ABSTRACT
The venom of the banded krait (Bungarus multicinctus), one of the major venomous species in Taiwan, contains neurotoxic venom proteins (B. multicinctus proteins) that pose a serious medical problem in tropical and subtropical countries. Even though horse-derived serum is an efficient therapy against snake venom, it is associated with a high cost and side effects. Therefore, developing a more cost-effective alternative treatment option is highly envisaged. In this study, chickens were immunized with B. multicinctus proteins, and polyclonal immunoglobulin Y (IgY) antibodies were purified from eggs. IgY showed a binding activity to B. multicinctus proteins that was similar to horse antivenin, and its titer in chickens lasted for at least 6 months. We constructed two antibody libraries by phage display antibody technology, which contain 1.0 × 107 and 2.9 × 108 transformants, respectively. After biopanning, a phage-based enzyme-linked immunosorbent assay (ELISA) indicated that specific clones were enriched. Thirty randomly selected clones expressing monoclonal single-chain variable-fragment (scFv) antibodies were classified into four groups with a short linker and two with a long linker. These selected scFv antibodies showed specific binding activities to B. multicinctus proteins but not to the venomous proteins of other snakes. Most importantly, polyclonal IgY demonstrated a similar neutralization efficiency as did horse-derived antivenin in mice that were injected with a minimum lethal dosage (MLD) of venom proteins. A mixture of several monoclonal anti-B. multicinctus scFv antibodies was also able to partially inhibit the lethal effect on mice. We profoundly believe that IgY and scFv antibodies can be applied in developing diagnostic agents for wound exudates and as an alternative treatment for snakebite envenomation in the future.
IMPORTANCE Snake envenomation is one of the global medical issues of concern. Horse-derived antivenin is an effective way to treat snakebites, but it is costly and occasionally causes severe side effects. In this study, we first generated and characterized IgY antibodies with neutralization activity in chickens. Subsequently, we generated a panel of monoclonal scFv antibodies using phage display antibody technology. A mixture of scFv antibodies was able to partially inhibit the lethal effect in mice that were injected with lethal dosages of venom proteins and prolong their survival time. We believe that chicken-derived IgY and scFv antibodies have great potential for the development of diagnostic agents for wound exudates and therapeutic agents against snake envenomation in the future.
INTRODUCTION
Envenomation caused by venomous snakes is considered to be a medical issue worldwide, especially in tropical and subtropical regions. Approximately 2,700 species of snakes exist in the tropical regions of the world, 500 of which are venomous (1). The venomous snakes have been classified into the Viperidae, Elapidae, Colubridae, and Atractaspididae families. Most of the poisonous components of snake venoms are toxins that are classified into hemotoxins, neurotoxins, and myotoxins based on the biological effects on snake bite victims. The Bungarus multicinctus snake of the Elapidae family, which is also called the banded krait, is one of the major causes of snake envenomation in Taiwan. Neurotoxic venom proteins from B. multicinctus contain different toxins, enzymes, and components that damage the central nervous system and cause neuromuscular blockage, which results in different symptoms, such as ptosis and especially paralysis of the respiratory muscles leading to death (2, 3). Among these components, bungarotoxins, which belong to a three-finger toxin family, are considered the major lethal components in B. multicinctus proteins. Their main function is to block neuromuscular junctions, which ultimately results in death (4, 5). However, other biologically active components, such as phospholipase A2 in crude venom, may have autonomous effects or synergistic effects with other components (6, 7). Thus, developing therapeutic agents for specific components has some limitations.
Currently, antibody immunotherapy derived from hyperimmunized horse serum is the most effective treatment against snake poisoning. However, the high cost of generating antibodies in horses and side effects, such as serum sickness, are bona fide problems (8). To partially solve these problems, extraction of immunoglobulin Y (IgY) antibodies from chicken eggs provides an alternative cost-effective and convenient strategy to substitute for using mammalian antibodies from serum (9, 10). Chicken IgY, lacking the evocative function on the mammalian complement system or Fc receptor, has been reported as passive immunization for clinical and experimental treatments (11–14). Additional advantages of using chickens include inexpensive rearing, the high yield of IgY antibodies (∼100 to ∼150 mg per egg), and a strong immune response elicited by a small amount of antigen, which is most beneficial when collection of venom proteins is difficult (15, 16). Thus, hens supply a more hygienic, convenient, and cost-effective platform to generate neutralizing antibodies against snake envenomation than horses (14, 17).
Although polyclonal antibodies are an effective treatment for snakebites, they are less specific and cannot be decisively used to develop rapid diagnostic reagents for wound exudates or serum for snakebite victims. In contrast, monoclonal antibodies, such as the single-chain variable-fragment (scFv) antibody that recognizes a single antigenic epitope, have a higher propensity to be used as potential diagnostic and therapeutic agents. Huse et al. broke through the stereotypes and opened up new prospects for the generation of monoclonal antibodies and the development of targeted therapies by constructing antibody libraries using phage display technology (18). As for animal immunization, chickens are relatively cost-effective hosts to construct antibody libraries to select specific antibodies against various targets (19, 20). Several studies have indicated that recombinant scFv antibodies show promise in developing agents against snake envenomation (21, 22). Even though monoclonal antibodies recognize one specific epitope, a combination of different monoclonal antibodies can potentially reduce symptoms of snakebites and increase survival rates. In addition, specific monoclonal antibodies can potentially be used as rapid diagnostic agents, ascertaining the type of snakebites for selecting accurate therapeutic agents within the golden time.
In our study, we attempted to generate polyclonal IgY and monoclonal scFv antibodies with high specificity and neutralization activity against B. multicinctus proteins. After immunizing chickens with attenuated B. multicinctus proteins, IgY antibodies were purified from eggs and scFv antibodies were selected from libraries constructed by phage display technology. Antibodies were characterized for specific binding activity, and the in vivo neutralization efficacy was also determined. These specific IgY and scFv antibodies can be applied in developing diagnostic agents and remedies for deadly envenomation in the future.
MATERIALS AND METHODS
Chickens and mice.
Animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Taipei Medical University before the study was initiated. Female chickens (Gallus domesticus) at 6 months of age and ICR mice weighing ∼12 to ∼14 g were purchased and maintained in the animal facility of Taipei Medical University.
Chicken immunization.
B. multicinctus protein powders were provided by the Centers for Disease Control (CDC), Taiwan, and were dissolved in phosphate-buffered saline (PBS) to a final concentration of 10 mg/ml. Ten micrograms of B. multicinctus proteins was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing condition and visualized with Coomassie brilliant blue staining. After attenuation using 0.125% glutaraldehyde in the dark for 1 h at room temperature (25°C), 100 μg of B. multicinctus proteins was mixed with an equal volume of Freund's complete adjuvant for the first intramuscular immunization. Eighty micrograms of B. multicinctus proteins with Freund's incomplete adjuvant was used for subsequent immunizations at 7-day intervals. Eggs were collected before chicken immunization and a week after each immunization for 6 months to purify polyclonal IgY antibodies as described previously (23, 24).
