Abstract
Traditional recruitment methods for microbicide efficacy trials are labor intensive and may fail to reach high-risk hard-to-reach populations. We report duration of recruitment and lessons learned from a two-stage process to recruit female sex workers (FSWs) into a placebo microbicide trial, and examined characteristics associated with successful recruitment of peers who screened for and enrolled in the trial. FSWs were first recruited via respondent-driven sampling (RDS) to complete a survey and subsequently invited to screen for enrollment into a placebo microbicide trial taking place at a local clinic. It took 6 months to enroll 267 participants into the trial. Successful recruiters of peers who enrolled were more likely to have enrolled themselves (AOR 2.0, CI 1.3–2.9) and less likely to visit Nellore city (AOR 0.5, CI 0.3–0.9). Recruitment of FSWs via a two-stage recruitment strategy with RDS can be a good option for future clinical trials.
Keywords: Microbicides, HIV prevention trial, Respondent-driven sampling, Research subject recruitment, Female sex workers
Introduction
HIV prevention randomized clinical trials (RCTs) must be conducted among populations having high HIV incidence to be adequately powered. India has the third largest number of HIV-infected people in the world; within India, the state of Andhra Pradesh has the second highest HIV prevalence rate—0.75 % compared with the national average of 0.27 % [1]. An estimated 1.26 million female sex workers (FSWs) live and work in the state and researchers believe this an adequate population of “at risk” women for HIV prevention trials [2, 3].
For RCTs, if the target sample size is not reached, statistical power to test outcome variables is compromised [4]. RCTs typically utilize multiple recruitment methods including holding community meetings, posting fliers, broadcasting radio and television advertisements, engaging community outreach workers and/or recruiting directly from clinics. Notwithstanding these efforts, RCTs and microbicide trials in particular have been challenged by recruitment problems [4–6]; given limited resources, additional costs related to recruitment delays could result in fewer novel interventions being evaluated. Furthermore, these methods, while appropriate for RCTs that seek to recruit participants from the general population, are not ideal for trials seeking to enroll hidden populations such as FSWs who are marginalized and who engage in behaviors that are stigmatized and/or illegal [2]. Additionally, FSWs in India experience high levels of police harassment by way of threats, arrests and fines [7, 8], presenting additional barriers to recruitment.
Respondent-driven sampling (RDS) is a modified peer-referral sampling that is now commonly used to recruit hidden and hard-to-reach populations for studies [9–16]. The method relies on peer referral to recruit participants. To initiate the recruitment process, the research staff recruits a small group of initial subjects who are referred to as “seeds.” Seeds are given recruitment coupons that have unique numbers, which they use when they recruit eligible peers. Each referred peer is then given additional numbered coupons to distribute to peers who in turn refer other eligible peers. Participants are compensated both for study participation and for recruiting eligible peers. The number of peers that participants can recruit is limited, peers are not identified by name (thus maintaining confidentiality), information on social network sizes is collected, and the process is documented through the numbered coupon system linking the recruiter and the recruit. Recruitment using RDS can yield a large and diverse sample size including otherwise inaccessible target population members [14, 17–19]. Whereas many observational and surveillance studies have used RDS to recruit hard-to-reach participants for HIV prevention interventions and studies [13–16, 20, 21], there is no literature suggesting that RDS has been used to recruit participants for a microbicide clinical trial.
We evaluated the recruitment of FSWs for a placebo microbicide gel clinical trial in Andhra Pradesh (AP), India, employing a two-stage strategy: first using RDS to recruit participants for a survey, and subsequently inviting survey participants to screen for a four-month placebo microbicide gel clinical trial. Because participation in a clinical trial involves a time commitment and undergoing invasive procedures, we felt that recruiting potential trial participants first to a noninvasive study visit not requiring any clinical procedures would help earn participant trust prior to participation in a clinical trial. The objectives of this paper are to: (a) assess the time it would take to implement a two-stage recruitment strategy to recruit microbicide trial participants and document lessons learned, and (b) determine the characteristics of recruiters whose recruits were more likely to be screened for and enrolled in the trial.
Methods
Study Population
FSWs from the Nellore (AP), India area were recruited via RDS to complete a survey that collected data on demographics, HIV-related risk factors, and hypothetical willingness to participate in an HIV prevention trial. Afterwards, participants were informed about a four-month placebo microbicide gel trial and invited to screen for the trial at the YRG-CARE Community Health Clinic located near the Nellore city center. Details of the trial have previously been described [22].
Women aged 18–45 years were eligible to complete the survey if they lived or worked as an FSW in the Nellore area; reported vaginal, oral or anal sex in exchange for money, goods or help at least once in the past month; gave informed consent; and arrived at the survey site with a valid recruitment coupon. FSWs were excluded if they appeared to be mentally impaired or under the influence of drugs/alcohol, reported or appeared to have been coerced to participate, or falsely identified as an FSW.
Recruitment Process
Formative research of FSW catchment areas was conducted by both YRG-CARE outreach staff and the Population Council team. Accordingly, survey sites were located in areas known to be frequented by and easily accessible to FSWs: Buchi, Rajupalem and Nellore city center (located approximately 19.5, 15.1 and 2.1 km, respectively, from the trial clinic). To maximize access and comfort for FSWs and minimize potential negative community attention, sites were neutral venues (e.g., not located in health clinics or identifiable as being related to FSW or HIV/AIDS). Although one site was attached to an NGO that provided services for drug users, the other two sites were not linked to any NGO or clinic.
Outreach workers identified potential seed participants who had large FSW networks, were highly regarded by FSW peers, met eligibility criteria, and were willing to recruit their FSW peers. Recruitment was initiated by three seeds diversified on characteristics including age, place of residence, and type of sex worker (e.g., home-, street-, or brothel-based). Seeds were given two recruitment coupons to recruit FSW peers, and the process was repeated with subsequent recruits until the sample size for the clinical trial was achieved. The number of peers that participants could recruit was limited at first to two to keep the volume of potential participants arriving at the community health clinic for the clinical trial at a manageable level. Upon arrival at the survey site, informed consent was obtained and eligibility was determined. To ensure that potential participants were truly engaged in sex work, the interviewer asked a few questions from a list of screening questions, such as: how much they charge for different sex acts, how they find clients, and the names of specific places where they find clients. An Excel spreadsheet was used by a designated coupon manager to track compensation and recruitment (i.e., to link recruiters and recruits). To make sure that participants did not register more than once for the study, their name and mother's name, number of siblings and first letter of their birth village were collected and stored in a secure database. Non-sensitive questions were administered by trained interviewers; sexual and drug use behavioral questions were administered via audio computer-assisted self-interview (ACASI).
