Abstract
Background
There has been growing interest in understanding how neighborhoods may be related to cardiovascular risk. Neighborhood effects on sleep apnea could be one contributing mechanism. We investigated whether neighborhood walking environment and personal activity levels are related to OSA.
Methods
Data were analyzed from a subpopulation of the Multi-Ethnic Study of Atherosclerosis (MESA), including subjects who participated in both the MESA Sleep and Neighborhood studies (N = 1,896). Perceived neighborhood walking environment and subjects’ objective activity were evaluated in multivariate, multilevel models to determine any association with sleep apnea severity as defined by using the apnea-hypopnea index. Sex, race/ethnicity, and obesity were examined as moderators.
Results
Residing in the lowest quartile walking environment neighborhoods (score < 3.75) was associated with more severe sleep apnea (mean, 2.7 events/h greater AHI [95% CI, 0.7 to 4.6]), after adjusting for demographic characteristics, BMI, comorbidities, health behaviors, neighborhood socioeconomic status, and site. Associations were stronger among obese and male individuals. Approximately 1 SD greater objective activity in men was associated with a lower AHI (mean, –2.4 events/h [95% CI, –3.5 to –1.3]). This association was partially mediated by BMI (P < .001).
Conclusions
Living in neighborhoods with a low walking environment score is associated with greater severity of sleep apnea, especially in male and obese individuals. In men, greater activity level is associated with less severe sleep apnea, independent of BMI, comorbidities, and socioeconomic status. Neighborhood-level interventions that increase walkability and enable increased physical activity may potentially reduce the severity of sleep apnea.
Key Words: activity, neighborhood, OSA
Abbreviations: AHI, apnea-hypopnea index; WE, walking environment
OSA is strongly associated with hypertension, diabetes, stroke, and ischemic heart disease.1, 2, 3 Neighborhood-built environmental features, independent of race and individual socioeconomic status, are also associated with cardiovascular health.4, 5, 6, 7, 8 OSA could represent one pathway through which neighborhood factors influence cardiovascular outcomes.
The built environment may lead to OSA by promoting obesity and reducing physical activity. Perceptions of safety, density of fast food restaurants, and neighborhood disorder have been associated with BMI,9, 10, 11, 12 possibly by affecting health behaviors and cultivating psychological distress,13 which leads to obesity14 and thus promotes OSA. Walkability is negatively associated with BMI.15, 16 Neighborhood features that promote physical activity, including street connectivity, mixed land use, and green space,17, 18, 19 may be inversely associated with OSA. Walkability is associated with greater physical activity levels,20, 21 and exercise interventions have been found to reduce the risk and severity of sleep apnea, independent of inducing weight loss.22, 23 Recent studies have found an association between walkability and incident diabetes,24, 25 which has risk factors similar to those of OSA.
Neighborhood features that limit physical activity may foster sedentary habits.26, 27 Peripheral edema may be more prominent in those with sedentary lifestyles. Research indicates that lower extremity edema can exacerbate OSA by serving as a reservoir of extravascular fluid that can redistribute to the upper airway during sleep, causing airway narrowing.28 Variation in edema has been correlated to interindividual variation in the apnea-hypopnea index (AHI), with greater edema associated with more severe OSA.29 Increased physical activity has been shown to reduce both leg edema and AHI.30
Few studies have investigated the relationship between neighborhood environment and OSA in adults. Neighborhood disadvantage has been associated with OSA in children.31, 32 Neighborhood-level crowding, however, has been associated with greater severity of OSA in adults, which seems to be mediated by BMI.33 Previous studies have examined associations of the neighborhood-built environment with subjective sleep disturbance in adults but did not include objective measures of sleep disorders.34
In the present study, we hypothesized that living in a neighborhood with lower walking environment (WE) scores would be associated with a greater severity of OSA, as assessed by using the AHI, and that this association would be mediated by objective activity level. We also hypothesized that a greater objective activity level would be inversely associated with OSA severity and mediated by BMI. The study explored if sex, obesity, and race/ethnicity moderate these associations as walking behaviors and if the impact of exercise would differ among the groups.
Material and Methods
Data
The Multi-Ethnic Study of Atherosclerosis (MESA) is a longitudinal trial of cardiovascular disease among adults aged 45 to 84 years. Subjects were recruited from the community in six US cities (Los Angeles, CA; New York, NY; Chicago, IL; Winston-Salem, NC; St. Paul, MN; and Baltimore, MD).35 The current sample was restricted to those who agreed to participate in the ancillary MESA Neighborhood Study and the MESA Sleep Study (N = 1,896), which occurred at the 10-year MESA follow-up (examination 5 in 2010-2012). The study was approved by the institutional review boards at each site, and all participants provided written informed consent (e-Appendix 1).
