Abstract
Background
Obstructive sleep apnoea is a common disorder with under-rated clinical impact, which is increasingly being recognised as having a major bearing on global disease burden. Men are especially vulnerable and become a priority group for preventative interventions. However, there is limited information on prevalence of the condition in Australia, its co-morbidities, and potential risk factors.
Methods
We used data from 13,423 adult men included in the baseline wave of Ten to Men, an Australian national study of the health of males, assembled using stratified cluster sampling with oversampling from rural and regional areas. Those aged 18–55 years self-completed a paper-based questionnaire that included a question regarding health professional-diagnosed sleep apnoea, physical and mental health status, and health-related behaviours. Sampling weights were used to account for the sampling design when reporting the prevalence estimates. Odds ratios were used to describe the association between health professional-diagnosed sleep apnoea and potential correlates while adjusting for age, country of birth, and body-mass index (BMI).
Results
Prevalence of self-reported health professional-diagnosed sleep apnoea increased from 2.2 % in age 18–25 years to 7.8 % in the age 45–55 years. Compared with those without sleep apnoea, those with sleep apnoea had significantly poorer physical, mental, and self-rated health as well as lower subjective wellbeing and poorer concentration/remembering (p < 0.001 for all). Sleep apnoea was significantly associated with older age (p < 0.001), unemployment (p < 0.001), asthma (p = 0.011), chronic obstructive pulmonary disease/chronic bronchitis (p = 0.002), diabetes (p < 0.001), hypercholesterolemia (p < 0.001), hypertension (p < 0.001), heart attack (p < 0.001), heart failure (p < 0.001), angina (p < 0.001), depression (p < 0.001), post-traumatic stress disorder (p < 0.001), other anxiety disorders (p < 0.001), schizophrenia (p = 0.002), overweight/obesity (p < 0.001), insufficient physical activity (p = 0.006), smoking (p = 0.005), and high alcohol consumption (p < 0.001).
Conclusion
Health professional-diagnosed sleep apnoea is relatively common, particularly in older males. Associations between sleep apnoea and cardiovascular, metabolic, respiratory, and psychiatric disorders have important clinical and public health implications. As men are especially vulnerable to sleep apnoea as well as some of its chronic co-morbidities, they are potentially a priority group for health interventions. Modifiable lifestyle related factors such as smoking, alcohol consumption, level of physical activity and BMI are possible key foci for interventions.
Background
Chronic respiratory disorders contribute significantly to the global burden of disease, the recognition of which prompted their inclusion among the four priorities of the 2008–2013 World Health Organization’s action plan on non-communicable diseases [1]. Chronic obstructive pulmonary disease (COPD) is the 4th leading cause of death, and others such as asthma are increasing in prevalence affecting hundreds of millions at some stage in their lives [2], while obstructive sleep apnoea (the prevalence of the other type of sleep apnoea, i.e., central sleep apnoea is very low in the population [3–5]) is also a common but under-diagnosed chronic respiratory disorder [6–8]. It is increasingly being recognised as having a significant impact on global burden of disease [9]. However, the evidence on the prevalence and associations of obstructive sleep apnoea in Australian (and New Zealand) populations are limited [10–14]. A recent study showed that one in ten Australians suffer from undiagnosed obstructive sleep apnoea [13]; in the males aged 40–69 years this could be as high as 49 % and in males aged >70 years, as high as 62 % [10]. It is becoming a major public health concern with its increasing prevalence and complications [9, 15–18].
Most advances in knowledge of obstructive sleep apnoea have taken place only recently [19]; it is now suggested that it may overlap with other chronic respiratory diseases; a substantial overlap occurs between obstructive sleep apnoea and COPD or asthma [20, 21]. The prevalence of obstructive sleep apnoea varies in different populations and different age groups, becoming markedly increased in older age [22, 23]. It is commoner in obese individuals, as well as in those with underlying comorbidities (such as cardiovascular diseases, cerebrovascular diseases, and metabolic abnormalities), people with a family history of obstructive sleep apnoea, and those with anatomically compromised upper airways. Men are more likely to develop obstructive sleep apnoea and the diseases that it complicates [9, 17, 23, 24]. There is, however, a dearth of evidence on prevalence of obstructive sleep apnoea and its co-morbidities in Australian men.
Using cross-sectional data from the baseline wave of Ten to Men (The Australian Longitudinal Study on Male Health) we aimed to describe the prevalence of self-reported, doctor-/health professional- diagnosed sleep apnoea (hereafter referred to as sleep apnoea) and its overlap with other chronic respiratory diseases, its association with measures of health status and quality of life, and potential correlates among Australian men.
Methods
Study design and setting
Ten to Men is a longitudinal cohort study of Australian males aged between 10 and 55 years at the time of recruitment (October 2013 to July 2014). Participants were recruited from major cities as well as regional and rural areas. The target population was all Australian male citizens or permanent residents living in private dwellings except those residing in areas classified as remote or very remote.
Sample and sampling process
We used multi-stage stratified cluster sampling with oversampling of regional areas to increase their representation within the cohort. We approached 104,884 dwellings, of which 32 % met eligibility criteria. Of these, 13,697 (41 %) households returned usable data. Of the 45,510 males confirmed as meeting eligibility criteria, 15,988 (35 %) returned usable data. The setting, design, sampling, study instruments, data collection process, and participants’ characteristics of the Ten to Men study is described elsewhere in this issue [25].
Study instruments
Adult participants answered a self-completed paper-based questionnaire, a copy of which is available at the Ten to Men website [26]. The questionnaire sought details of the participants’ socio-demographic characteristics, physical and mental health status, health-related behaviours, family and social life, and health service use. Wherever possible, the items were from validated scales or questions used in other large health studies.
Data collection
Interviewers made up to three attempts (including at least once at a weekend) to contact each household and ascertain the presence of eligible males. A study pack, including consent forms and questionnaires, was left for each eligible individual in a household, which were collected about a week later.
Definitions
We defined sleep apnoea as self-reported, doctor-or other health professional- diagnosed sleep apnoea during life. This was based on the question “Has a doctor or other health professional ever told you that you had this condition? (Sleep apnoea)”. We used the same question to define the other chronic respiratory diseases (asthma, COPD (“emphysema or chronic obstructive pulmonary disease”), and chronic bronchitis).
Self-rated health was defined using the question “In general, would you say your health is excellent/very good/good/fair/poor” from the Short Form 12 (SF12) [27]. Cognition was assessed by the response to the question “Do you have difficulty remembering or concentrating?” taken from the Washington Group on Disability Statistics’ Short set of Questions on Disability [28]. We used the items of the SF-12 and Personal Wellbeing Index for Adults (English PWI-A, 4th edition) [29] to measure participants’ physical and mental health and their subjective wellbeing, respectively. Presence/absence of non-respiratory co-morbidities (eczema, diabetes, high cholesterol, high blood pressure, heart attack, heart failure, angina, stroke, depression, post-traumatic stress disorder, other anxiety disorders, schizophrenia, and cancer) was ascertained in the same manner as for sleep apnoea using the same question. As this paper is exploratory in nature, we included all reported cardiovascular, metabolic, respiratory, and hypersensitivity disorders as well as cancer in the analysis of correlates. We used self-reported height and weight to derive body-mass index (BMI). Level of physical activity was assessed using the Active Australia survey [30] and sitting time by questions on time spent sitting on work and non-work days [31].
