Abstract
Understanding the genetic factors underlying brain structural connectivity is a major challenge in imaging genetics. Here, we present results from genome-wide association studies (GWASs) of whole-brain white matter (WM) fractional anisotropy (FA), an index of microstructural coherence measured using diffusion tensor imaging. Data from independent GWASs of 355 Swedish and 250 Norwegian healthy adults were integrated by meta-analysis to enhance power. Complementary GWASs on behavioral data reflecting processing speed, which is related to microstructural properties of WM pathways, were performed and integrated with WM FA results via multimodal analysis to identify shared genetic associations. One locus on chromosome 17 (rs145994492) showed genome-wide significant association with WM FA (meta P value = 1.87 × 10−08). Suggestive associations (Meta P value <1 × 10−06) were observed for 12 loci, including one containing ZFPM2 (lowest meta P value = 7.44 × 10−08). This locus was also implicated in multimodal analysis of WM FA and processing speed (lowest Fisher P value = 8.56 × 10−07). ZFPM2 is relevant in specification of corticothalamic neurons during brain development. Analysis of SNPs associated with processing speed revealed association with a locus that included SSPO (lowest meta P value = 4.37 × 10−08), which has been linked to commissural axon growth. An intergenic SNP (rs183854424) 14 kb downstream of CSMD1, which is implicated in schizophrenia, showed suggestive evidence of association in the WM FA meta-analysis (meta P value = 1.43 × 10−07) and the multimodal analysis (Fisher P value = 1 × 10−07). These findings provide novel data on the genetics of WM pathways and processing speed, and highlight a role of ZFPM2 and CSMD1 in information processing in the brain.
Electronic supplementary material
The online version of this article (doi:10.1007/s00429-016-1194-0) contains supplementary material, which is available to authorized users.
Keywords: Imaging genetics, DTI, GWAS, Processing speed, Fractional anisotropy, Cognition
Introduction
There is a strong genetic influence on brain structure (Thompson et al. 2001, 2014). The genome wide association study (GWAS) approach has proven to be successful in identifying specific genes related to individual differences in cortical and subcortical grey matter volumes (Potkin et al. 2009; Rimol et al. 2010; Bakken et al. 2012; Stein et al. 2012; Hibar et al. 2015). For white matter (WM) pathways that are crucial for speed of information processing (Kail and Salthouse 1994) studies revealed high heritability (Kochunov et al. 2010a, 2016; Jahanshad et al. 2013a). There are conflicting reports on few candidate genes, such as BDNF (Chiang et al. 2011), APOE (Heise et al. 2011; Westlye et al. 2012; Nyberg and Salami 2014), ADRB2 (Penke et al. 2010), GRM3 (Mounce et al. 2014), and ZNF804A (Voineskos et al. 2011; Wei et al. 2012; Fernandes et al. 2014) among others. In addition, several groups have undertaken a genome wide approach (Lopez et al. 2012; Jahanshad et al. 2013b; Sprooten et al. 2014). Lopez et al. (2012) used a global measure of white matter tract integrity (g FA) and identified suggestive evidence for ADAMTS18, LOC388630. Five SNPs reaching a genome-wide significance were identified in a GWAS of whole brain fractional anisotropy (FA) (Sprooten et al. 2014). This study implicated GNA13, HTR7, and CCDC91 genes to influence brain structure and emphasized the role for g-protein signaling in WM development and maintenance. A study by Jahanshad et al. (2013b) identified genome-wide significant association between a variant in the SPON1 gene and brain connectivity.
Microstructural variation in WM pathways has been linked to measures of information processing speed in both younger adults (Gold et al. 2007; Turken et al. 2008) and in samples of heterogeneous age (Kennedy and Raz 2009; Kochunov et al. 2010b; Penke et al. 2010; Madden et al. 2012; Salami et al. 2012). Increased myelination and axonal diameter is crucial for information processing in the brain (Tessier-Lavigne and Goodman 1996; Haász et al. 2013). Further, same genetic factors mediate the correlation between WM integrity and intellectual performance indicating common physiological mechanism for both (Chiang et al. 2009). The correlation between WM integrity and processing speed although complex (Tuch et al. 2005; Fjell et al. 2011; Tamnes et al. 2012) is consistent not just in healthy subjects but also in patients with psychiatric disorders such as schizophrenia (Karbasforoushan et al. 2015; Wright et al. 2015). This gives a strong rationale to study the potential sources of shared genetic contributions. Notably, recent study on old order amish families and the human connectome project (Kochunov et al. 2016) showed high heritability for both the traits with a high genetic correlation between the two suggesting common genes influencing joint variation in WM microstructure and processing speed. Support for this notion also comes from findings that some genes associated with WM microstructure also associate with processing speed, such as BDNF (McAllister et al. 2012), and APOE (Luciano et al. 2009). Notably, a recent large scale GWAS study (Ibrahim-Verbaas et al. 2015) on processing speed [letter–digit substitution (LDS)/digit–symbol substitution (DSS) tests] implicated CADM2, DRD2, and PAX3.
The main purpose of the present study was to identify genes that jointly influence WM microstructural coherence as indexed by whole-brain WM fractional anisotropy (FA) (Pierpaoli and Basser 1996) derived from diffusion tensor imaging (DTI) data and processing speed. FA reflects the directional coherence of water molecules. In WM, diffusion perpendicular to the tract is constrained by the axons and myelin sheaths (Thomason and Thompson 2011), and can thus be used to characterize tissue integrity. In a first step we performed meta-analyses of GWAS data from two independent samples of healthy adults to identify genetic associations with FA and speed tasks (Nilsson et al. 1997, 2004; Espeseth et al. 2012). Thereafter, we tested for genetic associations shared by WM and processing speed, by means of statistical integration of the meta-analysis results.
Materials and methods
Participants
The Betula sample examined here was part of a larger prospective cohort study of memory, health and aging (Nilsson et al. 1997, 2004). The current Betula sub-sample consisted of 360 participants (191 females and 169 males) aged between 25 and 80 years (mean = 62.3; SD = 13.3). Of these, 355 had DTI data (188 females and 167 males; age range 25–80 years with mean = 62.3; SD = 13.4). Sample demographics are summarized in Supplementary Table 1. All participants were native speakers of Swedish. The age distribution was skewed towards older participants, with 304 subjects out of 360 between ages 55 and 80 years. None of the participants had any history of severe neurological illness or events; all had normal or corrected to normal vision, and were in good general health. They were non-demented based on an extensive neuropsychological examination and clinical evaluation of data obtained at the test occasions and reviews of medical records starting from adulthood. All participants signed informed consent, in accordance with the guidelines of the Swedish Council for Research in Humanities and Social Sciences.
The Norwegian Cognitive NeuroGenetics (NCNG) sample examined here consisted of 250 participants (166 females and 84 males) aged 18–77 years (mean = 48.8; SD = 16.9) with available DTI data. 220 of these had data available for processing speed (143 females and 77 males, mean age 51.1 ranging from 19 to 77 years). Detailed sample demographics are presented in Supplementary Table 1. The sample was recruited by advertisements in a local newspaper to take part in a larger community-based study on the genetics of cognition (Espeseth et al. 2012). All participants read an information sheet and signed a statement of informed consent approved by the regional committee for Medical and Health Research Ethics (South-East Norway) (Project ID: S-03116). All participants were native speakers of Norwegian and had completed basic education with no history of learning deficits. All participants were interviewed for past or present neurological or psychiatric illnesses known to affect the CNS. Any person with a history of treatment for any of these conditions was excluded from the sample. Furthermore, persons with a depression inventory score indicating undiagnosed depressive illness were excluded. All participants were also interviewed at each visit according to a ‘Life events questionnaire’, which included questions on health, alcohol consumption, smoking habits, physical exercise, and positive and negative life events.
Genotyping and quality control
Genotyping was performed using commercially available Illumina arrays on DNA isolated from blood samples. The genotyping for both cohorts was performed at the Department of Genomics, Life and Brain Center, University of Bonn, Germany. Betula samples were genotyped using Illumina Omni Express and Omni 1S Bead chip kits. Genotyping and preprocessing was performed using Illumina GenomeStudio software. Manual examination and editing of a subset of the genotype clusters was performed according to the Illumina user guidelines. The following sample and genotyping quality checks were performed using PLINK (Purcell et al. 2007) and GenABEL (Aulchenko et al. 2007) software tools. Samples with call rates <0.97, with high autosomal heterozygosity (FDR < 0.01) or with sex discrepancies were excluded. Since we aimed at a genetically homogeneous sample, the population structure was assessed by multi-dimensional scaling (MDS) analysis using 250 K random SNPs to exclude samples with possible non-Swedish ancestry. Cryptic relatedness was assessed using identity-by-state estimates—IBS (as implemented in GenABEL). The individuals with the higher call rate among the pairs of individuals showing an IBS ≥0.85 were retained. Of the 371 individuals originally genotyped, a total of 10 individuals were excluded: two on the basis of sex discrepancy, four as a result of falling outside the MDS clustering based on the first three components, and four on the basis of cryptic relatedness. This resulted in a data set of 361 individuals. Further, SNPs were filtered and excluded from the analysis if they had a call rate <0.95, minor allele frequency (MAF) <0.01 and Hardy–Weinberg equilibrium (HWE) exact test P value <0.001. The final clean data set consisted of 1.4 million SNPs. The same genotyping quality control thresholds were applied to the NCNG sample which was described earlier by Espeseth et al. (2012).
Genotype imputation
Genotype imputation in the two samples was carried out using the same imputation protocol provided by the enhancing neuroimaging genetics through meta-analysis (ENIGMA), which is accessible at http://enigma.ini.usc.edu/wp-content/uploads/2012/07/ENIGMA2_1KGP_cookbook_v3.pdf. The 1000 Genomes Project Phase I reference haplotype data sets for the European populations (EUR) available at http://www.sph.umich.edu/csg/abecasis/MACH/download/ were used. The protocol used can be summarized as follows. First, using the ChunkChromosome tool (http://genome.sph.umich.edu/wiki/ChunkChromosome), each chromosome was split into manageable pieces of 5000 SNPs, each with an overlap of 500. Each chromosomal chunk was then phased into haplotypes using MaCH (Li et al. 2009, 2010) with 20 rounds and 200 states. The phased haplotypes were then imputed to the reference using minimac (Howie et al. 2012) run for five rounds and 200 states. SNPs with an imputation quality estimate Rsq value >0.5, which is an estimated squared correlation between imputed and true genotypes, are considered to be successfully imputed as recommended by the software developers. The most likely genotypes were then derived from the dosage values, which were rounded to the nearest whole number, and converted to the appropriate genotype. Further quality checks on the genotype files were performed in PLINK to exclude SNPs with a call rate <0.95, minor allele frequency <0.01 and Hardy–Weinberg Equilibrium (exact test) P value <0.001. At this stage the SNP overlap between the two samples was assessed and the overlapping SNPs were retained for further analysis. Finally, the following were removed: SNPs with ambiguous alleles between the two samples (i.e. A/T and G/C SNPs), tri allelic SNPs with ambiguous alleles between the two samples, and single base insertions/deletions. This resulted in a final data set of 6.1 million overlapping SNPs between the two samples.