After completion of the survey, participants were issued uniquely numbered recruitment coupons; the coupons and recruitment procedures were explained to participants by trained staff. Both text and graphics were utilized to show survey participants that they were to keep one-half of the recruitment coupon and give the other half to their peer(s); the locations of the three survey sites were also indicated on the coupons. All participants received a reimbursement of Rs 100 (approximately US$2.00) for completing the survey and Rs 50 (approximately US$1.00) for each peer successfully recruited into the survey. After successfully recruiting peers, survey participants were asked to return to the survey site for reimbursement and to complete a brief questionnaire about the peers who accepted or refused the recruitment coupons. These data were not analyzed, however, because only 166 participants completed the questionnaire.
FSWs were also given a brochure explaining the purpose of the placebo microbicide gel trial and that the gel did not prevent HIV or other STIs. Staff reviewed the information in the brochure with FSWs and referred them to the trial clinic to learn more about the trial and to be screened. No other advertising about the trial or recruiting was conducted in the community, and recruiters were not aware of the eligibility criteria for the clinical trial. They did, however, know from the brochure that screening for the trial would involve a physical exam and STI/HIV testing. Because the study was also designed to identify factors associated with willingness to participate in the trial [23], outreach staff were instructed not to contact participants between the survey visit and screening visit.
Upon arrival at the trial clinic, FSWs were given detailed information about the trial and informed consent was obtained before screening. Trial eligibility criteria mirrored those required for a clinical trial of an active microbicide product. Eligibility for the trial was assessed via interviews; medical history; and physical and gynecological exams, including a Pap smear, pregnancy test, and STI and HIV tests. Women returned to the clinic two weeks later to receive test results and to enroll in the trial, if eligible. When clinically indicated, participants were treated for STIs, and were either rescreened after completing treatment, or were simultaneously treated and enrolled [22].
To enroll a target of 250–300 participants into the trial, an initial target sample size of 500 FSWs was set for the survey, assuming that 50–60 % would enroll. To accelerate enrollment, four additional seeds were identified (one added after 1 month, two added after 2 months, and one added after 3 months from the start of data collection) and the number of coupons distributed to each participant was increased to three (4 months after the start of data collection). Coupon distribution ceased during the last month of data collection because we were nearing the target sample size. The survey sites remained open 2 weeks beyond the completion of the last survey in order for participants to receive reimbursement for recruiting peers.
Variables
Analyses were based on survey data, and trial screening and enrollment rates. The screening rate was the proportion of survey participants who completed the screening visit for the trial. The enrollment rate was the proportion of survey participants who met the eligibility criteria and elected to enroll in the trial. Dependent variables were successful recruitment of: (1) at least one FSW peer who completed the trial screening visit, and (2) at least one FSW peer who enrolled in the trial.
Sex work was defined as engaging in vaginal, oral or anal sex in exchange for money, goods or other help, at least once in the past week. FSWs were asked where they typically found paying partners: in brothels (“brothel-based”), in public places such as railway/bus stations, parks, or cinemas (“street-based”), or based in their homes, either through word of mouth or by mobile phone (“home-based”). Condom use was determined by FSWs’ response to how often they generally used condoms with paying partners. For socioeconomic status (SES), a composite asset score was generated based on access to 14 household goods or services, such as toilets and electricity, and categorized into tertiles. An “HIV Risk Index” was created using five self-reported survey items: inconsistent condom use with a paying partner, anal intercourse with a paying partner in the last month, forced sex in the last year, any drug use in the last year, and experiencing at least one STI symptom in the last month. The index was categorized into a dichotomous variable: “lower risk” (two or fewer activities) and “higher risk” (three or more activities).
Statistical Analysis
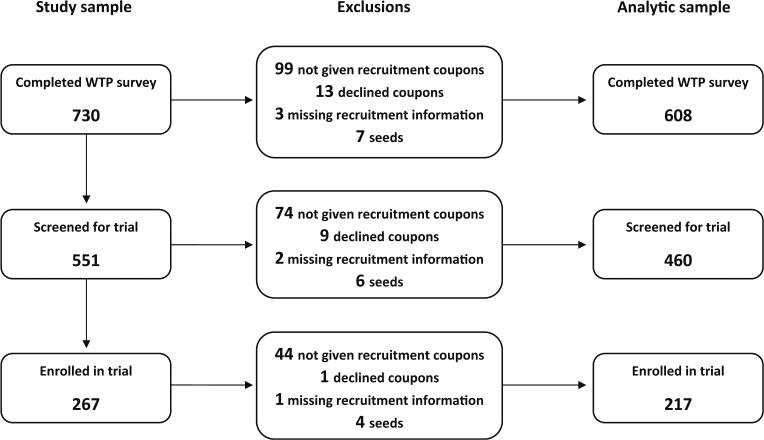
A total of 734 FSWs were recruited to the survey site: four were ineligible and all eligible FSWs agreed to participate. Of the 730 FSWs who completed the survey, our analysis was restricted to 608 FSWs. The analysis excluded 13 participants who declined recruitment coupons, 3 FSWs having missing recruitment information, 99 FSWs who were not given coupons because survey data collection was nearing completion, and 7 seed participants. Descriptive statistics and regression analyses do not include seed participants (N = 7) and were analyzed in Stata (Version 11).
Demographic and behavioral data and data regarding willingness to participate were analyzed in relation to two different outcome variables: successful recruitment of peers who (1) screened for, and (2) enrolled into the trial. Variables associated with successful recruitment in bivariate analysis as well as other variables determined a priori based on a review of the literature were included in the models [7, 24, 25].
This protocol was approved by the Institutional Review Boards of the Population Council, New York; YRG CARE, Chennai; and the Indian Council of Medical Research, New Delhi.