Statistical methods
Stata SE 13.1 (Stata Corp LP, College Station, TX, USA) was used for data analysis. When estimating prevalence, we accounted for the multi-stage sampling, stratification and selection probabilities (including varying response fractions by primary sampling unit) by using Stata’s “survey” commands and sampling weights [32]. For associations between sleep apnoea and other characteristics, no account was taken of the clustering, stratification and sampling weights [32]. We compared the distribution of other variables among those with and without sleep apnoea using bivariate statistics (χ 2 test and t-test). We used logistic regression to estimate crude odds ratios to measure associations between possible correlates and sleep apnoea, and odds ratios adjusted for the possible confounding effects of age, country of birth, and BMI. Because the prevalence of sleep apnoea was less than 10 %, the prevalence odds ratios are reasonably accurate estimates of prevalence ratios.
Results
Prevalence of sleep apnoea and overlap with other chronic respiratory disorders
Of the 13,423 respondents, 710 reported as being diagnosed to have sleep apnoea by a doctor or another health professional. The prevalence of sleep apnoea increased with age from 2.0 % (95 % CI 1.4 %-2.9 %) in those aged 18–25 years to 3.7 % (95 % CI 2.8 %–4.8 %), 6.6 % (95 % CI 5.6 %–7.8 %), and 7.8 % (95 % CI 6.7 %–9.0 %) in those aged 26–35, 36–45, and 46–55 years, respectively. Sleep apnoea without other comorbid chronic respiratory disorders increased from 1.4 % in 18–25 years age-group to 6.1 % in 46–55 years age-group (Fig. 1). Combination of sleep apnoea and asthma increased from 0.6 % in the youngest to 1.2 % in the oldest age-group. The overlap of sleep apnoea and COPD/chronic bronchitis was relatively low, occurring only in those aged over 35 years. The proportion with sleep apnoea and both above other conditions was also very low (<0.1 % in all ages). Of those with sleep apnoea, 14.2 % had concurrent asthma but only 0.01 % and 0.02 %, respectively, had concurrent COPD/Chronic bronchitis and both asthma and COPD/Chronic bronchitis.
Fig. 1.
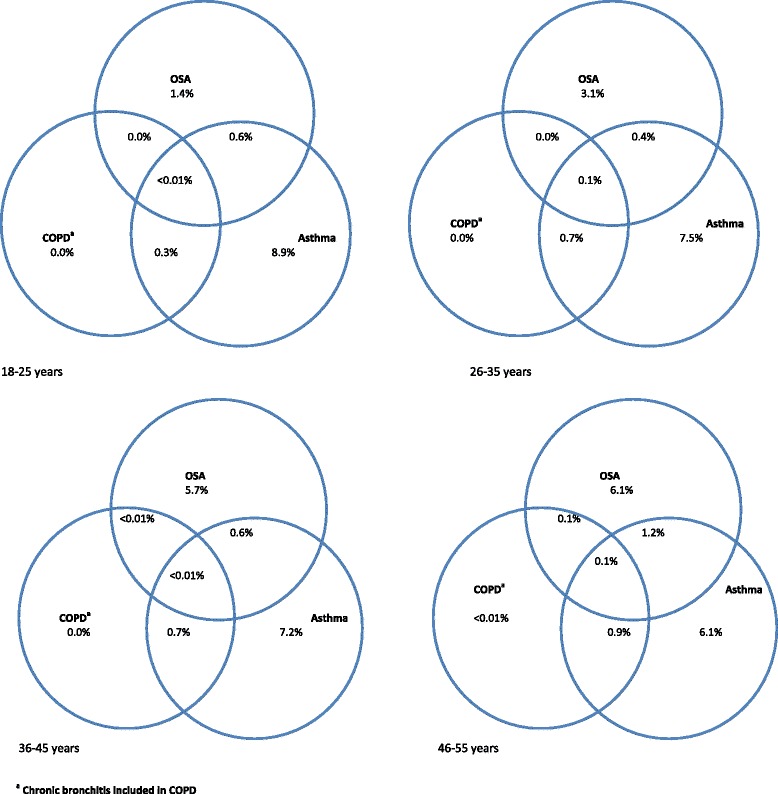
Lifetime prevalence of sleep apnoea and other chronic respiratory diseases adjusted for the sampling design
The impact of sleep apnoea on health and wellbeing
Men with doctor-/health professional- diagnosed sleep apnoea were more likely to report fair or poor health status and difficulty in remembering or concentrating than those without sleep apnoea (Table 1). Those with sleep apnoea also had lower SF-12 physical health and mental health summary scores as well as subjective wellbeing scores compared with those without sleep apnoea.
Table 1.
Differences in self-rated health and wellbeing by sleep apnoea status
| Health and wellbeing measure | Lifetime doctor- or other health professional- diagnosed sleep apnoea | ||
|---|---|---|---|
| Sleep apnoea | No sleep apnoea | ||
| Health | |||
| N (% within sleep apnoea category) | p | ||
| Self-rated health status | |||
| Excellent/very good/good | 524 (74.3 %) | 11669 (92.0 %) | <0.001a |
| Fair/Poor | 181 (25.7 %) | 1009 (8.0 %) | |
| Difficulty in remembering or concentrating | |||
| No difficulty | 365 (51.8 %) | 8736 (69.2 %) | <0.001a |
| Some difficulty | 282 (40.1 %) | 3485 (27.6 %) | |
| A lot of difficulty or cannot do at all | 57 (8.1 %) | 409 (3.2 %) | |
| Mean (SD) | |||
| SF-12 Physical Component Score | 50.0 (9.9) | 54.2 (7.1) | <0.001b |
| SF-12 Mental Component Score | 46.3 (10.6) | 50.1 (9.1) | <0.001b |
| Wellbeing | |||
| Subjective Wellbeing Score | 63.6 (19.2) | 70.8 (16.9) | <0.001b |
aBased on χ 2 test
bBased on t test
Correlates of sleep apnoea
Compared with those aged 18–25 years, the association between doctor-/health professional- diagnosed sleep apnoea and age showed a 1.6, 2.4, and 3.1 fold increased odds, respectively, in those aged 26–35, 36–45, and 46–55 years (Table 2). Although being born in an Asian country had significantly lower odds of sleep apnoea in the univariate analysis, this was no longer significant when adjusted for age and BMI. Marital status and level of education were not significantly associated with sleep apnoea. Significantly more men with sleep apnoea (11.0 %) were neither working nor looking for work compared with those without sleep apnoea (5.6 %). Among those who were employed, the number of hours worked and shift work did not significantly differ between sleep apnoea and non-sleep apnoea groups.
Table 2.