In order to check the accuracy of our genotyping (between the two genotyping experiments) and of the imputation, 11 individuals from the NCNG sample were genotyped along with the Betula sample. Thus these 11 individuals were first genotyped on the Illumina Human610Quad, then run through imputation using the 1000 Genomes reference sample, and finally genotyped on the OmniExpress + Omni1S arrays along with the Betula sample. To calculate the genotyping accuracy between experiments, we compared N = 373,105 SNPs that overlapped between the two genotyping experiments. The genotyping reproducibility was 99.97 %. To calculate the imputation accuracy, we compared 666,027 SNPs that had been imputed in these 11 NCNG samples (based on the Illumina Human610Quad genotyping and imputation with the1000 Genomes reference sample) and that were also genotyped with the OmniExpress + Omni1S arrays. The accuracy of the imputation compared to the genotyping was 99.67 %.
Diffusion MRI protocol and data processing
A detailed description of the DTI MRI methods and subsequent data analysis for the Betula sample is available elsewhere (Salami et al. 2012). In brief, all the MRI data were acquired at Umeå Center for Functional Brain Imaging (UFBI) using the same 3T GE scanner with a 32-channel head coil. Diffusion-weighted data were acquired in three repetitions of 32 independent directions (b = 1000 s/mm2) and six non-gradient (b = 0 s/mm2) images. The data matrix was interpolated to a 256 × 256 matrix with an up-sampled spatial resolution of 0.98 × 0.98 × 2 mm. The three runs were then averaged and corrected for head movement and eddy current distortions. The first volume within the averaged volume that did not have a gradient applied was used to generate a binary brain mask. Finally, DTI fit (Behrens et al. 2003) was used to fit a diffusion tensor to each voxel included in the brain mask, yielding a voxel-wise FA volume for each subject.
The data and processing scheme for the NCNG data was performed as previously described (Westlye et al. 2012). Imaging was performed on a 12-channel head coil on a 1.5-T Siemens Avanto scanner (Siemens Medical Solutions, Erlangen, Germany) at Oslo University Hospital, Rikshospitalet. For diffusion weighted imaging a single-shot twice-refocused spin-echo echo planar imaging sequence with the following parameters was used: repetition time (TR)/echo time (TE) = 8590 ms/87 ms, b value = 1000 s/mm2, voxel size = 2.0 × 2.0 × 2.0 mm, and 64 axial slices. The sequence was repeated twice with 10 b = 0 and 60 diffusion-weighted volumes per run. DTI datasets were processed using the FMRIB Software Library (FSL) (Smith et al. 2004). Each volume was affine registered to the first b = 0 volume using FMRIB’s linear image registration tool (FLIRT) (Jenkinson et al. 2002) to correct for motion and eddy currents. After removal of non-brain tissue, FA (Basser and Pierpaoli 1996), eigenvectors, and eigenvalue maps were computed by linearly fitting a diffusion tensor to the data.
Both samples were analyzed using the same processing pipeline. FA volumes were transformed into a common space and skeletonized using tract skeleton generation program as employed in tract based spatial statistics (TBSS) (Smith et al. 2006). All volumes were nonlinearly warped to the FMRIB58_FA template by use of local deformation procedures performed by FMRIB’s nonlinear image registration tool (FNIRT) (Andersson et al. 2007). Next, a mean FA volume of all subjects was generated and thinned to create a mean FA skeleton representing the centers of all common tracts. We thresholded and binarized the mean skeleton at FA >0.2. Finally, each subject’s FA map was projected onto the common skeleton, yielding subject-specific FA skeleton maps. Whole-brain FA, computed by averaging FA values across the entire skeleton for each subject, was used in the GWAS.
Measures of speed of processing
In Betula, a revised version of the Wechsler (1981) DSS test was used: the letter–digit substitution (LDS) test (Nilsson et al. 2005). Briefly, it consists of rows of blank squares, each paired with a letter in a random sequence. A key pairing each letter with a number (1–9) is printed above these rows. Following ten practice trials, participants are asked to fill in the correct number in the blank squares, according to the key, as quickly and accurately as possible. The final test score is the number of correct responses given within a period of 60 s (max score = 125).
In NCNG the digit–symbol substitution (DSS) test from WAIS-R (Wechsler 1981), which has similar basic structure and sensitivity, was used. Briefly, it consists of rows of blank squares, each paired with a number from one to nine in a random sequence. A key pairing each number with a nonsense symbol is printed above these rows. Following seven practice trials, participants are asked to fill in the correct nonsense symbol in the blank squares, according to the key, as quickly and accurately as possible. The final test score is the number of correct responses given within a period of 90 s.
Correlation between FA and measures of speed of processing
WM FA and speed task measures showed a negative correlation with age in both the samples. For WM FA the correlation with age was r = −0.64 and r = −0.56, and for the speed task it was r = −0.56 and r = −0.53 for the Betula and NCNG samples, respectively. The unadjusted correlation between FA and the speed task was r = 0.48 in the Betula and r = 0.38 in the NCNG sample. When adjusted for age, age2 and gender the correlations were modest at r = 0.18 in the Betula sample and r = 0.11 in the NCNG sample.
GWAS association testing
We tested for single-marker allelic association under an additive model using linear regression, as implemented in the –linear option in the PLINK software. Age and sex were included as covariates. In addition, the age2 term was added to the FA GWAS to account for potential nonlinear relationships between age and WM changes in the brain (Bartzokis et al. 2010; Westlye et al. 2010). Let a SNP has AA, AB and BB as genotypes, and S be the number of B alleles in an individual. Linear regression allows us to include covariates such as gender, age, etc.
where, . We compared the distribution of P values obtained under the additive model to that expected under the null hypothesis of no association across the genome and report the quantile–quantile plot to verify the absence of systematic biases due to experimental or other confounding factors such as population stratification. The inflation factor (λ) and corresponding standard errors (SE) for the distribution of P values were estimated using the estlambda function in the GenABEL software. Manhattan and q–q plots were generated using the tool available at https://github.com/stephenturner/qqman/blob/master/qqman.r.
Meta-analysis
The overall measure of association in the two samples tested was obtained by meta-analysis, using the inverse variance weighted model from the METAL software package (Willer et al. 2010). The inverse variance based meta-analysis takes inputs: , effect size estimate for study i; , standard error for study i, with intermediate statistics: , , . Overall Z score: , overall P value: We further applied stringent filters to the meta-analysis results by only retaining SNPs showing the same direction of effect in both the samples and meta-analysis P values (meta P values) that were smaller, or more significant, than the two individual P values. SNPs with a P value below the traditional GWAS threshold (P value ≤5 × 10−8) were considered genome-wide significant. Additionally, SNPs showing suggestive evidence of association (meta P value ≤10−6) are also reported in this paper.
Since imputed data were used in this analysis, a high level of linkage disequilibrium (LD) was expected between SNPs showing suggestive evidence of significance. Thus, pair-wise LD for these markers was estimated using—ld option in PLINK, applying the 1000 Genomes Project EUR genotype data release April 2012 as reference and independent loci (as defined by pairwise r 2 < 0.2 or distance >250 kb) with at least one SNP showing suggestive evidence of significant association. Then, for each of the LD-independent signals, locus-specific plots (Pruim et al. 2010) were generated.
Genetic overlap test between the traits
To assess the genetic overlap between the two GWAS results we used gene set enrichment analysis (GSEA). GSEA, originally developed for interpreting gene expression studies (Song and Black 2008; Ackermann and Strimmer 2009), is now also applied to GWAS data (Ersland et al. 2012; Fernandes et al. 2013) to test if specific gene sets of interest are enriched for association in a GWAS. First, P values from the two meta-analyses were assigned to genes and used to calculate gene-based scores, using the R package LDsnpR (Christoforou et al. 2012) with ENSEMBL66 gene definitions (±10 kb). Since the datasets used were imputed at a high-density level, no additional LD parameters were included. Each ENSEMBL gene was then scored based on the lowest P value observed in the gene and corrected for the total number of SNPs in the gene using a modified Sidak’s correction (Saccone et al. 2007). This gene scoring method was found to correlate highly with minimal P value permutation-based scoring (Christoforou et al. 2012). Gene set enrichment analysis was performed using the GSEA tool (Mootha et al. 2003; Subramanian et al. 2005) provided for download at http://www.broadinstitute.org/gsea/index.jsp. Given an a priori defined set of genes S, the goal of GSEA is to determine whether the members of S are randomly distributed throughout L or primarily found at the extremes (top or bottom). An enrichment score (ES) is calculated reflecting the degree of over representation that corresponds to a Kolmogorov–Smirnov-like statistic. Statistical significance (nominal P value) of the ES is estimated using an empirical phenotype-based permutation test. When a database of gene sets is evaluated, the estimated significance level is adjusted for multiple hypothesis testing. First, the ES for each gene set is normalized to account for the size of the set and then control the proportion of false positives by calculating the false discovery rate (FDR). The analysis was performed on the pre-ranked list based on the Saccone-corrected gene scores with 1000 permutations. The test parameters were kept at the default settings.
To avoid artifacts in the enrichment test due to regions with high LD, the test sets (i.e., top 50 to top 2000) were pruned to contain only one gene in LD with the same markers (for details, see Fernandes et al. 2013).
The GSEA method used in the present study tests whether the top hits in one trait (top 50, 100, 150, 250, 500, 750, 1000 and 2000 genes) are enriched in the second trait and vice versa. Each GSEA was run three times and a test gene set was considered as significantly enriched only if the nominal P value from the GSEA analysis was smaller than 0.05. For each gene set that showed a significant P value, random mimic gene sets (N = 100) were generated, with each random set having the same number of SNPs and number of genes as the test gene set. GSEA was run for each significant gene set along with its random gene sets and the results were ranked according to the enrichment score (Ersland et al. 2012; Fernandes et al. 2013). The gene set was considered to show truly significant enrichment only if it passed this random gene set test by being in the top 5 % of the ranked list.