Neighborhood WE was assessed as part of the MESA Neighborhood Study. Participants completed a four-item questionnaire during MESA examination 5, responding on a 5-point scale ranging from “strongly agree” to “strongly disagree.” Questions included: “It is pleasant to walk in my neighborhood,” “In my neighborhood it is easy to walk to places,” “I often see other people walking in my neighborhood,” and “I often see other people exercise in my neighborhood.” An identical questionnaire was administered to a random community sample identified via random-digit dialing or list-based sampling. These participants (n = 4,212), referred to as the Community Survey, resided in the same census tracts as MESA subjects in 2011 and 2012.
The WE scale was calculated by taking the mean of the responses for all respondents from both the MESA and Community Survey samples living within a 1-mile buffer of the participant’s home address, excluding the subject’s survey response. The WE scale has been previously validated (Cronbach alpha, 0.79).36 A higher score on a range of 1 to 5 indicates a better WE. We compared those subjects with a WE score < 3.75 (25% of the sample) vs those residing in neighborhoods with a WE score ≥ 3.75.
Sleep metrics were made through the ancillary MESA Sleep Study. Eligible participants (not on positive airway pressure, an oral appliance, or oxygen) were invited to participate, and 60% agreed. As described previously, participants underwent in-home full polysomnography with a 15-channel monitor (Somte System; Compumedics Ltd.) enabling scoring of sleep stage and respiratory events. Participants wore an Actiwatch Spectrum actigraph (Philips Respironics) on their nondominant wrist for 1 week.37 Our main outcome was the AHI calculated by using the American Academy of Sleep Medicine’s hypopnea scoring criterion of ≥ 3% desaturation or an arousal.38 Objective activity level was derived from 7-day wrist actigraphy findings. The activity status for each 30-s epoch was computed by using the Actiware-Sleep version 5.59 (Mini-Mitter Co. Inc.) scoring algorithm. Sleep periods were identified by technicians using sleep diaries, light levels, event markers, and activity levels.39 Activity counts per minute divided by 100, approximating the SD, were averaged for periods scored as active (excluding sleep periods and nonwear time) over the 7-day recording.
The sociodemographic and comorbidity data were obtained from the MESA health and medical history surveys and examination. Subjects reported race/ethnicity (white non-Hispanic, black non-Hispanic, Hispanic, and Chinese), cigarette smoking status (never, current/former), education level (dichotomized according to college degree or higher), and household income (categorized as less than $25,000, $25,000-75,000, or greater than $75,000). Depressive symptoms were based on a Center for Epidemiologic Studies-Depression scale score > 16.40 Diabetes status was categorized based on the 2003 American Diabetes Association criteria (impaired fasting glucose, untreated diabetes, and treated diabetes).41 Hypertension was defined as BP ≥ 140/90 mm Hg or use of antihypertensive medication. Measured weight and height were used to calculate BMI category (< 25, 25-29.9, 30-40, and > 40 kg/m2). To account for neighborhood socioeconomic status, a factor scale for neighborhood socioeconomic status was used, generated from census tract-level data from the American Community Survey 5-year estimates from 2007 through 2011. These data comprised information on median household value, percentage of subjects with college education, percent with a managerial/professional occupation, percent with a high school diploma, and median household income.9
Analysis
The WE score was dichotomized after identifying a threshold effect for the lowest quartile with our outcome of AHI and a lack of a linear or dose-response relationship. Subjects living in neighborhoods with the lowest quartile WE (< 3.75) were compared with the rest of the cohort by using sample characteristics; χ2 tests were used for categorical variables and t tests for continuous variables.
We then examined the associations of AHI with neighborhood WE score and with actigraphy-derived activity counts by using two linear mixed, two-level models with random effect to account for clustering according to census tract as a proxy for neighborhood. Models adjusted for age, race/ethnicity, sex (except in stratified analyses), household income, education, study site, cigarette smoking, diabetes, hypertension, depressive symptoms, and neighborhood socioeconomic status. Observations with any missing covariates were excluded. In secondary analyses, multiple imputation was applied to account for missing data (e-Appendix 1).42, 43 BMI was included in select models. Effect modification was evaluated according to sex and obesity (BMI ≥ 30 kg/m2; when BMI was not identified as a mediator) by adding an interaction term and performing a stratified analysis; in exploratory analysis, effect modification was evaluated according to race/ethnicity.