Results
It took approximately 6 months to recruit 730 respondents for the survey. A total of 1,484 coupons were distributed to seeds and peers, yielding a coupon return rate of 49.2 %. The maximum recruitment wave lengths of the seven seeds were 1, 3, 7, 9, 17, 19, and 24. Wave 1 refers to the seeds’ recruits, wave 2 refers to the recruits’ recruits, and so forth. It took 6 months to enroll 267 participants into the trial. Of the 608 survey participants included in our analysis, 460 (75.7 %) completed a screening visit for the trial. Of those screened, 299 (65.0 %) participants were eligible for enrollment into the trial; 217 (47.2 %) ultimately enrolled (Fig. 1). The median interval between the index FSW's survey visit and her recruit's survey visit was 5 days (interquartile range [IQR]: 1–16). The median interval between the survey visit and the screening visit was 1 day (IQR: 0–2) and between the screening visit and enrollment visit was 14 days (IQR: 14–17).
Fig. 1.
Constituents of study sample and analytic sample
Background Characteristics
Table 1 shows the socio-demographic characteristics of the analytic sample, as well as screening, enrollment, and recruitment rates. The median age was 30 years (IQR: 25–36). More than two-thirds were Hindu (68.6 %) and currently married (68.3 %). One-third (32.3 %) lived at least 40 km away from the trial clinic, primarily in Gudur, which is easily accessible to and from the city of Nellore by bus and train and a “hot spot” for FSWs. The mean network size was 3.8 peers.
Table 1.
Characteristics of female sex workers who were eligible to recruit other female sex workers
| Characteristics | Sample proportions % (n) |
|---|---|
| Age | |
| 18–24 | 18.8 (114) |
| 25–34 | 42.4 (258) |
| 35–45 | 38.8 (236) |
| Marital status | |
| Married | 68.3 (415) |
| Never married/previously marrieda | 31.7 (193) |
| Religion | |
| Hindu | 68.6 (417) |
| Muslim | 18.4 (112) |
| Christian/other | 13.0 (79) |
| Current subdistrict | |
| Gudur | 31.6 (192) |
| Other subdistricts | 68.4 (416) |
| Distance to Nellore clinic | |
| 0–10 km | 42.4 (258) |
| 10.1–10 km | 25.3 (154) |
| 40 + km | 32.2 (196) |
| Frequency of visits to Nellore | |
| Not at all/less than once a week | 52.0 (316) |
| At least once a week | 24.3 (148) |
| Every day/almost every day | 23.7 (144) |
| Moves in past year | |
| None | 80.8 (491) |
| At least one | 19.2 (117) |
| Number of children | |
| None | 8.4 (51) |
| 1–2 | 65.6 (399) |
| 3 or more | 26.0 (158) |
| Children under 12 living with participant | |
| No | 39.8 (242) |
| Yes | 60.2 (366) |
| SES group | |
| Lower SES | 34.7 (211) |
| Mid SES | 32.4 (197) |
| Higher SES | 32.9 (200) |
| Sex work in past week | |
| No | 10.0 (60) |
| Yes | 90.0 (541) |
| Usual way of finding clients | |
| Brothel | 25.7 (155) |
| From home | 61.1 (369) |
| In public places | 13.3 (80) |
| Worried about contracting HIV/AIDS | |
| Not at all worried | 44.6 (271) |
| Very/a little worried | 55.4 (337) |
| HIV testing | |
| Tested | 51.2 (311) |
| Never tested/don't know if tested | 48.9 (297) |
| Drug use past year | |
| No | 69.1 (415) |
| Yes | 31.0 (186) |
| Anal sex in past month with paying partner | |
| No | 45.9 (274) |
| Yes | 54.1 (323) |
| Forced sex in past year | |
| No | 51.8 (311) |
| Yes | 48.2 (289) |
| Condom use with paying partner | |
| Sometimes/rarely/never | 52.0 (313) |
| Always | 48.0 (289) |
| HIV risk index | |
| Low risk | 54.6 (326) |
| High risk | 45.4 (271) |
| Experience with HIV/community outreach services | |
| No | 57.4 (341) |
| Yes | 42.6 (259) |
| Perceived income (to support self, others and pay debts) | |
| Enough money | 13.2 (80) |
| Not enough money | 86.8 (528) |
| Would not participate in trial because husband/partner or family would not allow it | |
| Agree | 26.0 (158) |
| Disagree | 74.0 (450) |
| Would not participate in trial because of fear of trouble with police | |
| Disagree | 67.8 (412) |
| Agree | 32.2 (196) |
| Would not participate in trial because people in the community might think she has HIV | |
| Disagree | 87.0 (529) |
| Agree | 13.0 (79) |
| Participant screened for trial | |
| No | 24.3 (148) |
| Yes | 75.7 (460) |
| Participant enrolled in trial | |
| No | 64.3 (391) |
| Yes | 35.7 (217) |
| Success at recruiting peer to screening | |
| None | 51.0 (310) |
| Recruited at least one peer | 49.0 (298) |
| Success at recruiting peer into trial | |
| None | 69.7 (424) |
| Recruited at least one peer | 30.3 (184) |
We include in the never married group women who report being married but for whom no “guana” was performed. Guana is a practice whereby girls are promised in marriage but may not live with the husband until they reach puberty and the marriage ceremony is performed
The majority (90.0 %) reported engaging in sex work at least once in the past week. Home-based sex work was most common (61.1 %) versus brothel-based (25.7 %) or street-based (13.3 %). One-half (48.2 %) experienced forced sex and 31.0 % used drugs in the past year. One-half (51.2 %) reported ever having an HIV test; 55.4 % indicated they were very worried or a little worried about contracting HIV.
When asked why they might not participate in an HIV prevention clinical trial, 13.0 % said they feared people would think they were HIV-positive, 26.0 % said it was because their husbands/partners or family members would not allow it, and 32.2 % feared that participation might cause conflicts with the police.
Associations with Successful Recruitment of Peers for Screening into the Trial
Of those given coupons to recruit peers, 49.0 % recruited at least one peer who screened for the trial. Successful recruitment was independently associated in multivariate analysis with greater likelihood of the recruiter herself having screened for the trial (Adjusted Odds Ratio [AOR]: 2.4), and being in the middle (AOR: 1.8) and higher (AOR: 1.6) SES group (Table 2).
Table 2.