Antibody library construction.
Antibody libraries were constructed as reported in a previous study (25). Briefly, total RNA molecules were isolated from the spleens of chickens and used as templates to synthesize cDNA using reverse transcriptase, which was subsequently used to amplify the immunoglobulin of light-chain variable regions (VL) and heavy-chain variable regions (VH). VL and VH were joined by a 7- or 18-amino acid peptide linker to form a full-length scFv with a short linker (scFv-S) or with a long linker (scFv-L) through an overlapping extension PCR. After SfiI (New England BioLabs, USA) digestion, scFv genes were cloned into the pComb3X phagemid vector, and the resultant recombinant DNAs were transformed into Escherichia coli strain ER2738 by electroporation (MicroPulser; Bio-Rad, USA). A portion of the transformed E. coli was plated on an LB agar plate containing 50 μg/ml of ampicillin (Amp) to calculate the library size. The remaining culture was added into 100 ml of super broth and infected with 1012 PFU of the VCS-M13 helper phage. After centrifugation to remove the bacterial pellets on the following day, recombinant phages in the supernatant were precipitated using 4% polyethylene glycol 8000 and 3% NaCl on ice for 30 min. After further centrifugation, recombinant phage pellets were resuspended in PBS containing 1% bovine serum albumin (BSA) and 20% glycerol and stored at −20°C.
Biopanning of antibody library.
Enzyme-linked immunosorbent assay (ELISA) plates were coated with B. multicinctus proteins (0.5 μg/well) at 4°C overnight and blocked using 3% BSA for 1 h at 37°C. A total of ∼1011 to ∼1012 PFU of recombinant phages were added and incubated at 37°C for 2 h. After washing out unbound phages using PBS containing 0.05% Tween 20 (PBST), bound phages were eluted with 0.1 M glycine-HCl (pH 2.2) followed by the addition of 2 M Tris base buffer. Eluted phages were used to infect E. coli ER2738, and their titers were determined on LB agar plate containing 50 μg/ml Amp. Amplified phages in the overnight culture were collected as described above for the next round of biopanning. Four rounds of biopanning were carried out. The recombinant phagemid DNAs were purified from the E. coli after the fourth biopanning and transformed into the E. coli Top10F′ strain for analysis of scFv antibody expression.
Expression, purification, and gene sequence analysis of scFv antibodies.
An individual clone that was randomly selected from the LB agar plate was grown in super broth containing 20 mM MgCl2 and 50 μg/ml of Amp at 37°C for 8 h. After adding 1 mM isopropyl-β-d-thiogalactopyranoside overnight, E. coli cells were collected by centrifugation, resuspended in a histidine-binding buffer (20 mM sodium phosphate, 0.5 M NaCl, and 20 mM imidazole [pH 7.4]), and lysed via sonication. The scFv antibodies in the supernatant were purified using Ni2+ Sepharose according to the manufacturer's recommendations (GE Healthcare Bio-Sciences AB) and further concentrated/dialyzed in PBS using Amicon Ultra-4 centrifugal filter devices (Merck Millipore, Germany). Nucleotide sequences of scFv-expressing clones were determined using the ompseq (5′-AAGACAGCTATCGCGATTGCAGTG-3′) primer, and their putative amino acid sequence VL and VH genes were aligned with those of the chicken immunoglobulin germ line gene (25).
Western blotting.
B. multicinctus proteins were immobilized on polyvinylidene difluoride (PVDF) membranes, which were later blocked with PBS containing 5% skim milk at 25°C for 1 h. Therapeutic horse IgG antivenin or IgY from chickens immunized 7 times was added at 1:1,000 dilution and incubated at 25°C for 1 h. After washing three times with PBST, bound antibodies were detected using horseradish peroxidase (HRP)-conjugated goat anti-horse Fab (Jackson ImmunoResearch, USA) or HRP-conjugated donkey anti-chicken IgY (Jackson ImmunoResearch, USA) for another 1 h. After three washes, the membranes were developed using diaminobenzidine (DAB) substrate until the desired clarity was attained. To analyze their binding specificity, purified scFv antibodies (5 μg/ml) were individually added to the membranes containing the venom proteins of Deinagkistrodon acutus, B. multicinctus, Trimeresurus stejnegeri, Trimeresurus mucrosquamatus, Naja naja atra, and Daboia russellii formosensis and detected using goat anti-chicken light-chain (Bethyl, USA) and HRP-conjugated donkey anti-goat antibodies (Jackson ImmunoResearch, USA). Blocking, washing, incubation, and color development were carried out following the same conditions as described above.
ELISA and competitive ELISA.
B. multicinctus or BSA proteins were coated on ELISA wells (0.5 μg/well) at 37°C for 1 h, which were blocked with PBS containing 5% skim milk for another 1 h. IgY antibodies from preimmunized chickens or from chickens immunized 7 times diluted 2-fold serially from 4,000× to 256,000× were added and incubated at 37°C for 1 h. After washing six times with PBST, bound IgY was detected by HRP-conjugated donkey anti-chicken IgY at 37°C for another 1 h. After washing as above, the binding reactivities were visualized using tetramethylbenzidine (TMB), stopped by HCl, and measured at 450 nm. For phage-based ELISA, each round of amplified phages containing 1011 to 1012 PFU was added and detected using HRP-conjugated mouse anti-M13 phage antibodies (Amersham Biosciences, USA). For specific binding assay, purified scFv antibodies (5 μg/ml) were incubated with six venom proteins in ELISA wells and detected using goat anti-chicken light-chain and HRP-conjugated donkey anti-goat antibodies. For competitive ELISA, B. multicinctus proteins diluted 2-fold serially from 400 μg/ml to 0.40 μg/ml were mixed with the scFv antibodies (10 μg/ml) at a 1:1 volume ratio. After incubation at 25°C for 1 h, the mixtures were added to the wells, which were coated with B. multicinctus proteins, and incubated at 37°C for another 1 h. The blocking, washing, incubation, and color development were performed following the same conditions as those described above. All ELISA results were represented as the mean plus or minus the standard deviation (SD) of duplicated determinations.
Neutralization effect of antibodies.