Associations between socio-demographic characteristics and sleep apnoea
| Characteristic | Lifetime doctor- or other health professional- diagnosed sleep apnoea | Odds ratio (95 % CI) | p | Adjusteda Odds ratio (95 % CI) | p | |
|---|---|---|---|---|---|---|
| Sleep apnoea | Total | |||||
| N (%b) | ||||||
| Age | ||||||
| 18–25 years | 48 (2.2 %) | 2219 (100.0 %) | 1.0 | 1.0 | ||
| 26–35 years | 112 (3.6 %) | 3158 (100.0 %) | 1.7 (1.2, 2.3) | 0.004 | 1.6 (1.1, 2.4) | 0.016 |
| 36–45 years | 246 (6.0 %) | 4095 (100.0 %) | 2.9 (2.1, 4.0) | <0.001 | 2.4 (1.6, 3.4) | <0.001 |
| 46–55 years | 304 (7.7 %) | 3951 (100.0 %) | 3.8 (2.8, 5.1) | <0.001 | 3.1 (2.1, 4.4) | <0.001 |
| Country of birth | ||||||
| Australia | 584 (5.7 %) | 10258 (100.0 %) | 1.0 | 1.0 | ||
| New Zealand, Polynesia, Micronesia, or Melanesia | 23 (4.6 %) | 503 (100.0 %) | 0.7 (0.5, 1.2) | 0.289 | 0.7 (0.5, 1.2) | 0.193 |
| Central, East, South or South-East Asia | 35 (3.0 %) | 1175 (100.0 %) | 0.5 (0.4, 0.7) | <0.001 | 0.7 (0.5, 1.1) | 0.098 |
| Middle-East (West Asia) or Africa | 14 (3.6 %) | 387 (100.0 %) | 0.6 (0.4, 1.1) | 0.085 | 0.8 (0.4, 1.3) | 0.356 |
| Europe or North America | 54 (5.3 %) | 1025 (100.0 %) | 0.9 (0.7, 1.2) | 0.575 | 0.8 (0.6, 1.2) | 0.315 |
| South America, Central America or Caribbean | 0 (0.0 %) | 64 (100.0 %) | - | |||
| Marital status | ||||||
| Never married | 121 (3.4 %) | 3508 (100.0 %) | 1.0 | 1.0 | ||
| Widowed/divorced/separated | 63 (7.3 %) | 865 (100.0 %) | 2.2 (1.6, 3.0) | <0.001 | 1.2 (0.8, 1.8) | 0.297 |
| Married/de facto relationship | 522 (5.8 %) | 8950 (100.0 %) | 1.7 (1.4, 2.1) | <0.001 | 1.2 (0.9, 1.5) | 0.278 |
| Highest Educational Level | ||||||
| Completed secondary school or less | 176 (5.4 %) | 3279 (100.0 %) | 1.0 | 1.0 | ||
| Tertiary certificate, diploma, trade qualifications or other similar qualifications | 341 (5.7 %) | 6020 (100.0 %) | 1.0 (0.9, 1.3) | 0.550 | 0.9 (0.8, 1.2) | 0.513 |
| Graduate or post-graduate | 149 (4.5 %) | 3299 (100.0 %) | 0.8 (0.7, 1.0) | 0.112 | 1.0 (0.8, 1.3) | 0.906 |
| Employment Status | ||||||
| Employed | 566 (5.0 %) | 11286 (100.0 %) | 1.0 | 1.0 | ||
| Unemployed and looking for work | 58 (5.3 %) | 1093 (100.0 %) | 1.1 (0.8, 1.4) | 0.674 | 1.2 (0.9, 1.7) | 0.265 |
| Neither working nor looking for work | 77 (9.9 %) | 777 (100.0 %) | 2.1 (1.6, 2.7) | <0.001 | 1.9 (1.5, 2.6) | <0.001 |
| Number of hours worked per week | ||||||
| Up to 20 h | 27 (4.0 %) | 681 (100.0 %) | 1.00 | 1.00 | ||
| 21–40 h | 205 (4.7 %) | 4377 (100.0 %) | 1.2 (0.8, 1.8) | 0.405 | 1.0 (0.6, 1.5) | 0.896 |
| More than 40 h | 257 (5.1 %) | 5054 (100.0 %) | 1.3 (0.9, 1.9) | 0.207 | 1.0 (0.6, 1.5) | 0.860 |
| Main job has night shift | ||||||
| No | 418 (4.9 %) | 8536 (100.0 %) | 1.0 | 1.0 | ||
| Yes | 138 (5.3 %) | 2595 (100.0 %) | 1.1 (0.9, 1.3) | 0.389 | 1.1 (0.8, 1.3) | 0.611 |
aAdjusted for age, country of birth and BMI
bRow percentages
Those with doctor-/health professional- diagnosed sleep apnoea had significantly higher odds of a number of co-morbidities (Table 3). The means (and standard deviations) of the total number of lifetime chronic respiratory diseases, cardiovascular or metabolic diseases, and psychiatric disorders in those with and without sleep apnoea were 0.4 (0.6) vs 0.3 (0.5), 0.9 (1.2) vs 0.4 (0.7), and 0.8 (1.0) vs 0.3 (0.7), respectively (p < 0.001 for all). Sleep apnoea group had significantly increased odds of all co-morbidities included in univariate analyses (Table 3), and all these associations with the exception of stroke and cancer remained significantly high after adjusting for age, country of birth and BMI. Sleep apnoea was associated with a 4.5 fold increased odds of heart failure and over 2 fold increased odds of psychiatric disorders, heart attack, angina, and diabetes. The association between higher BMI and sleep apnoea remained significant after adjusting for country of birth and age. The strength of this association consistently increased from OR 1.7 in the overweight group to OR 10.3 in the morbidly obese (Obesity III) group.
Table 3.
Associations between self-reported comorbidities and sleep apnoea
| Co-morbidities | Lifetime doctor- or other health professional- diagnosed sleep apnoea status Mean (SD) |
OR (95 % CI) | p | Adjusteda OR (95 % CI) | p | |
|---|---|---|---|---|---|---|
| Sleep apnoea N (%b) |
Total | |||||
| Lifetime disease status | ||||||
| Eczema | 103 (7.4 %) | 1395 (100.0 %) | 1.5 (1.2, 1.9) | <0.001 | 1.6 (1.3, 2.0) | <0.001 |
| Asthma | 194 (6.6 %) | 2944 (100.0 %) | 1.4 (1.2, 1.6) | <0.001 | 1.3 (1.0, 1.6) | 0.011 |
| COPD or chronic bronchitis | 72 (10.5 %) | 683 (100.0 %) | 2.3 (1.8, 3.0) | <0.001 | 1.6 (1.2, 2.1) | 0.002 |
| Diabetes | 87 (18.2 %) | 477 (100.0 %) | 4.4 (3.5, 5.7) | <0.001 | 2.4 (1.8, 3.2) | <0.001 |
| High cholesterol | 220 (10.0 %) | 2202 (100.0 %) | 2.5 (2.1, 2.9) | <0.001 | 1.7 (1.4, 2.1) | <0.001 |
| High blood pressure | 235 (10.7 %) | 2197 (100.0 %) | 2.7 (2.3, 3.2) | <0.001 | 1.6 (1.4, 2.0) | <0.001 |
| Heart attack | 34 (21.7 %) | 157 (100.0 %) | 5.2 (3.5, 7.7) | <0.001 | 2.8 (1.8, 4.4) | <0.001 |
| Heart failure | 23 (28.8 %) | 80 (100.0 %) | 7.6 (4.6, 124) | <0.001 | 4.5 (2.6, 8.0) | <0.001 |
| Angina | 29 (17.8 %) | 163 (100.0 %) | 4.1 (2.7, 6.1) | <0.001 | 2.6 (1.6, 4.1) | <0.001 |
| Stroke | 13 (15.1 %) | 86 (100.0 %) | 3.3 (1.8, 6.0) | <0.001 | 1.9 (0.9, 3.9) | 0.096 |
| Depression | 297 (11.3 %) | 2629 (100.0 %) | 3.2 (2.8, 3.8) | <0.001 | 2.7 (2.3, 3.2) | <0.001 |
| PTSD | 63 (14.6 %) | 433 (100.0 %) | 3.3 (2.5, 4.4) | <0.001 | 2.6 (1.9, 3.5) | <0.001 |
| Other anxiety disorders | 183 (11.7 %) | 1567 (100.0 %) | 2.9 (2.4, 3.5) | <0.001 | 2.7 (2.2, 3.3) | <0.001 |
| Schizophrenia | 14 (14.9 %) | 94 (100.0 %) | 3.2 (1.8, 5.7) | <0.001 | 2.9 (1.5, 5.7) | 0.002 |
| Cancer | 34 (9.3 %) | 366 (100.0 %) | 1.9 (1.3, 2.8) | <0.001 | 1.4 (0.9, 1.2) | 0.092 |
| BMI category (kg/m2) | ||||||
