Abstract
Objective
Self-reported sleep disturbances may confer elevated risk for suicidal ideation, suicide attempts, and death. However, limited research has evaluated polysomnography (PSG)-determined sleep disturbance as an acute physiological risk factor for suicidal thoughts. This study sought to investigate the relationship between nocturnal wakefulness in association with next-day suicidal ideation using overnight PSG assessment from data collected between 2006 and 2013.
Method
Participants with DSM-IV-diagnosed major depressive disorder (MDD) or bipolar depression underwent overnight PSG monitoring in a sleep laboratory. The Hamilton Depression Rating Scale (HAM-D) was administered the morning after PSG recording to assess next-day suicidal ideation, severity of depressive symptoms, and subjective sleep disturbances.
Results
Using a generalized linear mixed model, a significant time-by-ideation interaction was found indicating greater nocturnal wakefulness at 4:00 AM among participants with suicidal ideation (F(4,136) = 3.65, p = .007). Increased time awake during the 4:00 AM hour (4:00 to 4:59) was significantly associated with elevated suicidal thoughts the next day (standardized β = .31, p = .008). This relationship persisted after controlling for age, gender, diagnosis, and severity of depressive symptoms.
Conclusion
Greater nocturnal wakefulness, particularly in the early morning hours, was significantly associated with next-day suicidal thoughts. PSG-documented sleep disruption at specific times of night may represent an acute risk factor of suicidal ideation that warrants additional research.
Clinical Trials Identifier
Keywords: suicide, depression, sleep, polysomnography (PSG)
Introduction
Every year, nearly 900,000 individuals worldwide die by suicide1. Recent epidemiological and clinical research has focused on insomnia, nightmares, and other sleep disturbances as potentially modifiable risk factors for suicide. This association between sleep disturbance and suicidal thoughts, behaviors, and death has been demonstrated in large epidemiological and national samples2, 3 and in veterans and military service members4. In one analysis, the relationship between sleep disturbance and suicidal thoughts persisted when controlling for shared genetic and environmental influences in adolescent monozygotic twins5. Both a meta-analysis6 and a systematic review7 noted that subjective sleep disturbances, including insomnia and nightmares, are evidence-based risk factors for suicidal thoughts, behaviors, and death, independent of depressed mood. This relationship is further supported by results from the National Violence Death Reporting System demonstrating that suicide deaths are most likely to occur between midnight and 4:59 AM when accounting for the proportion of the population who is awake, with a peak incidence of death in the 2:00–2:59 hour8. These promising epidemiological and clinical results have led to ongoing clinical trials investigating the use of sleep treatments—both pharmacologic and psychotherapeutic—for potential use with suicidal patients (see NCT01770587 and NCT01689909).
Despite findings from self-reported data, relatively few analyses have investigated potential time-related factors over the course of the night that may underlie the relationship between impaired sleep and suicide. Studies of sleep architecture using polysomnography (PSG) suggest that percentage of increased rapid eye movement (REM) sleep, REM activity, or REM duration are all associated with suicidal thoughts or behaviors in depressed adults, depressed young adults, and psychotic/schizophrenia patients9, 10. PSG-assessed decreased sleep efficiency has also been associated with number of lifetime suicide attempts10. Other investigations have suggested that altered chronobiological activity is related to both suicidal thoughts11 and deaths12. The EEG sleep literature and systematic investigations evaluating time of day effects on suicide risk are relatively sparse, however, and analyses often do not adjust for depressive symptoms or use current suicidal thoughts as an outcome measure. Further evaluation of the epidemiologically and clinically reported link between “sleep disturbances” and current suicidal ideation, using techniques such as PSG that can detect temporal variations in wakefulness over the course of the night, is warranted. Such analyses can evaluate the extent of sleep disruption and its temporal distribution over the course of the night to assess etiology and guide the development of targeted treatments. Analyses can also be used to guide future research into biological mechanisms for the relationship between sleep and suicide risk, including changes in cortisol, melatonin, or clock gene expression.
Given the existing literature linking sleep disturbance and suicide, this study explored the extent to which EEG-assessed sleep disturbance may serve as an acute risk factor of suicidal thoughts among a sample with treatment-resistant major depressive disorder (MDD) or bipolar disorder (BD). We hypothesized that whole-night wakefulness after sleep onset, assessed via PSG as a physiological correlate of disturbed sleep, would be associated with suicidal thoughts the next morning. We also investigated variation in wakefulness by hour from midnight to 4:59 AM, due to the potential importance of the midnight to 4:59 AM time period as a high-risk time for suicide death8. We further hypothesized that the relationship between sleep disturbance and suicidal thoughts would occur independently of the severity of depressive symptoms, in line with previous meta-analyses and systematic literature reviews. The main strength of this approach is the use of PSG to measure wakefulness, which allows us to accurately detect wakefulness as well as its temporal variation over the course of the night13. Current suicidal ideation was chosen as the primary outcome because acute suicidal thoughts are a clinical psychiatric emergency and because of its relationship with future suicidal behavior14. Given the relationship between REM sleep and suicidal thoughts and behaviors9, 10, we also performed this analysis using minutes of REM sleep to identify potential differences in sleep architecture between suicidal ideators and non-ideators.
