Abstract
Repeated encounters with the same event typically lead to decreased activation in the medial temporal lobe (MTL) and dopaminergic midbrain, a phenomenon known as repetition suppression. In contrast, encountering an event that overlaps with prior experience leads to increased response in the same regions. Such increased responding is thought to reflect an associative novelty signal that promotes memory updating to resolve differences between current events and stored memories. Here, we married these ideas to test whether event overlap significantly modulates MTL and midbrain responses—even when events are repeated and expected—to promote memory updating through integration. While undergoing high-resolution functional MRI, participants were repeatedly presented with objects pairs, some of which overlapped with other, intervening pairs and some of which contained elements unique from other pairs. MTL and midbrain regions showed widespread repetition suppression for non-overlapping pairs containing unique elements; however, the degree of repetition suppression was altered for overlapping pairs. Entorhinal cortex, perirhinal cortex (PRc), midbrain, and PRc—midbrain connectivity showed repetition-related increases across overlapping pairs. Notably, increased PRc activation for overlapping pairs tracked individual differences in the ability to reason about the relationships among pairs—our behavioral measure of memory integration. Within hippocampus, activation increases across overlapping pairs were unique to CA1, consistent with its hypothesized comparator function. These findings demonstrate that event overlap engages MTL and midbrain functions traditionally implicated in novelty processing, even when overlapping events themselves are repeated. Our findings further suggest that the MTL—midbrain response to event overlap may promote integration of new content into existing memories, leading to the formation of relational memory networks that span experiences. Moreover, the results inform theories about the division of labor within MTL, demonstrating that the role of PRc in episodic encoding extends beyond familiarity processing and item-level recognition.
Keywords: memory integration, hippocampus, perirhinal cortex, novelty, encoding
Introduction
The regions within the medial temporal lobe (MTL) comprise a memory system specialized in detecting novelty (Tulving et al., 1996; Ranganath and Rainer, 2003). Repeated presentation of the same stimulus leads to decreased MTL activation, with the degree of repetition suppression predicting successful memory for repeated stimuli (Gonsalves et al., 2005; Rand-Giovannetti et al., 2006; Vannini et al., 2013; Ward et al., 2013). The presence or absence of repetition suppression effects can indicate whether a current stimulus is treated as the same or different as one encountered previously, thereby providing an indirect measure of the encoding demand for the current stimulus. Repetition suppression indicates that the stimulus is the same as a previous event, with minimal need for encoding. The absence of repetition suppression indicates that the stimulus is novel and requires new encoding.
Previous work using repetition suppression to index stimulus novelty has proved a fruitful window into MTL subregional function (Aggleton and Brown, 2005; Kumaran and Maguire, 2009; Turk-Browne et al., 2012). For instance, perirhinal cortex (PRc) is sensitive to changes in the presentation of individual objects, whereas hippocampus demonstrates sensitivity to changes in the configurations of objects, suggesting that these regions play dissociable roles in memory for items and memory for relations (Köhler et al., 2005). Moreover, and of particular relevance to the current study, repeating events with alterations to their temporal (Kumaran and Maguire, 2006) or configural (Chen et al., 2011; Duncan et al., 2011) structure results in increased engagement rather than repetition suppression in the hippocampus (Sullivan Giovanello et al., 2004; Köhler et al., 2005; Kumaran and Maguire, 2006; 2007; Howard et al., 2011), and in some instances perirhinal cortex (PRc) (Chen et al., 2011; Davis et al., 2012a). In these studies, the repeated event elements themselves would not require new encoding, but changes in associative structure would. Increased activation in the face of repeated event elements but novel event structure is therefore thought to reflect an associative novelty signal that triggers memory updating (Kumaran and Maguire, 2007; Hupbach et al., 2008; Duncan et al., 2009; Iordanova et al., 2011; Bridge and Voss, 2014). In this study, we use repetition suppression to determine how MTL subregions respond not only to event alterations, but how they promote encoding of the relationships among events to support flexible behavior.
Within the hippocampus, CA1 in particular is sensitive to configural changes across repetitions of events (Chen et al., 2011; Duncan et al., 2011), consistent with its proposed comparator function that detects mismatches between current events and memory-based expectations (Lisman and Otmakhova, 2001a). Theoretical models of novelty-triggered encoding (Lisman and Otmakhova, 2001b; Lisman and Grace, 2005; Lisman et al., 2011) propose that CA1 associative novelty signals project to midbrain—another region that preferentially responds to novel events (Bunzeck and Duzel, 2006)—triggering dopaminergic innervation of the hippocampus. Dopaminergic release within the hippocampus increases plasticity within the circuit to facilitate new encoding (Otmakhova and Lisman, 1998; Li et al., 2003; Lemon and Manahan-Vaughan, 2006; Shohamy and Adcock, 2010; Lemon and Manahan-Vaughan, 2012). Human neuroimaging work has further shown that MTL—midbrain activation and connectivity during novel events are associated with an encoding advantage (Schott, 2004; Wittmann et al., 2005; Adcock et al., 2006; Bunzeck and Duzel, 2006; Wittmann et al., 2007; 2008; Kuhl et al., 2010; Zweynert et al., 2011; Chowdhury et al., 2012; Wolosin et al., 2012).
Moreover, recent data (Larkin et al., 2014) suggest that increased hippocampal plasticity in response to associative novelty promotes memory integration (Shohamy and Wagner, 2008; Schlichting and Preston, 2015), whereby existing memories are updated to incorporate new, related information. Midbrain interactions with MTL regions may critically modulate such novelty-driven memory integration (Shohamy and Adcock, 2010). Consistent with this view, Shohamy and Wagner (2008) observed increases in hippocampal—midbrain interactions across repeated presentations of overlapping events that correlated with individuals’ ability to form connections among related experiences. Interestingly, these effects were observed even though the same overlapping events were repeated many times. This finding thus suggests that events do not have to comprise unexpected configurations to elicit MTL—midbrain engagement; rather, repeated instances of event overlap may recruit the MTL—midbrain circuit to promote a memory updating process that binds related events together in memory. However, the lack of a non-overlapping control condition in that work makes it difficult to fully ascribe increased MTL responding during event overlap to a comparator signal per se.
A further goal of the present study is to test current theories of functional specialization within MTL. Leading theories propose that hippocampus supports associative and relational memory while the adjacent perirhinal cortex supports familiarity and item memory (Aggleton and Brown, 2005; Davachi, 2006; Diana et al., 2007; Aggleton et al., 2012). While the item vs. relational memory division of labor between PRc and hippocampus has been supported by several studies (Yonelinas et al., 2007; Staresina and Davachi, 2008; Diana et al., 2010; Tompary et al., 2015), recent high-resolution functional magnetic resonance imaging (fMRI) studies have challenged this view by detecting enhanced PRc responding in the face of changed relationships among events (Chen et al., 2011; Davis et al., 2012a). In addition to testing hypotheses regarding hippocampal—midbrain responses during event overlap, we also seek to determine whether PRc memory function extends beyond processing of individual items.
Here, we used high-resolution fMRI and a repetition suppression paradigm to test how MTL and midbrain respond to event overlap and how this response relates to memory integration. Participants encoded non-overlapping and overlapping object pairs across three repetitions during scanning. First, we measured how subregional responses to repetition of object pairs are modulated by event overlap. We tested whether such modulation is specific to CA1 and midbrain, as predicted by theoretical models, or whether sensitivity to event overlap extends to PRc. We next tested whether MTL—midbrain connectivity is modulated by event overlap. Finally, we tested whether MTL—midbrain responses to event overlap promote participants’ ability to infer new relationships across events.
Materials and Methods
Participants
Twenty-nine healthy, English-speaking individuals (18 females, ages 18-27 years, mean age = 21) participated in the experiment after giving informed consent in accordance with a protocol approved by the Institutional Review Boards of Stanford University and The University of Texas at Austin. Participants received $20/h for their involvement. Data from seven participants were excluded from analysis due to excessive head motion (4 participants), anatomical anomalies (1 participant), equipment malfunction (1 participant), and failure to comply with task instructions (1 participant). Consequently, data from twenty-two participants (15 females, mean age = 21) were included in the analyses.