Multimodal analysis
To identify the commonality between the GWAS results from FA and the speed task measure we used a Fisher’s combined probability test for combining the P values from the two meta-analyses (Fisher 1932). The idea is that if the ‘k’ null hypotheses are all correct, the P values will be uniformly distributed on [0, 1] independently of each other. Then, with following a from which a P value for the global hypothesis can easily be obtained. Since this test does not account for the direction of effect, only those SNPs that showed the same direction of effect in the two meta-analyses were included in the analysis. The results from the combined analysis were also filtered to retain those SNPs that showed a Fisher P value that was smaller than the two individual meta P values, with each meta P value being <0.05.
Voxel-wise analysis
In keeping with some past reports (e.g., Sprooten et al. 2013), we considered a whole-WM FA-measure in the main analyses. Previous studies suggest that this global measure is a good approximation for relations between specific WM tracts and other variables, such as chronological age (Westlye et al. 2010; Salami et al. 2012). We had no a priori reason to expect this to be different for relations with genes, although some recent data indicate that there may be some tract-specific genetic relations (Kochunov et al. 2015). Based on the top hit from the WM FA analysis (rs149603240 in the ZFPM2 gene) we conducted some preliminary analyses of general versus local relations between WM and genetic variation by voxel-wise analyses using non-parametric permutation-based statistics estimated via a randomization algorithm implemented in the FSL. Initial data processing and TBSS analysis was performed jointly on both samples up to the point of final statistical analysis, thus ensuring that the analysis was performed using a common mask. The statistical analyses were performed for each sample individually, testing the effect of the relevant allele status on FA while including age and sex as covariates. Ten thousand permutations were run for each contrast (testing positive and negative associations with allele carrier status, respectively), and threshold-free cluster-enhancement (TFCE) (Smith and Nichols 2009) was used for statistical inference to avoid arbitrary initial cluster-forming thresholds. P values <0.05 (two-tailed, permutation-based TFCE-corrected) were regarded significant, corrected for multiple comparisons across space. Note that these voxel-wise analyses were only performed for alleles which showed a significant association with mean skeleton FA in both samples to characterize the spatial distribution of the effects, and the voxel-wise correction for family-wise errors should thus be regarded as relatively conservative.
Results
GWAS of WM FA
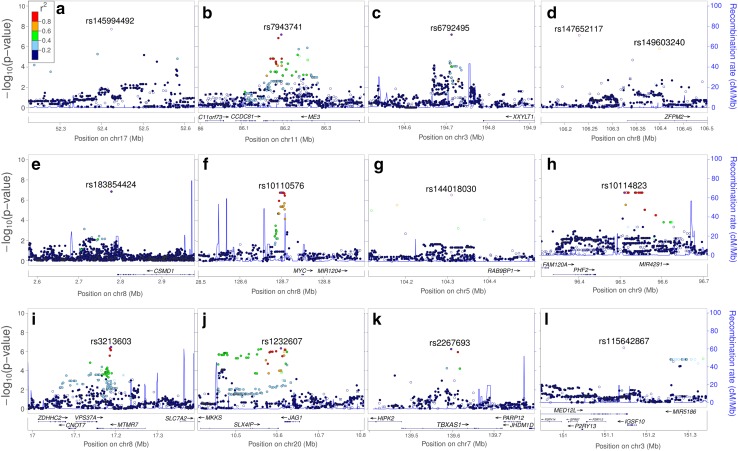
Using an additive model with age, age2 and sex as covariates, associations between 6.1 million SNPs and mean skeletal FA were tested in the two samples followed by meta-analysis. The q–q plot showing the P value distribution from the meta-analysis is shown in Fig. 1a (left panel), and the Manhattan plot for the same analysis is presented in Fig. 1b (upper panel). The q–q and Manhattan plots for the individual samples are shown in the Supplementary Figure. One SNP (rs145994492) surpassed the conventional threshold for genome-wide significance of 5 × 10−8 in the GWAS of mean skeletal FA. A total of 50 other SNPs showed a meta P value ≤10−6. Table 1a shows the different genomic loci (12 in total) represented by these SNPs and the most significant SNP(s) in each locus after pruning for LD at r 2 < 0.8. The SNPs were annotated to the following genes: ME3, MTMR7, JAG1, SLX4IP, TBXAS1, IGSF10 and MED12L (Fig. 2). One of the top hits, rs147652117, was found to be in strong linkage disequilibrium with an intronic SNP, rs149603240 (pairwise r 2 = 0.73), in the closest gene ZFPM2. The marker rs183854424, which showed suggestive evidence of association, was annotated to the nearest gene, CSMD1 (14 kb downstream). Several regions with suggestive levels of association were located in intervals that were not near any known genes (intergenic regions; see Table 1). The genes ZFPM2, MTMR7, and JAG1 have been implicated in CNS-related functions (Mochizuki and Majerus 2003; Ables et al. 2011; Nielsen et al. 2013). The CSMD1 gene has been implicated in schizophrenia (Håvik et al. 2011; Ripke et al. 2014) and in neuropsychological deficits in a mouse model (Steen et al. 2013). Further, the GWAS data were mined for polymorphisms implicated in previous reports on candidate genes and recent genome-wide studies of WM FA (Lopez et al. 2012; Jahanshad et al. 2013b; Sprooten et al. 2014) and the findings from two large GWASs of brain volumetric measures (Stein et al. 2012; Hibar et al. 2015) (Supplementary Table 2). None of the genes surpass the suggestive level of significance of meta P value <1 × 10−06. The lowest P value was observed for the gene ERBB4 (meta P value = 0.0005).
Fig. 1.
Genome-wide plots for the meta-analysis results of whole-brain skeletal FA and processing speed. a q–q plots of meta-analysis results of FA (λ = 1.02, SE = 5.42 × 10−6), and processing speed (λ = 1.01, SE = 3.56 × 10−6) with the diagonal line representing the expectation under the null hypothesis of no association. b Distribution of log-transformed P values (Y-axis) from the meta-analysis of FA and processing speed for 6.12 million SNPs tested plotted against the chromosomal positions (X-axis). The red line represents the genome-wide significant threshold of 5 × 10−8 and the blue line represents the threshold for suggestive evidence of 10−6
Table 1.
Genome-wide significant and suggestive SNPs from meta-analysis of whole-brain skeleton FA (a), Processing Speed (b), and the multimodality approach (c)
| a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | CHR | BP | LD | A1 | A2 | Betula (N = 355) | ||||
| Geno | Rsq | MAF | Beta | P | ||||||
| rs145994492 | 17 | 52423691 | G | A | Imp | 0.89 | 0.032 | –0.0172 | 3.68 × 10−06 | |
| rs7943741 | 11 | 86195270 | G | A | Imp | 1.00 | 0.365 | –0.0048 | 0.00035 | |
| rs6792495 | 3 | 194713051 | C | T | Gen | 1.00 | 0.095 | –0.0093 | 2.98 × 10−05 | |
| rs147652117 | 8 | 106230390 | A | G | Imp | 0.91 | 0.020 | –0.0194 | 4.89 × 10 −05 | |
| rs149603240 | 8 | 106398958 | 0.73 | T | C | Imp | 0.96 | 0.025 | –0.0153 | 0.00029 |
| rs183854424 | 8 | 2778557 | G | T | Imp | 0.80 | 0.076 | –0.0104 | 2.77 × 10−05 | |
| rs10110576 | 8 | 128699454 | A | G | Gen | 1.00 | 0.288 | 0.0042 | 0.00278 | |
|
rs144018030
rs139701334 |
5
5 |
104310291
104517549 |
0.56 |
A
A |
G
C |
Imp
Imp |
0.81
0.66 |
0.014
0.014 |
–0.0146
–0.0146 |
0.00927
0.00927 |
| rs10114823 | 9 | 96510399 | T | C | Imp | 0.99 | 0.028 | –0.0108 | 0.00692 | |
| rs3213603 | 8 | 17188911 | G | A | Imp | 1.00 | 0.203 | –0.0051 | 0.00094 | |
|
rs1232607
rs640336 rs1051421 |
20
20 20 |
10609594
10561369 10620275 |
0.57 0.6 |
T
C T |
C
T C |
Imp
Imp Gen |
1.00
1.00 1.00 |
0.370
0.256 0.283 |
0.0054
0.0067 0.0064 |
8.82 × 10
−05
2.29 × 10−05 3.23 × 10−05 |
| rs2267693 | 7 | 139597043 | G | A | Imp | 0.99 | 0.020 | –0.0171 | 0.0003 | |
| rs115642867 | 3 | 151145469 | G | A | Gen | 1.00 | 0.013 | –0.0217 | 0.00022 | |
| b | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SNP | CHR | BP | LD | A1 | A2 | Betula (N = 360) | ||||
| Geno | Rsq | MAF | Beta | P | ||||||
| rs6972739 | 7 | 149550942 | T | G | Imp | 0.98 | 0.107 | –3.363 | 2.23 × 10 −06 | |
| rs3823698 | 7 | 149563894 | 0.66 | G | A | Gen | 0.93 | 0.094 | –3.508 | 5.32 × 10−06 |
| rs117443760 | 7 | 149570724 | 0.56 | C | A | Imp | 1.00 | 0.086 | –3.597 | 5.95 × 10−06 |