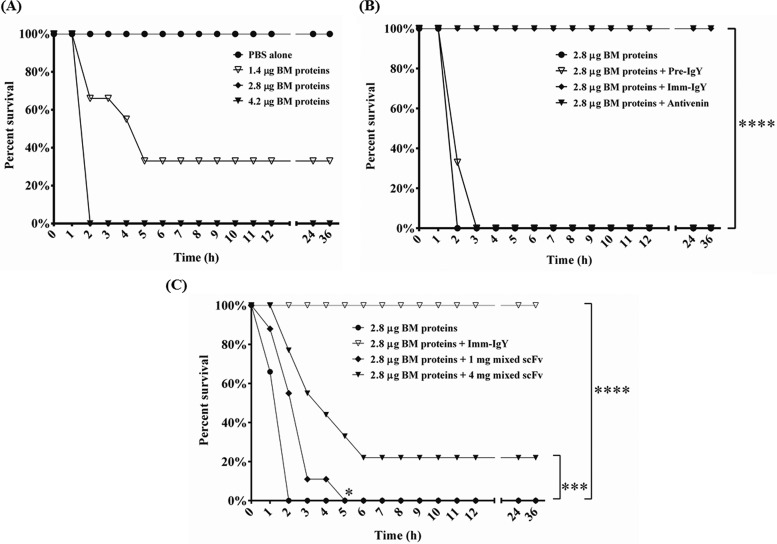
ICR mice were randomly distributed into groups of nine. To determine the minimum lethal dose (MLD), 1.4, 2.8, or 4.2 μg of B. multicinctus proteins in 200 μl PBS after incubating at 37°C for 1 h was injected intraperitoneally into mice, respectively. To test the neutralization effect of antibodies, the B. multicinctus proteins of 1× MLD were individually mixed with 4 mg of horse IgG antivenin, IgY from preimmunized or immunized chickens, and 1 or 4 mg of combinations of six anti-B. multicinctus scFv antibodies in 200 μl PBS. The mixtures were incubated at 37°C for 1 h and then injected intraperitoneally into mice. The survival rate of the mice was monitored at 1-h intervals for 36 h.
Statistical analyses.
For statistical comparison, the ELISA data were analyzed and expressed as means plus or minus standard errors via multiple t tests. The neutralization assays in mice were analyzed via the Gehan-Breslow-Wilcoxon test using GraphPad Prism 6 software (La Jolla, CA, USA). A P value of <0.05 was considered statistically significant.
RESULTS
Characterization of anti-B. multicinctus IgY antibodies.
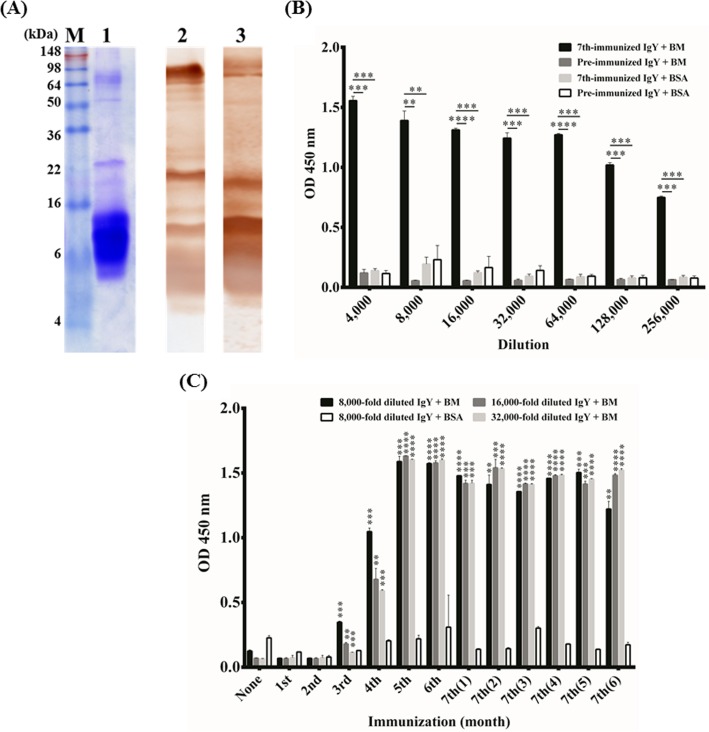
B. multicinctus venom proteins were analyzed by Coomassie brilliant blue-stained SDS-PAGE (Fig. 1A). The results showed that B. multicinctus venom was composed of complicated proteins, most of which have molecular masses of less than 16 kDa (Fig. 1A, lane 1). The IgY antibodies purified from chickens immunized 7 times were examined for their binding activity to B. multicinctus proteins on Western blots. The results indicated that the specific anti-B. multicinctus IgY antibodies were elicited significantly and were able to react with various B. multicinctus proteins (Fig. 1A, lane 3). The recognized protein patterns are similar to those recognized by horse-derived antivenin (Fig. 1A, lane 2). Interestingly, in addition to the major recognition patterns, antivenin showed a strong binding signal to a protein with a molecular mass of around 80 kDa. In contrast, IgY antibodies showed a stronger reaction to a protein of around 10 kDa. The results indicated that the humoral antibody responses are different in horses and chickens immunized with B. multicinctus proteins. Additional experiments need to be carried out to unveil the distinct patterns and natures of these identified proteins. The strong humoral responses were further demonstrated by ELISA analysis (Fig. 1B). The anti-B. multicinctus IgY had a significant binding activity for optical densities (ODs) of >1.0 at a 1:128,000 dilution, but no reactive signal was detected in wells coated with BSA. In contrast, IgY antibodies from chickens before immunization showed little binding reaction to B. multicinctus or BSA proteins. To further monitor the process of the antibody response in chickens, IgY antibodies were purified from chickens before immunization, after weekly immunization, and each month after the 7th immunization for examination on ELISA. Compared to preimmunization, these results indicated that the immune response was significantly elicited after the 3rd or 4th immunization to reach a stage of plateau and last for at least 6 months after the 7th immunization (Fig. 1C).
FIG 1.
Characterization of polyclonal antibodies against Bungarus multicinctus proteins. (A) B. multicinctus proteins were visualized by Coomassie blue-stained SDS-PAGE (lane 1) and were recognized by horse antivenin (lane 2) and immunoglobulin Y (IgY) from chickens immunized 7 times (lane 3) on Western blots. (B) Purified IgY antibodies from chickens immunized 7 times were 2-fold serially diluted (4,000× to 256,000×) to test the binding activity to B. multicinctus (BM) proteins on ELISA plates. (C) The humoral immune response in chickens was monitored after the 7th immunization for 6 months. The IgY antibodies from chickens after each immunization were compared with those from preimmunized chickens. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Construction of scFv antibody libraries and selection of biopanning.
