Abstract
Background
Wilms’ tumor is an embryonal neoplasm of the kidney that accounts for approximately 6 % of all childhood tumors. The chemokine CXCL12 (C-X-C chemokine ligand 12) and its ligand CXCR4 (C-X-C chemokine receptor type 4) are involved in the development of several organs, including the kidney, and are also associated with tumor growth and metastasis. FOXP3 (forkhead transcription factor 3) was initially described as a marker for regulatory T cells; however, its expression in several types of tumor cells has already been described and may have prognostic significance. The aim of the present study was to analyze rs3761548 and rs2232365 FOXP3 polymorphisms, as well as evaluate rs1801157 CXCL12 polymorphism in Wilms’ tumor samples.
Methods
Polymorphisms were evaluated in 32 patients and 78 neoplasia-free controls. Genotypes of rs1801157 were determined using PCR-restriction fragment length polymorphism (PCR-RFLP) method, and genotypes of rs2232365 and rs3761548 were determined using allele-specific PCR (AS-PCR).
Results
The case-control study indicated a significant association for allele A carriers of rs1801157 polymorphism in relation to Wilms’ tumor susceptibility (OR = 5.261; 95 % CI 2.156 to 12.84; p = 0.0002). The opposite was observed in male carriers of G allele for rs2232365 polymorphism (OR 0.1164; 95 % CI 0.0227 to 0.5954; p = 0.0091) or when male and female subjects were analyzed (OR = 0.1304; 95 % CI 0.05013 to 0.3394; p < 0.0001).
Conclusions
All in all, these markers may contribute to this neoplasia susceptibility and progression; however, further studies are needed to real clarify their role in Wilms’ tumor pathogenesis.
Keywords: Wilms’ tumor, FOXP3, CXCL12, Genetic polymorphism
Background
Childhood cancers differ from adult malignant neoplasia in several aspects, such as in primary and histological origins and, also, in clinical outcomes, suggesting they have to be studied independently from adult cancer [1]. Besides, their early onset suggest a low exposition to risk factors, indicating that genetic alterations may have major influences in childhood tumor development [2].
The Wilms’ tumor (WT) develops from nephroblastic remnants, and it is characterized as an embryonal tumor, composed of persistent blastema, dysplastic tubules, and supporting mesenchyme or stroma [3]. It accounts for approximately 6 % of all childhood tumors [4], and its incidence corresponds to 1 in 10,000 children. The majority of WT are usually unilateral and sporadic, with only 1 % considered hereditary [5].
The tumor microenvironment is composed of neoplastic and stromal cells and a great number of immune cells. Interactions among tumor microenvironment components are an emerging issue in tumor progression, influencing growth, invasiveness, and metastatic process [6]. Understanding these complex networks is extremely important for prognostic markers discovery and development of new therapeutic strategies [7].
Chemokines play a major role in several homeostatic [8], pathological [9], and developmental processes [10]. Among them, C-X-C chemokine ligand 12 (CXCL12) and its receptor C-X-C chemokine receptor type 4 (CXCR4) seem to be involved in the development of several organs [11, 12], including kidney [13], and they are also related to tumor growth [14] and metastatic process in many types of cancer [15]. Some authors have investigated polymorphisms of CXCL12 in disease pathogenesis, including cancer, [16] but its value as a susceptibility marker is not well determined.
The forkhead box protein 3 (FOXP3) is a transcription factor that has a fundamental role on the regulation and development of the immune system [17, 18]. Although it was first described as restricted to hematopoietic lineages, recent studies have shown FOXP3 expression in several tissues, including tumor cells [19–21], and it has also been suggested a nuclear or cytoplasmic localization, which can be related with patient prognosis [22].
Genetic analysis of some diseases like psoriasis [23] and breast cancer [24] showed significant association with the single nucleotide polymorphisms (SNP) rs3761548 (−3279 C/A) and rs2232365 (−924 A/G) of FOXP3 gene [25]. The study of these allelic variants can elucidate the role of such polymorphisms in several pathologies, including cancer, concerning to susceptibility, and prognosis.