| rs73168071 | 7 | 149541502 | 0.46 | T | C | Imp | 1.00 | 0.165 | –2.902 | 2.87 × 10−06 |
| rs16930911 | 12 | 26660336 | C | T | Imp | 1.00 | 0.031 | 5.552 | 1.54 × 10−05 | |
| rs1467356 | 12 | 16338172 | G | A | Gen | 0.96 | 0.1859 | –2.884 | 8.65 × 10−07 | |
| rs73785576 | 5 | 126636244 | C | T | Gen | 1.00 | 0.060 | 4.664 | 5.22 × 10−06 | |
| rs61642959 | 4 | 4961319 | T | C | Imp | 0.97 | 0.186 | –2.582 | 5.56 × 10−06 | |
| rs4779527 | 15 | 31736091 | T | C | Gen | 1.00 | 0.110 | –3.705 | 9.61 × 10−07 | |
| rs10776903 | 9 | 137689481 | A | G | Imp | 1.00 | 0.407 | 1.89 | 7.55 × 10−05 | |
| c | |||||||
|---|---|---|---|---|---|---|---|
| SNP | CHR | BP | A1 | A2 | Betula | ||
| Geno | Rsq | MAF | |||||
| rs183854424 | 8 | 2778557 | G | T | Imp | 0.80 | 0.076 |
| rs149603240 | 8 | 106398958 | T | C | Imp | 0.96 | 0.025 |
| rs74887000 | 17 | 41695496 | A | G | Gen | 0.92 | 0.034 |
| a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP | NCNG (N = 250) | Meta | HGNC Symbol | ||||||
| Geno | Rsq | MAF | Beta | P | Effect | SE | P | ||
| rs145994492 | Imp | 0.54 | 0.014 | –0.0199 | 0.00213 | 0.0179 | 0.0032 | 1.87 × 10−08 | Intergenic |
| rs7943741 | Gen | 1.00 | 0.348 | –0.0062 | 6.03 × 10−05 | 0.0054 | 0.001 | 6.49 × 10−08 | ME3 |
| rs6792495 | Imp | 0.64 | 0.084 | –0.0096 | 0.00089 | 0.0094 | 0.0017 | 6.54 × 10−08 | Intergenic |
|
rs147652117
rs149603240 |
Imp
Imp |
0.77
0.73 |
0.012
0.016 |
–0.0240
–0.0189 |
0.00053
0.00185 |
–0.0209
–0.0165 |
0.0039
0.0034 |
7.44 × 10
−08
1.57 × 10−06 |
ZFPM2
ZFPM2 |
| rs183854424 | Imp | 0.58 | 0.036 | –0.0121 | 0.00197 | 0.0109 | 0.0021 | 1.43 × 10−07 | Closest gene CSMD1 (14 kb downstream) |
| rs10110576 | Imp | 1.00 | 0.41 | 0.0064 | 1.44 × 10−05 | 0.0053 | 0.001 | 1.67 × 10−07 | Intergenic |
|
rs144018030
rs139701334 |
Imp
Imp |
0.83
0.66 |
0.012
0.012 |
–0.0331
–0.0331 |
1.80 × 10
−06
1.80 × 10−06 |
–0.0221
–0.0221 |
0.0043
0.0043 |
2.87 × 10
−07
2.87 × 10−07 |
Intergenic |
| rs10114823 | Imp | 0.97 | 0.03 | –0.0200 | 6.24 × 10−06 | –0.015 | 0.0029 | 2.91 × 10−07 | Intergenic |
| rs3213603 | Imp | 0.97 | 0.204 | –0.0074 | 8.87 × 10−05 | 0.006 | 0.0012 | 3.29 × 10−07 | MTMR7 |
|
rs1232607
rs640336 rs1051421 |
Imp
Imp Gen |
1.00
0.99 1.00 |
0.338
0.21 0.262 |
0.0048
0.0049 0.0045 |
0.00195
0.00754 0.0058 |
0.0051
–0.0059 0.0055 |
0.001
0.0012 0.0011 |
4.54 × 10
−07
5.42 × 10−07 6.51 × 10−07 |
JAG1, SLX4IP
JAG1, SLX4IP JAG1, SLX4IP |
| rs2267693 | Imp | 0.96 | 0.026 | –0.0163 | 0.00069 | 0.0167 | 0.0033 | 5.42 × 10−07 | TBXAS1 |
| rs115642867 | Imp | 0.81 | 0.014 | –0.0206 | 0.00131 | 0.0212 | 0.0043 | 7.57 × 10−07 | IGSF10, MED12L |
| b | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP | NCNG (N = 220) | Meta | HGNC Symbol | ||||||
| Geno | Rsq | MAF | Beta | P | Effect | SE | P | ||
| rs6972739 | Imp | 0.96 | 0.102 | –4.838 | 0.00692 | –3.5613 | 0.6504 | 4.37 × 10 −08 | ZNF862, SSPO, ATP6V0E2 |
| rs3823698 | Gen | 1.00 | 0.096 | –5.386 | 0.00327 | 3.7885 | 0.6999 | 6.21 × 10−08 | ZNF862, SSPO, ATP6V0E2 |
| rs117443760 | Imp | 0.93 | 0.096 | –5.386 | 0.00327 | 3.8785 | 0.7183 | 6.69 × 10−08 | ZNF862, SSPO, ATP6V0E2 |
| rs73168071 | Imp | 0.91 | 0.148 | –3.07 | 0.04028 | –2.9262 | 0.5645 | 2.18 × 10−07 | ZNF862, SSPO, ATP6V0E2 |
| rs16930911 | Imp | 0.96 | 0.042 | 7.117 | 0.003792 | –5.8857 | 1.123 | 1.60 × 10−07 | ITPR2 |
| rs1467356 | Gen | 0.98 | 0.228 | –1.602 | 0.1709 | 2.6327 | 0.5163 | 3.41 × 10−07 | SLC15A5 |
| rs73785576 | Imp | 1.00 | 0.036 | 5.142 | 0.06126 | –4.7212 | 0.9457 | 5.97 × 10−07 | MEGF10 |
| rs61642959 | Imp | 0.98 | 0.178 | –2.34 | 0.06923 | –2.5432 | 0.5131 | 7.17 × 10−07 | Intergenic |
| rs4779527 | Gen | 1.00 | 0.084 | –1.434 | 0.4005 | –3.3415 | 0.6809 | 9.24 × 10−07 | KLF13 |
| rs10776903 | Imp | 1.00 | 0.41 | 2.846 | 0.003372 | 2.076 | 0.4235 | 9.49 × 10−07 | COL5A1 |
| c | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | NCNG | Meta FA | Meta speed task | Fisher–P | HGNC symbol | ||||||
| Geno | Rsq | MAF | Effect | SE | P | Effect | SE | P | |||
| rs183854424 | Imp | 0.58 | 0.036 | 0.0109 | 0.0021 | 1.43 × 10−07 | 1.7805 | 0.8432 | 0.03472 | 1.00 × 10−07 | Closest gene CSMD1 (14 kb downstream) |
| rs149603240 | Imp | 0.74 | 0.016 | –0.0165 | 0.0034 | 1.57 × 10−06 | –3.0633 | 1.4161 | 0.03053 | 8.56 × 10−07 | ZFPM2 |
| rs74887000 | Imp | 0.78 | 0.022 | –0.0098 | 0.003 | 0.00099 | –4.8929 | 1.207 | 5.04E−05 | 8.93 × 10−07 | Intergenic |
A SNP was assigned to a gene if it falls within ±10kbp of the gene based on annotation from ENSEMBL release 66
CHR chromosome, BP base pair position on the GRC human genome assembly 37, LD pair-wise linkage disequilibrium (LD) with the most significant SNP in the close vicinity (bold faced), A1 first allele for the marker, A2 second allele for the marker, Geno shows whether the SNP has directly been genotyped (Geno) or imputed (Imp), Rsq imputation quality estimate r-square value, MAF minor allele (A1) frequency, P P value, effect overall estimated effect size for A1 in the meta-analysis, SE standard error for the overall estimated effect size for A1 in the meta-analysis, P P value, HGNC symbol genes to which the SNPs were assigned
Fig. 2.
Locus-specific plots highlighting the loci implicated by SNPs reaching a significance threshold of meta P value <10−6 for FA. a–i Each plot shows the −log10 P value (Y-axis) of SNPs arranged according to their chromosomal positions (X-axis). The locus-specific plots include the genes ME3 (b), ZFPM2 (d), MTMR7 (i), JAG1 and SLX4IP(j), TBXAS1 (k), and IGSF10 and MED12L (l). The blue lines show estimated recombination rates calculated from the HapMap data. The arrows represent the genomic locations of genes based on the NCBI Build 37 human assembly. SNP color represents LD with the SNP showing highest association in the locus. The SNP annotation is represented as follows: circles no annotation; squares synonymous or 3′ UTR; triangles non-synonymous; asterisks TFBScons (in a conserved region predicted to be a transcription factor binding site); squares with an X, MCS44 placental (in a region highly conserved in placental mammals)
GWAS of processing speed
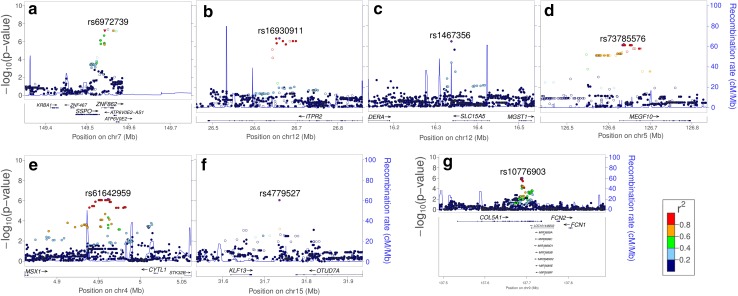
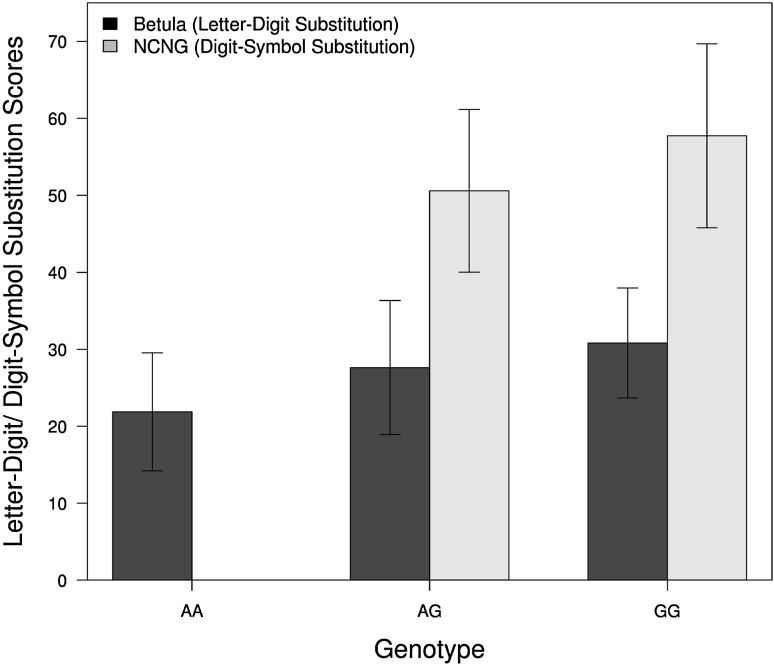
For processing speed, scores from the speed tasks (letter–digit-/digit–symbol substitution) were used to test for genetic association in the GWAS (Fig. 1a right panel and 1b lower panel). The q–q and Manhattan plots for the individual samples are shown in the Supplementary Figure. Table 1b shows the top hits from the analysis of genes related to speed of processing (meta P value ≤10−6). Of the top hits, rs6972739 surpassed the conventional threshold for genome-wide significance, and a total of 47 other SNPs showed a meta P value ≤10−6. Table 1b shows the genomic loci represented by SNPs with meta P value ≤ 10−6 after LD pruning (r 2 < 0.8). Together these pruned SNPs represent seven different genomic locations (Fig. 3), which include the genes SSPO, ZNF862, ATP6V0E2, ITPR2, SLC15A5, MEGF10, KLF13, and COL5A1. Of these, SSPO, ITPR2, and MEGF10 have been implicated in CNS-related functions (van Es et al. 2007; Singh et al. 2010; Scheib et al. 2012; Grondona et al. 2012). For illustration, Fig. 4 shows genetic mean differences in performance of the processing speed task in both samples for rs6972739. This SNP is the most strongly associated with processing speed in our meta-analysis and is located in the SSPO locus. The GWAS data was mined for polymorphisms implicated in a recent large scale study on processing speed using the LDS/DSS tasks (Ibrahim-Verbaas et al. 2015) (Supplementary Table 2). None of the genes surpass the suggestive level of significance of meta P value <1 × 10−06. However, nominal significance was observed for CADM2, DRD2, PAX3, and WDR72 implicated by this study.