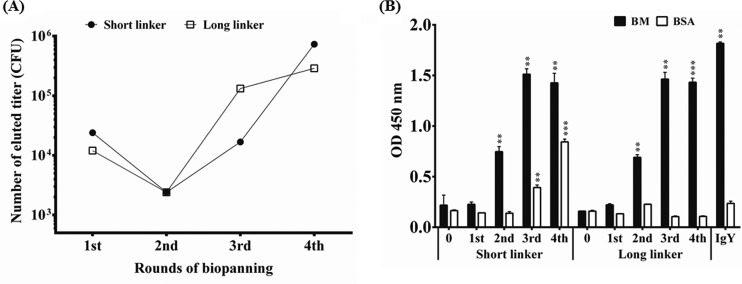
The cDNA was synthesized using total RNA extracted from chicken spleens and was used to amplify the VL and VH genes. The amplified VL and VH products were linked by a short or a long peptide linker to form the full-length of scFv gene fragments and were then cloned into a phagemid vector. We constructed two antibody libraries, scFv-S and scFv-L, containing 1.0 × 107 and 2.9 × 108 transformants, respectively. After infection by M13 helper phages, the recombinant phages displaying scFv antibodies were used for four rounds of biopanning (Fig. 2A). The eluted phage titers were determined as around 104 CFU in the first round, decreased 10-fold in the second round, and increased to a range of 104 to 105 CFU in the third and fourth rounds. The phenomenon was commonly observed in our previous studies, suggesting that specific binders were successfully enriched throughout the biopanning procedure (26). We further confirmed the speculated results of the biopanning process using a phage-based ELISA. The amplified phages were added to the wells coated with B. multicinctus proteins. Compared with those of phages from the original libraries, these results indicated that the binding activity of harvested phages was significantly enhanced after the second panning and reached a stage of plateau after the third round (Fig. 2B). Even though the nonspecific binding activity of the total phages harvested from the library with the short linker after the final panning also reacted with BSA (Fig. 2B), these results had no substantial effect on the screening of the specific anti-B. multicinctus scFv antibodies demonstrated in Fig. 4, 5, and 6.
FIG 2.
Effect of biopanning. (A) Antibody libraries constructed with short and long linkers were applied for the biopanning procedure. The titers of eluted phages were determined after each round of panning and monitored for four rounds. (B) Amplified phages from each round of panning were used to test the binding ability to B. multicinctus (BM) proteins on ELISA plates. Anti-B. multicinctus IgY antibodies from chickens immunized 7 times were used as a positive control. The binding activities of the amplified phages from each round of biopanning were compared with those of the phages from original libraries (0). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
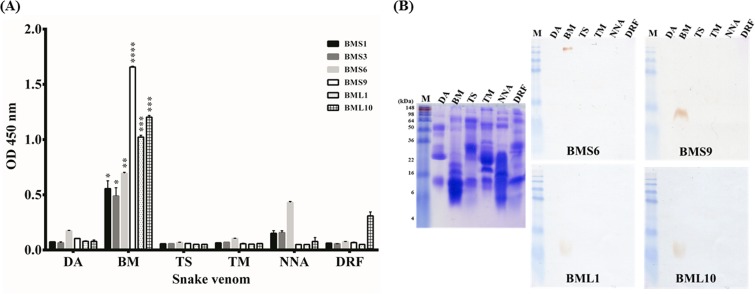
FIG 4.
Determination of binding specificity of scFv antibodies to various snake venom proteins. Anti-B. multicinctus scFv antibodies (BMS1, BMS3, BMS6, BMS9, BML1, and BML10) were purified and examined for their binding activity to six snake venom proteins (D. acutus [DA], B. multicinctus [BM], T. stejnegeri [TS], T. mucrosquamatus [TM], N. naja atra [NNA], and D. russellii formosensis [DRF]) immobilized on ELISA plates (A) or on PVDF membranes (B). BMS9, BML1, and BML10 scFv antibodies showed significant binding specificity. Moreover, they recognized a protein with approximately 12 kDa in molecular mass. Please see the text for details. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
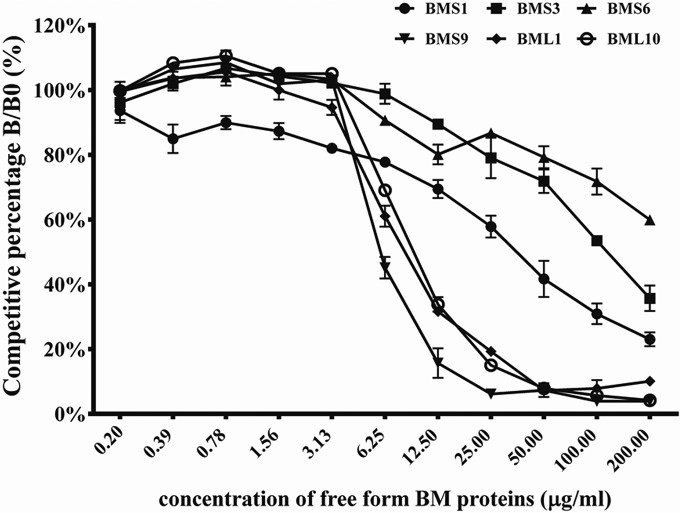
FIG 5.
Competitive inhibition assay of scFv antibodies against B. multicinctus (BM) proteins. Purified scFv antibodies were mixed with different concentrations of soluble B. multicinctus proteins, which were later added to the ELISA wells immobilized with B. multicinctus proteins as described in the text. The competitive percentage was shown as B/B0, representing the amounts of bound scFv in the presence or absence of B. multicinctus proteins, respectively. The data were represented as the means of duplicated experiments.
FIG 6.
The neutralization efficiency of IgY or scFv antibodies against B. multicinctus (BM) proteins in vivo. ICR mice were challenged with B. multicinctus proteins (1.4, 2.8, or 4.2 μg/each mouse) intraperitoneally to test the minimum lethal dose (MLD). PBS was used as a control. (B) IgY antibodies from preimmunized chickens (Pre-IgY), chickens immunized 7 times (Imm-IgY), or horse-derived antivenin (antivenin) were incubated individually with 2.8 μg of B. multicinctus proteins for 1 h, and the mixtures were subsequently intraperitoneally injected into mice. Imm-IgY showed significant protective activity compared to that of horse-derived IgG antivenin. (C) One milligram or 4 mg of combinations of six anti-B. multicinctus scFv antibodies was mixed with B. multicinctus proteins for 1 h and subsequently intraperitoneally injected into mice. The survival statuses of the mice were monitored hourly for 36 h. The survival rates were analyzed via Gehan-Breslow-Wilcoxon test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Analysis, expression, and purification of selected scFv antibodies.