Recently, a crosstalk between FOXP3 and CXCR4 has been described by Douglass et al. [26]. They demonstrated that downregulated FOXP3 cells have increased CXCR4 expression, and their migration toward CXCL12 gradient is higher when compared with cells who expressed higher FOXP3 levels.
The present study aimed to analyze two polymorphisms in FOXP3 and one polymorphism in CXCL12 in WT samples, in a search for new possible molecular markers to this childhood neoplasia.
Methods
Human subjects
A total of 32 paraffin-embedded samples containing normal and tumor tissues was obtained at University Hospital of the State University of Londrina, Londrina, Paraná, Brazil. Clinical data presented (age, tumor size, and gender) were obtained from clinical pathological reports. For control group, blood samples from 78 neoplasia-free individuals were collected at the same region, with an informed consent signed by their parents. This study was conducted following approval from the Human Ethics Committee of State University of Londrina (CEP/UEL 189/2013 – CAAE 17123113400005231), which was in compliance with the declaration of Helsinki.
DNA extraction
Genomic DNA was isolated from formalin-fixed paraffin-embedded samples, according to innuPREP DNA Mini Kit (Analytik Jena AG, Jena, Germany) protocol, following manufacturer’s instructions. For neoplasia-free control group, DNA was obtained from peripheral blood white cells using the extraction kit Mini Spin (Biometrix, Curitiba, PR, Brazil), according to manufacturer’s instructions. All DNA samples were quantified in NanoDrop 2000® (NanoDrop Technologies, Wilmington, DE, USA).
Genotyping of CXCL12 and FOXP3 polymorphisms
Genotypes of rs1801157 were determined using polymerase chain reaction (PCR)-restriction fragment length polymorphism (PCR-RFLP) method, and genotypes of rs2232365 and rs3761548 were determined using allele-specific PCR (AS-PCR) [23, 27]. Reactions were performed with 100 ng of genomic DNA, 100 μM dNTP, 150 ρM of each primer (Table 1), MgCl2 1.5 mM, buffer 10 %, and 1.25 units of Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA), in a thermocycler A200 Gradient Thermal Cycler (LongGene, Hangzhou, China). PCR products of CXCL12 were subjected to restriction digestion by incubation with MspI (10 U) (Promega, Madison, WI, USA) during 4 h at 37 °C. All PCR products were analyzed by electrophoresis on polyacrylamide gel (10 %) and detected using a silver staining method.
Table 1.
Primer sequences of FOXP3 and CXCL12 genes
| Primer sequence | PCR product | ||
|---|---|---|---|
| RFLP-PCR | rs1801157 | 5′-CAGTCAACCTGGGCAAAGCC-3′ | 293 bp |
| CXCL12 | 5′-CCTGAGAGTCCTTTTGCGGG-3′ | ||
| AS-PCR | 5′-CTGGCTCTCTCCCCAACTGA-3′ | Allele A 334 bp | |
| rs3761548 | 5′-ACAGAGCCCATCATCAGACTCTCTA-3′ | ||
| FOXP3 | 5′-TGGCTCTCTCCCCAACTGC-3′ | Allele C 333 bp | |
| 5′-ACAGAGCCCATCATCAGACTCTCTA-3′ | |||
| 5′-CCCAGCTCAAGAGACCCCA-3′ | Allele A 442 bp | ||
| rs2232365 | 5′-GGGCTAGTGAGGAGGCTATTGTAAC-3′ | ||
| FOXP3 | 5′-CCAGCTCAAGAGACCCCG-3′ | Allele G 427 bp | |
| 5′-GCTATTGTAACAGTCCTGGCAAGTG-3′ | |||
| Sequencing | rs3761548 | 5′-TCTCCGTGCTCAGTGTAGAA-3′ | 330 bp |
| FOXP3 | 5′-AACTAGGCCTCCTGACCTATG-3′ | ||
| rs2232365 | 5′-AGAAGGAGTGGGCATTTGAG-3′ | 284 bp | |
| FOXP3 | 5′-GCAGGTGTAGATAGACATGAAGAG-3′ |
AS-PCR for FOXP3 polymorphisms were confirmed by randomly sequencing in 15 % of the samples. After amplification, PCR products were purified using PureLink™ PCR Purification Kit (Invitrogen), following manufacturer instructions. The sequencing reaction was performed using BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems®, Foster City, CA, USA), 50 ng of DNA template and 5 ρM of primer (forward or reverse) in a final volume of 10 μl. PCR conditions were as follows: 5 min denaturing at 95 °C, 30 cycles of 20 s at 95 °C, 20 s at 50 °C, and 1 min at 60 °C. The amplicons were sequenced in a 24-capillary 3500xl Genetic Analyzer (Applied Biosystems).
