Abstract
Individuals with internet gaming disorder (IGD) often have impaired risky decision-making abilities, and IGD-related functional changes have been observed during neuroimaging studies of decision-making tasks. However, it is still unclear how feedback (outcomes of decision-making) affects the subsequent risky decision-making in individuals with IGD. In this study, twenty-four adolescents with IGD and 24 healthy controls (HCs) were recruited and underwent functional magnetic resonance imaging while performing the balloon analog risk task (BART) to evaluate the effects of prior outcomes on brain activity during subsequent risky decision-making in adolescents with IGD. The covariance between risk level and activation of the bilateral ventral medial prefrontal cortex, left inferior frontal cortex, right ventral striatum (VS), left hippocampus/parahippocampus, right inferior occipital gyrus/fusiform gyrus and right inferior temporal gyrus demonstrated interaction effects of group by outcome (P < 0.05, AlphaSim correction). The regions with interactive effects were defined as ROI, and ROI-based intergroup comparisons showed that the covariance between risk level and brain activation was significantly greater in adolescents with IGD compared with HCs after a negative outcome occurred (P < 0.05). Our results indicated that negative outcomes affected the covariance between risk level and activation of the brain regions related to value estimation (prefrontal cortex), anticipation of rewards (VS), and emotional-related learning (hippocampus/parahippocampus), which may be one of the underlying neural mechanisms of disadvantageous risky decision-making in adolescents with IGD.
Keywords: Bart, fMRI, Internet gaming disorder, Risky decision-making
Highlights
-
•
Evaluating effects of outcome on subsequent risk decision making in IGDs
-
•
Negative feedback affects risk decision making related brain activity in IGDs.
-
•
Altered response to negative feedback contributes to adverse decision making in IGDs.
1. Introduction
Internet gaming disorder (IGD) is defined as persistent and recurrent use of the internet to engage in games, which may lead to significant psychological distress and interfere with daily social life (Young, 1999). IGD is the most prevalent form of internet addiction disorder in Asia (Dong et al., 2012b, Tang et al., 2014, Wu et al., 2013). Reduced risky decision-making ability is one of the most significant behavioral impairments in IGD individuals (Pawlikowski and Brand, 2011, Yao et al., 2015). Risky decision-making is essential for human survival because risk is ubiquitous in the natural world and human life (Hastie, 2001). However, IGD individuals tend to exhibit disadvantageous risky decision-making behavior, partly because of failure to utilize feedback (Pawlikowski and Brand, 2011, Yao et al., 2014). IGD adolescents continue to use internet games despite negative long-term consequences in the social or work domains of life (Pawlikowski and Brand, 2011); which may lead to physical dependence (Brand, 2008, Ersche et al., 2005), and eventually cause psychological, social, and/or work problems similar to other addictions (Young, 1999). Therefore, it is important to investigate the neural mechanisms underlying feedback processing impairments during decision-making in individuals with IGD.
Optimal decision-making often requires the ability to learn from the outcomes of previous choices, rewards or punishments and adjust future choices accordingly (O'Doherty et al., 2003). The neural circuits related to the ability to interpret this feedback in health adults include the dorsal and ventral prefrontal cortex (PFC), striatum, anterior cingulate cortex (ACC), nucleus accumbens and insula (Balasubramani et al., 2015, Ernst and Steinhauser, 2015, Hauser et al., 2015, Kohno et al., 2015, Liu et al., 2011, Rao et al., 2014, Tanabe et al., 2013). An impaired ability to learn from outcomes has been demonstrated in individuals with substance dependence disorders (Balconi et al., 2014, Tanabe et al., 2013, Worbe et al., 2014). Studies using the Iowa gambling task (IGT) have identified decreased task performance in substance-dependent individuals, which may indicate deficits in learning from feedback (Balconi et al., 2014, Tanabe et al., 2013). While performing the IGT, activation of the ventral striatum (VS) and medial orbitofrontal cortex (OFC) was found to be deceased in substance-dependent individuals compared to healthy subjects, which may be the underlying neuronal mechanism of these deficits in learning from feedback (Tanabe et al., 2013). A study by Worbe et al. (2014) using an anticipatory risk-taking task found that binge drinkers showed a higher number of risky choices in high-risk losses, and the high-risk attitude in the loss condition was associated with greater activity in the dorsal lateral prefrontal cortex (DLPFC), the lateral OFC, and the superior parietal cortices. Failure to utilize feedback has also been identified in individuals with IGD by both behavioral and neuroimaging studies (Dong et al., 2013, Pawlikowski and Brand, 2011, Yao et al., 2014). For instance, a behavioral study by Yao et al. (2014) found that IGD individuals made more disadvantageous choices during the Game of Dice Task compared with healthy controls (HCs) and that individuals with IGD could not utilize feedback to optimize their decision-making and improve their performance. A fMRI study using a continuous wins-and-losses task found that subjects with IGD exhibited enhanced sensitivity to win and decreased sensitivity to loss (Dong et al., 2013). Although preliminary studies have demonstrated that outcomes may affect brain activation during decision-making in individuals with IGD, no previous studies have focused on the effect of different outcomes on the covariance between risk level and brain activation during the risky decision-making processing in individuals with IGD.