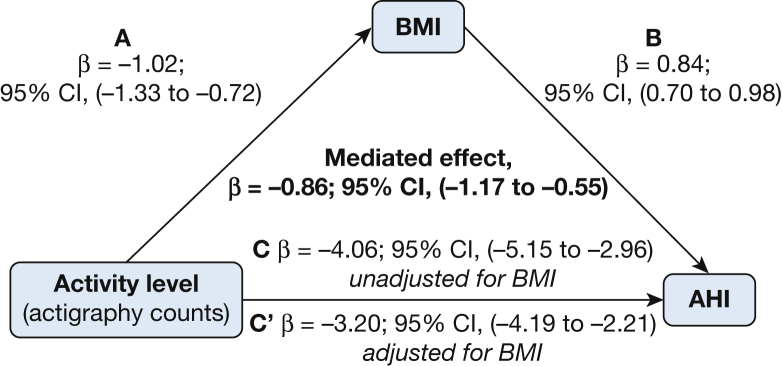
We examined if activity level mediated the association between WE and AHI, as well if BMI mediated the association between activity level and AHI. Using methods described by Baron and Kenny,44 a series of regression models were fit (Fig 1); mixed effects models were used with the same clustering according to census tract. The mediated effect point estimate was calculated by using methods described by Krull and MacKinnon45 using boot-strapping to calculate the 95% CIs.
Figure 1.
A-C, Mediation analysis demonstrating that the association of activity level on AHI is mediated by BMI. Activity level is associated with both (A) BMI and (C) AHI. (B) BMI is also strongly associated with AHI. The mediated effect is C-C′ or the difference in the point estimate of activity level on AHI without and with adjustment for BMI. AHI = apnea-hypopnea index.
Results
Of the 1,896 MESA subjects with sleep and neighborhood data included in the analysis, the mean ± SD age was 68.5 ± 9.1 years and BMI was 28.6 ± 5.5 kg/m2, with racial/ethnic and geographic diversity (Table 1). The median WE score for the subject neighborhood was 3.9 (interquartile range, 3.75-4.15); the median AHI was 14.4 (interquartile range, 6.9-27.2). Of those residing in neighborhoods with a lower WE score (< 3.75), a significantly greater proportion of subjects were black, hypertensive, diabetic, obese, had a household income less than $25,000, had an AHI ≥ 30, and were from the Baltimore, Maryland, site. A lower proportion of subjects were white, college educated, had a household income greater than $75,000, and were from the St. Paul, Minnesota, site.
Table 1.
MESA Neighborhood and Sleep Cohort Characteristics, N = 1,896
| Characteristic | All Subjects (n = 1,896) | WE ≥ 3.75 (n = 1,424) | WE < 3.75 (n = 472) |
|---|---|---|---|
| BMI, mean ± SD, kg/m2a | 28.6 ± 5.5 | 28.3 ± 5.4 | 29.6 ± 5.7 |
| Age, mean ± SD, y | 68.5 ± 9.1 | 68.3 ± 9.0 | 69.0 ± 9.3 |
| Male sex | 45.6 (864) | 45.5 (648) | 45.8 (216) |
| Racea | |||
| White | 35.7 (677) | 41.0 (584) | 19.7 (93) |
| Chinese-American | 12.2 (231) | 12.6 (180) | 10.8 (51) |
| Black | 28.4 (538) | 22.5 (321) | 46.0 (217) |
| Hispanic | 23.7 (450) | 23.8 (339) | 23.5 (111) |
| College educationa,b (bachelor degree and higher) | 39.6 (750) | 44.0 (626) | 26.3 (124) |
| Hypertensiona,b | 57.0 (1080) | 54.4 (774) | 64.8 (306) |
| Diabetesa,b | 39.9 (750) | 38.6 (545) | 44.0 (205) |
| Depression (CES-D ≥ 16)b | 14.5 (269) | 14.4 (201) | 14.8 (68) |
| Obese (BMI ≥ 30 kg/m2)a,b | 34.7 (656) | 32.2 (457) | 42.3 (199) |
| Smokerb | 45.7 (860) | 45.6 (646) | 45.9 (214) |
| Household income, $a,b | |||
| < 25,000 | 26.6 (489) | 24 (335) | 34 (154) |
| 25,000-75,000 | 45 (829) | 44 (612) | 48 (217) |
| > 75,000 | 28.4 (524) | 32 (444) | 18 (80) |
| Sitea | |||
| Wake Forest University (Winston-Salem, NC) | 14.9 (282) | 13.4 (191) | 19.3 (91) |
| Columbia University (NY, NY) | 18.5 (351) | 19.1 (272) | 16.7 (79) |
| Johns Hopkins University (Baltimore, MD) | 14.3 (271) | 11.4 (162) | 23.0 (109) |
| University of Minnesota (St. Paul, MN) | 17.1 (324) | 20.7 (295) | 9.0 (29) |
| Northwestern University (Chicago, IL) | 19.0 (360) | 20.7 (295) | 13.8 (65) |
| University of California, Los Angeles (Los Angeles, CA) | 16.2 (308) | 14.7 (209) | 21.0 (99) |
| Neighborhood scales | |||
| Neighborhood WE score, median (IQR) | 3.9 (3.75-4.15) | 4.0 (3.9-4.3) | 3.6 (3.5-3.7) |