Relationship between selected demographic and behavioral characteristics and successful recruitment of peers who screened for the placebo trial
| Characteristics | Successfully recruited peers who screened for the trial: % (95 % CI) n = 608 | Odds ratios (95 % CI) n = 608 | Adjusted odds ratios (95 % CI) n = 594 | ||
|---|---|---|---|---|---|
| Age | |||||
| 18–24 | 43.9 (34.7–53.0) | 1.0 | 1.0 | ||
| 25–34 | 49.2 (43.1–55.3) | 1.2 | (0.8–1.9) | 1.3 | (0.8–2.1) |
| 35–15 | 51.3 (44.9–57.7) | 1.4 | (0.9–2.1) | 1.3 | (0.8–2.2) |
| Marital status | |||||
| Never/previously married | 51.8 (44.7–58.9) | 1.0 | 1.0 | ||
| Married | 47.7 (42.9–52.5) | 0.9 | (0.6–1.2) | 0.9 | (0.6–1.2) |
| Current subdistrict | |||||
| Other subdistricts | 48.3 (43.5–53.1) | 1.0 | 1.0 | ||
| Gudur | 50.5 (43.4–57.6) | 1.1 | (0.8–1.5) | 0.9 | (0.6–1.4) |
| Frequency of visits to Nellore | |||||
| Not at all/less than once a week | 50.0 (44.5–55.5) | 1.0 | 1.0 | ||
| At least once a week | 50.0 (41.9–58.1) | 1.0 | (0.7–1.5) | 1.0 | (0.6–1.5) |
| Everyday/almost every day | 45.8 (37.7–54.0) | 0.9 | (0.6–1.3) | 0.8 | (0.5–1.3) |
| Children under 12 living with participant | |||||
| No | 49.6 (43.3–55.9) | 1.0 | 1.0 | ||
| Yes | 48.6 (43.5–53.8) | 1.0 | (0.7–1.3) | 1.1 | (0.8–1.7) |
| SES group | |||||
| Lower SES | 41.2 (34.6–47.9) | 1.0 | 1.0 | ||
| Mid SES | 54.8 (47.8–61.8) | 1.7** | (1.2–2.6) | 1.8** | (1.2–2.7) |
| Higher SES | 51.5 (44.5–58.5) | 1.5* | (1.0–2.2) | 1.6* | (1.1–2.4) |
| Sex work in past week | n = 601 | n = 601 | |||
| No | 41.7 (29.1–54.3) | 1.0 | 1.0 | ||
| Yes | 49.7 (45.5–53.9) | 1.4 | (0.8–2.4) | 1.2 | (0.7–2.2) |
| Usual way of finding clients | n = 604 | n = 604 | |||
| Brothel | 49.7 (41.8–57.6) | 1.0 | 1.0 | ||
| From home | 48.5 (43.4–53.6) | 1.0 | (0.7–1.4) | 1.0 | (0.6–1.5) |
| In public places | 51.3 (40.2–62.3) | 1.1 | (0.6–1.8) | 1.1 | (0.6–1.9) |
| Worried about contracting HIV/AIDS | |||||
| Not at all worried | 49.8 (43.8–55.8) | 1.0 | 1.0 | ||
| Very/a little worried | 48.4 (43.0–53.7) | 0.9 | (0.7–1.3) | 0.9 | (0.6–1.3) |
| HIV testing | |||||
| Tested | 48.2 (42.7–53.8) | 1.0 | 1.0 | ||
| Never tested/don't know if tested | 49.8 (44.1–55.5) | 1.1 | (0.8–1.5) | 1.2 | (0.8–1.8) |
| HIV risk index | n = 597 | n = 597 | |||
| Low risk | 47.9 (42.4–53.3) | 1.0 | 1.0 | ||
| High risk | 50.9 (44.9–56.9) | 1.1 | (0.8–1.6) | 1.1 | (0.7–1.5) |
| Experience with HIV/community outreach services | |||||
| No | 46.7 (41.5–52.0) | 1.0 | 1.0 | ||
| Yes | 52.1 (46.0–58.2) | 1.2 | (0.9–1.7) | 1.4 | (0.9–2.0) |
| Perceived income (to support self, others and pay debts) | |||||
| Enough money | 48.8 (37.7–59.8) | 1.0 | 1.0 | ||
| Not enough money | 49.1 (44.8–53.3) | 1.0 | (0.6–1.6) | 1.0 | (0.6–1.7) |
| Would not participate in trial because husband/partner or family would not allow it | |||||
| Disagree | 48.2 (43.6–52.9) | 1.0 | 1.0 | ||
| Agree | 51.3 (43.4–59.1) | 1.1 | (0.8–1.6) | 1.1 | (0.7–1.7) |
| Would not participate in trial because of fear of trouble with police | |||||
| Disagree | 48.8 (43.9–53.6) | 1.0 | 1.0 | ||
| Agree | 49.5 (42.5–56.5) | 1.0 | (0.7–1.5) | 1.0 | (0.7–1.5) |
| Would not participate in trial because people in the community might think she has HIV | |||||
| Disagree | 49.0 (44.7–53.2) | 1.0 | 1.0 | ||
| Agree | 49.4 (38.2–60.5) | 1.0 | (0.6–1.6) | 0.9 | (0.5–1.6) |
| Participant screened for trial | |||||
| No | 32.4 (24.8–40.0) | 1.0 | 1.0 | ||
| Yes | 54.3 (49.8–58.9) | 2.5*** | (1.7–3.7) | 2.4*** | (1.6–3.6) |
p < 0.001
p < 0.01
p < 0.05
Associations with Successful Recruitment of Peers who Enrolled in the Trial
Among eligible recruiters, 30.3 % recruited at least one peer who subsequently enrolled in the trial. Successful recruitment was associated in multivariate analysis with older age (25–34 years: AOR: 1.6), greater likelihood of the recruiter herself enrolling in the trial (AOR: 2.0), and lower likelihood of visiting Nellore city center every day or almost every day (AOR: 0.5) (Table 3).
Table 3.
