Abstract
Glaucoma is characterized by axonal degeneration of retinal ganglion cells (RGCs) and apoptotic death of their cell bodies, and lowering intraocular pressure is associated with an attenuation of progressive optic nerve damage. Nevertheless, intraocular pressure (IOP) reduction alone was not enough to inhibit the progression of disease, which suggests the contribution of other factors to the glaucoma pathogenesis. In this study, we investigated the cytoprotective effect of geranylgeranylacetone (GGA) on RGCs degeneration using a normal tension glaucoma (NTG) mouse model, which lacks glutamate/aspartate transporter (GLAST) and demonstrates spontaneous RGC and optic nerve degeneration without elevated intraocular pressure (IOP). Three-week-old GLAST+/− mice were given oral administration of GGA at 100, 300, or 600 mg/kg/day or vehicle alone, and littermate control mice were given vehicle alone for 14 days, respectively. At 5 weeks after birth, the number of RGCs was counted in paraffin sections of retinal tissues stained with hematoxylin and eosin. In addition, retrograde labeling technique was also used to quantify the number of RGC. Expression and localization of heat shock protein 70 (HSP70) in retinas were evaluated by reverse transcription polymerase chain reaction and immunohistochemistry, respectively. Activities of caspase-9 and -3 in retinas were also assessed. The number of RGCs of GLAST+/− mice significantly decreased, as compared to that of control mice. RGC loss was significantly suppressed by administration of GGA at 600 mg/kg/day, compared with vehicle alone. Following GGA administration, HSP70 was significantly upregulated together with reduction in the activities of caspase-9 and -3. Our studies highlight HSP70 induction in the retina is available to suppress RGC degeneration, and thus GGA may be applicable for NTG as a promising therapy.
Keywords: Cell biology, Neuroscience
1. Introduction
Glaucoma is one of the leading causes of blindness worldwide (Quigley et al., 2006), characterized by axonal degeneration of retinal ganglion cells (RGCs) and apoptotic death of their cell bodies. Typically, glaucoma is associated with chronic elevation in intraocular pressure (IOP), and lowering IOP is associated with an attenuation of progressive optic nerve damage (Kass et al., 2010; Collaborative Normal-Tension Glaucoma Study Group, 1998; The Advanced Glaucoma Intervention Study, 2000). Nevertheless, a growing body of evidence have demonstrated that IOP reduction alone was not enough to inhibit the progression of disease (Koseki et al., 1997; Shigeeda et al., 2002), which suggests the contribution of other factors to the glaucoma pathogenesis. In Japan where normal tension glaucoma (NTG) comprises the majority of glaucoma (Iwase et al., 2004), it is of special importance to elucidate IOP-independent factors and explores alternative therapeutic strategy such as cytoprotection of RGCs.
Heat shock proteins (HSPs), the major molecular chaperons, comprise a family of stress-induced proteins, highly conserved and rapidly induced in response to stress to counteract the formation of aberrantly folded proteins (Young et al., 2004; Itoh and Tashima, 1991). Up to date, multiple lines of evidence have revealed that HSP with a molecular mass of 70 kDa (HSP70), plays a critical role in cytoprotection, preventing apoptosis and allowing cellular adaptation in stress conditions (Rajdev et al., 2000; Kawana et al., 2000; McClellan and Frydman, 2001; Zhan et al., 2010).
Recently, numerous studies have demonstrated that systemic administration of geranylgeranylacetone (GGA), an acylic isoprenoid, show cytoprotective effects in various pathological conditions, including retinal detachment (Kayama et al., 2011), experimental autoimmune uveoretinitis (Kitamei et al., 2007), ischemia-induced injury in the central nervous system (Kitamei et al., 2007; Yasuda et al., 2005; Fujiki et al., 2006), liver transplantation (Fudaba et al., 2001) and myocardium (Ooie et al., 2001), via upregulation of HSP70 and/or suppression of apoptosis. In addition, GGA has been also demonstrated to be effective in protecting RGCs in a rat glaucoma model (Ishii et al., 2003). Considering GGA has been widely used in clinical practice for decades with its cytoprotective effect but few adverse effects, it imposes the potential to be a promising approach to treat NTG.
Recent studies have shown that loss of a glutamate transporter, glutamate/aspartate transporter (GLAST) or excitatory amino-acid carrier 1 (EAAC1), in mice leads to RGC degeneration similar to human NTG (Harada et al., 2007a; Harada et al., 2007b; Harada et al., 2010; Semba et al., 2014; Kimura et al., 2015; Nakano et al., 2016). In these mice, not only glutamate neurotoxicity but also oxidative stress is involved in their mechanisms. Glutamate neurotoxicity and oxidative stress have been proposed to contribute to retinal damage in various eye diseases including glaucoma (Osborne, 2008). Together with the decrease of glutamate transporters and glutathione levels observed in glaucoma patients (Gherghel et al., 2013; Naskar et al., 2000; Goyal et al., 2014), these mice seem to be useful as the animal models of NTG (Harada et al., 2007a; Harada et al., 2007b; Harada et al., 2010; Semba et al., 2014; Kimura et al., 2015; Nakano et al., 2016). In this study, we investigated the cytoprotective effect of oral GGA on RGC loss observed in GLAST heterozygous (GLAST+/−) mice, as well as underlying molecular mechanisms.