In this study, twenty-four IGD adolescents and 24 HCs were enrolled, and fMRI data were obtained from the participants while performing the Balloon Analog Risk Task (BART) (Lejuez et al., 2002) to investigate the manner in which different outcomes affected the covariance between risk level and brain activation during decision-making processes in adolescents with IGD. The risk level in the BART was represented by the probability of balloon explosion, and task performance depended on to what extent participants learned from different previous outcomes (Kohno et al., 2015, Rao et al., 2014). Thus, the BART may then be adapted to evaluate the effect of previous outcomes on the covariance between brain activation and risk level during subsequent risky decision-making processes. Based on the previous studies that the subjects with IGD failed to utilize feedback(Yao et al., 2014) and exhibited different sensitivity to different outcomes (Dong et al., 2013), we hypothesized that different outcomes would cause different effects on the covariance between risk level and brain activation in feedback-related brain regions, mainly including the PFC and striatum in adolescents with IGD. This study may bring new insights into the understanding of the underlying neuronal mechanisms of impaired risky decision-making ability in adolescents with IGD.
2. Material and method
2.1. Participant selection
In this study, twenty-four male adolescents with IGD and 24 age- and education-matched HCs were recruited. Only male adolescents were enrolled because the prevalence of IGD is substantially higher in men than in women. All participants were recruited based on the findings of diagnostic interviews by a senior psychiatrist. Adolescents with IGD were defined according to the following criteria: five or more “yes” responses to the eight questions on the Young Diagnostic Questionnaire for Internet Addiction (YDQ) (Young, 1998), a score ≥ 50 on Young's Online Internet Addiction Test (IAT), spending an average of four or more hours per day playing internet games, right-handed, no alcohol or drug abuse, no neurologic or psychiatric diseases, and medication-free. The MINI-International Neuropsychiatric Interview (MINI) was used to exclude adolescents with diagnosis of a DSM-IV Axis I disorder. HCs were defined as adolescents not fitting the criteria for an YDQ diagnosis, spending < 2 h/day on the internet, and having an IAT score was < 50. Other selection criteria for the HCs were the same as those for the adolescents with IDG. The standard Raven's Progressive Matrices (SPM) was used to test the intelligence quotient (IQ) of all participants. The Barratt Impulsivity Scale (BIS) was used to test the impulsivity of all participants. The Self-Rating Anxiety Scale (SAS) and Self-Rating Depression Scale (SDS) were used to test the levels of anxiety and depression among participants, respectively. Detailed clinical information for the two groups is listed in Table 1.
Table 1.
Demographic and clinical characteristics of subjects (Mean ± SD).
| IGD (N = 24) | HCs (N = 24) | t | P | |
|---|---|---|---|---|
| Age (year) | 17.17 ± 3.51 | 17.42 ± 3.05 | − 2.263 | 0.793 |
| Education (year) | 10.08 ± 2.98 | 11.25 ± 2.88 | − 1.380 | 0.174 |
| IQ (SPM) | 48.92 ± 6.79 | 48.58 ± 6.26 | 0.177 | 0.860 |
| IAT score | 70.71 ± 10.76 | 33.42 ± 7.75 | 13.852 | < 0.001 |
| BIS | 68.79 ± 11.83 | 54.13 ± 8.05 | 5.022 | < 0.001 |
| SAS | 43.13 ± 8.90 | 35.42 ± 5.02 | 3.680 | 0.001 |
| SDS | 49.57 ± 5.02 | 39.38 ± 9.16 | 3.442 | 0.001 |
Two-sample two-tailed t-tests. Significant level is set as P < 0.05.
BIS, Barratt impulsivity scale; HCs, healthy controls; IAT, internet addiction test; IGD, internet game disorder; IQ, intelligence quotient; SAS, Self-Rating Anxiety Scale; SDS, Self-rating depression scale; SPM, standard Raven's progressive matrices.
The protocol of this study was approved by the Ethical Committee of Tianjin Medical University General Hospital, and written informed consent was obtained from each subject according to institutional guidelines.
2.2. Task and procedure
The fMRI-adapted version of the BART used in this study was guided by prior imaging work (Rao et al., 2008). The details of the experimental task have previously been described (Qi et al., 2015). Briefly, the participants were presented a virtual balloon and asked to press one of two buttons to either inflate (pump) the balloon or cash out. Before the experiment, participants were informed that they would receive the equivalent amount of money earned during the experiment. As the balloon was inflated, both the monetary reward and the probability of explosion (risk level) increased. The value of the wagers corresponded to the various balloon sizes, and the cumulative earnings for the tasks were displayed underneath the balloon stimuli. In a trial, participants could stop inflating the balloon at any point to win the wager or keep inflating until the balloon exploded (loss). The maximum number of pumps that participants could use for each balloon was 12. The time of inflation was controlled by a cue (the color of a small circle changing from red to green). After the participants successfully pressed a button, the small circle immediately turned red at a random interval of between 1.5 and 2.5 s and then turned green again to indicate the next inflation. There was a varying 2–4 s interval between trials. Text indicating whether the trial was a “win” or “loss” was presented for 1.5 s. The picture of the exploded balloon was presented for 20 ms. The number of balloons depended on response speed rather than being pre-determined in the experiment. After the experiment, the participants received the equivalent amount of money earned during the experiment.
2.3. Data acquisition
The functional MRI was conducting using a Siemens 3.0 T scanner (Magnetom Verio, Siemens, Erlangen, Germany). A gradient-recalled echo-planar imaging sequence was used with the following parameters: repetition time (TR) = 2000 ms; echo time (TE) = 30 ms; field of view = 220 × 220 mm; matrix = 64 × 64; slice thickness = 4 mm; and slice gap = 1 mm. Through a mirror mounted on the head-coil, the participants viewed the stimuli that was projected onto a screen in front of the magnet bore. The participants were asked to respond to the task by pressing a button on the fMRI-compatible response box. A 10-minute BART fMRI was performed on all participants after they learned and practiced the tasks.