Materials
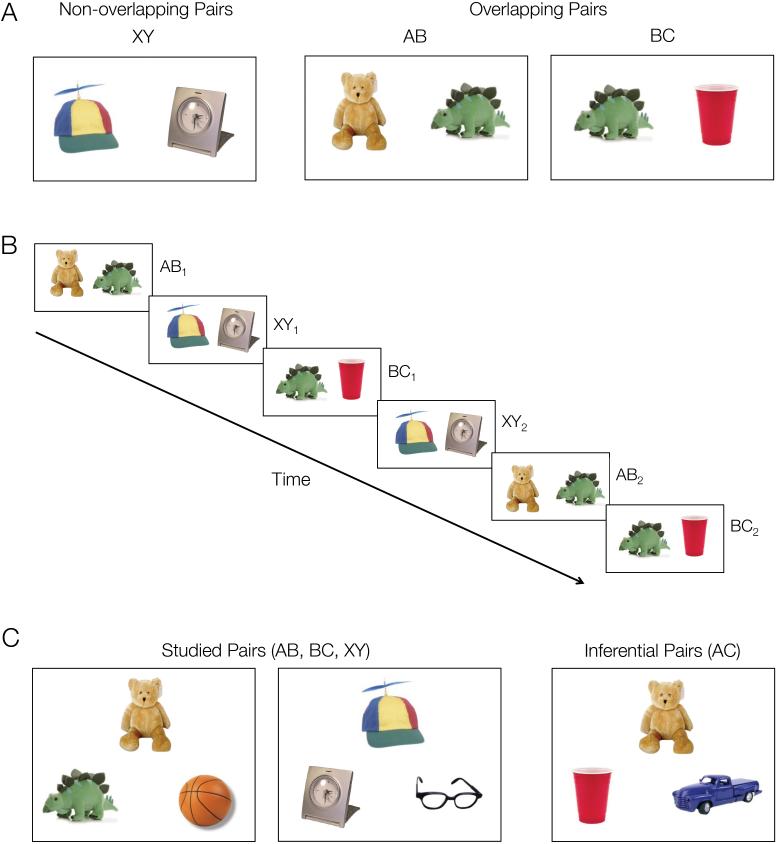
Stimuli consisted of 240 color images of common objects arranged into 96 overlapping object pairs (48 AB, 48 BC) and 48 unique, non-overlapping objects pairs (XY) (Fig. 1A). Overlapping AB and BC pairs were composed such that two objects (A and C) shared a common association with a third, overlapping object (B), thus creating a “triad” of related objects (ABC). Non-overlapping XY pairs were composed of two unique objects that shared no associations with other pairings.
Figure 1.
Experimental design. A. Participants studied overlapping (AB, BC) and non-overlapping (XY) object pairs while undergoing high-resolution functional MRI. B. Each object pair was presented three times. Trials were randomly intermixed with a restriction that overlapping pairs from the same ABC triad were presented in an interleaved manner (AB1, BC1, AB2, BC2, AB3, BC3). C. Memory tests. After scanning, participants’ memory for studied pairs (AB, BC, XY) as well as inferential (AC) relationships was tested using 2-alternative forced-choice.
Procedure
The task employed an associative inference paradigm (Preston et al., 2004; Zeithamova and Preston, 2010), consisting of two phases: (1) an encoding phase during which participants intentionally learned associations between object pairs (AB, BC, XY; Fig. 1A,B), and (2) a test phase during which participants were tested on trained associations (AB, BC, XY) as well as inferential relationships among overlapping pairs (AC) (Fig. 1C). High-resolution fMRI data were collected during the encoding phase. Participants were instructed that they would see repeated overlapping object pairings and would be tested on directly learned associations (AB, BC, XY) as well as indirectly related inferential (AC) associations. Participants were given the opportunity to practice both the encoding and test phases of the experiment prior to scanning.
During each encoding run, participants learned eight associations of each type (AB, BC, XY), across three repetitions of each pair. Pairs from each condition were presented in a pseudo-random order: AB and BC trials from the same ABC triad were presented in an interleaved manner (AB1, BC1, AB2, BC2, AB3, BC3) (Fig. 1B). Each encoding trial consisted of a 3.5 second stimulus presentation followed by a 0.5 second fixation period. The left-right position of the two objects was randomized across trials. Participants were instructed to use an elaborative encoding strategy in which they generated a story linking the two items in a pair. While each object pair was on the screen, participants were asked to rate the quality of their story using a 4-point scale (1 = no story, 2 = poor story, 3 = good story, 4 = best story). Ratings were collected primarily to encourage an elaborative encoding strategy and ensure participants were attending the stimuli during encoding.
Trial onsets were jittered with a variable number (0, 2, 4, 6, or 8) of odd/even digit baseline trials using a sequence optimization program (Dale, 1999) to allow for event-related analyses. Baseline trials consisted of a 2-second presentation of a single digit between 1 and 8 during which participants indicated whether the digit was odd or even. Participants completed six encoding runs, for a total of 48 pairs per condition. To ensure that any observed differences between conditions are not due to idiosyncratic properties of specific stimulus pairings or presentation schedule, we generated six different stimulus presentation schedules, each with different unique object pairings, timing, and stimulus order. Each participant completed one of these schedules.
After the six encoding runs, participants were tested outside the scanner on directly learned associations (48 of each AB, BC, XY) and inferential (48 AC) associations using a 2-alternative forced-choice paradigm (Fig. 1C). In the course of each self-paced test trial, a single cue object (e.g., an A object) was presented at the top of the screen and two object choices—the correct paired associate and a foil object that was studied with a different item than the cue—were presented at the bottom of the screen. The identity of the cue stimulus was randomized across trials; for instance, an AB pair was equally likely to be cued by the A stimulus or the B stimulus. Participants were required to make a decision indicating which of the two choice objects was associated with the cue. Because foils were familiar items encountered in the context of an object distinct from the cue, correct responses required successful retrieval of associative information and could not rely on familiarity-based judgments regarding the two choices. To enable testing of all encoded associations, the same objects appeared twice during the test, once as the correct choice and once as a foil for another pair.
For inferential AC associations, participants were instructed that the association between the cue and the correct choice was indirectly mediated through the shared common object (i.e., B). Foils for these trials were other familiar objects studied during the encoding phase that did not share a common association with the cue. For instance, when an AC trial was cued with the A object, the incorrect choice was a C object from a different ABC triad. As for the directly learned associations, each object served as a response option twice during the inference test, once as the correct choice and once as a foil for another inference trial. Individual inferential AC trials were always presented before the corresponding AB and BC associations to prevent participants from learning AC relationships during the test phase.
fMRI data acquisition
Imaging data were collected on a 3.0 T GE Signa whole-body MRI system (GE Medical Systems, Milwaukee, WI, USA) with an 8-channel head coil. High-resolution, T2-weighted, flow-compensated spin-echo structural images (TR = 3s, TE = 68 ms, 0.43 × 0.43 inplane resolution) were acquired in advance of functional scanning using 20 3-mm thick slices perpendicular to the main axis of the hippocampus to enable visualization of hippocampal subfields and MTL cortical subregions. A high-resolution T2*-sensitive gradient echo spiral in/out pulse sequence (Glover and Law, 2001) was used to gather functional images with the same slice locations as the structural images (TR = 4s, TE = 34 ms, flip angle = 80°, FOV = 22 cm, 1.7 × 1.7 × 3.0 mm resolution). This imaging prescription provided coverage of our regions of interest—MTL and midbrain—at the expense of whole-brain coverage. Therefore, analyses focused solely on the MTL and midbrain. A high-order shimming procedure based on spiral acquisitions was employed to reduce B0 heterogeneity prior to functional scanning.