Relationship between selected demographic and behavioral characteristics and successful recruitment of peers who enrolled in the placebo trial
| Characteristics | Successfully recruited peers who enrolled in trial: % (95 % CI) n = 608 | Odds ratios (95 % CI) n = 608 | Adjusted odds ratios (95 % CI) n = 594 | ||
|---|---|---|---|---|---|
| Age | |||||
| 18–24 | 21.9 (14.3–29.6) | 1.0 | 1.0 | ||
| 25–34 | 29.8 (24.2–35.5) | 1.5 | (0.9–2.5) | 1.6† | (0.9–2.8) |
| 35–45 | 34.7 (28.6–40.8) | 1.9* | (1.1–3.2) | 1.6 | (0.9–2.9) |
| Marital status | |||||
| Never/previously married | 31.1 (24.5–37.6) | 1.0 | 1.0 | ||
| Married | 29.9 (25.5–34.3) | 0.9 | (0.7–1.4) | 1.0 | (0.6–1.5) |
| Current subdistrict | |||||
| Other subdistricts | 27.4 (23.1–31.7) | 1.0 | 1.0 | ||
| Gudur | 36.5 (29.6–43.3) | 1.5* | (1.1–2.2) | 1.2 | (0.8–1.9) |
| Frequency of visits to Nellore | |||||
| Not at all/less than once a week | 34.5 (29.2–39.8) | 1.0 | 1.0 | ||
| At least once a week | 31.8 (24.2–39.3) | 0.9 | (0.6–1.3) | 1.1 | (0.7–1.9) |
| Everyday/almost every day | 19.4 (12.9–25.9) | 0.5** | (0.3–0.7) | 0.5* | (0.3–0.9) |
| Children under 12 living with participant | |||||
| No | 33.9 (27.9–39.9) | 1.0 | 1.0 | ||
| Yes | 27.9 (23.3–32.5) | 0.8 | (0.5–1.1) | 0.9 | (0.6–1.3) |
| SES Index | |||||
| Lower SES | 29.9 (23.7–36.1) | 1.0 | 1.0 | ||
| Mid SES | 36.0 (29.3–42.8) | 1.3 | (0.9–2.0) | 1.4 | (0.9–2.2) |
| Higher SES | 25.0 (19.0–31.0) | 0.8 | (0.5–1.2) | 0.9 | (0.5–1.4) |
| Sex work in past week | n = 601 | n = 601 | |||
| No | 20.0 (9.8–30.2) | 1.0 | 1.0 | ||
| Yes | 31.1 (27.1–35.0) | 1.8 | (0.9–3.5) | 1.8† | (0.9–3.7) |
| Usual way of finding clients | n = 604 | n = 604 | |||
| Brothel | 33.5 (26.1–41.0) | 1.0 | 1.0 | ||
| From home | 29.8 (25.1–34.5) | 0.8 | (0.6–1.3) | 1.0 | (0.6–1.5) |
| In public places | 26.3 (16.5–36.0) | 0.7 | (0.4–1.3) | 0.7 | (0.4–1.4) |
| Worried about contracting HIV/AIDS | |||||
| Not at all worried | 30.6 (25.1–36.1) | 1.0 | 1.0 | ||
| Very/a little worried | 30.0 (25.1–34.9) | 1.0 | 1.0 | (0.7–1.5) | |
| HIV testing | |||||
| Tested | 25.1 (20.2–29.9) | 1.0 | (0.7–1.4) | 1.0 | |
| Never tested/don't know if tested | 35.7 (30.2–41.2) | 1.7** | (1.2–2.4) | 1.5† | (1.0–2.3) |
| HIV risk index | n = 597 | n = 597 | |||
| Low risk | 28.2 (23.3–33.1) | 1.0 | 1.0 | ||
| High risk | 32.5 (26.9–38.1) | 1.2 | (0.9–1.7) | 1.0 | (0.7–1.5) |
| Experience with HIV/community outreach services | |||||
| No | 31.2 (26.4–36.1) | 1.0 | 1.0 | ||
| Yes | 29.0 (23.4–34.5) | 0.9 | (0.6–1.3) | 1.2 | (0.8–1.8) |
| Perceived income (to support self, others and pay debts) | |||||
| Enough money | 25.0 (15.4–34.6) | 1.0 | 1.0 | ||
| Not enough money | 31.1 (27.1–35.0) | 1.4 | (0.8–2.3) | 1.5 | (0.8–2.6) |
| Would not participate in trial because husband/partner or family would not allow it | |||||
| Disagree | 32.0 (27.7–36.3) | 1.0 | 1.0 | ||
| Agree | 25.3 (18.5–32.1) | 0.7 | (0.5–1.1) | 0.9 | (0.5–1.4) |
| Would not participate in trial because of fear of trouble with police | |||||
| Disagree | 32.0 (27.5–36.6) | 1.0 | 1.0 | ||
| Agree | 26.5 (20.3–32.7) | 0.8 | (0.5–1.1) | 0.7† | (0.4–1.0) |
| Would not participate in trial because people in the community might think she has HIV | |||||
| Disagree | 31.2 (27.2–35.2) | 1.0 | 1.0 | ||
| Agree | 24.1 (14.5–33.6) | 0.7 | (0.4–1.2) | 1.0 | (0.5–2.0) |
| Participant enrolled in trial | |||||
| No | 24.0 (19.8–28.3) | 1.0 | 1.0 | ||
| Yes | 41.5 (34.9–48.1) | 2.2*** | (1.6–3.2) | 2.0** | (1.3–2.9) |
p < 0.001
p < 0.01
p < 0.05
p < 0.1
Discussion
Targeted and novel strategies are needed to improve recruitment of high-risk and hidden populations into microbicide clinical trials. We were able to use a two-stage recruitment strategy to recruit FSWs for a placebo microbicide clinical trial: first recruiting participants via RDS to participate in a survey and then inviting survey participants to screen for the trial. The rate of trial enrollment was modest compared with that of screening, in part because of reproductive health and STI morbidity [26]. However, recruitment for the trial took approximately 6 months, which was within our expected timeframe. We reviewed publications that report on trial recruitment challenges, microbicide trials in India, and microbicide and vaccine trials among female commercial sex workers [6, 27–40]. Only some studies provide the time required to achieve the target sample; when provided, such information was not specific to Indian or high-risk female populations [34, 36, 41]. Other studies included multiple sites or both men and women, reporting on the combined populations. Van Damme (2008) [38] included the time for all five sites (two in India, three in Africa) to enroll a target sample size of FSWs (1.5 years); the two India sites contributed to less than 20 % of the overall enrolled population. Solomon (2006) [36] reported that it took 6 months to enroll a “high-risk” sample of 500 men and women (630 screened). Other studies are Phase 1 trials, which are limited to low-risk participants [28–30, 32, 33, 40]. As such, we were not able to compare this recruitment rate to other trials. The use of a two-stage sampling strategy allowed potential trial participants to participate in a survey that required no invasive clinical procedures before being invited to participate in a clinical study requiring more time and trust of FSWs; this may have developed trust and confidence in the study. Other uses of the “foot-in-the-door” approach have been successful in enrolling participants into interventions and clinical trials [42, 43].