| Normal or underweight (up to 24.9) | 81 (2.1 %) | 3910 (100.0 %) | 1.0 | |||
| Overweight (25.0–29.9) | 224 (4.4 %) | 5062 (100.0 %) | 2.2 (1.7, 2.8) | <0.001 | 1.7 (1.4, 2.4) | <0.001 |
| Obese 1 (30.0–34.9) | 167 (8.6 %) | 1951 (100.0 %) | 4.4 (3.4, 5.8) | <0.001 | 3.6 (2.7, 4.7) | <0.001 |
| Obese 2(35.0–39.9) | 93 (15.4 %) | 606 (100.0 %) | 8.6 (6.3, 11.7) | <0.001 | 7.0 (5.1, 9.6) | <0.001 |
| Obese 3(> = 40) | 58 (20.7 %) | 280 (100.0 %) | 12.4 (8.6, 17.8) | <0.001 | 10.3 (7.1, 14.9) | <0.001 |
aAdjusted for age, country of birth and BMI
bRow percentages
Insufficient physical activity and sitting time significantly increased the odds of doctor-/health professional- diagnosed sleep apnoea (Table 4). Each one hour increase in sitting time per day was associated with an increased odds of 1.04 for sleep apnoea (95 % CI 1.02, 1.07; p < 0.001). This odds remained significantly increased after adjusting for age, country of birth and BMI (OR 1.03; 95 % CI 1.005, 1.06; p = 0.023). When the average sitting time was dichotomised as less than 6 h vs. 6 h or more [33], the odds of sleep apnoea for those with more sitting time increased by 1.2 fold. Despite the significantly increased odds of sleep apnoea when being an ex-smoker and a current smoker, the strength of association between sleep apnoea and lifetime smoking exposure showed no discernible pattern across different levels of lifetime smoking exposure (Table 4). The association between sleep apnoea and high alcohol use months was also significant.
Table 4.
Associations of lifestyle factors and health-related behaviour with sleep apnoea
| Characteristic | Proportion with lifetime sleep apnoea status | OR (95 % CI) | p | Adjusteda OR (95 % CI) | p | |
|---|---|---|---|---|---|---|
| Sleep apnoea | Total | |||||
| Lifestyle-related factors | N (%b) | |||||
| Level of Physical activity | ||||||
| Sufficient | 352 (4.5 %) | 7773 (100.0 %) | 1.0 | 1.0 | ||
| Sedentary/Insufficient | 304 (7.0 %) | 4330 (100.0 %) | 1.6 (1.4, 1.9) | <0.001 | 1.3 (1.1, 1.5) | 0.006 |
| Sitting time | ||||||
| < 6 h per day | 277 (4.7 %) | 5949 (100.0 %) | 1.0 | 1.0 | ||
| > = 6 h per day | 318 (5.7 %) | 5590 (100.0 %) | 1.2 (1.0, 1.5) | 0.012 | 1.2 (1.0, 1.4) | 0.036 |
| Smoking status | ||||||
| Never smoked | 319 (4.3 %) | 7364 (100.0 %) | 1.0 | |||
| Ex-smoker | 220 (6.8 %) | 3227 (100.0 %) | 1.6 1.4, 1.9) | <0.001 | 1.2 (1.0, 1.5) | 0.021 |
| Currently smoking | 163 (6.2 %) | 2636 (100.0 %) | 1.5 (1.2, 1.8) | <0.001 | 1.4 (1.1, 1.7) | 0.005 |
| Lifetime smoking exposure | ||||||
| Never smoked | 319 (4.3 %) | 7364 (100.0 %) | 1.0 | 1.0 | ||
| Up to 5 pack-years | 74 (4.3 %) | 1716 (100.0 %) | 1.0 (0.8, 1.3) | 0.971 | 1.1 (0.8, 1.4) | 0.593 |
| 6–10 pack-years | 49 (4.8 %) | 1014 (100.0 %) | 1.1 (0.8, 1.5) | 0.466 | 1.0 (0.7, 1.4) | 0.971 |
| 11–20 pack-years | 103 (8.0 %) | 1290 (100.0 %) | 1.9 (1.5, 2.4) | <0.001 | 1.6 (1.3, 2.1) | <0.001 |
| 21–40 pack-years | 86 (7.6 %) | 1139 (100.0 %) | 1.8 (1.4, 2.3) | <0.001 | 1.2 (0.9, 1.6) | 0.167 |
| > 40 pack-years | 47 (13.3 %) | 354 (100.0 %) | 3.4 (2.4, 6.7) | <0.001 | 1.9 (1.3, 2.8) | 0.001 |
| Alcohol use months | ||||||
| Low (<8) | 433 (5.0 %) | 8660 (100.0 %) | 1.0 | 1.0 | ||
| Medium (8–15) | 184 (5.2 %) | 3515 (100.0 %) | 1.0 (0.9, 1.2) | 0.593 | 1.1 (0.9, 1.3) | 0.291 |
| High (> = 16) | 87 (7.6 %) | 1137 (100.0 %) | 1.6 (1.2, 2.0) | <0.001 | 1.7 (1.3, 2.2) | <0.001 |
aAdjusted for age, country of birth and BMI
bRow percentages
Discussion
The estimated population prevalence of doctor-/health professional- diagnosed sleep apnoea among Australian men aged 18–25 years to 46–55 years increased from 2.0 % to 7.8 %. Men with sleep apnoea had poorer mental, physical, and self-rated health as well as low subjective wellbeing and difficulty in concentration or remembering. Presence of thirteen of the fifteen co-morbidities examined were significantly increased with sleep apnoea, as were insufficient physical activity, sitting time, smoking, and high alcohol consumption.
Our study has several limitations. While it was designed to be representative of the Australian male population aged 10–55 years, the response fraction was low. Although the sampling weights we used can account to some extent for non-response, bias is still possible when estimating population prevalence. The ‘sleep apnoea’ definition we used did not discriminate between obstructive vs. central sleep apnoea. Therefore, it is difficult to compare our findings directly with previous studies that are predominantly on obstructive sleep apnoea. However, as the population prevalence of central sleep apnoea is known to be very low [3–5], our definition is unlikely to have a major effect on our findings. The cross sectional associations identified in our study do not allow any conclusions about causation or temporality. In addition, there is also the possibility that our inability to accurately determine whether the reported doctor-/health professional- diagnosed sleep apnoea is being treated might have introduced a bias we cannot account for as there are some evidence that treatment of obstructive sleep apnoea can help better control some co-morbidities [34–36]. However, we do not expect this to be a major limitation as the determination of both sleep apnoea and the co-morbidities were based on ‘ever-having’ the disease rather than being a current event. We did not objectively measure sleep apnoea or other co-morbidities, which are likely to have influenced the prevalence estimates as well as the associations between sleep apnoea and co-morbidities. Although we used the variable ‘country of birth’ as a crude proxy for ethnicity in adjusting the odds ratios for associations, it may not necessarily be a reliable marker of ethnicity. Furthermore, our study also lacked some key indices of sleep apnoea burden such as sleepiness and motor vehicle crashes.