2.4. Behavioral analysis
In the fMRI experiment, the behavioral variables collected during the BART included the trial number, total and mean number of pumps, number of wins and losses, adjusted number of pumps (defined as the average number of pumps excluding the balloons that exploded), adjusted number of pumps after wins or losses, the reward collection rate (the number of win trials divided by the number of total trials), and average response time (RT). A two-sample t-test was used to compare behavioral data between the two groups. Statistical analyses were conducted with SPSS 21.0, and the significance level was set at P < 0.05.
2.5. Functional MRI data preprocessing
Statistical Parametric Mapping software (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8) was used to preprocess functional MRI data. First, the functional images of each subject were corrected for the acquisition time delay between different slices. Second, geometric displacements due to estimated head movement were corrected by realigning all images to the first volume. Participants who had a maximum displacement in any direction (x, y, or z) > 2 mm or > 2° of angular rotation (x, y, or z) were excluded from the study. Third, all realigned images were spatially normalized to the Montreal Neurological Institute (MNI) EPI template and resampled to 3 × 3 × 3 mm3. Finally, normalized images were smoothed with a Gaussian kernel of 6 mm full-width at half-maximum (FWHM).
2.6. Statistical analysis
In the general linear model (GLM), preprocessed fMRI data were modeled using a standard hemodynamic response function (HRF) with a time derivative. The head movement parameters of each participant were introduced as covariates of no-interest.
In our BART experiment, three events resulting from a button press could be modeled: an inflation of the balloon, a win outcome, or a loss outcome. Because we aimed to investigate the effect of prior outcomes on the brain activity during subsequent decision-making, four regressors were included in the GLM: win outcome, inflation after win, loss outcome, and inflation after loss. The risk level associated with each inflation (i.e., the probability of explosion, orthogonalized by a mean central correction) was also entered into the model as a linear parametric modulation of the balloon inflation regressor. For each subject, the β estimates for the parametric modulation of the risk of balloon inflation after wins and after losses represented the covariance between the risk and brain activation.
Second level random effect analyses were conducted using a 2 (group: IGD and HCs) × 2 (post-outcome: “after win” and “after loss”) ANOVA on the covariance between risk and brain activation with full factorial analysis, embedded in SPM8. The covariance between risk and brain activation “after win” and “after loss” within the same participant were processed as repeated measures. In this study, the main aim was to evaluate the intergroup difference in the different effects of outcome on the covariance between risk and brain activation during decision-making following outcomes. Therefore, the interaction effect of group by post-outcome, HCs (“after loss” - “after win”) - IGD (“after loss” - “after win”), was analyzed in this study. Additionally, the main effects of group and post-outcome were also tested. A correction for multiple comparisons was performed using the Monte Carlo simulation method, resulting in a corrected threshold of P < 0.05 (AlphaSim program, parameters including: single voxel P = 0.005; 1000 simulations; full width at half maximum = 6 mm; cluster connection radius r = 5 mm; and the mask of global gray matter). The brain regions with interaction effects were defined as regions of interest (ROI). The average β estimates within ROIs were extracted and a ROI-based intergroup comparison was conducted using t-tests. Pearson's correlation analysis was performed to test the correlations between the average β estimates within ROIs and behavioral performance and IAT scores in adolescents with IGD. The significance level was set at P < 0.05.
3. Results
3.1. Behavioral results
Table 2 shows the behavioral results obtained during the fMRI experiment. The two-sample t-test revealed that the average RT was significantly shorter (P = 0.033) and the number of the total pumps was significantly greater in adolescents with IGD than in HCs (P = 0.003). There was no significant intergroup difference in the adjusted number of pumps; mean numbers of pumps after wins, losses, or overall; trial number; number of wins/losses, or reward collection rate (P > 0.05).
Table 2.
The behavioral results of the BART during fMRI experiment (Mean ± SD).
| IGD (N = 24) | HCs (N = 24) | t | P | |
|---|---|---|---|---|
| Mean pumps | 7.77 ± 1.01 | 7.32 ± 0.93 | 1.621 | 0.112 |
| Adjusted pumps | 6.28 ± 1.12 | 5.71 ± 1.10 | 1.783 | 0.081 |
| Mean pumps after win | 6.91 ± 1.05 | 6.53 ± 1.03 | 1.266 | 0.212 |
| Mean pumps after loss | 6.42 ± 1.21 | 5.82 ± 1.15 | 1.777 | 0.082 |
| Total pumps | 210.67 ± 9.77 | 199.21 ± 15.16 | 3.112 | 0.003 |
| Trial number | 31.21 ± 5.00 | 31.58 ± 5.03 | − 0.259 | 0.797 |
| Win trials | 23.21 ± 5.97 | 25.00 ± 7.02 | − 0.952 | 0.346 |
| Pop trials | 8.00 ± 3.17 | 6.58 ± 2.81 | 1.636 | 0.109 |
| Reward collection rate | 0.74 ± 0.11 | 0.78 ± 0.12 | − 1.233 | 0.224 |
| Response time (ms) | 494.29 ± 71.84 | 553.21 ± 110.23 | − 2.194 | 0.033 |
Two-sample two-tailed t-tests. Significant level is set as P < 0.05.