Using RDS for the first stage of recruitment also helped disseminate information about the survey and subsequent trial to a large, diverse subset of potential trial participants who might have otherwise been missed with sampling strategies typically used to recruit FSWs such as targeted or time-location sampling. Our high screening rate may have been a result of FSWs’ positive experience with the survey or, as reported elsewhere, that FSWs were interested in being screened for a clinical trial because of the clinical services they would receive [23]. Lower enrollment rates into the trial might have resulted from the high levels of STI and gynecological morbidity among FSWs who came for screening, which made them less likely to meet the eligibility criteria for the trial [26]. In fact, we previously reported that FSWs who came for the screening visit were more likely to have reported symptoms than those who did not come for screening [23]. Additionally, trial enrollment may have been slower because trial participation required a commitment that included repeated travel to the trial clinic, using a vaginal gel daily, and undergoing a variety of clinical procedures.
The two-stage recruitment strategy could be considered for trials seeking to enroll other at-risk populations, including drug users and men who have sex with men. Peer recruitment was a key component to the recruitment. Recruitment within social networks using peer referral has been successful for studies and interventions among populations who engage in illegal and stigmatized activities; peers have been found to influence each other more than outsiders [9, 11, 44–48].
Successful Recruitment of Peers Who Screened
Previous studies have examined willingness to participate in microbicide trials and motivations for trial participation [6, 49–51]; however this was one of the first studies to examine these factors in the context of clinical trial recruitment via RDS. FSWs in the higher SES groups were found to be successful recruiters of those who screened for the trial. Having assets such as a television or water source (that other FSWs might not have) may have made them more influential. FSWs having a higher SES may have also been those who acted as madams/brokers (arrange clients), and thus may have had a larger network of FSWs and more influence. Finally, FSWs who successfully recruited peers who screened for the trial were more likely to have screened for the trial themselves, again indicating the influence of peers.
Successful Recruitment of Peers Who Enrolled
The infrequency of visits to Nellore city by successful recruiters of peers who enrolled was unexpected; we anticipated regular visitors of Nellore city to be better recruiters. FSWs who frequented Nellore city less often were likely to recruit others who visited Nellore city less often, and women who frequented Nellore city less often were also more likely to indicate receiving health care as their main reason for participating in a clinical trial. The majority of hospitals and clinics in Nellore were located in or near the city center; FSWs who visited Nellore city less regularly would have less access to clinical services and therefore the clinical procedures surrounding trial participation might have influenced participation.
One of the strongest indicators of a successful recruiter of FSWs who enrolled was whether the recruiter herself enrolled in the trial. We posit that eligible women who chose to enroll did so partly because of their positive experience at the clinic, which in turn may have influenced peers to enroll in the trial. We would recommend adding a third stage to recruitment efforts, asking enrolled women to distribute additional coupons or brochures to their peers.
Limitations
First, our results are based on a placebo gel trial; recruitment may be different for a trial using an active product. Second, we did not include a comparison recruitment strategy, and as noted above, published recruitment rates for a similar population were unavailable. Future research should compare the two-stage recruitment strategy with traditional recruitment approaches.
Recommendations
Given the challenges in recruiting the large number of high-risk participants needed for large-scale HIV prevention trials, this study has implications for mitigating recruitment challenges in future clinical trials. Our findings suggest that a two-stage sampling strategy involving RDS recruitment into a survey followed by a clinical trial may be a good option. This strategy may be particularly useful when trials involve recruitment of hidden populations that require a more targeted recruitment strategy.
Supplementary Material
Acknowledgments
The authors would like to thank Ulrike Rawiel, as well as all YRG CARE study staff, particularly: K.R. Hari, Rajeswari and outreach volunteers; Durga, Jayanthi, Issaiah Kumari, Sowjanya, Sarojini, Prema Joythi, Dr. C.S. Shalini, Vijaya Lakshmi, K. Aparna, and Sasi J., for their dedicated work on this project. This manuscript is made possible by the generous support of the American and Indian people through the United States Agency for International Development (USAID), Bureau for Global Health, Office of Population and Reproductive Health, under the terms of Award No. GPOA-00-04-00019; the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), under the terms of Award No. R21 HD060270; and the Indian Council of Medical Research (ICMR), under the terms of Award No. Indo-US/54/2007-ECD II. The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the U.S. or Indian governments.
Contributor Information
Waimar Tun, HIV and AIDS Program, Population Council, 4301 Connecticut Avenue, NW, Suite 80, Washington, DC 20008, USA.
Lauren L. Katzen, HIV and AIDS Program, Population Council, New York, USA
Sharon A. Abbott, HIV and AIDS Program, Population Council, New York, USA
Aylur K. Srikrishnan, YRG Centre for AIDS Research and Education (YRG CARE), Chennai, India
Christine A. Kelly, Poverty, Gender, and Youth Program, Population Council, New York, USA
Avina Sarna, HIV and AIDS Program, Population Council, New Delhi, India.
Barbara A. Friedland, HIV and AIDS Program, Population Council, New York, USA
Suniti Solomon, YRG Centre for AIDS Research and Education (YRG CARE), Chennai, India.