Fig. 3.
Locus-specific plots highlighting the gene(s) represented by SNPs reaching a genome-wide significance threshold of meta P value ≤10−6 for processing speed. a–f Each plot shows the −log10 P value (Y-axis) of SNPs (localized in the genic region) arranged according to their chromosomal positions (X-axis). The locus-specific plots include the genes SSPO, ZNF862 and ATP6V0E2 (a), ITPR2 (b), SLC15A5 (c), MEGF10 (d), KLF13 (f), and COL5A1 (g). The blue lines show estimated recombination rates calculated from the HapMap data. The arrows represent the genomic locations of genes based on the NCBI Build 37 human assembly. SNP color represents LD with the SNP showing highest association in the locus. The SNP annotation is represented as follows: circles no annotation; squares synonymous or 3′ UTR; triangles non-synonymous; asterisks TFBScons (in a conserved region predicted to be a transcription factor binding site); squares with an X, MCS44 placental (in a region highly conserved in placental mammals)
Fig. 4.
Genotype means for the processing speed task measures in the Betula and NCNG samples for the SNP rs6972739. The X-axis shows the three genotypes and the Y-axis represents scores from the letter digit substitution and digit symbol substitution tests in Betula and NCNG samples, respectively. Error bars indicate one standard deviation from the mean. Number of individuals in each sample that were used to generate the plots: N = 360 for Betula and N = 220 for NCNG
Genetic overlap between two traits: results from GSEA
The top gene sets associated with processing speed were significantly enriched for association in the FA GWAS (Table 2). Random gene set testing validated this finding: these gene sets ranked in the top 5 % when tested along with 100 random genes sets. No significant positive enrichment of top FA hits in processing speed was observed.
Table 2.
Gene set enrichment analysis of top ranked FA gene sets in processing speed and vice versa
| Gene set | FA gene sets in processing speed | Processing speed gene sets in FA | ||||||
|---|---|---|---|---|---|---|---|---|
| Size | NES | NOM P value | Validation | Size | NES | NOM P value | Validation | |
| Ranked 1–50 | 47 | 0.84 | 0.848 | n.d. | 49 | 1.3 | 0.017 | At 5 % |
| Ranked 1–100 | 96 | 0.9 | 0.812 | n.d. | 98 | 1.2 | 0.041 | At 5 % |
| Ranked 1–150 | 143 | 0.86 | 0.917 | n.d. | 148 | 1.21 | 0.015 | At 5 % |
| Ranked 1–250 | 241 | 0.87 | 0.953 | n.d. | 246 | 1.14 | 0.027 | At 5 % |
| Ranked 1–500 | 486 | 1 | 0.511 | n.d. | 485 | 1.14 | 0.004 | At 1 % |
| Ranked 1–750 | 735 | 1.01 | 0.441 | n.d. | 725 | 1.09 | 0.03 | At 5 % |
| Ranked 1–1000 | 980 | 0.98 | 0.691 | n.d. | 966 | 1.09 | 0.013 | At 5 % |
| Ranked 1–2000 | 1965 | 0.97 | 0.828 | n.d. | 1931 | 1.04 | 0.083 | n.d. |
Size number of genes in the set, NES normalized enrichment score, NOM P value nominal P value, validation rank when tested along with one hundred random gene sets mimicking the test set in number of genes in the set and number of SNPs in each gene, n.d. not determined
Values below significant threshold are in italics
Identification of shared genetic associations for FA and processing speed
Table 1c shows the three top hits from the analysis of genes related to both FA and speed of processing (P value ≤10−6). Two of the SNPs identified in the joint analysis, rs183854424 (14 kb downstream of CSMD1) and rs149603240 (in an intron of ZFPM2) were also among the top hits in the analysis of FA-related genes (Table 1a). The third marker, rs74887000, is an intergenic SNP newly identified in this analysis.
Spatial distribution of FA effect
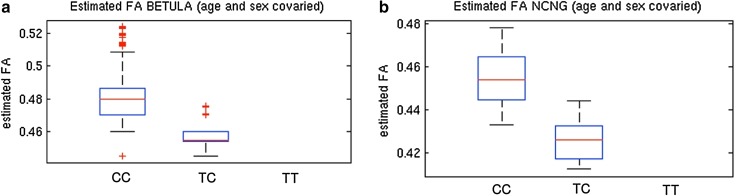
We carried out voxel-wise analyses for the ZFPM2 intronic SNP rs149603240, which shows a suggestive level of association with WM FA and is significant in the joint analysis with processing speed. A strong effect was observed in both samples, with TC carriers showing decreased FA. Figure 5 depicts the estimated marginal means of global WM FA per allelic group. The effect was anatomically non-specific, covering WM pathways in large parts of the brain (Fig. 6).
Fig. 5.
Box plot showing the distribution of FA values for the two genotypes observed for the ZFPM2 SNP (rs149603240). FA values (covaried for age, age2 and sex) are plotted on the Y-axis and the two observed genotypes CC and TC on the X-axis. Number of individuals included: N = 355 for Betula and N = 250 for NCNG
Fig. 6.
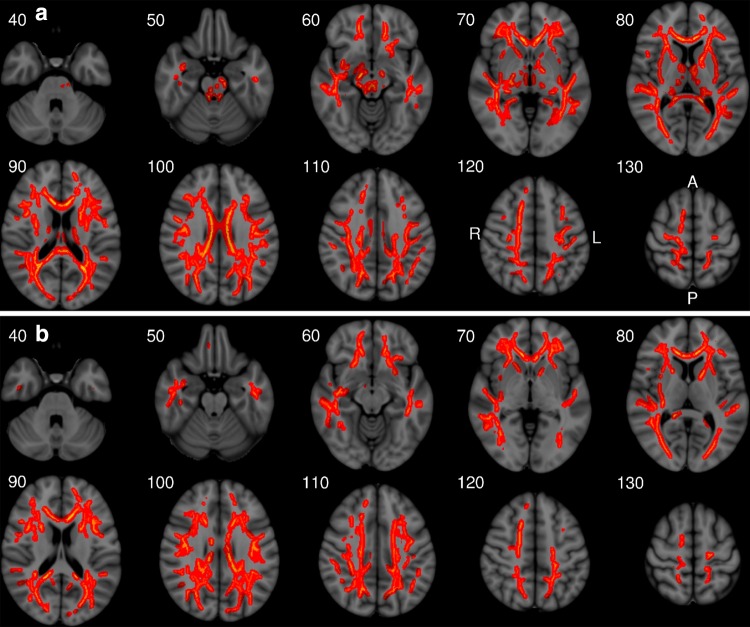
Illustration of voxel-wise analysis of (a) Betula and (b) NCNG samples using TBSS and permutation-based testing for rs149603240. Red denotes voxels with significantly decreased FA in TC carriers compared to CC carriers of rs149603240 (P < 0.05, 2-tailed, corrected for multiple comparisons across space using 10 k permutations and TFCE), covarying for main effects of age and sex. Results from tract-based spatial statistics are superimposed on a Montreal Neurological Institute (MNI) common brain template. The numbers refer to the corresponding MNI coordinate for each axial slice. A anterior; P posterior; L left side; R right side of the brain
Discussion
This study revealed novel associations between genetic variants in specific loci and mean skeletal FA, a whole-brain index of microstructural coherence. A genome-wide significant association (meta P value = 1.87 × 10−08) with WM FA was observed for an intergenic SNP (rs145994492), located on chromosome 17. This genetic variant appears to be isolated as no other SNP in the region show suggestive levels of association. Given the low allele frequency (<5 %) of this SNP one should be cautious in the interpretation of this signal. Similarly, several other suggestive association signals were not annotated to genes (intergenic). Genome-wide suggestive significance (meta P value ≤10−6) was observed for ME3. This gene encodes mitochondrial malic enzyme 3, NADP(+) dependent, which catalyzes the oxidative decarboxylation of malate to pyruvate. Interestingly, a copy number variant which includes the ME3 gene has been associated previously with brain volume (Boutte et al. 2012). The third most significant association in the present study was observed for ZFPM2 (Zinc Finger Protein, FOG Family Member 2), a cofactor for the GATA family of transcription factors, which regulates the expression of GATA target genes. ZFPM2 is expressed predominantly in the brain, heart, and testis (Lu et al. 1999) and is relevant in the specification of corticothalamic neurons during neuronal development (Kwan et al. 2008; Nielsen et al. 2013; Deck et al. 2013). Our voxel-wise findings indicate a wide-ranging role of genetic variants in ZFPM2 in widely distributed WM pathways (Fig. 6). Further, ZFPM2 is a negative regulator of the PI3K-Akt pathway (Hyun et al. 2009) which in turn is implicated in neurogenesis, neuronal survival, and synaptic plasticity (Spencer 2008), as well as differentiation of oligodendrocytes (Pérez et al. 2013). Other top signals included MTMR7, JAG1, SLX4IP, TBXAS1, IGSF10 and MED12L. JAG1 has been related to many CNS functions such as synaptic plasticity and axon guidance (Ables et al. 2011), which is in accordance with the present findings.