A total of 648 volumes were acquired for each participant. To obtain a field map for the correction of magnetic field heterogeneity, the echo time of the first time frame of the functional timeseries was 2 ms longer than all subsequent frames. The map for each slice was calculated from the phase of the first two time frames and applied as a first order correction during reconstruction of the functional images, thereby minimizing blurring and geometric distortion on a per-slice bases. Correction for off-resonance due to breathing was applied on a per-time-frame basis as well via phase navigation (Pfeuffer et al., 2002). This initial volume, together with following two volumes of each scan (a total of 12s), was then discarded to allow for T1 stabilization.
fMRI data processing
Data were preprocessed and analyzed using SPM5 (Wellcome Department of Cognitive Neurology, London, UK) and custom MATLAB (MathWorks, MA) routines. Differences in functional image slice acquisition times were corrected for by interpolating the voxel time series using sinc interpolation and resampling the time series with the center slice as a reference point. To account for motion, functional images were realigned to the first volume of the time series. The realigned data were filtered with a 128s high-pass filter, converted to percent signal, and concatenated across the five study runs. Structural images were coregistered to the mean functional image computed during realignment.
First-level, individual participant analyses were performed in the native space of each participant. To allow for optimal spatial localization of group activation patterns within our regions of interest, we used a landmark-based Advanced Normalization Techniques (ANTS: http://picsl.upenn.edu/ANTS) before proceeding with second-level group analyses. First, a model template (1.5 × 1.5 × 1.5 mm resolution) was created from a composite of participants’ structural data centered at the anterior commissure. We then manually segmented an MTL mask—inclusive of bilateral hippocampus and bilateral entorhinal (ERc), perirhinal (PRc), and parahippocampal (PHc) cortices—on each participant’s structural image. The MTL mask guided the generation of linear and non-linear transformation matrices from the participant space to the template space. Transformed structural images were then visually inspected to verify that they provided good alignment of both MTL and midbrain regions across participants. Finally, the transformation parameters were applied to first-level contrast and beta images for group analysis.
Group-level statistical maps were created using an voxel-wise threshold of p < 0.05. Small-volume correction was employed to establish a cluster-level corrected threshold of FDR p < 0.05 to correct for multiple comparisons within our two regions of interest: MTL and dopaminergic midbrain inclusive of ventral tegmental area (VTA) and substantia nigra (SN). For the purpose of thresholding, a group-level MTL mask was created by segmenting the template brain using the same procedures implemented for the segmentation of individual participants’ structural images. Additionally, a group-level midbrain mask was created. Because there are not clear anatomical boundaries that demarcate VTA/SN on MRI scans, we created a mask within anterior midbrain, spanning the posterior end of the red nucleus and the anterior boundary of the midbrain, between the superior and inferior end of the red nucleus (Oades and Halliday, 1987; D'Ardenne et al., 2008; Wolosin et al., 2012). Activation loci within this midbrain mask were then visually inspected to confirm localization. Small-volume correction was determined through Monte Carlo simulations run via the AlphaSim tool in AFNI. Simulations were performed for MTL and midbrain bilaterally. Cluster sizes that occurred with probability less than 0.05 across 5000 simulations were considered significant.
Because we were interested in fine-grain activation patterns within individual MTL subregions, we also manually segmented MTL subregions (ERc, PRc, PHc, and hippocampus) on the template brain based on landmarks proposed for MRI image segmentation based on post-mortem histology (Frankó et al., 2012). When a group-level activation cluster spanned multiple MTL regions, we split that cluster into the individual subregions along the drawn boundaries, and interrogated condition-related responses within each subregion separately. Homologous regions from left and right hemispheres were tested for interregional interaction and collapsed into a single bilateral ROI when no significant condition × region interaction existed. Finally, to test the hypothesis that the CA1 subfield may be preferentially responsive to event overlap, we further manually drew hippocampal subfield boundaries using established procedures (Pruessner et al., 2000; Zeineh et al., 2003; Preston et al., 2010; Wolosin et al., 2013; Schlichting et al., 2014). This segmentation delineated three subregions within the body of the hippocampus in each hemisphere: CA1, a combined dentate gyrus/CA2-3 region (DG/CA2,3), and subiculum. Additionally, we drew anterior hippocampus and posterior hippocampus masks spanning all subfields on the most anterior and posterior slices. Group-level hippocampal activation clusters isolated from our contrasts of interest (see fMRI data analysis below) were then masked with these hippocampal subregions to interrogate the nature of each subfield response.
fMRI data analysis
First-level, individual participant analyses proceeded under the assumptions of the general linear model (Worsley and Friston, 1995). Nine regressors—the first, second, and third repetition of AB, BC, and XY stimulus pairs—along with their temporal derivatives were constructed as stick functions at stimulus onset convolved with a canonical hemodynamic response.
Analysis logic
The current experimental manipulation allowed us to examine how repetition-related changes in activation are modulated by event overlap. Repeated presentations of non-overlapping events would be expected to result in activation decreases throughout MTL (repetition suppression). However, we hypothesized that presentation of shared information across repetitions of overlapping pairs would trigger recruitment of MTL comparison processes that would eliminate repetition suppression or lead to repetition-related signal increases.
We specifically focused on repetition changes across overlapping AB and non-overlapping XY trials. The first presentations of the overlapping (AB) and non-overlapping (XY) pairs are indistinguishable from the participants’ perspective – each pairing represents an association of two novel objects. Subsequent presentations of non-overlapping XY trials are exact repetitions of the first presentation. As XY pairs are encoded, the decreasing novelty of the pairing would therefore be expected to result in repetition suppression within MTL because the encoding demand would be lessened. In contrast, our main hypothesis was that subsequent presentations of AB pairs would not be treated as simply decreasingly novel events. Because of the interleaved presentation of overlapping information (BC), subsequent presentations of AB pairs would recruit additional processes in response to overlapping information. Namely, the shared content across the AB and BC pairs was hypothesized to trigger a comparator/mismatch response within MTL and midbrain that would alter the repetition suppression signal to promote memory integration. Comparing repetition-related activation changes for XY pairs versus AB pairs can thus assess the effect of event overlap on repetition suppression signals in our targeted regions.
Predictions regarding repetition-related changes across BC pairs and their interpretation are less apparent. The first BC presentation, which consists of one novel (C) and one familiar object (B from the first AB presentation), would elicit different processes than the first presentation of AB and XY pairs, both of which consist of two novel objects. Thus, BC is not comparable to either AB or XY. Moreover, because BC pairs are overlapping events from their first instance, we would expect BC pairs to elicit response in regions that are sensitive to event overlap during all presentations, including the first. It is unclear, however, whether engagement of such regions should increase, decrease, or remain constant across BC repetitions. Therefore, we focus our analyses on repetition-related responses for the AB and XY conditions in the main body of the manuscript, with the term “overlapping” from here out referring to AB trials specifically. Exploratory analyses of repetition-related response during BC trials are reported in Supplementary Materials; these analyses show that MTL and midbrain response during BC are intermediate to those observed for AB and XY trials.
Measurement of repetition-related signal change
To assess how event-overlap modulates response to repetition, we took several approaches. First, we used a last versus first presentation contrast to construct a statistical parametric map of repetition-related activation changes (increases or decreases), separately for each condition. In parallel, we also performed an anatomical region of interest (ROI) voxel count analysis, counting the number of voxels that showed significant repetition suppression and repetition enhancement separately within each MTL subregion (without imposing a cluster extent threshold that may mask effects in smaller hippocampal subfields). We then used a Χ2 test to determine whether the number of voxels showing repetition suppression vs. enhancement is modulated by condition.
Isolating the impact of event overlap on repetition-related response
To further quantify the effect of event overlap on repetition suppression, we constructed an interaction contrast testing for the hypothesized difference in repetition-related signal for overlapping and non-overlapping pairs [(XY1 − XY3) + (AB3 − AB1) > 0]. Because multiple activation patterns may give rise to a significant interaction in this contrast (e.g., XY decreases and AB increases, XY decreases and no changes across AB, etc.), we also interrogated each resulting ROI in a follow-up analysis to determine the specific pattern. Beta-weights within each region were extracted for the first and last repetition of overlapping and non-overlapping pairs. For each participant and condition separately, we subtracted activation during the last presentation from activation during first repetition. Across the group, one-sample t-tests were used to assess whether there the activation changes in each condition were significant. We used a paired t-test to confirm that the pattern of activation change across repetitions was different between the overlapping and non-overlapping conditions (which would be expected given that these regions were extracted using the interaction contrast). To address our a prior hypothesis regarding the role of CA1 in the response to overlap, clusters within hippocampus that spanned multiple subfields were further masked by manually drawn hippocampal subfield boundaries and interrogated within each subfield.