Method
Participants
Sixty-five participants (ages 18–65) with treatment-resistant depression, evaluated between 2006 and 2013, were included in the study (see Table 1 for demographic information). All participants had been admitted to the NIMH Experimental Therapeutics and Pathophysiology Branch in Bethesda, MD, USA and provided written informed consent. The NIH Combined Central Nervous System (CNS) Institutional Review Board approved this protocol (NCT00024635). Diagnoses of MDD or BD were confirmed via Structured Clinical Interview for Axis I Diagnostic and Statistical Manual (DSM)–IV Disorders, patient version (SCID-P)15. Participants were required to meet criteria for a moderately severe depressive episode (defined as 18 on the Hamilton Depression Rating Scale (HAM-D-17)16, or 20 on the Montgomery-Asberg Depression Rating Scale (MADRS))17. To qualify as treatment-resistant, they must not have responded to at least two previous adequate antidepressant trials. The MDD participants were medication-free for at least two weeks prior to PSG; the BD participants received only lithium or valproate at therapeutic levels. No participants met criteria for active substance use disorder for the three months prior to screening, but were permitted use of nicotine and caffeine. Participants were not excluded for insomnia symptoms, but all had a primary mood disorder diagnosis. Sleep disorders, such as sleep apnea or restless leg syndrome, were not exclusion criteria for the study, unless the participant was not able to withdraw from their treatment (i.e. they were not able stop their medications or use of a CPAP for the duration of the study). Participants were determined to be in good physical health as assessed by medical history, physical examination, blood labs, electrocardiogram (ECG), chest x-ray, urinalysis, and toxicology. Females were excluded if they were pregnant or nursing.
Table 1.
Demographic and Clinical Characteristics of Participant Sample by Suicidal Ideation
| Total Sample (n= 65) | Ideators HAM-D suicide item: >0 (n = 39) | Non-Ideators HAM-D suicide item: 0 (n = 26) | |||
|---|---|---|---|---|---|
| N(%) | N(%) | N(%) | X2 | p | |
|
| |||||
| Male Gender | 30(46) | 22(56) | 8(31) | 4.13 | .04 |
| Lifetime History of Suicide Attempt | 27(42) | 22(56) | 5(14) | 8.88 | .003 |
| Bipolar I or II Diagnosis | 24(37) | 9(23) | 15(42) | .20 | .75 |
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | t | p | |
|
| |||||
| Age | 43.8(12.7) | 44.0(13.1) | 43.5(12.3) | −.13 | .89 |
| Illness duration | 25.5(11.8) | 25.8(12.3) | 25.1(12.3) | −.23 | .82 |
| Depression/Sleep HAM-D Items | |||||
| Early Insomnia | 1.2(.9) | 1.1(.9) | 1.2(.9) | .55 | .58 |
| Middle Insomnia | 1.0(.8) | 1.0(.8) | 1.1(.7) | .53 | .60 |
| Late Insomnia | .6(.8) | .7(.8) | .6(.7) | −.46 | .65 |
| Remaining HAM-D Items | 17.6(3.2) | 18.3(3.2) | 16.4(2.7) | −2.56 | .01 |
Abbreviations: HAM-D: Hamilton Depression Rating Scale.
Most participants were consented into the study and then underwent a medication taper before the PSG. They were not eligible for initial consent if they had acute suicidal thoughts (a score of 4 or more on the MADRS item 10 or as determined by clinical judgement). Thus, acutely suicidal individuals were not consented into study and then withdrawn from their medications. Individuals who developed suicidal thoughts over the course of the medication taper or study procedures were not systematically excluded from participation.
Electroencephalography (EEG) Procedure and Analysis
Participants completed whole-night sleep recordings on the night before clinical ratings; they were adapted on a prior night to the sleep room and equipment. Participants were permitted to sleep any time after 10:00 PM with a final wake-up time of 7:00 AM. Within this window, participants self-selected their bedtime and communicated the time of lights off to an attending staff member. Naps were not permitted on days of electroencephalography (EEG) recordings. Participants were inpatients on a voluntary psychiatric unit on the days before and after EEG recordings.