The analysis of genetic variants related to processing speed suggested associations with some candidate genes previously linked to various brain-related phenotypes (SSPO, ITPR2, MEGF10). In humans, the SSPO gene encodes the SCP-spondin protein which contributes to commissural axon growth, notably in the posterior commissure (Grondona et al. 2012). The integrity of inter-hemispheric pathways is critical for cognitive information processing speed (Bergendal et al. 2013). The present findings associate the G allele of rs6972739, the most significant SNP in this locus, with faster performance in the processing speed task (Fig. 4). ITPR2 has been identified as a susceptibility gene in sporadic amyotrophic lateral sclerosis, possibly via its role in glutamate-mediated neurotransmission (van Es et al. 2007).
Although well-designed individual GWA studies like ours have the capacity to identify novel loci, candidate genes and SNPs from previously published GWASs failed to reach nominal significance in our study (Supplementary Table 2). Possible explanations for this inconsistency include limited sample size, polygenic inheritance of complex traits and genetic heterogeneity among different study groups (Liu et al. 2008; Pei et al. 2014).
The primary purpose of the present study was to test the hypothesis that some genes influence both WM microstructure and processing speed. This kind of multi-modal approach (Thompson et al. 2014) has been proposed as a way of enhancing the chances of identifying significant top hits. GSEA between the two traits revealed a significant positive enrichment of the top processing speed genes in the FA GWAS. This implies that the genetic associations identified in the FA GWAS were also enriched for associations relevant in processing speed. The Fisher’s combined P value method identified the highest significance (Fisher P value = 1 × 10−07) for a SNP 14 kb downstream of the CSMD1 gene, which has been implicated in schizophrenia (Håvik et al. 2011; Ripke et al. 2014). CSMD1 is also relevant at the functional level, since it has been implicated in neuropsychological deficits in the mouse (Steen et al. 2013). In addition, an intronic SNP in ZFPM2 was identified in the analysis of shared associations. The CSMD1 and ZFPM2 SNPs were both among the top hits in the analysis of FA alone, but were not top hits in the analysis of speed-related genes. Thus, the putative role of the ZFPM2 or CSMD1 loci in information processing speed as revealed here would not have been discovered had processing speed been the only phenotype included. As noted above, the ZFPM2 gene is relevant for the specification of corticothalamic neurons during neuronal development. Corticothalamic neurons are crucial for processing and transmission of sensory information (Alitto and Usrey 2003; Bruno and Sakmann 2006; Briggs and Usrey 2008). A role for ZFPM2 in mediating processing speed is consistent with these prior observations.
None of the top hits in the processing speed analysis came out as strong hits in the joint analysis. Thus the enrichment effect of adding a phenotype to the multimodal analysis was not symmetrical. This impression is further underscored by results from gene-set enrichment analysis where we observed that the genes associated with processing speed show an enrichment of association in FA, but there is no reciprocal enrichment. We speculate that this asymmetry might be due to the fact that performance in the behavioral task reflects processes other than information processing speed, such as attention and working memory. Another possible explanation is that FA only accounts for a limited amount of the variance in processing speed.
In our study we have successfully identified one genome-wide significant SNP each for WM FA and processing speed, and we have observed multiple loci that show suggestive evidence that the phenotype is affected by many genetic variants (polygenicity). Interestingly, this is consistent with only five novel loci surpassing the genome wide significance in the recent large GWAS (30,177 individuals from 50 different cohorts) of brain subcortical volumetric measures (Hibar et al. 2015). However, our study is limited by several factors, which call for caution in the interpretation. First, it must be noted that even though our sample is among the largest reported for GWAS of FA, the sample size is still rather limited for a GWAS study. It is a general observation in complex trait/disease genetics that common variants (MAF ≥0.01) explain a large proportion of the phenotypic variance with small or modest effects, and large sample sizes are needed for genome-wide significance to be achieved. In addition, the sample composition was biased towards older adults, and this could potentially influence the results, for example if some genes exert a stronger effect at younger ages (but see McClearn et al. 1997 for a suggestion that the same genes contribute to individual differences early as well as later in life). Our study is also limited by the lack of a replication sample. Rather than using one sample as the discovery sample and the second sample as the replication sample, we chose to perform a meta-analysis since this has been shown to be more powerful than to use one sample for discovery and the other for replication (Skol et al. 2006). Although the samples are comparable in terms of phenotypes and genetic origin, there might be sample-specific differences that we would not be able to control for in a mega-analysis, which is why we chose to perform meta-analyses rather than mega-analyses to correct for sample-specific differences. We stress here that the integration of findings from the two samples should be valid as the samples were quite homogeneous from a population perspective, they were genotyped and imputed on the same platform, and the imaging phenotyping and speed tasks were highly comparable. Still, between-site procedural differences did exist, particularly for the imaging procedures. Another point to consider critically is the use of Fisher’s combined P value method, which might produce inflated P values when the test statistics are correlated. We took measures to counteract this effect, such as matching the directionality of effect and including only those SNPs with meta P values < 0.05 prior to testing, and only keeping Fisher P values smaller than the test P values. For the gene set-based analysis, the method can be prone to bias due to gene set size, gene length, and LD patterns (Wang et al. 2011). To address the issue of variable numbers of SNPs and LD around the markers we applied a modified Sidak’s correction (Saccone et al. 2007) when scoring the genes, which is highly correlated with the gold standard of permutation correction. We pruned our test gene sets to avoid potential intergenic-LD biasing the test statistics. In addition, a non-random distribution of gene size with respect to function has previously been reported; for example, brain-expressed genes are relatively large (Raychaudhuri et al. 2010). This poses a challenge to the gene set-based analysis of GWAS data sets, and we have no control for it in the present study. Another limitation is that WM hyperintensities (WMHIs) are common neuroradiological observations, in particular in samples of older adults, with no obvious clinical or functional implications e.g., (Söderlund et al. 2003). It has been shown that incidental WMHIs might affect FA estimates (Iverson et al. 2011). We did not control for WMHIs in our study, which might be seen as a limitation. Finally, the behavioral and imaging measures have limitations. MRI-derived indices of WM microstructural coherence as indexed by whole-brain FA are indirect measures, so the observed associations could be influenced by additional factors unrelated to or only partly related to brain WM. Relatedly, with the exception of a targeted voxel-wise analysis, we focused on a global index of WM integrity. While there is data to suggest that this is a good proxy for WM status in the brain e.g., (Salami et al. 2012; Sprooten et al. 2013), there is also evidence for heterogeneity among WM tracts in relation to genetic variation (Kochunov et al. 2015). By the latter view, we may well have missed detecting genetic associations that are selective for certain tracts. Similarly, although the letter–digit-/digit–symbol substitution tasks are clearly tapping information processing speed, other cognitive components such as working memory capacity could also influence performance.
In conclusion, the present analyses provide novel data on the genetics of brain WM as well as processing speed, and in particular highlight a key role of ZFPM2 and CSMD1 in information processing in the brain. Considering the aforementioned limitations, it will be necessary to validate these findings by analyzing the same phenotypes in further samples.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by a Wallenberg Scholar grant from the Knut and Alice Wallenberg (KAW) Foundation and a grant from Torsten and Ragnar Söderbergs Foundation to LN, a grant from HelseVest RHF (Grant 911554) to SLH, grants from the Bergen Research Foundation and the University of Bergen to SLH, grants from the Dr Einar Martens Fund and the K.G. Jebsen Foundation to SLH and VMS, the Research Council of Norway to TE (Grant 177458/V 50) and LTW (Grant 204966/F 20). We thank the Centre for Advanced Study (CAS) at the Norwegian Academy of Science and Letters in Oslo for hosting collaborative projects and workshops between Norway and Sweden in 2011–2012.