Relationship between repetition enhancement and inference
In regions demonstrating repetition enhancement instead of repetition suppression for overlapping pairs, we tested the hypothesis such response enhancement promotes integration across related events. To do so, we performed an across subject correlation assessing the relationship between activation increases across overlapping events and individual differences in AC inference success—our behavioral index of memory integration.
MTL—midbrain connectivity during event overlap
Finally, we examined how interactions between midbrain and MTL regions are modulated by event overlap using psychophysiological interaction (PPI) analysis. First, we reverse-normalized midbrain regions modulated by event-overlap (isolated by the interactions contrast described above) into the native space of each participant using ANTS. After this reverse normalization step, localization of midbrain was visually confirmed for each individual participant. We then used PPI with the midbrain region as a seed to assess changes in connectivity between midbrain and MTL regions across repetitions of overlapping and non-overlapping events. The first level PPI model contained 19 regressors: the mean timecourse of the midbrain seed region, nine regressors for each task condition, and nine regressors for the PPI interaction with midbrain for each task condition. An interaction contrast was created to identify regions with differential midbrain coupling across repetitions of overlapping and non-overlapping events [(XY1 − XY3) + (AB3 − AB1) > 0, involving PPI regressors rather than task condition regressors]. Normalization of the first level contrast and beta images, group level analysis, and interrogation of the resulting clusters for the specific pattern of interaction used the same procedures as described above.
Results
Story ratings during encoding
Story ratings were primarily collected to encourage an elaborative encoding strategy; analysis of these ratings was thus exploratory. The majority of participants rarely used a rating of 4 (best story) during the first presentation or a rating of 1 and 2 (no story or poor story) during the last presentation, suggesting they were successfully elaborating on their story across repetitions. Mean ratings for each repetition and condition (overlapping, non-overlapping) were entered into a repeated measures ANOVA that revealed that ratings linearly increased with repetition (mean rating for presentation one: 2.22; two: 2.64; three: 2.86; F(2,21) = 61.7, p < 0.001). Story ratings did not significantly differ between the overlapping (mean = 2.61) and non-overlapping (mean = 2.54) pairs on average (F(1,21) = 3.26, p = 0.085); however, there was an interaction between condition and repetition, with ratings for overlapping pairs increasing more with repetition than non-overlapping pairs (last-first rating difference: overlapping mean = 0.70, non-overlapping mean = 0.59; F(2,21) = 4.50, p = 0.017). The individual differences in rating increases across overlapping pairs tracked subsequent inference performance (r = 0.37, p = 0.043). While the ratings were initially collected to ensure attention during encoding, in light of these behavioral findings, we opted to perform exploratory analyses relating the repetition-related ratings changes to repetition-related neural changes. We did not find a relationship between ratings and neural response.
Memory performance
All participants successfully learned the trained object pairs (mean = 97% correct, SD = 4%; t(21) = 54.4, p < 0.001). Performance on the inferential associations was also significantly above chance (mean = 89% correct, SD = 13%; t(21) = 14.3, p < 0.001). Average median reaction time for trained associations (mean = 1636 ms, SD = 391 ms) was significantly faster than for inferential associations (mean = 2999 ms, SD = 1325 ms; t(21) = 5.05, p < .001). While memory for the trained associations was consistently high across participants (range 84-100%, only 3 participants below 95%), inferential performance varied widely (range 56-100%). This variability allowed us to investigate the relationship between individual differences in inference performance and individual differences in repetition-related brain activation.
Repetition-related activation changes during encoding of non-overlapping and overlapping events
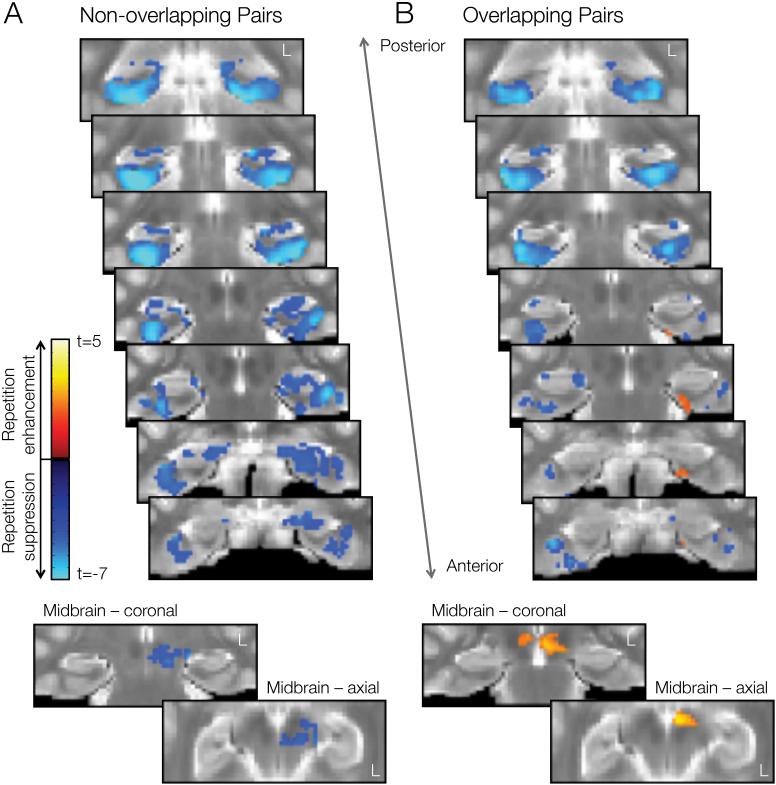
FDR-corrected statistical parametric maps representing regions that showed significant changes in activation across repetitions of non-overlapping events and overlapping events are presented in Figure 2. For non-overlapping events (Fig. 2a), we observed activation decreases in the midbrain and throughout MTL subregions. There were no clusters showing activation increases across repetitions of non-overlapping events. In contrast, for overlapping events (Fig. 2b), activation decreases were less pronounced, in particular within ERc, PRc, and hippocampus. We also observed clusters within ERc, PRc, and midbrain that showed significant activation increases across repetition of overlapping events.
Figure 2.
Activation changes in MTL and midbrain across repeated presentations of object pairs, as measured by differences between the last minus first presentation. Repetition suppression effects (first > last) are depicted in cool colors; repetition enhancements (last > first) in hot colors. A. Repetition-related activation changes for non-overlapping (XY) events. B. Repetition-related activation changes for overlapping (AB) events. Activation patterns are overlaid on coronal slices of the group template image. Left hemisphere is on the right side of the image. Color bars indicate voxelwise t-value.
To complement the statistical parametric maps, we also performed a complementary anatomical ROI analysis, for which we counted voxels that showed significant repetition-related changes (increases or decreases) in each condition and region without imposing a cluster extent threshold. The results are presented in Table 1. As in Fig. 2, we found that event overlap leads to reduced repetition suppression and the emergence of repetition enhancements in ERc, PRc, and midbrain (all Χ2(1) > 133, p < 0.0001). A similar pattern was observed within hippocampus (Χ2(1) = 278.8, p < 0.0001). To address our hypothesis that CA1 should be particularly sensitive to event overlap, we further performed a voxel count analysis in each hippocampal subfield. Increased activation across repetition of overlapping events was primarily driven by changes in CA1 responses (pairwise pattern × region interaction with all other hippocampal subfields, all Χ2(2) > 41.1 , p < 0.0001).
Table 1.