A Nihon–Kohden system (Neurofax v. 05–50) and Polysmith Acquisition and Review software (v. 4.0.25.0) were used to record EEGs (C3/A2 and C4/A1), electrooculograms (EOGs) to monitor eye movements, and submental electromyograms (EMGs) to monitor muscle activity. EEG electrodes were placed according to the EEG 10–20 montage (Central 3, 4 and Frontal 3, 4). EEG readings were scored in 30-second epochs by a reviewer blind to participant diagnosis, clinical ratings, and study purpose18.
For this analysis, three variables were used as general measures of disrupted sleep over the entire night, as determined by PSG: 1) Total Sleep Time (TST); 2) Wake After Sleep Onset (WASO; minutes of wakefulness after falling asleep); and 3) Sleep Efficiency (SE; percentage of time asleep to time spent in bed). In order to examine wakefulness over the course of the night, 30-second epochs were binned by clock hour. For each hour (e.g. from 1:00:00 to 1:59:30 AM), a total sum of minutes awake was calculated for each participant. For the purposes of this analysis, this sum is termed “minutes awake” or “nocturnal wakefulness” in order to distinguish it from WASO, which includes the entire night and is not connected to a specific time. Minutes of REM sleep per hour were also calculated using the same method to assess whether REM distribution across the night, or REM fragmentation, was associated with next-day suicidal ideation.
Assessment
Rating instruments to assess suicidal ideation and depressive symptoms were administered on the morning after EEG recordings were completed. Participants were administered the HAM-D, which includes one item that evaluates suicidal thoughts and actions on a scale from 0 to 4 (Item 3)16. The HAM-D also includes several items of subjective sleep quality: early insomnia, middle insomnia, and late insomnia (Items 6, 7, and 8). To evaluate correspondence between objective and subjective sleep items, these sleep items were incorporated into analyses for comparison. The remaining HAM-D items (with suicide or sleep items removed) were used to measure severity of depressive symptoms to avoid collinearity and to be consistent with past research2, 19, 20.
The MADRS, which includes a suicide item rated from 0 to 6 (Item 10)17, and the Scale for Suicide Ideation (SSI)21 were also administered and included in analyses for confirmatory purposes. The SSI includes a five-item screen; a participant must receive a specific score before the remaining 14 items are administered. For the purposes of this analysis, only the first five items were included, as they were consistently administered to all participants. The SSI was administered to 61 of the 65 participants.
Statistical Analysis
Univariate analyses were used to compare the baseline characteristics of participants who reported suicidal ideation (a HAM-D score on the suicide item of 1 or greater) against those who reported no suicidal ideation. Spearman correlations evaluated the relationship between suicidal ideation, the whole-night PSG sleep variables TST (minutes), WASO (minutes), and SE (%), and subjective HAM-D sleep variables (early, middle, and late insomnia) due to the non-normal distribution of sleep variables in the study sample. The HAM-D suicide item was considered the primary measure of suicidal ideation because it is commonly used in clinical trials of depression22 and because it was administered to all participants; the MADRS suicide item and the five-item SSI were included to confirm significant findings.
Due to the distribution of wakefulness, a negative binomial generalized linear mixed model with a log linking function was used to evaluate minutes awake by hour of night between individuals who did and did not report suicidal ideation the next day. The time for this model was limited to midnight to 4:59 AM due to the focus on wakefulness after sleep onset. Time, suicidal ideation, and the interaction between time and suicidal ideation were included in this model. A first-order autoregressive structure was used in accordance with Schwarz’s Bayesian criteria for best fit. A Satterthwaite approximation was used to determine degrees of freedom due to the smaller sample size. Bonferroni post-hoc tests were used to follow up significant omnibus effects. To manage violations of model assumptions, robust estimation of fixed effects and coefficients were used. This model was run again, adjusting for effects of key covariates, including age, gender, diagnosis (MDD vs. BD), and severity of depression (the HAM-D with suicide and sleep items removed) in separate models, in order to account for potential confounders of sleep time and suicidal thoughts. As a post-hoc analysis, the model was run with consecutive minutes awake as an outcome to determine if the sleep disturbance was due to multiple brief awakenings, as compared to more prolonged episodes of wakefulness. The model was also run with suicide attempts, rather than suicidal ideation, as a factor, to determine potential state vs. trait markers of sleep disturbance.
As another post-hoc analysis, the same model was run for the time period between 11:00 PM and 6:59 AM, which encompasses the majority of the time the participants were asleep, to ensure that findings were not due to the timeframe of interest. This model was then re-run with REM sleep as the outcome variable, given that REM episodes increase in frequency over the course of the night.