Statistical analysis
Case-control study association was assessed through odds ratio (OR) analysis, adopting 95 % confidence interval (CI), and Fisher’s exact test, performed using Prism 6 for Windows (GraphPad Software, San Diego, CA, USA). Since FOXP3 gene in humans is located in the p-arm of the X-chromosome at Xp.11.23, polymorphism analysis was performed separately for genders. p value <0.05 was considered statistically significant.
Results
This retrospective study evaluated 32 tissue samples of pathologically confirmed patients diagnosed with WT between January 1990 and December 2013. The mean age at diagnosis was 45 months (range 1 year–13 years), and more than 76 % of cases diagnosed before the age of 5 years old, which is in accordance with literature data [5].
Nineteen (59.37 %) tumoral tissues were obtained from female patients and 13 (40.63 %) were from male patients. Information regarding tumor size was recovered from 25 (78.12 %) samples, once records available through the hospital were not necessarily historically complete or present. This parameter ranged from 6 to 20.5 cm, with an average of 8 cm, which was used to perform the analysis in relation to genetic variants. From 25 samples, seven (28 %) had tumor size less than or equal to 8 cm and 18 (72 %) had tumor size larger than 8 cm.
Likewise, not all data regarding capsular invasion (n = 17), metastasis (n = 11), lymph node involvement (n = 18), and staging (n = 22) were available. Such parameters are summarized in Table 2.
Table 2.
Histopathological parameters of Wilms’ tumor samples
| Capsular invasion | Presence | 11 |
|---|---|---|
| Absence | 6 | |
| Metastasis | Presence | 7 |
| Absence | 4 | |
| Lymph node involvement | Presence | 5 |
| Absence | 13 | |
| Tumor staging | I | 6 |
| II | 6 | |
| III | 3 | |
| IV | 6 | |
| V | 1 |
Considering WT histology, ten samples were classified as blastemal, six samples presented mixed type, four samples were epithelial, and two monophasic tumors (cells with vesicular nuclei, visible nucleoli, and acidic cytoplasm). It has not been possible to obtain ten samples data regarding tumor histological classification. The preoperative chemotherapy was used in four samples.
In the control group, children and adolescents (age average 12 years old) were included according to negative hematological, biochemical, and serological tests for infectious or chronic diseases and consisted of 37 (47.4 %) females and 41 (52.6 %) males.
CXCL12 genetic polymorphism
The electrophoretic profile of rs1801157 CXCL12 polymorphism is represented in Fig. 1a, b. Figure 1a shows the PCR fragment of 293 bp and the MspI enzyme cut amplicons without the polymorphic variant. Thus, the CXCL12 GG genotype produces 100 and 193 bp products; the AA genotype produces a 293 bp, and the heterozygote genotype GA produces three distinct fragments (Fig. 1b).
Fig. 1.

CXCL12 rs1801157 polymorphism. a Electrophoretic profile of CXCL12 polymorphism. C+ positive control, L ladder 100 bp, S1, S2, S3, S4 samples, NTC no template control. b Electrophoretic profile of MspI treatment products. C+ positive control for both alleles (GA), L ladder 100 bp, GG wild-type homozygote, AA mutant homozygote, GA heterozygote. c Genotype distribution of healthy control (HC) and Wilms’ tumor (WT) individuals. d Odds ratio analysis
The genotype frequency observed for CXCL12 polymorphism for WT patients and controls is represented in Fig. 1c, in which 78 % (22/32) are carriers of the variant allele A. The case-control study indicated a strong positive association of more than fivefold, for A allele carriers, with WT susceptibility (p = 0.0002) (Fig. 1d).
