. G2350A)-INS/DEL at intron 16 | 17 | 1 and 2 | Caucasian - Slovene, Danish; Japanese, Multi-ethnic, Iranian, Japanese, Chinese, Pakistani | D allele possibly associated with DR in T2DM in Chinese, but inconsistent finding and weak association in other populations, associated with NPDR in Pakistani |
[62–66] |
| GSTT1 | Glutathione S-transferase T1 | Detoxifying enzyme—conjugation of reduced glutathione to a compounds | Null genotype | 22 | 2 | Caucasian - Slovenian | Greater risk of DR | [67] |
| GSTM1 | Glutathione S-transferase M1 | Detoxifying enzyme—conjugation of reduced glutathione to a compounds | Null genotype | 1 | 2 | Caucasian - Slovenian | Lower risk of DR | [67] |
| SOD2/MnSOD | Mitochondrial manganese superoxide dismutase | Decrease of ROS production (transformation to to peroxide and oxide) | rs4880 (c. C47T, p. A16V) | 6 | 1 and 2 | Slovene (Caucasian), Finnish, Indian | C allele reduces risk of DR, not confirmed in Indian population | [15, 36, 56, 65, 68, 69] |
| eNOS3 | Endothelial nitric oxide synthases | Synthesis of nitric oxide (vasodilatation) | rs3138808 (27 VNTR intron 4 a/b) | 7 | 2 | Indians, West African, Caucasian - Brazilian | 4a allele protective effect against DR | [15, 37, 70–73] |
| rs1799983 (c. C894T) | 7 | 2 | Caucasian—Brazilian, Danish, Multi-ethnic | G allele increases risk but weak association | [15, 37, 50, 60, 72, 74] | |||
| rs41322052 (c. T-784C) | 7 | 1&2 | Caucasian—Brazilian, Multi-ethnic | Inconsistent finding and weak association | [37, 71, 72, 75] | |||
| rs2297518 (c. G-954C) | 7 | 2 | Caucasian | Protective factor against NPDR | [48] | |||
| RXRA | Retinoid X receptor alpha | Nuclear receptor—retinoic acid-mediated gene activation (antoxidants properties) |
rs3132300 | 9 | 1 | African American | Associated with progression of DR | [76, 77] |
| RXRG | Retinoid X receptor gamma | nuclear receptor—retinoic acid-mediated gene (antiproliferative effects) | rs3818569 | 9 | Taiwanese | G allele associated with development of DR | [78] | |
| UCP1 | Uncoupling protein-1 | Mitochondrial anion carrier protein (thermogenesis), protection againt oxidative stress | rs1800592 (c. A-3826G) | 4 | 1 and 2 | Brazilian, Chinese, Danish | G allele associated with increased risk of PDR | [60, 75, 79] |
| UCP2 | Uncoupling protein-2 | Mitochondrial anion carrier protein (thermogenesis), control of ROS production | rs660339 (p. A55V, 45 bp INS/DEL) | 11 | 1 and 2 | Brazilian | Risk factor for PDR | [77, 80] |
| TLR4 | Toll-like receptor 4 | Pathogen recognition and activation of innate immunity | rs10759931, rs1927914 | 9 | 2 | Indian, Chinese | A,T alleles positively modulate the risk of DR, rs1927914 associated with susceptibility to DR in a Han Chinese population | [3, 55] |
| rs4986790, rs4986791 (p. D299G) | 9 | 2 | Polish | G allele associated with early onset of DR | [81] | |||
| CFH | Complement factor H | Regulator of complement activation | rs800292 (p. I62V) | 1 | 2 | Chinese | Associated with DR | [82] |
| CFB | Complement factor B | Regulator of complement activation | rs1048709 | 6 | 2 | Chinese | Associated with DR | [82, 83] |
| MCP-1/CCL2 | Monocyte chemoattractant protein-1 | Cytokine—activation of monocytes, macrophages and lymphocytes | rs1024611 (c. A-2518G) | 17 | 2 | Chinese, Korean, Japanese | G allele associated with susceptibility to DR and specifically PDR in Koreans | [84–86] |
| TGF-β1 | Transforming growth factor-beta 1 | Control of cell growth, proliferation, differentiation and apoptosis | c. T869C (p. L10P) | 19 | 2 | Multi-ethnic | Potential protect factor against DR | [15, 83] |
| c. G915C (p. R25P) | 19 | 2 | Slovak | Strong risk factor for PDR | [87] | |||
| ICAM1 | Intercellular adhesion molecule 1 | Stabilization of cell–cell interactions and facilitation of leukocyte endothelial transmigration | rs13306430 | 19 | 2 | Multi-ethnic | G allele confers protection | [15, 77] |
| rs5498 (p. K469E) | 19 | 2 | Chinese, Indian, Japanese, Caucasian - Slovene | Inconsistent finding, discrepancy maybe caused by ethnicities | [55, 88–93] | |||
| SLC2A1/GLUT1 | Solute carrier family 2, member 1 | Transport of glucose across the plasma membranes | rs841846 (c. A26177G) | 1 | 1 and 2 | African American, Malaysian | Significant associations with severe DR, associated with progression of DR, not confirmed in Malaysian | [77, 94] |
| rs841853 | 1 | 1 | Malaysian, Multi-ethnic | Weak association | [77, 94] | |||
| SLC2A11 | Solute carrier family 2, member 11 | Transport of glucose across the plasma membranes | rs4822441 | 22 | 1 | African American | Associated with progression of DR | [77] |
| SLC24A3 | Solute carrier family 24, member 3 | Sodium-calcium exchanger | rs2294895 | 20 | 1 | African American | Associated with progression of DR | [77] |
| PPARγ | Peroxisome proliferator-activated receptor γ | Nuclear receptor—regulation of fatty acid storage and glucose metabolism, role in vascular permeability, inflammation, angiogenesis, neovascularization, and insulin resistance | rs1801282 (c. C34G, p. P12A) | 3 | 1 and 2 | Caucasian - Poland; Chinese, Danish, Multi-ethnic | G allele confers protection against DR in Caucasian but finding inconsistent - protective effect against only PDR during T2DM in Pakistan population, not for Asian patients | [60, 79, 95, 96] |
| rs10510419 | 3 | 1 | African American | Associated with progression of DR | [15, 95] | |||
| TCF7L2/TCF4 | Transcription factor 7-like 2 | Transcription factor for several genes (Wnt signaling pathway), vascular development | rs7903146, rs7901695, rs12255372 | 10 | 2 | Caucasian—Italian, Chinese, Multi-ethnic | Associated with DR, cardiovascular disease and coronary artery disease, rs7903146 associated with DR risk in Caucasian | [15, 79, 96–98] |
| OPG/OCIF | Osteoprotegerin/osteoclastogenesis inhibitory factor | Cytokine receptor | rs2073618, rs3134069 | 8 | 2 | Caucasian - Slovenian | CA haplotype increase risk of DR | [99] |
| PAI-1 | Plasminogen activator inhibitor-1 | Serine protease inhibitor—inhibitor of plasminogen activation, tissue repair and remodeling | rs1799768 (4G/5G INS/DEL) | 7 | 2 | Indian, Caucasian, Euro-Brazilian, Multi-ethnic, Pakistani | 4G/5G allele increases risk but finding inconsistent, ethnicity discrepancies | [28, 55, 66, 100, 101] |
| MMP-2 | Matrix metalloproteinase-2 | Breakdown of extracellular matrix | c. C-1306T | 16 | 2 | Chinese | T allele associated with PDR | [7] |
| ANGPT1 | Angiopoietin 1 | Vascular development and angiogenesis | rs1283649 | 8 | 1 | African American | Significant associations with severe DR | [77] |
| APOE | Apolipoprotein E | Transportation of lipoproteins, fat-soluble vitamins, and cholesterol | E2/E3/E4 | 19 | 1 and 2 | Mexicans, Multi-ethnic | Inconsistent finding and weak association | [102, 103] |
| BBS2 | Bardet-Biedl syndrome 2 protein | Unknown function and link to DR | rs4784675 | 16 | 1 | African American | Significant associations with severe DR | [15] |
| CPVL/CHN2 | Carboxypeptidase, vitellogenic-like; chimerin 2 | Carboxypeptidase—unknown function/regulation of a cell growth, proliferation, and migration | rs39059 | 7 | 2 | Chinese | Increases risk of DR, significant in meta-analysis | [77, 104] |
| rs1002630 | 7 | 2 | Taiwanese | Associated with DR and NPDR | [142] | |||
| CTSH | Cathepsin H | Lysosomal cysteine proteinase - degradation of lysosomal proteins, putative role in microcirculation changes | rs3825932 | 15 | 1 | Danish | T allele associated with reduced risk of progression to PDR | [60] |
| DRD2 | Dopamine receptor D2 | Dopamine receptor—regulation of vasodilatation, aldosterone production and insulin secretion | rs7131056 | 11 | 1 | African American | Significant associations with severe DR | [77] |
| EDN1 | Endothelin-1 | Vasoconstriction | rs5370 (p. K198N) | 6 | 2 | Chinese | Reduced risk in Chinese | [77, 105] |
| ENPP1 | Ectonucleotide pyrophosphatase/phosphodiesterase 1 | Insulin resistance, interaction with integrins | rs1409181 | 6 | 1 | African American | Significant associations with severe DR | [77] |
| ERBB3/HER3 | Human epidermal growth factor receptor 3 | Protein-tyrosine kinase—activation of downstream signaling pathways, unknown link to DR | rs2292239 | 12 | 1 | Danish | T allele associated with reduced risk of progression to PDR | [60] |
| FLT1/VEGFR1 | FMS-like tyrosine kinase 1/vascular endothelial growth factor receptor 1 | Protein-tyrosine kinase—control of cell proliferation and differentiation | rs622227 | 13 | 1 | African American | Associated with progression of DR | [77] |
| FRMD3 | FERM domain containing 3 | Maintaining cellular shape, putative TSG, unknown link to DR | rs10868025 | 9 | 2 | Chinese | Weak association with DR | [104] |
| HLA-B | Major histocompatibility complex, class I, B | Regulation of the immune system—presenting peptides on the cell surface | rs2523608 | 6 | 1 | African American | Significant associations with severe DR, associated with progression of DR | [77] |