2. Material and methods
2.1. Animals and reagents
Experiments were performed using 3-week-old C57 BL/6J mice (WT; CLEA Japan, Tokyo, Japan) and GLAST+/− mice (Harada et al., 2007a; Harada et al., 2007b), both of which were fed at standard laboratory chow and allowed free access to water in an air-conditioned room. All animal experiments were approved by the Hokkaido University Animal Use Committee and conducted in accordance with the ARVO (Association of Research in Vision and Ophthalmology) Statement for the Use of Animals in Ophthalmic and Vision Research. GGA (teprenone), vitamin E, and Arabic gum were purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). Anesthesia was induced by intraperitoneal injection of pentobarbital (0.05 mg/g body weight).
2.2. Administration of GGA
GGA was emulsified with 0.5% Arabic gum and distilled water containing 0.2% vitamin E. For a vehicle-treated control, the same component without GGA was prepared and emulsified. Using a feeding needle, 3-week-old GLAST+/− mice received oral administration of GGA at 100, 300, or 600 mg/kg/day or vehicle alone for 14 days until the end of the 5th week, while WT mice received vehicle alone during the same period.
2.3. Histological and morphometric analyses
At the end of the 5th week (i.e., following the 14-day administration of GGA or vehicle), eyes were enucleated from the mice following euthanasia with an overdose anesthetic and fixation by intracardiac perfusion of 4% paraformaldehyde in phosphate-buffered saline (PBS). Paraffin sections (5-μm thick) of retinal specimens were cut through the optic nerve and stained with hematoxylin and eosin (H&E). The number of neurons in the ganglion cell layer (GCL) was counted from one ora serrata through the optic nerve to the other ora serrata, and the results of 3 serial sections were averaged for each sample in each group. Eight eyes from 4 animals were used for each group.
To further confirm the results of H&E staining, RGCs were retrogradely labeled from the superior colliculus with Fluoro-Gold (Biotium, Hayward, CA), and RGC density was determined as previously described (Harada et al., 2006; Harada et al., 2007a; Harada et al., 2007b). Briefly, four standard areas (0.04 mm2) of each whole-mounted retina at the point of 0.1 mm from the optic disc were randomly chosen, and labeled cells were counted.
2.4. Real-time polymerase chain reaction (PCR) for HSP70 mRNA expression
Total RNA was extracted using TRIzol Reagent (Life Technologies, Carlsbad, CA) from the retinas of 3-week-old WT and GLAST+/− mice 24 hours after vehicle or GGA was administrated. Reverse transcription was performed with GoScrip Reverse Transcriptase (Promega, Madison, WI) and oligo dT(20) primers following the manufacturer’s instructions. The TaqMan probe for mouse HSP70 was purchased from Life Technologies. Real-time PCR was performed using the GoTaq qPCR Master Mix (Promega), THUNDERBIRD Probe qPCR Mix (TOYOBO, Tokyo, Japan) and StepOnePlus™ Real-Time PCR System (Life Technologies). β-actin (sense 5′-CAT CCG TAA AGA CCT CTA TGC CAA C-3′, anti-sense 5′-ATG GAG CCA CCG ATC CAC A-3′) was used as endogenous control. Threshold cycle (CT) was determined automatically and relative changes in mRNA expression were calculated using the ΔΔCT values (Livak and Schmittgen, 2001). All PCR reactions were repeated in triplicate and the average values were used in statistical analyses.
2.5. Immunohistochemistry for HSP70
To examine retinal HSP70 accumulation at the end of the 5th week, paraffin sections of mouse retinas were dewaxed in xylene, dehydrated in ethanol at various concentrations, followed by microwave-based antigen retrieval using Dako Target Retrieval Solution (Dako North America, Inc, Carpinteria, CA) for 15 minutes and inactivation of endogenous peroxidase by incubation with Dako Protein Block (Dako North America) for 30 minutes. Then, the sections were incubated with the rabbit anti-HSP70 antibody (1:100, ab31010, Abcam, Tokyo, Japan) for 2 hours, followed by incubation with the biotinylated secondary antibody (1:500 dilution; Jackson ImmunoResearch Lab., West Grove, PA) at room temperature for 1 hour. Subsequently, the sections were incubated with VECTASTAIN ABC-AP Kit (Vector Lab., Burlingame, CA) for 30 minutes and in alkaline phosphatase substrate solution (BCIP/NBT AP Substrate Kit IV, Vector Lab.) for 40 minutes, to develop blue reaction products according to the manufacturer’s protocols. Finally, sections were dehydrated with ethanol and xylene, mounted with a mounting medium (MP500, Matsunami Corporation, Osaka, Japan) and coverslipped. All the sections were examined by microscopy (BIOREVO, Keyence, Osaka, Japan). The average brightness value of HSP70 in whole neural retina was calculated using the analyzing unit installed in the microscopy (BZII analyzer, Keyence). Since stronger immunoreactivity shows darker blue, low brightness value means large amounts of HSP70. To make the results easier to understand, the results were expressed as the ratio to mean 1/brightness value of WT mice retinas. Six samples were enrolled from each group. For all immunostaining procedure, negative controls were used to confirm the immunostaining was specific, using PBS instead of the primary antibody.