3.2. Imaging results
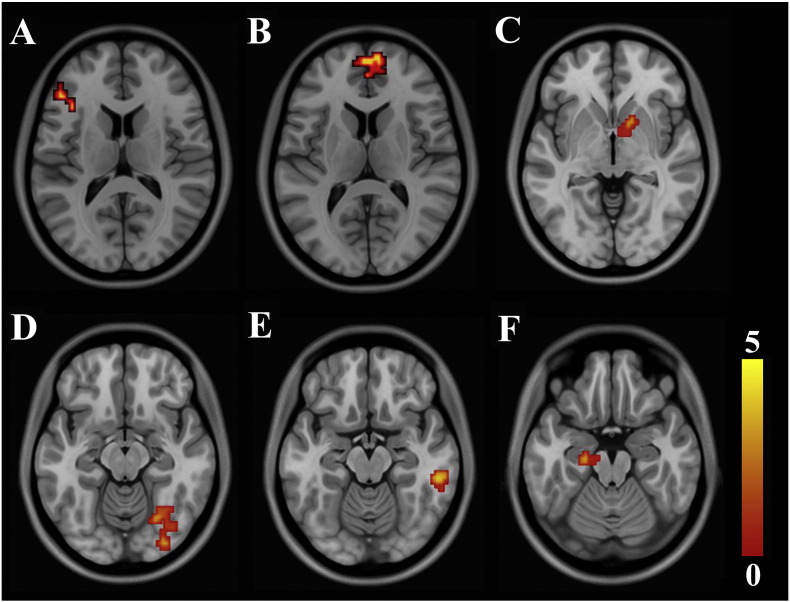
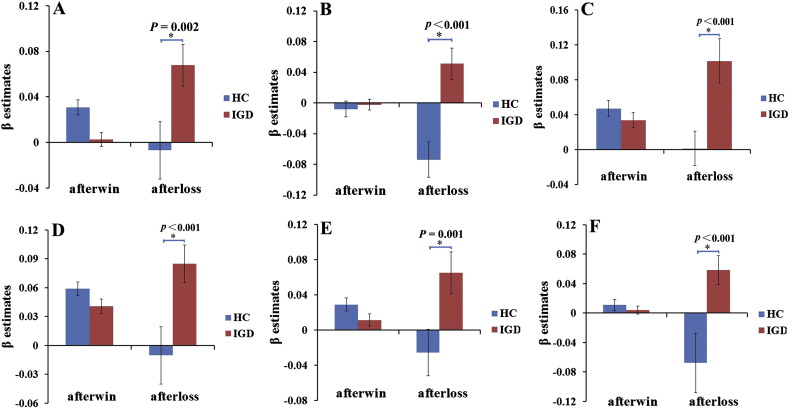
A repeated ANOVA revealed a significant interaction effect of group (IGD and HCs) by post-outcome (“after win” and “after loss”) on the covariance between risk level and activation of the bilateral ventral medial prefrontal cortex (VMPFC), left IFC, right VS, left hippocampus/parahippocampus, right inferior occipital gyrus/fusiform and right inferior temporal gyrus (P < 0.05, AlphaSim correction) (Table 3) (Fig. 1). ROI-based intergroup comparisons revealed that the reason for the interaction effects was that the covariance between risk level and brain activation in these regions had no significant intergroup differences after wins, whereas higher covariance was found in the adolescents with IGD after losses (Fig. 2). The main effects of group and outcome were insignificant after correction for multiple comparisons (P > 0.05, AlphaSim correction).
Table 3.
Brain regions exhibited a significant interaction effect of group by outcome on the risk-related activation.
| Brain regions | Cluster size (voxels) | Peak T value | MNI coordinates of cluster centroid (mm) |
||
|---|---|---|---|---|---|
| x | y | z | |||
| VMPFC | 99 | 3.983 | 3 | 57 | 15 |
| IFC (L) | 25 | 3.489 | − 48 | 33 | 12 |
| VS (R) | 22 | 3.165 | 15 | 12 | − 3 |
| Hippocampus/parahippocampus (L) | 70 | 3.562 | − 15 | − 21 | − 15 |
| Inferior occipital gyrus/fusiform (R) | 50 | 3.378 | 30 | − 87 | − 12 |
| Inferior temporal gyrus (R) | 28 | 4.078 | 54 | − 33 | − 15 |
MNI: Montreal Neurological Institute; R: right side; L: left side. IFC, inferior frontal cortex; VMPFC, ventral medial prefrontal cortex; VS, ventral striatum.
Fig. 1.
Brain regions with a significant interaction effect of group by post-outcome on the covariance between the risk level and the brain activation. A, left IFC; B, VMPFC; C, right ventral striatum; D, right inferior occipital cortex/fusiform; E, right inferior temporal gyrus; F, left hippocampus/parahippocampus. T value ranges from 0 to 5 presented by color bar.
Fig. 2.
ROI-based post hoc analysis of the brain regions with a significant interaction effect of group by post-outcome on the covariance between the risk level and the brain activation. A, left IFC; B, VMPFC; C, right ventral striatum; D, right inferior occipital gyrus/fusiform; E, right inferior temporal gyrus; F, left hippocampus/parahippocampus. The covariance between the risk level and the brain activation of these regions after loss significantly increased in IGD group compared with the HCs group.
There was no significant correlation between mean β estimates within ROIs and behavioral performance or IAT scores in adolescents with IGD (P > 0.05).
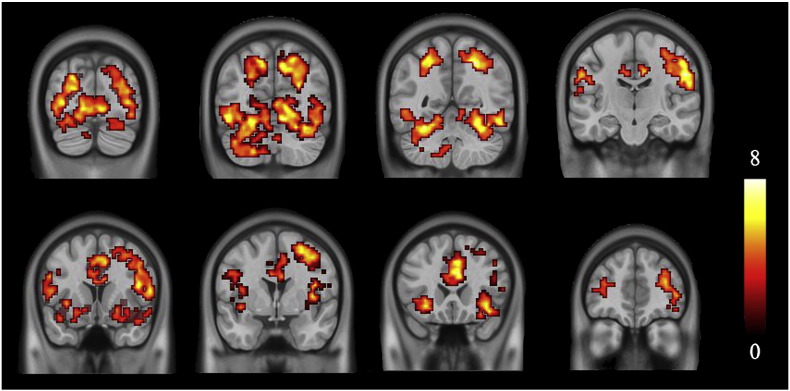
Brain activations that co-varied with the risk levels in HCs are illustrated in Fig. 3. A threshold of whole brain false discovery rate (FDR) corrected P < 0.005 and cluster size larger than 50 voxels was used to identify activation areas.