FOXP3 genetic polymorphisms
In the present study, we also investigated two single nucleotide polymorphisms (SNPs) on the promoter region of FOXP3 gene. The electrophoretic profile for polymorphism rs3761548 can be observed in Fig. 2a. As illustrated in Fig. 2b—left panel, genotype frequencies of rs3761548 for female patients and controls were as follows: 47.4 % (9/19) and 54.1 % (20/37) for CC homozygote, 26.3 % (5/19) and 13.5 % (5/37) for CA heterozygote, and 26.3 % (5/19) and 32.4 % (12/37) for AA homozygote, respectively. Male patients and control genotype frequencies for this same polymorphism were as follows: 69.2 % (9/13) and 61.0 % (25/41) for C hemizygote and 30.8 % (4/13) and 39.0 % (16/41) for A hemizygote (Fig. 2b—right panel).
Fig. 2.

Analysis of FOXP3 rs3761548 polymorphism. a Electrophoretic profile. C+ positive control for allele C and A, L ladder 100 bp, CC wild-type homozygote genotype, AA variant homozygote, CA heterozygote genotype, NTC no template control. b Genotype frequency of healthy control (HC) and Wilms’ tumor (WT) individuals, in female individuals (left) and male individuals (right). c Odds ratio analysis
In this work, no significant association was observed for AA homozygotes or A allele carriers in relation to WT susceptibility (p > 0.05; Fig. 2c). Moreover, A allelic frequency of rs3761548 was higher in WT patients (62.5 %) than in the control group (58.97 %).
Regarding the FOXP3 rs2232365 polymorphism, electrophoretic profile is represented in Fig. 3a. The genotype frequency observed was 63.2 % (12/19) and 37.8 % (14/37) for AA homozygotes, 21.0 % (4/19) and 43.3 % (16/37) for AG heterozygotes, and 15.8 % (3/19) and 18.9 % (7/37) for GG homozygote, for female patients and controls, respectively. The genotype frequencies for male patients and controls were as follows, respectively: 85.6 % (11/13) and 39.0 % (16/41) for A hemizygotes and 15.4 % (2/13) and 61.0 % (25/41) for G hemizygotes (Fig. 3b). Some products of AS-PCR for rs3761548 and rs2232365 were confirmed by direct automated sequencing of PCR products using BigDye terminator chemistry kit and 3500 Genetic Analyzer.
Fig. 3.
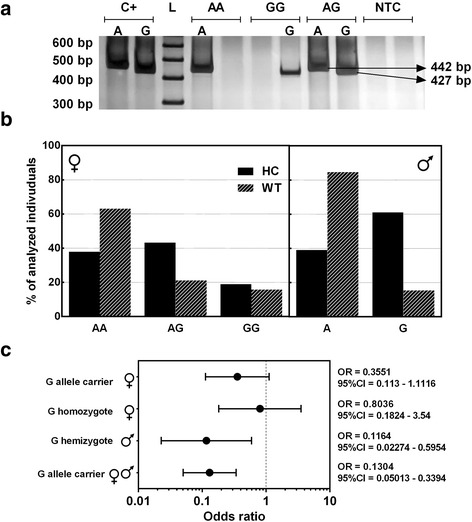
Analysis of FOXP3 rs2232365 polymorphism. a Electrophoretic profile. C+ positive control for allele A and G, L ladder 100 bp, AA wild-type homozygote genotype, GG mutant homozygote genotype, AG heterozygote genotype, NTC no template control. b Genotype frequency of healthy control (HC) and Wilms’ tumor (WT) individuals, in female individuals (left) and male individuals (right). c Odds ratio analysis
The case-control study indicated that G allele carriers of FOXP3 polymorphism rs2232365 were negatively associated with WT susceptibility, comparing male individuals (OR 0.1164; 95 % CI 0.0227 to 0.5954; p = 0.0091), and when male and female subjects were analyzed together (OR = 0.1304; 95 % CI 0.05013 to 0.3394; p < 0.0001) (Fig. 3c).