2.6. Measurement of caspase-9 and -3 activities
At the end of the 4th week, activities of caspase-9 and -3 were measured in the retinas obtained from WT mice and GLAST+/− mice treated with vehicle or GGA. They were measured using a commercially available caspase-9 and -3 colorimetric protease assay kits (MBL, Nagoya, Japan), according to the manufacturer’s instructions. Six to 8 eyes were enrolled from each group.
2.7. Statistics
Results are presented as mean ± SEM. Statistical differences (P < 0.05) were determined by ANOVA followed by Fisher’s PLSD as the post-hoc test using BellCurve for excel software.
3. Results
3.1. Dose-dependent inhibition of RGC loss by GGA administration
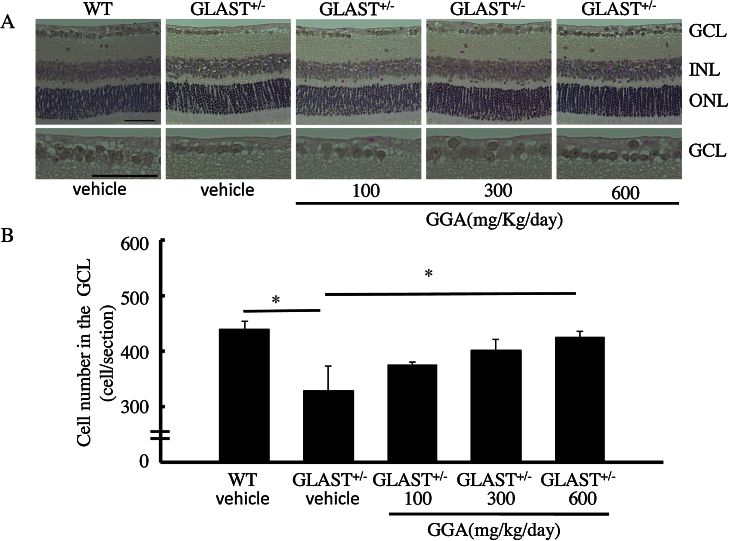
RGC degeneration in GLAST+/− mice starts at 3 weeks old (Kayama et al., 2011). To determine whether GGA inhibits RGC loss, 3-week-old mice received oral administration of GGA at 100, 300, or 600 mg/kg/day or vehicle alone for 14 days. Following the 14-day administration (i.e., at the end of the 5th week), the number of RGCs of GLAST+/− mice significantly decreased (328 ± 45, n = 8), as compared with that of WT mice (435 ± 13, n = 8. p < 0.01; Fig. 1). Importantly, RGC loss tended to be suppressed by GGA administration at 100 mg/kg/day (375 ± 5, n = 8) and 300 mg/kg/day (401 ± 20, n = 8) and was significantly suppressed by administration at 600 mg/kg/day (423 ± 12, n = 8. p < 0.05; Fig. 1). These results suggest that GGA administration protects RGCs.
Fig. 1.
Dose-dependent inhibition of RGC loss by GGA administration. (A) H&E staining of retinal sections. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. Scale bars: 50 μm. (B) Quantification of cell number per section in the GCL. *, p < 0.05, **, p < 0.01.