| HTR1B | Serotonin receptor 1B | GPCR for serotonin—regulation of the serotonin, dopamine, and acetylcholine release, putative regulator of retinal blood flow | rs1228814 | 6 | 1 | African American | Significant associations with severe DR | [77] |
| HTRA1/ARMS2 | HtrA serine peptidase 1/age-related maculopathy susceptibility 2 | Serine protease—regulation of insulin-like growth factors, putative regulator of cell growth and neovascularization | rs11200638, rs10490924 | 10 | 2 | Indian | Marginal association with DR | [55] |
| IL-10 | Interleukin-10 | Cytokine—pleiotropic effects in immunoregulation and inflammation | n. A-1082G | 1 | 2 | Indian | G allele is risk factor for PDR | [113] |
| INSR | Insulin receptor | Activation of the insulin signaling pathway | rs10500204 | 19 | 1 | African American | Associated with progression of DR | [77] |
| ITGA2B1 | Integrin α2β1 | Cell–cell and cell-extracellular matrix interactions | RFLP - Bgl II | 5/10 | 2 | Japanese, Caucasian | Risk factor for DR | [15, 106, 143] |
| ITGB5 | Integrin β5 | Cell–cell and cell-extracellular matrix interactions | rs9865359 | 3 | 1 | African American | Associated with progression of DR | [15] |
| MTHFR | Methylenetetrahydrofolate reductase | Remethylation of homocysteine to methionine | rs1801133 (c. C677T) | 1 | 2 | Japanese, Euro-Brazilian, Multi-ethnic, Turkish | Controversial findings, T allele possible increases risk of DR because of hyperhomocysteinemia | [28, 77, 107, 108] |
| NPY | Neuropeptide Y (p. L7P) | Vasoconstriction, angiogenesis | rs16139 | 7 | 2 | Finnish | C allele increases risk but weak association | [144] |
| OLR1 | Oxidited low-density lipoprotein (lectin-like) receptor 1 | Recognition, internalization and degradation of oxidized low-density lipoprotein, putative regulator of Fas-induced apoptosis | rs2742115 | 12 | 1 | African American | Associated with progression of DR | [77] |
| PEDF/SERPINF1 | Pigment epithelium derived factor/serpin peptidase inhibitor, clade F member 1 (alpha-2 antiplasmin) | Antioxidative properties, inhibition of angiogenesis, neurotrophic factor (neuronal differentiation in retinoblastoma cells) | rs12150053, rs12948385, rs8697961, rs1126287 | 17 | 2 | Multi-ethnic | Not associated with DR | [8, 15] |
| PON1 | Paraoxonase 1 | Cellular antioxidant—inhibition of HDL oxidation | rs662 (p. Q192R) | 7 | 2 | Multi-ethnic | Inconsistent finding and weak association | [15, 109] |
| rs854560 (p. L55M) | 7 | 1 and 2 | Multi-ethnic | Associated with DR | [109] | |||
| PON2 | Paraoxonase 2 | Cellular antioxidant, hydrolytic activity—a putative role in defense responses to pathogenic bacteria | rs7493 (p. S311C) | 7 | 1 and 2 | Multi-ethnic | Inconsistent and weak association | [77] |
| s12026 (p. A148G) | 7 | 1 and 2 | Multi-ethnic | Inconsistent and weak association | [109] | |||
| PROS1 | Protein S | Cofactor for the anticoagulant protease | rs13062355 | 3 | 1 | African American | Significant associations with severe DR | [77] |
| PSMD9 | Proteasome 6S subunit, non-ATPase, 9 | Part of multicatalytic proteinase complex (proteasome) | rs74421874, rs14259, rs3825172 | 12 | 2 | Italian | Associated with DR | [110] |
| ROBO2 | Roundabout, axon guidance receptor, homologue 2 | Axon guidance and cell migration, unknown link to DR | rs10865559 | 3 | 1 | African American | Significant associations with severe DR | [77] |
| ROCK2 | Rho-associated, coiled-coil containing protein kinase 2 | Serine/threonine kinase—regulation of cytokinesis, smooth muscle contraction, the formation of actin stress fibers and focal adhesions, and the activation of the c-fos serum response element | p. T431N, p. R83K | 2 | 1 and 2 | Turkish | No association | [15, 111] |
| Romo-1 | Reactive oxygen species modulator 1 | Mitochondrial membrane protein—increase of the level of reactive oxygen species in cells | rs6060566 | 20 | 2 | Caucasian | Independent risk factor for DR | [112] |
| TF | Transferrin | Transportation iron from the intestine, reticuloendothelial system, and liver parenchymal cells to all proliferating cells in the body | rs3811647 | 3 | 1 | Associated with progression of DR | [77] | |
| TNF-α | Tumor necrosis factor-alfa | Multifunctional proinflammatory cytokine (cell proliferation, differentiation, apoptosis, lipid metabolism, and coagulation) | rs361525 (c. G-238A), rs1800629 (c. G-308A), rs1799724 (c. C-857T) | 6 | 2 | Indian, Caucasian - Brazilians | AA genotype of rs361525 confers risk for pathogenesis of PDR in Indian, rs1800629 associated with PDR in Caucasian - Brazilians | [113–115] |
| TNF-β/LTA | Tumor necrosis factor-beta (lymphotoxin-alpha) | Cytokine— inflammatory, immunostimulatory, and antiviral responses, the formation of secondary lymphoid organs, apoptosis | NcoI | 6 | 2 | Caucasian - Slovak | β2 allele is genetic factor for incidence of PDR in T2DM | [15, 116] |
| (GT)n microsatellite | 6 | 2 | Asian Indian | Allele 4 (103 bp) is a low risk for developing retinopathy, allele 8 (111 bp) is associated with PDR | [114, 115] | |||
| VDR | Vitamin D receptor | Nuclear hormone receptor for vitamin D3, associated with insulin secretion and sensitivity, anti-proliferative and anti-angiogenic effect, regulator of apoptoses | rs10735810 | 12 | 1 and 2 | Multi-ethnic | T allele increases risk but weak association | [15] |
| rs2228570 | 12 | 2 | Han Chinese | T allele increases risk of DR onset | [117] | |||
| rs1544410 | 12 | 2 | Polish, Korean | Protective effect against DR in Korean | [118, 119] |
Ch. chromoseme, Ref. references, Multi-ethnic findings of studies regardless of ethnicity or from meta-analyses, TSG tumor suppressor gene, GPCR G protein-coupled receptor
The mitochondrial aldehyde dehydrogenase 2 (ALDH2), expressed in vasculature, detoxifies reactive aldehydes formed from glucose and lipids, also prevents creation of AGE (advanced glycation end products) [32]. Morita et al. have reported a substantial relation between the ALDH2*2 allele and the incidence of DR in their study.
Growth factors with role in DR
Vascular endothelial growth factor (VEGF) is one of the major factors in angiogenesis and influences vascular permeability of endothelial cells. VEGF is activated by microvascular changes induced by hypoxia during DM and also by hyperglycaemia [120]. Activation of VEGF leads to the destruction of the blood retinal barrier (BRB), the development of diabetic macular oedema and neovascularization typical for PDR. At the same time, elevated serum and vitreous levels of VEGF have also been described in eyes of patients with PDR [121]. Anti-VEGF therapies have led to the improvement of the patients´ condition and to the deceleration of retinal vessels proliferation [122]. Studies have revealed several polymorphisms in the VEGF promoter (rs2010963, rs25648, rs1570360, rs3095039, rs35569394, rs699947, rs13207351, rs735286, rs2146323, rs833061, rs302502, rs10434, rs833068 and rs833070) with possible associations with DR [15, 33, 42, 44]. Rs2010963 (−634C/G) has been associated with DR in Japanese and Indian populations [34, 35] whereas G allele of rs2010963 has significant protective effect against NPDR in patients with T2DM. Rs2010963 is also associated with higher risk of macular oedema in Japanese population [123]. There are constantly emerging studies identifying new polymorphisms in VEGF gene with possible connections to DR which underlines importance of this gene in the development of DR.
Other growth factors with a possible function in the pathology of DR are the basic fibroblast growth factor (bFGF) and insulin-like growth factor 1 (IGF-1). The bFGF is important for tissue repair and is angiogenic factor. Studies have revealed increased level of bFGF in patients with PDR and it seems to stimulate VEGF production. IGF-1 regulates the proliferation and differentiation of several cell types. Levels of intravitreal IGF-1 were found to be significantly increased in the eyes of patients with PDR compared to those of controls [124]. Variants identified to date are summarized in the Table 1.
Erythropoietin (EPO) plays an important role in stimulation of bone marrow stem cells, erythropoiesis, proliferation, migration, and angiogenesis in hypoxic vascular endothelial cellsStudy has reported a elevated concentration of EPO in the vitreous of DM and PDR patients compared to controls [52]. There are two studies which have reported the association of rs1617640, rs507392, and rs551238 with the development of DR, but these studies report different findings. Tong et al. have determined the TTA haplotype as a risk contributor in European American population, whereas Abhary et al. have associated the GCC haplotype with DR in Australian population [52, 53].
Interaction of various growth factors, cytokines, cell signalling molecules and extracellular matrix are essential for angiogenesis during DR [125] while VEGF plays crucial part [126] (Fig. 3).
Fig. 3.