References
- Ables JL, Breunig JJ, Eisch AJ, Rakic P. Not(ch) just development: notch signalling in the adult brain. Nat Rev Neurosci. 2011;12(5):269–283. doi: 10.1038/nrn3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackermann M, Strimmer K. A general modular framework for gene set enrichment analysis. BMC Bioinformatics. 2009;10:47. doi: 10.1186/1471-2105-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitto HJ, Usrey WM. Corticothalamic feedback and sensory processing. Curr Opin Neurobiol. 2003;13(4):440–445. doi: 10.1016/S0959-4388(03)00096-5. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S (2007) Non-linear registration aka Spatial normalisation FMRIB Technical Report TR07JA2. Oxford
- Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007;23(10):1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
- Bakken TE, Roddey JC, Djurovic S, Akshoomoff N, Amaral DG, Bloss CS, Carlson H, et al. Association of common genetic variants in GPCPD1 with scaling of visual cortical surface area in humans. Proc Natl Acad Sci USA. 2012;109(10):3985–3990. doi: 10.1073/pnas.1105829109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31(9):1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50(5):1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Bergendal G, Martola J, Stawiarz L, Kristoffersen-Wiberg M, Fredrikson S, Almkvist O. Callosal atrophy in multiple sclerosis is related to cognitive speed. Acta Neurol Scand. 2013;127(4):281–289. doi: 10.1111/ane.12006. [DOI] [PubMed] [Google Scholar]
- Boutte D, Calhoun VD, Chen J, Sabbineni A, Hutchison K, Liu J. Association of genetic copy number variations at 11 q14.2 with brain regional volume differences in an alcohol use disorder population. Alcohol. 2012;46(6):519–527. doi: 10.1016/j.alcohol.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F, Usrey WM. Emerging views of corticothalamic function. Curr Opin Neurobiol. 2008;18(4):403–407. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RM, Sakmann B. Cortex is driven by weak but synchronously active thalamocortical synapses. Science. 2006;312(5780):1622–1627. doi: 10.1126/science.1124593. [DOI] [PubMed] [Google Scholar]
- Chiang M-C, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009;29(7):2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M-C, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, Wright MJ, Thompson PM. Genetics of white matter development: a DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage. 2011;54(3):2308–2317. doi: 10.1016/j.neuroimage.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforou A, Dondrup M, Mattingsdal M, Mattheisen M, Giddaluru S, Nöthen MM, Rietschel M, Cichon S, Djurovic S, Andreassen OA, Jonassen I, Steen VM, Puntervoll P, Le Hellard S. Linkage-disequilibrium-based binning affects the interpretation of GWASs. Am J Hum Genet. 2012;90(4):727–733. doi: 10.1016/j.ajhg.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deck M, Lokmane L, Chauvet S, Mailhes C, Keita M, Niquille M, Yoshida M, Yoshida Y, Lebrand C, Mann F, Grove EA, Garel S. Pathfinding of corticothalamic axons relies on a rendezvous with thalamic projections. Neuron. 2013;77(3):472–484. doi: 10.1016/j.neuron.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersland KM, Christoforou A, Stansberg C, Espeseth T, Mattheisen M, Mattingsdal M, Hardarson GA, Hansen T, Fernandes CPD, Giddaluru S, Breuer R, Strohmaier J, Djurovic S, Nöthen MM, Rietschel M, Lundervold AJ, Werge T, Cichon S, Andreassen OA, Reinvang I, Steen VM, Le Hellard S. Gene-based analysis of regionally enriched cortical genes in GWAS data sets of cognitive traits and psychiatric disorders. PLoS One. 2012;7(2):e31687. doi: 10.1371/journal.pone.0031687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeseth T, Christoforou A, Lundervold AJ, Steen VM, Le Hellard S, Reinvang I. Imaging and cognitive genetics: the Norwegian Cognitive NeuroGenetics sample. Twin Res Hum Genet. 2012;15(3):442–452. doi: 10.1017/thg.2012.8. [DOI] [PubMed] [Google Scholar]
- Fernandes CPD, Christoforou A, Giddaluru S, Ersland KM, Djurovic S, Mattheisen M, Lundervold AJ, Reinvang I, Nöthen MM, Rietschel M, Ophoff RA, Hofman A, Uitterlinden AG, Werge T, Cichon S, Espeseth T, Andreassen OA, Steen VM, Le Hellard S. A genetic deconstruction of neurocognitive traits in schizophrenia and bipolar disorder. PLoS One. 2013;8(12):e81052. doi: 10.1371/journal.pone.0081052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes CPD, Westlye LT, Giddaluru S, Christoforou A, Kauppi K, Adolfsson R, Nilsson L-G, Nyberg L, Lundervold AJ, Reinvang I, Steen VM, Le Hellard S, Espeseth T. Lack of association of the rs1344706 ZNF804A variant with cognitive functions and DTI indices of white matter microstructure in two independent healthy populations. Psychiatry Res Neuroimaging. 2014;222(1–2):60–66. doi: 10.1016/j.pscychresns.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Fisher RA. Statistical Methodsfor Research Workers. 4. Edinburgh: Oliver and Boyd; 1932. [Google Scholar]
- Fjell AM, Westlye LT, Amlien IK, Walhovd KB. Reduced white matter integrity is related to cognitive instability. J Neurosci. 2011;31(49):18060–18072. doi: 10.1523/JNEUROSCI.4735-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA. Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: evidence from diffusion tensor imaging. Neuropsychologia. 2007;45(11):2439–2446. doi: 10.1016/j.neuropsychologia.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondona JM, Hoyo-Becerra C, Visser R, Fernández-Llebrez P, López-Ávalos MD (2012) The subcommissural organ and the development of the posterior commissure. In: International review of cell and molecular biology. pp 63–137 [DOI] [PubMed]
- Haász J, Westlye ET, Fjær S, Espeseth T, Lundervold A, Lundervold AJ. General fluid-type intelligence is related to indices of white matter structure in middle-aged and old adults. Neuroimage. 2013;83:372–383. doi: 10.1016/j.neuroimage.2013.06.040. [DOI] [PubMed] [Google Scholar]
- Håvik B, Le Hellard S, Rietschel M, Lybæk H, Djurovic S, Mattheisen M, Mühleisen TW, Degenhardt F, Priebe L, Maier W, Breuer R, Schulze TG, Agartz I, Melle I, Hansen T, Bramham CR, Nöthen MM, Stevens B, Werge T, Andreassen OA, Cichon S, Steen VM. The complement control-related genes CSMD1 and CSMD2 associate to schizophrenia. Biol Psychiatry. 2011;70(1):35–42. doi: 10.1016/j.biopsych.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Heise V, Filippini N, Ebmeier KPMC. The APOE ɛ4 allele modulates brain white matter integrity in healthy adults. Mol Psychiatry. 2011;16(9):908–916. doi: 10.1038/mp.2010.90. [DOI] [PubMed] [Google Scholar]
- Hibar D, Stein J, Renteria M, Arias-Vasquez A, Desrivières S, Jahanshad N, Medland S, et al. Common genetic variants influence human subcortical brain structures. Nature. 2015;520(7546):224–229. doi: 10.1038/nature14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44(8):955–959. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun S, Lee JH, Jin H, Nam J, Namkoong B, Lee G, Chung J, Kim VN. Conserved MicroRNA miR-8/miR-200 and its target USH/FOG2 control growth by regulating PI3K. Cell. 2009;139(6):1096–1108. doi: 10.1016/j.cell.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Ibrahim-Verbaas CA, Bressler J, Debette S, Schuur M, Smith AV, Bis JC, Mosley TH, et al. GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol Psychiatry. 2015 doi: 10.1038/mp.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson GL, Hakulinen U, Wäljas M, Dastidar P, Lange RT, Soimakallio S, Öhman J. To exclude or not to exclude: white matter hyperintensities in diffusion tensor imaging research. Brain Inj. 2011;25(13–14):1325–1332. doi: 10.3109/02699052.2011.608409. [DOI] [PubMed] [Google Scholar]
- Jahanshad N, Kochunov P, Sprooten E, Mandl RC, Nichols TE, Almassy L, Blangero J, Brouwer RM, Curran JE, de Zubicaray GI, Duggirala R, Fox PT, Hong LE, Landman BA, Martin NG, McMahon KL, Medland SE, Mitchell BD, Olvera RL, Peterson CP, Starr JM, Sussmann JE, Toga AW, Wardlaw JM, Wright MJ, Hulshoff Pol HE, Bastin ME, McIntosh AM, Deary IJ, Thompson PM, Glahn DC. Multi-site genetic analysis of diffusion images and voxelwise heritability analysis: a pilot project of the ENIGMA-DTI working group. Neuroimage. 2013;81:455–469. doi: 10.1016/j.neuroimage.2013.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshad N, Rajagopalan P, Hua X, Hibar DP, Nir TM, Toga AW, Jack CR, Saykin AJ, Green RC, Weiner MW, Medland SE, Montgomery GW, Hansell NK, Mcmahon KL, De Zubicaray GI, Martin NG, Wright MJ, Thompson PM, Alzheimer’s Disease Neuroimaging Initiative Genome-wide scan of healthy human connectome discovers SPON1 gene variant influencing dementia severity. Proc Natl Acad Sci USA. 2013;110(12):4768–4773. doi: 10.1073/pnas.1216206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Kail R, Salthouse TA. Processing speed as a mental capacity. Acta Psychol (Amst) 1994;86:199–225. doi: 10.1016/0001-6918(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Karbasforoushan H, Duffy B, Blackford JU, Woodward ND. Processing speed impairment in schizophrenia is mediated by white matter integrity. Psychol Med. 2015;45(1):109–120. doi: 10.1017/S0033291714001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Raz N. Aging white matter and cognition: differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47(3):916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Glahn DC, Lancaster JL, Winkler AM, Smith S, Thompson PM, Almasy L, Duggirala R, Fox PT, Blangero J. Genetics of microstructure of cerebral white matter using diffusion tensor imaging. Neuroimage. 2010;53(3):1109–1116. doi: 10.1016/j.neuroimage.2010.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, Bartzokis G, Stanley J, Royall D, Schlosser AE, Null M, Fox PT. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage. 2010;49(2):1190–1199. doi: 10.1016/j.neuroimage.2009.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Jahanshad N, Marcus D, Winkler A, Sprooten E, Nichols TE, Van Essen DC, et al. Heritability of fractional anisotropy in human white matter: a comparison of human connectome project and ENIGMA-DTI data. Neuroimage. 2015;111:300–311. doi: 10.1016/j.neuroimage.2015.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Thompson PM, Winkler A, Morrissey M, Fu M, Coyle TR, Du X, Muellerklein F, Savransky A, Gaudiot C, Sampath H, Eskandar G, Jahanshad N, Patel B, Rowland L, Nichols TE, O’Connell JR, Shuldiner AR, Mitchell BD, Hong LE. The common genetic influence over processing speed and white matter microstructure: evidence from the Old Order Amish and Human Connectome Projects. Neuroimage. 2016;125:189–197. doi: 10.1016/j.neuroimage.2015.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Lam MMS, Krsnik Z, Kawasawa YI, Lefebvre V, Sestan N. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci USA. 2008;105(41):16021–16026. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009;10:387–406. doi: 10.1146/annurev.genom.9.081307.164242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34(8):816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Papasian CJ, Liu JF, Hamilton J, Deng HW. Is replication the gold standard for validating genome-wide association findings? PLoS One. 2008;3(12):e4037. doi: 10.1371/journal.pone.0004037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez LM, Bastin ME, Maniega SM, Penke L, Davies G, Christoforou A, Valdés Hernández MC, Royle NA, Tenesa A, Starr JM, Porteous DJ, Wardlaw JM, Deary IJ. A genome-wide search for genetic influences and biological pathways related to the brain’s white matter integrity. Neurobiol Aging. 2012;33(8):1847.e1–1847.e14. doi: 10.1016/j.neurobiolaging.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Lu JR, McKinsey TA, Xu H, Wang DZ, Richardson JA, Olson EN. FOG-2, a heart- and brain-enriched cofactor for GATA transcription factors. Mol Cell Biol. 1999;19(6):4495–4502. doi: 10.1128/MCB.19.6.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Gow AJ, Harris SE, Hayward C, Allerhand M, Starr JM, Visscher PM, Deary IJ. Cognitive ability at age 11 and 70 years, information processing speed, and APOE variation: the Lothian Birth Cohort 1936 study. Psychol Aging. 2009;24(1):129–138. doi: 10.1037/a0014780. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen N-K, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822(3):386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister TW, Tyler AL, Flashman LA, Rhodes CH, McDonald BC, Saykin AJ, Tosteson TD, Tsongalis GJ, Moore JH. Polymorphisms in the brain-derived neurotrophic factor gene influence memory and processing speed one month after brain injury. J Neurotrauma. 2012;29(6):1111–1118. doi: 10.1089/neu.2011.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClearn GE, Johansson B, Berg S, Pedersen NL, Ahern F, Petrill SA, Plomin R. Substantial genetic influence on cognitive abilities in twins 80 or more years old. Science. 1997;276(5318):1560–1563. doi: 10.1126/science.276.5318.1560. [DOI] [PubMed] [Google Scholar]