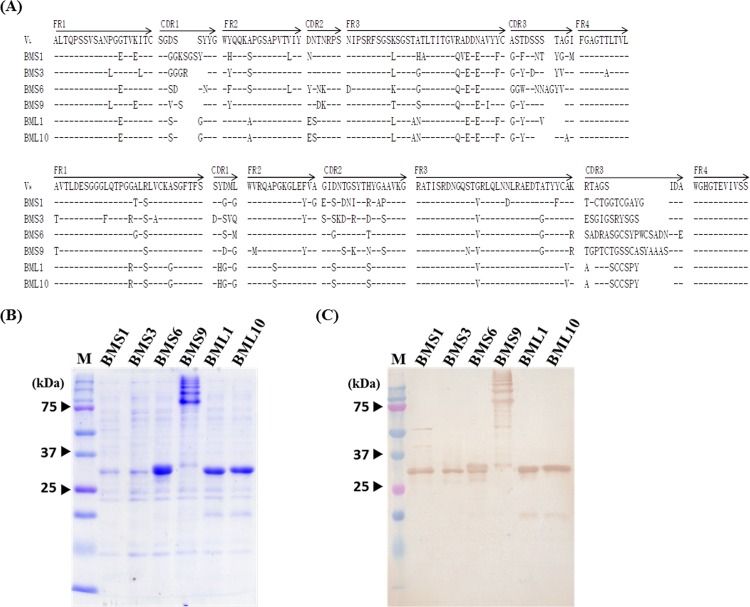
We first randomly selected 15 clones from each library to analyze the nucleotide sequences of their VH and VL genes. Sequence comparison showed that four distinct groups of scFv antibody clones were identified in the screened library with short linkers, which were represented by BMS1 (6/15; 40%), BMS3 (7/15; 46.7%), BMS6 (1/15; 6.67%), and BMS9 (1/15; 6.67%). Similarly, two different groups were identified in the screened library with long linkers as represented by BML1 (14/15; 93.3%) and BML10 (1/15; 6.67%). The predicted amino acid sequences of these six anti-B. multicinctus scFv antibodies were further compared with those of the chicken germ line gene (Fig. 3A). The overall mutation rates of amino acid residues in complementary-determining regions (CDRs) ranged from 25% to 54% in VL regions and 35% to 61% in VH regions. Otherwise, the mutation rates in the framework regions (FRs) ranged from 10% to 17% in VL regions and 6% to 9% in VH regions (Table 1). Taken together, the analysis of amino acid sequence homology showed that much greater variation was seen in the CDRs than in the FRs, especially in the CDR3 regions of the VL and VH genes with ∼22% to ∼90%. The results suggested that these scFv clones were selected from immunized B cells but not directly from naive B cells, which is well known as an antigen-driven humoral antibody response after immunization. Intriguingly, BML1 and BML10 had the same sequence of VH paired with divergent VL to form functional scFv molecules. However, the biological significance of the same VH gene usage is not presently understood.
FIG 3.
Sequence analysis and purification of selected single-chain variable fragment (scFv) antibodies. (A) Fifteen clones expressing scFv antibodies with short or long linkers were randomly selected to determine nucleotide sequences of light and heavy chain variable regions (VL and VH), and their predicted amino acid sequences were compared with that of the chicken germ line. Sequence gaps were introduced to maximize the alignment using blank spaces. Dashes indicate identical sequences. Framework region (FR) and complementary-determining region (CDR) boundaries are indicated above the germ line gene sequences. Six representative clones were identified. (B) The scFv antibodies expressed by these six clones were purified by Ni2+ Sepharose and analyzed on SDS-PAGE. (C) The identities of purified scFv antibodies were further confirmed by Western blotting as described in the text.
TABLE 1.
Amino acid mutation rates of VL and VH genes used by anti-B. multicinctus scFv antibodies
| scFv clone | Region | Dif/Tot (%)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CDR1 | CDR2 | CDR3 | Total CDRs | FR1 | FR2 | FR3 | FR4 | Total FRs | ||
| BMS1 | VL | 7/12 (58) | 1/7 (14) | 7/11 (64) | 15/30 (50) | 2/20 (10) | 3/16 (19) | 8/32 (25) | 0/10 (0) | 13/76 (17) |
| VH | 2/5 (40) | 8/17 (47) | 11/15 (73) | 21/37 (57) | 2/30 (7) | 2/14 (14) | 3/32 (9) | 0/11 (0) | 7/87 (8) | |
| BMS3 | VL | 4/8 (50) | 0/7 (0) | 5/11 (45) | 9/26 (35) | 2/20 (10) | 2/16 (13) | 6/32 (19) | 1/10 (10) | 11/78 (14) |
| VH | 4/5 (80) | 7/17 (41) | 11/14 (79) | 22/36 (61) | 5/30 (17) | 1/14 (7) | 2/32 (6) | 0/11 (0) | 8/87 (9) | |
| BMS6 | VL | 3/8 (38) | 3/7 (43) | 9/13 (69) | 15/28 (54) | 1/20 (5) | 3/16 (19) | 6/32 (19) | 0/10 (0) | 10/78 (13) |
| VH | 2/5 (40) | 2/17 (12) | 18/20 (90) | 22/42 (52) | 2/30 (7) | 0/14 (0) | 3/32 (9) | 0/11 (0) | 5/87 (6) | |
| BMS9 | VL | 2/8 (25) | 2/7 (29) | 2/9 (22) | 6/24 (25) | 2/20 (10) | 1/16 (6) | 5/32 (16) | 0/10 (0) | 8/78 (10) |
| VH | 2/5 (40) | 4/17 (24) | 17/20 (85) | 23/42 (55) | 2/30 (7) | 2/14 (14) | 4/32 (13) | 0/11 (0) | 8/87 (9) | |
| BML1 | VL | 2/8 (25) | 2/7 (29) | 3/9 (33) | 7/24 (29) | 1/20 (5) | 1/16 (6) | 6/32 (19) | 0/10 (0) | 8/78 (10) |
| VH | 3/5 (60) | 2/17 (12) | 7/12 (58) | 12/34 (35) | 3/20 (10) | 1/14 (7) | 2/32 (6) | 0/11 (0) | 6/87 (7) | |
| BML10 | VL | 2/8 (25) | 2/7 (29) | 3/9 (33) | 7/24 (29) | 1/20 (5) | 1/16 (6) | 7/32 (22) | 0/10 (0) | 9/78 (12) |
| VH | 3/5 (60) | 2/17 (12) | 7/12 (58) | 12/34 (35) | 3/30 (10) | 1/14 (7) | 2/32 (6) | 0/11 (0) | 6/87 (7) | |
Dif/Tot, no. of differences/total no.
After expression and purification, the purity and identity of these six scFv antibodies were confirmed with SDS-PAGE and Western blotting. All scFv antibodies contained a protein band of ∼25 to ∼37 kDa as expected (Fig. 3B). Nonetheless, in addition to the major band, the purified BMS9 scFv showed a ladder of other proteins with larger molecular masses. The character of these proteins was not exactly known, but their recognition by anti-chicken light-chain antibodies on Western blots suggested that they may be the different aggregate forms (Fig. 3C).
Specific binding assay of scFv antibodies and competitive ELISA.
Purified anti-B. multicinctus scFv antibodies were used to detect six venom proteins of commonly found venomous snakes in Taiwan. The ELISA showed that these scFv antibodies had binding activities to B. multicinctus proteins at different degrees (Fig. 4A). BMS1 and BMS3 scFv had weaker binding activities (ODs = 0.5), BMS6 had a moderate activity (OD = 0.7), and BMS9, BML1, and BML10 had stronger binding activities (ODs > 1.0). Compared with the other snake venom proteins, all of the anti-B. multicinctus scFv antibodies had significant binding activity to B. multicinctus proteins (Fig. 4A). However, BMS6 showed a weak binding activity (OD = 0.4) to N. naja atra venom containing neurotoxic proteins, and BML10 showed a weak binding activity (OD = 0.3) to D. russellii formosensis venom containing both hemotoxic and neurotoxic proteins. The Western blots showed that purified BMS6 scFv recognized a protein with a molecular mass of around 98 kDa while BMS9, BML1, and BML10 recognized a protein with a molecular mass of around 12 kDa (Fig. 4B). BMS1 and BMS3 showed no binding signals on Western blots (data not shown). All together, these results indicated that BMS6, BMS9, BML1, and BML10 scFv antibodies recognized B. multicinctus proteins with high specificity. Moreover, the antigenic epitope recognized by the BMS6 scFv is clearly distinct from those recognized by BMS9, BML1, and BML10.