In addition, when times differed significantly between ideators and non-ideators, the relationship between sleep at this time point and suicidal ideation ratings the next morning was evaluated. This analysis took into account the range of suicidal thoughts on the next day. Due to the nonlinear distribution of the data, all variables were ranked and linear regression was conducted using these ranked data. This relationship was evaluated using severity of depression (HAM-D with suicide and sleep items removed) as a covariate. Similar analyses were run using the HAM-D sleep items as dependent variables. All statistics were conducted with SPSS version 21 (IBM Corp). Significance was considered at p<.05, two-tailed.
Results
Initial descriptive statistics comparing individuals with and without suicidal ideation (as measured by the HAM-D suicide item) are presented in Table 1. Participants reporting suicidal ideation were more likely to be male (p = .04), have a lifetime history of suicide attempt (p = .003), and report a higher number of depressive symptoms (p = .01). Of the 39 participants who reported suicidal ideation, six received a score of 3 on the HAM-D suicide item (“suicidal ideas or gestures”). As threshold values for insomnia symptoms, 51% of the sample (n = 33) reported early insomnia (HAM-D item: “complains of nightly difficulty falling sleep”), 29% (n = 19) reported middle insomnia (HAM-D item: “waking during the night –any getting out of bed (except to void)”), and 17% (n = 11) reported late insomnia (HAM-D item: “unable to fall asleep again if gets out of bed”).
Whole Night Analyses
Table 2 displays correlations of measures of suicidal ideation with overall sleep variables over the course of the night, both objective and reported; WASO (r = .28 to .30, p < .05) and SE (r = −.26 to −.28, p .05) were significantly correlated with suicidal ideation. An additional analysis of the relationship between subjective sleep measures from the HAM-D and suicidal ideation was not significant.
Table 2.
Spearman Correlations between EEG Sleep Variables, Next-Day Suicidal Ideation, Reported Sleep, and Depression
| Suicidal Ideation
|
Reported Sleep and Depression
|
||||||
|---|---|---|---|---|---|---|---|
| HAM-D Suicide Item | MADRS Suicide Item | SSI 5-item | HAM-D Early Insomnia | HAM-D Middle Insomnia | HAM-D Late Insomnia | Remaining HAM-D items | |
|
|
|
||||||
| Total Sleep Time | −.05 | −.03 | −.08 | −.24 | −.16 | −.14 | .02 |
|
|
|
||||||
| Wake After Sleep Onset | .30* | .29* | .28* | .13 | .30* | .19 | −.08 |
|
|
|
||||||
p < .05, two-tailed.
HAM-D: Hamilton Depression Rating Scale; MADRS: Montgomery-Asberg Depression Rating Scale; SSI: Scale for Suicide Ideation; Wake After Sleep Onset: minutes of wakefulness after falling asleep
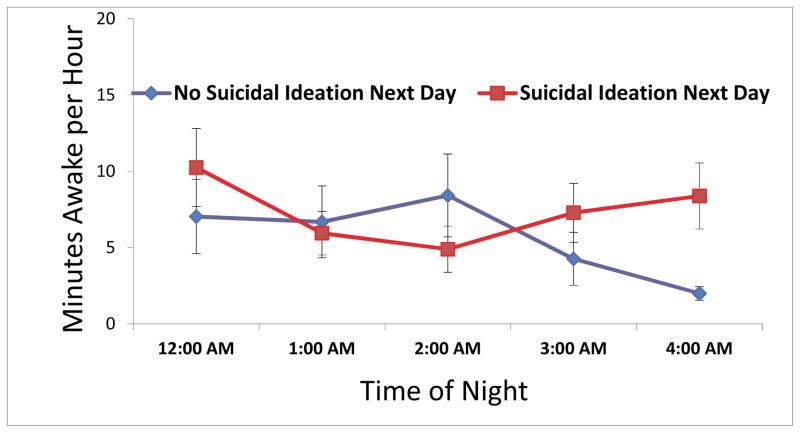
Hour-by-Hour Analyses from midnight to 4:59 AM
Figure 1 displays the minutes awake by hour from midnight to 4:59 AM in participants with and without next-day suicidal ideation. In the negative binomial mixed model of total time awake by hour from midnight to 4:59 AM, the main effect of ideation was not significant (F(1,47) = 1.27, p = .27), but a significant time-by-ideation interaction was observed (F(4,136) = 3.65, p = .007; Figure 2). Post-hoc analyses revealed significant differences in minutes awake between ideators and non-ideators from 4:00 to 4:59 AM (F(1,257) = 4.36, p = .004). Specifically, results revealed that increased nocturnal wakefulness during this clock hour was significantly associated with next-day suicidal ideation. Importantly, this time-by-ideation interaction remained significant in each model when controlling for central covariates: gender (F(4,137) = 3.68, p = .007), age (F(4,138) = 3.67, p = .007), diagnosis (F(4,136) = 3.52, p = .009), and severity of depressive symptoms (F(4, 112) = 3.48, p = .010).