- Mochizuki Y, Majerus PW. Characterization of myotubularin-related protein 7 and its binding partner, myotubularin-related protein 9. Proc Natl Acad Sci USA. 2003;100(17):9768–9773. doi: 10.1073/pnas.1333958100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- Mounce J, Luo L, Caprihan A, Liu J, Perrone-Bizzozero NI, Calhoun VD. Association of GRM3 polymorphism with white matter integrity in schizophrenia. Schizophr Res. 2014;155(1–3):8–14. doi: 10.1016/j.schres.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JV, Thomassen M, Møllgård K, Noraberg J, Jensen NA. zbtb20 defines a hippocampal neuronal identity through direct repression of genes that control projection neuron development in the isocortex. Cereb Cortex. 2013;24(5):1216–1229. doi: 10.1093/cercor/bhs400. [DOI] [PubMed] [Google Scholar]
- Nilsson L-G, Bäckman L, Erngrund K, Nyberg L, Adolfsson R, Bucht G, Karlsson S, Widing M, Winblad B. The betula prospective cohort study: memory, health, and aging. Aging Neuropsychol Cogn. 1997;4(1):1–32. doi: 10.1080/13825589708256633. [DOI] [Google Scholar]
- Nilsson L-G, Adolfsson R, Bäckman L, de Frias CM, Molander B, Nyberg L. Betula : a prospective cohort study on memory, health and aging betula: a prospective cohort study on memory, health and aging. Aging Neuropsychol Cogn. 2004;11(2–3):134–148. doi: 10.1080/13825580490511026. [DOI] [Google Scholar]
- Nilsson L-G, Söderlund H, Berger K, Breteler M, de Ridder M, Dufouil C, Fuhrer R, Giampaoli S, Hofman A, Pajak A, Sans S, Schmidt R, Launer LJ. Cognitive test battery of CASCADE: tasks and data. Aging Neuropsychol Cogn. 2005;12(1):32–56. doi: 10.1080/13825580590925099. [DOI] [Google Scholar]
- Nyberg L, Salami A. The APOE ε4 allele in relation to brain white-matter microstructure in adulthood and aging. Scand J Psychol. 2014;55(3):263–267. doi: 10.1111/sjop.12099. [DOI] [PubMed] [Google Scholar]
- Pei YF, Zhang L, Papasian CJ, Wang YP, Deng HW. On individual genome-wide association studies and their meta-analysis. Hum Genet. 2014;133(3):265–279. doi: 10.1007/s00439-013-1366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke L, Muñoz Maniega S, Houlihan LM, Murray C, Gow AJ, Clayden JD, Bastin ME, Wardlaw JM, Deary IJ. White matter integrity in the splenium of the corpus callosum is related to successful cognitive aging and partly mediates the protective effect of an ancestral polymorphism in ADRB2. Behav Genet. 2010;40(2):146–156. doi: 10.1007/s10519-009-9318-4. [DOI] [PubMed] [Google Scholar]
- Pérez MJ, Fernandez N, Pasquini JM. Oligodendrocyte differentiation and signaling after transferrin internalization: a mechanism of action. Exp Neurol. 2013;248:262–274. doi: 10.1016/j.expneurol.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Guffanti G, Lakatos A, Turner JA, Kruggel F, Fallon JH, Saykin AJ, Orro A, Lupoli S, Salvi E, Weiner M, Macciardi F. Hippocampal atrophy as a quantitative trait in a genome-wide association study identifying novel susceptibility genes for Alzheimer’s disease. PLoS One. 2009;4(8):e6501. doi: 10.1371/journal.pone.0006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, Boehnke M, Abecasis GR, Willer CJ. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26(18):2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raychaudhuri S, Korn JM, McCarroll SA, Altshuler D, Sklar P, Purcell S, Daly MJ. Accurately assessing the risk of schizophrenia conferred by rare copy-number variation affecting genes with brain function. PLoS Genet. 2010;6(9):e1001097. doi: 10.1371/journal.pgen.1001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Panizzon MS, Fennema-Notestine C, Eyler LT, Fischl B, Franz CE, Hagler DJ, Lyons MJ, Neale MC, Pacheco J, Perry ME, Schmitt JE, Grant MD, Seidman LJ, Thermenos HW, Tsuang MT, Eisen SA, Kremen WS, Dale AM. Cortical thickness is influenced by regionally specific genetic factors. Biol Psychiatry. 2010;67(5):493–499. doi: 10.1016/j.biopsych.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Neale BM, Corvin A, Walters JTR, Farh K-H, Holmans PA, O’Donovan MC, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511(7510):421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PAF, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet. 2007;16(1):36–49. doi: 10.1093/hmg/ddl438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Eriksson J, Nilsson L-G, Nyberg L. Age-related white matter microstructural differences partly mediate age-related decline in processing speed but not cognition. Biochim Biophys Acta. 2012;1822(3):408–415. doi: 10.1016/j.bbadis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Scheib JL, Sullivan CS, Carter BD. Jedi-1 and MEGF10 signal engulfment of apoptotic neurons through the tyrosine kinase Syk. J Neurosci. 2012;32(38):13022–13031. doi: 10.1523/JNEUROSCI.6350-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh TD, Park S-Y, Bae J, Yun Y, Bae Y-C, Park R-W, Kim I-S. MEGF10 functions as a receptor for the uptake of amyloid-β. FEBS Lett. 2010;584(18):3936–3942. doi: 10.1016/j.febslet.2010.08.050. [DOI] [PubMed] [Google Scholar]
- Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006;38(2):209–213. doi: 10.1038/ng1706. [DOI] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Söderlund H, Nyberg L, Adolfsson R, Nilsson L-G, Launer LJ. High prevalence of white matter hyperintensities in normal aging: relation to blood pressure and cognition. Cortex. 2003;39:1093–1105. doi: 10.1016/S0010-9452(08)70879-7. [DOI] [PubMed] [Google Scholar]
- Song S, Black MA. Microarray-based gene set analysis: a comparison of current methods. BMC Bioinformatics. 2008;9:502. doi: 10.1186/1471-2105-9-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JPE (2008) Flavonoids: modulators of brain function?. Br J Nutr 99 E Suppl:ES60–77. doi: 10.1017/S0007114508965776 [DOI] [PubMed]
- Sprooten E, Fleming KM, Thomson PA, Bastin ME, Whalley HC, Hall J, Sussmann JE, McKirdy J, Blackwood D, Lawrie SM, McIntosh AM. White matter integrity as an intermediate phenotype: exploratory genome-wide association analysis in individuals at high risk of bipolar disorder. Psychiatry Res. 2013;206(2–3):223–231. doi: 10.1016/j.psychres.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Sprooten E, Knowles EE, McKay DR, Göring HH, Curran JE, Kent JW, Carless MA, Dyer TD, Drigalenko EI, Olvera RL, Fox PT, Almasy L, Duggirala R, Kochunov P, Blangero J, Glahn DC. Common genetic variants and gene expression associated with white matter microstructure in the human brain. Neuroimage. 2014;97:252–261. doi: 10.1016/j.neuroimage.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen VM, Nepal C, Ersland KM, Holdhus R, Nævdal M, Ratvik SM, Skrede S, Håvik B. Neuropsychological deficits in mice depleted of the schizophrenia susceptibility gene CSMD1. PLoS One. 2013;8(11):e79501. doi: 10.1371/journal.pone.0079501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Medland SE, Vasquez AA, Hibar DP, Senstad RE, Winkler AM, Thompson PM, et al. Identification of common variants associated with human hippocampal and intracranial volumes. Nat Genet. 2012;44(5):552–561. doi: 10.1038/ng.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis : a knowledge-based approach for interpreting genome-wide. PNAS. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamnes CK, Fjell AM, Westlye LT, Østby Y, Walhovd KB. Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci. 2012;32(3):972–982. doi: 10.1523/JNEUROSCI.4779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The Molecular Biology of Axon Guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thomason ME, Thompson PM. Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol. 2011;7:63–85. doi: 10.1146/annurev-clinpsy-032210-104507. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lönnqvist J, Standertskjöld-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat Neurosci. 2001;4(12):1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Drevets W, et al. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 2014;8(2):153–182. doi: 10.1007/s11682-013-9269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JJ, Zaleta AK, Hevelone ND, Rosas HD. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. Proc Natl Acad Sci USA. 2005;102(34):12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken AU, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JDE. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es MA, Van Vught PW, Blauw HM, Franke L, Saris CG, Andersen PM, Van Den Bosch L, de Jong SW, van’t Slot R, Birve A, Lemmens R, de Jong V, Baas F, Schelhaas HJ, Sleegers K, Van Broeckhoven C, Wokke JHJ, Wijmenga C, Robberecht W, Veldink JH, Ophoff RA, van den Berg LH (2007) ITPR2 as a susceptibility gene in sporadic amyotrophic lateral sclerosis: a genome-wide association study. Lancet Neurol 6:869–77. doi: 10.1016/S1474-4422(07)70222-3 [DOI] [PubMed]
- Voineskos AN, Lerch JP, Felsky D, Tiwari A, Rajji TK, Miranda D, Lobaugh NJ, Pollock BG, Mulsant BH, Kennedy JL. The ZNF804A gene: characterization of a novel neural risk mechanism for the major psychoses. Neuropsychopharmacology. 2011;36(9):1871–1878. doi: 10.1038/npp.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Jia P, Wolfinger RD, Chen X, Zhao Z. Gene set analysis of genome-wide association studies: methodological issues and perspectives. Genomics. 2011;98(1):1–8. doi: 10.1016/j.ygeno.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981) WAIS-R manual: Wechsler adult intelligence scale-revised. Psychological Corporation
- Wei Q, Kang Z, Diao F, Guidon A, Wu X, Zheng L, Li L, Guo X, Hu M, Zhang J, Liu C, Zhao J. No association of ZNF804A rs1344706 with white matter integrity in schizophrenia: a tract-based spatial statistics study. Neurosci Lett. 2012;532:64–69. doi: 10.1016/j.neulet.2012.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, Grydeland H, Tamnes CK, Ostby Y, Fjell AM. Life-span changes of the human brain white matter: diffusion tensor imaging (DTI) and volumetry. Cereb Cortex. 2010;20(9):2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Westlye LT, Reinvang I, Rootwelt H, Espeseth T. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79(19):1961–1969. doi: 10.1212/WNL.0b013e3182735c9c. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26(17):2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S, Hong L, Winkler A, Chiappelli J, Nugent K, Muellerklein F, Du X, Rowland L, Wang D, Kochunov P. Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Hum Brain Mapp. 2015;36(10):3793–3804. doi: 10.1002/hbm.22878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.