To further confirm the binding specificities of these scFv antibodies, competitive ELISAs were carried out. Before adding to the ELISA wells, six scFv antibodies were premixed with free form B. multicinctus proteins individually. Absorbance values in the absence of free form B. multicinctus proteins were used as 100% to calculate the percentage of inhibitory effects against when different concentrations of free form B. multicinctus proteins were present. As shown in Fig. 5, the binding activities of these scFv antibodies to B. multicinctus proteins were inhibited in a dose-dependent manner. As calculated, 58%, 64%, 40%, 55%, 68%, and 66% decreases in the binding activities of BMS1, BMS3, BMS6, BMS9, BML1, and BML10 scFv antibodies were detected when 50, 200, 200, 6.25, 12.5, and 12.5 μg/ml of free form B. multicinctus proteins were used in the mixture, respectively. Taken as a whole, all of the results suggested BMS9 scFv had the strongest binding specificity while BMS6 was the weakest binder.
Neutralization assay in mice.
We determined the MLD of B. multicinctus proteins for the survival period of mice by administrating 1.4, 2.8, and 4.2 μg intraperitoneally (Fig. 6A). PBS alone was used as a control. The results showed that three mice expired within 2 h, one expired within 4 h, two expired within 5 h, and three survived for the experimental duration when 1.4 μg was challenged. In contrast, all mice died within 2 h when 2.8 and 4.2 μg were used, while a 100% survival rate was observed in the PBS-challenged group. Thus, 2.8 μg of B. multicinctus proteins was defined as 1× MLD and was used for subsequent studies. First, we tested the neutralization efficacy of polyclonal IgY antibodies (Fig. 6B). IgY antibodies from chickens before immunization had no protective effect, resulting in 100% death of mice within 3 h. In contrast, anti-B. multicinctus IgY antibodies from B. multicinctus-immunized chickens provided complete protection to all of the mice treated by 1× the MLD of B. multicinctus proteins; these results were comparable to those observed in mice challenged with antivenin from a horse immunized with B. multicinctus proteins (Fig. 6B). Furthermore, we analyzed the neutralization activity of a mixture of monoclonal antibodies containing 1 or 4 mg of six anti-B. multicinctus scFv (Fig. 6C). As seen in the results, although all of the mice died when challenged with 1 mg of mixed scFv antibodies in the end, the survival times of mice were significantly prolonged compared with those of the PBS-challenged group. Accordingly, when challenged with 4 mg of mixed scFv antibodies, two mice died within 2 h, two survived for 1 h longer, one survived for 2 h longer, one survived for 3 h longer, one survived for 4 h longer, and two survived for the experimental duration (Fig. 6C). These results suggested that the mixture of anti-B. multicinctus scFv antibodies provided partial and significant protective activities in B. multicinctus-administered mice.
DISCUSSION
At present, poisoning from venomous snakes with high mortality is an often-discussed global health problem. Compared to horse-derived agents, with their high costs and side effects, chickens can provide an alternative source of antibodies that is cost-effective and convenient (8, 14). In our study, we demonstrated that chickens are suitable to elicit humoral immune responses, as we previously observed, and last for 6 months at least (Fig. 1) (26). Purification of IgY antibodies is also noninvasive and more accessible than that of IgG antibodies from horse serum. Most importantly, we used only about 800 μg of B. multicinctus venom proteins for chicken immunization. The amount was 75-fold less than that (60 mg at least) used for horse immunization to generate neutralization antibodies (27). Furthermore, chicken-derived IgY antibodies did not activate mammalian complement components or bind to Fc receptors, protein A, protein G, and rheumatoid factor as often occurred during use of horse-derived IgG antibodies (28). Although the application of anti-B. multicinctus IgY antibodies against snakebites is not feasible presently, our results offer an alternative way that the generation of antibodies with neutralizing activities in chickens is viable and more cost-effective than that in horses, in particular when the immunogens are not readily available.
Phage display technology is a powerful tool for identifying specific antibodies against a broad range of targets, including tumor-associated antigens or snake venom proteins (22, 29, 30). We constructed two libraries to select specific antibodies and obtained six scFv antibodies against B. multicinctus proteins after four rounds of biopanning (Fig. 4). The libraries from hyperimmunized chickens offered a more effective and feasible way to select specific antibodies from the antibody repertoire as suggested by the results of phage-based ELISA (Fig. 2B) (31). In contrast, antigen-specific antibodies were generally obtained after more than four rounds of biopanning when naive libraries constructed from nonimmunized chickens were applied. In addition, the entire procedure required to identify specific antibodies was 1 to 2 months using a phage display system, which is much more time-effective than traditional hybridoma technology that requires 3 to 4 months (32). Thus, considering the expense and time, phage display technology is further confirmed to be superior to hybridoma technology for selecting specific anti-B. multicinctus scFv antibodies in chickens, although the promiscuous pairing of VL and VH genes that occurred in the E. coli host may be disputable (25, 33). This problem can be answered only by carrying out further experiments. However, since the main purpose of our study was to obtain a protein molecule(s) with binding activity to B. multicinctus proteins, it may be more appropriate to nominate these E. coli-derived anti-B. multicinctus scFv molecules as “antibody-like molecules” or “recombinant B. multicinctus-binding proteins” to avoid such confusion.
In chickens, the functional variable VH and VL regions of antibody molecules are generated via various V-J region recombination (additional D for VH), somatic mutation, and assorted heavy chains with light chains to create antibody diversity in most vertebrates (34, 35). Among all of the CDRs of antibodies, CDR3 in the VH fragment is commonly considered to be the most divergent and the most important region against target proteins (36). After comparing the amino acid sequences of selected anti-B. multicinctus scFv clones with that of the chicken germ line, we found that the total mutation rates of CDRs in both VL and VH fragments were 25% to 54% and 35% to 61%, respectively, while the total mutation rates of FRs in both VL and VH were 10% to 17% and 6% to 9% (Table 1; Fig. 3A). It is notable that the mutation rates found in CDR3 of the VH fragments of BMS6 and BMS9 scFv antibodies were 90% and 85%, the highest numbers identified in all CDR regions of six analyzed scFv clones. Our results were in accordance with those reported in previous studies, indicating that somatic hypermutations to increase antibody affinity occurred more often in the CDR than in the FR of the rearranged V gene (34, 37). These observations also suggested that the selected anti-B. multicinctus antibodies were generated by an antigen-driven immune response rather than being directly from the naive immunoglobulin repertoire.