Figure 1.
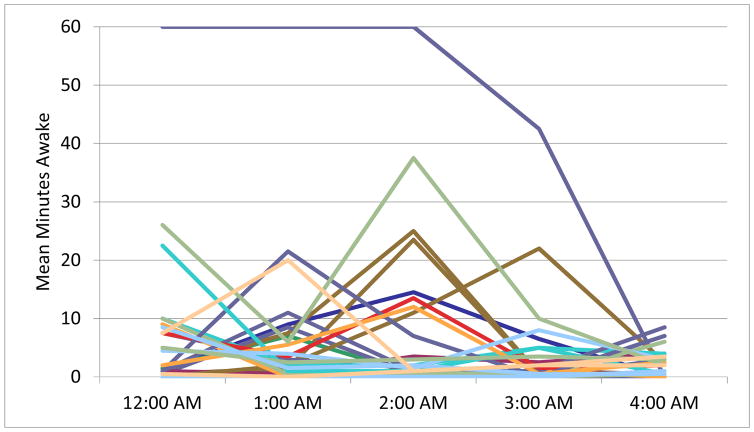
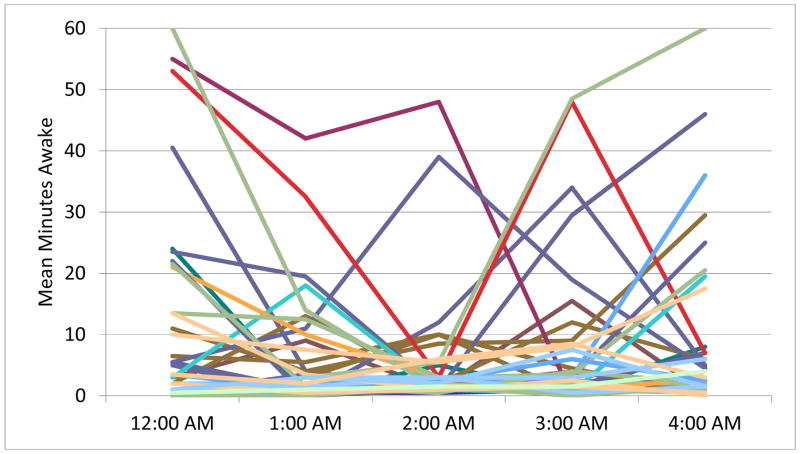
Polysomnography (PSG) documented minutes awake per hour. Each line represents the sleep of one participant. Figure 1A depicts the sleep of participants who reported no suicidal ideation the next day. Figure 1B depicts participants who reported suicidal ideation the next day. Time periods represent the entire hour of wakefulness (i.e. midnight is equivalent to 00:00 to 00:59).
Figure 2.
Average time awake from midnight to 4:00 AM, by ideation or no ideation the next day.
*p < .05, two-tailed.
Post-Hoc Analyses of Consecutive Minutes Awake and Suicide Attempts
When the model was run with consecutive minutes awake as the outcome variable, the time by ideation interaction was significant (F(4,96) = 5.40, p = .001), with a significant difference at the 4:00 to 4:59 AM hour.
When the model for minutes awake (not consecutive minutes awake) was re-run with suicide attempt history as a variable instead of suicidal thoughts, results were not significant for effect of suicide attempt history (F(1,65) = 0.31, p = .58) or time by attempt interaction (F(4, 146) = 2.25, p = .07).
Hour-by-Hour Analyses from 11:00 PM to 6:59 AM
When a similar mixed model was run using a timeframe from 11:00 PM to 6:59 AM, the time-by-ideation interaction remained significant (F(7, 185) = 4.13, p < .001), with a significant difference in minutes awake between ideators and non-ideators from 4:00 to 4:59 AM (F(1, 167) = 8.33, p = .004). The main effect of ideation on minutes awake was not significant. In a model evaluating minutes spent in REM sleep over the same extended timeframe, neither the main effect of suicidal ideation nor the time-by-ideation interaction was significant (p > .05). As a result, no further analyses were pursued using REM sleep.
Linear Regression of Wakefulness in 4:00 to 4:59 AM Hour
On linear regression of ranked variables, time spent awake in the 4:00 to 4:59 AM hour predicted suicidal ideation the next day using the HAM-D suicide item (standardized β = .31, p = .008) when controlling for severity of depressive symptoms as measured by the remaining HAM-D items. Similar results were found for the suicide item from the MADRS (standardized β = .31, p = .01), as well as the five-item SSI (standardized β = .30, p = .02), also controlling for severity of depressive symptoms. Wakefulness from 4:00–4:59 AM was not correlated with reported next-day HAM-D-assessed subjective sleep disturbances, such as early (standardized β =-.18, p = .16) or middle (standardized β = .19, p = .13) insomnia; however, results for late insomnia symptoms approached significance (standardized β = .22, p = .08).