The age-specific prevalence of sleep apnoea in our study is much lower than both the age-specific [12, 37, 38] and age-standardised [13] prevalence of obstructive sleep apnoea that has been reported in other previous studies. As some people with sleep apnoea can be asymptomatic, and as only those who are symptomatic would generally seek treatment leading to diagnosis by a health professional, the proportion of health professional-diagnosed sleep apnoea tends to be less than the actual population prevalence at any given time. Therefore, the ‘self-reported doctor-/health professional- diagnosed’ definition of sleep apnoea used in our study compared with the sleep measurement-based objective definitions used in the above studies is likely to have led to lower estimate in our study, reflecting under-reporting related to under-diagnosis of obstructive sleep apnoea, which has been found by others to be highly prevalent [13]. The prevalence of 7.8 % among those aged 46–55 years in our study is also lower than 11.2 % reported for previously diagnosed obstructive sleep apnoea in another cohort of Australian men aged >40 years, likely due to inclusion of comparatively older men in that cohort (including those aged >70 years) [10]. Given this gradual increase in the sleep apnoea prevalence with advancing age, it would be important to study the older age groups further to determine the prevalence among them and resulting burden of disease. Studies on prevalence and co-morbidities among females will also provide evidence needed to better plan the possible preventative interventions.
Prevalence of concurrent asthma or COPD among those with doctor-/health professional- diagnosed sleep apnoea was lower in our study than what has been previously reported for those with obstructive sleep apnoea [39–42]. This difference could be explained by the participants in those studies being older than our sample on the average, as most chronic respiratory diseases increase with age [43–45]. Lower prevalence in our study may also be due to possible under-reporting resulting from the use of aforementioned definition for sleep apnoea and other diseases. If this lower prevalence does reflect under-diagnosis, it could indicate an increase in future burden of the disease as sleep apnoea concurrent with asthma or COPD leads to higher morbidity and mortality [41, 46] and underdiagnosed diseases usually present later with more complications.
Our results confirm the previously reported positive associations between obstructive sleep apnoea and ageing [14, 37, 47–49] and BMI [8, 50–52]. However, our results on the positive association between sleep apnoea and heart failure are in contrast to the recent findings from the Atherosclerosis Risk in the Communities Study and the Sleep Heart Health Study [52] as well as the Osteoporotic Fractures in Men Study [53], which showed no association between obstructive sleep apnoea/sleep-disordered breathing and heart failure. Those studies were on older men, and therefore, are not comparable with the younger age distribution in our sample. Other literature, however, suggests that obstructive sleep apnoea is a risk factor for heart failure [54–56] concurring with our findings. It is also possible that some of the reported doctor-/health professional- diagnosed sleep apnoea in our study is central sleep apnoea (rather than obstructive sleep apnoea), which is known to be a consequence of heart failure [56]. However, 60–78 % of sleep apnoea in heart failure is reported in recent studies to be obstructive sleep apnoea [57, 58].
The associations we found between sleep apnoea and other co-morbidities also confirm the positive associations of obstructive sleep apnoea which previously have been reported for cardiovascular diseases [55, 59–63], metabolic disorders [64–68], and psychiatric disorders [69–72]. Although the association with schizophrenia (OR 2.9) is difficult to interpret due to the wide confidence interval, the association with depression and anxiety disorders (OR 2.7) are robust and may indicate an impending compound burden on the healthcare system as the prevalence of these latter diseases themselves are high and increasing [73–77].
The association between doctor-/health professional- diagnosed sleep apnoea and stroke in our study, nevertheless, was not significant after adjusting for age, country of birth, and BMI. Even with similar adjustments, the significant association between the obstructive sleep apnoea and stroke had remained in the Sleep Heart Health Study participants [78]. Participants in that study, however, were aged approximately 50 years or more when this association was assessed. As stroke is more a disease of older ages [79–81], inclusion of younger men in our study as well as inadequate power to detect an association due to the low frequency of stroke would have attenuated its association with sleep apnoea.
Our study also adds to the existing evidence on some putative associations. The previous evidence for the association between smoking and obstructive sleep apnoea has been equivocal, the positive association found by smaller studies [82–84] being disputed by findings of much larger studies [85]. Overall, the evidence favours a positive association, and our findings confirm this. Similar conflicts of evidence exist with regard to the association between obstructive sleep apnoea and alcohol consumption [38, 86–89], which overall favours a positive association, again concurring with our findings.
Conclusion
The estimated prevalence of doctor-/health professional- diagnosed sleep apnoea and its associations with cardiovascular, metabolic, respiratory, and psychiatric disorders have important clinical and public health implications for Australia. As men are especially vulnerable to some of the chronic diseases that are associated with sleep apnoea, they represent a relevant group for targeted health interventions. While it is not possible to make any direct causal inferences from our study, it is reasonable to consider modifiable lifestyle-related factors such as smoking, alcohol consumption, level of physical activity and BMI to be potential foci for such interventions. These findings may help inform public health policy and clinical disease screening protocols.
Acknowledgements
The research on which this paper is based on was conducted as part of the Australian Longitudinal Study on Male Health by the University of Melbourne. We are grateful to the Australian Government Department of Health for funding and to the boys and men who provided survey data.
Declaration
Publication of this article was funded by the Ten to Men Study. This article has been published as part of BMC Public Health Vol 16 Suppl 3, 2016: Expanding the knowledge on male health: findings from the Australian Longitudinal Study on Male Health (Ten to Men). The full contents of the supplement are available online at https://bmcpublichealth.biomedcentral.com/articles/supplements/volume-16-supplement-3.
Availability of data and materials
Ten to Men response data are available to researchers via a request and review process. Information on accessing Ten to Men data is available at http://www.tentomen.org.au/index.php/researchers.html. Copies of Wave 1 questionnaires, Wave 1 data books, and the Ten to Men Data User’s Manual are also available at that site.
Enquires about potential collaborations including sub-studies involving members of the Ten to Men cohort can be addressed to the Study Coordinator at info@tentomen.org.au.
Authors’ contributions
CS, GH, JP, MM and SD were responsible for the analytical design. SS undertook data analysis. CS, DE, DC, JP, AL, CL, MR, MM, GH and CD were involved in interpreting the analysis. CS drafted the manuscript. All authors undertook critical revision of the manuscript and have approved this manuscript version for submission.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The Australian Longitudinal Study on Male Health was approved by the University of Melbourne Human Research Ethics Committee (HREC 1237897 & 1237376). Participants provided written consent for their participation.
Abbreviations
- BMI
Body-mass index
- COPD
Chronic obstructive pulmonary disease
Footnotes
Garun S. Hamilton and Shyamali C. Dharmage are equal senior authors.
Contributor Information
Chamara Visanka Senaratna, Email: chamaravs@sjp.ac.lk.
Dallas R. English, Email: d.english@unimelb.edu.au
Dianne Currier, Email: dianne.currier@unimelb.edu.au.
Jennifer L. Perret, Email: j.perret@student.unimelb.edu.au
Adrian Lowe, Email: lowea@unimelb.edu.au.