Fig. 3.
Brain activations covaried with the level of risk in HCs. T value ranges from 0 to 8 presented by color bar.
4. Discussion
In the present study, the effects of outcome on the covariance between the brain activation and risk level during subsequent risky decision-making in adolescents with IGD were evaluated using BART-fMRI. Our findings in the HCs demonstrated that the BART was a reliable method for evaluating changes in brain activation during risky decision-making. There was a significant interaction effect of group by post-outcome (“after win” and “after loss”) on the subsequent covariance between risk level and activation of the bilateral VMPFC, left IFC, right VS, left hippocampus/parahippocampus, right inferior occipital gyrus/fusiform and right inferior temporal gyrus. The cause of this interaction may have been that there was no significant intergroup difference in the covariance between brain activation and risk level after wins, while adolescents with IGD showed significantly higher covariance between brain activation and risk level after losses compared with HCs.
It has been demonstrated that various behaviors that repeatedly reinforce the reward circuitry are part of the disease of addiction (Grant et al., 2010, Karim and Chaudhri, 2012, Love et al., 2015). When a negative outcome occurs, the resulting negative emotional state leads to activation and dysregulation of brain reward systems, which leads to further negative reinforcement as the individual continues to engage in the addictive behavior to avoid the negative affect (Koob and Volkow, 2010, Volkow et al., 2011). Therefore, a negative emotional state is an important component in the process of behavioral addiction. This may be the reason why the covariance between risk level and brain activation in adolescents was affected more dramatically after losses in our study.
Previous studies have demonstrated that the IFC and VMPFC are involved in risk-evaluation and value calculation (Cardinal, 2006, Christopoulos et al., 2009, Rushworth et al., 2011, Schonberg et al., 2012, Tobler et al., 2007). The VMPFC is responsible for processing negative performance outcomes and receiving punishment feedback (Elliott et al., 2000, O'Doherty et al., 2003, O'Doherty et al., 2001, Rushworth et al., 2011, van Leijenhorst et al., 2006), and activation of the VMPFC decreased in proportion to the value of an anticipated loss (Tom et al., 2007). A previous BART study found robust activation of the VMPFC when participants chose to continue inflating the balloon (risky option) (Fukunaga et al., 2012). The IFC is responsible for the modulation between reward and risky level (Christopoulos et al., 2009, Lin et al., 2015). A previous study by Christopoulos et al. (2009) found a positive correlation between the activation of the IFC and the risk aversion. A study by Dong et al. (2013) found that subjects with IGD showed increased activation of the inferior frontal gyrus after continuous wins or losses. In present study, the covariance between risk level and activation of the left IFC and bilateral VMPFC increased after negative feedback during decision-making, which may suggest that the higher the risk level was, the higher brain activation in adolescent with IGD. In our study, the average RT was shorter in adolescents with IGD, and the number of total pumps was significantly higher in adolescents with IGD. The number of mean pumps after a loss in adolescents with IGD was higher than in HCs. Additionally, the maximum number of possible balloon pumps in our BART experiment was 12; only approximately 30 balloon trials were performed during the BOLD scanning, which may have decreased the sensitivity of detecting intergroup differences in behavioral performance (Rao et al., 2010). It is cannot be excluded that the intergroup difference in behavioral performance would become more significant with an increase in the number of possible balloon pumps. Combined, these suggest that the adolescents with IGD were more impulsive than HCs, which was consistent with the observed higher scores on the BIS in adolescents with IGD. Our results indicated that adolescents with IGD and high impulsivity might need to recruit more PFC resources to evaluate the risk values of the subsequent options after receiving negative feedback.
The VS is a key structure of the brain's reward system, and has been implicated in anticipating and processing different types of rewards, as well as producing learning signals known as prediction errors (Dong et al., 2013, Haber and Knutson, 2010, McClure et al., 2003, Seymour et al., 2007, van Duijvenvoorde et al., 2014). Neuroimaging studies have revealed increased reward-related activation of the VS in individuals with substance dependence and behavioral addiction. For instance, fMRI studies have found hyperactivity in the VS when processing reward stimuli (Garcia-Garcia et al., 2014) and risky decision-making (Yamamoto et al., 2015) in substance-dependent individuals. In addition, activity of the VS increased in pathological gambling patients during loss anticipation in a monetary incentive delay task (Romanczuk-Seiferth et al., 2015). In our study, the covariance between risk level and activation of the VS increased after receiving negative feedback, which indicated that adolescents with IGD might experience higher anticipation of rewards during subsequent risky decision-making after a loss. Moreover, previous studies revealed strong interconnections between the VS and several parts of the medial PFC, namely the fronto-striatal circuits, which are associated to the addictive behavior, and the PFC exerts top-down control of the VS that has been found to be involved in reward-processing (Ashby et al., 2010, Haber and Knutson, 2010). Therefore, it is also plausible that the increased covariance between risk level and activation of the PFC after receiving negative feedback might be due to the need to control VS activation.
The hippocampus is a key structure in the memory system and has been implicated in encoding recent experiences (Ferbinteanu and Shapiro, 2003). The parahippocampus provides a contextual representation function and is an important afferent pathway to the hippocampus (Rudy, 2009). Human decisions cannot be explained solely by rational imperatives but are strongly influenced by emotion (Coricelli et al., 2007). Previous studies have found that hippocampal activation was affected by emotionality and regret (Addis et al., 2004, Coricelli et al., 2007). The hippocampus/parahippocampus involvement in the process of learning from experiences (Eichenbaum, 2004, Steidl et al., 2006) might play a role in the recollection of a previous decision-making experiences, especially the emotional experiences (Ferbinteanu and Shapiro, 2003, Kohno et al., 2015). Thus, after a negative feedback, the lesson to be learnt is: ‘in the future, pay more attention to the potential consequences of your choices’ (Coricelli et al., 2007). In our study, the increased covariance between risk level and activation of the hippocampus/parahippocampus in the adolescents with IGD after negative feedback might be associated with learning following emotional experiences during risky decision-making.