Number of voxels showing significant activation change (either increase or decrease) across repetitions of non-overlapping and overlapping events (a cluster extent threshold was not applied). SUB = subiculum, aHIP = anterior hippocampus, = posterior hippocampus.
| Midbrain | MTL subregions | Hippocampal subfields | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| ERc | PRc | PHc | HIP | CA1 | DG/CA2,3 | SUB | aHIP | pHIP | ||
| Non-overlapping | ||||||||||
|
| ||||||||||
| Increase | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Decrease | 227 | 71 | 733 | 1889 | 950 | 114 | 275 | 141 | 305 | 115 |
|
| ||||||||||
| Overlapping | ||||||||||
|
| ||||||||||
| Increase | 173 | 77 | 110 | 10 | 71 | 60 | 0 | 9 | 2 | 0 |
| Decrease | 1 | 4 | 439 | 1402 | 186 | 11 | 115 | 18 | 12 | 30 |
Regions for which repetition-related response was impacted by event overlap
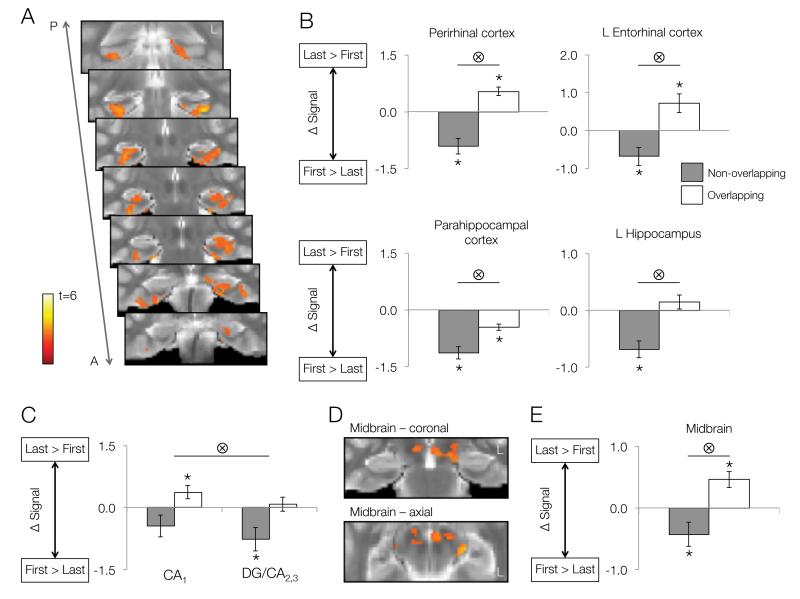
To further isolate regions modulated by event overlap, we also used an interaction contrast [(XY1 − XY3) + (AB3 − AB1) > 0]. The interaction contrast yielded four clusters within MTL (Fig. 3A): left hippocampus (spanning all subfields), left ERc, bilateral PRc, and bilateral PHc. All of these clusters showed the repetition × condition interaction (all F(2,20) > 9.46, p < 0.006) as would be expected. However, the pattern of responses giving rise to the interaction (e.g., repetition-related changes in one condition but not the other) may differ among regions. To specify the pattern of the interaction, we extracted repetition-related responses for the two conditions and for each cluster (Fig. 3B).
Figure 3.
The interaction between repetition and event overlap. A. MTL regions showing a significant interaction between repetition-related activation changes and condition. B. Follow-up ROI analyses illustrating repetition-related activation changes within MTL subregions separately for non-overlapping (gray bars) and overlapping (white bars) events. Bar heights represent group means; error bars represent standard error. Positive values reflect repetition enhancement (last > first), while negative values represent repetition suppression (first > last). Asterisks above or below bars indicate significant repetition-related activation changes for each condition at p < 0.05. Bars with tensor symbols designate a significant interaction between repetition-related activation changes and condition at p < 0.05 (as would be expected based on the contrast). A = Anterior, P = Posterior. C. CA1 and DG/CA2,3 responses to repetition of overlapping and non-overlapping events. Tensor symbol indicates main effect of region. D. Midbrain region showing a significant repetition × condition interaction. E. Follow-up ROI analyses illustrating repetition-related activation changes within midbrain separately for non-overlapping and overlapping events.
In bilateral PRc and left ERc clusters, significant repetition suppression for non-overlapping events was accompanied by repetition enhancements for overlapping events (all t(21) > 2.87, p < 0.01). This pattern indicates that the same voxels within PRc and ERc exhibited repetition suppression or enhancement, depending on whether repeated events shared content with other experiences. In contrast, bilateral PHc showed significant repetition suppression for both overlapping and non-overlapping events (both t(21) > 4.9, p < 0.001). However, the degree of repetition suppression was significantly greater for non-overlapping relative to overlapping events (t(21) = 4.48, p < 0.001). Therefore, while PHc predominantly showed repetition suppression, it was also sensitive to event overlap. An additional interaction region was found in midbrain (Fig. 3D). This region showed similar pattern to the left ERc and bilateral PRc clusters, with significant decreases across repetitions of non-overlapping events (t(21) = 2.14, p = 0.044) alongside significant increases across overlapping events (t(21) = 3.43, p = 0.003; Fig. 3E).
Within hippocampus, a cluster in the left hemisphere showed significant repetition suppression for non-overlapping events (t(21) = 2.91, p = 0.008) and non-significant repetition enhancement for overlapping pairs (t(21) = 1.17, p = 0.25). To investigate whether patterns of responses differed among hippocampal subregions comprising the hippocampal cluster (CA1, DG/CA2,3, subiculum, anterior hippocampus), we further segmented the cluster using manually drawn subregional boundaries. We then submitted the activation changes (last-first presentation) in both conditions and each subregion into a 2 × 4 repeated measures ANOVA. As would be expected, we found a significant main effect of condition on repetition related response across regions (F(1,21) = 8.99, p = 0.007). We also observed a main effect of subregion (F(3,63) = 4.86, p = 0.004). While all subregions showed significant condition × repetition interaction (all F(2,20) > 4.68, p < 0.015), CA1 responses were shifted compared to each of other subregion, as evidenced by a significant main effect of subregion for all pairwise subregion × condition ANOVAs that included CA1 as one subregion and another subfield (DG/CA2,3, subiculum, anterior hippocampus) as a second subregion (all F(1,21) > 6.10, p < 0.022). Specifically, CA1 showed significant repetition enhancement for overlapping events and did not show repetition suppression for non-overlapping events. All other regions showed a different pattern, with significant repetition suppression for non-overlapping events and no change in response across repetitions of overlapping events (see Fig. 3C for CA1 and DG/CA2,3 comparison).
Relationship between repetition-related activation and memory integration
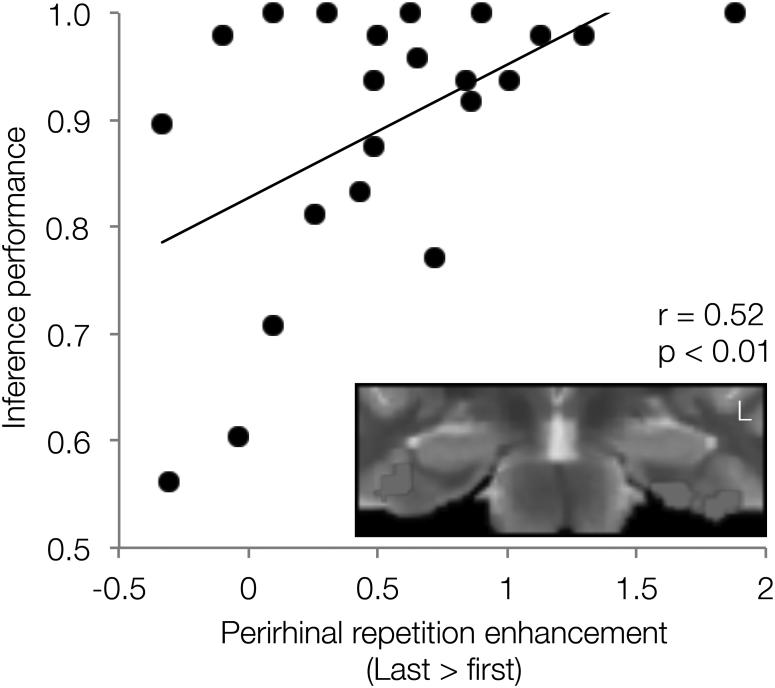
Next, we tested the hypothesis that repetition enhancements unique to overlapping events may promote memory updating processes that integrate information across overlapping events. We performed an across subject correlation assessing the relationship between individual differences in AC inference success—our behavioral index of memory integration—and activation changes across overlapping events in the three regions that showed significant repetition enhancement (bilateral PRc, left ERc, midbrain). Activation increases in bilateral PRc were positively correlated with AC performance (Fig. 4); participants who demonstrated greater repetition enhancement for overlapping events showed superior inference performance (r = 0.52, p = 0.01, p < 0.05 corrected for multiple comparisons). The relationship was not driven by any one participant and remains significant if any one participant is left out (lowest r = 0.42, p = 0.03). Midbrain and left ERc clusters showed a trend in a similar direction but did not reach significance (left ERc r = 0.31, uncorrected p > 0.15; midbrain r = 0.27, uncorrected p > 0.22). Finally, because of our a priori focus on CA1, we also tested the CA1 portion of the left hippocampal cluster and found no relationship between activation increases across overlapping events and inference performance (r = 0.05, p > 0.88).