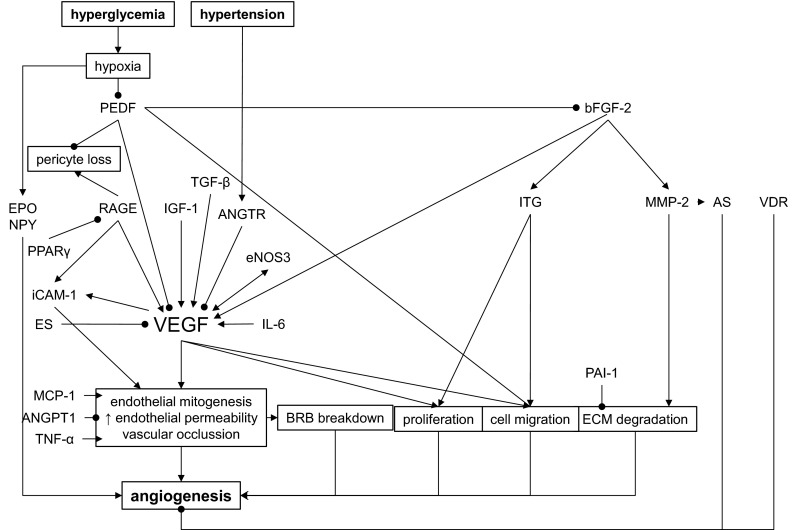
Genes harboring DNA polymorphisms involved in angiogenesis during diabetic retinopathy (DR). AS angiostatin, ES endostatin, BRB blood retinal barrier, ECM extracellular matrix, • inhibition
Receptor for advanced glycation end products and cytokines
Hyperglycaemia causes nonenzymatic glycation of proteins and lipids and the creation of AGE. Accumulation of AGE leads to tissue damage by the formation of a covalent crosslinks between proteins, which alter structure and function of proteins. Another feature of AGE is its ability to interact with different surface receptors, such as the receptor for advanced glycation end products (RAGE). RAGE is a immunoglobulin and its activation leads to cytokine secretion. Cytokines accelerate the advance of diabetic complications by supporting proinflammatory processes and increasing endothelial permeability [127]. AGEs are found in the retinal vessels of diabetic patients where their levels correlate with those in the serum as well as with severity of retinopathy [128]. The c. T–374A (rs1800624), p. Gly82Ser (rs2070600) and c. T-429C (rs1800625) polymorphisms in the RAGE gene are associated with DR in Caucasians and Asian Indians [54, 57, 129, 130], but the association was not confirmed in Chinese [131].
Dysregulation of RAAS system
The rennin-angiotensin-aldosterone system (RAAS) is an endocrine system involved in the regulation of blood pressure and fluid balance. Patients with diabetes show dysregulation of RAAS system, namely angiotensin converting enzymes I and II (ACEI, ACEII) and angiotensin receptors which are upregulated in retina during PDR independently of blood pressure [132]. ACE converts angiotensin I (ATI) to angiotensin II (ATII) which mediates its haemodynamic effects through the angiotensin receptor ANGTR1 and ANGTR2. ATII in the eye regulates promotion of capillary growth, cell growth, intraocular blood flow and pressure,, enhances vascular permeability, increases oxidative stress and via the expression of several growth factors including VEGF, IGF-1 and PDGF [15]. ACE inhibitors, angiotensin receptor blockers prevents neovascularization, reduce the incidence and progression of DR in T1DM. Studies have proposed that ACEII is also involved in PKC activation [133]. Meta-analysis suggested that ACE I/D polymorphism (insertion/deletion of a 287 bp Alu sequence in intron 16) may be associated with PDR [62].
The other polymorphisms modulating risk of DR
The retina is very sensitive to damage by oxidative stress. Oxidative stress is strongly implicated in the pathogenesis of DR, therefore the role of detoxifying enzymes, such as glutathione S-transferases (GST), was considered in the development of DR. Studies have shown that GSTT1-null genotype is found more frequently in the cases with DR in Caucasians patients with T2DM compared to controls, so the GSTT1-null genotype can be a risk factor for DR The individuals homozygous for the GSTT1-null allele had more generalized vasculopathy that leads to increased risk of sight threatening DR. In contrast, the GSTM1-null genotype may confer protection against development of DR in people with T2DM [67], but at the same time this polymorphism confers elevated risk for lung cancer [134]. There are reports that deficiency in GSTM1 leads to slower excretion of isothiocyanates. Isothiocyanates also suppress expression of VEGF which is the main inductor of retinal neovascularization in diabetes [67].
Oxidative stress induces a large amount of ROS and is assumed to damage the mitochondrial DNA. Mitochondrial manganese superoxide dismutase (MnSOD) prevents an excessive production of ROS by dismutation of superoxide radicals into hydrogen peroxide and hence defends the retinal endothelial cells from oxidative damage. The polymorphism rs4880 (c. C47T, p. A16V) affects a mitochondrial processing efficiency under oxidative stress and has been associated with DR in some studies [36, 68, 69].
Regarding PDR, presence of the 4a/4a genotype of the VNTR polymorphism for endothelial nitric oxide synthase (eNOS) has been associated with 3.4 times increased risk of PDR in Caucasian patients with T2DM [135]. In contrast, other studies have proposed that the 4a allele has a protective effect against DR [70, 71]. NO synthesized by eNOS is an endogenous vasodilator and has a role in induction of angiogenesis and regulation of VEGF expression. NO levels are significantly elevated in PDR patients relative to nondiabetic subject.
A study in 2008 revealed that retinoid-X receptor alpha (RXRA) possessess antioxidants properties and is associated with the development of DR [76]. Polymorphism rs3132300 has been linked with a progression of DR in T2DM in African American population. Also, polymorphism rs3818569 of the retinoid-X receptor gamma (RXRG) has been found to be connected with an increased DR risk in the Taiwanese population [78].
Uncoupling protein 1 (UCP 1) is the mitochondrial inner membrane electron carrier that has a part in protection against oxidative stress. It has been proposed that UCP 1 and its product play role in insulin resistance when oxidative stress pathways are activated. SNP rs1800592, which is located in the promoter of the UCP1, has been shown to be associated with glucose homeostasis, adiposity and obesity, as well as changes in the body mass index (BMI) and body weight, resulting from metabolic disorders. UCP 1 has been implicated as a candidate marker for a risk factor of DR and the rs1800592 (c. A-3826G) polymorphism has been associated with PDR [75, 79]. Uncoupling protein 2 (UCP2) regulates production of reactive oxygen species (ROS) by mitochondria. Overproduction of ROS is associated with diabetic retinopathy (DR), thereby UCP2 gene polymorphisms can be involved in the development of this complication. rs660339 can be a relevant risk factor associated with PDR in both type 2 and 1 of diabetes [77, 80].
The inflammatory processes are a major part of the DR pathophysiology. They are often regulated by inadequate activation of members of the immune system. Toll-like receptor 4 (TLR4) takes part in the activation of a pro-inflammatory response by the ligand-depended activation of the nuclear factor-κB (NF-κB) pathway. Any deregulation of TLR4 signaling due to single nucleotide polymorphisms (SNPs) in the extracellular domain of TLR4 may alter the ligand binding capacity and hence disturb the balance of pro- and anti-inflammatory cytokines [81]. It has been reported that rs4986790, rs4986791, rs10759931 and rs1927914 in TLR4 positively modulate the risk of DR [3, 81, 136].
There is increasing evidence from in vitro and in vivo studies that suggests a pathogenic role of the complement system in the development of diabetic angiopathy. In these studies, increased expression of several complement factors, namely, complement factor H (CFH), complement factor B (CFB), component 3 (C3), and component 5 (C5), has been observed in the vitreous of DR patients. CFH and CFB (an antagonist of CFH) contribute to the regulation of the activation of complement cascade. Polymorphism rs800292 (p.I62V) in CFH affects protein-binding affinity with C3b and subsequently activation of the complement alternative pathway. A synergy effect between CFH rs800292 and CFB rs1048709 conferring a significantly increased risk for DR has been identified in the study of Wang [82].
Studies have reported significantly increased levels of monocyte chemotactic protein 1 (MCP-1) in aqueous and vitreous conditions in DR patients. MCP-1 has an ability to activate monocytes, macrophages and lymphocytes. Hyperglycaemia accelerates MCP-1 production in vascular endothelial cells and retinal epithelial cells which can lead to neovascularization and increased permeability of retinal vessels typical for PDR. Moreover rs1024611 polymorphism has been associated with DR in the Japanese, Korean, and Chinese populations [84–86].
Transforming growth factor β1 (TGF-β1) has an important role in angiogenesis, endothelial cell proliferation, adhesion and the deposition of extracellular matrix. The TGF-β1 gene may be involved in the development of DR through induction of angiogenesis and BRB breakdown. C. T869C (p. L10P) polymorphism has been associated with a protective effect against DR [15, 83].
Intercellular adhesion molecule-1 (ICAM-1) has a major role in mediating the adhesion of circulating leukocytes to the blood vessel wall and transendothelial migration to the vascular intima. The increased retinal expression of ICAM-1 is thought to play a key role in leukostasis-mediated BRB breakdown, capillary occlusion and endothelial cell damage in DR [88]. Polymorphisms of ICAM-1 gene might have a role in modulation of its own gene expression but findings about p. K469E polymorphism (rs5498) are inconsistent across multiple studies [55, 88–91]. The G allele of rs13306430 could confer protection against DR in T2DM patients [15].
Solute carrier family 2(SLC2A1), also known as facilitated glucose transporter (GLUT1) is expressed in endothelial cells of the BRB where SLC2A1 is the prevalent glucose transporter. Functional loss of BRB is typical for DR and studies have shown that patients with DR have high expression of GLUT1 in endothelial cells. SLC2A1 c. A26177G polymorphism has been associated with DR in a study of Ng [94].
The role of peroxisome proliferator-activated receptor γ (PPARγ) in DR pathogenesis has come to forefront mainly because of the protein’s role in vascular permeability, inflammation, angiogenesis, neovascularization, and insulin resistance, all of which contribute to the onset and severity of DR. However, the studies describing associations of PPARγ polymorphisms and DR have been inconsistent [15, 95].