Caroline Lodge, Email: clodge@unimelb.edu.au.
Melissa Russell, Email: melissar@unimelb.edu.au.
Sashane Sahabandu, Email: sashane.sahabandu@unimelb.edu.au.
Melanie C. Matheson, Email: mcmat@unimelb.edu.au
Garun S. Hamilton, Email: garun.hamilton@monashhealth.org
Shyamali C. Dharmage, Email: s.dharmage@unimelb.edu.au
References
- 1.Bousquet J, Kiley J, Bateman ED, Viegi G, Cruz AA, Khaltaev N, Aït Khaled N, Baena-Cagnani CE, Barreto ML, Billo N, et al. Prioritised research agenda for prevention and control of chronic respiratory diseases. Eur Respir J. 2010;36(5):995–1001. doi: 10.1183/09031936.00012610. [DOI] [PubMed] [Google Scholar]
- 2.Ferkol T, Schraufnagel D. The global burden of respiratory disease. Ann Am Thorac Soc. 2014;11(3):404–406. doi: 10.1513/AnnalsATS.201311-405PS. [DOI] [PubMed] [Google Scholar]
- 3.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: Effects of gender. Am J Respir Crit Care Med. 2001;163(3 I):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 4.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: I. Prevalence and severity. Am J Respir Crit Care Med. 1998;157(1):144–148. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 5.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 6.Fuhrman C, Fleury B, Nguyên X-L, Delmas M-C. Symptoms of sleep apnea syndrome: high prevalence and underdiagnosis in the French population. Sleep Med. 2012;13(7):852–858. doi: 10.1016/j.sleep.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Johansson K, Hemmingsson E, Harlid R, Trolle Lagerros Y, Granath F, Rössner S, Neovius M. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. BMJ. 2011;342(7809). http://dx.doi.org/10.1136/bmj.d3017. [DOI] [PMC free article] [PubMed]
- 8.Heinzer R, Vat S, Marques-Vidal P, Marti-Soler H, Andries D, Tobback N, Mooser V, Preisig M, Malhotra A, Waeber G, et al. Prevalence of sleep-disordered breathing in the general population: THE HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appleton SL, Vakulin A, McEvoy RD, Vincent A, Martin SA, Grant JF, Taylor AW, Antic NA, Catcheside PG, Wittert GA, et al. Undiagnosed obstructive sleep apnea is independently associated with reductions in quality of life in middle-aged, but not elderly men of a population cohort. Sleep Breath. 2015;19(4):1309–1316. doi: 10.1007/s11325-015-1171-5. [DOI] [PubMed] [Google Scholar]
- 11.Bearpark H, Elliott L, Grunstein R, Hedner J, Cullen S, Schneider H, Althaus W, Sullivan C. Occurrence and correlates of sleep disordered breathing in the Australian town of Busselton: a preliminary analysis. Sleep. 1993;16(8 Suppl):S3–5. [PubMed] [Google Scholar]
- 12.Mihaere KM, Harris R, Gander PH, Reid PM, Purdie G, Robson B, Neill A. Obstructive sleep apnea in new zealand adults: prevalence and risk factors among Maori and non-Maori. Sleep. 2009;32(7):949–956. doi: 10.1093/sleep/32.7.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simpson L, Hillman DR, Cooper MN, Ward KL, Hunter M, Cullen S, James A, Palmer LJ, Mukherjee S, Eastwood P. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath. 2013;17(3):967–973. doi: 10.1007/s11325-012-0785-0. [DOI] [PubMed] [Google Scholar]
- 14.Olson LG, King MT, Hensley MJ, Saunders NA. A community study of snoring and sleep-disordered breathing: Prevalence. Am J Respir Crit Care Med. 1995;152(2):711–716. doi: 10.1164/ajrccm.152.2.7633731. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton GS, Solin P, Naughton MT. Obstructive sleep apnoea and cardiovascular disease. Intern Med J. 2004;34:420–426. doi: 10.1111/j.1445-5994.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 16.Pretto JJ, Gyulay SG, Hensley MJ. Trends in anthropometry and severity of sleep-disordered breathing over two decades of diagnostic sleep studies in an Australian adult sleep laboratory. Med. J. Aust. 2010;193(4):213–216. doi: 10.5694/j.1326-5377.2010.tb03870.x. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-de-la-Torre M, Campos-Rodriguez F, Barbé F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir Med. 2013;1(1):61–72. doi: 10.1016/S2213-2600(12)70051-6. [DOI] [PubMed] [Google Scholar]
- 18.Punjabi N, Sorkin J, Katzel L, Goldberg A, Schwartz A, Smith P. Sleep-disordered breathing and insulin resistance in middle-aged and overweight men. Am J Respir Crit Care Med. 2002;165(5):677–682. doi: 10.1164/ajrccm.165.5.2104087. [DOI] [PubMed] [Google Scholar]
- 19.Pack AI. Advances in sleep-disordered breathing. Am J Respir Crit Care Med. 2006;173(1):7–15. doi: 10.1164/rccm.200509-1478OE. [DOI] [PubMed] [Google Scholar]
- 20.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 21.Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap Syndrome. Proc Am Thorac Soc. 2008;5(2):237–241. doi: 10.1513/pats.200706-077MG. [DOI] [PubMed] [Google Scholar]
- 22.Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33(4):907–914. doi: 10.1183/09031936.00180108. [DOI] [PubMed] [Google Scholar]
- 23.Redline S. Epidemiology of Sleep-Disordered Breathing. Semin. Respir. Crit. Care Med. 1998;19(2):113–122. doi: 10.1055/s-2007-1009388. [DOI] [Google Scholar]
- 24.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 25.Currier D, Schlichthorst M, Koelmeyer R, English D, Pirkis J. The Australian Longitudinal Study on Male Health – Methods. BMC Public Health. 2016.doi:10.1186/s12889-016-3698-1. [DOI] [PMC free article] [PubMed]
- 26.Ten to Men: Data Documentation; Questionnaires for Wave 1. http://www.tentomen.org.au/index.php/Researchers/data-doc.html]. Accessed 22 Nov 2015.
- 27.Ware J, Jr, Kosinski M, Turner-Bowker D, Gandek B. How to score version 2 of the SF-12 Health Survey (with a supplement documenting version 1) Lincoln: QualityMetric Incoprorated; 2002. [Google Scholar]
- 28.Short set of Questions on Disability: Census Questions on Disability Endorsed by the Washington Group. https://www.cdc.gov/nchs/data/washington_group/wg_short_measure_on_disability.pdf. Accessed 22 Nov 2015.