Figure 4.
PRc repetition enhancement for overlapping events tracks subsequent inference. Scatterplot showing the relationship between PRc repetition enhancement for overlapping events and inference (AC) performance. The bilateral PRc region identified as showing a significant interaction between repetition and condition is shown on the insert (see also Fig. 3A).
Functional interactions between MTL and midbrain during encoding of overlapping events
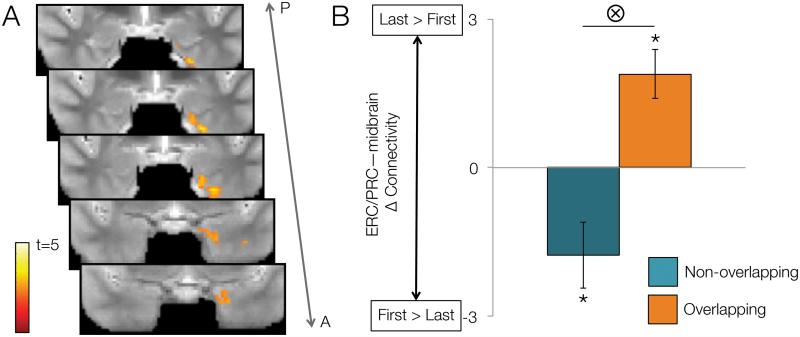
To directly test the notion that MTL—midbrain interactions are preferentially driven by event overlap, we performed a PPI connectivity analysis. Specifically, we sought to identify MTL regions whose connectivity with midbrain showed differential changes across encoding of non-overlapping relative to overlapping pairs. We found a single region spanning left PRc and ERc, whose activation was functionally coupled with that of the midbrain region identified in the interaction contrast (Fig. 5A). Follow-up analysis revealed that connectivity between the PRc/ERc cluster and midbrain significantly increased across repetitions of overlapping associations (t(21) = 3.80, p = 0.001), whereas it significantly decreased across repetitions of non-overlapping associations (t(21) = 4.51, p < 0.001; Fig. 5B).
Figure 5.
Psychophysiological interaction analysis showing connectivity changes between MTL and midbrain as a function of repetition and condition. A. A region spanning left PRc and ERc, showed significantly greater connectivity change for overlapping than non-overlapping pairs (as indexed by differences between first and last repetitions) with a seed midbrain region. Color bar indicates voxelwise t-value. B. Follow-up ROI analyses revealed decreasing connectivity between midbrain and ERc/PRc across repetitions of non-overlapping pairs (green bar), in contrast to increasing connectivity across repetition of overlapping pairs (blue bar). Bar heights represent group means; error bars represent standard error. Asterisks indicate significant repetition-related changes in connectivity at p < 0.05; bar with a tensor symbol designates a significant interaction between repetition-related connectivity changes and condition at p < 0.05.
Discussion
In the present study, we show that engagement of MTL encoding processes depends on how events relate to existing memories. When events were repeated across learning, we found widespread repetition suppression across MTL subregions, consistent with reduced encoding demands for familiar events. However, for events overlapping with other experience, we showed increased responding in PRc, ERc, and CA1, reflecting the need to accommodate new information. These distinct patterns of repetition-related response may reflect two broad MTL memory functions (Olsen et al., 2012): (1) associative binding and (2) memory-based comparison. In particular, increased MTL responses in the face of partial event overlap may reflect a comparator response that signals a mismatch between the current experience and memory-based expectation (Kumaran and Maguire, 2006; 2007; Chen et al., 2011; Duncan et al., 2011). Recent theories propose that mismatch—or associative novelty—signals trigger a specialized memory integration process, whereby existing memories are updated to incorporate new, related information (Shohamy & Wagner, 2008; Preston & Eichenbaum, 2013). Consistent with this hypothesis, we found that PRc repetition enhancement tracked participants’ ability to infer relationships among overlapping events—our behavioral index of integration. Furthermore, we showed that midbrain responses and connectivity with PRc was enhanced across repetitions of overlapping events relative to non-overlapping events. Collectively, these findings indicate that event overlap recruits an MTL—midbrain functional loop typically associated with novelty detection (Lisman and Grace, 2005; Wittmann et al., 2007; Lisman et al., 2011; Kafkas and Montaldi, 2015) to promote new encoding.
The MTL regions demonstrating distinct repetition-related responses were often in close proximity, highlighting the utility of high-resolution fMRI in dissociating distinct MTL subregional computations. Moreover, our ability to isolate responses related to associative binding and memory-based comparison may resolve conflicting findings, which have shown both hippocampal activation increases (Shohamy and Wagner, 2008) and decreases (Zeithamova et al., 2012) related to memory integration. We propose that different patterns of hippocampal engagement in those studies reflect the different fundamental processes ascribed to the hippocampus, with activation decreases reflecting associative binding and activation increases reflecting comparison between present events and related experience. Both processes may be essential to memory integration; once a mismatch is detected, binding processes would serve to link new content to existing representations (Schlichting & Preston, 2015). Notably, these prior studies on memory integration used standard-resolution fMRI techniques combined with analysis strategies that may have prevented detection of multiple, simultaneously engaged processes. For instance in one study (Zeithamova et al., 2012), activation changes were measured across the entire anatomically defined hippocampus, which would conceal heterogeneous response patterns within the region. In another study (Shohamy and Wagner, 2008), the analysis was limited to a single directional hypothesis focused on a comparison signal, wherein the authors searched for regions with activation increases from early to late learning. Here, our experimental manipulation combined with high-resolution fMRI allowed us to dissociate binding and comparison signals during overlapping event encoding to reveal how they engage different MTL subregions.
CA1 in particular has been associated with the hypothesized comparator function of the MTL (Vinogradova, 2001; Vago and Kesner, 2008; Chen et al., 2011; Duncan et al., 2011); this region receives converging input from ERc and CA3, allowing information about current sensory experience from ERc to be processed in reference to memory-based expectations derived from CA3 pattern completion (Lisman & Grace, 2005). When current information differs from stored memory, recent rodent data indicate that CA1 comparator signals trigger memory updating (Lisman and Grace, 2005; Larkin et al., 2014). Human neuroimaging has further shown that CA1 activation during encoding of overlapping events uniquely predicts inference about the relationship among experiences (Schlichting et al., 2014). The present findings extend this work by demonstrating that increased CA1 response during overlapping events reflects a comparator signal specific to overlapping event encoding. Notably, prior studies linking human CA1 responses to a memory-based comparator explicitly required participants to detect physical changes (i.e., mismatches) between study and test probes (Chen et al., 2011; Duncan et al., 2011). For instance, in one such study, CA1 activation linearly tracked the number of altered object configurations within a studied environment (Duncan et al., 2011). Here, we show a CA1 comparator response during repeated presentations of the same physical event, without explicit instruction to detect changes across trials. Our data thus suggest that the CA1 comparator process can be engaged automatically by event overlap.
Interestingly, the present data also show a strong response to event overlap in PRc, a finding that would not be predicted by several current models of MTL function (Brown and Aggleton, 2001; Vinogradova, 2001; Lisman and Grace, 2005). However, our findings converge with other recent fMRI studies, which show associative memory signals in PRc when using object or face stimuli (Chen et al., 2011; Duncan et al., 2011; Davis et al., 2012a). Notably, prior studies that observed hippocampal but not PRc encoding activation during memory integration tasks used pairs of stimuli that included scenes: either objects and scenes (Zeithamova et al., 2012) or faces and scenes (Shohamy and Wagner, 2008). One high-resolution fMRI study that directly compared associative mismatch responses for different types of stimuli observed increased CA1 activation when mismatches were scenes, in contrast to increased PRc activation when mismatches were faces (Chen et al., 2011).