Transcription factor 7-like 2 (TCF7L2/TCF4) is a key component in the regulation of fundamental processes such as vascular development. It has been found to mediate pathological neovascularization in PDR. Common variant rs7903146 in TCF7L2 has been reported to be strongly associated with T2DM and also with PDR in Caucasian [96]. An Italian study observed associations between TCF7L2 variants (rs7903146, rs7901695 and rs12255372) and DR, cardiovascular disease and coronary artery disease [97].
Osteoprotegerin (OPG), also called the osteoclastogenesis inhibitory factor (OCIF), is an important regulatory molecule in the vasculature. Rs2073618, rs3134069 polymorphisms have been linked with DR [99].
Plasminogen activator inhibitor-1 (PAI-1) is an inhibitor of plasminogen activation and is involved in tissue repair and remodeling. PAI-1 plays a crucial part in the regulation of intravascular fibrinolysis which is part of DR pathophysiology. Studies have investigated the connection between PAI-1 4G/5G and DR risk but findings have been inconsistent, maybe due to ethnicity discrepancies [15, 100, 101].
Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade extracellular matrix (ECM) components. MMPs also regulate cell proliferation, neovasculogenesis and tissue remodelling because degradation of the extracellular matrix (ECM) proteins of the basement membrane is necessary for endothelial cells to migrate, proliferate, and to form capillaries. Increased expression of MMP-2 may expedite degradation of the type IV collagen and the gap junction protein, accelerating the vascular complications of diabetes. It has been reported that c. C-1306T polymorphism seems to be genetic susceptibility factor for the development of DR [7].
In this review, we discussed candidate genes and polymorphisms with the highest genetic association with DR, or those most frequently analysed in studies in different population. The other genes and their polymorphisms associated with DR are summarized in Table 1 in alphabetical order. Studies concerning these genes have reported very weak or borderline associations, had small sample sizes and most of them have failed replication in other populations [137]. We did not include studies and polymorphisms that have been done on only one population and showed no associations with DR. None of the polymorphisms identified by candidate gene studies have achieved widespread acceptance as a marker of high risk of diabetic retinopathy. In part, this may be because of the complexity of DR which probably has more multifactorial, polygenic and environmental contributors to its pathophysiology.
In our review, we have included a number of genes in which multiple alleles or nearby SNPs have varying strengths of association or significance in relation to diabetic retinopathy. Possible explanation for this is variable association between the SNPs themselves with the causative change depended also on populations.
Another one of the possible reasons for the setback of the gene candidate approach is focusing on single SNPs when the linkage disequilibrium does not have to manifest [16]. It would be potentially useful to focus on haplotypes associated with DR instead of solely on SNPs. In addition, insufficient sample sizes to detect the modest effect of polymorphisms, incomplete coverage of variation in candidate genes and incorrect hypotheses about genes in the pathophysiology of DR are some of the reasons for controversial success of candidate genes studies. Based on these weaknesses of standard candidate gene studies, two recent studies have examined a higher number of candidate genes for DR in sample sizes larger than those used previously, in an approach that mimics a genome-wide approach [77, 138]. The first study, which examined 193 candidate genes with DR of type 1 diabetic African-Americans, found genetic associations in 13 genes with progression of DR and the polymorphisms are listed in Table 1 [77]. Identified genes are involved in pathways related to glucose metabolism, inflammatory processes, angiogenesis/vascular permeability, insulin signalling, retinal development, or blood pressure regulation. The second study, the Candidate gene Association Resource (CARe) has not confirmed connection between previously associated gene from numerous previous independent studies and DR [138]. The most interesting findings from this study are the variants in the P-selectin (SELP) (rs6128) and in the iduronidase (rs6856425) that have shown to be significantly linked with DR in European Americans, but were not seen in African-Americans, Hispanic Americans, or Asian Americans.
It is possible that the potential success of candidate gene studies lies in better characterization and definition of clinical phenotypes of DR, represented by specific patterns of severity and progression of DR. Only then, studies of candidate genes are worth pursuing, involving appropriately well-defined subgroups of patients [139]. Furthermore, it would be worthwhile to expand studies to those genes mutations in which are known to initiate hyperglycaemia [140] and indirectly lead to development of DM and DR.
Conclusion
DR remains to be one of the most complex, heterogeneous, multifactorial disorders in any genetic studies. It is one of the leading causes of blindness and visual impairment in the world and treatments options are limited. Research worldwide is focused on understanding the pathogenic mechanisms in DR with the key goal to prevent this disease and developing new drugs for treatments. There is a common consensus that the susceptibility to DR is contingent to a great amount of relatively common allelic variants with a modest effect, and how these genes interact among themselves and with environmental influences. Each of the allelic variants increased risk of DR by a small portion in overall susceptibility. The identification of genetic susceptibility loci for DR by genetic studies has not proved notably successful thus far, given the often contradictory and inconclusive results. It is obvious that the study of the DR genetics is still poorly developed and stands against numerous challenges. The most common approaches in studying this complex disease are insufficient for elucidating pathology of DR. At the same time, most studies of new possible treatments disregarded genetic background of the patietns, which could contribute to the treatment setback. Possible new NextGen sequencing methods and approaches based on interconnection of various omics, such as genomics, especially pharmacogenetics, transcriptomics, proteomics and metabolomics, will bring new breakthrough findings in the future. At this time, we can surely state that there is a long way ahead to fully understand this complex disease.
Acknowledgments
The authors acknowledge the funding provided by Research and Development Operational Programme ITMS 26240120038, 2013/4.1/04-SORO.
References
- 1.Petrovič D. Candidate genes for proliferative diabetic retinopathy. Biomed Res Int. 2013;2013:540416. doi: 10.1155/2013/540416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Dieren S, Beulens JW, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic. Eur J Cardiovasc Prev Rehabil. 2010;17(1):S3–S8. doi: 10.1097/01.hjr.0000368191.86614.5a. [DOI] [PubMed] [Google Scholar]
- 3.Singh K, Kant S, Singh VK, Agrawal NK, Gupta SK, Singh K. Toll-like receptor 4 polymorphisms and their haplotypes modulate the risk of developing diabetic retinopathy in type 2 diabetes patients. Mol Vis. 2014;20:704–713. [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122:1631–1640. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 5.Lim LS, Wong TY. Lipids and diabetic retinopathy. Expert Opin Biol Ther. 2012;12:93–105. doi: 10.1517/14712598.2012.641531. [DOI] [PubMed] [Google Scholar]
- 6.Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Fan XH, Guan YQ, Li Y, Sun W, Yang XZ, et al. MMP-2 gene polymorphisms in type 2 diabetes mellitus diabetic retinopathy. Int J Ophthalmol. 2010;3:137–140. doi: 10.3980/j.issn.2222-3959.2010.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304:649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong TY, Liew G, Tapp RJ, Schmidt MI, Wang JJ, Mitchell P, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet. 2008;371:736–743. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo JZ, Wong TY, Rotter JI. Challenges in elucidating the genetics of diabetic retinopathy. JAMA Ophthalmol. 2014;132:96–107. doi: 10.1001/jamaophthalmol.2013.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leslie RD, Pyke DA. Diabetic retinopathy in identical twins. Diabetes. 1982;31:19–21. doi: 10.2337/diab.31.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Looker HC, Nelson RG, Chew E, Klein R, Klein BE, Knowler WC, et al. Genome-wide linkage analyses to identify loci for diabetic retinopathy. Diabetes. 2007;56:1160–1166. doi: 10.2337/db06-1299. [DOI] [PubMed] [Google Scholar]