Discussion
Studies have shown that WT cells express markers of early kidney development [28, 29]. In addition, several studies have highlighted the presence and importance of CXCL12 and CXCR4 during kidney maturation [13, 30–32]. In this context, genotype frequencies of CXCL12 polymorphism rs1801157 have been investigated in order to address its possible role in tumor pathogenesis in different conditions, including acute lymphoblastic leukemia [16], chronic myelogenous leukemia [33], breast cancer [34, 35], and Hodgkin’s lymphoma and non-Hodgkin’s lymphoma [35]. However, there was no study in literature indicating the frequency of this polymorphism in WT patients. In the present case-control study, it was verified a strong positive association for A allele carriers and WT susceptibility (Fig. 1).
Polymorphisms in regulatory regions can change protein expression and may be associated with susceptibility to certain diseases [27]. In fact, the rs1801157 polymorphism is located at a regulatory region of CXCL12; however, there are conflicting results about the influence of this polymorphism in protein expression. Some studies have shown that A allele carriers have increased CXCL12 protein levels [27, 36]; on the other hand, de Oliveira et al. [34] observed that A allele carriers had low levels of CXCL12 messenger RNA (mRNA) compared to GG genotype.
These contrast CXCL12 expression patterns might represent different techniques (serum ELISA, mRNA expression, blot analysis) or biological samples tested (peripheral blood, cultured cells). Moreover, prospective studies should be developed in order to provide rational conclusions on how CXCL12 rs1801157 genotypes would influence gene transcription and/or translation.
It is known that spatial and temporal relationship between CXCL12- and CXCR4-positive cells are required for a regular kidney development [13]. In light of our results, the authors would suggest that A allele carriers, which may express altered CXCL12 levels, could be more susceptible to kidney development disruption.
FOXP3 is an X-linked gene that encodes a transcription factor, which is essential in CD4+CD25+FOXP3 regulatory T (Treg) cells [37]. Treg cells may contribute to tumorigenesis by suppressing immune responses from host, and mutations of this gene have already been reported in cancer patients [38]. To date, there are no studies investigating FOXP3 polymorphisms in WT patients. Regarding the abovementioned, investigation of possible association of FOXP3 genetic variants in WT may shed light on the molecular pathogenesis of this neoplasia, opening up new paths to screening susceptible individuals.
Although AA homozygotes for rs3761548 FOXP3 polymorphism have been considered susceptible to breast neoplasia [24], no significant association was observed for AA homozygotes or A allele carriers in relation to WT susceptibility (Fig. 2c). Furthermore, allelic distribution of rs3761548 A allele in WT patients was slightly different from that in the control group. Concerning FOXP3 rs2232365 polymorphism, the case-control study indicated that G allele is negatively associated with WT susceptibility in male individuals and when males and females subjects were analyzed together (Fig. 3c).
The FOXP3 rs2232365 polymorphism is located within a putative DNA-binding site of another transcription factor, GATA-3, that directly regulates FOXP3 expression, in addition to controlling Treg cell function via interaction with the regulatory regions of the FOXP3 locus. GATA-3 is essential to Th2 immune response [39] and can only bind the FOXP3 promoter region if the A allele is present [40]. The GG genotype of rs2232365 was observed to decrease FOXP3 expression, affecting Treg cell function by disruption of the Th1/Th2 balance [40]. Conventionally, Th2-mediated immunity has been considered to favor tumor growth, by promoting angiogenesis as well inhibiting cell-mediated immunity and tumor cell killing [41, 42]. Hence, we inferred that high frequencies of G allele might affect Treg function and decrease Th2 immune response, leading to a protective effect against tumor development.
FOXP3 transcription factor has different expression patterns in a great variety of cell types, and its role in cancer remains unclear. Nowadays, it is well established that this protein can be expressed by different cell types, aside from its expression in Tregs, which include normal [21] and tumor [20, 43] cells. Studies have supported that FOXP3 protein also has different roles, acting as a tumor suppressor protein [21], or as evading mechanisms for tumors, when expressed by Tregs [44]. In breast cancer, the FOXP3 has been described as a transcriptional repressor of genes involved in tumor development, like HER2 and SKP2 [21], and also in cancer progression, like CXCR4 [26].