| Actigraphy data | |||
| Activity counts, median (IQR), per min during active intervals | 218.2 (172.1-275.6) | 219.1 (173.4-275.5) | 216.3 (168.4-275.6) |
| PSG data | |||
| AHI ≥ 15 | 47.8 (894) | 47.6 (672) | 48.4 (223) |
| AHI (hypopnea defined as 3% desaturation or cortical arousal), median (IQR) | 14.4 (6.9-27.2) | 14.3 (6.8-26.6) | 14.6 (7.2-29.2) |
| AHI ≥ 30a | 20.8 (390) | 19.7 (278) | 24.3 (112) |
Unless otherwise indicated, data are presented as % (no.). AHI = apnea-hypopnea index; CES-D = Center for Epidemiologic Studies-Depression; IQR = interquartile range; PSG = polysomnography; WE = walking environment.
Significant difference between lowest quartile neighborhood WE and all other quartiles, P < .05, via χ2 test for categorical variables and Wilcoxon rank sum test for continuous variables.
Missing values: smoking (n = 14), education (n = 4), BMI (n = 4), depression (n = 35), hypertension (n = 5), diabetes (n = 18), household income (n = 54), actigraphy-derived activity counts (n = 81), and neighborhood socioeconomic status (n = 46). Details of imputation are provided in e-Appendix 1.
Living in the lowest quartile WE neighborhoods was associated with higher AHI, (mean, 3.21 more events/h [95% CIs, 1.13 to 5.29]) in adjusted models (Table 2, model 1). The association was attenuated after adjusting for BMI but remained significant (model 2). Obesity (BMI ≥ 30 kg/m2) and sex seemed to modify the association between WE and AHI (Pint = .05 for obesity; Pint = .06 for sex), with a significant association between WE and AHI among obese subjects and men. Results of stratified exploratory analyses indicate that the association between low WE and AHI was strongest among black participants (e-Table 1).
Table 2.
Mean Difference in AHI According to Low WE Score (< 3.75) in Adjusted Models
| WE Score | Model 1, N = 1,732 |
Model 2, n = 1,730 |
Model (Men), n = 787 | Model 3B (Women), n = 943 | Model 4A (BMI ≥ 30 kg/m2), n = 598 |
Model 4B (BMI < 30 kg/m2), n = 1132 |
|---|---|---|---|---|---|---|
| < 3.75 | 3.21 (1.13 to 5.29) | 2.73 (0.74 to 4.63) | 3.74 (0.24 to 7.24) | 0.81 (–1.46 to 3.07) | 5.21 (1.42 to 9.01) | 1.02 (–1.25 to 3.29) |
Data are presented as ß (95% confidence interval), N = 1,732. All models were adjusted for age, race/ethnicity, sex (except model 3), BMI (except models 1 and 4), education, site, diabetes, hypertension, depression, smoking status, household income, and neighborhood socioeconomic status with two-level mixed effects linear model with random effect for census tract. See Table 1 legend for expansion of abbreviations.
Living in the lowest quartile WE neighborhood was not associated with activity level in mixed-level models, and activity level did not mediate the association between low WE and AHI. Higher activity level was associated with lower AHI; approximately 1 SD greater activity was associated with an average decrease in AHI by 3.3 events/h (Table 3). This association was attenuated after adjusting for BMI and low WE score, but it was still statistically significant. The effect of activity on AHI differed by sex (Pint = .04), with the association observed among men (AHI mean difference, –3.61 [95% CI, –5.41 to –1.81]) but not women. Similarly, a significant association was seen among black and Hispanic subjects but not among white or Chinese-American subjects (e-Table 2) when stratified.
Table 3.