- 14.Kinoshita JH. A thirty year journey in the polyol pathway. Exp Eye Res. 1990;50:567–573. doi: 10.1016/0014-4835(90)90096-d. [DOI] [PubMed] [Google Scholar]
- 15.Abhary S, Hewitt AW, Burdon KP, Craig JE. A systematic meta-analysis of genetic association studies for diabetic retinopathy. Diabetes. 2009;58:2137–2147. doi: 10.2337/db09-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng DPK. Human genetics of diabetic retinopathy: current perspectives. J Ophthalmol. 2010 doi: 10.1155/2010/172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2009;298:902–916. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 18.Amano S, Yamagishi SI, Koda Y, Tsuneoka M, Soejima M, Okamoto T, et al. Polymorphisms of sorbitol dehydrogenase (SDH) gene and susceptibility to diabetic retinopathy. Med Hypotheses. 2003;60:550–551. doi: 10.1016/s0306-9877(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 19.Szaflik JP, Majsterek I, Kowalski M, Rusin P, Sobezuk A, Borucka AI, Szaflik J, Blasiak J. Association between sorbitol dehydrogenase gene polymorphisms and type 2 diabetic retinopathy. Exp Eye Res. 2008;86:647–652. doi: 10.1016/j.exer.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Ko BC, Lam KS, Wat NM, Chung SS. An (A-C)n dinucleotide repeat polymorphic marker at the 5′ end of the aldose reductase gene is associated with early-onset diabetic retinopathy in NIDDM patients. Diabetes. 1995;44:727–732. doi: 10.2337/diabetes.44.7.727. [DOI] [PubMed] [Google Scholar]
- 21.Fujisawa T, Ikegami H, Kawaguchi Y, Yamato E, Nakagawa Y, Shen GQ, et al. Length rather than a specific allele of dinucleotide repeat in the 5′ upstream region of the aldose reductase gene is associated with diabetic retinopathy. Diabet Med. 1999;16:1044–1047. doi: 10.1046/j.1464-5491.1999.00192.x. [DOI] [PubMed] [Google Scholar]
- 22.Ichikawa F, Yamada K, Ishiyama-Shigemoto S, Yuan X, Nonaka K. Association of an (A-C)n dinucleotide repeat polymorphic marker at the 5′-region of the aldose reductase gene with retinopathy but not with nephropathy or neuropathy in Japanese patients with type 2 diabetes mellitus. Diabet Med. 1999;16:744–748. doi: 10.1046/j.1464-5491.1999.00155.x. [DOI] [PubMed] [Google Scholar]
- 23.Ikegishi Y, Tawata M, Aida K, Onaya T. Z-4 allele upstream of the aldose reductase gene is associated with proliferative retinopathy in Japanese patients with NIDDM, and elevated luciferase gene transcription in vitro. Life Sci. 1999;65:2061–2070. doi: 10.1016/s0024-3205(99)00329-x. [DOI] [PubMed] [Google Scholar]
- 24.Lee SC, Wang Y, Ko GT, Critchley JA, Ng MC, Tong PC, et al. Association of retinopathy with a microsatellite at 5′ end of the aldose reductase gene in Chinese patients with lateonset type 2 diabetes. Ophthalmic Genet. 2001;22:63–67. doi: 10.1076/opge.22.2.63.2230. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Ng MC, Lee SC, So WY, Tong PC, Cockram CS, et al. Phenotypic heterogeneity and associations of two aldose reductase gene polymorphisms with nephropathy and retinopathy in type 2 diabetes. Diabetes Care. 2003;26:2410–2415. doi: 10.2337/diacare.26.8.2410. [DOI] [PubMed] [Google Scholar]
- 26.Abhary S, Burdon KP, Laurie KJ, Thorpe S, Landers J, Goold L, et al. Aldose reductase gene polymorphisms and diabetic retinopathy susceptibility. Diabetes Care. 2010;33:1834–1836. doi: 10.2337/dc09-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richeti F, Noronha RM, Waetge RT, de Vasconcellos JP, de Souza OF, Kneipp B, Assis N, Rocha MN, Calliari LE, Longui CA, Monte O, de Melo MB. Evaluation of AC(n) and C(–106)T polymorphisms of the aldose reductase gene in Brazilian patients with DM1 and susceptibility to diabetic retinopathy. Mol Vis. 2007;13:740–745. [PMC free article] [PubMed] [Google Scholar]
- 28.Santos KG, Tschiedel B, Schneider J, SOuto K, Roisenberg I. Diabetic retinopathy in Euro-Brazilian type 2 diabetic patients: relationship with polymorphisms in the aldose reductase, the plasminogen activator inhibitor-1 and the methylenetetrahydrofolate reductase genes. Diabetes Res Clin Pract. 2003;61:133–136. doi: 10.1016/s0168-8227(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 29.Katakami N, Kaneto H, Takahara M, Matsuoka TA, Imamura K, Ishibashi F, et al. Aldose reductase C-106 T gene polymorphism is associated with diabetic retinopathy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2011;92:e57–e60. doi: 10.1016/j.diabres.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Song ZD, Tao Y, Han N, Wu YZ. Association of the aldose reductase-106TT genotype with increased risk for diabetic retinopathy in the Chinese han population: an updated meta-analysis. Curr Eye Res. 2015;18:1–5. doi: 10.3109/02713683.2015.1084642. [DOI] [PubMed] [Google Scholar]
- 31.Ferreira FN, Crispim D, Canani LH, Gross JL, dos Santos KG. Association study of sorbitol dehydrogenase–888G >C polymorphism with type 2 diabetic retinopathy in Caucasian-Brazilians. Exp Eye Res. 2013;115:140–143. doi: 10.1016/j.exer.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 32.Morita K, Saruwatari J, Miyagawa H, Uchiyashiki Y, Oniki K, Sakata M, Kajiwara A, Yoshida A, Jinnouchi H, Nakagawa K. Association between aldehyde dehydrogenase 2 polymorphisms and the incidence of diabetic retinopathy among Japanese subjects with type 2 diabetes mellitus. Cardiovasc Diabetol. 2013;12:132. doi: 10.1186/1475-2840-12-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abhary S, Burdon KP, Gupta A, et al. Common sequence variation in the VEGFA gene predicts risk of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:5552–5558. doi: 10.1167/iovs.09-3694. [DOI] [PubMed] [Google Scholar]
- 34.Awata T, Inoue K, Kurihara S, Ohkubo T, Watanabe M, Inukai K, et al. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes. 2002;51:1635–1639. doi: 10.2337/diabetes.51.5.1635. [DOI] [PubMed] [Google Scholar]
- 35.Uthra S, Raman R, Mukesh BN, Rajkumar SA, Padmaja KR, Paul PG, et al. Association of VEGF gene polymorphisms with diabetic retinopathy in a south Indian cohort. Ophthalmic Genet. 2008;29:11–15. doi: 10.1080/13816810701663527. [DOI] [PubMed] [Google Scholar]
- 36.Kangas-Kontio T, Vavuli S, Kakko SJ, Penna J, Savolainen ER, Liinamaa Polymorphism of the manganese superoxide dismutase gene but not of vascular endothelial growth factor gene is a risk factor for diabetic retinopathy. Br J Ophthalmol. 2009;93:14011406. doi: 10.1136/bjo.2009.159012. [DOI] [PubMed] [Google Scholar]
- 37.Suganthalakshmi B, Anand R, Kim R, Mahalakshmi R, Karthiprakash S, Namperumalsamy P, et al. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol Vis. 2006;12:336–341. [PubMed] [Google Scholar]
- 38.Buraczynska M, Ksiazek P, Baranowicz-Gaszczyk I, Jozwiak L. Association of the VEGF gene polymorphism with diabetic retinopathy in type 2 diabetes patients. Nephrol Dial Transplant. 2007;22:827–832. doi: 10.1093/ndt/gfl641. [DOI] [PubMed] [Google Scholar]
- 39.Szaflik JP, Wysocki T, Kowalski M, Majsterek I, Borucka AI, Blasiak J, Szaflik J. An association between vascular endothelial growth factor gene promoter polymorphisms and diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2008;246:39–43. doi: 10.1007/s00417-007-0674-6. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura S, Iwasaki N, Funatsu H, Kitano S, Iwamoto Y. Impact of variants in the VEGF gene on progression of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2009;247:21–26. doi: 10.1007/s00417-008-0915-3. [DOI] [PubMed] [Google Scholar]
- 41.Qiu M, Xiong W, Liao H, Li F. VEGF–634G >C polymorphism and diabetic retinopathy risk: a meta-analysis. Gene. 2013;518:310–315. doi: 10.1016/j.gene.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Churchill AJ, Carter JG, Ramsden C, Turner SJ, Yeung A, Brenchley PE, et al. VEGF polymorphisms are associated with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3611–3616. doi: 10.1167/iovs.07-1383. [DOI] [PubMed] [Google Scholar]
- 43.Gong JY, Sun YH. Association of VEGF gene polymorphisms with diabetic retinopathy: a meta-analysis. PloS One. 2013;8:84069. doi: 10.1371/journal.pone.0084069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakanishi K, Watanabe C. Single nucleotide polymorphisms of vascular endothelial growth factor gene intron 2 are markers for early progression of diabetic retinopathy in Japanese with type 1 diabetes. Clin Chim Acta. 2009;402:171–175. doi: 10.1016/j.cca.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Chun MY, Hwang HS, Cho HY, Chun HJ, Woo JT, Lee KW, et al. Association of vascular endothelial growth factor polymorphisms with nonproliferative and proliferative diabetic retinpathy. J Clin Endocrinol Metab. 2010;95:3547–3551. doi: 10.1210/jc.2009-2719. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Deng Y, Gu H, Lim A, Altankhuyag A, Jia W, et al. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol Vis. 2011;17:3088–3096. [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng Y, Dai F, Yang K, Tang Y, Xu M, Zhou Y. Association between a vascular endothelial growth factor gene polymorphism (rs2146323) and diabetic retinopathy: a meta-analysis. BMC Ophthalmol. 2015;15:163. doi: 10.1186/s12886-015-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porojan MD, Cătană A, Popp RA, Dumitrascu DL, Bala C. The role of NOS2A–954G/C and vascular endothelial growth factor +936C/T polymorphisms in type 2 diabetes mellitus and diabetic nonproliferative retinopathy risk management. Ther Clin Risk Manag. 2015;11:1743–1748. doi: 10.2147/TCRM.S93172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beránek M, Kolar P, Tschoplova S, Kankova K, Vasku A. Genetic variation and plasma level of the basis fibroblast growth factor in proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2008;79:362–367. doi: 10.1016/j.diabres.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Petrovič MG, Krkovič M, Osredkar J, Hawlina M, Petrovič D. Polymorphisms in the promoter region of the basic fibroblast growth factor gene and proliferative diabetic retinopathy in Caucasians with type 2 diabetes. Clin Experiment Ophthalmol. 2008;36:168–172. doi: 10.1111/j.1442-9071.2007.01647.x. [DOI] [PubMed] [Google Scholar]