It was also revealed that the CDR3 regions in the VH fragments contained 8 to 32 (mean, 16.2 ± 3.2) amino acids in chickens, 89% of which contained 15 to 23 amino acids. This figure was similar to those in humans, containing 5 to 37 amino acids (mean, 16.1 ± 4.1) (38). However, BMS3, BML1, and BML10 had 14, 12, and 12 amino acids, respectively. Thus, 50% of our selected scFv antibodies had shorter CDR3 fragments than those reported in the previous studies. Additionally, the other 50% of scFv antibodies, including BMS1, BMS6, and BMS9 with 15, 20, and 20 amino acids in the CDR3 regions, respectively, were in the range (Table 1; Fig. 3A). It is interesting to observe that the six scFv antibodies showed various levels of specific binding activity to B. multicinctus proteins. Furthermore, BMS6 exhibited weak cross-reactivity (OD = 0.4) to the neurotoxic N. naja atra proteins while BML10 had weak cross-reactivity (OD = 0.3) to D. russellii formosensis proteins (Fig. 4A). All of the results together suggested that the length of anti-B. multicinctus scFv antibodies may play a limited role in their binding activity and specificity.
However, it is remarkable that BML1 and BML10 had the same VH but divergent VL usage as shown by the sequence alignment (Fig. 3A). BML1 and BML10 scFv exhibited divergent OD readings on ELISAs (Fig. 4A) and binding signals on Western blots (Fig. 4B). These results suggested that the divergent VL fragments used by these two antibodies may make significant contributions to their cross binding specificity to D. russellii formosensis proteins and binding activity to B. multicinctus proteins. BMS6 recognized a protein band (85 kDa), which was distinct from that recognized by BMS9, BML1, and BML10 (12 kDa), suggesting they were elicited by two separated antigenic epitopes. Even though their identities need further characterization, the two proteins were estimated to be 0.5% and 33% in crude B. multicinctus venom proteins as analyzed by ImageJ software (39). The amounts of B. multicinctus proteins that were required to reach 50% inhibitory effects on the binding activities of BMS6, BMS9, BML1, and BML10 were 13.82, 2.61, 3.07, and 3.31 μg/ml, respectively (Fig. 5). Thus, the dissociation constant (Kd) values of these four scFv antibodies were calculated to be 1.6 × 10−7, 2.18 × 10−7, 2.56 × 10−7, and 2.76 × 10−7 M based on the Klotz plot method (40).
The mixture of the selected scFv antibodies provided partial protective effects in mice, suggesting that they possessed partial neutralization activity against the MLD of B. multicinctus proteins (Fig. 6C). These results may be partially explained by the following descriptions: (i) previous studies reported that the bungarotoxin group in B. multicinctus venom is the major group of lethal proteins (4, 5, 41); (ii) homologous bungarotoxins have different levels of neurotoxicity or reacted with other components to synergistically increase the neurotoxicity (4); (iii) knowing the molecular mass of the bungarotoxins (<15 kDa), combined with the data shown in Fig. 4B, it was reasoned that anti-B. multicinctus scFv antibodies may only neutralize the neurotoxicity of a fraction of bungarotoxins (4). Since the polyclonal anti-B. multicinctus IgY antibodies provided complete protection in mice (Fig. 6B), it is believed that additional anti-B. multicinctus scFv antibodies in the original antibody libraries with neutralizing activities against the neurotoxicity of other bungarotoxins may be identified. Accordingly, as mentioned above, all of the anti-B. multicinctus scFv antibodies together would have great potential for the development of therapeutic treatments against snake envenomation in the future.
ACKNOWLEDGMENTS
We are grateful to Meng-Huei Liang for her help with SDS-PAGE and Western blotting.
We declare that we have no conflicts of interest.
REFERENCES
- 1.Chippaux J-P. 2006. Clinic and treatment of envenomations. Krieger Publishing Company, Malabar, FL. [Google Scholar]
- 2.Hung HT, Hojer J, Du NT. 2009. Clinical features of 60 consecutive ICU-treated patients envenomed by Bungarus multicinctus. Southeast Asian J Trop Med Public Health 40:518–524. [PubMed] [Google Scholar]
- 3.Jiang Y, Li Y, Lee W, Xu X, Zhang Y, Zhao R, Wang W. 2011. Venom gland transcriptomes of two elapid snakes (Bungarus multicinctus and Naja atra) and evolution of toxin genes. BMC Genomics 12:1. doi: 10.1186/1471-2164-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khow O, Chanhome L, Omori-Satoh T, Ogawa Y, Yanoshita R, Samejima Y, Kuch U, Mebs D, Sitprija V. 2003. Isolation, toxicity and amino terminal sequences of three major neurotoxins in the venom of Malayan krait (Bungarus candidus) from Thailand. J Biochem 134:799–804. doi: 10.1093/jb/mvg187. [DOI] [PubMed] [Google Scholar]
- 5.Chang L-S, Kao P-H. 2009. Origin of functional diversities in Taiwan banded krait (Bungarus multicinctus) three-finger proteins. Fooyin J Health Sci 1:57–64. doi: 10.1016/S1877-8607(10)60001-X. [DOI] [Google Scholar]
- 6.Ouyang C, Teng CM, Huang TF. 1990. Characterization of snake venom principles affecting blood coagulation and platelet aggregation. Adv Exp Med Biol 281:151–163. doi: 10.1007/978-1-4615-3806-6_15. [DOI] [PubMed] [Google Scholar]
- 7.Burke JE, Dennis EA. 2009. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50(Suppl):S237–S242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold BS, Dart RC, Barish RA. 2002. Bites of venomous snakes. N Engl J Med 347:347–356. doi: 10.1056/NEJMra013477. [DOI] [PubMed] [Google Scholar]
- 9.Dias da Silva W, Tambourgi DV. 2010. IgY: a promising antibody for use in immunodiagnostic and in immunotherapy. Vet Immunol Immunopathol 135:173–180. doi: 10.1016/j.vetimm.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warr GW, Magor KE, Higgins DA. 1995. IgY: clues to the origins of modern antibodies. Immunol Today 16:392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 11.Carlander D. 2002. Avian IgY antibody: in vitro and in vivo. Ph.D. dissertation Uppsala University, Uppsala, Sweden. [Google Scholar]
- 12.Carlander D, Larsson A. 2001. Avian antibodies can eliminate interference due to complement activation in ELISA. Ups J Med Sci 106:189–195. doi: 10.3109/2000-1967-145. [DOI] [PubMed] [Google Scholar]
- 13.Kruger C, Pearson SK, Kodama Y, Vacca Smith A, Bowen WH, Hammarstrom L. 2004. The effects of egg-derived antibodies to glucosyltransferases on dental caries in rats. Caries Res 38:9–14. [DOI] [PubMed] [Google Scholar]