Discussion
In an evaluation of individuals with both MDD and BD, participants with next-day suicidal ideation demonstrated a different pattern of nocturnal wakefulness than those without suicidal ideation. Specifically, nocturnal wakefulness during the 4:00 to 4:59 AM hour was associated with suicidal ideation the next day. This relationship persisted across multiple measures of suicidal thoughts and remained even after adjusting for age, gender, diagnosis, and severity of depressive symptoms. The results emphasize the importance of both reported and physiological approaches when evaluating this relationship; they also uncerscore the potential use of PSG sleep parameters as pathophysiological indicators of disturbed sleep and as acute risk factors for suicidal ideation. While the use of PSG will not be a feasible or appropriate monitor of acute suicide risk, these results can help clinicians conduct suicide risk assessments with their patients. The results also inform future research investigating possible biological mechanisms (discussed below) underlying the relationship between sleep disturbance and suicide.
Our results indicate that disrupted sleep and time spent awake, particularly in the early morning hours, may be a potential indicator of suicidal ideation. This may be associated with fundamental aspects of sleep/wake regulation, which may impact risk for suicidal thoughts and symptoms. Although nocturnal wakefulness is a correlate of insomnia, 4:00–6:00 AM corresponds to the human core body temperature nadir and the highest degree of circadian propensity for sleep23. It is possible that being awake during this time could perpetuate changes in next-day mood regulation, executive function, or risk for suicidal thoughts. Interestingly, REM sleep, which is hypothesized to maintain affective homeostasis in the brain, did not differ overnight in participants with and without suicidal ideation. Further analyses are warranted to replicate this finding and investigate other potential neurobiological factors that may define this subgroup. In particular, biological markers such as cortisol have been linked to suicide risk24; it is possible that cortisol changes over the course of the night could be related to the relationship between sleep and suicide. Furthermore, recent analyses of clock genes have found altered circadian patterns of clock gene expression in individuals with MDD compared to heathy controls25. Future work is indicated to investigate whether clock gene expression contributes to suicide risk.
Sleep restriction and deprivation studies highlight the confluence of symptoms that may arise when an individual has insufficient sleep. One functional magnetic resonance imaging (fMRI) study of healthy controls found that restricted sleep (four hours per night over five days) was associated with increased anxiety and amygdala activity in response to fearful faces26. Sleep loss in healthy controls has also been associated with disinhibition, including altered brain activity in the nucleus accumbens in response to risky decision-making27, as well as increased negative affect in response to mild stress28. Each of these clinical outcomes has been associated with suicidal behavior29.
Paradoxically, 36 hours of complete or partial sleep deprivation is a well-known, rapid-acting treatment for depressive symptoms30. Sleep deprivation in conjunction with lithium and light therapy has also been demonstrated to rapidly reduce suicidal thoughts in BD patients31 but, to our knowledge, no systematic studies of the ability of sleep deprivation alone to reduce suicidal thoughts has been conducted32. Even with these seemingly contradictory findings, the sleep deprivation literature in both healthy volunteers and depressed patients underscores the importance of sleep in suicide risk and the potential of sleep-focused interventions for the treatment of suicidal patients.
These results are consistent with previous research implicating disrupted sleep in suicide risk, and suicidal thoughts in particular2–4. Strengths of our current approach include studying a sample of treatment-resistant depressed participants who were assessed for depressive symptoms and suicidal thoughts using multiple measures the morning after EEG sleep recording. We were able to assess current suicidal thoughts as well as subjective measures of sleep quality. PSG recordings were scored by a reviewer blind to participant diagnosis, clinical ratings, and study purpose, ensuring the objectivity of the recordings. Other strengths include the fact that participants were medication-free (for MDD) or on minimal psychotropics (valproate or lithium) for BD, thus minimizing the confounding effects that drugs may have on sleep architecture parameters.
Nevertheless, the study is also associated with some limitations. First, the study excluded individuals at acute risk of suicide; additional analyses are indicated in an acutely suicidal sample. Second, suicidal thoughts can occur outside the context of a depressive episode. Third, the findings may not necessarily apply to patients with non-treatment-resistant depression. Fourth, other components of sleep architecture, such as REM latency and slow-wave sleep, may also influence the relationship between sleep and suicide. Fifth, analyses may have benefited from formal diagnoses of DSM-V Insomnia Disorder or other sleep disorders to evaluate any possible relationships between these diagnoses and the results. Sixth, it is possible that the relationship between sleep disturbance and suicidal thoughts may be mediated by clinical factors, such as melancholic depression, which has been linked to suicide risk33, as well as disinhibition; further analyses are indicated to identify potential mediators or mechanisms. Finally, prospective analyses of the relationship between wakefulness over the night and suicidal ideation are warranted to replicate findings and further explore how and why sleep disruptions lead to suicidal thoughts.