Mean Difference in AHI According to Activity Level, Standardized by Approximately 1 SD of Actigraphy-Measured Counts Per Minute
| Level | Model 1 N = 1,781 |
Model 2 n = 1,781 |
Model 3A (Men), n = 820 |
Model 3B (Women), n = 908 |
Model 4,a n = 1,656 |
|---|---|---|---|---|---|
| Activity count (per min/100) | –3.32 (–4.43 to –2.20) | –2.40 (–3.48 to –1.32) | –3.61 (–5.41 to –1.81) | –0.81 (–2.09 to 0.47) | –2.12 (–3.25 to –1.00) |
Data are presented as ß (95% confidence interval), N = 1,781 in adjusted models. All models were adjusted for age, race/ethnicity, sex (except model 3), BMI (except model 1) site, diabetes, hypertension, depression, smoking status, household income, education, and neighborhood socioeconomic status with two-level mixed effects linear model with random effect for census tract.
Lowest quartile neighborhood walking environment score included in model 4.
The association of activity level with AHI was partially mediated by BMI. The mediated effect of BMI was –0.86 event/h (95% CI, –1.17 to –0.55) (Fig 1). BMI explained 21% of this association.
Sensitivity analyses using imputed data for the sample with missing covariate data revealed modest attenuation of the main effects. For the fully adjusted models, the mean AHI change associated with low WE was 1.89 events/h (95% CI, 0.02 to 3.80), and with activity level, it was –1.92 events/h (95% CI, –3.01 to –0.82) (e-Tables 3 and 4).
Discussion
This study provides novel data linking neighborhood WE with sleep apnea. We found that living in the least walkable neighborhoods was associated with greater severity of OSA, specifically in male and obese subjects. The association persisted after adjusting for comorbidities and sociodemographic characteristics. The mean difference of a 2.7 AHI associated with neighborhood is comparable to a 5% to 10% weight loss or 20% of the effect of using a mandibular advancement device or undergoing uvulopalatopharyngoplasty, depending on baseline severity.46, 47, 48 Living in a less walkable environment may promote a sedentary lifestyle and favor vehicular transportation.27 Reduced walking might also promote peripheral edema, which has been identified as a risk factor for sleep apnea.28 Although higher neighborhood walkability has not been associated with less sitting time,49, 50 greater walkability may encourage greater physical activity and promote social interaction and time spent outdoors.51
A greater objectively recorded activity level was associated with lower AHI independent of WE. This association was only partially mediated by BMI, suggesting a beneficial effect of physical activity on OSA independent of BMI. This finding supports previous studies that reported a reduction in AHI and a decrease in incident OSA with exercise interventions that did not result in weight loss.22, 23, 30, 52 Potential mechanisms include reduction in dependent edema and rostral fluid shift to the pharynx during the night.30 In addition, exercise may lead to a reduction in visceral adipose tissue, which might also reduce the risk of OSA.53 The stronger association between activity level and AHI in men may be due to sex differences in the distribution of fluid, including a male predisposition to accumulate neck fluid volume when supine.54
A stronger association between WE and a lower AHI was observed in men compared with women. Neighborhood attributes may also have less of an impact on walking behavior in women due to a greater underlying fear of crime than men.17
There was limited power to detect modification of associations by race/ethnicity. However, stratified analyses suggest that associations were strongest in black subjects (for both WE and activity level) and Hispanic subjects (for activity level). These data suggest the potential beneficial role of environmental and behavioral interventions in minority groups at increased risk for OSA.
No association was observed between WE and objective activity level; accordingly, there was no evidence of mediation according to activity level as hypothesized. The WE score reflects perceived environment for walking and may only roughly reflect actual walking/exercise behavior.55, 56, 57 Misclassification of physical activity level with use of wrist actigraphy may bias the association toward the null.
The strengths of the present study include the diversity of the subjects (both in racial/ethnic background and geography), the large sample size gathered from the community, and the use of objective sleep and activity measures. A well-validated neighborhood WE scale was used, which provided information unbiased by the participant’s own response, reducing the effect of same source bias.9, 11, 25 This study confirms previous findings of the relationship of neighborhood features to sleep disturbance35 but with objective measures of sleep apnea and actigraphy to provide an objective measure of wake-time activity.
Limitations of the study include the cross-sectional and observational nature of the analysis, restricting evaluation of causality and the direction of association. The use of mediation analysis with cross-sectional data should be viewed cautiously.58 We also only captured a 1-week snapshot of activity, considering current neighborhood and BMI, and did not account for previous neighborhoods and factors leading to the development of obesity and OSA. Our WE measure did not include geographical information systems data on street connectivity, population density, or recreational facilities to better assess walkability20, 56, 58 and perhaps walking behavior. In addition, the sample of MESA participants in the present study cohort had a mean age of 68.5 years, and our findings may thus not be generalizable to younger individuals.