Notwithstanding, AA homozygous samples for rs3761548 and rs2232365 of FOXP3 polymorphisms, considered variant and ancestral genotypes, respectively, presented larger tumor size (>8 cm). This could suggest that certain genotypes of FOXP3 gene might contribute, in some way, to disease prognosis.
In another study [24], the variant genotype AA of FOXP3 was also positively associated with tumor size, in triple negative breast cancer. Taken together, these results may indicate a role for this marker in cancer progression, raising new possibilities for research, targeting FOXP3.
Conclusions
In conclusion, the present study demonstrated that FOXP3 rs2232365 is negatively and CXCL12 rs1801157 is positively associated with WT susceptibility. Although the number of WT patients in this case-control study was small, the incidence of this cancer is relatively rare in population. Thus, this study demonstrated, for the first time, an association between FOXP3 and CXCL12 genetic polymorphisms with this cancer, demonstrating that these markers are, somehow, involved in WT pathogenesis. Further studies are needed to define the precise roles in this process.
Acknowledgements
The authors would like to acknowledge Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Araucária do Paraná, Secretaria da Ciência, Tecnologia e Ensino Superior (SETI), Fundo Estadual para a Infância e Adolescência (FIA/PR), Secretaria da Família e Desenvolvimento Social (SEDS), and Pró reitoria de Pós-Graduação da Universidade Estadual de Londrina (PROPPG-UEL).
Authors’ contributions
PMMO collected the samples, performed the PCR-RFLP and AS-PCR, analyzed the results, and wrote the manuscript. CBA collected the samples, performed the statistical analysis, and wrote the manuscript. RLG, ALG, CECO, and MOK interpreted the clinical data and contributed to the writing of the manuscript. BKBH and DLP performed the PCR-RFLP and AS-PCR and wrote the manuscript. MAEW, an advisor, designed the structure of the manuscript, supervised the details, and wrote the final version of the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Informed consent was obtained from all parents of individuals included in the control group of this study.
Ethical approval
The protocol was approved by the Institutional Human Research Ethics Committee of the State University of Londrina, Paraná, Brazil (n°.171231134.0000.5231).
Abbreviations
- AS-PCR
Allele-specific PCR
- CXCL12
C-X-C chemokine ligand 12
- CXCR4
C-X-C chemokine receptor type 4
- FOXP3
Forkhead box protein 3
- PCR
Polymerase chain reaction
- PCR-RFLP
PCR-restriction fragment length polymorphism
- SNP
Single nucleotide polymorphisms
- Treg
Regulatory T cell
- WT
Wilms’ tumor
References
- 1.Malkin D. Cancer of childhood. In: Vita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 5. New York: Lippincott-Raven; 1997. [Google Scholar]
- 2.Davidoff AM. Wilms tumor. Adv Pediatr. 2012;59:247–267. doi: 10.1016/j.yapd.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckwith JB. Renal pathology with clinical and functional correlations. Philadelphia: J B. Lippincott Company; 1994. [Google Scholar]
- 4.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, et al. SEER cancer statistics review, 1975-2001. Bethesda: National Cancer Institute; 2004. [Google Scholar]
- 5.Breslow NE, Beckwith JB, Perlman EJ, Reeve AE. Age distributions, birth weights, nephrogenic rests, and heterogeneity in the pathogenesis of Wilms tumor. Pediatr Blood Cancer. 2006;47:260–267. doi: 10.1002/pbc.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaqub S, Aandahl EM. Inflammation versus adaptive immunity in cancer pathogenesis. Crit Rev Oncog. 2009;15:43–63. doi: 10.1615/CritRevOncog.v15.i1-2.20. [DOI] [PubMed] [Google Scholar]