In summary, we found that wakefulness in the early morning hours, particularly 4:00 to 4:59 AM, was associated with next-day suicidal thoughts in a treatment-resistant combined sample of participants with MDD or BD. This highly specific time-dependent finding emerged even after controlling for severity of depressive symptoms, suggesting that risk may be conferred relatively independently of depressed mood. This is the first known report to evaluate PSG sleep parameters as an acute indicator of risk for next-day suicidal ideation. The findings highlight particular night-time intervals that may enhance risk detection and inform potential intervention targets for the prevention and treatment of suicidal ideation.
Clinical Points.
Sleep problems have been associated with suicide risk over the long-term, but may also represent an acute risk factor for suicide.
Wakefulness over the course of the night, particularly around 4:00 AM, was associated with increased suicidal thoughts the next morning in depressed patients.
Sleep problems may be an important element of suicide risk assessment.
Acknowledgments
Financial Support: Funding for this work was provided in part by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH) (NCT00024635; 04-M-0222), by a NARSAD Independent Investigator to CAZ, by a Brain & Behavior Mood Disorders Research Award to CAZ, and by grants from the National Institutes of Health (K23MH093490) to RAB. The NIMH, NIH, NARSAD, and the Brain & Behavior Research Foundation had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
The authors thank the 7SE research unit and staff for their support. Ioline Henter, MA (NIMH) provided invaluable editorial assistance.
Footnotes
Declaration of Interest: Dr. Zarate is listed as a co-inventor on a patent for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the US government but will share a percentage of any royalties that may be received by the government. The NIMH has filed a use patent for the use of scopolamine in the treatment of depression, and Dr. Furey is identified as a co-inventor on this pending patent application in the US and an existing patent in Europe. This work was completed while Dr. Furey was a staff scientist at the National Institute of Mental Health; she is now a full-time employee at Janssen Pharmaceuticals, Neuroscience Research and Development, La Jolla, CA. All other authors have no conflict of interest to report, financial or otherwise.
References
- 1.World Health Organization. Preventing Suicide: A Global Imperative. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 2.Bernert RA, Turvey CL, Conwell Y, Joiner TE., Jr Association of poor subjective sleep quality with risk for death by suicide during a 10-year period: a longitudinal, population-based study of late life. JAMA Psychiatry. 2014;71:1129–1137. doi: 10.1001/jamapsychiatry.2014.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunnell D, Chang SS, Tsai MK, Tsao CK, Wen CP. Sleep and suicide: an analysis of a cohort of 394,000 Taiwanese adults. Soc Psychiatry Psychiatr Epidemiol. 2013;48(9):1457–1465. doi: 10.1007/s00127-013-0675-1. [DOI] [PubMed] [Google Scholar]
- 4.Pigeon WR, Britton PC, Ilgen MA, Chapman B, Conner KR. Sleep disturbance preceding suicide among veterans. Am J Public Health. 2012;102(Suppl 1):S93–97. doi: 10.2105/AJPH.2011.300470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matamura M, Tochigi M, Usami S, et al. Associations between sleep habits and mental health status and suicidality in a longitudinal survey of monozygotic twin adolescents. J Sleep Res. 2014;23(3):290–294. doi: 10.1111/jsr.12127. [DOI] [PubMed] [Google Scholar]
- 6.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- 7.Bernert RA, Kim JS, Iwata NG, Perlis ML. Sleep disturbances as an evidence-based suicide risk factor. Curr Psychiatry Rep. doi: 10.1007/s11920-015-0554-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlis ML, Chaudhary NS, Grandner MA, et al. When accounting for wakefulness, completed suicides ehibit an increased likelihood during circadian night SLEEP 2014. The 28th Annual Meeting of the Associated Professional Sleep Societies, LLC (APSS); Minneapolis, MN. 2014. [Google Scholar]
- 9.Agargun MY, Cartwright R. REM sleep, dream variables and suicidality in depressed patients. Psychiatry Res. 2003;119(1–2):33–39. doi: 10.1016/s0165-1781(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 10.Sabo E, Reynolds CF, 3rd, Kupfer DJ, Berman SR. Sleep, depression, and suicide. Psychiatry Res. 1991;36(3):265–277. doi: 10.1016/0165-1781(91)90025-k. [DOI] [PubMed] [Google Scholar]