Conclusions
This study provides strong evidence that neighborhood WE is associated with severity of sleep apnea, with associations strongest in men and among obese as well as black subjects. Urban planning may be an important tool of public health to improve cardiovascular and sleep health, potentially reducing OSA and health disparities. Being more active during the day may reduce the severity of OSA. Public health measures that increase walking behaviors could potentially reduce the severity of sleep disorders and resulting cardiovascular disease. Further studies are needed to assess for longitudinal changes in OSA severity as neighborhood walkability evolves. Ultimately, large-scale public health studies are need to evaluate if neighborhood-level interventions aimed at improving walkability and activity levels lead to reductions in OSA severity and improved sleep health.
Acknowledgments
Author contributions: M. E. B. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. D. A. J. and K. M. contributed substantially to data analysis and interpretation; and G. S., S. R. P., S. R., and A. V. D. R. contributed substantially to the study design, data interpretation, and the writing of the manuscript.
Financial/nonfinancial disclosure: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
This material has been reviewed by the Walter Reed Army Institute of Research, and there is no objection to its presentation and/or publication. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the position of the Department of the Army of the Department of Defense.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This research was supported by the National Heart, Lung and Blood Institute (NHLBI) at the National Institutes of Health through contracts N01-HC-95159 through N01-HC-95169 and by grants UL1-TR-000040 and UL1-TR-001079 from the National Center for Research Resources. Additional funding for the ancillary MESA Neighborhood Study was provided by NHLBI grant R01 HL071759 (A. V. D. R.) and for the ancillary MESA Sleep Study from NHLBI grant R01 L098433 (S. R.).
Supplementary Data
References
- 1.Marin J.M., Carrizo S.J., Vicente E., Agusti A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 2.Young T., Peppard P. Sleep-disordered breathing and cardiovascular disease: epidemiologic evidence for a relationship. Sleep. 2000;23(suppl 4):S122–S126. [PubMed] [Google Scholar]
- 3.Yaggi H.K., Concato J., Kernan W.N., Lichtman J.H., Brass L.M., Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 4.Diez Roux A.V., Merkin S.S., Arnett D. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 5.Clark C.J., Guo H., Lunos S. Neighborhood cohesion is associated with reduced risk of stroke mortality. Stroke. 2011;42(5):1212–1217. doi: 10.1161/STROKEAHA.110.609164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Augustin T., Glass T.A., James B.D., Schwartz B.S. Neighborhood psychosocial hazards and cardiovascular disease: the Baltimore Memory Study. Am J Public Health. 2008;98(9):1664–1670. doi: 10.2105/AJPH.2007.125138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerber Y., Benyamini Y., Goldbourt U., Drory Y. Israel Study Group on First Acute Myocardial Infarction. Neighborhood socioeconomic context and long-term survival after myocardial infarction. Circulation. 2010;121(3):375–383. doi: 10.1161/CIRCULATIONAHA.109.882555. [DOI] [PubMed] [Google Scholar]
- 8.Lisabeth L.D., Diez Roux A.V., Escobar J.D., Smith M.A., Morgenstern L.B. Neighborhood environment and risk of ischemic stroke: the Brain Attack Surveillance in Corpus Christi (BASIC) Project. Am J Epidemiol. 2007;165(3):279–287. doi: 10.1093/aje/kwk005. [DOI] [PubMed] [Google Scholar]
- 9.Moore K., Diez Roux A.V., Auchincloss A. Home and work neighbourhood environments in relation to body mass index: the Multi-Ethnic Study of Atherosclerosis (MESA) J Epidemiol Community Health. 2013;67(10):846–853. doi: 10.1136/jech-2013-202682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fish J.S., Ettner S., Ang A., Brown A.F. Association of perceived neighborhood safety with [corrected] body mass index. Am J Public Health. 2010;100(11):2296–2303. doi: 10.2105/AJPH.2009.183293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auchincloss A.H., Mujahid M.S., Shen M., Michos E.D., Whitt-Glover M.C., Diez Roux A.V. Neighborhood health-promoting resources and obesity risk (the Multi-Ethnic Study of Atherosclerosis) Obesity (Silver Spring) 2013;21(3):621–628. doi: 10.1038/oby.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdette H.L., Wadden T.A., Whitaker R.C. Neighborhood safety, collective efficacy, and obesity in women with young children. Obesity (Silver Spring) 2006;14(3):518–525. doi: 10.1038/oby.2006.67. [DOI] [PubMed] [Google Scholar]
- 13.Diez Roux A.V., Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 14.Lovasi G.S., Hutson M.A., Guerra M., Neckerman K.M. Built environments and obesity in disadvantaged populations. Epidemiol Rev. 2009;31:7–20. doi: 10.1093/epirev/mxp005. [DOI] [PubMed] [Google Scholar]
- 15.Lovasi G.S., Neckerman K.M., Quinn J.W., Weiss C.C., Rundle A. Effect of individual or neighborhood disadvantage on the association between neighborhood walkability and body mass index. Am J Public Health. 2009;99(2):279–284. doi: 10.2105/AJPH.2008.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mujahid M.S., Diez Roux A.V., Shen M. Relation between neighborhood environments and obesity in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167(11):1349–1357. doi: 10.1093/aje/kwn047. [DOI] [PubMed] [Google Scholar]