- 29.The International Wellbeing Group . Personal Wellbeing Index. Melbourne: Australian Centre on Quality of Life, Deakin University; 2006. [Google Scholar]
- 30.Australian Institute of Health and Welfare . The Active Australia Survey: a guide and manual for implementation, analysis and reporting. Canberra: Australian Institute of Health and Welfare; 2003. [Google Scholar]
- 31.Women's Health Australia . Women's Health Australia (2010 Mid Survey No 6) Newcastle and Brisbane: The University of Newcastle Australia and The University of Queensland Australia; 2010. [Google Scholar]
- 32.Spittal MJ, Carlin JB, Currier D, Downes M, English D, Gordon I, Pirkis J, Gurrin L. Ten to Men sampling design and survey weighting: Implications for analysis and interpretation of clustered data. BMC Public Health. 2016. in press. [DOI] [PMC free article] [PubMed]
- 33.Hadgraft NT, Lynch BM, Clark BK, Healy GN, Owen N, Dunstan DW. Excessive sitting at work and at home: Correlates of occupational sitting and TV viewing time in working adults. BMC Public Health. 2015;15:899. doi: 10.1186/s12889-015-2243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu X, Fan J, Chen S, Yin Y, Zrenner B. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 2015;17(3):215–222. doi: 10.1111/jch.12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen L, Pei JH, Chen HM. Effects of continuous positive airway pressure treatment on glycaemic control and insulin sensitivity in patients with obstructive sleep apnoea and type 2 diabetes: a meta-analysis. Arch Med Sci. 2014;10(4):637–642. doi: 10.5114/aoms.2014.44854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kasai T, Yumino D, Redolfi S, Su MC, Ruttanaumpawan P, Mak S, Newton GE, Floras JS, Bradley TD. Overnight Effects of Obstructive Sleep Apnea and Its Treatment on Stroke Volume in Patients With Heart Failure. Can J Cardiol. 2015;31(7):832–838. doi: 10.1016/j.cjca.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LRA. Obstructive Sleep Apnea Syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Bearpark H, Elliott L, Grunstein R, Cullen S, Schneider H, Althaus W, Sullivan C. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med. 1995;151(5):1459–1465. doi: 10.1164/ajrccm.151.5.7735600. [DOI] [PubMed] [Google Scholar]
- 39.Soler X, Gaio E, Powell FL, Ramsdell JW, Loredo JS, Malhotra A, Ries AL. High prevalence of obstructive sleep apnea in patients with moderate to severe Chronic Obstructive Pulmonary Disease. Ann Am Thorac Soc. 2015;12(8):1219–1225. doi: 10.1513/AnnalsATS.201407-336OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zamarrón C, Paz VG, Morete E, del Campo MF. Association of chronic obstructive pulmonary disease and obstructive sleep apnea consequences. Int J Chron Obstruct Pulmon Dis. 2008;3(4):671–682. doi: 10.2147/COPD.S4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Budhiraja R, Siddiqi TA, Quan SF. Sleep disorders in chronic obstructive pulmonary disease: etiology, impact, and management. J Clin Sleep Med. 2015;11(3):259–270. doi: 10.5664/jcsm.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Julien JY, Martin JG, Ernst P, Olivenstein R, Hamid Q, Lemiere C, Pepe C, Naor N, Olha A, Kimoff RJ. Prevalence of obstructive sleep apnea-hypopnea in severe versus moderate asthma. J. Allergy Clin. Immunol. 2009;124(2):371–376. doi: 10.1016/j.jaci.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Oraka E, Kim HJ, King ME, Callahan DB. Asthma prevalence among US elderly by age groups: age still matters. J Asthma. 2012;49(6):593–599. doi: 10.3109/02770903.2012.684252. [DOI] [PubMed] [Google Scholar]
- 44.Lindberg A, Eriksson B, Larsson LG, Ronmark E, Sandstrom T, Lundback B. Seven-year cumulative incidence of COPD in an age-stratified general population sample. Chest. 2006;129(4):879–885. doi: 10.1378/chest.129.4.879. [DOI] [PubMed] [Google Scholar]
- 45.Jarad N. Chronic obstructive pulmonary disease (COPD) and old age? Chron Respir Dis. 2011;8(2):143–151. doi: 10.1177/1479972311407218. [DOI] [PubMed] [Google Scholar]
- 46.Teodorescu M, Broytman O, Curran-Everett D, Sorkness RL, Crisafi G, Bleecker ER, Erzurum S, Gaston BM, Wenzel SE, Jarjour NN. Obstructive Sleep Apnea risk, asthma burden, and lower airway inflammation in adults in the Severe Asthma Research Program (SARP) II. J Allergy Clin Immunol Pract. 2015;3(4):566–575.e561. doi: 10.1016/j.jaip.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee SD, Kang SH, Ju G, Han JW, Kim TH, Lee CS, Kim T, Kim KW, Yoon IY. The prevalence of and risk factors for sleep-disordered breathing in an elderly Korean population. Respiration. 2014;87(5):372–378. doi: 10.1159/000358442. [DOI] [PubMed] [Google Scholar]
- 48.Plywaczewski R, Bednarek M, Jonczak L, Zielinski J. Sleep-disordered breathing in a middle-aged and older Polish urban population. J Sleep Res. 2008;17(1):73–81. doi: 10.1111/j.1365-2869.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 49.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cui R, Tanigawa T, Nakano H, Sakurai S, Yamagishi K, Ohira T, Iso H. Associations between weight change since 20 years of age and sleep-disordered breathing among male truck drivers. Int J Obes (Lond) 2009;33(12):1396–1401. doi: 10.1038/ijo.2009.192. [DOI] [PubMed] [Google Scholar]
- 51.Togeiro SM, Carneiro G, Ribeiro Filho FF, Zanella MT, Santos-Silva R, Taddei JA, Bittencourt LRA, Tufik S. Consequences of obstructive sleep apnea on metabolic profile: A population-based survey. Obesity. 2013;21(4):847–851. doi: 10.1002/oby.20288. [DOI] [PubMed] [Google Scholar]
- 52.Roca GQ, Redline S, Claggett B, Bello N, Ballantyne CM, Solomon SD, Shah AM. Sex-Specific association of sleep apnea severity with subclinical myocardial injury, ventricular hypertrophy, and heart failure risk in a community-dwelling cohort: The Atherosclerosis Risk in Communities-Sleep Heart Health Study. Circulation. 2015;132(14):1329–1337. doi: 10.1161/CIRCULATIONAHA.115.016985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Javaheri S, Blackwell T, Ancoli-Israel S, Ensrud KE, Stone KL, Redline S. Sleep Disordered Breathing and incident heart failure in older men. Am J Respir Crit Care Med. 2015;193(5):561–568. [DOI] [PMC free article] [PubMed]
- 54.Vijayan VK. Morbidities associated with obstructive sleep apnea. Expert Rev Respir Med. 2012;6(5):557–566. doi: 10.1586/ers.12.44. [DOI] [PubMed] [Google Scholar]
- 55.Monahan K, Redline S. Role of obstructive sleep apnea in cardiovascular disease. Curr Opin Cardiol. 2011;26(6):541–547. doi: 10.1097/HCO.0b013e32834b806a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasai T. Sleep apnea and heart failure. J Cardiol. 2012;60(2):78–85. [DOI] [PubMed]
- 57.Herrscher TE, Akre H, Overland B, Sandvik L, Westheim AS. High prevalence of sleep apnea in heart failure outpatients: even in patients with preserved systolic function. J Card Fail. 2011;17(5):420–425. doi: 10.1016/j.cardfail.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Ferreira S, Marinho A, Patacho M, Santa-Clara E, Carrondo C, Winck J, Bettencourt P. Prevalence and characteristics of sleep apnoea in patients with stable heart failure: Results from a heart failure clinic. BMC Pulm Med. 2010;10:9. doi: 10.1186/1471-2466-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, Redline S, Resnick HE, Tong EK, Diener-West M, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. 2010;122(4):352–360. doi: 10.1161/CIRCULATIONAHA.109.901801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shah NA, Yaggi HK, Concato J, Mohsenin V. Obstructive sleep apnea as a risk factor for coronary events or cardiovascular death. Sleep Breath. 2010;14(2):131–136. doi: 10.1007/s11325-009-0298-7. [DOI] [PubMed] [Google Scholar]