- 7.Fridman WH, Galon J, Dieu-Nosjean MC, Cremer I, Fisson S, Damotte D, et al. Immune infiltration in human cancer: prognostic significance and disease control. Curr Top Microbiol Immunol. 2011;344:1–24. doi: 10.1007/82_2010_46. [DOI] [PubMed] [Google Scholar]
- 8.Viola A, Luster AD. Chemokines and their receptors: drug targets in immunity and inflammation. Annu Rev Pharmacol Toxicol. 2008;48:171–197. doi: 10.1146/annurev.pharmtox.48.121806.154841. [DOI] [PubMed] [Google Scholar]
- 9.Balkwill F. Chemokine biology in cancer. Semin Immunol. 2003;15:49–55. doi: 10.1016/S1044-5323(02)00127-6. [DOI] [PubMed] [Google Scholar]
- 10.Klein RS, Rubin JB, Gibson HD, DeHaan EN, Alvarez-Hernandez X, Segal RA, et al. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128:1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 11.Nagasawa T, Tachibana K, Kishimoto T. A novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: their functions in development, hematopoiesis and HIV infection. Semin Immunol. 1998;10:179–185. doi: 10.1006/smim.1998.0128. [DOI] [PubMed] [Google Scholar]
- 12.Salcedo R, Oppenheim JJ. Role of chemokines in angiogenesis: CXCL12/SDF-1 and CXCR4 interaction, a key regulator of endothelial cell responses. Microcirculation. 2003;10:359–370. doi: 10.1080/mic.10.3-4.359.370. [DOI] [PubMed] [Google Scholar]
- 13.Takabatake Y, Sugiyama T, Kohara H, Matsusaka T, Kurihara H, Koni PA, et al. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20:1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 16.de Lourdes PA, Guembarovski RL, Oda JM, Lopes LF, Ariza CB, Amarante MK, et al. CXCL12 and TP53 genetic polymorphisms as markers of susceptibility in a Brazilian children population with acute lymphoblastic leukemia (ALL) Mol Biol Rep. 2013;40:4591–4596. doi: 10.1007/s11033-013-2551-1. [DOI] [PubMed] [Google Scholar]
- 17.Coffer PJ, Burgering BM. Forkhead-box transcription factors and their role in the immune system. Nat Rev Immunol. 2004;4:889–899. doi: 10.1038/nri1488. [DOI] [PubMed] [Google Scholar]
- 18.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 19.Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68:3001–3009. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- 20.Karanikas V, Speletas M, Zamanakou M, Kalala F, Loules G, Kerenidi T, et al. Foxp3 expression in human cancer cells. J Transl Med. 2008;6:19. doi: 10.1186/1479-5876-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–1286. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T, et al. FOXP3 expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1:625–632. doi: 10.3892/mco.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao L, Li K, Li F, Li H, Liu L, Wang L, et al. Polymorphisms in the FOXP3 gene in Han Chinese psoriasis patients. J Dermatol Sci. 2010;57:51–56. doi: 10.1016/j.jdermsci.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Lopes LF, Guembarovski RL, Guembarovski AL, Kishima MO, Campos CZ, Oda JM, et al. FOXP3 transcription factor: a candidate marker for susceptibility and prognosis in triple negative breast cancer. Biomed Res Int. 2014;2014:341654. doi: 10.1155/2014/341654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song P, Wang XW, Li HX, Li K, Liu L, Wei C, et al. Association between FOXP3 polymorphisms and vitiligo in a Han Chinese population. Br J Dermatol. 2013;169:571–578. doi: 10.1111/bjd.12377. [DOI] [PubMed] [Google Scholar]
- 26.Douglass S, Meeson AP, Overbeck-Zubrzycka D, Brain JG, Bennett MR, Lamb CA, et al. Breast cancer metastasis: demonstration that FOXP3 regulates CXCR4 expression and the response to CXCL12. J Pathol. 2014;234:74–85. doi: 10.1002/path.4381. [DOI] [PubMed] [Google Scholar]
- 27.Winkler C, Modi W, Smith MW, Nelson GW, Wu X, Carrington M, et al. Genetic restriction of AIDS pathogenesis by an SDF-1 chemokine gene variant. ALIVE Study, Hemophilia Growth and Development Study (HGDS), Multicenter AIDS Cohort Study (MACS), Multicenter Hemophilia Cohort Study (MHCS), San Francisco City Cohort (SFCC) Science. 1998;279:389–393. doi: 10.1126/science.279.5349.389. [DOI] [PubMed] [Google Scholar]