3.2. GGA-mediated protection of RGCs detected by retrograde labeling
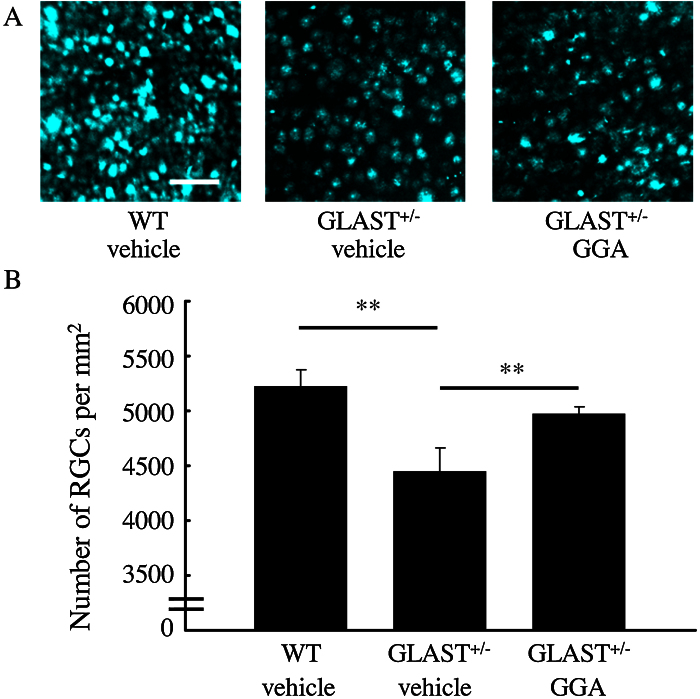
To confirm protection of RGCs by 600-mg/kg/day GGA administration, we further examined RGC density by the method of retrograde labeling. Similarly, RGC density of GLAST+/− mice retinas (4445 ± 220/mm2, n = 6.) was significantly lower than that of WT mice (5224 ± 153/mm2, n = 6. p < 0.01), whereas a decrease in RGC density was suppressed by 600-mg/kg/day GGA administration (4978 ± 64/mm2, n = 6. p < 0.01; Fig. 2). Collectively, these data confirm the protective effect of GGA on RGCs loss in GLAST+/− mice.
Fig. 2.
GGA-mediated protection of RGCs detected by retrograde labeling. (A) Retrogradely labeled RGCs. Scar bar: 50 μm. (B) Quantification of retrogradely labeled RGCs. Note that RGC number was significantly lower in GLAST+/− mice than control mice, while loss of RGC was suppressed by 600 mg/kg/day GGA administration. **, p < 0.01.
3.3. Upregulation of retinal HSP70 expression after GGA administration
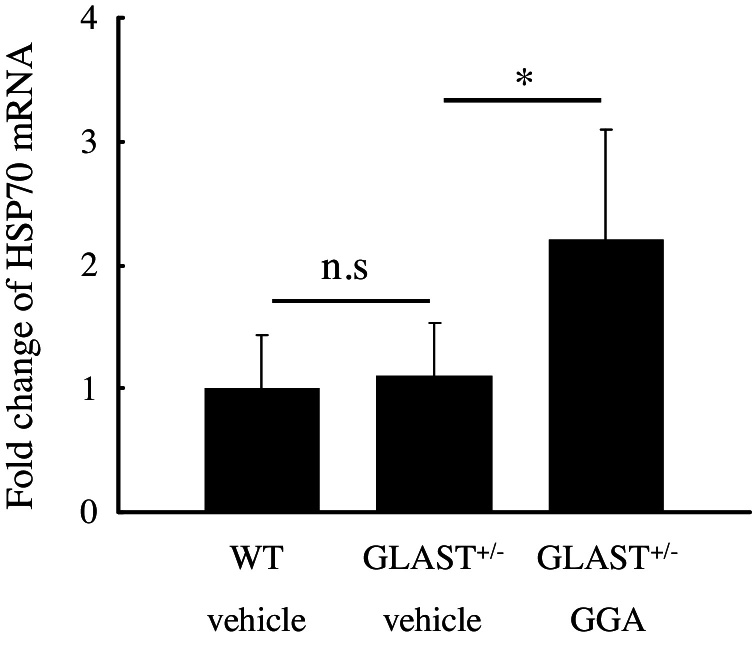
To confirm whether GGA induces the expression of HSP70 in the retinas of GLAST+/− mice, we first performed quantitative real-time PCR analysis. Twenty-four hours after pretreatment of GGA or vehicle, total RNA was isolated from the retinas of WT or GLAST+/− mice. As shown in Fig. 3, GGA treatment significantly upregulated HSP70 expression in GLAST+/− mice retinas by twofold, while no significant difference in HSP70 expression could be observed between WT mice and GLAST+/− mice treated with vehicle.
Fig. 3.
Upregulation of retinal HSP70 expression after GGA administration. Note that no significant difference in retinal HSP70 expression could be identified between GLAST+/− mice and WT mice treated with vehicle, while retinal HSP70 expression was upregulated following GGA administration to GLAST+/− mice. **, p < 0.01.