- 17.Foster S., Giles-Corti B. The built environment, neighborhood crime and constrained physical activity: an exploration of inconsistent findings. Prev Med. 2008;47(3):241–251. doi: 10.1016/j.ypmed.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Müller-Riemenschneider F., Pereira G., Villanueva K. Neighborhood walkability and cardiometabolic risk factors in Australian adults: an observational study. BMC Public Health. 2013;13:755. doi: 10.1186/1471-2458-13-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brownson R.C., Hoehner C.M., Day K., Forsyth A., Sallis J.F. Measuring the built environment for physical activity: state of the science. Am J Prev Med. 2009;36(4 suppl):S99–S123. doi: 10.1016/j.amepre.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sundquist K., Eriksson U., Kawakami N., Skog L., Ohlsson H., Arvidsson D. Neighborhood walkability, physical activity, and walking behavior: the Swedish Neighborhood and Physical Activity (SNAP) study. Soc Sci Med. 2011;72(8):1266–1273. doi: 10.1016/j.socscimed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez D.A., Evenson K.R., Diez Roux A.V., Brines S.J. Land use, residential density, and walking. The Multi-Ethnic Study of Atherosclerosis. Am J Prev Med. 2009;37(5):397–404. doi: 10.1016/j.amepre.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Awad K.M., Malhotra A., Barnet J.H., Quan S.F., Peppard P.E. Exercise is associated with a reduced incidence of sleep-disordered breathing. Am J Med. 2012;125(5):485–490. doi: 10.1016/j.amjmed.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kline C.E., Crowley E.P., Ewing G.B. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631–1640. doi: 10.5665/sleep.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sundquist K., Eriksson U., Mezuk B., Ohlsson H. Neighborhood walkability, deprivation and incidence of type 2 diabetes: a population-based study on 512,061 Swedish adults. Health Place. 2015;31:24–30. doi: 10.1016/j.healthplace.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christine P.J., Auchincloss A.H., Bertoni A.G. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA) JAMA Intern Med. 2015;175(8):1311–1320. doi: 10.1001/jamainternmed.2015.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Dyck D., Cerin E., Conway T.L. Associations between perceived neighborhood environmental attributes and adults' sedentary behavior: findings from the U.S.A., Australia and Belgium. Soc Sci Med. 2012;74(9):1375–1384. doi: 10.1016/j.socscimed.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kozo J., Sallis J.F., Conway T.L. Sedentary behaviors of adults in relation to neighborhood walkability and income. Health Psychol. 2012;31(6):704–713. doi: 10.1037/a0027874. [DOI] [PubMed] [Google Scholar]
- 28.White L.H., Bradley T.D. Role of nocturnal rostral fluid shift in the pathogenesis of obstructive and central sleep apnoea. J Physiol. 2013;591(5):1179–1193. doi: 10.1113/jphysiol.2012.245159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White L.H., Lyons O.D., Yadollahi A., Ryan C.M., Bradley T.D. Night-to-night variability in obstructive sleep apnea severity: relationship to overnight rostral fluid shift. J Clin Sleep Med. 2015;11(2):149–156. doi: 10.5664/jcsm.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Redolfi S., Bettinzoli M., Venturoli N. Attenuation of obstructive sleep apnea and overnight rostral fluid shift by physical activity. Am J Respir Crit Care Med. 2015;191(7):856–858. doi: 10.1164/rccm.201412-2192LE. [DOI] [PubMed] [Google Scholar]
- 31.Brouillette R.T., Horwood L., Constantin E., Brown K., Ross N.A. Childhood sleep apnea and neighborhood disadvantage. J Pediatr. 2011;158(5):789–795.e1. doi: 10.1016/j.jpeds.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 32.Spilsbury J.C., Storfer-Isser A., Kirchner H.L. Neighborhood disadvantage as a risk factor for pediatric obstructive sleep apnea. J Pediatr. 2006;149(3):342–347. doi: 10.1016/j.jpeds.2006.04.061. [DOI] [PubMed] [Google Scholar]
- 33.Johnson D.A., Drake C., Joseph C.L., Krajenta R., Hudgel D.W., Cassidy-Bushrow A.E. Influence of neighbourhood-level crowding on sleep-disordered breathing severity: mediation by body size. J Sleep Res. 2015;24(5):559–565. doi: 10.1111/jsr.12305. [DOI] [PubMed] [Google Scholar]
- 34.Desantis A.S., Diez Roux A.V., Moore K., Baron K.G., Mujahid M.S., Nieto F.J. Associations of neighborhood characteristics with sleep timing and quality: the Multi-Ethnic Study of Atherosclerosis. Sleep. 2013;36(10):1543–1551. doi: 10.5665/sleep.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bild D.E., Bluemke D.A., Burke G.L. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 36.Mujahid M.S., Diez Roux A.V., Morenoff J.D., Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–867. doi: 10.1093/aje/kwm040. [DOI] [PubMed] [Google Scholar]
- 37.Chen X., Wang R., Zee P. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA) Sleep. 2015;38(6):877–888. doi: 10.5665/sleep.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry R.B., Budhiraja R., Gottlieb D.J. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oakley NR. Validation with polysomnography of the Sleepwatch Sleep/Wake Scoring Algorithm used by the Actiwatch Activity Monitoring System. Technical report. Bend, OR: Mini-Mitter; 1997.