- 28.Li CM, Guo M, Borczuk A, Powell CA, Wei M, Thaker HM, et al. Gene expression in Wilms’ tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am J Pathol. 2002;160:2181–2190. doi: 10.1016/S0002-9440(10)61166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li W, Kessler P, Williams BR. Transcript profiling of Wilms tumors reveals connections to kidney morphogenesis and expression patterns associated with anaplasia. Oncogene. 2005;24:457–468. doi: 10.1038/sj.onc.1208228. [DOI] [PubMed] [Google Scholar]
- 30.Ueland J, Yuan A, Marlier A, Gallagher AR, Karihaloo A. A novel role for the chemokine receptor Cxcr4 in kidney morphogenesis: an in vitro study. Dev Dyn. 2009;238:1083–1091. doi: 10.1002/dvdy.21943. [DOI] [PubMed] [Google Scholar]
- 31.Grone HJ, Cohen CD, Grone E, Schmidt C, Kretzler M, Schlondorff D, et al. Spatial and temporally restricted expression of chemokines and chemokine receptors in the developing human kidney. J Am Soc Nephrol. 2002;13:957–967. doi: 10.1681/ASN.V134957. [DOI] [PubMed] [Google Scholar]
- 32.Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM, et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12:1081–1087. doi: 10.1038/nm1460. [DOI] [PubMed] [Google Scholar]
- 33.de Oliveira CE, Cavassin GG, Perim Ade L, Nasser TF, de Oliveira KB, Fungaro MH, et al. Stromal cell-derived factor-1 chemokine gene variant in blood donors and chronic myelogenous leukemia patients. J Clin Lab Anal. 2007;21:49–54. doi: 10.1002/jcla.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Oliveira KB, Guembarovski RL, Guembarovski AM, da Silva do Amaral Herrera AC, Sobrinho WJ, Ariza CB, et al. CXCL12, CXCR4 and IFNgamma genes expression: implications for proinflammatory microenvironment of breast cancer. Clin Exp Med. 2013;13:211–219. doi: 10.1007/s10238-012-0194-5. [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira KB, Oda JM, Voltarelli JC, Nasser TF, Ono MA, Fujita TC, et al. CXCL12 rs1801157 polymorphism in patients with breast cancer, Hodgkin’s lymphoma, and non-Hodgkin’s lymphoma. J Clin Lab Anal. 2009;23:387–393. doi: 10.1002/jcla.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirata H, Hinoda Y, Kikuno N, Kawamoto K, Dahiya AV, Suehiro Y, et al. CXCL12 G801A polymorphism is a risk factor for sporadic prostate cancer susceptibility. Clin Cancer Res. 2007;13:5056–5062. doi: 10.1158/1078-0432.CCR-07-0859. [DOI] [PubMed] [Google Scholar]
- 37.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 38.Li W, Wang L, Katoh H, Liu R, Zheng P, Liu Y. Identification of a tumor suppressor relay between the FOXP3 and the Hippo pathways in breast and prostate cancers. Cancer Res. 2011;71:2162–2171. doi: 10.1158/0008-5472.CAN-10-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, Yamane H, Cote-Sierra J, Guo L, Paul WE. GATA-3 promotes Th2 responses through three different mechanisms: induction of Th2 cytokine production, selective growth of Th2 cells and inhibition of Th1 cell-specific factors. Cell Res. 2006;16:3–10. doi: 10.1038/sj.cr.7310002. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, You Z, Zhang C, Li Z, Su X, Zhang X, et al. Association between functional polymorphisms of Foxp3 gene and the occurrence of unexplained recurrent spontaneous abortion in a Chinese Han population. Clin Dev Immunol. 2012;2012:896458. doi: 10.1155/2012/896458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212–222. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 43.Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, et al. Foxp3 expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–8350. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- 44.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]