3.4. Retinal HSP70 accumulation after GGA administration
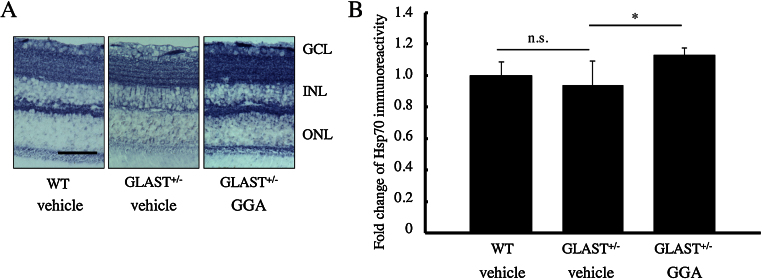
To examine whether GGA treatment increases protein levels of HSP70 in retinas, we performed immunohistochemistry. Indeed, GGA treatment increased the deposition of HSP70 throughout the retina, especially in the GCL and the inner nuclear layer (INL; Fig. 4A). Further quantification of HSP70 deposition in retinas showed that retinal HSP70 expression was similar in WT and GLAST+/− mice, whereas a significant increase by 13% was detected following GGA treatment to GLAST+/− mice (p < 0.05; Fig. 4B).
Fig. 4.
Retinal HSP70 accumulation after GGA administration. (A) Immunohistochemistry of HSP70 in retinas. GCL: ganglion cell layer; INL: inner nuclear layer; ONL: outer nuclear layer. Scale bar: 50 μm. (B) Quantification of HSP70 accumulation in retinas. Note that accumulation of HSP70 was almost equal in retinas of GLAST+/− mice and WT mice treated with vehicle, while GGA administration significantly increased HSP70 accumulation in GLAST+/− mice. *, p < 0.05.
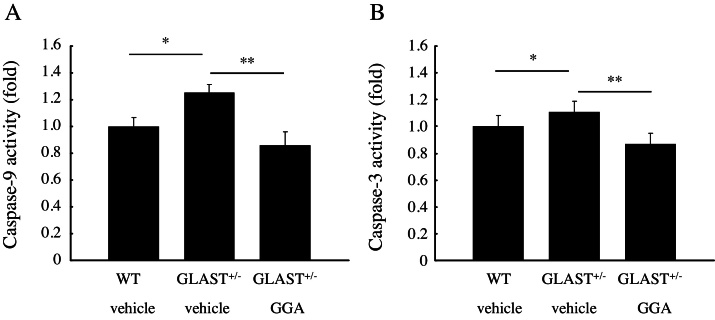
3.5. Retinal caspase-9 and -3 deactivation after GGA administration
Next, to further explore the underlying mechanism through which GGA inhibited RGCs loss, we performed activity assays to investigate the levels of retinal caspase-9 and -3 activities following GGA or vehicle treatment at the 4th week. As compared with WT mice (n = 8), caspase-9 and -3 activities increased by 25% and 11%, respectively, in GLAST+/− mice treated with vehicle (n = 6. p < 0.05 for both; Fig. 5A,B), but were suppressed by 15% and 13%, respectively, following GGA treatment to GLAST+/− mice (n = 6. p < 0.01 for both; Fig. 5A,B).
Fig. 5.
Retinal caspase-9 and -3 deactivation after GGA administration. Note that both caspase-9 and -3 activities increased in GLAST+/− mice as compared to WT mice, but were significantly suppressed following GGA administration. *, p < 0.05, **, p < 0.01.
4. Discussion
GGA has been demonstrated to suppress apoptosis via induction of HSP70 protein in various ocular disorders, including uveitis (Kitamei et al., 2007), ultraviolet-induced photokeratitis (Lennikov et al., 2013), retinal detachment (Kayama et al., 2011), and glaucoma with laser-induced IOP elevation (Ishii et al., 2003). However, to the best of our knowledge, there is no report about the applicability of GGA in the treatment for NTG. In the current study, we confirmed RGC degeneration in GLAST+/− mice, consistent with the previous report (Harada et al., 2007a; Harada et al., 2007b), and we demonstrated that the degeneration could be inhibited by the systemic administration of GGA. Previously, HSP70 expression was shown to be upregulated 24 hours after the systemic administration of GGA in experimental autoimmune uveoretinitis (Kitamei et al., 2007). In this study, using real-time PCR, we detected the upregulation of HSP70 expression in the retinas of GLAST+/− mice 24 hours after the systemic administration of GGA, and also demonstrated increased retinal HSP70 accumulation using immunohistochemistry.