Barbara S. Mensch, Poverty, Gender, and Youth Program, Population Council, New York, USA
References
- 1.National AIDS Control Organization, Department of AIDS Control, Ministry of Health and Family Welfare, Government of India . Annual Report 2012–2013. New Delhi, India: 2013. [Google Scholar]
- 2.Joshi S, Solomon S, Mayer K, Mehendale S. Preparing for efficacy trials of vaginal microbicides in Indian women. Indian J Med Res. 2005;121(4):502–9. [PubMed] [Google Scholar]
- 3.National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India . Annual Report 2010–2011. New Delhi: 2011. [Google Scholar]
- 4.Toerien M, Brookes ST, Metcalfe C, et al. A review of reporting of participant recruitment and retention in RCTs in six major journals. Trials. 2009;10:52. doi: 10.1186/1745-6215-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell MK, Snowdon C, Francis D, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess. 2007;11(48):iii–ix. doi: 10.3310/hta11480. [DOI] [PubMed] [Google Scholar]
- 6.Guest G, Severy L, von Mollendorf C, Van Damme L. Overcoming recruitment challenges: lessons learned from a safety and feasibility study of a diaphragm/microbicide combination in South Africa. J Acquir Immune Defic Syndr. 2007;45(4):481–2. doi: 10.1097/QAI.0b013e318093de8a. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee P. AIDS in India: police powers and public health. The Lancet. 2006;367:805–6. doi: 10.1016/S0140-6736(06)68319-0. [DOI] [PubMed] [Google Scholar]
- 8.Biradavolu MR, Burris S, George A, Jena A, Blankenship KM. Can sex workers regulate police? Learning from an HIV prevention project for sex workers in southern India. Soc Sci Med. 2009;68(8):1541–7. doi: 10.1016/j.socscimed.2009.01.040. [DOI] [PubMed] [Google Scholar]
- 9.Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–99. [Google Scholar]
- 10.Scott G. “They got their program, and I got mine”: a cautionary tale concerning the ethical implications of using respondent-driven sampling to study injection drug users. Int J Drug Policy. 2008;19(1):42–51. doi: 10.1016/j.drugpo.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Heckathorn D, Semaan S, Broadhead RS, Hughes J. Extensions of respondent-driven sampling: a new approach to the study of injection drug users aged 18–25. AIDS Behav. 2002;6(1):55–67. [Google Scholar]
- 12.Magnani R, Sabin K, Saidel T, Heckathorn D. Review of sampling hard-to-reach and hidden populations for HIV surveillance. Aids. 2005;19(2):S67–72. doi: 10.1097/01.aids.0000172879.20628.e1. [DOI] [PubMed] [Google Scholar]
- 13.Erausquin JT, Reed E, Blankenship KM. Police-related experiences and HIV risk among female sex workers in Andhra Pradesh, India. J Infect Dis. 2011;204(5):S1223–8. doi: 10.1093/infdis/jir539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnston LG, Sabin K, Mai TH, Pham TH. Assessment of respondent driven sampling for recruiting female sex workers in two Vietnamese cities: reaching the unseen sex worker. J Urban Health. 2006;83(6 Suppl):i16–28. doi: 10.1007/s11524-006-9099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wattana W, van Griensven F, Rhucharoenpornpanich O, et al. Respondent-driven sampling to assess characteristics and estimate the number of injection drug users in Bangkok, Thailand. Drug Alcohol Depend. 2007;90(2–3):228–33. doi: 10.1016/j.drugalcdep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Yeka W, Maibani-Michie G, Prybylski D, Colby D. Application of respondent driven sampling to collect baseline data on FSWs and MSM for HIV risk reduction interventions in two urban centres in Papua New Guinea. J Urban Health. 2006;83(6):i60–72. doi: 10.1007/s11524-006-9103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston LG, Sabin K. Sampling hard-to-reach populations with respondent driven sampling. Methodological Innovations Online. 2010;5(2):38–48. [Google Scholar]
- 18.Kendall C, Kerr LRFS, Gondim RC, et al. An empirical comparison of respondent-driven sampling, time location sampling, and snowball sampling for behavioral surveillance in men who have sex with men, Fortaleza, Brazil. AIDS Behav. 2008;12:S97–104. doi: 10.1007/s10461-008-9390-4. [DOI] [PubMed] [Google Scholar]
- 19.Malekinejad M, Johnston LG, Kendall C, Kerr LR, Rifkin MR, Rutherford GW. Using respondent-driven sampling methodology for HIV biological and behavioral surveillance in international settings: a systematic review. AIDS Behav. 2008;12(4):S105–30. doi: 10.1007/s10461-008-9421-1. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins R. Recruiting substance-using men who have sex with men into hiv prevention research: current status and future directions. AIDS Behav. 2012;16(6):1411–9. doi: 10.1007/s10461-011-0037-5. [DOI] [PubMed] [Google Scholar]
- 21.Medhi GK, Mahanta J, Paranjape RS, Adhikary R, Laskar N, Ngully P. Factors associated with HIV among female sex workers in a high HIV prevalent state of India. AIDS Care. 2012;24(3):369–76. doi: 10.1080/09540121.2011.608787. [DOI] [PubMed] [Google Scholar]
- 22.Abbott SA, Friedland BA, Sarna A, et al. An evaluation of methods to improve the reporting of adherence in a placebo gel trial in Andhra Pradesh, India. AIDS Behav. 2013;17(6):2222–36. doi: 10.1007/s10461-012-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mensch BS, Friedland BA, Abbott SA, et al. Characteristics of female sex workers in southern india willing and unwilling to participate in a placebo gel trial. AIDS Behav. 2013;17(2):585–97. doi: 10.1007/s10461-012-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parivartan P. A Summary Report. Yale University, CARE India, Avahan; May, 2007. Results of a Cross-sectional Survey of Female Sex Workers in Rajahmundry, Andhra Pradesh. [Google Scholar]
- 25.Dandona R, Dandona L, Gutierrez JP, et al. High risk of HIV in non-brothel based female sex workers in India. [October 2007];BMC Public Health. 2005 5(87) doi: 10.1186/1471-2458-5-87. Available from: http://www.biomedcentral.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarna A, Friedland BA, Srikrishnan AK, et al. Sexually transmitted infections and reproductive health morbidity in a cohort of female sex workers screened for a microbicide feasibility study in Nellore, India. Glob J Health Sci. 2013;5(3):139–49. doi: 10.5539/gjhs.v5n3p139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barroso PF, de Souza MB, do Lago RF. Barriers to recruit female commercial sex workers for HIV vaccine trials: the Rio de Janeiro experience. J Acquir Immune Defic Syndr. 2009;50(1):116–7. doi: 10.1097/QAI.0b013e31818d5e3f. [DOI] [PubMed] [Google Scholar]