Here, PRc may play an important role in memory-based comparison because all stimuli were common objects (Graham et al., 2010; Watson et al., 2012). In particular, the correlation between the PRc response to event overlap and subsequent inference suggests that PRc can form flexible representations that anticipate the relationships among objects experienced at different times. These findings extend prior work highlighting the role of PRc in representing complex relationships within (Barense et al., 2012; Erez et al., 2016) and among (Alvarado and Bachevalier, 2005; Saksida et al., 2007) objects. In particular, human neuroimaging work has implicated PRc function in the formation of conceptual knowledge about object stimuli (O'Kane et al., 2005; Davis et al., 2012b; 2014). The PRc-mediated integration processes observed in the present study may represent one mechanism through which semantic networks of related concepts are formed.
Midbrain activation and ERc/PRc—midbrain connectivity were also enhanced during presentation of overlapping events. Together with prior work (Shohamy and Wagner, 2008), this finding highlights the role of MTL—midbrain interactions in the formation of flexible memory traces that link experiences. Stereotypically, MTL—midbrain interactions during memory encoding have been studied with paradigms using explicit rewards (Wittmann et al., 2005; Adcock et al., 2006; Wolosin et al., 2012) or feedback-based learning (Shohamy and Wagner, 2008). In particular, midbrain comparator responses have been linked to a “reward prediction error” that signals differences between expected and actual rewards during reinforcement learning (Schultz et al., 1997). Here, we demonstrate overlap-driven midbrain responses in the absence of either reward or explicit feedback, thus providing additional evidence for the general role of this region in novelty detection (Bunzeck and Duzel, 2006) and episodic prediction (Shohamy and Adcock, 2010). While current models of MTL—midbrain interactions emphasize midbrain connections with hippocampus (Lisman and Grace, 2005), dopaminergic modulation of PRc memory function has been observed in non-human primates (Liu et al., 2004), consistent with the pattern of PRc—midbrain connectivity observed in the present study.
The present findings provide further evidence that MTL novelty responses afford a window to MTL subregional functions in memory. By dissociating novelty responses for two different kinds of events, we show that multiple MTL computations operate simultaneously to enable the formation of flexible memory representations. Our data also evince the proposition that memory-based comparison, here supported by CA1 and PRc, plays a key role in memory integration by triggering new encoding and memory updating (Schlichting & Preston, 2015). Moreover, the observed interactions between midbrain and PRc driven by event overlap further extend and refine an influential model of MTL—midbrain interactions during novelty-driven encoding (Lisman and Grace, 2005).
Supplementary Material
Acknowledgments
Grant sponsor: NSF Grant number: CAREER (BCS 1056019)
Grant sponsor: NIH Grant number: R01 MH100121
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-Motivated Learning: Mesolimbic Activation Precedes Memory Formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005;58:218–233. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW, Albasser MM. Contrasting brain activity patterns for item recognition memory and associative recognition memory: insights from immediate-early gene functional imaging. Neuropsychologia. 2012;50:3141–3155. doi: 10.1016/j.neuropsychologia.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Bachevalier J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J Neurosci. 2005;25:1599–1609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Groen IIA, Lee ACH, Yeung L-K, Brady SM, Gregori M, Kapur N, Bussey TJ, Saksida LM, Henson RNA. Intact memory for irrelevant information impairs perception in amnesia. Neuron. 2012;75:157–167. doi: 10.1016/j.neuron.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge DJ, Voss JL. Hippocampal Binding of Novel Information with Dominant Memory Traces Can Support Both Memory Stability and Change. Journal of Neuroscience. 2014;34:2203–2213. doi: 10.1523/JNEUROSCI.3819-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron. 2006;51:369–379. doi: 10.1016/j.neuron.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Chen J, Olsen RK, Preston AR, Glover GH, Wagner AD. Associative retrieval processes in the human medial temporal lobe: Hippocampal retrieval success and CA1 mismatch detection. Learning & Memory. 2011;18:523–528. doi: 10.1101/lm.2135211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R, Guitart-Masip M, Bunzeck N, Dolan RJ, Duzel E. Dopamine modulates episodic memory persistence in old age. J Neurosci. 2012;32:14193–14204. doi: 10.1523/JNEUROSCI.1278-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ardenne K, McClure SM, Nystrom LE, Cohen JD. BOLD Responses Reflecting Dopaminergic Signals in the Human Ventral Tegmental Area. Science. 2008;319:1264–1267. doi: 10.1126/science.1150605. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davis T, Love BC, Preston AR. Striatal and hippocampal entropy and recognition signals in category learning: simultaneous processes revealed by model-based fMRI. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2012a;38:821–839. doi: 10.1037/a0027865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T, Love BC, Preston AR. Learning the exception to the rule: model-based FMRI reveals specialized representations for surprising category members. Cereb Cortex. 2012b;22:260–273. doi: 10.1093/cercor/bhr036. [DOI] [PubMed] [Google Scholar]
- Davis T, Xue G, Love BC, Preston AR, Poldrack RA. Global Neural Pattern Similarity as a Common Basis for Categorization and Recognition Memory. Journal of Neuroscience. 2014;34:7472–7484. doi: 10.1523/JNEUROSCI.3376-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Medial Temporal Lobe Activity during Source Retrieval Reflects Information Type, not Memory Strength. Journal of Cognitive Neuroscience. 2010;22:1808–1818. doi: 10.1162/jocn.2009.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Curtis C, Davachi L. Distinct Memory Signatures in the Hippocampus: Intentional States Distinguish Match and Mismatch Enhancement Signals. Journal of Neuroscience. 2009;29:131–139. doi: 10.1523/JNEUROSCI.2998-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: A high-resolution fMRI study of the human hippocampus. Hippocampus. 2011;22:389–398. doi: 10.1002/hipo.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez J, Cusack R, Kendall W, Barense MD. Conjunctive Coding of Complex Object Features. Cereb Cortex. 2016;26:2271–2282. doi: 10.1093/cercor/bhv081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankó E, Insausti AM, Artacho-Pérula E, Insausti R, Chavoix C. Identification of the human medial temporal lobe regions on magnetic resonance images. Hum Brain Mapp. 2012;35:248–256. doi: 10.1002/hbm.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Memory strength and repetition suppression: multimodal imaging of medial temporal cortical contributions to recognition. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee ACH. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Howard LR, Kumaran D, Ólafsdóttir HF, Spiers HJ. Double dissociation between hippocampal and parahippocampal responses to object-background context and scene novelty. J Neurosci. 2011;31:5253–5261. doi: 10.1523/JNEUROSCI.6055-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Hardt O, Gomez R, Nadel L. The dynamics of memory: Context-dependent updating. Learning & Memory. 2008;15:574–579. doi: 10.1101/lm.1022308. [DOI] [PubMed] [Google Scholar]
- Iordanova MD, Good M, Honey RC. Retrieval-Mediated Learning Involving Episodes Requires Synaptic Plasticity in the Hippocampus. Journal of Neuroscience. 2011;31:7156–7162. doi: 10.1523/JNEUROSCI.0295-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkas A, Montaldi D. Striatal and midbrain connectivity with the hippocampus selectively boosts memory for contextual novelty. Hippocampus. 2015;25:1262–1273. doi: 10.1002/hipo.22434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S, Danckert S, Gati JS, Menon RS. Novelty responses to relational and non-relational information in the hippocampus and the parahippocampal region: A comparison based on event-related fMRI. Hippocampus. 2005;15:763–774. doi: 10.1002/hipo.20098. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Shah AT, DuBrow S, Wagner AD. Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nature Publishing Group. 2010;13:501–506. doi: 10.1038/nn.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. An Unexpected Sequence of Events: MismatchDetection in the Human Hippocampus. Plos Biol. 2006;4:2372–2382. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Match Mismatch Processes Underlie Human Hippocampal Responses to Associative Novelty. Journal of Neuroscience. 2007;27:8517–8524. doi: 10.1523/JNEUROSCI.1677-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. Novelty signals: a window into hippocampal information processing. Trends in Cognitive Sciences. 