- 51.Kytnarova J, Vesela K, Zlatohlavkova B, Dohnalova A, Fedorova M, Krsek M, et al. Cytosine-adenosine (CA)n repeats polymorphim in IGF-I gene and early growth in infants born appropriate and small for gestational age. Neuro Endocrinol Lett. 2009;30:501–505. [PubMed] [Google Scholar]
- 52.Abhary S, Burdon KP, Casson RJ, Goggin M, Petrovsky NP, Craig JE. Association between erythropoietin gene polymorphisms and diabetic retinopathy. Arch Ophthalmol. 2010;128:102–106. doi: 10.1001/archophthalmol.2009.355. [DOI] [PubMed] [Google Scholar]
- 53.Tong Z, Yang Z, Patel S, Chen H, Gibbs D, Yang X, et al. Promoter polymorphism of the erythropoietin gene in severe diabetic eye and kidney complications. Proc Natl Acad Sci USA. 2008;105:6998–7003. doi: 10.1073/pnas.0800454105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hudson BI, Stickland MH, Futers TS, Grant PJ. Effects of novel polymorphisms in the RAGE gene on transcriptional regulation and their association with diabetic retinopathy. Diabetes. 2001;50:1505–1511. doi: 10.2337/diabetes.50.6.1505. [DOI] [PubMed] [Google Scholar]
- 55.Balasubbu S, Sundaresan P, Rajendran A, Ramasamy K, Govindarajan G, Perumalsamy N, et al. Association analysis of nine candidate gene polymorphisms in Indian patients with type 2 diabetic retinopathy. BMC Med Genet. 2010;11:158. doi: 10.1186/1471-2350-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vanita V. Association of RAGE (p.Gly82Ser) and MnSOD (p.Val16Ala) polymorphisms with diabetic retinopathy in T2DM patients from north India. Diabetes Res Clin Pract. 2014;104:155–162. doi: 10.1016/j.diabres.2013.12.059. [DOI] [PubMed] [Google Scholar]
- 57.Kumaramanickavel G, Ramprasad VL, Sripriya S, Upadyay NK, Paul PG, Sharma T. Association of Gly82Ser polymorphism in the RAGE gene with diabetic retinopathy in type II diabetic Asian Indian patients. J Diabetes Complications. 2002;16:391–394. doi: 10.1016/s1056-8727(02)00187-3. [DOI] [PubMed] [Google Scholar]
- 58.Yang L, Wu Q, Li Y, Fan X, Hao Y, Sun H, et al. Association of the receptor for advanced glycation end products gene polymorphisms and circulating RAGE levels with diabetic retinopathy in Chinese population. J Diabetes Res. 2013;2013:264579. doi: 10.1155/2013/264579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Globocnik Petrovic M, Steblovnik K, Peterlin B, Petrovic D. The –429T/C and –374T/A gene polymorphisms of the receptor of advanced glycation end products gene are not risk factors for diabetic retinopathy in Caucasians with type 2 diabetes. Klin Monbl Augenheilkd. 2003;220:873–876. doi: 10.1055/s-2003-812553. [DOI] [PubMed] [Google Scholar]
- 60.Thorsen SU, Sandahl K, Nielsen LB, Broe R, Rasmussen ML, Peto T, Grauslund J, Andersen ML, Mortensen HB, Pociot F, Olsen BS, Brorsson C. Polymorphisms in the CTSH gene may influence the progression of diabetic retinopathy: a candidate-gene study in the Danish Cohort of Pediatric Diabetes 1987 (DCPD1987) Graefes Arch Clin Exp Ophthalmol. 2015;253:1959–1965. doi: 10.1007/s00417-015-3118-8. [DOI] [PubMed] [Google Scholar]
- 61.Ng ZX, Kuppusamy UR, Poh R, Tajunisah I, Koay A, Fong K, et al. Lack of association between Gly82Ser, 1704G/T and 2184A/G RAGE gene polymorphisms and retinopathy susceptibility in Malaysian diabetic patients. Genet Mol Res. 2012;11:455–461. doi: 10.4238/2012.March.1.2. [DOI] [PubMed] [Google Scholar]
- 62.Zhou JB, Yang JK. Angiotensin-converting enzyme gene polymorphism is associated with proliferative diabetic retinopathy: a meta-analysis. Acta Diabetol Suppl. 2010;1:187–193. doi: 10.1007/s00592-009-0160-1. [DOI] [PubMed] [Google Scholar]
- 63.Lu Y, Ge Y, Hu Q, Shi Y, Xue C, Shi Y, et al. Association between angiotensin-converting enzyme gene polymorphism and diabetic retinopathy in the Chinese population. J Renin Angiotensin Aldosterone Syst. 2012;13:289–295. doi: 10.1177/1470320311432187. [DOI] [PubMed] [Google Scholar]
- 64.Liang S, Pan M, Hu N, Wu YY, Chen H, Zhu J, et al. Association of angiotensin-converting enzyme gene 2350 G/A polymorphism with diabetic retinopathy in Chinese Han population. Mol Biol Rep. 2013;40:463–468. doi: 10.1007/s11033-012-2081-2. [DOI] [PubMed] [Google Scholar]
- 65.Nikzamir A, Rashidi A, Esteghamati A, Nakhjavani M, Golmohammadi T, Khalilzadeh O. The relationship between ACE gene insertion/deletion polymorphism and diabetic retinopathy in Iranian patients with type 2 diabetes. Ophthalmic Genet. 2010;31:108–113. doi: 10.3109/13816810.2010.482554. [DOI] [PubMed] [Google Scholar]
- 66.Saleem S, Azam A, Maqsood SI, Muslim I, Bashir S, Fazal N, et al. Role of ACE and PAI-1 Polymorphisms in the Development and Progression of Diabetic Retinopathy. PLoS One. 2015;10:e0144557. doi: 10.1371/journal.pone.0144557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cilenšek I, Mankoč S, Petrovič MG, Petrovič D. GSTT1 null genotype is a risk factor for diabetic retinopathy in Caucasians with type 2 diabetes, whereas GSTM1 null genotype might confer protection against retinopathy. Dis Markers. 2012;32:93–99. doi: 10.3233/DMA-2011-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrovič MG, Cilenšek I, Petrovič D. Manganese superoxide dismutase gene polymorphism (V16A) is associated with diabetic retinopathy in Slovene (Caucasians) type 2 diabetes patients. Dis Markers. 2008;24:59–64. doi: 10.1155/2008/940703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tian C, Fang S, Du X, Jia C. Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: a meta-analysis. Diabetologia. 2011;54:803–811. doi: 10.1007/s00125-010-2004-5. [DOI] [PubMed] [Google Scholar]
- 70.Cheema BS, Kohli HS, Sharma R, Bhansali A, Khullar M. Endothelial nitric oxide synthase gene polymorphism and type 2 diabetic retinopathy among Asians Indians. Acta Diabetol. 2012;49:481–488. doi: 10.1007/s00592-012-0437-7. [DOI] [PubMed] [Google Scholar]
- 71.Zhao S, Li T, Zheng B, Zheng Z. Nitric oxide synthase 3 (NOS3) 4b/a, T-786C and G894T polymorphisms in association with diabetic retinopathy susceptibility: a meta-analysis. Ophthalmic Genet. 2012;33:200–2007. doi: 10.3109/13816810.2012.675398. [DOI] [PubMed] [Google Scholar]
- 72.Santos KG, Crispim D, Canani LH, Ferrugem PT, Gross JL, Roisenberg I. Relationship of endothelial nitric oxide synthase (eNOS) gene polymorphisms with diabetic retinopathy in Caucasians with type 2 diabetes. Ophtalmic Genet. 2012;33:23–27. doi: 10.3109/13816810.2011.620057. [DOI] [PubMed] [Google Scholar]
- 73.Chen Y, Huang H, Zhou JM, Doumatey A, Lashley K, Chen G, et al. Polymorphism of the endothelial nitric oxide synthase gene is associated with diabetic retinopathy in a cohort of West Africans. Mol Vis. 2007;26:2142–2147. [PubMed] [Google Scholar]
- 74.Bazzaz JT, Amoli MM, Pravica V, Chandrasecaran R, Boulton AJ, Larijani B, Hutchinson IV. eNOS gene polymorphism association with retinopathy in type 1 diabetes. Ophthalmic Genet. 2010;31:103–107. doi: 10.3109/13816810.2010.482553. [DOI] [PubMed] [Google Scholar]
- 75.Brondani LA, de Souza BM, Duarte GCK, Kliemann LM, Esteves JF, Marcon AS, et al. The UCP1–3826A/G polymorphism is associated with diabetic retinopathy and increased UCP1 and MnSOD2 gene expression in human retina. Invest Ophthalmol Vis Sci. 2012;53:7449–7457. doi: 10.1167/iovs.12-10660. [DOI] [PubMed] [Google Scholar]
- 76.Chai D, Wang B, Shen L, Pu J, Zhang XK, He B. RXR agonists inhibit high-glucose-induced oxidative stress by repressing PKC activity in human endothelial cells. Free Radic Biol Med. 2008;44:1334–1347. doi: 10.1016/j.freeradbiomed.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 77.Roy M, Hallman M, Fu Y, Machado M, Hanis CL. Assesment of 193 candidate genes for retinopathy in African Americans with type 1 diabetes. Arch Ophthalmol. 2009;127:605–612. doi: 10.1001/archophthalmol.2009.48. [DOI] [PubMed] [Google Scholar]