HSP70 inhibits apoptosis through several pathways, including inhibition of caspase activities. Previous reports have shown that, in response to various stimuli, pro-apoptotic proteins such as Bcl-2 family members mediate mitochondrial outer-membrane permeabilization, leading to cytochrome c release through the mitochondrial pore to the cytoplasm (Chipuk and Green, 2008; Youle and Strasser, 2008). Subsequently, cytochrome c contributes to the formation of apoptosome through activating apoptotic protease activating factor (Apaf)-1 (Saleh et al., 1999; Li et al., 1997), which in turn recruits, cleaves and activates caspase-9 and the effector caspases-3 (Li et al., 1997). In GLAST+/− mice, both increased oxidative stress and glutamate toxicity contribute to RGC apoptosis (Harada et al., 2007a; Harada et al., 2007b Harada et al., 2010; Semba et al., 2014; Kimura et al., 2015; Nakano et al., 2016). In this study, we confirmed increased activities of both caspase-9 and -3 in GLAST+/− mice compared to normal littermates, indicating increased caspase activities were involved in RGC apoptosis as the pathogenesis of NTG. On the other hand, HSP70 inhibits the formation of apoptosome via 1) preventing cytochrome c release from mitochondria by directly binding with proapoptotic Bcl-2 family members, such as Bax (Stankiewicz et al., 2005; Gotoh et al., 2004), 2) preventing recruitment of procaspase-9 to the Apaf-1 apoptosome by directly binding with Apaf-1 (Beere et al., 2000), thus inhibiting the subsequent activation of caspase cascade causing RGC apoptosis. Given that HSP70 also inhibits apoptosis through intervening with multiple apoptosis-associated signaling pathways (Park et al., 2001; Matsumori et al., 2005; Ravagnan et al., 2001), future studies are required to clarify the possibility of other targets.
Although lowering IOP in glaucoma is associated with an attenuation of progressive optic nerve damage (Gordon et al., 2002), a variety of IOP-independent factors, such as oxidative stress (Osborne, 2008), aging (Buono et al., 2002), glutamate toxicity (Zhang et al., 2012), and vascular factors (Meyer et al., 1996) are thought to play some roles, underscoring the importance of clarifying the precise mechanisms in RGC loss and exploring alternative approach for future treatments of glaucoma, especially NTG. In this study, we showed GGA could inhibit RGC apoptosis through induction of HSP70 protein, using GLAST mutant mice. Initially developed as an antiulcer drug in Japan, GGA has been widely used in the world for decades, which proved its high level of safety. Including this study, vast evidence has shown that GGA contributes to cytoprotection through introduction of HSP70. Taking together, GGA may be useful as an alternative therapy for the treatment of NTG.
Declarations
Author contribution statement
Zhenyu Dong: Performed the experiments; Wrote the paper.
Yasuhiro Shinmei: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Yoko Dong, Saori Inafuku, Junichi Fukuhara, Ryo Ando, Atsuhiro Kanda, Kohichi Tanaka, Kousuke Noda, Shinki Chin, Susumu Ishida: Wrote the paper.
Nobuyoshi Kitaichi, Takayuki Harada: Conceived and designed the experiments; Wrote the paper.
Competing interest statement
The authors declare no conflict of interest.
Funding statement
This work was supported by JSPS Grant-in-Aid for Scientific Research: 23592548.
Additional information
No additional information is available for this paper.
Acknowledgments
We thank S. Yoshida, and I. Hirose for their technical assistance.
References
- Beere H.M., Wolf B.B., Cain K., Mosser D.D., Mahboubi A., Kuwana T., Tailor P., Morimoto R.I., Cohen G.M., Green D.R. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Buono L.M., Foroozan R., Sergott R.C., Savino P.J. Is normal tension glaucoma actually an unrecognized hereditary optic neuropathy? New evidence from genetic analysis. Curr. Opin. Ophthalmol. 2002;13:362–370. doi: 10.1097/00055735-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Chipuk J.E., Green D.R. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Normal-Tension Glaucoma Study Group The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am. J. Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- Fudaba Y., Ohdan H., Tashiro H., Ito H., Fukuda Y., Dohi K., Asahara T. Geranylgeranylacetone a heat shock protein inducer, prevents primary graft nonfunction in rat liver transplantation. Transplantation. 2001;72:184–189. doi: 10.1097/00007890-200107270-00003. [DOI] [PubMed] [Google Scholar]
- Fujiki M., Hikawa T., Abe T., Uchida S., Morishige M., Sugita K., Kobayashi H. Role of protein kinase C in neuroprotective effect of geranylgeranylacetone, a noninvasive inducing agent of heat shock protein, on delayed neuronal death caused by transient ischemia in rats. J. Neurotrauma. 2006;23:1164–1178. doi: 10.1089/neu.2006.23.1164. [DOI] [PubMed] [Google Scholar]