In this study, adolescents with IGD also showed increased covariance between risk level and activation of the right inferior occipital gyrus/fusiform and the right inferior temporal gyrus after receiving negative feedback during risky decision-making. The occipitotemporal area is related to visual perceptual learning, spatial attention and perceptual judgments (LaBar et al., 1999, Li et al., 2012), and the inferior temporal gyrus/fusiform are a component of the fronto-temporal circuits, which are associated with working memory and attention processing. The increased covariance between risk level and activation of the occipitotemporal areas in adolescents with IGD after receiving negative feedback might indicate that these adolescents made more attempts to remember previous feedback and focus on subsequent risky decision-making simultaneously; however, which should be confirmed in future studies.
There was no significant correlation between the average β estimates within ROIs and behavioral performance or IAT scores in adolescents with IGD. One possible explanation is that altered covariance between risk level and brain activation is a trait index rather than state index, which means that the changed index has no quantitative relationship with the extent of disease. It's also worth noting that the adolescent brain is still developing compared to the adult brain, so risk decision-making strategies and the responses to feedback may differ between the two (Bjork et al., 2004, Smith et al., 2015, van Leijenhorst et al., 2006). Thus, the developmental heterogeneity of adolescents might be a factor that affects decision-making-related brain activity, and a longitudinal study would be warranted.
Several limitations need be noted in the present study. First, a relative small sample size may have reduced the statistical power. Second, our results are limited to male adolescents because of the substantially higher IGD prevalence in young males; conditions in female adolescents and other age groups need to be evaluated in future studies. Third, the length and amount of experience across video game genres were not controlled. Finally, although the widely used YDQ and YIAT were adopted as diagnostic criteria, other standards have been adopted by other researchers (Dong et al., 2012a, Ko et al., 2014, Yao et al., 2014), which may limit the comparability among the studies to some extent. With the IGD now listed in the DSM-V as a disorder requiring further research (Association AP, 2013), this problem may be corrected in the near future.
5. Conclusions
To the best of our knowledge, this is the first study to investigate the effect of previous outcomes on the covariance between brain activation and risk level during risky decision-making processing adolescents with IGD using the BART-fMRI. Our results indicated that negative outcomes affected the covariance between risk level and activation of the brain regions related to value estimation (PFC), anticipation of rewards (VS), and emotion-related learning (hippocampus/parahippocampus), which may be one of the underlying neural mechanisms of disadvantageous risky decision-making in adolescents with IGD.
Author contributions
X.Q., Y.Y., X.L. and Q.Z. designed research; X.Q., S.D., P.G., Y.Z., X.D. and Q.Z. performed research; Y.Y., S.D., P.G. was involved in the clinical assessment; X.Q., Y.Z., G.D., and Q.Z. analyzed data; X.Q., Y.Z., X.L., Y.Y. and Q.Z. wrote the paper.
Disclosure
All authors have reported no conflicts of interest.
Contributor Information
Xiaodong Li, Email: lxd8199819@126.com.
Quan Zhang, Email: zhangquan0912@163.com.
References
- Addis D.R., Moscovitch M., Crawley A.P., McAndrews M.P. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Ashby F.G., Turner B.O., Horvitz J.C. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn. Sci. 2010;14:208–215. doi: 10.1016/j.tics.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association AP . fifth ed. American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- Balasubramani P.P., Chakravarthy V.S., Ravindran B., Moustafa A.A. A network model of basal ganglia for understanding the roles of dopamine and serotonin in reward-punishment-risk based decision making. Front. Comput. Neurosci. 2015;9:76. doi: 10.3389/fncom.2015.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balconi M., Finocchiaro R., Campanella S. Reward sensitivity, decisional bias, and metacognitive deficits in cocaine drug addiction. J. Addict. Med. 2014;8:399–406. doi: 10.1097/ADM.0000000000000065. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J. Neurosci. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. Does the feedback from previous trials influence current decisions? A study on the role of feedback processing in making decisions under explicit risk conditions. J. Neuropsychol. 2008;2:431–443. doi: 10.1348/174866407x220607. [DOI] [PubMed] [Google Scholar]
- Cardinal R.N. Neural systems implicated in delayed and probabilistic reinforcement. Neural Netw. 2006;19:1277–1301. doi: 10.1016/j.neunet.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Christopoulos G.I., Tobler P.N., Bossaerts P., Dolan R.J., Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. J. Neurosci. 2009;29:12574–12583. doi: 10.1523/JNEUROSCI.2614-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coricelli G., Dolan R.J., Sirigu A. Brain, emotion and decision making: the paradigmatic example of regret. Trends Cogn. Sci. 2007;11:258–265. doi: 10.1016/j.tics.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Dong G., DeVito E., Huang J., Du X. Diffusion tensor imaging reveals thalamus and posterior cingulate cortex abnormalities in internet gaming addicts. J. Psychiatr. Res. 2012;46:1212–1216. doi: 10.1016/j.jpsychires.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Devito E.E., Du X., Cui Z. Impaired inhibitory control in ‘internet addiction disorder’: a functional magnetic resonance imaging study. Psychiatry Res. 2012;203:153–158. doi: 10.1016/j.pscychresns.