- 78.Hsieh CH, Pei D, Hung YJ, Hsiao FC. Association between retinoid-X receptor-gamma genetic polymorphisms and diabetic retnopathy. Genet Mol Res. 2011;10:3545–3551. doi: 10.4238/2011.December.5.4. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Y, Meng N, Lv Z, Li H, Qu Y. The gene polymorphisms of UCP1 but not PPARγ and TCF7L2 are associated with diabetic retinopathy in Chinese type 2 diabetes mellitus cases. Acta Ophthalmol. 2015;93:e223–e229. doi: 10.1111/aos.12542. [DOI] [PubMed] [Google Scholar]
- 80.Crispim D, Faqundes NJ, dos Santos KG, Rheinheimer J, Bouças AP, de Souza BM, et al. Polymorphisms of the UCP2 gene are associated with proliferative diabetic retinopathy in patients with diabetes mellitus. Clin Endocrinol. 2010;72:612–619. doi: 10.1111/j.1365-2265.2009.03684.x. [DOI] [PubMed] [Google Scholar]
- 81.Buraczynska M, Baranowicz-Gaszczyk I, Tarach J, Ksiazek A. Toll-like receptor 4 gene polymorphism and early onset of diabetic retinopathy in patients with type 2 diabetes. Hum Immunol. 2009;70:121–124. doi: 10.1016/j.humimm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 82.Wang J, Yang MM, Li YB, Liu GD, Teng Y, Liu XM. Association of CFH and CFB gene polymorphisms with retinopathy in type 2 diabetic patients. Mediators Inflamm. 2013;2013:748435. doi: 10.1155/2013/748435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu L, Jiao J, Wang Y, Wu J, Huang D, Teng W, et al. TGF-beta1 gene polymorphism in association with diabetic retinopathy susceptibility: a systematic review and meta-analysis. PLoS One. 2014;9:e94160. doi: 10.1371/journal.pone.0094160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Imamura K, Ishibashi F, et al. Monocyte chemoattractant protein-1 (MCP-1) gene polymorphism as a potentional risk factor for diabetic retinopathy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;89:e9–e12. doi: 10.1016/j.diabres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Jeon HJ, Choi HJ, Park BH, Lee YH, Oh T. Association on monocyte chemoattractant protein-1(MCP-1) 2518A/G with proliferative diabetic retinopathy in Korean type 2 diabetes. Yonsei Med J. 2013;54:621–625. doi: 10.3349/ymj.2013.54.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dong L, Iv XY, Wang BJ, Wang YQ, Mu H, Feng ZL, et al. Association of monocyte chemoattractant protein-1 (MCP-1) 2518A/G polymorphism with proliferative diabetic retinopathy in nirthern Chinese type 2 diabetes. Graefes Arch Clin Exp Ophtalmol. 2014;252:1921–1926. doi: 10.1007/s00417-014-2651-1. [DOI] [PubMed] [Google Scholar]
- 87.Beránek M, Kanková K, Benes P, Izakovicová-Hollá L, Znojil V, Hájek D, et al. Polymorphism R25P in the gene encoding transforming growth factor-beta (TGF-beta1) is a newly identified risk factor for proliferative diabetic retinopathy. Am J Med Genet. 2002;109:278–283. doi: 10.1002/ajmg.10372. [DOI] [PubMed] [Google Scholar]
- 88.Sun H, Cong X, Sun R, Wang C, Wang X, Liu Y. Association between the ICAM-1K469E polymorphism and diabetic retinopathy in type 2 diabetes mellitus: a meta-analysis. Diabetes Res Clin Pract. 2014;104:46–49. doi: 10.1016/j.diabres.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 89.Liu L, Yu Q, Wang H, Zhang SX, Huang C, Chen X. Association of intercellular adhesion molecule 1 polymorphisms with retinopathy in Chinese patients with type 2 diabetes. Diabet Med. 2006;23:643–648. doi: 10.1111/j.1464-5491.2006.01884.x. [DOI] [PubMed] [Google Scholar]
- 90.Petrovič MG, Osredkar J, Saraga-Babić M, Petrovič D. K469E polymorphism of the intracellular adhesion molecule 1 gene is associated with proliferative diabetic retinopathy in Caucasians with type 2 diabetes. Clin Experiment Ophthalmol. 2008;36:468–472. doi: 10.1111/j.1442-9071.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 91.Zhu J, Gao SH, Chi XD, Zhang YS, Lai XZ, Zhong WL. Relationship between diabetic retinopathy and K469E gene polymorphism of intracellular adhesion molecule-1. J Fujian Med Univ. 2010;44:190–193. [Google Scholar]
- 92.Su X, Chen X, Lui L, Chang X, Yu X, Sun K. Intracellular adhesion molecule-1K469E gene polymorphim and risk of diabetic microvascular complications: a meta-analysis. PloS One. 2013;8:e69940. doi: 10.1371/journal.pone.0069940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lv Z, Li Y, Wu Y, Qu Y. Association of ICAM-1 and HMGA1 gene variants with retinopathy in type 2 diabetes mellitus among Chinese individuals. Curr Eye Res. 2015;30:1–5. doi: 10.3109/02713683.2015.1094093. [DOI] [PubMed] [Google Scholar]
- 94.Ng ZX, Kuppusamy UR, Tajunisah I, Fong KCS, Chua KH. Investigation of SLC2A1 26177A/G gene polymorphism via high resolution melting curve analysis in Malaysian patients with diabetic retinopathy. J Diabetes Complications. 2012;26:388–392. doi: 10.1016/j.jdiacomp.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 95.Tariq K, Malik SB, Ali SH, Maqsood SE, Azam A, Muslim I, et al. Association of Pro12Ala polymorphism in peroxisome proliferator activated receptor gamma with proliferative diabetic retinopathy. Mol Vis. 2013;19:710–717. [PMC free article] [PubMed] [Google Scholar]
- 96.Luo J, Zhao L, Chen AY, Zhang X, Zhu J, Zhao J, et al. TCF7L2 variation and proliferative diabetic retinopathy. Diabetes. 2013;62(7):2613–2617. doi: 10.2337/db12-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ciccacci C, Di Fusco D, Cacciotti L, Morganti R, D´Amato C, Novelli G, et al. TCF7L2 gene polymorphisms and type 2 diabetes: association with diabetic retinopathy and cardiovascular autonomic neuropathy. Acta Diabetol. 2012;50(5):789–799. doi: 10.1007/s00592-012-0418-x. [DOI] [PubMed] [Google Scholar]
- 98.Ding Y, Hu Z, Yuan S, Xie P, Liu Q. Association between transcription factor 7-like 2 rs7903146 polymorphism and diabetic retinopathy in type 2 diabetes mellitus: a meta-analysis. Diab Vasc Dis Res. 2015;12:436–444. doi: 10.1177/1479164115598274. [DOI] [PubMed] [Google Scholar]
- 99.Ramuš SM, Kumše T, Petrovič MG, Petrovič D, Cilenšek I. SNP 2073618 of the osteoprotegerin gene is associated with diabetic retinopathy in Slovenian patients with type 2 diabetes. Biomed Research Internat. 2013 doi: 10.1155/2013/364073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagi DK, McCormack LJ, Mohamed-Ali V, Yudkin JS, Knowler WC, Grant PJ. Diabetic retinopathy, promoter (4G/5G) polymorphism of PAI-1 gene, and PAI-1 activity in Pima Indians with type 2 diabetes. Diabetes Care. 1997;20(8):1304–1309. doi: 10.2337/diacare.20.8.1304. [DOI] [PubMed] [Google Scholar]
- 101.Zhang T, Pang C, Li N, Zhou E, Zhao K. Plasminogen activator inhibitor-1 4G/5G polymorphism and retinopathy risk in type 2 diabetes: a meta-analysis. BMC Med. 2013 doi: 10.1186/1741-7015-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Santos A, Salguero ML, Gurrola C, Munoz F, Roig-Melo E, Panduro A. The epsilon4 allele of apolipoprotein E gene is a potential risk factor for the severity of macular edema in type 2 diabetic Mexican patients. Ophthalmic Genet. 2002;23:13–19. doi: 10.1076/opge.23.1.13.2203. [DOI] [PubMed] [Google Scholar]
- 103.Liew G, Shankar A, Wang JJ, Klein R, Bray MS, Couper DJ, et al. Apolipoprotein E gene polymorphisms are not associated with diabetic retinopathy: the atherosclerosis risk in communities study. Am J Ophthalmol. 2006;142:105–111. doi: 10.1016/j.ajo.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 104.Hu C, Zhang R, Yu W, Wang J, Wang C, Pang C, et al. CPVL/CHN2 genetic variant is associated with diabetic retinopathy in Chinese type 2 diabetic patients. Diabetes. 2011;60:3085–3089. doi: 10.2337/db11-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li H, Louey JW, Choy KW, Liu DT, Chan WM, Chan YM, et al. EDN1 Lys198Asn is associated with diabetic retinopathy in type 2 diabetes. Mol Vis. 2008;14:1698–1704. [PMC free article] [PubMed] [Google Scholar]
- 106.Matsubara Y, Murata M, Maruyama T, Handa M, Yamagata N, Watanabe G, Saruta T, Ikeda Y. Association between diabetic retinopathy and genetic variations in alpha2beta1 integrin, a platelet receptor for collagen. Blood. 2000;95:1560–1564. [PubMed] [Google Scholar]
- 107.Yoshioka K, Yoshida T, Takakura Y, Kogure A, Umekawa T, Toda H, Yoshikawa T. No association between the MTHFR gene polymorphism and diabetic retinopathy in type 2 diabetic patients without overt nephropathy. Diabetes Care. 2003;26:1947–1948. doi: 10.2337/diacare.26.6.1947. [DOI] [PubMed] [Google Scholar]
- 108.Maeda M, Yamamoto I, Fukuda M, Nishida M, Fujitsu J, Nonen S, Igarashi T, Motomura T, Inaba M, Fujio Y, Azuma J. MTHFR gene polymorphism as a risk for diabetic retinopathy in type 2 diabetic patients without serum creatinine elevation. Diabetes Care. 2003;26:547–548. doi: 10.2337/diacare.26.2.547-a. [DOI] [PubMed] [Google Scholar]
- 109.Wang J, Yang MM, Rong SS, Ng TK, Li YB, Liu XM. Association of paraoxonase gene polymorphisms with diabetic nephropathy and retinopathy. Mol Med Rep. 2013;8:1845–1851. doi: 10.3892/mmr.2013.1710. [DOI] [PubMed] [Google Scholar]
- 110.Gragnoli C. Proteasome modulator 9 gene is linked to diabetic and non-diabetic retinopathy in T2D. Ophthalmic Genet. 2011;32:228–230. doi: 10.3109/13816810.2011.592174. [DOI] [PubMed] [Google Scholar]