- Gherghel D., Mroczkowska S., Qin L. Reduction in blood glutathione levels occurs similarly in patients with primary-open angle or normal tension glaucoma. Invest. Ophthalmol. Vis. Sci. 2013;54:3333–3339. doi: 10.1167/iovs.12-11256. [DOI] [PubMed] [Google Scholar]
- Gordon M.O., Beiser J.A., Brandt J.D., Heuer D.K., Higginbotham E.J., Johnson C.A., Keltner J.L., Miller J.P., Parrish R.K., 2nd, Wilson M.R. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- Gotoh T., Terada K., Oyadomari S., Mori M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004;11:390–402. doi: 10.1038/sj.cdd.4401369. [DOI] [PubMed] [Google Scholar]
- Goyal A., Srivastava A., Sihota R., Kaur J. Evaluation of oxidative stress markers in aqueous humor of primary open angle glaucoma and primary angle closure glaucoma patients. Curr. Eye Res. 2014;39:823–829. doi: 10.3109/02713683.2011.556299. [DOI] [PubMed] [Google Scholar]
- Harada C., Namekata K., Guo X., Yoshida H., Mitamura Y., Matsumoto Y., Tanaka K., Ichijo H., Harada T. ASK1 deficiency attenuates neural cell death in GLAST-deficient mice: a model of normal tension glaucoma. Cell Death Differ. 2010;17:1751–1759. doi: 10.1038/cdd.2010.62. [DOI] [PubMed] [Google Scholar]
- Harada C., Nakamura K., Namekata K., Okumura A., Mitamura Y., Iizuka Y., Kashiwagi K., Yoshida K., Ohno S., Matsuzawa A. Role of apoptosis signal-regulating kinase 1 in stress-induced neural cell apoptosis in vivo. Am. J. Pathol. 2006;168:261–269. doi: 10.2353/ajpath.2006.050765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada C., Nakamura K., Guo X., Kitaichi N., Mitamura Y., Yoshida K., Ohno S., Yoshida H., Harada T. Neuroprotective effect of geranylgeranylacetone against ischemia-induced retinal injury. Mol. Vis. 2007;13:1601–1607. [PubMed] [Google Scholar]
- Harada T., Harada C., Nakamura K., Quah H.M., Okumura A., Namekata K., Saeki T., Aihara M., Yoshida H., Mitani A., Tanaka K. The potential role of glutamate transporters in the pathogenesis of normal tension glaucoma. J. Clin. Invest. 2007;117:1763–1770. doi: 10.1172/JCI30178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii Y., Kwong J.M., Caprioli J. Retinal ganglion cell protection with geranylgeranylacetone a heat shock protein inducer, in a rat glaucoma model. Invest. Ophthalmol. Vis. Sci. 2003;44:1982–1992. [PubMed] [Google Scholar]
- Itoh H., Tashima Y. The stress (heat shock) proteins. Int. J. Biochem. 1991;23:1185–1191. doi: 10.1016/0020-711x(91)90214-8. [DOI] [PubMed] [Google Scholar]
- Iwase A., Suzuki Y., Araie M., Yamamoto T., Abe H., Shirato S., Kuwayama Y., Mishima H.K., Shimizu H., Tajimi Study Group, Japan Glaucoma Society The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–1648. doi: 10.1016/j.ophtha.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Kass M.A., Gordon M.O., Gao F., Heuer D.K., Higginbotham E.J., Johnson C.A., Keltner J.K., Miller J.P., Parrish R.K., Wilson M.R., Ocular Hypertension Treatment Study Group Delaying treatment of ocular hypertension: the ocular hypertension treatment study. Arch. Ophthalmol. 2010;128:276–287. doi: 10.1001/archophthalmol.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawana K., Miyamoto Y., Tanonaka K., Han-no Y., Yoshida H., Takahashi M., Takeo S. Cytoprotective mechanism of heat shock protein 70 against hypoxia/reoxygenation injury. J. Mol. Cell. Cardiol. 2000;32:2229–2237. doi: 10.1006/jmcc.2000.1250. [DOI] [PubMed] [Google Scholar]
- Kayama M., Nakazawa T., Thanos A., Morizane Y., Murakami Y., Theodoropoulou S., Abe T., Vavvas D., Miller J.W. Heat shock protein 70 (HSP70) is critical for the photoreceptor stress response after retinal detachment via modulating anti-apoptotic Akt kinase. Am. J. Pathol. 2011;178:1080–1091. doi: 10.1016/j.ajpath.2010.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura A., Guo X., Noro T., Harada C., Tanaka K., Namekata K., Harada T. Valproic acid prevents retinal degeneration in a murine model of normal tension glaucoma. Neurosci. Lett. 2015;588:108–113. doi: 10.1016/j.neulet.2014.12.054. [DOI] [PubMed] [Google Scholar]
- Kitamei H., Kitaichi N., Yoshida K., Nakai A., Fujimoto M., Kitamura M., Iwabuchi K., Miyazaki A., Namba K., Ohno S. Association of heat shock protein 70 induction and the amelioration of experimental autoimmune uveoretinitis in mice. Immunobiology. 2007;212:11–18. doi: 10.1016/j.imbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Koseki N., Araie M., Shirato S., Yamamoto S. Effect of trabeculectomy on visual field performance in central 30 degrees field in progressive normal-tension glaucoma. Ophthalmology. 1997;104:197–201. doi: 10.1016/s0161-6420(97)30334-0. [DOI] [PubMed] [Google Scholar]