- 40.Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 41.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26 Suppl 1:S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 42.Comulada W.S. Model specification and bootstrapping for multiply imputed data: an application to count models for the frequency of alcohol use. Stata J. 2015;15(3):833–844. [PMC free article] [PubMed] [Google Scholar]
- 43.Sterne J.A., White I.R., Carlin J.B. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baron R.M., Kenny D.A. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 45.Krull J.L., MacKinnon D.P. Multilevel mediation modeling in group-based intervention studies. Eval Rev. 1999;23(4):418–444. doi: 10.1177/0193841X9902300404. [DOI] [PubMed] [Google Scholar]
- 46.Lim J., Lasserson T.J., Fleetham J., Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006;(1):CD004435. doi: 10.1002/14651858.CD004435.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boyd S.B., Walters A.S., Song Y., Wang L. Comparative effectiveness of maxillomandibular advancement and uvulopalatopharyngoplasty for the treatment of moderate to severe obstructive sleep apnea. J Oral Maxillofac Surg. 2013;71(4):743–751. doi: 10.1016/j.joms.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peppard P.E., Young T., Palta M., Dempsey J., Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 49.Barnett A., Cerin E., Ching C.S., Johnston J.M., Lee R.S. Neighbourhood environment, sitting time and motorised transport in older adults: a cross-sectional study in Hong Kong. BMJ Open. 2015;5(4):e007557. doi: 10.1136/bmjopen-2014-007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Dyck D., Cardon G., Deforche B., Owen N., Sallis J.F., De Bourdeaudhuij I. Neighborhood walkability and sedentary time in Belgian adults. Am J Prev Med. 2010;39(1):25–32. doi: 10.1016/j.amepre.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 51.Ross C.E., Mirowsky J. Neighborhood disadvantage, disorder, and health. J Health Soc Behav. 2001;42(3):258–276. [PubMed] [Google Scholar]
- 52.Iftikhar I.H., Kline C.E., Youngstedt S.D. Effects of exercise training on sleep apnea: a meta-analysis. Lung. 2014;192(1):175–184. doi: 10.1007/s00408-013-9511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goedecke J.H., Micklesfield L.K. The effect of exercise on obesity, body fat distribution and risk for type 2 diabetes. Med Sport Sci. 2014;60:82–93. doi: 10.1159/000357338. [DOI] [PubMed] [Google Scholar]
- 54.Yadollahi A., Singh B., Bradley T.D. Investigating the dynamics of supine fluid redistribution within multiple body segments between men and women. Ann Biomed Eng. 2015;43(9):2131–2142. doi: 10.1007/s10439-015-1264-0. [DOI] [PubMed] [Google Scholar]
- 55.Duncan G.E., Cash S.W., Horn E.E., Turkheimer E. Quasi-causal associations of physical activity and neighborhood walkability with body mass index: a twin study. Prev Med. 2015;70:90–95. doi: 10.1016/j.ypmed.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dannenberg A.L., Frumkin H., Jackson R. Island Press; Washington, DC: 2011. Making Healthy Places: Designing and Building for Health, Well-Being, and Sustainability. [Google Scholar]
- 57.Hirsch J.A., Moore K.A., Clarke P.J. Changes in the built environment and changes in the amount of walking over time: longitudinal results from the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2014;180(8):799–809. doi: 10.1093/aje/kwu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maxwell S.E., Cole D.A. Bias in cross-sectional analyses of longitudinal mediation. Psychol Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.