2009;13:47–54. doi: 10.1016/j.tics.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Larkin MC, Lykken C, Tye LD, Wickelgren JG, Frank LM. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus. 2014;24:773–783. doi: 10.1002/hipo.22268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors gate the acquisition of novel information through hippocampal long-term potentiation and long-term depression. J Neurosci. 2006;26:7723–7729. doi: 10.1523/JNEUROSCI.1454-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon N, Manahan-Vaughan D. Dopamine D1/D5 receptors contribute to de novo hippocampal LTD mediated by novel spatial exploration or locus coeruleus activity. Cereb Cortex. 2012;22:2131–2138. doi: 10.1093/cercor/bhr297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Cullen WK, Anwyl R, Rowan MJ. Dopamine-dependent facilitation of LTP induction in hippocampal CA1 by exposure to spatial novelty. Nat Neurosci. 2003;6:526–531. doi: 10.1038/nn1049. [DOI] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends in Neurosciences. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: Elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001a;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Otmakhova NA. Storage, recall, and novelty detection of sequences by the hippocampus: elaborating on the SOCRATIC model to account for normal and aberrant effects of dopamine. Hippocampus. 2001b;11:551–568. doi: 10.1002/hipo.1071. [DOI] [PubMed] [Google Scholar]
- Liu Z, Richmond BJ, Murray EA, Saunders RC, Steenrod S, Stubblefield BK, Montague DM, Ginns EI. DNA targeting of rhinal cortex D2 receptor protein reversibly blocks learning of cues that predict reward. Proceedings of the National Academy of Sciences. 2004;101:12336–12341. doi: 10.1073/pnas.0403639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Kane G, Insler RZ, Wagner AD. Conceptual and perceptual novelty effects in human medial temporal cortex. Hippocampus. 2005;15:326–332. doi: 10.1002/hipo.20053. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Research. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Olsen RK, Moses SN, Riggs L, Ryan JD. The hippocampus supports multiple cognitive processes through relational binding and comparison. Front Hum Neurosci. 2012;6 doi: 10.3389/fnhum.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otmakhova NA, Lisman JE. D1/D5 dopamine receptors inhibit depotentiation at CA1 synapses via cAMP-dependent mechanism. Journal of Neuroscience. 1998;18:1270–1279. doi: 10.1523/JNEUROSCI.18-04-01270.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer J, Van de Moortele P-F, Ugurbil K, Hu X, Glover GH. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magn Reson Med. 2002;47:344–353. doi: 10.1002/mrm.10065. [DOI] [PubMed] [Google Scholar]
- Preston AR, Bornstein AM, Hutchinson JB, Gaare ME, Glover GH, Wagner AD. High-resolution fMRI of content-sensitive subsequent memory responses in human medial temporal lobe. Journal of Cognitive Neuroscience. 2010;22:156–173. doi: 10.1162/jocn.2009.21195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Shrager Y, Dudukovic NM, Gabrieli JDE. Hippocampal contribution to the novel use of relational information in declarative memory. Hippocampus. 2004;14:148–152. doi: 10.1002/hipo.20009. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiol Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Rainer G. Neural mechanisms for detecting and remembering novel events. Nat Rev Neurosci. 2003;4:193–202. doi: 10.1038/nrn1052. [DOI] [PubMed] [Google Scholar]
- Saksida LM, Bussey TJ, Buckmaster CA, Murray EA. Impairment and facilitation of transverse patterning after lesions of the perirhinal cortex and hippocampus, respectively. Cerebral Cortex. 2007;17:108–115. doi: 10.1093/cercor/bhj128. [DOI] [PubMed] [Google Scholar]
- Schlichting ML, Preston AR. Memory integration: neural mechanisms and implications for behavior. Current Opinion in Behavioral Sciences. 2015;1:1–8. doi: 10.1016/j.cobeha.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichting ML, Zeithamova D, Preston AR. CA 1subfield contributions to memory integration and inference. Hippocampus. 2014;24:1248–1260. doi: 10.1002/hipo.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH. Activation of Midbrain Structures by Associative Novelty and the Formation of Explicit Memory in Humans. Learning & Memory. 2004;11:383–387. doi: 10.1101/lm.75004. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends in Cognitive Sciences. 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Shohamy D, Wagner AD. Integrating Memories in the Human Brain: Hippocampal-Midbrain Encoding of Overlapping Events. Neuron. 2008;60:378–389. doi: 10.1016/j.neuron.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan Giovanello K, Schnyer DM, Verfaellie M. A critical role for the anterior hippocampus in relational memory: Evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Tompary A, Duncan K, Davachi L. Consolidation of Associative and Item Memory Is Related to Post-Encoding Functional Connectivity between the Ventral Tegmental Area and Different Medial Temporal Lobe Subregions during an Unrelated Task. J Neurosci. 2015;35:7326–7331. doi: 10.1523/JNEUROSCI.4816-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cerebral Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Simon MG, Sederberg PB. Scene representations in parahippocampal cortex depend on temporal context. J Neurosci. 2012;32:7202–7207. doi: 10.1523/JNEUROSCI.0942-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago DR, Kesner RP. Disruption of the direct perforant path input to the CA1 subregion of the dorsal hippocampus interferes with spatial working memory and novelty detection. Behav Brain Res. 2008;189:273–283. doi: 10.1016/j.bbr.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannini P, Hedden T, Sullivan C, Sperling RA. Differential functional response in the posteromedial cortices and hippocampus to stimulus repetition during successful memory encoding. Hum Brain Mapp. 2013;34:1568–1578. doi: 10.1002/hbm.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradova OS. Hippocampus as comparator: role of the two input and two output systems of the hippocampus in selection and registration of information. Hippocampus. 2001;11:578–598. doi: 10.1002/hipo.1073. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Chun MM, Kuhl BA. Repetition suppression and multi-voxel pattern similarity differentially track implicit and explicit visual memory. J Neurosci. 2013;33:14749–14757. doi: 10.1523/JNEUROSCI.4889-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson HC, Wilding EL, Graham KS. A role for perirhinal cortex in memory for novel object-context associations. J Neurosci. 2012;32:4473–4481. doi: 10.1523/JNEUROSCI.5751-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Bunzeck N, Dolan RJ, Duzel E. Anticipation of novelty recruits reward system and hippocampus while promoting recollection. NeuroImage. 2007;38:194–202. doi: 10.1016/j.neuroimage.2007.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann BC, Schiltz K, Boehler CN, Duzel E. Mesolimbic interaction of emotional valence and reward improves memory formation. Neuropsychologia. 2008;46:1000–1008. doi: 10.1016/j.neuropsychologia.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze H-J, Duzel E. Reward-Related fMRI Activation of Dopaminergic Midbrain Is Associated with Enhanced Hippocampus-Dependent Long-Term Memory Formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Reward Modulation of Hippocampal Subfield Activation during Successful Associative Encoding and Retrieval. Journal of Cognitive Neuroscience. 2012;24:1532–1547. doi: 10.1162/jocn_a_00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosin SM, Zeithamova D, Preston AR. Distributed hippocampal patterns that discriminate reward context are associated with enhanced associative binding. Journal of Experimental Psychology: General. 2013;142:1264–1276. doi: 10.1037/a0033609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Friston KJ. Analysis of fMRI time-series revisited--again. NeuroImage. 1995;2:173–181. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Widaman K, Mungas D, Reed B, Weiner MW, Chui HC. Memory in the aging brain: doubly dissociating the contribution of the hippocampus and entorhinal cortex. Hippocampus. 2007;17:1134–1140. doi: 10.1002/hipo.20341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. Hippocampal and Ventral Medial Prefrontal Activation during Retrieval-Mediated Learning Supports Novel Inference. Neuron. 2012;75:168–179. doi: 10.1016/j.neuron.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeithamova D, Preston AR. Flexible Memories: Differential Roles for Medial Temporal Lobe and Prefrontal Cortex in Cross-Episode Binding. Journal of Neuroscience. 2010;30:14676–14684. doi: 10.1523/JNEUROSCI.3250-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweynert S, Pade JP, Wüstenberg T, Sterzer P, Walter H, Seidenbecher CI, Richardson-Klavehn A, Duzel E, Schott BH. Motivational salience modulates hippocampal repetition suppression and functional connectivity in humans. Front Hum Neurosci. 2011;5:144. doi: 10.3389/fnhum.2011.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.