- 11.Verkes RJ, Kerkhof GA, Beld E, Hengeveld MW, van Kempen GM. Suicidality, circadian activity rhythms and platelet serotonergic measures in patients with recurrent suicidal behaviour. Acta Psychiatr Scand. 1996;93(1):27–34. doi: 10.1111/j.1600-0447.1996.tb10615.x. [DOI] [PubMed] [Google Scholar]
- 12.Hiltunen L, Suominen K, Lonnqvist J, Partonen T. Relationship between daylength and suicide in Finland. J Circadian Rhythms. 2011;9:10. doi: 10.1186/1740-3391-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 14.Kuo WH, Gallo JJ, Tien AY. Incidence of suicide ideation and attempts in adults: the 13-year follow-up of a community sample in Baltimore, Maryland. Psychol Med. 2001;31(7):1181–1191. doi: 10.1017/s0033291701004482. [DOI] [PubMed] [Google Scholar]
- 15.First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV TR Axis I Disorders, Research Version, Patient Edition. New York: New York State Psychiatric Institute, Biometrics Research; 2001. [Google Scholar]
- 16.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 18.Rechtschaffen AKA. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Washington, D.C: 1968. [DOI] [PubMed] [Google Scholar]
- 19.Wong MM, Brower KJ. The prospective relationship between sleep problems and suicidal behavior in the National Longitudinal Study of Adolescent Health. J Psychiatr Res. 2012;46:953–959. doi: 10.1016/j.jpsychires.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCall WV, Blocker JN, D'Agostino RJ, et al. Insomnia severity is an indicator of suicidal ideation during a depression clinical trial. Sleep Med. 2010;11:822–827. doi: 10.1016/j.sleep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47(2):343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 22.Desseilles M, Perroud N, Guillaume S, et al. Is it valid to measure suicidal ideation by depression rating scales? J Affect Disord. 2012;136(3):398–404. doi: 10.1016/j.jad.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Lack LC, Lushington K. The rhythms of human sleep propensity and core body temperature. J Sleep Res. 1996;5:1–11. doi: 10.1046/j.1365-2869.1996.00005.x. [DOI] [PubMed] [Google Scholar]
- 24.Chatzittofis A, Nordstrom P, Hellstrom C, Arver S, Asberg M, Jokinen J. CSF 5-HIAA cortisol and DHEAS levels in suicide attempters. Eur Neuropsychopharmacol. 2013;23:1280–1287. doi: 10.1016/j.euroneuro.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Li JZ, Bunney BG, Meng F, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci USA. 2013;110:9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motomura Y, Kitamura S, Oba K, et al. Sleep debt elicits negative emotional reaction through diminished amygdala-anterior cingulate functional connectivity. PLoS One. 2013;8(2):e56578. doi: 10.1371/journal.pone.0056578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatraman V, Chuah YM, Huettel SA, Chee MW. Sleep deprivation elevates expectation of gains and attenuates response to losses following risky decisions. Sleep. 2007;30(5):603–609. doi: 10.1093/sleep/30.5.603. [DOI] [PubMed] [Google Scholar]
- 28.Minkel JD, Banks S, Htaik O, et al. Sleep deprivation and stressors: evidence for elevated negative affect in response to mild stressors when sleep deprived. Emotion. 2012;12(5):1015–1020. doi: 10.1037/a0026871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Heeringen K. Stress-diathesis model of suicidal behavior. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press, LLC; 2012. [Google Scholar]
- 30.Bunney BG, Bunney WE. Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms. Biol Psychiatry. 2013;73:1164–1171. doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Benedetti F, Riccaboni R, Locatelli C, Poletti S, Dallaspezia S, Colombo C. Rapid treatment response of suicidal symptoms to lithium, sleep deprivation, and light therapy (chronotherapeutics) in drug-resistant bipolar depression. J Clin Psychiatry. 2014;75(2):133–140. doi: 10.4088/JCP.13m08455. [DOI] [PubMed] [Google Scholar]
- 32.Sahlem GL, Kalivas B, Fox JB, et al. Adjunctive triple chronotherapy (combined total sleep deprivation, sleep phase advance, and bright light therapy) rapidly improves mood and suicidality in suicidal depressed inpatients: An open label pilot study. J Psychiatr Res. 2014 doi: 10.1016/j.jpsychires.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grunebaum MF, Galfalvy HC, Oquendo MA, Burke AK, Mann JJ. Melancholia and the probability and lethality of suicide attempts. Br J Psychiatry. 2004;184:534–535. doi: 10.1192/bjp.184.6.534. [DOI] [PubMed] [Google Scholar]