- 28.Dutta S, Joglekar N, Joshi S, Steven JR. Experience of conducting a phase I safety & acceptability clinical trial of a candidate vaginal microbicide & lessons learned. Indian J Med Res. 2008;128(2):212–3. [PubMed] [Google Scholar]
- 29.Joglekar N, Joshi S, Kakde M, et al. Acceptability of PRO2000 vaginal gel among HIV un-infected women in Pune, India. AIDS Care. 2007;19(6):817–21. doi: 10.1080/09540120601133576. [DOI] [PubMed] [Google Scholar]
- 30.Joglekar NS, Joshi SN, Navlakha SN, Katti UR, Mehendale SM. Acceptability of Praneem polyherbal vaginal tablet among HIV uninfected women & their male partners in Pune, India-Phase I study. Indian J Med Res. 2006;123(4):547–52. [PubMed] [Google Scholar]
- 31.Joglekar NS, Joshi SN, Deshpande SS, Parkhe AN, Katti UR, Mehendale SM. Acceptability and adherence: findings from a Phase II study of a candidate vaginal microbicide, ‘Praneem polyherbal tablet’, in Pune, India. Trans R Soc Trop Med Hyg. 2010;104(6):412–5. doi: 10.1016/j.trstmh.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Joshi S, Joglekar N, Ghate M, et al. Phase I safety & preliminary acceptability of nonoxynol-9 vaginal pessary as a vaginal microbicide in low risk women in Pune, India. Indian J Med Res. 2003;117:152–7. [PubMed] [Google Scholar]
- 33.Joshi SN, Katti U, Godbole S, et al. Phase I safety study of Praneem polyherbal vaginal tablet use among HIV-uninfected women in Pune, India. Trans R Soc Trop Med Hyg. 2005;99(10):769–74. doi: 10.1016/j.trstmh.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Mauck C, Joshi S, Schwartz J, Callahan M, Walsh T. Reddy female condom: functional performance of a 90-mm shaft length in two clinical studies. Contraception. 2011;83(5):466–71. doi: 10.1016/j.contraception.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Mehendale S, Deshpande S, Kohli R, Tsui S, Tolley E. Acceptability of coitally-associated versus daily use of 1% tenofovir vaginal gel among women in Pune, India. Int Health. 2012;4:63–9. doi: 10.1016/j.inhe.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Solomon SS, Solomon S, Masse BR, et al. Risk reduction counseling is associated with decreased hiv transmission-associated behaviors in high-risk indian heterosexuals. JAIDS J Acquir Immune Defic Syndr. 2006;42(4):478–83. doi: 10.1097/01.qai.0000221684.83057.2f. [DOI] [PubMed] [Google Scholar]
- 37.Van Damme L, Chandeying V, Ramjee G, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II Study Group. AIDS. 2000;14(1):85–8. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 38.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N Engl J Med. 2008;359(5):463–72. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 39.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360(9338):971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 40.van De Wijgert J, Fullem A, Kelly C, et al. Phase 1 trial of the topical microbicide BufferGel: safety results from four international sites. J Acquir Immune Defic Syndr. 2001;26(1):21–7. doi: 10.1097/00126334-200101010-00003. [DOI] [PubMed] [Google Scholar]
- 41.Mehendale SM, Ghate MV, Kishore Kumar B, et al. Low HIV-1 Incidence Among Married Serodiscordant Couples in Pune, India. JAIDS J Acquir Immune Defic Syndr. 2006;41(3):371–3. doi: 10.1097/01.qai.0000209905.35620.48. doi:10.1097/1001.qai.0000209905.0000235620.0000209948. [DOI] [PubMed] [Google Scholar]
- 42.Kanouse D, Bluthenthal R, Bogart L, Iguchi M, Perry S, Sand K. Recruiting drug-using men who have sex with men into behavioral interventions: a two-stage approach. J Urban Health. 2005;82:i109–19. doi: 10.1093/jurban/jti030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grov C, Bux D, Jr, Parsons JT, Morgenstern J. Recruiting hard-to-reach drug-using men who have sex with men into an intervention study: lessons learned and implications for applied research. Subst Use Misuse. 2009;44(13):1855–71. doi: 10.3109/10826080802501570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrow KM, Fava JL, Rosen RK, Christensen AL, Vargas S, Barroso C. Willingness to use microbicides varies by race/ethnicity, experience with prevention products, and partner type. Health Psychol. 2007;26(6):777–86. doi: 10.1037/0278-6133.26.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Broadhead R, Heckathorn D, Weakliem D, et al. Harnessing peer networks as an instrument for AIDS prevention: results from a peer-driven intervention. Public Health Rep. 1998;113(1):42–57. [PMC free article] [PubMed] [Google Scholar]
- 46.Broadhead R, Volkanevsky V, Rydanovab T, et al. Peer-driven HIV interventions for drug injectors in Russia: first year impact results of a field experiment. Int J Drug Policy. 2006;17:379–92. [Google Scholar]
- 47.Broadhead R, Heckathorn D, Grund J, Stern L, Anthony D. Drug users versus outreach workers in combating AIDS: preliminary results of a peer-driven intervention. J Drug Issues. 1995;25(3):531–64. [Google Scholar]
- 48.Broadhead R, Heckathorn D, Altice F, et al. Increasing drug users’ adherence to HIV treatment: results of a peer-driven intervention feasibility study. Soc Sci Med. 2002;55(2):235–46. doi: 10.1016/s0277-9536(01)00167-8. [DOI] [PubMed] [Google Scholar]
- 49.Gross M, Buchbinder SP, Celum C, Heagerty P, Seage GR., III Rectal microbicides for U.S. gay men: Are clinical trials needed? Are they feasible? Sex Transm Dis. 1998;25(6):296–302. doi: 10.1097/00007435-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 50.Tharawan K, Manopaiboon C, Ellertson C, et al. Women's willingness to participate in microbide trials in northern Thailand. J Acquir Immune Defic Syndr Human Retrovirol. 2001;28(2):180–6. doi: 10.1097/00126334-200110010-00011. [DOI] [PubMed] [Google Scholar]
- 51.van de Wijgert J, Coetzee N, de Kock A, Blanchard K, Jones H. Assessing selection bias in a microbicide trial.. Paper presented at: Poster presentation at the International Congress of Sexually Transmitted Infections—ISSTDR/IUSTI; June 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.