- 14.Kovacs-Nolan J, Mine Y. 2012. Egg yolk antibodies for passive immunity. Annu Rev Food Sci Technol 3:163–182. doi: 10.1146/annurev-food-022811-101137. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarty D, Bhattacharyya D, Sarkar HS, Lahiri SC. 1993. Purification and partial characterization of a haemorrhagin (VRH-1) from Vipera russelli russelli venom. Toxicon 31:1601–1614. doi: 10.1016/0041-0101(93)90344-I. [DOI] [PubMed] [Google Scholar]
- 16.Mine Y, Kovacs-Nolan J. 2002. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. J Med Food 5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- 17.de Almeida CM, da Silva CL, Couto HP, Escocard RDC, da Rocha DG, Sentinelli LDP, Kipnis TL, da Silva WD. 2008. Development of process to produce polyvalent IgY antibodies anti-African snake venom. Toxicon 52:293–301. doi: 10.1016/j.toxicon.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Huse WD, Sastry L, Iverson SA, Kang AS, Alting-Mees M, Burton DR, Benkovic SJ, Lerner RA. 1989. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science 246:1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- 19.Finlay WJ, Shaw I, Reilly JP, Kane M. 2006. Generation of high-affinity chicken single-chain Fv antibody fragments for measurement of the Pseudonitzschia pungens toxin domoic acid. Appl Environ Microbiol 72:3343–3349. doi: 10.1128/AEM.72.5.3343-3349.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park KJ, Park DW, Kim CH, Han BK, Park TS, Han JY, Lillehoj HS, Kim JK. 2005. Development and characterization of a recombinant chicken single-chain Fv antibody detecting Eimeria acervulina sporozoite antigen. Biotechnol Lett 27:289–295. doi: 10.1007/s10529-005-0682-8. [DOI] [PubMed] [Google Scholar]
- 21.Kulkeaw K, Sakolvaree Y, Srimanote P, Tongtawe P, Maneewatch S, Sookrung N, Tungtrongchitr A, Tapchaisri P, Kurazono H, Chaicumpa W. 2009. Human monoclonal ScFv neutralize lethal Thai cobra, Naja kaouthia, neurotoxin. J Proteomics 72:270–282. doi: 10.1016/j.jprot.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Oliveira JG, Soares SG, Soares AM, Giglio JR, Teixeira JE, Barbosa JE. 2009. Expression of human recombinant antibody fragments capable of partially inhibiting the phospholypase activity of Crotalus durissus terrificus venom. Basic Clin Pharmacol Toxicol 105:84–91. doi: 10.1111/j.1742-7843.2008.00322.x. [DOI] [PubMed] [Google Scholar]
- 23.Akita EM, Nakai S. 1993. Production and purification of Fab' fragments from chicken egg yolk immunoglobulin Y (IgY). J Immunol Methods 162:155–164. doi: 10.1016/0022-1759(93)90380-P. [DOI] [PubMed] [Google Scholar]
- 24.Akita EM, Nakai S. 1993. Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. J Immunol Methods 160:207–214. doi: 10.1016/0022-1759(93)90179-B. [DOI] [PubMed] [Google Scholar]
- 25.Andris-Widhopf J, Rader C, Steinberger P, Fuller R, Barbas CF III. 2000. Methods for the generation of chicken monoclonal antibody fragments by phage display. J Immunol Methods 242:159–181. doi: 10.1016/S0022-1759(00)00221-0. [DOI] [PubMed] [Google Scholar]
- 26.Leu SJ, Lee YC, Shih NY, Huang IJ, Liu KJ, Lu HF, Huang SY, Yang YY. 2010. Generation and characterization of anti-alpha-enolase single-chain antibodies in chicken. Vet Immunol Immunopathol 137:251–260. doi: 10.1016/j.vetimm.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO/SEARO. 1999. WHO/SEARO guidelines for the clinical management of snake bites in the Southeast Asian region. Southeast Asian J Trop Med Public Health 30(Suppl):S1–S85. [PubMed] [Google Scholar]
- 28.Carlander D, Kollberg H, Wejaker PE, Larsson A. 2000. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol Res 21:1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebrahimizadeh W, Rajabibazl M. 2014. Bacteriophage vehicles for phage display: biology, mechanism, and application. Curr Microbiol 69:109–120. doi: 10.1007/s00284-014-0557-0. [DOI] [PubMed] [Google Scholar]
- 30.Stewart CS, MacKenzie CR, Hall JC. 2007. Isolation, characterization and pentamerization of alpha-cobrotoxin specific single-domain antibodies from a naive phage display library: preliminary findings for antivenom development. Toxicon 49:699–709. doi: 10.1016/j.toxicon.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Gao C, Mao S, Kaufmann G, Wirsching P, Lerner RA, Janda KD. 2002. A method for the generation of combinatorial antibody libraries using pIX phage display. Proc Natl Acad Sci U S A 99:12612–12616. doi: 10.1073/pnas.192467999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandey S. 2010. Hybridoma technology for production of monoclonal antibodies. Hybridoma 1:017. [Google Scholar]
- 33.Hammers CM, Stanley JR. 2014. Antibody phage display: technique and applications. J Invest Dermatol 134:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parvari R, Ziv E, Lantner F, Heller D, Schechter I. 1990. Somatic diversification of chicken immunoglobulin light chains by point mutations. Proc Natl Acad Sci U S A 87:3072–3076. doi: 10.1073/pnas.87.8.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynaud CA, Dahan A, Anquez V, Weill JC. 1989. Somatic hyperconversion diversifies the single Vh gene of the chicken with a high incidence in the D region. Cell 59:171–183. doi: 10.1016/0092-8674(89)90879-9. [DOI] [PubMed] [Google Scholar]
- 36.Xu JL, Davis MM. 2000. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity 13:37–45. doi: 10.1016/S1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 37.Gearhart PJ, Bogenhagen DF. 1983. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A 80:3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu L, Oficjalska K, Lambert M, Fennell BJ, Darmanin-Sheehan A, Ni Shuilleabhain D, Autin B, Cummins E, Tchistiakova L, Bloom L, Paulsen J, Gill D, Cunningham O, Finlay WJ. 2012. Fundamental characteristics of the immunoglobulin VH repertoire of chickens in comparison with those of humans, mice, and camelids. J Immunol 188:322–333. doi: 10.4049/jimmunol.1102466. [DOI] [PubMed] [Google Scholar]
- 39.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friguet B, Chaffotte AF, Djavadi-Ohaniance L, Goldberg ME. 1985. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J Immunol Methods 77:305–319. doi: 10.1016/0022-1759(85)90044-4. [DOI] [PubMed] [Google Scholar]
- 41.Chang L-S, Chung C, Wu B-N, Yang C-C. 2002. Characterization and gene organization of Taiwan banded krait (Bungarus multicinctus) γ-bungarotoxin. J Prot Chem 21:223–229. doi: 10.1023/A:1019760401692. [DOI] [PubMed] [Google Scholar]