- 111.Demiryurek AT, Erbagci I, Oztuzcu S, Alasehirli B, Ozkara E, Seker M, et al. Lack of association between the Thr431Asn and Arg83Lys polymorphisms of the ROCK2 gene and diabetic retinopathy. Curr Eye Res. 2010;35:1128–1134. doi: 10.3109/02713683.2010.507903. [DOI] [PubMed] [Google Scholar]
- 112.Petrovič MG, Kružliak P, Petrovič D. The rs6060566 of the reactive oxygen species modulator 1 (Romo-1) gene affects Romo-1 expression and the development of diabetic retinopathy in Caucasians with type 2 diabetes. Acta Ophthalmol. 2015;93:e654–e657. doi: 10.1111/aos.12723. [DOI] [PubMed] [Google Scholar]
- 113.Paine SK, Sen A, Choudhuri S, Mondal LK, Cowdhury IH, Basu A, et al. Association of tumor necrosis factor α, interleukin 6, and interleukin 10 promoter polymorphism with proliferative diabetic retinopathy in type 2 diabetic subjects. Retina. 2012;32:1197–1203. doi: 10.1097/IAE.0b013e31822f55f3. [DOI] [PubMed] [Google Scholar]
- 114.Kumaramanickavel G, Sripriya S, Vellanki RN, Upadyay NK, Badrinath SS, Arokiasamy T, et al. Tumor necrosis factor allelic polymorphim with diabetic retinopathy in India. Diabetes Res Clin Pract. 2001;54:89–94. doi: 10.1016/s0168-8227(01)00269-8. [DOI] [PubMed] [Google Scholar]
- 115.Sesti LF, Crispim D, Canani LH, Polina ER, Rheinheimer J, Carvalho PS, Gross JL, Santos KG. The –308G> a polymorphism of the TNF gene is associated with proliferative diabetic retinopathy in Caucasian Brazilians with type 2 diabetes. Invest Ophthalmol Vis Sci. 2015;56:1184–1190. doi: 10.1167/iovs.14-15758. [DOI] [PubMed] [Google Scholar]
- 116.Kanková K, Muzík J, Karásková J, Beránek M, Hájek D, Znojil V, et al. Duration of non-insulin-dependent diabetes mellitus and the TNF-beta NcoI genotype as predictive factors in proliferative diabetic retinopathy. Ophtalmologica. 2001;215:294–298. doi: 10.1159/000050877. [DOI] [PubMed] [Google Scholar]
- 117.Zhong X DU Y, Lei Y, Liu N, Guo Y, Pan T. Effects of vitamin D receptor gene polymorphism and clinical characteristics on risk of diabetic retinopathy in Han Chinese type 2 diabetes patients. Gene. 2015;566:212–216. doi: 10.1016/j.gene.2015.04.045. [DOI] [PubMed] [Google Scholar]
- 118.Cyganek K, Mirkiewicz-Sieradzka B, Malecki MT, Wolkow P, Skupien J, Bobrek J, Czogala M, Klupa T, Sieradzki J. Clinical risk factors and the role of VDR gene polymorphisms in diabetic retinopathy in Polish type 2 diabetes patients. Acta Diabetol. 2006;43:114–119. doi: 10.1007/s00592-006-0225-3. [DOI] [PubMed] [Google Scholar]
- 119.Hong YJ, Kang ES, Ji MJ, Choi HJ, Oh T, Koong S-S, Jeon HJ. Association between Bsm1 polymorphism in vitamin D receptor gene and diabetic retinopathy of type 2 Diabetes in Korean population. Endocrinol Metab (Seoul) 2015;30:469–474. doi: 10.3803/EnM.2015.30.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simó-Servat O, Hernández C, Simó R. Genetics in diabetic retinopathy: current concepts and new insights. Curr Genomics. 2013;14:289–299. doi: 10.2174/13892029113149990008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sydorova M, Lee MS. Vascular endothelial growth factor levels in vitreous and serum of patients with either proliferative diabetic retinopathy or proliferative vitreoretinopathy. Ophthalmic Res. 2005;37:188–190. doi: 10.1159/000086594. [DOI] [PubMed] [Google Scholar]
- 122.Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96:1157–1158. doi: 10.1136/bjophthalmol-2011-300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Awata T, Kurihara S, Takata N, Neda T, Iizuka H, Ohkubo T, et al. Functional VEGF C-634G polymorphism is associated with development of diabetic macular edema and correlated with macula rretinal thickness in type 2 diabetes. Biochem Biophys Res Commun. 2005;333:679–685. doi: 10.1016/j.bbrc.2005.05.167. [DOI] [PubMed] [Google Scholar]
- 124.Semeraro F, Cancarini A, dell´Omo R, Rezzola S, Romano MR, Costagliola C. Diabetic retinopathy: vascular and inflammatory disease. J Diabetes Res Suppl. 2015;2015:582060. doi: 10.1155/2015/582060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Quazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abcouwer SF. Angiogenic factors and cytokines in diabetic retinopathy. J Clin Cell Immunol. 2013 doi: 10.4172/2155-9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tarr JM, Kaul K, Chopra M, Kohner EM, Chibber R. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2012;2013:343560. doi: 10.1155/2013/343560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zong H, Ward M, Stitt AW. AGEs, RAGE, and diabetic retinopathy. Curr Diabetes Rep. 2011;11:244–252. doi: 10.1007/s11892-011-0198-7. [DOI] [PubMed] [Google Scholar]
- 129.Lindholm E, Bakhtadze E, Sjogren M, Cilio CM, Agardh E, Groop L, et al. The –374T/A polymorphism in the gene encoding RAGE is associated with diabetic nephropathy and retinopathy in type 1 diabetic patients. Diabetologica. 2006;49:2745–2755. doi: 10.1007/s00125-006-0412-3. [DOI] [PubMed] [Google Scholar]
- 130.Ramprasad S, Radha V, Mathias RA, Majumder PP, Rao MR, Rema M. Rage gene promoter polymorphisms and diabetic retinopathy in a clinic-based population from South India. Eye. 2007;21:395–401. doi: 10.1038/sj.eye.6702239. [DOI] [PubMed] [Google Scholar]
- 131.JiXiong X, BiLin X, MingGong Y, ShuQin L. –429T/C and –374T/A polymorphisms of RAGE gene promoter are not associated with diabetic retinopathy in Chinese patients with type 2 diabetes. Diabetes Care. 2003;26:2696–2697. doi: 10.2337/diacare.26.9.2696. [DOI] [PubMed] [Google Scholar]
- 132.Wilkinson-Berka JL. Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol. 2006;38:752–765. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 133.Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression ofretinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 134.Matakova T, Sivonova M, Halasova E, Mistuna D, Dzian A, Berzinec P, et al. Gene polymorphisms of biotransforming enzymes (GSTs) and their association with lung cancer in the Slovakian population. Eur J Med Res Suppl. 2009;4:275–9. doi: 10.1186/2047-783X-14-S4-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cilenšek I, Mankoc S, Petrovic MG, Petrovic D. The 4a/4a genotype of the VNTR polymorphism for endothelial nitric oxide synthase (eNOS) gene predicts risk for proliferative diabetic retinopathy in Slovenian patients (Caucasians) with type 2 diabetes mellitus. Mol Biol Rep. 2012;39:7061–7067. doi: 10.1007/s11033-012-1537-8. [DOI] [PubMed] [Google Scholar]
- 136.Xu Y, Jiang Z, Huang J, Menq Q, Coh P, Tao L. The association between toll-like receptor 4 polymorphisms and diabetic retinopathy in Chinese patients with type 2 diabetes. Br J Ophthalmol. 2015;99:1301–1305. doi: 10.1136/bjophthalmol-2015-306677. [DOI] [PubMed] [Google Scholar]
- 137.Liew G, Klein R, Wong TY. The role of genetics in susceptibility to diabetic retinopathy. Int Ophthalmol Clin. 2009;49:35–52. doi: 10.1097/IIO.0b013e31819fd5d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sobrin L, Green T, Sim X, Jensen RA, Tai ES, Tay WT, et al. Candidate gene association study for diabetic retinopathy in persons with type 2 diabetes: the Candidate gene Association Resources (CARE) Invest Ophthalmol Vis Sci. 2011;52:7593–7602. doi: 10.1167/iovs.11-7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cunha-Vaz J, Ribeiro L, Lobo C. Phenotypes and biomarkers of diabetic retinopathy. Personalized medicine for diabetic retinopathy: the Weisenfeld award. Invest Ophthalmol Vis Sci. 2014;55:5412–5419. doi: 10.1167/iovs.14-14884. [DOI] [PubMed] [Google Scholar]
- 140.Gašperíková D, Tribble ND, Staník J, Hučková M, Mišovicová N, van de Bunt M, et al. Identification of a novel β-cell glucokinase (GCK) promoter mutation (–71G>C) that modulates GCK gene expression through loss of allele-specific sp1 binding causing mild fasting hyperglycaemia in humans. Diabetes. 2009;58:1929–1935. doi: 10.2337/db09-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang HM, Chen LL, Wang L, Liao YF, Wu ZH, Ye F, et al. Association of 1704G/T and G82S polymorphisms in the receptor for advanced glycation end products gene with diabetic retinopathy in Chinese population. J Endocrinol Invest. 2009;32:258–262. doi: 10.1007/BF03346463. [DOI] [PubMed] [Google Scholar]
- 142.Chen M, Lin W, Lu C, Chen C, Huang Y, Liao W, Tsai F. Chimerin 2 genetic polymorphisms are associated with non-proliferative diabetic retinopathy in Taiwanese type 2 diabetic patients. J Diabetes Complications. 2014;28:460–463. doi: 10.1016/j.jdiacomp.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 143.Petrovic MG, Hawlina M, Peterlin B, Petrovic D (2003) BglII gene polymorphism of the α2β1 integrin gene is a risk factor for diabetic retinopathy in Caucasians with type 2 diabetes. J Hum Genet 48(9):457–460 [DOI] [PubMed]
- 144.Niskanen L, Voutilainen-Kaunisto R, Teräsvirta M, Karvonen MK, Valve R, Pesonen U, Laakso M, Uusitupa MIJ, Koulu M (2000) Leucine 7 to proline 7 polymorphism in the neuropeptide y gene is associated with retinopathy in type 2 diabetes. Exp Clin Endocrinol Diabetes 108(3):235–236 [DOI] [PubMed]