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Hu Y., Lin X., Lu Q. What makes Internet addicts continue playing online even when faced by severe negative consequences? Possible explanations from an fMRI study. Biol. Psychol. 2013;94:282–289. doi: 10.1016/j.biopsycho.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Elliott R., Friston K.J., Dolan R.J. Dissociable neural responses in human reward systems. J. Neurosci. 2000;20:6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B., Steinhauser M. Effects of invalid feedback on learning and feedback-related brain activity in decision-making. Brain Cogn. 2015;99:78–86. doi: 10.1016/j.bandc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Ersche K.D., Roiser J.P., Clark L., London M., Robbins T.W., Sahakian B.J. Punishment induces risky decision-making in methadone-maintained opiate users but not in heroin users or healthy volunteers. Neuropsychopharmacology. 2005;30:2115–2124. doi: 10.1038/sj.npp.1300812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J., Shapiro M.L. Prospective and retrospective memory coding in the hippocampus. Neuron. 2003;40:1227–1239. doi: 10.1016/s0896-6273(03)00752-9. [DOI] [PubMed] [Google Scholar]
- Fukunaga R., Brown J.W., Bogg T. Decision making in the Balloon Analogue Risk Task (BART): anterior cingulate cortex signals loss aversion but not the infrequency of risky choices. Cogn. Affect. Behav. Neurosci. 2012;12:479–490. doi: 10.3758/s13415-012-0102-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia I., Horstmann A., Jurado M.A., Garolera M., Chaudhry S.J., Margulies D.S., Villringer A., Neumann J. Reward processing in obesity, substance addiction and non-substance addiction. Obes. Rev. 2014;15:853–869. doi: 10.1111/obr.12221. [DOI] [PubMed] [Google Scholar]
- Grant J.E., Potenza M.N., Weinstein A., Gorelick D.A. Introduction to behavioral addictions. Am. J. Drug Alcohol Abuse. 2010;36:233–241. doi: 10.3109/00952990.2010.491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie R. Problems of judgment and decision making. Annu. Rev. Psychol. 2001;52:653–683. doi: 10.1146/annurev.psych.52.1.653. [DOI] [PubMed] [Google Scholar]
- Hauser T.U., Hunt L.T., Iannaccone R., Walitza S., Brandeis D., Brem S., Dolan R.J. Temporally dissociable contributions of human medial prefrontal Subregions to reward-guided learning. J. Neurosci. 2015;35:11209–11220. doi: 10.1523/JNEUROSCI.0560-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim R., Chaudhri P. Behavioral addictions: an overview. J. Psychoactive Drugs. 2012;44:5–17. doi: 10.1080/02791072.2012.662859. [DOI] [PubMed] [Google Scholar]
- Ko C.H., Hsieh T.J., Chen C.Y., Yen C.F., Chen C.S., Yen J.Y., Wang P.W., Liu G.C. Altered brain activation during response inhibition and error processing in subjects with Internet gaming disorder: a functional magnetic imaging study. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264:661–672. doi: 10.1007/s00406-013-0483-3. [DOI] [PubMed] [Google Scholar]
- Kohno M., Ghahremani D.G., Morales A.M., Robertson C.L., Ishibashi K., Morgan A.T., Mandelkern M.A., London E.D. Risk-taking behavior: dopamine d2/d3 receptors, feedback, and frontolimbic activity. Cereb. Cortex. 2015;25:236–245. doi: 10.1093/cercor/bht218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K.S., Gitelman D.R., Parrish T.B., Mesulam M. Neuroanatomic overlap of working memory and spatial attention networks: a functional MRI comparison within subjects. NeuroImage. 1999;10:695–704. doi: 10.1006/nimg.1999.0503. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Read J.P., Kahler C.W., Richards J.B., Ramsey S.E., Stuart G.L., Strong D.R., Brown R.A. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) J. Exp. Psychol. Appl. 2002;8:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Li S., Mayhew S.D., Kourtzi Z. Learning shapes spatiotemporal brain patterns for flexible categorical decisions. Cereb. Cortex. 2012;22:2322–2335. doi: 10.1093/cercor/bhr309. [DOI] [PubMed] [Google Scholar]
- Lin X., Zhou H., Dong G., Du X. Impaired risk evaluation in people with Internet gaming disorder: fMRI evidence from a probability discounting task. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2015;56:142–148. doi: 10.1016/j.pnpbp.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Liu X., Hairston J., Schrier M., Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011;35:1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love T., Laier C., Brand M., Hatch L., Hajela R. Neuroscience of internet pornography addiction: a review and update. Behav. Sci. 2015;5:388–433. doi: 10.3390/bs5030388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure S.M., Berns G.S., Montague P.R. Temporal prediction errors in a passive learning task activate human striatum. Neuron. 2003;38:339–346. doi: 10.1016/s0896-6273(03)00154-5. [DOI] [PubMed] [Google Scholar]
- O'Doherty J., Kringelbach M.L., Rolls E.T., Hornak J., Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat. Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O'Doherty J., Critchley H., Deichmann R., Dolan R.J. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J. Neurosci. 2003;23:7931–7939. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlikowski M., Brand M. Excessive Internet gaming and decision making: do excessive World of Warcraft players have problems in decision making under risky conditions? Psychiatry Res. 2011;188:428–433. doi: 10.1016/j.psychres.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Qi X., Du X., Yang Y., Du G., Gao P., Zhang Y., Qin W., Li X., Zhang Q. Decreased modulation by the risk level on the brain activation during decision making in adolescents with internet gaming disorder. Front. Behav. Neurosci. 2015;9:296. doi: 10.3389/fnbeh.2015.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H., Korczykowski M., Pluta J., Hoang A., Detre J.A. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI study of the Balloon Analog Risk Task (BART) NeuroImage. 2008;42:902–910. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao H., Mamikonyan E., Detre J.A., Siderowf A.D., Stern M.B., Potenza M.N., Weintraub D. Decreased ventral striatal activity with impulse control disorders in Parkinson's disease. Mov. Disord. 2010;25:1660–1669. doi: 10.1002/mds.23147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao L.L., Zhou Y., Liang Z.Y., Rao H., Zheng R., Sun Y., Tan C., Xiao Y., Tian Z.Q., Chen X.P., Wang C.H., Bai Y.Q., Chen S.G., Li S. Decreasing ventromedial prefrontal cortex deactivation in risky decision making after simulated microgravity: effects of − 6 degrees head-down tilt bed rest. Front. Behav. Neurosci. 2014;8:187. doi: 10.3389/fnbeh.2014.00187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanczuk-Seiferth N., Koehler S., Dreesen C., Wüstenberg T., Heinz A. Pathological gambling and alcohol dependence: neural disturbances in reward and loss avoidance processing. Addict. Biol. 2015;20:557–569. doi: 10.1111/adb.12144. [DOI] [PubMed] [Google Scholar]
- Rudy J.W. Context representations, context functions, and the parahippocampal-hippocampal system. Learn. Mem. 2009;16:573–585. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F., Noonan M.P., Boorman E.D., Walton M.E., Behrens T.E. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Schonberg T., Fox C.R., Mumford J.A., Congdon E., Trepel C., Poldrack R.A. Decreasing ventromedial prefrontal cortex activity during sequential risk-taking: an fMRI investigation of the balloon analog risk task. Front. Neurosci. 2012;6:80. doi: 10.3389/fnins.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour B., Daw N., Dayan P., Singer T., Dolan R. Differential encoding of losses and gains in the human striatum. J. Neurosci. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.R., Steinberg L., Strang N., Chein J. Age differences in the impact of peers on adolescents' and adults' neural response to reward. Dev. Cogn. Neurosci. 2015;11:75–82. doi: 10.1016/j.dcn.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl S., Mohi-uddin S., Anderson A.K. Effects of emotional arousal on multiple memory systems: evidence from declarative and procedural learning. Learn. Mem. 2006;13:650–658. doi: 10.1101/lm.324406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J., Reynolds J., Krmpotich T., Claus E., Thompson L.L., Du Y.P., Banich M.T. Reduced neural tracking of prediction error in substance-dependent individuals. Am. J. Psychiatry. 2013;170:1356–1363. doi: 10.1176/appi.ajp.2013.12091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Yu Y., Du Y., Ma Y., Zhang D., Wang J. Prevalence of internet addiction and its association with stressful life events and psychological symptoms among adolescent internet users. Addict. Behav. 2014;39:744–747. doi: 10.1016/j.addbeh.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Tobler P.N., O'Doherty J.P., Dolan R.J., Schultz W. Reward value coding distinct from risk attitude-related uncertainty coding in human reward systems. J. Neurophysiol. 2007;97:1621–1632. doi: 10.1152/jn.00745.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tom S.M., Fox C.R., Trepel C., Poldrack R.A. The neural basis of loss aversion in decision-making under risk. Science. 2007;315:515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- van Duijvenvoorde A.C., Op de Macks Z.A., Overgaauw S., Gunther Moor B., Dahl R.E., Crone E.A. A cross-sectional and longitudinal analysis of reward-related brain activation: effects of age, pubertal stage, and reward sensitivity. Brain Cogn. 2014;89:3–14. doi: 10.1016/j.bandc.2013.10.005. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L., Crone E.A., Bunge S.A. Neural correlates of developmental differences in risk estimation and feedback processing. Neuropsychologia. 2006;44:2158–2170. doi: 10.1016/j.neuropsychologia.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.J., Fowler J.S., Tomasi D., Telang F. Addiction: beyond dopamine reward circuitry. Proc. Natl. Acad. Sci. U. S. A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbe Y., Irvine M., Lange I., Kundu P., Howell N.A., Harrison N.A., Bullmore E.T., Robbins T.W., Voon V. Neuronal correlates of risk-seeking attitudes to anticipated losses in binge drinkers. Biol. Psychiatry. 2014;76:717–724. doi: 10.1016/j.biopsych.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Chen X., Han J., Meng H., Luo J., Nydegger L., Wu H. Prevalence and factors of addictive Internet use among adolescents in Wuhan, China: interactions of parental relationship with age and hyperactivity-impulsivity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto D.J., Woo C.W., Wager T.D., Regner M.F., Tanabe J. Influence of dorsolateral prefrontal cortex and ventral striatum on risk avoidance in addiction: a mediation analysis. Drug Alcohol Depend. 2015;149:10–17. doi: 10.1016/j.drugalcdep.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y.W., Chen P.R., Chen C., Wang L.J., Zhang J.T., Xue G., Deng L.Y., Liu Q.X., Yip S.W., Fang X.Y. Failure to utilize feedback causes decision-making deficits among excessive Internet gamers. Psychiatry Res. 2014;219:583–588. doi: 10.1016/j.psychres.2014.06.033. [DOI] [PubMed] [Google Scholar]
- Yao Y.W., Chen P.R., Li S., Wang L.J., Zhang J.T., Yip S.W., Chen G., Deng L.Y., Liu Q.X., Fang X.Y. Decision-making for risky gains and losses among college students with internet gaming disorder. PLoS One. 2015;10 doi: 10.1371/journal.pone.0116471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young K. Internet addiction: the emergence of a new clinical disorder. CyberPsychology & Behavior. 1998;1:237–244. [Google Scholar]
- Young K. Internet addiction: symptoms, evaluation, and treatment. Innovations in Clinical Practice: A Source Book. 1999;17:19–31. [Google Scholar]