- 62.Mohsenin V. Obstructive sleep apnea and hypertension: a critical review. Curr Hypertens Rep. 2014;16(10):482. doi: 10.1007/s11906-014-0482-4. [DOI] [PubMed] [Google Scholar]
- 63.Tamura A, Kawano Y, Ando S, Watanabe T, Kadota J. Association between coronary spastic angina pectoris and obstructive sleep apnea. J Cardiol. 2010;56(2):240–244. doi: 10.1016/j.jjcc.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 64.Kawano Y, Tamura A, Kadota J. Association between the severity of obstructive sleep apnea and the ratio of low-density lipoprotein cholesterol to high-density lipoprotein cholesterol. Metab. Clin. Exp. 2012;61(2):186–192. doi: 10.1016/j.metabol.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Xu H, Guan J, Yi H, Zou J, Meng L, Tang X, Zhu H, Yu D, Zhou H, Su K, et al. Elevated low-density lipoprotein cholesterol is independently associated with obstructive sleep apnea: evidence from a large-scale cross-sectional study. Sleep Breath. 2015;20(2):627–34. [DOI] [PubMed]
- 66.Kent BD, Grote L, Ryan S, Pepin JL, Bonsignore MR, Tkacova R, Saaresranta T, Verbraecken J, Levy P, Hedner J, et al. Diabetes mellitus prevalence and control in sleep-disordered breathing: The European Sleep Apnea Cohort (ESADA) study. Chest. 2014;146(4):982–990. doi: 10.1378/chest.13-2403. [DOI] [PubMed] [Google Scholar]
- 67.Pamidi S, Tasali E. Obstructive sleep apnea and type 2 diabetes: is there a link? Front. Neurol. 2012;3:126. doi: 10.3389/fneur.2012.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rajan P, Greenberg H. Obstructive sleep apnea as a risk factor for type 2 diabetes mellitus. J Nat Sci Sleep. 2015;7:113–125. doi: 10.2147/NSS.S90835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rezaeitalab F, Moharrari F, Saberi S, Asadpour H, Rezaeetalab F. The correlation of anxiety and depression with obstructive sleep apnea syndrome. J Res Med Sci. 2014;19(3):205–210. [PMC free article] [PubMed] [Google Scholar]
- 70.Ejaz SM, Khawaja IS, Bhatia S, Hurwitz TD. Obstructive Sleep Apnea and Depression: A Review. Innov Clin Neurosci. 2011;8(8):17–25. [PMC free article] [PubMed] [Google Scholar]
- 71.Annamalai A, Palmese LB, Chwastiak LA, Srihari VH, Tek C. High rates of obstructive sleep apnea symptoms among patients with schizophrenia. Psychosomatics. 2015;56(1):59–66. doi: 10.1016/j.psym.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Peppard PE, Szklo-Coxe M, Hla KM, Young T. Longitudinal association of sleep-related breathing disorder and depression. Arch Intern Med. 2006;166(16):1709–1715. doi: 10.1001/archinte.166.16.1709. [DOI] [PubMed] [Google Scholar]
- 73.Kilkkinen A, Kao-Philpot A, O'Neil A, Philpot B, Reddy P, Bunker S, Dunbar J. Prevalence of psychological distress, anxiety and depression in rural communities in Australia. Aust. J. Rural Health. 2007;15(2):114–119. doi: 10.1111/j.1440-1584.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 74.Hidaka BH. Depression as a disease of modernity: explanations for increasing prevalence. J Affect Disord. 2012;140(3):205–214. doi: 10.1016/j.jad.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Andersen I, Thielen K, Bech P, Nygaard E, Diderichsen F. Increasing prevalence of depression from 2000 to 2006. Scand J Public Health. 2011;39(8):857–863. doi: 10.1177/1403494811424611. [DOI] [PubMed] [Google Scholar]
- 76.Haller H, Cramer H, Lauche R, Gass F, Dobos GJ. The prevalence and burden of subthreshold generalized anxiety disorder: a systematic review. BMC Psychiatry. 2014;14:128. doi: 10.1186/1471-244X-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Starcevic V. Review: worldwide lifetime prevalence of anxiety disorders is 16.6%, with considerable heterogeneity between studies. Evid Based Ment Health. 2006;9(4):115. doi: 10.1136/ebmh.9.4.115. [DOI] [PubMed] [Google Scholar]
- 78.Redline S, Yenokyan G, Gottlieb DJ, Shahar E, O'Connor GT, Resnick HE, Diener-West M, Sanders MH, Wolf PA, Geraghty EM, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182(2):269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gupta A, Shukla G, Mohammed A, Goyal V, Behari M. Restless legs syndrome, a predictor of subcortical stroke: a prospective study in 346 stroke patients. Sleep Med. 2015. http://dx.doi.org/10.1016/j.sleep.2015.05.025. [DOI] [PubMed]
- 80.Zhao Y, Condon J, You J, Guthridge S, He V. Assessing improvements in survival for stroke patients in the Northern Territory 1992-2013: a marginal structural analysis. Aust Health Rev. 2015;39(4):437–43. [DOI] [PubMed]
- 81.Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, Flaherty ML, Khatri P, Ferioli S, De Los Rios La Rosa F, et al. Age at stroke: Temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79(17):1781–1787. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kashyap R, Hock LM, Bowman TJ. Higher prevalence of smoking in patients diagnosed as having obstructive sleep apnea. Sleep Breath. 2001;5(4):167–172. doi: 10.1055/s-2001-18805. [DOI] [PubMed] [Google Scholar]
- 83.Kim KS, Kim JH, Park SY, Won HR, Lee HJ, Yang HS, Kim HJ. Smoking induces oropharyngeal narrowing and increases the severity of obstructive sleep apnea syndrome. J Clin Sleep Med. 2012;8(4):367–374. doi: 10.5664/jcsm.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wetter DW, Young TB, Bidwell TR, Badr MS, Palta M. Smoking as a risk factor for sleep-disordered breathing. Arch Intern Med. 1994;154(19):2219–2224. doi: 10.1001/archinte.1994.00420190121014. [DOI] [PubMed] [Google Scholar]
- 85.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 86.Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982;45(4):353–359. doi: 10.1136/jnnp.45.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Udwadia ZF, Doshi AV, Lonkar SG, Singh CI. Prevalence of sleep-disordered breathing and sleep apnea in middle-aged urban Indian men. Am J Respir Crit Care Med. 2004;169(2):168–173. doi: 10.1164/rccm.200302-265OC. [DOI] [PubMed] [Google Scholar]
- 88.Jennum P, Sjol A. Snoring, sleep apnoea and cardiovascular risk factors: the MONICA II Study. Int J Epidemiol. 1993;22(3):439–444. doi: 10.1093/ije/22.3.439. [DOI] [PubMed] [Google Scholar]
- 89.Peppard PE, Austin D, Brown RL. Association of alcohol consumption and sleep disordered breathing in men and women. J Clin Sleep Med. 2007;3(3):265–270. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Ten to Men response data are available to researchers via a request and review process. Information on accessing Ten to Men data is available at http://www.tentomen.org.au/index.php/researchers.html. Copies of Wave 1 questionnaires, Wave 1 data books, and the Ten to Men Data User’s Manual are also available at that site.
Enquires about potential collaborations including sub-studies involving members of the Ten to Men cohort can be addressed to the Study Coordinator at info@tentomen.org.au.