- Lennikov A., Kitaichi N., Kase S., Noda K., Horie Y., Nakai A., Ohno S., Ishida S. Induction of heat shock protein 70 ameliorates ultraviolet-induced photokeratitis in mice. Int. J. Mol. Sci. 2013;14:2175–2189. doi: 10.3390/ijms14012175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Nijhawan D., Budihardjo I., Srinivasula S.M., Ahmad M., Alnemri E.S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Matsumori Y., Hong S.M., Aoyama K., Fan Y., Kayama T., Sheldon R.A., Vexler Z.S., Ferriero D.M., Weinstein P.R., Liu J. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J. Cereb. Blood Flow Metab. 2005;25:899–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- Meyer J.H., Brandi-Dohrn J., Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br. J. Ophthalmol. 1996;80:864–867. doi: 10.1136/bjo.80.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano N., Ikeda H.O., Hasegawa T., Muraoka Y., Iwai S., Tsuruyama T., Nakano M., Fuchigami T., Shudo T., Kakizuka A. Neuroprotective effects of VCP modulators in mouse models of glaucoma. Heliyon. 2016;19:e00096. doi: 10.1016/j.heliyon.2016.e00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClellan A.J., Frydman J. Molecular chaperones and the art of recognizing a lost cause. Nat. Cell Biol. 2001;3:E51–53. doi: 10.1038/35055162. [DOI] [PubMed] [Google Scholar]
- Naskar R., Vorwerk C.K., Dreyer E.B. Concurrent downregulation of a glutamate transporter and receptor in glaucoma. Invest. Ophthalmol. Vis. Sci. 2000;41:1940–1944. [PubMed] [Google Scholar]
- Ooie T., Takahashi N., Saikawa T., Nawata T., Arikawa M., Yamanaka K., Hara M., Shimada T., Sakata T. Single oral dose of geranylgeranylacetone induces heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001;104:1837–1843. doi: 10.1161/hc3901.095771. [DOI] [PubMed] [Google Scholar]
- Osborne N.N. Pathogenesis of ganglion cell death in glaucoma and neuroprotection: focus on ganglion cell axonal mitochondria. Prog. Brain Res. 2008;173:339–352. doi: 10.1016/S0079-6123(08)01124-2. [DOI] [PubMed] [Google Scholar]
- Park H.S., Lee J.S., Huh S.H., Seo J.S., Choi E.J. Hsp72 functions as a natural inhibitory protein of c-Jun N-terminal kinase. EMBO J. 2001;20:446–456. doi: 10.1093/emboj/20.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev S., Hara K., Kokubo Y., Mestril R., Dillmann W., Weinstein P.R., Sharp F.R. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann. Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- Ravagnan L., Gurbuxani S., Susin S.A., Maisse C., Daugas E., Zamzami N., Mak T., Jäättelä M., Penninger J.M., Garrido C. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat. Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- Saleh A., Srinivasula S.M., Acharya S., Fishel R., Alnemri E.S. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J. Biol. Chem. 1999;274:17941–17945. doi: 10.1074/jbc.274.25.17941. [DOI] [PubMed] [Google Scholar]
- Semba K., Namekata K., Kimura A., Harada C., Mitamura Y., Harada T. Brimonidine prevents neurodegeneration in a mouse model of normal tension glaucoma. Cell Death Dis. 2014;5:e1341. doi: 10.1038/cddis.2014.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeeda T., Tomidokoro A., Araie M., Koseki N., Yamamoto S. Long-term follow-up of visual field progression after trabeculectomy in progressive normal-tension glaucoma. Ophthalmology. 2002;109:766–770. doi: 10.1016/s0161-6420(01)01009-0. [DOI] [PubMed] [Google Scholar]
- Stankiewicz A.R., Lachapelle G., Foo C.P., Radicioni S.M., Mosser D.D. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J. Biol. Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- The AGIS Investigators The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Shichinohe H., Kuroda S., Ishikawa T., Iwasaki Y. Neuroprotective effect of a heat shock protein inducer: geranylgeranylacetone in permanent focal cerebral ischemia. Brain Res. 2005;1032:176–182. doi: 10.1016/j.brainres.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Young J.C., Agashe V.R., Siegers K., Hartl F.U. Pathways of chaperone-mediated protein folding in the cytosol. Nat. Rev. Mol. Cell Biol. 2004;5:781–791. doi: 10.1038/nrm1492. [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhang L., Weinreb R.N. Ophthalmic drug discovery: novel targets and mechanisms for retinal diseases and glaucoma. Nat. Rev. Drug Discov. 2012;11:541–559. doi: 10.1038/nrd3745. [DOI] [PubMed] [Google Scholar]
- Zhan X., Ander B.P., Liao I.H., Hansen J.E., Kim C., Clements D., Weisbart R.H., Nishimura R.N., Sharp F.R. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41:538–543. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]